Abstract
D-Serine is a co-agonist at forebrain N-methyl-D-aspartate receptors (NMDAR) and is synthesized by serine racemase (SR). Although D-serine and SR were originally reported to be localized to glia, recent studies have provided compelling evidence that under healthy physiologic conditions both are localized primarily in neurons. However, in pathologic conditions, reactive astrocytes can also express SR and synthesize D-serine. Since cultured astrocytes exhibit features of reactive astrocytes, we have characterized D-serine synthesis and the expression of enzymes involved in its disposition in primary glial cultures. The levels of SR were quite low early in culture and increased markedly in all astrocytes with the duration in vitro. The concentration of D-serine in the culture medium increased in parallel with SR expression in the astrocytes. Microglia, identified by robust expression of Iba1, did not express SR. While the levels of glial fibrillary acidic protein (GFAP), glycine decarboxylase (GLDC) and phosphoglycerate dehydrogenase (PHGDH), the initial enzyme in the pathway converting glycine to L-serine, remained constant in culture, the expression of lipocalin-2, a marker for pan-reactive astrocytes, increased several-fold. The cultured astrocytes also expressed Complement-3a, a marker for a subpopulation of reactive astrocytes (A1). Astrocytes grown from mice with a copy number variant associated with psychosis, which have four copies of the GLDC gene, showed a more rapid production of D-serine and a reduction of glycine in the culture medium. These results substantiate the conclusion that A1 reactive astrocytes express SR and release D-serine under pathologic conditions, which may contribute to their neurotoxic effects by activating extra-synaptic NMDARs.
Keywords: Astrocytes, Copy Number Variant, D-serine, Glycine Decarboxylase, N-Methyl-D-Aspartate Receptor, Serine Racemase
Graphical Abstract
1. INTRODUCTION
The N-methyl-D-aspartate receptor (NMDAR) is a coincidence detector, requiring three simultaneous events to occur in order to transduce the cations, Na+ and Ca2: membrane depolarization, binding of the neurotransmitter, glutamate, to its receptor on the GluN2 subunit and the binding of glycine or D-serine to the glycine modulatory site (GMS) site located on the GluN1 subunit (1). The Ca2+ influx that it mediates drives expression of genes critical for functional and structural plasticity such as brain derived neurotrophic factor (BDNF; 2). Johnson and Ascher (3) first demonstrated that glycine was required for NMDAR function, but subsequent studies in a Xenopus expression system demonstrated that D-serine was actually more potent as a GMS agonist than glycine (4). The significance of this finding was solidified by the demonstration by Hashimoto et al. (5) that D-serine was concentrated in the mammalian forebrain. Electrophysiologic studies in acute hippocampal slices using glycine oxidase and D-amino acid oxidase in the perfusate to degrade extracellular glycine and D-serine, respectively, provided convincing evidence of the role of D-serine for NMDAR function (6).
The cellular source of D-serine has been hotly disputed. Initial immunocytochemical studies appeared to show that D-serine (7) and its synthetic enzyme, serine racemase (SR), are localized to astrocytes in the rat forebrain (8). SR expression in primary cultures of astrocytes reinforced this presumed localization (8). There is still a widely held belief that D-serine and SR are components of a glial transmitter system (9). Nevertheless, the glial synthesis of D-serine was called into question a decade ago by the report of Miya et al (10), who used SR−/− mice to control for immunospecificity and showed that SR immunoreactivity was localized exclusively to neurons: cortical pyramidal neurons, striatal medium spiny neurons and weakly in cerebellar Purkinje cells. Benneyworth and colleagues (11) conditionally silenced SR gene expression either in glia with an inducible Cre-recombinase driven by a glial fibrillary acidic protein (GFAP) promoter or in neurons with a constitutive Cre-recombinase driven by a CamKIIα promoter. This strategy revealed that SR was minimally expressed in astrocytes, but predominantly expressed in neurons in cortex and hippocampus. Balu et al. (12) used SR−/− mouse tissue as the negative control to confirm the localization of SR in pyramidal neurons and subsets of GABAergic neurons. Since SR−/− mice have less than ten percent of wild type (WT) levels of D-serine, they also served as an ideal control for D-serine immune-staining. A concentration of blocking L-serine 100-fold higher than that used in prior studies was required to eliminate false positive immune-staining in astrocytes, leaving D-serine immune-reactivity restricted to neurons in cortex.
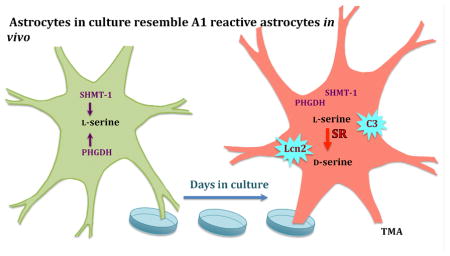
Although SR and D-serine are not physiologically produced by astrocytes, we recently showed that the reactive astrocytes, which proliferate in the hippocampus after traumatic brain injury (TBI) do express SR (13). Given this evidence that SR is expressed in reactive astrocytes in vivo, we have characterized primary glial cultures from mouse brain, which have features of reactive astrocytes. Reactive astrocytes can have neurotoxic (A1) or neurotrophic (A2) effects (14). Therefore, we have measured L- and D-serine and glycine levels, the expression of enzymes that are involved in their metabolism and markers for A1 and A2 astrocytes. The results indicate A1 reactive astrocytes express SR and release D-serine, which may contribute to their neurotoxic effects by acting at extra-junctional NMDARs.
