Abstract
The aim of this study was to evaluate the association between postural orthostatic tachycardia syndrome (POTS) and circulating antiganglionic acetylcholine receptor (gAChR) antibodies. We reviewed clinical assessments of Japanese patients with POTS, and determined the presence of gAChR antibodies in serum samples from those patients. Luciferase immunoprecipitation systems detected anti‐gAChR α3 and β4 antibodies in the sera from POTS (29%). Antecedent infections were frequently reported in patients in POTS patients. Moreover, autoimmune markers and comorbid autoimmune diseases were also frequent in seropositive POTS patients. Anti‐gAChR antibodies were detectable in significant number of patients with POTS, and POTS entailed the element of autoimmune basis.
Introduction
Orthostasis and fatigue with autonomic dysfunction frequently occur in autoimmune diseases.1 Postural orthostatic tachycardia syndrome (POTS) is the most common form of orthostatic intolerance characterized by lightheadedness, presyncope and palpitations, and nonorthostatic symptoms, such as fatigue, abnormalities of sleep, myofascial pain, nausea, and migraine headache.2 Gastrointestinal (GI) disturbances are common and prolonged in patients with POTS, and it remains unknown whether extravascular volume depletion and deconditioning contribute to POTS in patients with other autonomic symptoms.3, 4 The pathophysiology of POTS is heterogeneous and includes impaired sympathetically‐mediated vasoconstriction, excessive sympathetic drive, volume dysregulation, and deconditioning.2
An autoimmune basis has been suggested as a causal mechanism of POTS, and several autoreactive IgGs have been identified, including ganglionic acetylcholine receptor (gAChR), voltage‐gated potassium channel complex, cardiac lipid raft‐associated proteins, α1‐adrenergic receptor, as well as β1‐ and β2‐adrenergic receptors.5, 6, 7 Moreover, Blitshteyn recently determined the high prevalence of positive ANA and other autoantibodies and the high prevalence of comorbid autoimmune disorders that exist in patients with POTS.8, 9 Sandroni and Low demonstrated occasional positivity to gAChR antibodies in POTS patients.10 Previously, we reported the relationship among the anti‐gAChR antibodies, autonomic dysfunction, and autoimmune rheumatic diseases.11, 12, 13 We attempted to elucidate the autoimmune pathophysiology of POTS in this study as a part of our comprehensive approach. Therefore, we assessed the frequency of autonomic dysfunction in the patients with two highly prevalent orthostatic intolerances of POTS and neurally mediated syncope (NMS) using objective clinical indicators. And, we examined potential immune‐mediation of POTS by evaluating autoantibodies against gAChR, which have been implicated in both POTS and POTS‐associated autonomic dysfunction.
Patients and Methods
Standard protocol approvals, registrations, and patient consents
All participants provided written informed consent. The Ethics Committee of Nagasaki Kawatana Medical Center and Kumamoto University Hospital approved the study.
Study design
First analysis (Fig. 1A) tested for the presence of gAChR antibodies in serum samples from 34 patients with POTS, 19 patients with NMS, 34 patients with OND, and 73 healthy controls. The diagnosis of POTS and NMS were confirmed using the consensus statement by the American Autonomic Society, the European Federation of Autonomic Societies, the Autonomic Research Group of the World Federation of Neurology and the Autonomic Disorders section of the American Academy of Neurology, and 2015 heart rhythm society expert consensus statement.14, 15, 16 We compared the frequencies of autoantibodies against gAChR among four groups, and analyzed the clinical features between the POTS group and NMS group.
Figure 1.
