Abstract
Background
Household air pollution (HAP) from combustion of solid fuels is an important contributor to disease burden in low- and middle-income countries (LIC, and MIC). However, current HAP cardiovascular disease burden estimates are based on integrated exposure response curves that are not currently informed by quantitative HAP studies in LIC and MIC. While there is adequate evidence supporting causal relationships between HAP and respiratory disease, large cohort studies specifically examining relationships between quantitative measures of HAP exposure with cardiovascular disease are lacking.
Objective
We aim to improve upon exposure proxies based on fuel type, and to reduce exposure misclassification by quantitatively measuring exposure across varying cooking fuel types and conditions in diverse geographies and socioeconomic settings. We leverage technology advancements to estimate household and personal PM2.5 (particles below 2.5 microns in aerodynamic diameter) exposure within the large (N~250,000) multi-country (N~26) Prospective Urban and Rural Epidemiological [PURE] cohort study. Here, we detail the study protocol and the innovative methodologies being used to characterize HAP exposures, and their application in epidemiologic analyses.
Methods/Design
This study characterizes HAP PM2.5 exposures for participants in rural communities in ten PURE countries with >10% solid fuel use at baseline (Bangladesh, Brazil, Chile, China, Colombia, India, Pakistan, South Africa, Tanzania, and Zimbabwe). PM2.5 monitoring includes 48-hour cooking area measurements in 4,500 households and simultaneous personal monitoring of male and female pairs from 20% of the selected households. Repeat measurements occur in 20% of households to assess impacts of seasonality. Monitoring began in 2017, and will continue through 2019. The Ultrasonic Personal Aerosol Sampler (UPAS), a novel, robust, and inexpensive filter based monitor that is programmable through a dedicated mobile phone application is used for sampling. Pilot study field evaluation of cooking area measurements indicated high correlation between the UPAS and reference Harvard Impactors (r = 0.91; 95% CI: 0.84, 0.95; slope=0.95). To facilitate tracking and to minimize contamination and analytical error, the samplers utilize barcoded filters and filter cartridges that are weighed pre- and post-sampling using a fully automated weighing system. Pump flow and pressure measurements, temperature and RH, GPS coordinates and semi-quantitative continuous particle mass concentrations based on filter differential pressure are uploaded to a central server automatically whenever the mobile phone is connected to the internet, with sampled data automatically screened for quality control parameters. A short survey is administered during the 48-hr monitoring period. Post-weighed filters are further analyzed to estimate black carbon concentrations through a semi-automated, rapid, cost-effective image analysis approach. The measured PM2.5 data will then be combined with PURE survey information on household characteristics and behaviours collected at baseline and during follow-up to develop quantitative HAP models for PM2.5 exposures for all rural PURE participants (~50,000) and across different cooking fuel types within the 10 index countries. Both the measured (in the subset) and the modelled exposures will be used in separate longitudinal epidemiologic analyses to assess associations with cardiopulmonary mortality, and disease incidence.
Discussion
The collected data and resulting characterization of cooking area and personal PM2.5 exposures in multiple rural communities from 10 countries will better inform exposure assessment as well as future epidemiologic analyses assessing the relationships between quantitative estimates of chronic HAP exposure with adult mortality and incident cardiovascular and respiratory disease. This will provide refined and more accurate exposure estimates in global CVD related exposure-response analyses.
Introduction
Household air pollution (HAP) from cooking and heating with solid fuels (biomass and coal) remains a common phenomenon in low- and middle-income countries (LIC and MIC), and poses multiple health, environmental, and socioeconomic problems. Worldwide, the estimated health burden attributable to HAP is 2.6 million deaths annually (Abajobir et al.; World Health Organization 2012), primarily from respiratory and cardiovascular diseases (CVD) (Assad et al. 2016; Collaborators et al. 2015; World Health Organization 2014). The majority of this burden is thought to occur in LIC and MIC, including in several of the world’s most populous developing countries (India, Bangladesh, Nigeria, Pakistan, and China), where more than 75% of the rural population are estimated to cook primarily with solid fuels on traditional cookstoves/open fires (Bonjour et al. 2013; Collaborators et al. 2015; Smith et al. 2014; World Health Organization 2014).
While there is substantial evidence for causal relationships between HAP and respiratory diseases, firm conclusions have yet to be reached regarding CVD (Assad et al. 2016; Fatmi and Coggon 2016; Mitter et al. 2016; Smith et al. 2014). Currently, only a few epidemiological studies have examined HAP and CVD incidence or death (Abtahi et al. 2017; Alam et al. 2012; Fatmi and Coggon 2016; Lee et al. 2012; Mitter et al. 2016), and they have all relied solely on household cooking fuel types reported in surveys as a binary indicator for HAP exposure (Smith and Peel 2010; Smith et al. 2014). A population based study (Arku et al. 2017) and a few measurement studies, including small cookstove interventions (Alexander et al. 2015; Clark et al. 2013a; McCracken et al. 2007) and personal exposure studies in rural women, have reported associations between HAP and hypertension (Baumgartner et al. 2011; Norris et al. 2016; Quinn et al. 2016), a CVD risk factor, but large comprehensive epidemiological studies specifically examining associations between HAP from solid fuel use with cardiopulmonary events and mortality in LIC and MIC are lacking (Lee et al. 2012; Mitter et al. 2016; Painschab et al. 2013; Smith et al. 2014). Accordingly, HAP disease burden estimates such as the Global Burden of Disease (Gakidou et al. 2017) model HAP-CVD relationships using integrated exposure-response relationships (Burnett et al. 2014; Pope et al. 2009) incorporating epidemiologic effect estimates from outdoor air pollution and second-hand (and mainstream) tobacco smoke which are assumed to enclose the global HAP exposure range (Smith and Peel 2010). However, large within- and between-country variation in HAP burden exists (Dandona et al. 2017; Zhou et al. 2016), and requires multi-centric HAP exposure measurements instead of the current use of integrated exposure-response curves. While our primary aim is to estimate exposures for direct use in epidemiologic analyses of HAP and CVD and respiratory outcomes, these multi-country exposure estimates may also benefit future disease burden analyses at sub-national, national and global levels.
A major gap in the global study of the impact of HAP on cardiopulmonary outcomes has been the lack of quantitative exposure information for HAP. The use of binary or categorical proxy exposure information such as fuel type, as in existing literature, ignores the fact that HAP exposures are complex and vary by several other factors, including specific fuel type, stove type, fuel and stove stacking, quantity of fuel use, kitchen area ventilation, amount of time spent near/proximity to the cooking area, age, and gender (Balakrishnan et al. 2013; Meng et al. 2009), to name a few. This limitation substantially impacts health effect studies of HAP and calls for larger HAP health effect studies that include quantitative exposure measures across varying cooking fuel types and conditions in diverse geographies and socioeconomic settings in LIC and MIC. We expand upon previous efforts in which a limited number of household and personal PM2.5 (particles below 2.5 microns in aerodynamic diameter) measurements were collected in order to model long term HAP exposures (Balakrishnan et al. 2013; Balakrishnan et al. 2015; Pillarisetti et al. 2016) and apply this approach to a large international multi-centre cohort. A number of single country HAP studies have also used similar measurement and modelling approaches to characterize exposures for subsequent epidemiologic analyses (McCracken et al. 2009; Tonne et al. 2017).
Specifically, we leverage a large existing multi-country prospective cohort study and advancements in technology and modelling approaches to address the need to improve upon exposure proxies in order to reduce exposure misclassification in HAP health effect analyses. The Prospective Urban and Rural Epidemiological (PURE) Study (Corsi et al. 2013; Duong et al. 2013; Teo et al. 2009; Yusuf et al. 2014) is one of the largest multi-country epidemiologic cohort studies and is designed to collect comprehensive data on social, environmental, behavioral, biological, and other factors that contribute to the incidence of, and mortality from CVDs and other chronic diseases. The PURE cohort therefore provides a unique opportunity to comprehensively assess HAP exposures from multiple settings and its impact on a range of related health outcomes in LIC and MIC. Within PURE, we have implemented a HAP exposure assessment approach across multiple countries, and in multiple communities in each index country. This paper provides descriptive information, and details the study protocol and the innovative methodology being used to characterize both household and personal air pollution exposure in rural communities in 10 LIC and MIC within the PURE study for use in epidemiologic analyses. The paper also provides results from our pilot study evaluating a new PM monitoring device. The resulting measurements and exposure characterization will be used to develop novel quantitative HAP exposure models in support of epidemiological analysis in the PURE cohort as well as other global efforts to estimate the impacts of HAP on human health.