2. MATERIALS AND METHODS
2.1 Materials
Fetal bovine serum, penicillin-streptomycin, 2.5% Trypsin, 0.05% Trypsin-EDTA, DMEM high glucose (without glutamine, sodium pyruvate, HEPES), PBS, glutamine (200mM), sodium pyruvate (100mM), HEPES (1M) were obtained from Gibco. CMF-HBSS, 70-micron cell strainer, RIPA Lysis and Extraction Buffer were all purchased from Thermo Fisher Scientific. Trichloroacetic acid (TCA), Phthaldialdehyde reagent, and N-Isobutyryl-L-cysteine were from Sigma-Aldrich (Natick, MA). While methanol, acetonitrile, and sodium acetate were obtained from Fisher scientific. Normal goat serum (NGS) was from MP Biomedicals (Santa Ana, CA), LLC. 16% paraformaldehyde (PFA) was from Electron Microscopy Sciences Company (Hatfield, PA). All the chemicals were biological or HPLC grade.
2.2 Animals
SR+/− sires and dams were bred to produce SR+/+ and SR−/− offspring. For the copy number variant (CNV) mice, separate lines with loxP sites flanking the CNV region were generated by gene targeting and combined with an Hprt-Cre transgene (15; provided by Dr. Peter F. Nichol of the University of Wisconsin, Madison) in order to cause a trans-allelic recombination event leading to a genomic duplication from the Rln1 to the Gldc genes on mouse chromosome 19, which corresponds to human chromosome 9p24.1. Mice homozygous for this duplication have four copies of the genes in 9p24.1 (“triplication”). The generation of the 9p24.1 duplication allele will be described in more detail in a separate publication characterizing these mice. All mice were bred in the C57BL/6J background. All animal procedures were approved by the Mclean Hospital Institutional Animal Care and Use Committee.
2.3 Primary cell culture
Primary mixed glial cells were isolated from cortices of neonatal mice and cultured as previously described by McCarthy and de Vellis (16). Cerebral cortices were dissected from postnatal (p) day 1 pups, and the meninges were carefully removed under the sterile condition. The tissue was minced, and the cells were dissociated by 0.25% trypsin and then plated on 75cm flasks or 6 well plates. Culture medium was changed every week.
After two weeks, the cells formed two layers. Astrocytes grew tightly attached to the flask bottom while microglia and a few oligodendrocytes formed an upper layer, which could be easily to detached by vigorous shaking. Microglia cells were isolated by shaking cell flasks in a 37°C shaker, at 180rpm for two hours and re-plated on 24 well plates for Western-blotting or on poly-lysine coated 8-well culture slide for immunochemistry. The astrocyte cells were re-plated in 6-well plates and cultured in low glutamine (0.2mM, 2mM glutamine in normal DMEM medium) medium, L-Serine, D-serine and glycine levels in the cell culture medium was measured by high performance liquid chromatography (HPLC), and the cells were lysed for western-blots. Cells cultured in poly-lysine coated 8-well culture slides were used for immunochemistry assay.
2.4 Immunohistochemistry staining
Microglia or astrocyte cells were separately plated on 8-well culture slides and grown until 90% confluent or for periods of 3 days, 5 days, 7 days). The cells were fixed with fresh prepared 4% PFA for 10 minutes at room temperature and then were blocked with 5% NGS in PBS with 0.1% Triton X-100 for 1h.
Primary antibodies used for immunofluorescence were: rabbit monoclonal antibody to Iba1 (EPR16589) (Abcam catalog. #: ab178847, 1:100) (Cambridge, MA), mouse monoclonal anti-GFAP antibody (Millipore catalog #: MAB360, 1:400) (Burlington, MA), rabbit polyclonal antibody to GFAP (Abcam catalog #: ab7260, 1:600), mouse monoclonal antibody to serine racemase (SR) (BD Bioscience catalog #: 612052, 1:300), rabbit polyclonal antibody to GLDC (Abcam catalog #: ab97625, 1:200), and rabbit polyclonal antibody to PHGDH (Abcam catalog #: ab211365, 1:200) and rat monoclonal antibody against complement C3 (Abcam catalog #: 11862). The cells were washed and then incubated with Alexa Fluor conjugated secondary antibody (Molecular Probes; Eugene, OR). DAPI (Vector Laboratories; Burlingame, CA) was used to label nuclei. Images were obtained using a Zeiss Axiokop40 microscope.
2.5 Western blot analysis
Cells were collected at different time points (cell cultures for 3, 5 or, 7 days) and total protein was extracted using RIPA buffer (Thermo Scientific catalog #: 89901) with protease and phosphatase inhibitor cocktail (Thermo Scientific catalog #: 78440). Immunoblotting was performed as previously described. The following primary antibodies were used: goat polyclonal antibody to SR (Santa Cruz catalog #: sc15081, 1:500), rabbit polyclonal antibody to GLDC (Abcam catalog #: ab97625, 1:500), rabbit monoclonal antibody to Iba1 (EPR16589) (Abcam catalog #: ab178847, 1:1000), rabbit polyclonal antibody to β-actin (Abcam catalog #: ab8227, 1:10k), rabbit polyclonal antibody to PHGDH (Abcam catalog #: ab211365, 1:1000), anti-Glial Fibrillary Acidic Protein Antibody (Millipore catalog #: MAB3402, 1:1000), mouse anti-GAPDH antibody (Fitzgerald, catalog #: 10R-G109a; Acton, MA), rat monoclonal antibody (Abcam Catalog #: 11862) and rabbit anti-Lipocalin2 antibody (Abcam catalog #: ab63929, 1:1000) followed by incubation with horse radish peroxidase (HRP) conjugated secondary antibody (Molecular Probes). Each protein’s chemiluminescent values were normalized to β-actin chemiluminescent in the same sample.
