Abstract
Insomnia is prevalent in bipolar disorder (BD) even during periods of euthymic mood. We compared resting state brain activity and cognitive function between euthymic BD with and without insomnia, and secondarily to healthy individuals. BD patients with insomnia symptoms showed a significantly lower functional connectivity within the task-positive network, compared to those without insomnia. They also showed significantly slower cognitive processing speed. These two features of BD with insomnia appeared relatively independent of each other. Preliminary findings suggest that exploration of the mechanisms of sleep disturbance in BD could lead to improved understanding and treatment of inattention in BD.
Keywords: Bipolar disorder, Insomnia, Resting state
1. Introduction
Insomnia is prevalent in those with bipolar disorder (BD) and remains during euthymic periods (Harvey et al., 2005). Evidence suggests that sleep deprivation contributes to cognitive deficits, impulsivity, relapse, and quality of life (Harvey et al., 2009). Among those adverse outcomes, cognitive deficits are important for patients’ social and vocational functioning. Although the cognitive impairments found in persons with BD are often subtle, improving neuropsychological processing may dramatically improve psychosocial functioning in BD patients. However, the precise brain mechanisms underlying circadian rhythm disturbance and poor sleep in BD remain unclear, and very few studies consider sleep disturbance when interpreting imaging findings (McKenna and Eyler, 2012).
Resting-state functional connectivity (rsFC) measures the temporal correlation of spontaneous blood-oxygen-level-dependent (BOLD) signals between spatially remote brain regions during times when subjects are not performing attention-demanding cognitive tasks. Resting state analyses consistently identify two main networks: the default mode network (DMN), involved in self-referential processes including autobiographical memory; and the task positive network (TPN), involved in attentional control and behavioral response via the salience, dorsal attention, and ventral attention subnetworks. (Fox et al., 2005). Alterations in rsFC have been reported in both BD and insomnia.
Resting-state studies of BD suggest abnormal resting-state network function and connectivity, including aberrant DMN connectivity and mood state-related alterations in DMN and TPN. Ongur et al. (2010) reported that the left parietal cortex, left frontopolar cortex and left fusiform gyrus had significantly more coherence with the DMN network in manic and mixed state BD subjects. Another rsFC study showed a significant difference between euthymic BD and healthy controls (HC) in terms of connectivity between the medial prefrontal cortex and the right dorsolateral prefrontal cortex (Favre et al., 2014). Brady et al. (2017) reported greater rsFC between parietal, occipital, and frontal nodes within the dorsal attention network (DAN), one of the TPN, in mania compared to euthymic BD or HC, and hypoconnectivity between dorsal frontal nodes and the rest of the DMN in euthymic BD patients compared to manic BD subjects and HC. These findings suggest that some rsFC abnormalities in DMN and TPN might be related to BD pathogenesis; whereas, some changes in connectivity may be state-dependent.
Several resting-state functional connectivity studies have sought to determine whether patients with insomnia have connectivity alterations in the DMN or TPN. Li et al. (2014) reported that individuals with insomnia showed decreased functional connectivity within the DMN including the medial prefrontal cortex, the medial temporal lobe and the inferior parietal cortices. These are brain regions mediating attention and arousal. In addition, a structural connectivity study demonstrated reduced cortical thickness covariance between anterior and posterior regions of the DMN in patients with insomnia compared with good sleepers (Suh et al., 2016). Moreover, a study evaluating resting state networks in the setting of sleep deprivation found selective reductions in DMN functional connectivity and reduced anti-correlation between DMN and TPN (De Havas et al., 2012).
Several studies provide evidence that BD patients show specific cognitive deficits even during asymptomatic phases of illness. They exhibit cognitive impairments in executive function, attention span and verbal memory (Bostock et al., 2017). Executive function and cognitive processing speed, in particular, are closely related to daily functioning and quality of life (Tabares-Seisdedos et al., 2008). Altered connectivity in the TPN network is related to working memory and attention problems (Gordon et al., 2014), and DMN connectivity has also been related to cognitive performance (Anticevic et al., 2012). Therefore, we aimed to investigate whether euthymic BD patients with insomnia would show impairment in processing speed and in executive functions compared with BD patients without insomnia and healthy controls. We also examined if there was a relationship between insomnia-related rsFC alteration and cognitive function in euthymic BD. We hypothesized that intra- and inter-network functional connectivity of the DMN and TPN would be lower in euthymic BD with insomnia; and altered connectivity would underlie cognitive deficits.