Objectives
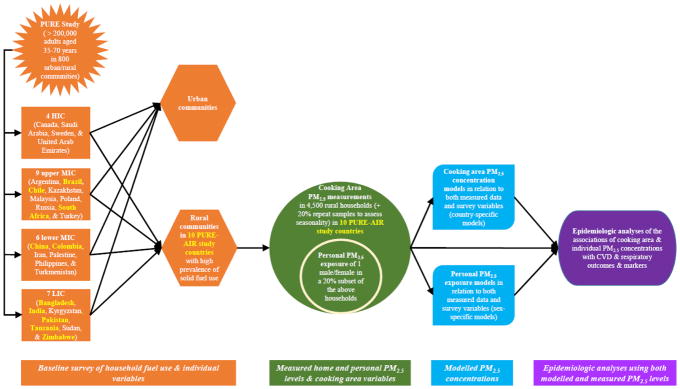
The main PURE study is examining cardiovascular risk factors and chronic disease incidence and mortality through a multi-country and community study (Teo et al. 2009). We have designed a sub-study with a key objective of addressing uncertainties in estimates of global impacts of HAP on cardiopulmonary health by quantitatively estimating HAP PM2.5 exposures among PURE participants in multiple communities within 10 LIC and MIC in the PURE study. Figure 1 illustrates the framing of the sub-study. The exposure estimation is based upon measurements of cooking area PM2.5 samples in ~4,500 homes of a subset of PURE study participants in rural communities where solid fuel was reported as the primary cooking fuel in at least 10% of households at baseline. In addition, we collect simultaneous personal samples on male and female pairs from 20% of these households of PURE participants. Building upon prior research (Balakrishnan et al. 2013), we will use these measurements along with a wide-ranging set of household/individual level variables from the PURE baseline and follow-up surveys to develop statistical models to estimate long term HAP PM2.5 exposures for all participants in the PURE study. Future epidemiological analyses in the PURE cohort will use both the measured (in the subset) and the modelled (in entire PURE LIC and MIC cohort) exposures to assess associations between HAP PM2.5 exposure with several CVD and respiratory outcomes (such as incident disease, mortality, blood pressure, and lung function) in both cross-sectional (baseline data) and longitudinal (at three-, six-, and nine-year follow-up) analyses.
Figure 1.
The main PURE study countries in relation to this air pollution sub-study and its design approach.
Methods and study design
PURE study location
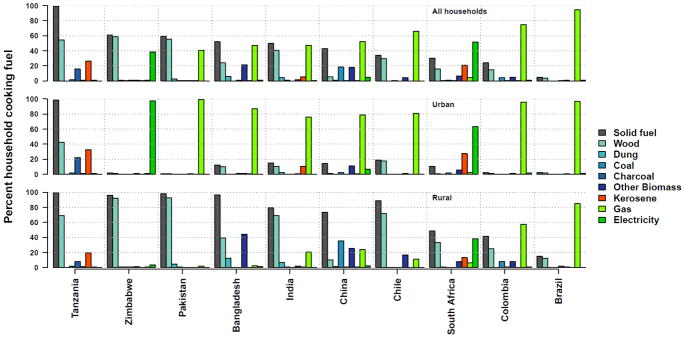
Selection of the main PURE study countries, communities, households, and individual participants are detailed elsewhere (Teo et al. 2009). Briefly, PURE currently involved ~250,000 adults aged 35–70 years residing in ~800 urban and rural communities in 26 countries, comprising 7 LIC (Bangladesh, India, Kyrgyzstan, Pakistan, Tanzania, Sudan, and Zimbabwe); 6 lower MIC (China, Colombia, Iran, Palestine, Philippines, and Turkmenistan); 9 upper MIC (Argentina, Brazil, Chile, Kazakhstan, Malaysia, Poland, Russia, South Africa, and Turkey); and 4 HIC (Canada, Saudi Arabia, Sweden, and United Arab Emirates) (Figure 1), according to the 2006 Word Bank classifications (http://web.worldbank.org). The countries were purposively selected to reflect varied income regions of the world, with more emphasis placed on LIC and MIC. Study communities in each country represent neighbourhoods in urban areas and small villages in rural areas; and multiple/cluster of communities in one geographical location constitute a study ‘center’. Of the 21 countries classified as LIC or MIC, 10 have at least 10% of its participants reported using solid fuel on traditional cookstove or open fires (Figures 1 and 2), and consisted of 194 rural and 175 urban communities assembled into 34 study centers and covered a range of ethnic groups. Study homes and individuals in the rural communities are the target of the PM measurements, as over 90% of solid fuel use for cooking within the PURE cohort occurs in rural areas.
Figure 2.
Distributions of household cooking fuel types and share of solid fuel use (percent households) by country, and stratified by rural/urban location.
Selection of household and personal sample for PM monitoring
The average number of PURE households in rural communities included in our study was 172, and were made up overall of about 60% women (Table 1). The PM2.5 monitoring includes ~4,500 households for cooking area measurements and ~1,800 individuals (i.e. 20% of the households) selected for personal sampling involving one male and one female from the selected households, with additional repeat sampling in 20% of the household and personal measurements. Priority was given to households having a pair of male and female members. Sample sizes by country were distributed according to the total number of participants enrolled in the main PURE study. In countries like China and India with multiple study centers, all centers within the country had equal number of households selected for measurement, but the total number of households by country differed in proportion to its total enrollment in the main PURE study (Table 1). In sum, the total number of households selected for monitoring across study centers ranged from 125 to 300.
Table 1.