2.6 D-serine Immunohistochemistry
Primary astrocyte cells were grown on poly-lysine coated 8 well culture slide until 90% confluent and fixed with the fixative containing 3% glutaraldehyde (25% stock; Fisher Scientific; Waltham, MA), 1% PFA, 0.2% sodium metabisulfite (Sigma Aldrich) for 10 minutes at room temperature. Cells were treated with freshly made 0.2 % sodium metabisulfite (Sigma Aldrich) and 0.5 % sodium borohydride (Sigma Aldrich) for 10 minutes to quench free aldehydes and then blocked for 60 minutes in 0.02 M Tris-buffered saline (TBS) containing 10 % normal goat serum and 0.1 % Triton X-100).
The primary D-serine antibody was diluted 1:60K in 10mM L-serine–glutaraldehyde–BSA conjugates and incubated for ~40 h at 4 °C (12). After 3 washes with TBS, cells were incubated with goat anti-rabbit IMMPRESS HRP reagent (Vector; ready-made working solution) for 60 minutes. Colorimetric detection was performed with IMMPACT DAB (Vector; 30ul concentration +1ml dilution buffer). In between each incubation step, cells were washed 3 times for 10 minutes each in 0.02 M TBS [except prior to the metabisulfite/borohydride incubation and colorimetric detection when only 0.1 M phosphate buffer (PB) was used]. Experimental and corresponding control samples were processed in parallel. Immunostaining was visualized on a Ziess Axioskop microscope using StereoInvestigator software (MBF Bioscience; Welliston, VT) to capture the digital images under constant conditions for subjects of each comparison.
2.7 HPLC analysis of D-serine and glycine of cell culture medium
Purified astrocyte cells were cultured on 6-well plate in 1ml low-glutamine (0.2mM) DMEM medium (2mM glutamine in normal DMEM medium). Cell medium was collected every day for 7 days and flash frozen on dry ice. The medium was prepared for HPLC by adding 5% TCA (final concentration) to precipitate protein and washed four times with water-saturated diethyl ether to remove the TCA. To measure D-serine, the derivatization reagent was prepared daily by dissolving 1mg OPA and 2mg IBC in 0.1ml methanol followed by the addition of 0.45ml 0.4M sodium borate buffer pH 9.0 and 0.45ml HPLC grade water. To measure glycine, 5mg of OPA in 1ml methanol with 11mM 2-mercaptoethanol was used as the derivatizer. Time programs were run on a Shimadzu HPLC machine (Kyoto, Japan) as previously described (17).
2.8 Real-time PCR
Total RNA was extracted from astrocytes using RNeasy plus mini kit (QIAGEN Company, Catalog #:74134; Redwood City, CA). cDNA for each RNA samples (800μg RNA input) was generated using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Catalog #:4368814). Real-time PCR were performed using TaqMan gene expression assays (Applied Biosystems) for GAPDH (Mm99999915_g1), SR (Mm01246014_m1), and DAAO (Mm00438378_m1). For relative quantification of mRNA expression, geometric means were calculated using the comparative 2–ΔΔCt method, with the housekeeping gene GAPDH used as the endogenous reference. Each sample was assayed in triplicate.
2.9 In situ hybridization
The cells were plated on poly-lysine coated 8-well culture slides and fixed with freshly prepared 4% PFA after culturing for 1, 3, or 7 days. Single molecule fluorescent in situ hybridization (smFISH; ACD Bio; Newark, CA) was performed according to the manufacturer’s protocol for cultured adherent cells (catalog #: 322500) using mRNA probes against SR (Catalog #: 486271-C2) and GFAP (catalog #: 322500) (18). Images were obtained from a Zeiss Axiokop40 microscope at 40X magnification.
3. RESULTS
3.1 Expression of enzymes involved in serine and glycine disposition
Since astrocytes are implicated in the disposition of both glycine and serine, we first assessed their expression using immunocytochemistry on primary cultures after 7 days in vitro (Figure 1). Consistent with prior results from primary astrocyte cultures, the cells robustly expressed GFAP in virtually every cell (19). Similarly, the astrocytes expressed SR as identified with antiserum validated against SR−/− tissue. D-serine is catabolized by D-amino acid oxidase (DAAO). Immunostaining revealed that many, but apparently not all, astrocytes appear to express DAAO (data not shown; 20). Notably, the absence of SR as a consequence of a null mutation did not appear to affect the expression of DAAO. The cultured astrocytes expressed both GLDC, which catabolizes glycine, and PHGDH, which is the rate-limiting step in the synthesis of L-serine from glycine.
Figure 1. Immunocytochemical demonstration of glial fibrillary acid protein and serine racemase in astrocytes after seven days of culture.