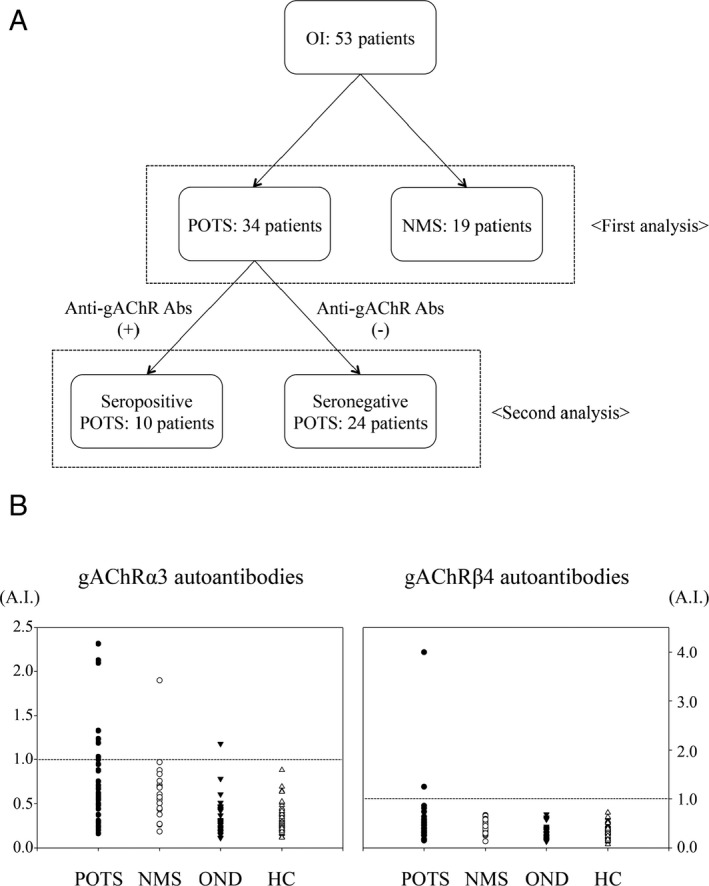
(A) Study design and participant information. Details regarding study design and participant recruitment for each subject group. OI, orthostatic intolerances; POTS, postural orthostatic tachycardia syndrome; NMS, neurally mediated syncope; anti‐gAChR Abs, antiganglionic acetylcholine receptor antibodies. (B) Anti‐gAChR α3 and β4 antibodies in sera from patients with postural orthostatic tachycardia syndrome (POTS). Anti‐gAChR α3 and β4 antibodies were assessed using luciferase immunoprecipitation system (LIPS) assays. The dotted line indicates the cut‐off. We tested sera from healthy controls (HC), as well as from patients with POTS and other neurologic diseases (OND). Autoantibodies against anti‐gAChR were detected in 29% (10 of 34) samples from patients with POTS.
In second analysis (Fig. 1A), we analyzed the clinical features between the seropositive and seronegative POTS group.
Study population
Serum samples from 53 patients with orthostatic intolerance were obtained from general and teaching hospitals throughout Japan between January 2012 and August 2016. The diagnosis of POTS and NMS were confirmed using the consensus statement on the definition of orthostatic hypotension, NMS, and POTS in 2011. Exclusion criteria are provided as Supplementary Information. The demographic data of 34 patients with POTS were as follows: median age, 22.2 ± 10.8 years old; male/female ratio, 10/24; onset age, 19.8 ± 10.8 years old. In addition, the demographic data of patients with 19 NMS were as follows: median age, 40.9 ± 23.9 years old; male/female ratio, 12/7; onset age, 38.4 ± 22.9 years old. Control groups included 34 patients with other neurological diseases (OND) with any autonomic symptoms (15 women, 19 men, mean age 56.3 years), and 73 healthy controls (HC) who donated blood for this study (42 women, 31 men, mean age 38.3 years).
Luciferase immunoprecipitation system assay for autoantibodies against gAChR
Serum gAChRα3 and β4 antibodies were detected using Luciferase immunoprecipitation system (LIPS) assays, as described previously.11 Antibody levels were expressed as the antibody index (A.I.). We established a normalized value for HC as <1.000 A.I. To evaluate the diagnostic accuracy of this assay, we verified the cut‐off points for all data collected in the previous study.12, 13
Clinical assessment of autonomic function
Comprehensive clinical, neurological, and serologic assessments were performed for all patients. We queried the presence or absence of each of the following functions that are controlled by the autonomic system as reported in our previous study: syncope or orthostatic hypotension (OH) for orthostatic intolerance (OI); arrhythmia; pupillary dysfunction; sicca complex; coughing episodes; skin dryness or hypohidrosis/anhidrosis for heat intolerance; upper GI system problems; diarrhea or constipation indicating dysfunction of the lower GI system; dysuria or urinary retention needing catheterization for bladder dysfunction; and sexual dysfunction.11 For the all patients, questionnaires were sent to the referring neurologists.