Number of subjects, cooking fuel types, and required cooking area and personal samples by study center and country
| Country/Center | No. Sample | Kerosene | Charcoal | Coal | Wood | Other biomass | Electricity | Gas | Solid fuel | Non-solid fuel | % solid fuel | HH Sampling | Personal Sampling |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All countries | 44632 | 498 | 316 | 8554 | 13891 | 7371 | 1887 | 12115 | 30132 | 14002 | 68.3 | 4625 | 1850 |
|
| |||||||||||||
| India | 11526 | 73 | 1 | 79 | 7885 | 611 | 13 | 2864 | 8576 | 2877 | 74.9 | 1000 | 400 |
| Bangalore | 5116 | 8 | 0 | 11 | 4538 | 25 | 13 | 521 | 4574 | 534 | 89.5 | 200 | 80 |
| Chennai | 1700 | 54 | 0 | 5 | 699 | 3 | 0 | 939 | 707 | 939 | 43.0 | 200 | 80 |
| Jaipur | 648 | 2 | 0 | 8 | 499 | 56 | 0 | 83 | 563 | 83 | 87.2 | 200 | 80 |
| Trivandrum | 2984 | 6 | 0 | 1 | 1932 | 0 | 0 | 1045 | 1933 | 1045 | 64.9 | 200 | 80 |
| Chandigarh | 1078 | 3 | 1 | 54 | 217 | 527 | 0 | 276 | 799 | 276 | 74.3 | 200 | 80 |
|
| |||||||||||||
| China | 22682 | 151 | 186 | 8157 | 2034 | 5969 | 576 | 5609 | 16346 | 6185 | 72.5 | 1500 | 600 |
| Yunnan | 932 | 0 | 43 | 2 | 813 | 22 | 28 | 24 | 880 | 52 | 94.4 | 125 | 50 |
| Qinghai | 924 | 22 | 1 | 330 | 16 | 524 | 0 | 31 | 871 | 31 | 96.6 | 125 | 50 |
| Beijing | 3383 | 18 | 37 | 433 | 248 | 772 | 36 | 1839 | 1490 | 1875 | 44.3 | 125 | 50 |
| Jiangsu | 3806 | 3 | 2 | 42 | 76 | 1902 | 40 | 1741 | 2022 | 1781 | 53.2 | 125 | 50 |
| Shandong | 3902 | 46 | 7 | 2318 | 15 | 25 | 269 | 1222 | 2365 | 1491 | 61.3 | 125 | 50 |
| Shanxi | 2909 | 29 | 11 | 2732 | 1 | 2 | 64 | 70 | 2746 | 134 | 95.3 | 125 | 50 |
| Shaanxi | 1848 | 15 | 9 | 1182 | 53 | 440 | 5 | 144 | 1684 | 149 | 91.9 | 125 | 50 |
| Liaoning | 998 | 0 | 0 | 19 | 1 | 771 | 46 | 161 | 791 | 207 | 79.3 | 125 | 50 |
| Jiangxi | 1026 | 1 | 1 | 182 | 17 | 612 | 18 | 195 | 812 | 213 | 79.2 | 125 | 50 |
| Inner M | 1099 | 13 | 4 | 258 | 91 | 601 | 37 | 95 | 954 | 132 | 87.8 | 125 | 50 |
| Xinjiang | 864 | 4 | 70 | 44 | 688 | 23 | 0 | 35 | 825 | 35 | 95.9 | 125 | 50 |
| Sichuan | 991 | 0 | 1 | 615 | 15 | 275 | 33 | 52 | 906 | 85 | 91.4 | 125 | 50 |
|
| |||||||||||||
| South Africa | 1467 | 182 | 8 | 12 | 555 | 17 | 598 | 95 | 592 | 693 | 46.1 | 200 | 80 |
| Cape Town | 606 | 77 | 3 | 11 | 163 | 14 | 308 | 30 | 191 | 338 | 36.1 | 200 | 80 |
|
| |||||||||||||
| Tanzania | 515 | 84 | 43 | 9 | 371 | 5 | 0 | 3 | 428 | 3 | 99.3 | 300 | 120 |
|
| |||||||||||||
| Colombia | 3260 | 3 | 37 | 292 | 904 | 28 | 22 | 1974 | 1261 | 1996 | 38.7 | 300 | 120 |
|
| |||||||||||||
| Zimbabwe | 535 | 1 | 5 | 0 | 497 | 8 | 20 | 4 | 510 | 24 | 95.5 | 300 | 120 |
|
| |||||||||||||
| Brazil | 1706 | 0 | 34 | 2 | 230 | 0 | 0 | 1440 | 266 | 1440 | 15.6 | 125 | 50 |
| Angatuba | 1074 | 0 | 29 | 2 | 151 | 0 | 0 | 892 | 182 | 892 | 16.9 | 125 | 50 |
| Guarei | 632 | 0 | 5 | 0 | 79 | 0 | 0 | 548 | 84 | 548 | 13.3 | ||
|
| |||||||||||||
| Chile | 592 | 0 | 0 | 2 | 513 | 1 | 1 | 75 | 516 | 76 | 87.2 | 300 | 120 |
|
| |||||||||||||
| Bangladesh | 1362 | 2 | 0 | 0 | 580 | 725 | 20 | 35 | 1305 | 55 | 96.0 | 300 | 120 |
|
| |||||||||||||
| Pakistan | 349 | 2 | 1 | 1 | 322 | 7 | 0 | 16 | 331 | 16 | 95.4 | 300 | 120 |
Across the rural communities in our sample, cooking fuel types included several solid fuels (agricultural residue, animal dung, charcoal, coal, and wood), kerosene, and clean fuels (electricity and gas). The prevalence of solid fuel use at baseline was more than 50% in seven of our 10 study countries, and ranged from less than 20% in Brazil to more than 90% in Bangladesh, Pakistan, Tanzania, and Zimbabwe, whereas the use of gas or electricity was above 40% in Brazil, Colombia, and South Africa (Figure 2). While there have been changes in primary fuel use in individual homes since study enrollment, our study households were selected to reflect the baseline fuel distribution in order to ensure a representative sample of cooking fuel types for each center to facilitate exposure modeling. In centers where the proportion of households cooking with clean fuel was more than 20%, households using solid fuels were oversampled such that only 20% of the sample from that center consisted of clean fuels. To account for differences in solid fuel use prevalence between communities within a center, we purposely included only communities with at least 20% solid fuel use in the sample frame of each center, and then randomly selected enough communities to make up the required sample size for that center. Overall, we selected enough communities in each center so that there was a backup sample of up to twice the number of required household samples.
Selected households and PURE participants’ identification numbers (IDs) were provided to the local study coordinators and their field staff, who would be responsible for the field measurements. Households will be visited prior to the start of measurement to explain the aims of the study and request participation through informed consent. Efforts will be made to find a replacement household of the same cooking fuel type as planned when a household switched fuel, refused, or is not available for monitoring. Thus, on a given fieldwork day, site coordinators arrange for collection of a specific number of cooking area and personal samples, taking into account cooking fuel type, participants’ availability, and the strength of their field staff.
PM measurement design
To implement a systematic and scalable protocol efficiently and effectively in multiple communities across our 10 countries, we utilized a new low cost PM2.5 monitoring device and simple sampling protocols. First, we describe the monitor and present evaluation data from a pilot study conducted in two PURE communities in India. Next, we document protocols for household and personal PM2.5 data collection.
PM monitoring device
The Ultrasonic Personal Aerosol Sampler (UPAS) (Volckens et al. 2017), a novel, robust, and inexpensive filter based monitor (https://accsensors.com/technologies/#UPAS), was used to measure both cooking area and personal PM2.5 concentrations (Figure 3). The UPAS monitor is a time-integrated filter sampler that uses a quiet solid-state piezoelectric micropump, and features a suite of onboard environmental sensors integrated with the pump to measure and record mass airflow [at between 0.5–3.0 liters per minute (lpm) (±5%)], temperature, pressure, and relative humidity. It is also equipped with global positioning system (GPS) tracking capability, which can be disabled depending on study/user requirements. In addition to being quiet and energy efficient, the mass flow sensor enables the device to maintain a constant sampling flow rate and measures changes in pressure drop across the filter media, giving the UPAS the advantage of having reliably steady flow rate over time. As part of our quality assurance procedure in the field, local study coordinators periodically check the mass flow sensors with external mass flow meters. In laboratory evaluations, the UPAS demonstrated very good agreement with the Personal Environmental Monitor (PEM) as well as a PM2.5 Federal Reference Monitor, indicating applicability for both household and personal monitoring (Volckens et al. 2017).
Figure 3.
The Ultrasonic Personal Aerosol Sampler (UPAS).
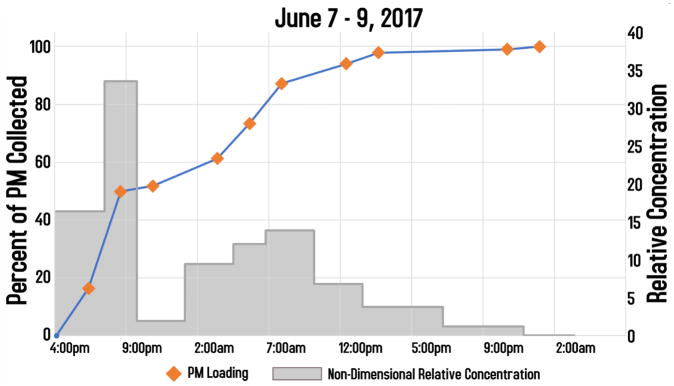
Further, the UPAS monitor provides semi-quantitative estimates of continuous particle concentrations. Using the differential pressure data that is continuously measured, equations can then be used to calculate estimates of time-resolved PM concentrations as shown in Figure 4. This information will be useful, for example, to count the number of cooking events that occur in a particular home during the 48-hour measurement period or to provide initial feedback to field coordinators and participants.