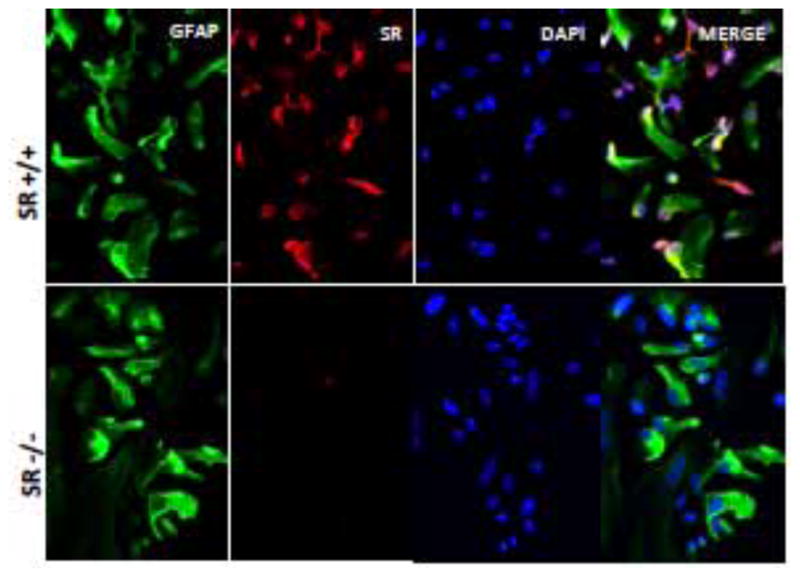
GFAP is green; SR is red; and the nuclei are stained blue with DAPI. Note the absence of SR in cultures from SR−/− mice.
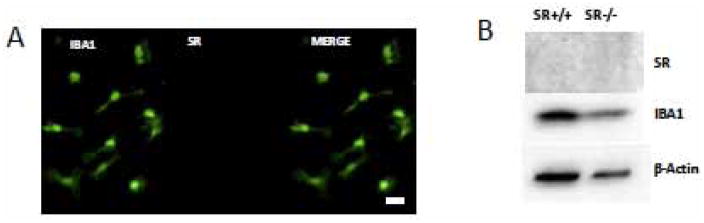
We “panned” the cultures to isolate microglia from the more adherent astrocytes and sub-cultured the microglia for seven additional days. The cells stained intensely for the microglial marker, ionized calcium-binding adapter molecule (Iba1; 21). In contrast to the astrocytes, the microglia did not express SR, although they did express DAAO (Figure 2).
Figure 2. Microglia do not express serine racemase.
Purified microglia were cultured on 24-well plates for western blots or immunocytochemistry. A. Ionized calcium binding adapter molecule (Iba1), a marker for microglia, exhibits green fluorescence. Nuclei are stained blue with DABI. Note the absence of SR immunoreactivity. B. Western blots demonstrate the presence of Iba1 but not SR in microglia lysates.
3.2 Expression of expression D-serine metabolic enzymes over time in culture
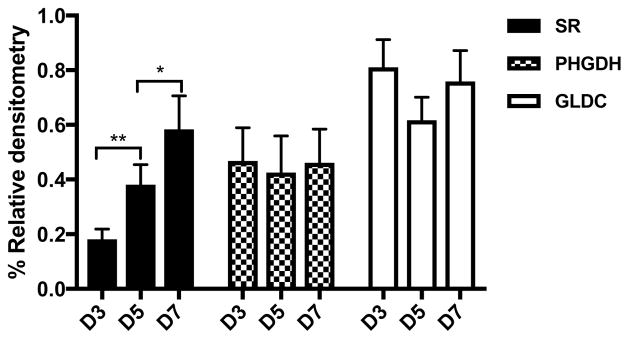
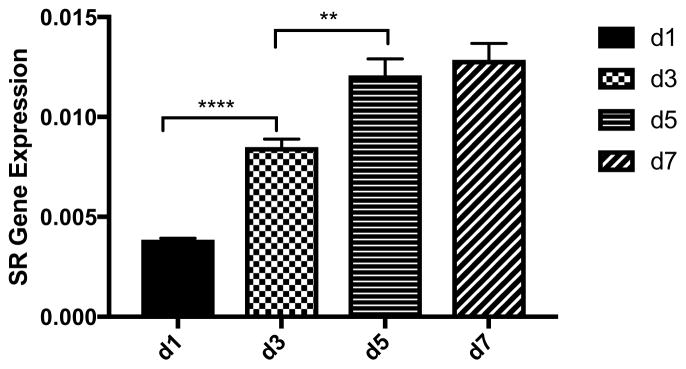
The expression of SR normalized to β-actin substantially increased between day three and day seven in culture (Figure 3). Immunocytochemical staining showed that this increase was due to an increased intensity of SR expression in all cells and not due to an increase in the number of SR positive cells. Furthermore, the SR results from the Western blots comport with the increased SR mRNA expression in the cells between days one and seven in culture as assessed by in situ hybridization (Figure 4). Comparing WT to SR−/− after seven days in culture revealed robust immunostaining for D-serine in WT, but not in SR−/− astrocytes. This increase in SR expression between three and seven days in culture was mirrored by an increase in the expression of lipocalin2, a marker for A1 astrocytes, between days three and seven in culture (Figure 5). In addition, Western blots of astrocyte lysates after seven days in culture revealed robust expression of Complement C3, another marker for A1 reactive astrocytes (data not shown). In contrast, the expression of GFAP, PHGDH and GLDC remained constant between days three and seven in culture as assessed by both Western blots and immunocytochemistry (Figure 3).
Figure 3. Expression of SR, PHGDH and GLDC as a function of time in culture.
Cells dissociated from WT mice cortices were directly cultured on 6-well plates and were collected for Western blots at days 1,3, 5 and 7. A. Quantitative levels of the expression of SR, GLDC and PHGDH in astrocyte lysates normalized to βactin. Results are the mean +/− S.E.M. of 5 cultures. B. Representative Western blots of GLDC, PHGDH, SR and βactin at 3, 5 and 7 days in culture. * p<0.05, ** p<0.01.