Statistical analyses
SigmaPlot® was used for data analyses. The normally distributed A.I. data were analyzed by one‐way analysis of variance between HC, POTS, NMS, and OND groups. Frequencies of autoantibodies and non‐normally distributed data were analyzed by one‐way analyses of variance of ranks. For all analyses, P < 0.05 was considered statistically significant.
Results
Frequencies of autoantibodies against gAChR in first analysis
Using the LIPS assay, we found that 29% (10 of 34) of patients with POTS were positive for autoantibodies. Specifically, anti‐gAChRα3 antibodies were detected in 8 samples (24%), and anti‐gAChRβ4 antibodies were detected in two samples (6%). Furthermore, no samples from the seropositive patients with POTS were positive for both antibodies. In contrast, we found that only one patient (5%) with NMS was positive for autoantibodies against gAChRα3. The frequencies of gAChRα3 antibodies in patients with POTS were statistically higher than those in patients with NMS (P = 0.041). However, no statistically significant difference was detected in between the POTS group and NMS group on the prevalence of anti‐gAChR antibodies (P = 0.479). In all 73 HC, anti‐gAChRα3 and anti‐gAChRβ4 antibodies were absent, and anti‐gAChRα3 antibodies were detected in the serum of one patient in the OND group, who had suspected amyloid neuropathy (Fig. 1B).
The mean A.I. value for anti‐gAChRα3 antibodies in the POTS group was 0.746, which was significantly greater than those obtained for the HC (0.305) or OND (0.336) groups (P < 0.001). The mean A.I. for anti‐gAChRβ4 antibodies in the POTS group was 0.536, which was also significantly greater than those obtained for the HC (0.367) or OND (0.302) groups (P = 0.007) (Fig. 1B). However, the mean A.I. value for anti‐gAChRα3 antibodies in the NMS group was 0.638, and the mean A.I. for anti‐gAChRβ4 antibodies in the NMS group was 0.421. No statistically significant difference was seen in between the POTS group and NMS group on the mean A.I. for anti‐gAChRα3 and gAChRβ4 antibodies (P = 0.774 and 0.744, respectively).
Clinical assessment of autonomic function in patients with POTS and NMS in first analysis
Table 1 summarizes the clinical characteristics and autonomic symptoms of patients with POTS and NMS. Age and age at onset in the patients with POTS were significantly younger than those in the patients with NMS (P = 0.004 and 0.013, respectively). Antecedent infections were reported in 10 patients (29%) in POTS group, and there was a difference between POTS group and NMS group (P = 0.010). Female was more frequently suffered in POTS group (24 patients, 71%) than in NMS group (7 patients, 37%) (P = 0.018).
Table 1.
Clinical features of patients with POTS and NMS
| POTS | NMS | P value | |
|---|---|---|---|
| Number of patients | 34 | 19 | |
| Age (year) | 22.2 ± 10.8 | 40.9 ± 23.9 | 0.004a |
| Age at onset (year) | 19.8 ± 10.8 | 38.4 ± 22.9 | 0.013a |
| Sex, female (%) | 24 (71) | 7 (37) | 0.018a |
| Anti‐gAChRα3 abs (A.I.) | 0.746 ± 0.549 | 0.638 ± 0.372 | 0.774 |
| Anti‐gAChRβ4 abs (A.I.) | 0.536 ± 0.653 | 0.421 ± 0.145 | 0.744 |
| Seropostive for anti‐gAChRα3 abs (%) | 8 (24) | 1 (5) | 0.041a |
| Seropostive for anti‐gAChRβ4 abs (%) | 2 (6) | 0 (0) | 0.479 |
| Onset (%)b |
Acute, subacute: 8 (24) Gradual: 26 (76) |
Acute, subacute: 5 (26) Gradual: 14 (74) |
0.832 |
| Antecedent infections (%) | 10 (29)c | 0 (0) | 0.010a |
| Orthostatic intolerance (%)d | 33 (97) | 19 (100) | 0.479 |
| Arrhythmia (%) | 6 (18) | 8 (42) | 0.901 |
| Pupil abnormalities (%) | 2 (6) | 0 (0) | 0.299 |
| Sicca complex (%) | 13 (38) | 5 (26) | 0.639 |
| Coughing episodes (%) | 1 (3) | 0 (0) | 0.479 |
| Heat intolerance and/or anhidrosis (%) | 14 (41) | 5 (26) | 0.289 |
| Upper gastrointestinal tract symptoms (%)e | 13 (38) | 4 (21) | 0.145 |
| Lower gastrointestinal tract symptoms (%)f | 14 (41) | 9 (47) | 0.674 |
| Bladder dysfunction (%) | 4 (12) | 2 (11) | 0.906 |
| Endocrine disorder complications (%) | 4 (12)g | 0 (0) | 0.129 |
| Autoimmune disease complications (%) | 8 (24)h | 4 (21)i | 0.979 |
P< 0.05
abs, antibodies.