2. Methods
The study included 52 subjects: 26 HC participants, 13 BD with no report of insomnia (BD), and 13 BD with insomnia (BD-IN). The study protocol was approved by the institutional review boards of the University of California, San Diego, and the San Diego Veterans Affairs Healthcare System. Patients were recruited both from the VA Hospital and from general community clinics and residences and using online advertisement. HC subjects were recruited by advertisements from the community. Written informed consent was obtained from all participants. All subjects were assessed by a trained rater using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (First, 1997). Patients were stable on medications for at least six weeks and were not currently experiencing a mood episode. Clinical rating scales were administered in our study as follows: Hamilton Depression Rating Scale (HAM-D), Positive and Negative Syndrome Scale (PANSS) and the Young Mania Rating Scale (YMRS).
All patients with BD were subtype I and were euthymic, as determined by HAM-D score < 7 and YMRS score < 6. The HC group was matched on age, gender, and education to 26 bipolar patients by propensity score matching analysis. Participants interested in this study were screened to ensure eligibility based on the following criteria: right-handed, no history of neurological (e.g. stroke), psychiatric, or substance use disorders, and did not have MRI contraindications (e.g. pacemaker or other implanted metallic devices).
Insomnia symptoms were assessed using three items on the HAM-D that evaluate early, middle, and late insomnia. Patients were placed in the BD-IN group if they endorsed one or more of the insomnia items on HAM-D (Nelson et al., 2006). All participants were administered the Trail Making Test (TMT) of Delis-Kaplan Executive Function System (D-KEFS) (Delis DC et al., 2001). A cognitive processing speed score was computed for each participant by averaging the raw scores of the Number Sequencing and Letter Sequencing conditions. Executive functioning was assessed with the Number-Letter Switching subtest, and the raw score was used in analyses.
Imaging data collection and analyses
Imaging data were acquired using a research-dedicated 3 Tesla General Electric Discovery MR750 MRI scanner with a 32-channel head coil. A high resolution anatomical T1-weighted MRI image was collected using a fast spoiled gradient echo pulse sequence (TE = 4 ms, FA = 8, TR = 600 ms, field FOV = 256 × 192 mm, 176 1-mm thick sagittal slices, voxel size 1×1×1 mm). The resulting images were utilized to localize the functional signal. Spin-echo field maps were also collected with the following parameters: TE: 90, TR: 10000, FOV: 256, Slice thickness: 4mm, In-plane resolution: 4×4mm. The BOLD signal for the resting state connectivity scan was measured with T2*-weighted echo planar images collected with eyes open (TR = 720ms, TE 33ms, FA = 52, FOV 180 × 208 mm, 90 × 104 matrix, 72 oblique axial 2 mm slices, voxel size 2×2×2 mm).
Raw fMRI data preprocessing was implemented with the National Institute of Health’s Analysis of Functional NeuroImages (AFNI) software package (Cox, 1996). Preprocessing of MR images included image reconstruction and correction for field inhomogeneities. Specifically, B0 distortions were corrected using the reversing gradient method (Chang and Fitzpatrick, 1992; Holland et al., 2010; Morgan et al., 2004), estimating the displacement field from separate spin-echo calibration scans that were adjusted for estimated head motion and applied to the series of gradient-echo images. This was followed by registration and automated motion correction with AFNI’s 3dvolreg. The first 10 images were discarded to allow for T1-equilibrium. Images were spatially blurred with a Gaussian kernel full width at half maximum of 3 mm. Linear regression was applied to remove sources of spurious variance in the data. In order to correct for confounds, the following nuisance regressors were included: linear and quadratic trends and six motion parameters estimated during image coregistration. The corrected BOLD time series were then low-pass filtered using a cut-off frequency of 0.08 Hz. Individual subject data were registered to Montreal Neurological Institute template using FSL FLIRT program. A visual inspection was conducted and remaining data points with excessive motion were rejected; these censored timepoints were not included in calculation of seed-to-seed correlations.
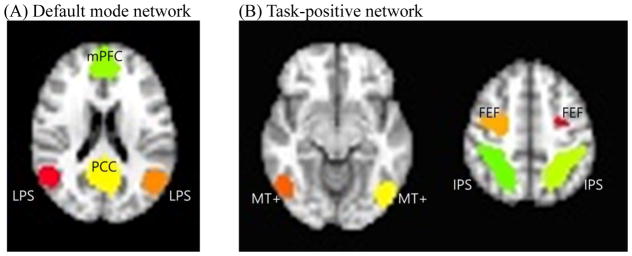
Time series within each of the DMN and TPN seeds were extracted and maps of voxel-wise correlations to each seed region’s time course were calculated (Fox et al., 2005). An averaged DMN and TPN group correlation map was created, and peak nodes were identified in order to locate more precise DMN and TPN seed locations in the current sample (Figure 1). The mean time course in each peak node region identified was extracted for each participant and correlated pairwise, using Pearson’s r, with all other nodes. Pairwise correlations were then converted to Fisher’s Z and an average connectivity score of each node with all other nodes was calculated. For internetwork connectivity, we averaged the Z-values for all the connections between each DMN node and each TPN node.