Figure 4. RT-PCR of serine racemase mRNA expression in WT astrocytes.
The results are the mean +/− S.E.M. from at least 4 cultures. ** p<0.01, *** p<0.001.
Figure 5. The expression of Lipocalin2 and GFAP in astrocyte cultures.
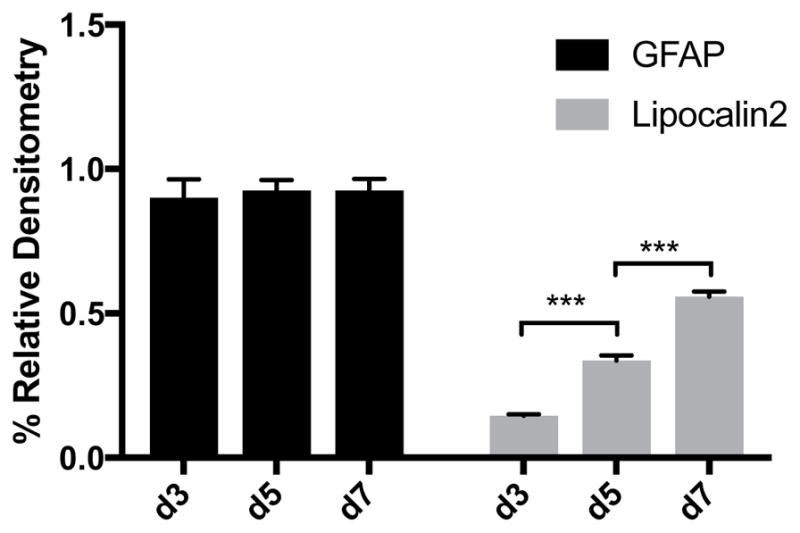
The Western blots were normalized to GAPDH. The results are the mean +/− S.E.M. from at least 4 cultures. *** p<0.001.
3.4 Effect of four copies of glycine decarboxylase
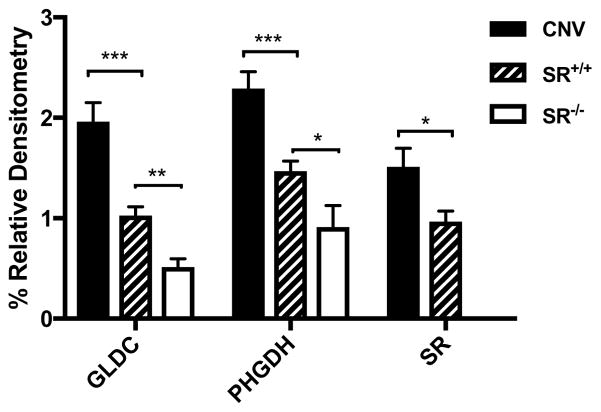
The GLDC gene is contained within a copy number variant (CNV) that is associated with a psychotic phenotype that is responsive to treatment with exogenous glycine (22). We cultured astrocytes obtained from newborn mice with a corresponding CNV. Western blots of the astrocytes cultured from the WT, CNV and SR−/− mice revealed a 4-fold increase in GLDC expression in the CNV-derived cultures (Figure 6). Notably, PHGDH and SR expression were also significantly increased in the CNV cells, whereas GLDC expression was modestly reduced in SR−/− astrocyte cultures.
Figure 6. The expression of GLDC, PHGDH and SR in astrocytes after 7 days in culture from WT, SR−/− and 9p24.1 CNV mice.
The Western blots were normalized to βactin and are the results of 4 or more cultures. * p<0.05, ** p<0.01, *** p<0.001
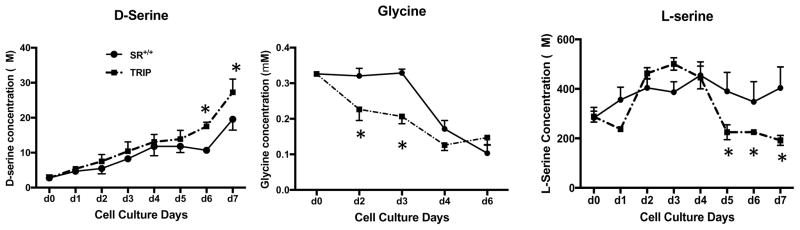
Measurements of the culture media from days one through seven showed increased levels of D-serine on days six and seven from the CNV cells as compared to those from WT cells (Figure 7). In contrast, the media from WT cultures had significantly higher levels of glycine than that from the CNV cultures on the second and third days in culture. The effects of the CNV on the medium were biphasic with substantial increases in L-serine from the second to fourth days in culture, followed by a decline to WT levels in subsequent days.
Figure 7. Secretion of D-serine, L-serine and glycine into the culture medium from astrocytes grown from WT and 9p24.1 CNV mice.