Subacute onset was defined as the reaching of the peak of autonomic failure within 3 months, and chronic was defined as reaching of the peak after 3 months.
Mycoplasma pneumonia; four patients of flu‐like symptom (fever, throat, and joint pain); three patients of upper respiratory tract (URT) infection; gastroenteritis; trauma.
Orthostatic intolerance = orthostatic hypotension and/or palpitation and/or syncope.
Upper gastrointestinal tract symptoms = appetite loss and/or nausea and/or vomiting and/or early satiety and/or postprandial abdominal pain.
Lower gastrointestinal tract symptoms = constipation and/or diarrhea and/or ileus.
Four patients of Amenorrhea.
Three patients of antinuclear antibody positive; two patients of Graves’ disease; Sjögren's syndrome; allergic bronchitis; rheumatoid arthritis and fibromyalgia.
Ulcerative colitis; type I diabetes; Hashimoto's disease; IgG4‐related disorder.
Clinical assessment of autonomic function in patients with seropositive POTS in second analysis
Table 2 summarizes the clinical characteristics and autonomic symptoms of patients with POTS, according to seroreactivity to anti‐gAChR antibodies. The age in seropositive POTS group was significantly higher than those in seronegative POTS group (P = 0.012). As for the age at onset, similar trend was observed, but we found no statistical significance (P = 0.052). Antecedent infections were reported in five patients (50%) with seropositive POTS, but there were no differences between seropositive and seronegative patients. For autonomic symptoms, pupil abnormality and lower gastrointestinal tract symptoms were most frequently observed (P = 0.029 and 0.032, respectively). Other autoimmune diseases were more common in anti‐gAChR antibody‐positive patients than in those who were antibody‐negative (P < 0.001). Three of 10 patients in the group of seropositive POTS were treated with intravenous methylprednisolone (IVMP) and resulted in clinical improvements. In the group of seronegative POTS, four of 24 patients were treated with IVMP. Three patients maintained improvements in OI, however, we found no clinical improvement in one patient.
Table 2.
Clinical characteristics of patients with POTS
| Patients with POTS Anti‐gAChR Abs positive | Patients with POTS Anti‐gAChR Abs negative | P value | |
|---|---|---|---|
| Number of patients | 10 | 24 | |
| Age (year) | 28.5 ± 12.0 | 19.5 ± 9.3 | 0.012a |
| Age at onset (year) | 25.6 ± 12.7 | 17.4 ± 9.2 | 0.052 |
| Sex, female (%) | 8 (80) | 16 (68) | 0.458 |
| Onset (%) |
Acute, subacute: 3 (30) Gradual: 7 (40) |
Acute, subacute: 5 (21) Gradual: 19 (79) |
0.589 |
| Antecedent infections (%) | 5 (50)b | 5 (21)c | 0.099 |
| Orthostatic intolerance (%) | 10 (100) | 23 (96) | 0.561 |
| ΔHeart rate (/min) | 39.8 ± 10.4 | 46.8 ± 13.8 | 0.084 |
| Arrhythmia (%) | 1 (10) | 2 (8) | 0.917 |
| Pupil abnormalities (%) | 2 (20) | 0 (0) | 0.029a |
| Sicca complex (%) | 5 (50) | 8 (33) | 0.381 |
| Coughing episodes (%) | 1 (10) | 0 (0) | 0.138 |
| Heat intolerance and/or anhidrosis (%) | 2 (20) | 12 (50) | 0.116 |
| Upper gastrointestinal tract symptoms (%) | 6 (60) | 7 (29) | 0.101 |
| Lower gastrointestinal tract symptoms (%) | 7 (70) | 7 (29) | 0.032a |
| Bladder dysfunction (%) | 1 (10) | 3 (13) | 0.866 |
| Endocrine disorder complications (%) | 2 (20)d | 2 (8)e | 0.361 |
| Autoimmune disease complications (%) | 6 (60)f | 2 (8)g | 0.001a |
P< 0.05
Mycoplasma pneumonia; Three patients of flu‐like symptom (fever, throat, and joint pain); URT infection.