Figure 4.
Relative PM2.5 concentrations by time of day as measured using the filter differential pressure from the data log files.
The differential pressure data from the log files are converted into estimates of PM concentrations for quality control and diagnostics purposes (e.g. to count the number of cooking events that occur in a particular home during the 48-hour measurement period)
Field evaluation pilot study
Fitted with interchangeable cyclone inlets, the UPAS has been shown to provide a close match to the US EPA PM2.5 mass criterion (within 5%) for device flows at either 1.0 or 2.0 lpm (Volckens et al. 2017). However, prior to rolling out the field measurement campaign, we conducted a separate field test in selected households to evaluate the performance of the first generation (v1.0) UPAS monitor against the established Harvard Impactors (Marple et al. 1987), and also to help refine our proposed sampling strategies and modelling approaches. Given our emphasis for the main monitoring protocol on cooking area measurements, and in the absence of a standard reference personal sampler, we did not conduct comparisons between the personal UPAS measurements and other personal PM2.5 samplers. Personal UPAS measurements were collected during the Pilot Study to evaluate battery life and sampling duration, assess participant compliance, and to identify participant concerns or potential weaknesses with the sampler.
This pilot study was conducted in two PURE rural communities in India: Andhra Pradesh (Bellupale) in southern India, and Haryana (Kheri) in northern India. The two communities differed in terms of the type of solid fuel used for cooking. Although liquefied petroleum gas (LPG) was the most common cooking fuel in both communities (~ 70%), wood was the predominant solid fuel type in the southern community, whereas a mix of wood and dung were popular in the northern community.
Between July and August 2015, we collected cooking area PM2.5 samples successfully in 43 households using the initial (v1.0) version UPAS monitors collocated with Harvard Impactors. The UPAS monitors were fitted with a 0.2 μm pore size 37mm Teflon coated glass fiber filters (Fiberfilm, Pall Corporation) and drew air at 1 lpm, whereas the Harvard Impactors used 0.2 μm pore size 37mm PTFE filters (Teflo, Pall Corporation) with a polyolefin support ring at a flow rate of 10 lpm. Sampling parameters on this first generation UPAS were not programmable, and thus, samples were collected at 100% duty cycle, whereas the Harvard Impactors sampled at 50% duty cycle to avoid overloading. Both monitors were placed in the kitchen area of participating households at ~one meter from the primary cookstove and ~one meter above the kitchen floor. Measurements for both monitors were conducted over both 24- and 48-h periods at a 2:1 ratio, respectively.
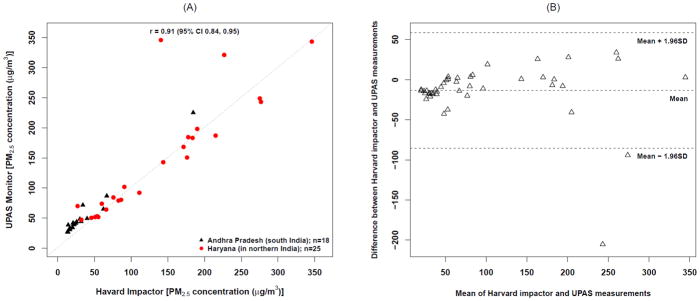
The measured PM2.5 concentrations did not differ significantly between the two sampling durations. The mean difference between the 24- (n=28) vs. 48-hr (n=15) samples by monitor types was 112 vs. 97 μg/m3 (95% CI: −43, 74) for the UPAS, and 97 vs. 88 μg/m3 (95% CI: −50, 67) for the Harvard Impactor; it was 104 vs. 92 μg/m3 (95% CI: −28, 52) across both monitors. The median household PM2.5 concentrations across both communities was 70 μg/m3 (IQR: 44–159) for the UPAS and 60 μg/m3 (IQR: 26–157) for the Harvard Impactor. Individual home levels ranged from 15 μg/m3 to more than 300 μg/m3. The UPAS recorded slightly higher average PM2.5 levels (107 μg/m3) when compared to the Harvard Impactor’s 93 μg/m3, but the confidence interval for the difference in means crossed the null (95% CI: −51, 24). We found high correlation between the samples from the UPAS and Harvard Impactors (r = 0.91; 95% CI: 0.84, 0.95), although there was noticeable deviation between monitor types at high concentrations (> 250 μg/m3) compared to low concentrations (Figure 5A). A Bland–Altman difference plot indicated agreement between the two monitor types as the majority of the individual mean differences showed no systematic bias and were contained within the 95% confidence interval (Figure 5B). Some of the minor biases in the UPAS measurements were thought to be due to an adsorption artifact on the filter media (Volckens et al. 2017), which has since been changed in the current (v2.0) version of the UPAS.
Figure 5.
The relationship between PM2.5 measurements from the UPAS and reference Harvard Impactor.
(A) Pearson correlation and coefficient. (B) Bland–Altman (Tukey mean-difference) plot of the UPAS and reference Harvard Impactor.
In addition to the household samples, we also collected successful personal samples on pairs of male and female participants in 60 households (31 males and 29 females) to evaluate battery life and sample duration, assess participant compliance, and to identify participant concerns or potential weaknesses with the sampler. Failed personal samples in the pilot study were primarily due to firmware or battery failures in the v1.0 UPAS; devices covered by clothing (e.g. saris); or to damaged filters. In our on-going field campaign using v2.0 UPAS, of the ~1700 samples collected to date, we have a failure rate of approximately 10%, with the majority due to unknown reasons resulting in the UPAS being shut off prior to the 48-hour sampling duration. However, as detailed below, we implement near real-time monitoring of UPAS errors using an automated program to identify any such invalid samples quickly and request re-sampling from field teams. The v1.0 UPAS used in the pilot study did not have GPS capability enabled, thus, we could not objectively assess participant compliance beyond verbal reports and field observations.
Following our field test, a number of changes were made in the subsequent generations of the UPAS monitors to improve use. These included the use of PTFE membrane filters with support rings to minimize any adsorption artifact, and barcoded filter cartridges to hold the filters instead of the bare filters used in the v1.0 version, which improves ease of use, protection, and tracking of filters. Also, barcoded PTFE filters are used to allow for automated weighing pre- and post-sampling. Additionally, the monitors now operate on a user-defined duty cycle, conserving battery life during sampling. Accordingly, the v2.0 UPAS monitors being used in the main field measurement campaign are set to sample PM2.5 mass at 1 lpm onto a barcoded 0.2-μm pore size 37mm PTFE filters contained in barcoded cartridges. With PTFE filter media, a flow rate of 1 lpm and a 50% duty cycle (15 seconds on, 15 seconds off) is used to ensure the UPAS’s battery life ranges between 48 to 55 hours, enabling collection of a 48-hour sample on a single battery charge, following a 10-hr charging period. Together, these changes significantly improved the performance and ease of use of the UPAS monitor.
Monitoring within the PURE Cohort
We began our main field monitoring campaign in the PURE cohort in June 2017, and monitoring will continue to 2019. During this time, we will monitor PM2.5 in ~4,500 households as well in ~1,800 individuals (i.e. 20% of the households) selected for personal sampling involving one male and one female from each household. Additional 20% repeat measurement will be collected to assess impacts of seasonality. Below are the descriptions of the monitoring protocol used in the main study.