Purified astrocyte cells were plated on 6-well plate in low glutamine (0.2mM) DMEM medium (2mM glutamine in normal DMEM medium) from WT and 9p24.1 CNV mice. Cell medium was collect every day over 7 days for HPLC assays. The results are the mean +/− S.E.M. obtained from 4 or more cultures. * p<0.05
4. DISCUSSION
Early publications on SR and D-serine indicated that they were localized to astrocytes in the brain. Electrophysiological studies showed that D-serine was the preferential NMDAR co-agonist in forebrain (6). The astrocytic source of D-serine seemed to be supported by the finding that fluoroacetate, a rather selective astrocyte toxin, reduced D-serine release and NMDAR function in slices (23). Furthermore, primary cultures of astrocytes prepared by the method of McCarthy and de Vellis (16) robustly expressed SR as demonstrated by immunocytochemistry (8). While it was recognized that cultured astrocytes have features more akin to reactive astrocytes, conditional expression of the schizophrenia risk gene, DISC1, in vivo and transfection of DISC1 into HEK-293 cells were used to argue that DISC1 exerts its schizophrenogenic effects by complexing with SR in astrocytes (24). Finally, immunocytochemical studies using low blocking concentrations of L-serine indicated that brain astrocytes stained intensely with antiserum to D-serine conjugated to bovine serum albumin with glutaraldehyde (7). Thus, D-serine became broadly accepted as a “gliotransmitter” (9).
This interpretation was first questioned when Miya et al. (10) used an SR antibody that did not show immunoreactivety in SR−/− tissue and observed SR immunoreactivity only in neurons and not in astrocytes, specifically pyramidal neurons and subsets of GABAergic neurons. Benneyworth and colleagues (11) used a different approach with transgenic mice to silence SR expression in specific cell types. They crossed mice whose first SR exon was flanked by Lox P sites, with mice having Cre-recombinase expression driven by the CAMKIIα promoter, and observed a major reduction in cortico-hippocampal SR expression. The CamKIIα promoter targets Cre-recombinase expression to neurons, particularly cortical glutamatergic neurons. In contrast, silencing SR expression in astrocytes with an inducible GFAP promoter did not reduce SR expression in cortex and only modestly in hippocampus, where many GFAP+ astrocytes are present. Functionally, eliminating SR from neurons impaired NMDAR function and long-term synaptic potentiation, but silencing SR in astrocytes did not (11, 13).
Balu and colleagues (12) used the SR−/− mice as negative controls and confirmed the findings of Miya et al. (10) that SR is located in cortico-hippocampal pyramidal neurons and subpopulations of cortical and striatal GABAergic neurons. SR exhibited a similar neuronal localization in human cortex as well. Since SR−/− mouse brains have less than ten percent of WT D-serine, the specificity of the D-serine immunocytochemistry could be established. Surprisingly, the concentration of blocking L-serine used in prior studies (7) left astrocytes intensely stained for “D-serine” in SR−/− mice. Increasing the blocking L-serine-conjugate 100-fold eliminated astroglial staining and left the pyramidal neurons stained in the cortex.
Wolosker and colleagues (25, 26) reviewed these discrepancies in the reported cellular localization of SR and D-serine. In studies ascribing their localization to astrocytes, the use of antiserum and biosensors that had not been validated against brain tissue from SR−/− mice could not preclude false positives. Studies that used the gliatoxin, fluoroacetate, to prevent D-serine release from astrocytes did not consider that astrocytes are the primary source for L-serine, which is taken up by neurons for D-serine synthesis (27). In contrast, TBI to the hippocampus caused a proliferation of GFAP-rich astrocytes in the hippocampus that express SR and synthesize D-serine (13). These SR-positive reactive astrocytes can clearly be distinguished from the vast majority of contiguous quiescent astrocytes that do not express SR.
The present studies demonstrate that the primary astrocyte cultures assume the characteristics of reactive astrocytes with increasing expression of SR and release of D-serine into the medium. In contrast, microglia as identified by their expression of Iba1 do not express SR under our experimental conditions. Wu and colleagues (28) similarly found that cultured microglia did not express SR until activated by exposure to pro-inflammatory stimuli such as amyloid Aβ. Liddelow and Barres (14) have described two types of reactive astrocytes: A1 reactive astrocytes that have toxic effects on adjacent neurons and A2 reactive astrocytes that have trophic effects on neurons. The A1 astrocytes are distinguished by their expression of complement C3. The cultured astrocytes, which differentiate in vitro to robustly express SR and synthesize D-serine, also express this A1 astrocyte marker. Although the toxin released by the A1 astrocytes is unidentified (14), we found that the reactive astrocytes that proliferate in the hippocampus after TBI express SR and synthesize D-serine. (13). D-serine released from these reactive astrocytes would preferentially act at extra-synaptic GluN2B – enriched NMDARs, which mediate excitotoxicity (30). Selectively silencing SR expression in these reactive astrocytes that proliferate after TBI prevents the cognitive and neurophysiologic deficits. Thus, these findings point to D-serine being one of the substances released by the A1 reactive astrocytes that mediates their neurotoxic effects.
Astrocytes are the primary source of L-serine synthesized in the brain with PHGDH being the initial and rate-limiting step in the conversion of glycine to L-serine (29). While the expression of GDLC and PHGDH remain constant during the duration of culture, GLDC is decreased in cultured astrocytes derived from SR−/− mice. This GLDC down-regulation may represent a compensation for the lack of D-serine, thereby reducing the catabolism of glycine, the other NMDAR co-agonist. The increased expression of GLDC in subjects with the 9p24.1 duplication/triplication (31) would have markedly reduced astrocytic glycine available to synthesize L-serine, as we demonstrated by the more rapid depletion of glycine in the culture medium of astrocytes grown from the 9p24.1 CNV mice. Since L-serine is transported into neurons to synthesize D-serine by the “serine shuttle” (27), the synaptic levels of both GMS agonists, glycine and D-serine, would be depressed in the 9p24.1 duplication, resulting in NMDAR hypofunction and symptoms of schizophrenia (32). This salient role of astrocytes in determining NMDAR co-agonist levels in the synaptic space may explain how co-agonist treatment with D-cycloserine reduces psychosis in patients with 9p24.1 duplication syndrome in spite of normal plasma L-serine levels (22).