Trauma; gastroenteritis; flu‐like symptom (fever, throat, and joint pain); Two patients of URT infection.
Amenorrhea.
Amenorrhea.
Three patients of antinuclear antibody positive; Graves’ disease; Sjögren's syndrome; rheumatoid arthritis; and fibromyalgia.
Graves’ disease; allergic bronchitis.
Discussion
In this study, we assessed presence or absence of autoantibodies against gAChR in patients with POTS and NMS. We found that anti‐gAChR antibodies were detected more frequently in patients with POTS compared to NMS and controls, suggesting that anti‐gAChR antibodies may be associated with POTS and its underlying autonomic dysfunction. POTS and NMS were clearly distinguishable by the prevalence of anti‐gAChR antibodies. On the basis of the acknowledgment, we verified widespread autonomic manifestations based on neurological assessments, and identified other autoimmune complications in POTS patients with seropositive for anti‐gAChR antibodies. However, the majority of seropositive samples did not have high levels of autoantibodies for gAChR. Therefore, elevated expression of antibodies to gAChR subunits may contribute to secondary autoimmune responses to ganglionic neuron damage in seropositive patients. The prevalence of anti‐gAChR antibodies in this study was higher than that in previous study.17 Anti‐gAChR antibodies may impair autonomic ganglionic synaptic transmission, and antibodies that interfere with ganglionic transmission may contribute to dysautonomia in patients with POTS.18 Although POTS is pathophysiologically categorized into four subtypes (neuropathic, hyperadrenergic, volume dysregulation, and physical deconditioning), it is not clear which subtype corresponds to gAChR autoimmunity‐related POTS, or whether anti‐gAChR antibodies have stimulatory functions that produce overactivity of sympathetic ganglia (i.e., type V hypersensitivity).2 Although POTS is a heterogeneous syndrome with various pathophysiologic mechanisms and complex symptomatology, we demonstrated POTS patients with autoimmune basis were present in significant proportions.2 In addition, we noted the possibility that anti‐gAChR antibodies are associated with autonomic dysfunction (e.g., POTS) in autoimmune rheumatic diseases.12, 13
This study is limited by an observational design and small sample size. Therefore, a subsequent study will be conducted in order to confirm the potential for immunotherapy to resolve the symptoms of autoimmune POTS and associated autonomic effects. A prospective, multicenter, clinical interventional study is necessary in order to confirm the relationships between levels of anti‐gAChR antibodies and the severity of POTS and autonomic symptoms.
In conclusion, we could demonstrate that the anti‐gAChR antibodies were detected more frequently in patients with POTS compared to NMS and controls. Seropositive POTS patients demonstrated autoimmune markers and comorbid autoimmune diseases were frequent. The results have important implications for our understanding of the pathophysiology of POTS and for the interpretation of humoral antibody responses in POTS.
Conflict of Interest
All authors have no disclosures to report.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Nakane had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. MW, SN, AM, HM, and YA conceived and designed the experiments. AM, YM (Yasuhiro Maeda), and OH performed the experiments. MW, SN, AM, MN, YM (Yukiko Mori), TM, KT, YK, and YA collected the samples. MW, SN, AM, MN, and YA analyzed the data. MW, SN, AM, HM, and YA wrote the paper.