Household and personal PM monitoring
Cooking area PM2.5 concentrations are collected using v2.0 UPAS samplers mounted on a one-meter-high monitor stand and placed ~1 meter from the primary cookstove in such a way as not to interfere with normal cooking activities (Figure 6). Together with the cooking area samples, personal PM2.5 exposures of a male and female household member are collected in 20% of selected households, with the personal samplers placed in a custom designed harness worn by the subjects. The harness design was refined based on participant feedback during the pilot study and holds the monitor on the participant’s upper body close to the breathing zone, in one of two configurations (body vs. armband) (Figure 7) to be adapted according to local socio-cultural preferences and climatic suitability. In the subset of homes where personal measurements are collected, cooking area and the personal monitoring is conducted concurrently. In cases where personal monitoring cannot be conducted on both male and female PURE participants in the same household, replacements are made in order to sample an equivalent number of males and females as planned for that center. All measurements are conducted over a 48-hour period in order to capture two sets of full daily cooking events. Field staff administer a short survey for each household and personal sample to collect information on household characteristics and behaviours during the 48-hour monitoring period. The field survey, which follows the same protocols as other PURE study surveys (involving standardized case report forms, translation where needed, and scanning for database entry), includes questions on household’s current and previous primary and secondary cooking fuel; length (years) of current and previous primary fuel use; cookstove type (open fire, mud stove, stove with chimney, stove with exhaust hood, stove with built in fan, and charcoal stove), kitchen design/location and ventilation; heating fuel and length of heating fuel use; occupation; tobacco exposure; and household size (See Appendix 1 for survey instrument).
Figure 6.
UPAS setup for monitoring cooking area PM2.5 concentrations
Figure 7.
A custom designed harness to hold the UPAS monitor to be worn by the subjects either over the shoulder or upper arm
It is not feasible to conduct repeated multi-season sampling for all households in the 10 countries. However, in order to facilitate modeling of seasonal variations in HAP exposures (Gurley et al. 2013; Ni et al. 2016), a repeat sampling campaign of 20% of household and personal measurements will be conducted six months after the original measurement campaign in one center from each of the following seven sampling clusters: South America (Brazil, Chile, Colombia); Sub-Saharan Africa (South Africa, Tanzania, Zimbabwe); South Asia (Bangladesh, Pakistan); South India; North India; East China; and West China. This will provide regional data on the impact of seasonality on household and personal exposures.
Filter preparation and management using an automated filter weighing systems
Weighing a large number of filters (~ 7,000) manually would be laborious and likely result in operator error from factors such as operation fatigue, weighing repeatability, poor judgment, and inconsistent accuracy. To address such problems associated with manual weighing, we are using pre-barcoded filters that are weighed pre- and post-sampling using an MTL fully automated robotic scale (http://www.mtlcorp.com/#/filter-weighing/) maintained in a temperature- and relative humidity-controlled laboratory at The University of British Columbia (UBC)’s School of Population and Public Health. Prior to weighing, the barcodes of the filters are scanned and time-stamped, and then placed in individual carriers and loaded into the MTL input silos to equilibrate to laboratory conditions. Filter weighing software, which controls the unit, is then activated to weigh each filter according to pre-programed parameters. During the weighing process, barometric pressure, temperature, and humidity are measured, allowing the software to apply a buoyancy correction. System generated weighing reports for each filter are issued for quality control purposes. The pre-weighed filters are again scanned, and then paired to and placed inside barcoded UPAS filter cartridges as shown in Figure 8. The preloaded filter cartridges are later sealed into individual packages for shipment. The filter cartridges are only removed from the packages and loaded directly into UPAS monitors immediately before sampling, and then removed and repackaged immediately after sampling. Thus, field staff are at no point in direct contact with the actual filters outside of their protective cartridges.
Figure 8.
Barcoded filter and a filter cartridge
Because black carbon aerosols are a known combustion-related component of PM2.5 and contribute to global climate change, all post-weighed filters are also analyzed to estimate black carbon concentrations through a semi-automated, rapid, cost-effective image analysis approach based on a method developed previously (Ramanathan et al. 2011). Several studies in the past have relied on the Smoke Stain Reflectometer (http://www.diffusion-systems.com; Model EEL 043) to measure optical reflectance as a surrogate for black carbon concentration. This approach is time consuming, susceptible to operator error, and prone to baseline drift. An image-based method can eliminate these problems (Ramanathan et al. 2011) and will be applied to all post-weighed filters. Preliminary results of our method show a high correlation between the image-based and the reflectometer methods (slope, β =1.02; R2 = 0.99).
Logistics and training
We purchased and assembled a total of 7 sets of sampling kits, with each kit containing 22 v2.0 UPAS monitors, 12 collapsible monitor stands, 3 smartphones, 12 adjustable monitor harnesses (6 body and 6 armbands), 200 filters plus 10% travel blanks, and other accessories. For ease of transportation, a kit consisted of 3 pieces of luggage, one dedicated to the filters, and the others for the monitors, stands, smartphones, and their accessories. Because there were multiple centers in China (12 centers) and India (5 centers), 3 and 2 kits, respectively, were sent to a central coordinating center, where all local center coordinators met for training, and planned center-specific sampling schedule in consultation with the project team. The remaining 2 of the 7 kits were sent to Tanzania and Chile. Upon completion of sampling in a center, the sampled filters will be sent back to the project team at UBC for analysis while the rest of the sampling kits (along with resupplied pre-weighed filters) will be rotated to other centers within the country. After sampling in a country, the kits will be sent to the next study country within that geographic region. For instance, kits in India will be sent to Bangladesh/Pakistan, whereas kits in Tanzania will go to Zimbabwe/South Africa, and same for countries in South America.
We developed a simplified field sampling protocol describing steps involved in pre-fieldwork, monitor preparation, field deployment, and post-sampling. We also produced short explanatory videos covering important steps in the protocol for the field staff. The manual and videos were provided to all country and local coordinators beforehand through a YouTube channel (https://www.youtube.com/channel/UCVU3EFv1EvWQUWkwFNRG9kA/videos?view=0&sort=dd&shelf_id=0). The videos were also loaded onto the project supplied smartphones so field staff could access them at any time without internet access. On-site training of center coordinators and field staff was also conducted in India and China.
Data management
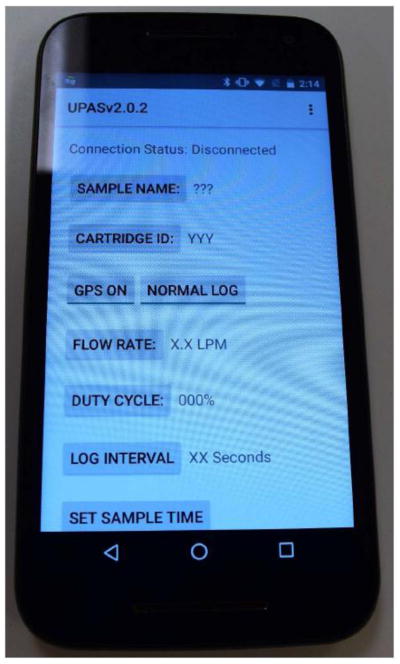
To aid our field operations, the manufacturer developed a dedicated mobile application (app) that is used to program and activate the UPAS monitors using Android smartphones supplied by the research project (Figure 9). Prior to the fieldwork, each UPAS monitor is paired and connected to the phone via Bluetooth technology. In the field, the app is used to program sample identifiers, including monitor, filter cartridge, and sample IDs, in addition to identifiers of PURE center, community, household, and participant IDs. As part of our quality assurance, and in order to protect some key parameters from being accidentally modified in the field, sampling flow rate, duty cycle, and logging intervals are pre-preprogramed and locked, and thus not user-defined in the field app.
Figure 9.
Mobile application for programing and activating the UPAS.
After sampling, the UPAS devices, through the app and Bluetooth connectivity, wirelessly transmits detailed sampling log information to their controlling smartphone, which will in turn relay this information by the internet to a central server at the study coordinating center at UBC. At the central server, we wrote an R script (R Project for Statistical Computing) (see Appendix 2) that scans arriving sample logs once every 24 hours to check for signs of error (e.g. consistent flow rate, sampling duration, and total volume sampled), and the error status is automatically emailed to the project team. When an error is detected, we immediately contact the corresponding local coordinator to remove the affected UPAS monitor from the field for repairs, and the associated filter cartridge/ID is flagged on the main server. Original sample logs, although transmitted wirelessly, are also retained on each device in removable microSD cards, which serve as backup, and can be manually transferred to the central computer if local internet connectivity is not available.