Figure 1.
Regions of interest within the default mode network and task positive network.
Note. FEF = frontal eye field region; IPS = intraparietal sulcus; LPS = lateral parietal sulcus; mPFC = medial prefrontal cortex; MT+, middle temporal region. PCC = posterior cingulate cortex.
Statistical analysis
The Statistical Package for Social Science, version 19.0 (SPSS Inc., Chicago, IL, USA), was used for statistical analyses. Significance was determined at the 0.05 level. ANOVAs with follow-up planned t-tests were used to examine group differences in demographic, cognitive and connectivity variables. A family-wise Bonferroni correction was applied if there were no pre-specified hypotheses. To account for testing six connections between four DMN seeds and fifteen connections between six TPN seed regions for the hypotheses of differences between BD-IN and BD groups, and between BD-IN and HC groups, the p-value was Bonferroni corrected and set at 0.05/12 = 0.004 (DMN) and 0.05/30 = 0.002 (TPN). To test the hypothesis that brain FC mediated the relationship of insomnia to cognitive function, causal mediation analyses with a bootstrap method were performed using R (version 3.0.1, The R Foundation for Statistical Computing) (MacKinnon et al., 2007).
3. Results
Age, gender, years of education, or estimated verbal IQ did not differ between the three groups. Symptoms of mania, depression and psychosis did not differ between BD-IN and BD (Supplementary Table 1). The BD-IN group showed slower processing speed compared to BD and HC groups (p’s < 0.014). There was no group difference on the executive function score.
Analyses revealed significant group differences between BD-IN and BD and between BD-IN and HC in average right intraparietal sulcus (IPS) connectivity to the right frontal eye field region (FEF) within the TPN and average medial prefrontal cortex (mPFC) connectivity strength with the posterior cingulate cortex (PCC) within the DMN. However, the differences in mPFC-PCC did not survive Bonferroni correction (Supplementary Table 2). Overall DMN-TPN internetwork connectivity strength was not different between groups (F=0.272, p=0.763).
Given the observed FC and processing speed differences between BD-IN and BD groups, a series of analyses was undertaken to determine whether the FC strength measurements mediated the relationship of subjective insomnia with processing speed in euthymic BD patients. We generated bootstrapped 95% confidence intervals for the mediation effect by calculating the difference between the coefficient for the direct effect of subjective insomnia (c) and the effect when adjusting for rIPS-rFEF connectivity (c′), resulting in the mediated effect (c-c′). Subjective insomnia has a significant direct effect (c) in the absence of mediator (p-value < 0.001). When the mediator is added (c′), the significance of direct effect is slightly reduced (p-value = 0.002). However, the indirect effect (c-c′) is weak (p-value = 0.881). Thus, there is no strong evidence that group differences in processing speed are explained by group differences in FC strength.
4. Discussion
Euthymic bipolar patients with insomnia symptoms showed aberrant functional connectivity within the TPN rather than within the DMN or between the DMN and TPN (figure 1). Our findings of IPS-FEF connectivity deficits in BD-IN and their relationships with processing speed are consistent with the literature that shows deficits of TPN connectivity during attentional tasks as a result of sleep deprivation (SD) in non-psychiatric samples (Chee et al., 2011). SD attenuated baseline signal elevations evoked by preparatory attention in the absence of visual stimulation within FEF and IPS in addition to visual extrastriate cortex. This result implies that SD affects higher cortical regions such as FEF and IPS and that these areas mediate endogenous attention. In addition, a functional imaging study revealed that decline in working memory after SD may be strongly influenced by degraded attention in that SD reduced the coupling between IPS activation and behavioral performance in the memory condition that was observed after normal sleep (Chee and Chuah, 2007).
BD-IN patients’ objective cognitive impairments were observed only in their processing speed score, but not in the executive functioning score. Cognitive processing speed, a relatively lower-level cognitive function, might be more easily disrupted by chronic partial sleep restriction than higher-level cognitive flexibility, particularly in BD where there are already known processing speed deficits. The observed processing speed deficits appeared to be relatively independent of the alteration in FC, as there was no strong evidence for mediation. Although TPN is highly associated with cognitive function, it does not seem to mediate the effect of insomnia on cognitive function. Instead, its effects could be mediated through other functional networks which were not tested in our study. Further investigation on other plausible networks will shed light on the pathway underlying processing speed disruptions in those reporting insomnia.