In summary, our studies solidify the evidence that SR is expressed in A1 reactive astrocytes, but not quiescent astrocytes. These results suggest that D-serine release from A1 astrocytes and the subsequent activation of extrasynaptic NMDARs contribute to their neurotoxic effects (30) and that astrocytic PHGDH plays a vital role in regulating the synaptic level of D-serine. Thus, astrocytes regulate the extracellular levels of D-serine in pathologic states, but not as a “gial transmitter” (26).
Acknowledgments
The research described in this manuscript was supported by RO1MH51290-18 and a subcontract of RO1NS098740-02 to JTC, 5R00MH099252-04 to DTB, by the Program for Advancing Strategic International Networks to Accelerate the Circulation of Talented Researchers (S2702) of the Japan Society for the Promotion of Science to YU, and by the following grants to UR: sub-award 6045285-5500000241 from the Broad Institute/Stanley Center for Psychiatric Research, a grant from the Shervert Frazier Research Institute at McLean Hospital, a Harvard Brain Science Initiative Bipolar Disorder Seed Grant, supported by Kent and Liz Dauten, and by grants R21MH104505 and R21MH087829 from NIH/NIMH.. JTC would like to thank Michael Williams, PhD, DSc for his faith in my expertise in having me as a consultant at Nova Pharm, Abbott Pharm, Genset and Cephalon. He manifests the highest standards of scientific integrity.
Abbreviations
- BDNF
Brain Derived Neurotrophic Factor
- CNV
Copy Number Variant
- DAAO
D-Amino Acid Oxidase
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- GFAP
Glial Fibrillary Acid Protein
- GLDC
Glycine Decarboxylase
- GMS
Glycine Modulatory Site
- HPLC
High Pressure Liquid Chromatography
- HRP
Horse Radish Peroxidase
- Iba1
Ionized calcium-binding adapter molecule 1
- Lcn2
Lipocalin-2
- NMDAR
N-Methyl-D-Aspartate Receptor
- PFA
Paraformade hyde
- PB
Phospahate Buffer
- PHGDH
Phosphoglycerate Dehydrogenase
- SHMT1
Serine Hydroxymethyl Transferase
- SR
Serine Racemase
- S.E.M
Standard Error of the Mean
- TBI
Traumatic Brain Injury
- TCA
Trichloroacetic Acid
- TBS
Tris-buffered saline
- WT
Wild Type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Seeburg PH, Burnashev N, Köhr G, Kuner T, Sprengel R, Monyer H. The NMDA receptor channel: molecular design of a coincidence detector. Recent Prog Horm Res. 1995;50:19–34. doi: 10.1016/b978-0-12-571150-0.50006-8. [DOI] [PubMed] [Google Scholar]
- 2.Harward SC, Hedrick NG, Hall CE, Parra-Bueno P, Milner TA, Pan E, Laviv T, Hempstead BL, Yasuda R, McNamara JO. Autocrine BDNF-TrkB signalling within a single dendritic spine. Nature. 2016 Oct 6;538(7623):99–103. doi: 10.1038/nature19766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5–11;325(6104):529–31. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- 4.Kleckner NW, Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988 Aug 12;241(4867):835–7. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto A, Nishikawa T, Hayashi T, Fujii N, Harada K, Oka T, Takahashi K. The presence of free D-serine in rat brain. FEBS Lett. 1992 Jan 13;296(1):33–6. doi: 10.1016/0014-5793(92)80397-y. [DOI] [PubMed] [Google Scholar]
- 6.Mothet JP, Parent AT, Wolosker H, Brady RO, Jr, Linden DJ, Ferris CD, Rogawski MA, Snyder SH. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 2000 Apr 25;97(9):4926–31. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schell MJ, Molliver ME, Snyder SH. D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci U S A. 1995 Apr 25;92(9):3948–52. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolosker H, Sheth KN, Takahashi M, Mothet JP, Brady RO, Jr, Ferris CD, Snyder SH. Purification of serine racemase: biosynthesis of the neuromodulator D-serine. Proc Natl Acad Sci U S A. 1999 Jan 19;96(2):721–5. doi: 10.1073/pnas.96.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papouin T, Henneberger C, Rusakov DA, Oliet SHR. Astroglial versus Neuronal D-Serine: Fact Checking. Trends Neurosci. 2017 Sep;40(9):517–520. doi: 10.1016/j.tins.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Miya K, Inoue R, Takata Y, Abe M, Natsume R, Sakimura K, Hongou K, Miyawaki T, Mori H. Serine racemase is predominantly localized in neurons in mouse brain. J Comp Neurol. 2008 Oct 20;510(6):641–54. doi: 10.1002/cne.21822. [DOI] [PubMed] [Google Scholar]