All used filter cartridges are shipped back to the project team at UBC along with the case report forms and microSD cards containing copies of UPAS’ sampling logged data. For tracking and quality assurance purposes, both the post-sampling cartridges and filters are rescanned and time-stamped before the filters are post-weighed as described earlier. The final data will then be compiled into a single data set by matching on relevant IDs and linked to PURE study baseline surveys, with each record representing a unique country, center, community, household, individual participants, and PM mass concentration as well as fuel types and other household/individual characteristics.
PM2.5 exposure models
Development of cooking area and personal PM2.5 exposure models
The collected measurements will provide exposure distributions for PURE communities to inform how household and personal PM2.5 exposures vary between and within countries. In addition, the measurements will be used to create predictive models on household PM2.5 concentrations. This approach follows Balakrishnan et al. (Balakrishnan et al. 2013), who used data from several rural homes in four states in India to demonstrate how national survey data containing records on cooking fuel type, ventilation, cooking location, and family size could be used to predict measured PM2.5 concentrations. This modelling approach offers substantial improvement over commonly used indicators for HAP exposure in epidemiologic analyses such as household cooking fuel types (Clark et al. 2013b), and thus the potential to reduce exposure misclassification for countries where no or little HAP monitoring data exist. Following Balakrishnan et al, (Balakrishnan et al. 2013), we will use the measured PM2.5 concentrations and information on a range of household and individual variables from the main PURE baseline household questionnaire to develop HAP PM2.5 exposure models in relation to these variables. We will also use the personal (male/female)-household measurement relationships as adjustment factors to predict male/female exposures in the cohort. For use in epidemiologic analyses we will model the relationship between individual fuel types and PM2.5 in order to estimate exposure levels for a given type of fuel used (including use of secondary fuels) at baseline under a given set of conditions as characterized by the information on the household questionnaire. We will also develop separate exposure estimates based on time-weighting of primary fuel types as reported at each follow-up period for homes that switched fuels since baseline. Together, these will allow us to assess either estimated exposures at baseline or cumulative/average exposures during follow-up after accounting for fuel switching and the resulting changes in (estimated) PM2.5 exposures. We will then be able to explore relationship between specific cardiopulmonary outcome and PM2.5 exposures due to specific cooking fuel type as well as the combined HAP PM2.5 exposures.
Cooking area model results from pilot data
To demonstrate the potential of such quantitative exposure models, we used the data from samples collected in pilot testing (described above) in two rural communities in India in the summer of 2015 in a simple proof of concept model. In addition to the 60 cooking area and 60 personal PM2.5 samples collected on the primary male and female residents using the v1.0 UPAS monitors, all participating households were assessed for a number of different features, including cooking locations (75% cooked inside), ventilation features (chimneys, windows), and home construction materials. This assessment followed the original PURE baseline questionnaire. Also, we collected information on cooking times and duration, tobacco use, household size, and types of cooking fuels (72% gas; 26% wood and dung) used during the monitoring period. Besides the PM2.5 mass concentration, the filters were analyzed for reflectance (Bond et al. 2007) using an EEL smoke stain reflectometer (Model 43D by Diffusion Systems Ltd.). We used the cooking area v1.0 UPAS PM data and reflectance measures to test the feasibility of building predictive household PM exposure models (Balakrishnan et al. 2013) by fitting a simple linear regression model to examine the association of average cooking area PM2.5 concentrations or reflectance with its potential household determinants. These models from the pilot data are presented as proof-of-concept and not intended to inform exposure relationships.
The basic models including household characteristics explained ~65% and ~45% of the variability in cooking area PM concentrations and reflectance, respectively. In this small sample and simple model, the results indicate that household cooking fuel, cooking area location (inside or outside), the presence of ventilation features, and household size may be important determinants of HAP PM exposures, as well as the measure of reflectance, which could serve as an indicator of various combustion sources in the home. However, this was a small sample and the model performance is likely driven by large differences in concentrations and fuel use patterns between the two villages where the pilot study was conducted.
From the personal samples collected in the pilot study, women on average had slightly higher personal PM2.5 exposures than men (geometric mean [GM]= 85 vs 76 μg/m3). While personal exposures in women were almost identical to cooking area levels, with a geometric mean personal-to-cooking area ratio of 0.98, exposures in men were higher than cooking area concentrations, resulting in geometric mean personal-to-cooking area ratio of 1.2. This was likely due to workplace exposures and we have added questions to the main monitoring protocol to assess workplace air pollution exposures as well as direct and second hand smoke exposures. The mean reflectance measure in men was also slightly higher than in women. Again, no firm conclusions regarding exposure levels or relationships should be drawn from the pilot results as they were intended to inform the main field measurement campaign.
Discussion and conclusion
Cooking with solid fuels is widespread in several LIC and MIC, particularly in rural areas, exposing household members to high pollutant concentrations with potentially serious health implications. However, there are major uncertainties in the global estimates of the health impact of HAP primarily due to the lack of quantitative exposure information and their application in epidemiologic analyses in general, and to CVD in particular. As yet, there are almost no comprehensive epidemiological studies that have examined the associations of HAP from solid fuel use with cardiopulmonary events and mortality in LIC and MIC. The overall aim of this work is an effort to provide refined and more accurate exposure estimates in HAP-CVD related exposure-response analyses.
Our study to characterize cooking area and personal PM2.5 air pollution exposure in several rural communities in 10 LIC and MIC represents one of the largest efforts to date to provide detailed data on HAP exposures and its impact on related cardiopulmonary health in developing country settings. Our design approach is to measure PM2.5 data in a subset of participants within the PURE study and combine the measured data with household/individual level variables from surveys in order to develop statistical models to estimate HAP PM2.5 exposures for all study participants (~ 50,000) across 10 LIC and MIC where solid fuel use is common in rural areas. The models will have adjustment factors for gender and other key factors like season, and geographic region. The estimated PM2.5 exposures will be used to assess their link with outcomes such as incident disease, mortality, blood pressure, and lung function in the large multi-country PURE cohort.
While this combined measurement and modelling approach allows for individual exposure estimation in this large multi-centre cohort, which improves upon survey-based exposure proxies such as solid fuel use, it falls short of full-scale repeated personal exposure monitoring employed in several single community or regional analyses (Balakrishnan et al. 2015; Balakrishnan et al. 2018; Baumgartner et al. 2011; Baumgartner et al. 2014). Our emphasis on cooking area measurements supplemented with personal monitoring in a sub-sample allows us to collect more samples and avoids complexities in differentiating personal measurements from the source of interest vs. other sources of PM exposure, such as those experienced at workplaces or in the community.
Further, our pilot study suggested that personal samples could be successfully collected with the UPAS but we did not directly assess compliance or the impact on time-activity patterns given an already high participant burden due to the many assessments in the parent PURE study. In the main study, however, we will review GPS data to qualitatively assess whether participants are actually wearing personal samplers per protocol. Our example preliminary models based on the pilot study demonstrated proof-of-concept and suggested that the measurement and modelling approach could offer the potential to differentiate between households/individuals using the same fuels. Although we anticipate a large variation in measured concentrations amongst all of the samples to be collected from across the diversity of settings in the 10 countries, the extent to which the modelling success from the pilot translates to the full study remains to be seen. Further, we may have limited ability to differentiate between households within the same community or centre as this will depend on variation in household factors (size of family, age of participants, assets/wealth, education, ventilation, etc.) and the strength of these as predictors of HAP levels. Still, even providing center or country specific estimates of PM2.5 exposure for the same fuel type is an advancement over the currently available simple proxies such as solid vs. clean fuel use or use of a specific fuel type where the same fuel type is treated similarly regardless of location and season.
Our overall approach to assessing HAP exposure in a large epidemiological study provides guidance for future epidemiological studies, and modelling of HAP exposures. We employ state of the art technologies and a novel study design that leverages a lightweight, quiet, energy efficient, and low cost filter-based PM monitor with a suite of onboard environmental sensors and other capabilities. The monitors are programmable using mobile apps with user-defined features that transmit log files for quality control. We follow simple sampling protocols, offer flexibility to field staff regarding sample collection, and rely on automation for filter weighing, reflectance measurements, and data management.