We did not find group differences in DMN connectivity that survived correction for multiple comparisons. Several previous studies have shown impaired DMN connectivity during manic and depressive state of bipolar disorder (Liu et al., 2012; Ongur et al., 2010). We previously found, in a subset of the participants in the current sample scanned with a high-temporal-resolution protocol, that average connectivity between mPFC and other DMN nodes was not different, but the temporal variability of mPFC to PCC connectivity was lower in euthymic BD (Nguyen et al., 2017). Our current study, using a standard resting state imaging protocol, also failed to show significant alterations in DMN connectivity in BD or BD-IN, suggesting that within-DMN connectivity might be state dependent or that any abnormalities during euthymia may be dynamic ones. We did not examine the relationship of mPFC signal to other non-DMN nodes. The mPFC has been shown to play a pivotal role in emotion regulation and PFC-limbic connections have been implicated in cognitive and emotional processing system (Phillips et al., 2003). In our previous review, we suggested that euthymic BD patients who have sleep disturbance are especially vulnerable to greater cognitive/emotional processing impairment and subsequent mood episodes due to an attenuation in this PFC-limbic connection (McKenna and Eyler, 2012). Therefore, further study is needed focusing on this functional connectivity between PFC and the limbic system.
This study has some limitations, including a small sample size, reliance on self-report for insomnia, and restricted sampling of the networks. Due to the small number of patients, the negative finding of the direct comparison between BD_IN and BD patients at the ROI level should be interpreted with caution. In regard to insomnia assessment, we had aimed to explore the effect of subjective insomnia on brain networks and cognitive function since subjective perception of sleep is important in the clinical setting. Furthermore, the subjective sleep questionnaires are for rating overall sleep quality, not sleep quality on a single night. Nevertheless, there are often significant discrepancies between subjective and objective ratings of sleep. Further study with objective sleep measures may reveal additional associations with cognition and brain function. All the patients were receiving psychotropic medications, which could affect functional connectivity, however this has not been convincingly demonstrated as a confound in the literature.
Disturbed sleep in bipolar disorder patients otherwise free of major symptoms appears to confer an increased risk for cognitive impairment, and, mostly independently, also reduces TPN connectivity. Several current hypotheses have been posited as to the importance of sleep in aspects of functioning such as cognitive deficits, impulsivity, substance use, and suicidal behavior. Importantly, insufficient sleep may be one modifiable contributor to a range of the adverse outcomes associated with bipolar disorder. The exploration of the basic mechanism of sleep disturbance in BD could provide the basis for improved understanding and treatment of BD. Future studies investigating bipolar disorder comorbid with insomnia could shed further light on the connections between insomnia and mood disorder.
Highlights.
Insomnia is prevalent in euthymic bipolar disorder.
Euthymic BD Patients with insomnia symptoms showed a significantly lower functional connectivity within the task positive network, compared to those without insomnia.
Euthymic BD patients with insomnia also showed significantly slower cognitive processing speed.
Acknowledgments
This research was supported by the National Institutes of Health (NIH) grant MH083968.