- 11.Benneyworth MA, Li Y, Basu AC, Bolshakov VY, Coyle JT. Cell selective conditional null mutations of serine racemase demonstrate a predominate localization in cortical glutamatergic neurons. Cell Mol Neurobiol. 2012 May;32(4):613–24. doi: 10.1007/s10571-012-9808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balu DT, Takagi S, Puhl MD, Benneyworth MA, Coyle JT. D-serine and serine racemase are localized to neurons in the adult mouse and human forebrain. Cell Mol Neurobiol. 2014 Apr;34(3):419–35. doi: 10.1007/s10571-014-0027-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez EJ, Tapanes SA, Loris ZB, Balu DT, Sick TJ, Coyle JT, Liebl DJ. Enhanced astrocytic d-serine underlies synaptic damage after traumatic brain injury. J Clin Invest. 2017 Aug 1;127(8):3114–3125. doi: 10.1172/JCI92300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liddelow SA, Barres BA. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity. 2017 Jun 20;46(6):957–967. doi: 10.1016/j.immuni.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Tang SH, Silva FJ, Tsark WM, Mann JR. A Cre/loxP-deleter transgenic line in mouse strain 129S1/SvImJ. Genesis. 2002 Mar;32(3):199–202. doi: 10.1002/gene.10030. [DOI] [PubMed] [Google Scholar]
- 16.McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980 Jun;85(3):890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashimoto A, Nishikawa T, Oka T, Takahashi K, Hayashi T. Determination of free amino acid enantiomers in rat brain and serum by high-performance liquid chromatography after derivatization with N-tert.-butyloxycarbonyl-L-cysteine and o-phthaldialdehyde. J Chromatogr. 1992 Nov 6;582(1–2):41–8. doi: 10.1016/0378-4347(92)80300-f. [DOI] [PubMed] [Google Scholar]
- 18.Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, Wu X, Vo HT, Ma XJ, Luo Y. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012 Jan;14(1):22–9. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison RS, De Vellis J, Lee YL, Bradshaw RA, Eng LF. Hormones and growth factors induce the synthesis of glial fibrillary acidic protein in rat brain astrocytes. J Neurosci Res. 1985;14(2):167–76. doi: 10.1002/jnr.490140202. [DOI] [PubMed] [Google Scholar]
- 20.Urai Y, Jinnouchi O, Kwak KT, Suzue A, Nagahiro S, Fukui K. Gene expression of D-amino acid oxidase in cultured rat astrocytes: regional and cell type specific expression. Neurosci Lett. 2002 May 17;324(2):101–4. doi: 10.1016/s0304-3940(02)00184-2. [DOI] [PubMed] [Google Scholar]
- 21.Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998 Jun 1;57(1):1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- 22.Levy D, Coleman M, Godfrey M, et al. Targeted treatment of a genetic mutation in glycine decarboxylase with d-cycloserine in psychotic disorders. Schiz Bull. 2017;43:S194–95. [Google Scholar]
- 23.Fossat P, Turpin FR, Sacchi S, Dulong J, Shi T, Rivet JM, Sweedler JV, Pollegioni L, Millan MJ, Oliet SH, Mothet JP. Glial D-serine gates NMDA receptors at excitatory synapses in prefrontal cortex. Cereb Cortex. 2012 Mar;22(3):595–606. doi: 10.1093/cercor/bhr130. [DOI] [PubMed] [Google Scholar]
- 24.Ma TM, Abazyan S, Abazyan B, Nomura J, Yang C, Seshadri S, Sawa A, Snyder SH, Pletnikov MV. Pathogenic disruption of DISC1-serine racemase binding elicits schizophrenia-like behavior via D-serine depletion. Mol Psychiatry. 2013 May;18(5):557–67. doi: 10.1038/mp.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolosker H, Balu DT, Coyle JT. The Rise and Fall of the d-Serine-Mediated Gliotransmission Hypothesis. Trends Neurosci. 2016 Nov;39(11):712–721. doi: 10.1016/j.tins.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolosker H, Balu DT, Coyle JT. Astroglial Versus Neuronal D-Serine: Check Your Controls! Trends Neurosci. 2017 Sep;40(9):520–522. doi: 10.1016/j.tins.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolosker H, Radzishevsky I. The serine shuttle between glia and neurons: implications for neurotransmission and neurodegeneration. Biochem Soc Trans. 2013 Dec;41(6):1546–50. doi: 10.1042/BST20130220. [DOI] [PubMed] [Google Scholar]
- 28.Wu SZ, Bodles AM, Porter MM, Griffin WS, Basile AS, Barger SW. Induction of serine racemase expression and D-serine release from microglia by amyloid beta-peptide. J Neuroinflammation. 2004 Apr 20;1(1):2. doi: 10.1186/1742-2094-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klomp LW, de Koning TJ, Malingré HE, van Beurden EA, Brink M, Opdam FL, Duran M, Jaeken J, Pineda M, Van Maldergem L, Poll-The BT, van den Berg IE, Berger R. Molecular characterization of 3-phosphoglycerate dehydrogenase deficiency--a neurometabolic disorder associated with reduced L-serine biosynthesis. Am J Hum Genet. 2000 Dec;67(6):1389–99. doi: 10.1086/316886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002 May;5(5):405–14. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- 31.Tcw J, Wang M, Pimenova AA, Bowles KR, Hartley BJ, Lacin E, Machlovi SI, Abdelaal R, Karch CM, Phatnani H, Slesinger PA, Zhang B, Goate AM, Brennand KJ. An Efficient Platform for Astrocyte Differentiation from Human Induced Pluripotent Stem Cells. Stem Cell Reports. 2017 Aug 8;9(2):600–614. doi: 10.1016/j.stemcr.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balu DT, Coyle JT. The NMDA receptor ‘glycine modulatory site’ in schizophrenia: D-serine, glycine, and beyond. Curr Opin Pharmacol. 2015 Feb;20:109–15. doi: 10.1016/j.coph.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]