The collection of detailed PM2.5 measurement data, combined with baseline and repeat survey data, will allow us to better estimate actual PM2.5 exposures resulting from HAP and its associations with chronic health outcomes. We will examine measured PM2.5 (in the subset with direct measurements) and the modelled exposures (in the entire PURE LIC and MIC cohort) in separate epidemiologic analyses to assess the associations of cooking area and individual PM2.5 concentrations with several CVD and respiratory outcomes, including incident disease, mortality, blood pressure, and lung function in both cross-sectional (baseline data) and longitudinal (at three-, six-, and nine-year follow-up) analyses. These analyses will involve multilevel models that account for a comprehensive set of individual, household, and community-level information collected at the main PURE Study entry and follow-ups (Teo et al. 2009; Yusuf et al. 2014).
In addition, within the context of the Global Burden of Disease, recently available sub-national baseline disease burden data, for example in China (Zhou et al. 2016) and India (Dandona et al. 2017), will benefit from characterization of sub-national variability in HAP exposures, and thus provide better understanding of the impact of HAP on CVD and respiratory outcomes in such complex exposure settings. Multi-centre HAP exposure measurements and models such as those being developed for PURE will be central to this effort, not only to better inform global HAP exposures but also the chronic health risks posed by these exposures across diverse geographies.
Overall, our study design and approach will overcome some of the major limitations of previous studies. This will be the largest, multinational model constructed, with identical cooking environment variables collected from administered questionnaires in each country to be included, also minimizing biases from aggregating Demographic Health Survey data across countries, which would be necessary to construct a multinational quantitative HAP-PM2.5 exposure model. Our questionnaire data and PM2.5 monitoring data are collected in the same study eliminating potential biases introduced by linking data from different study protocols. By nesting this study in the larger PURE cohort that has 10-year annual follow up on CVD events, we can assess the long-term effects of HAP on CVD outcomes, rather than solely acute CVD effects. This will also help inform future burden of disease assessments which focus on CVD incidence and mortality, but not acute events or sub-clinical indicators. The extensive information on potential confounding variables relevant to the relationship between HAP and CVD are also collected in PURE surveys for all countries, allowing for assessment of the risk attributed to HAP, while controlling for important confounders such as diet, exercise and socioeconomic indicators. The PURE study also includes monitoring of ambient air pollution at a community level. This will allow us to assess joint effects of ambient air pollution and HAP on various health outcomes to be examined, which will evaluate how both sources of PM2.5 exposures interact to adversely impact cardiovascular and respiratory health.
Supplementary Material
Highlights.
We aim to provide refined and more accurate exposure estimates in HAP-CVD related exposure-response analyses
We leverage technology advancements to estimate household and personal PM2.5 exposures in a large international cohort
PM2.5 levels are monitored using UPAS in ~4,500 households and ~1,800 male/female pairs from the selected households in 10 countries
The measured PM2.5 data will then be combined with household surveys to develop quantitative HAP models for PM2.5 exposures for entire cohort
Both the measured and the modelled exposures will be used in separate longitudinal epidemiologic analyses to assess associations with cardiopulmonary mortality, and disease incidence
Acknowledgments
Funding: This work was supported by a Michael Smith Foundation for Health Research Trainee Award to RA; by the Canadian Institutes of Health Research (CHIR) [grant #136893]; and by the Office of The Director, National Institutes of Health [Award Number DP5OD019850]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or CIHR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, Abdulle AM, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: A systematic analysis for the global burden of disease study 2016. The Lancet. 2017;390:1345–1422. doi: 10.1016/S0140-6736(17)32366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abtahi M, Koolivand A, Dobaradaran S, Yaghmaeian K, Mohseni-Bandpei A, Khaloo SS, et al. National and sub-national age-sex specific and cause-specific mortality and disability-adjusted life years (dalys) attributable to household air pollution from solid cookfuel use (hap) in iran, 1990–2013. Environ Res. 2017;156:87–96. doi: 10.1016/j.envres.2017.03.026. [DOI] [PubMed] [Google Scholar]
- Alam DS, Chowdhury MA, Siddiquee AT, Ahmed S, Hossain MD, Pervin S, et al. Adult cardiopulmonary mortality and indoor air pollution: A 10-year retrospective cohort study in a low-income rural setting. Glob Heart. 2012;7:215–221. doi: 10.1016/j.gheart.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Alexander D, Larson T, Bolton S, Vedal S. Systolic blood pressure changes in indigenous bolivian women associated with an improved cookstove intervention. Air Quality, Atmosphere & Health. 2015;8:47–53. [Google Scholar]
- Arku RE, Ezzati M, Baumgartner J, Fink G, Zhou B, Hystad P, et al. Elevated blood pressure and household solid fuel use in premenopausal women: Analysis of 12 demographic and health surveys (dhs) from 10 countries. Environ Res. 2017;160:499–505. doi: 10.1016/j.envres.2017.10.026. [DOI] [PubMed] [Google Scholar]
- Assad NA, Kapoor V, Sood A. Biomass smoke exposure and chronic lung disease. Curr Opin Pulm Med. 2016;22:150–157. doi: 10.1097/MCP.0000000000000246. [DOI] [PubMed] [Google Scholar]
- Balakrishnan K, Ghosh S, Ganguli B, Sambandam S, Bruce N, Barnes DF, et al. State and national household concentrations of pm2.5 from solid cookfuel use: Results from measurements and modeling in india for estimation of the global burden of disease. Environ Health. 2013;12:77. doi: 10.1186/1476-069X-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan K, Sambandam S, Ramaswamy P, Ghosh S, Venkatesan V, Thangavel G, et al. Establishing integrated rural-urban cohorts to assess air pollution-related health effects in pregnant women, children and adults in southern india: An overview of objectives, design and methods in the tamil nadu air pollution and health effects (taphe) study. BMJ Open. 2015;5:e008090. doi: 10.1136/bmjopen-2015-008090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan K, Ghosh S, Thangavel G, Sambandam S, Mukhopadhyay K, Puttaswamy N, et al. Exposures to fine particulate matter (pm2.5) and birthweight in a rural-urban, mother-child cohort in tamil nadu, india. Environ Res. 2018;161:524–531. doi: 10.1016/j.envres.2017.11.050. [DOI] [PubMed] [Google Scholar]
- Baumgartner J, Schauer JJ, Ezzati M, Lu L, Cheng C, Patz JA, et al. Indoor air pollution and blood pressure in adult women living in rural china. Environmental health perspectives. 2011;119:1390–1395. doi: 10.1289/ehp.1003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner J, Zhang Y, Schauer JJ, Huang W, Wang Y, Ezzati M. Highway proximity and black carbon from cookstoves as a risk factor for higher blood pressure in rural china. Proc Natl Acad Sci U S A. 2014;111:13229–13234. doi: 10.1073/pnas.1317176111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond TC, Bhardwaj E, Dong R, Jogani R, Jung S, Roden C, et al. Historical emissions of black and organic carbon aerosol from energy- related combustion, 1850–2000. Global Biogeochemical Cycles. 2007:21. [Google Scholar]