The authors thank Xin Tu, Ph. D. for performing the mediation analysis and Anders Dale, Ph.D. and Hauke Bartsch, Ph.D. for assisting with processing the resting state MRI data. H-K Yoon prepared this manuscript during his visiting scholar period.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 2012;16:584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock ECS, Kirkby KC, Garry MI, Taylor BVM. Systematic Review of Cognitive Function in Euthymic Bipolar Disorder and Pre-Surgical Temporal Lobe Epilepsy. Front Psychiatry. 2017;8:133. doi: 10.3389/fpsyt.2017.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady RO, Jr, Tandon N, Masters GA, Margolis A, Cohen BM, Keshavan M, Ongur D. Differential brain network activity across mood states in bipolar disorder. J Affect Disord. 2017;207:367–376. doi: 10.1016/j.jad.2016.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Fitzpatrick JM. A technique for accurate magnetic resonance imaging in the presence of field inhomogeneities. IEEE transactions on medical imaging. 1992;11:319–329. doi: 10.1109/42.158935. [DOI] [PubMed] [Google Scholar]
- Chee MW, Chuah YM. Functional neuroimaging and behavioral correlates of capacity decline in visual short-term memory after sleep deprivation. Proc Natl Acad Sci U S A. 2007;104:9487–9492. doi: 10.1073/pnas.0610712104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MW, Goh CS, Namburi P, Parimal S, Seidl KN, Kastner S. Effects of sleep deprivation on cortical activation during directed attention in the absence and presence of visual stimuli. Neuroimage. 2011;58:595–604. doi: 10.1016/j.neuroimage.2011.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- De Havas JA, Parimal S, Soon CS, Chee MW. Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. Neuroimage. 2012;59:1745–1751. doi: 10.1016/j.neuroimage.2011.08.026. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, JHK . D-KEFS examiner’s manual. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Favre P, Baciu M, Pichat C, Bougerol T, Polosan M. fMRI evidence for abnormal resting-state functional connectivity in euthymic bipolar patients. J Affect Disord. 2014;165:182–189. doi: 10.1016/j.jad.2014.04.054. [DOI] [PubMed] [Google Scholar]
- First MB. Structured clinical interview for DSM-IV axis I disorders SCID-I: clinician version, administration booklet. American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Breeden AL, Bean SE, Vaidya CJ. Working memory-related changes in functional connectivity persist beyond task disengagement. Hum Brain Mapp. 2014;35:1004–1017. doi: 10.1002/hbm.22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG, Schmidt DA, Scarna A, Semler CN, Goodwin GM. Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. Am J Psychiatry. 2005;162:50–57. doi: 10.1176/appi.ajp.162.1.50. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Talbot LS, Gershon A. Sleep Disturbance in Bipolar Disorder Across the Lifespan. Clin Psychol (New York) 2009;16:256–277. doi: 10.1111/j.1468-2850.2009.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland D, Kuperman JM, Dale AM. Efficient correction of inhomogeneous static magnetic field-induced distortion in Echo Planar Imaging. Neuroimage. 2010;50:175–183. doi: 10.1016/j.neuroimage.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang E, Zhang H, Dou S, Liu L, Tong L, Lei Y, Wang M, Xu J, Shi D, Zhang Q. Functional connectivity changes between parietal and prefrontal cortices in primary insomnia patients: evidence from resting-state fMRI. Eur J Med Res. 2014;19:32. doi: 10.1186/2047-783X-19-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Ma X, Li F, Wang YJ, Tie CL, Li SF, Chen TL, Fan TT, Zhang Y, Dong J, Yao L, Wu X, Wang CY. Regional homogeneity within the default mode network in bipolar depression: a resting-state functional magnetic resonance imaging study. PLoS One. 2012;7:e48181. doi: 10.1371/journal.pone.0048181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna BS, Eyler LT. Overlapping prefrontal systems involved in cognitive and emotional processing in euthymic bipolar disorder and following sleep deprivation: a review of functional neuroimaging studies. Clin Psychol Rev. 2012;32:650–663. doi: 10.1016/j.cpr.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PS, Bowtell RW, McIntyre DJ, Worthington BS. Correction of spatial distortion in EPI due to inhomogeneous static magnetic fields using the reversed gradient method. Journal of magnetic resonance imaging: JMRI. 2004;19:499–507. doi: 10.1002/jmri.20032. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Portera L, Leon AC. Assessment of outcome in depression. J Psychopharmacol. 2006;20:47–53. doi: 10.1177/1359786806066046. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Kovacevic S, Dev SI, Lu K, Liu TT, Eyler LT. Dynamic functional connectivity in bipolar disorder is associated with executive function and processing speed: A preliminary study. Neuropsychology. 2017;31:73–83. doi: 10.1037/neu0000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Lundy M, Greenhouse I, Shinn AK, Menon V, Cohen BM, Renshaw PF. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res. 2010;183:59–68. doi: 10.1016/j.pscychresns.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Suh S, Kim H, Dang-Vu TT, Joo E, Shin C. Cortical Thinning and Altered Cortico-Cortical Structural Covariance of the Default Mode Network in Patients with Persistent Insomnia Symptoms. Sleep. 2016;39:161–171. doi: 10.5665/sleep.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabares-Seisdedos R, Balanza-Martinez V, Sanchez-Moreno J, Martinez-Aran A, Salazar-Fraile J, Selva-Vera G, Rubio C, Mata I, Gomez-Beneyto M, Vieta E. Neurocognitive and clinical predictors of functional outcome in patients with schizophrenia and bipolar I disorder at one-year follow-up. J Affect Disord. 2008;109:286–299. doi: 10.1016/j.jad.2007.12.234. [DOI] [PubMed] [Google Scholar]