Supporting information
Exclusion Criteria
(1) Pregnant or lactating women; (2) the presence of failure of other organ systems affecting the autonomic nervous system or the patient's ability to participate in the study; (3) concomitant therapy that may alter autonomic function; and (4) clinically significant coronary artery disease.
Acknowledgments
This study was supported by the Ministry of Health, Labor, and Welfare, Japan, and the Ministry of Education, Culture, Sports, Science, and Technology of Japan (JSPS KAKENHI Grant Number 25461305 and 16K09695).
Funding Statement
This work was funded by Japanese Ministry of Health, Labor, and Welfare grant ; Ministry of Education, Culture, Sports, Science, and Technology of Japan grant ; JSPS KAKENHI grants 25461305 and 16K09695.
References
- 1. Streifler JY, Molad Y. Connective tissue disorders: systemic lupus erythematosus, Sjögren's syndrome, and scleroderma. Handb Clin Neurol 2014;119:463–473. [DOI] [PubMed] [Google Scholar]
- 2. Benarroch EE. Postural tachycardia syndrome: a heterogeneous and multifactorial disorder. Mayo Clin Proc 2012;87:1214–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology 1993;43:132–137. [DOI] [PubMed] [Google Scholar]
- 4. Loavenbruck A, Iturrino J, Singer W, et al. Disturbances of gastrointestinal transit and autonomic functions in postural orthostatic tachycardia syndrome. Neurogastroenterol Motil 2015;27:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang XL, Chai Q, Charlesworth MC, et al. Autoimmunoreactive IgGs from patients with postural orthostatic tachycardia syndrome. Proteomics Clin Appl 2012;6:615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang XL, Ling TY, Charlesworth MC, et al. Autoimmunoreactive IgGs against cardiac lipid raft‐associated proteins in patients with postural orthostatic tachycardia syndrome. Transl Res 2013;162:34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li H, Yu X, Liles C, et al. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc 2014;3:e000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blitshteyn S. Autoimmune markers and autoimmune disorders in patients with postural tachycardia syndrome (POTS). Lupus 2015;24:1364–1369. [DOI] [PubMed] [Google Scholar]
- 9. Dahan S, Tomljenovic L, Shoenfeld Y. Postural orthostatic tachycardia syndrome (POTS) – a novel member of the autoimmune family. Lupus 2016;25:339–342. [DOI] [PubMed] [Google Scholar]
- 10. Sandroni P, Low PA. Other autonomic neuropathies associated with ganglionic antibody. Auton Neurosci 2009;146:13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakane S, Higuchi O, Koga M, et al. Clinical features of autoimmune autonomic ganglionopathy and the detection of subunit‐specific autoantibodies to the ganglionic acetylcholine receptor in Japanese patients. PLoS ONE 2015;10:e0118312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maeda Y, Migita K, Higuchi O, et al. Association between anti‐ganglionic nicotinic acetylcholine receptor (gAChR) antibodies and HLA‐DRB1 alleles in the Japanese population. PLoS ONE 2016;11:e0146048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mukaino A, Nakane S, Higuchi O, et al. Insights from the ganglionic acetylcholine receptor autoantibodies in patients with Sjögren's syndrome. Mod Rheumatol 2016;12:1–40. [DOI] [PubMed] [Google Scholar]
- 14. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton Neurosci 2011;161:46–48. [DOI] [PubMed] [Google Scholar]
- 15. Sheldon RS, Grubb BP II, Olshansky B, et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015;12:e41–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jones PK, Shaw BH, Raj SR. Clinical challenges in the diagnosis and management of postural tachycardia syndrome. Pract Neurol 2016;16:431–438. [DOI] [PubMed] [Google Scholar]
- 17. Thieben MJ, Sandroni P, Sletten DM, et al. Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin Proc 2007;82:308–313. [DOI] [PubMed] [Google Scholar]
- 18. Wang Z, Low PA, Vernino S. Antibody‐mediated impairment and homeostatic plasticity of autonomic ganglionic synaptic transmission. Exp Neurol 2010;222:114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Exclusion Criteria
(1) Pregnant or lactating women; (2) the presence of failure of other organ systems affecting the autonomic nervous system or the patient's ability to participate in the study; (3) concomitant therapy that may alter autonomic function; and (4) clinically significant coronary artery disease.