- Bonjour S, Adair-Rohani H, Wolf J, Bruce NG, Mehta S, Pruss-Ustun A, et al. Solid fuel use for household cooking: Country and regional estimates for 1980–2010. Environmental health perspectives. 2013;121:784–790. doi: 10.1289/ehp.1205987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett RT, Pope CA, 3rd, Ezzati M, Olives C, Lim SS, Mehta S, et al. An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environmental health perspectives. 2014;122:397–403. doi: 10.1289/ehp.1307049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark ML, Bachand AM, Heiderscheidt JM, Yoder SA, Luna B, Volckens J, et al. Impact of a cleaner-burning cookstove intervention on blood pressure in nicaraguan women. Indoor Air. 2013a;23:105–114. doi: 10.1111/ina.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark ML, Peel JL, Balakrishnan K, Breysse PN, Chillrud SN, Naeher LP, et al. Health and household air pollution from solid fuel use: The need for improved exposure assessment. Environmental health perspectives. 2013b;121:1120–1128. doi: 10.1289/ehp.1206429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborators GBDRF. Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: A systematic analysis for the global burden of disease study 2013. Lancet. 2015;386:2287–2323. doi: 10.1016/S0140-6736(15)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi DJ, Subramanian S, Chow CK, McKee M, Chifamba J, Dagenais G, et al. Prospective urban rural epidemiology (pure) study: Baseline characteristics of the household sample and comparative analyses with national data in 17 countries. American heart journal. 2013;166:636–646. e634. doi: 10.1016/j.ahj.2013.04.019. [DOI] [PubMed] [Google Scholar]
- Dandona L, Dandona R, Kumar GA, Shukla D, Paul VK, Balakrishnan K, et al. Nations within a nation: Variations in epidemiological transition across the states of india, 1990–2016 in the global burden of disease study. The Lancet. 2017;390:2437–2460. doi: 10.1016/S0140-6736(17)32804-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong M, Islam S, Rangarajan S, Teo K, O’Byrne PM, Schünemann HJ, et al. Global differences in lung function by region (pure): An international, community-based prospective study. The Lancet Respiratory Medicine. 2013;1:599–609. doi: 10.1016/S2213-2600(13)70164-4. [DOI] [PubMed] [Google Scholar]
- Fatmi Z, Coggon D. Coronary heart disease and household air pollution from use of solid fuel: A systematic review. Br Med Bull. 2016;118:91–109. doi: 10.1093/bmb/ldw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gakidou E, Afshin A, Abajobir A. Gbd 2016 risk factors collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet. 2017;359:1345–1422. doi: 10.1016/S0140-6736(17)32366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley ES, Salje H, Homaira N, Ram PK, Haque R, Petri WA, Jr, et al. Seasonal concentrations and determinants of indoor particulate matter in a low-income community in dhaka, bangladesh. Environ Res. 2013;121:11–16. doi: 10.1016/j.envres.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Hang JQ, Zhang FY, Dai HL, Su L, Christiani DC. In-home solid fuel use and cardiovascular disease: A cross-sectional analysis of the shanghai putuo study. Environ Health. 2012;11:18. doi: 10.1186/1476-069X-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marple VA, Rubow KL, Turner W, Spengler JD. Low flow rate sharp cut impactors for indoor air sampling: Design and calibration. JAPCA. 1987;37:1303–1307. doi: 10.1080/08940630.1987.10466325. [DOI] [PubMed] [Google Scholar]
- McCracken JP, Smith KR, Diaz A, Mittleman MA, Schwartz J. Chimney stove intervention to reduce long-term wood smoke exposure lowers blood pressure among guatemalan women. Environmental health perspectives. 2007;115:996–1001. doi: 10.1289/ehp.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken JP, Schwartz J, Bruce N, Mittleman M, Ryan LM, Smith KR. Combining individual-and group-level exposure information: Child carbon monoxide in the guatemala woodstove randomized control trial. Epidemiology. 2009;20:127–136. doi: 10.1097/EDE.0b013e31818ef327. [DOI] [PubMed] [Google Scholar]
- Meng QY, Spector D, Colome S, Turpin B. Determinants of indoor and personal exposure to pm(2.5) of indoor and outdoor origin during the riopa study. Atmos Environ (1994) 2009;43:5750–5758. doi: 10.1016/j.atmosenv.2009.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitter SS, Vedanthan R, Islami F, Pourshams A, Khademi H, Kamangar F, et al. Household fuel use and cardiovascular disease mortality: Golestan cohort study. Circulation. 2016;133:2360–2369. doi: 10.1161/CIRCULATIONAHA.115.020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni K, Carter E, Schauer JJ, Ezzati M, Zhang Y, Niu H, et al. Seasonal variation in outdoor, indoor, and personal air pollution exposures of women using wood stoves in the tibetan plateau: Baseline assessment for an energy intervention study. Environ Int. 2016;94:449–457. doi: 10.1016/j.envint.2016.05.029. [DOI] [PubMed] [Google Scholar]
- Norris C, Goldberg MS, Marshall JD, Valois MF, Pradeep T, Narayanswamy M, et al. A panel study of the acute effects of personal exposure to household air pollution on ambulatory blood pressure in rural indian women. Environ Res. 2016;147:331–342. doi: 10.1016/j.envres.2016.02.024. [DOI] [PubMed] [Google Scholar]
- Painschab MS, Davila-Roman VG, Gilman RH, Vasquez-Villar AD, Pollard SL, Wise RA, et al. Chronic exposure to biomass fuel is associated with increased carotid artery intima-media thickness and a higher prevalence of atherosclerotic plaque. Heart. 2013;99:984–991. doi: 10.1136/heartjnl-2012-303440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillarisetti A, Alnes L, McCracken J, Canuz E, Smith K. Long-term pm2. 5 in kitchens cooking with wood: Implications for measurement strategies. Environmental Science and Technology. 2016;48:14525–14533. [Google Scholar]
- Pope CA, 3rd, Burnett RT, Krewski D, Jerrett M, Shi Y, Calle EE, et al. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: Shape of the exposure-response relationship. Circulation. 2009;120:941–948. doi: 10.1161/CIRCULATIONAHA.109.857888. [DOI] [PubMed] [Google Scholar]
- Quinn AK, Ae-Ngibise KA, Jack DW, Boamah EA, Enuameh Y, Mujtaba MN, et al. Association of carbon monoxide exposure with blood pressure among pregnant women in rural ghana: Evidence from graphs. Int J Hyg Environ Health. 2016;219:176–183. doi: 10.1016/j.ijheh.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan N, Lukac M, Ahmed T, Kar A, Praveen P, Honles T, et al. A cellphone based system for large-scale monitoring of black carbon. Atmospheric environment. 2011;45:4481–4487. [Google Scholar]
- Smith KR, Peel JL. Mind the gap. Environmental health perspectives. 2010;118:1643–1645. doi: 10.1289/ehp.1002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Bruce N, Balakrishnan K, Adair-Rohani H, Balmes J, Chafe Z, et al. Millions dead: How do we know and what does it mean? Methods used in the comparative risk assessment of household air pollution. Annu Rev Public Health. 2014;35:185–206. doi: 10.1146/annurev-publhealth-032013-182356. [DOI] [PubMed] [Google Scholar]
- Teo K, Chow CK, Vaz M, Rangarajan S, Yusuf S Group PI-W. The prospective urban rural epidemiology (pure) study: Examining the impact of societal influences on chronic noncommunicable diseases in low-, middle-, and high-income countries. American heart journal. 2009;158:1–7. e1. doi: 10.1016/j.ahj.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Tonne C, Salmon M, Sanchez M, Sreekanth V, Bhogadi S, Sambandam S, et al. Integrated assessment of exposure to pm2.5 in south india and its relation with cardiovascular risk: Design of the chai observational cohort study. Int J Hyg Environ Health. 2017;220:1081–1088. doi: 10.1016/j.ijheh.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Volckens J, Quinn C, Leith D, Mehaffy J, Henry CS, Miller-Lionberg D. Development and evaluation of an ultrasonic personal aerosol sampler. Indoor Air. 2017;27:409–416. doi: 10.1111/ina.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Mortality from household air pollution for 2012: Global health observatory (gho) data. Geneva, Switzerland: WHO; 2012. [Google Scholar]
- World Health Organization. Global health estimates: Deaths by cause, age, sex and country, 2000–2012. Geneva: WHO; 2014. [Google Scholar]
- Yusuf S, Rangarajan S, Teo K, Islam S, Li W, Liu L, et al. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med. 2014;371:818–827. doi: 10.1056/NEJMoa1311890. [DOI] [PubMed] [Google Scholar]
- Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu S, et al. Cause-specific mortality for 240 causes in china during 1990–2013: A systematic subnational analysis for the global burden of disease study 2013. The Lancet. 2016;387:251–272. doi: 10.1016/S0140-6736(15)00551-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.