Abstract
Background
Although pregnancy concentrations of some phenols have been associated with infant size at birth, there is limited data on the effect of preconception exposure.
Objective
We aimed to examine paternal and maternal preconception and maternal prenatal urinary phenol concentrations in relation to birth weight and head circumference.
Methods
We evaluated 346 singletons born to 346 mothers and 184 fathers (184 couples) from a prospective preconception cohort of subfertile couples from the Environment and Reproductive Health (EARTH) Study in Boston, USA. We used multiple urine samples collected before the index pregnancy in both men and women to estimate mean preconception urinary benzophenone-3, triclosan, butylparaben, propylparaben, methylparaben, or ethylparaben concentrations. We also estimated mean maternal prenatal urinary phenol concentrations by averaging trimester-specific urine samples. Birth weight and head circumference were abstracted from delivery records. We estimated the association of natural log-phenol concentrations with birth outcomes using multivariable linear regression models, adjusting for known confounders.
Results
In adjusted models, each log-unit increase in paternal preconception benzophenone-3 concentration was associated with a 137 g increase in birth weight (95% CI: 60, 214). Additional adjustment for prenatal benzophenone-3 concentration strengthened this association. None of the maternal preconception phenol concentrations were associated with birth weight. However, maternal prenatal triclosan concentrations were associated with a 38 g decrease in birth weight (95% CI: −76, 0). Few associations were observed between phenols and head circumference except for a decrease of 0.27 cm (95% CI: −54, 0) in relation to maternal preconception methylparaben concentration.
Conclusions
Although our findings should be interpreted in light of inherent study limitations, these results suggest potential evidence of associations between some paternal or maternal phenol concentrations and birth size.
INTRODUCTION
Birth size, a well-known marker of the intrauterine environment and fetal growth, is also one of the strongest predictors of neonatal morbidity and mortality (Basso et al. 2006; Calkins and Devaskar 2011; Kramer 1987; Wilcox 2001). Low birth weight infants (<2500 grams) have a significantly higher risk of death in the first year of life and a higher risk of disease and disability in childhood (Mathews and Driscoll 2017). Both low and high birth weight for gestational age are also associated with increased risk of chronic diseases later in life and premature adult death (Baker et al. 2008; Calkins and Devaskar 2011). Head circumference at birth – also a marker of fetal growth – is associated with poorer neurocognitive development and deficits in children (Cheong et al. 2008; Peterson et al. 2003). While many determinants of birth size are well established, including gestational age, maternal and paternal anthropometry, maternal nutritional status, pre-pregnancy weight and gestational weight gain, and tobacco use, others remain unknown (Kramer 1987; Valero De Bernabe et al. 2004). Exposure to some endocrine disrupting chemicals (EDCs) in utero may also influence fetal growth and consequently birth weight (Ferguson et al. 2016; Gore et al. 2015; Woodruff et al. 2008). However, important gaps remain including understanding the extent to which environmental chemicals impact birth size across different critical windows of exposure, including the preconception period.
Among the multitude of potential EDCs, there is widespread general population exposure to parabens, benzophenone-3, and triclosan which are used in many personal care products (Ferguson et al. 2017). Human exposure to these chemicals is ubiquitous in the United States, Europe, and elsewhere (Ferguson et al. 2017; Frederiksen et al. 2013; Guidry et al. 2015). Parabens, due to their anti-microbial and anti-fungal properties, are widely used as preservatives in products such as moisturizers, skin lotions, and other personal care products to increase shelf life (Giulivo et al. 2016). Benzophenone-3 is commonly used as an ultraviolet filter in sunscreens. The highest benzophenone-3 concentrations have been found in skin lotions (including sunscreens, face creams and body lotions), but also in makeup products (Liao and Kannan 2014). Triclosan is a multi-purpose antibacterial agent used in pharmaceuticals, household and personal care products such as hand-soap, mouthwash, and toothpaste (Dann and Hontela 2011). Concern over possible health effects of triclosan led the Food and Drug Administration to ban its use in over-the-counter antiseptic wash products in the United States in 2017 (Federal-Register 2016).
Accumulating epidemiologic evidence suggests that several EDCs such as some phthalates and phenols are associated with reproductive outcomes, including reduced birth weight, but also increased birth weight depending on the chemical (Lenters et al. 2016; Philippat et al. 2012; Wolff et al. 2008). Given that EDCs possess varied and complex mechanisms of action, both at the endocrine, neuro-endocrine, and metabolic levels (Mustieles et al. 2015), the relationship between EDC exposure and pregnancy and fetal-infant health is complex. Although a strong rationale for studying the prenatal window exists, the preconception period remains a potentially important but mostly unexplored critical window of exposure for perinatal and infant outcomes. Even less is known about the effects of paternal preconception exposure on the health of offspring. Our study therefore aimed to investigate whether paternal and maternal preconception phenol exposure, in addition to maternal prenatal exposure, was associated with birth weight and head circumference in a prospective cohort of couples undergoing treatment in a large fertility center.
METHODS
Study Cohort
The Environment and Reproductive Health (EARTH) Study is a prospective preconception cohort of couples from the Massachusetts General Hospital (MGH) Fertility Center. The EARTH Study – designed to assess the impact of environmental exposures and nutrition on fertility and pregnancy outcomes – has been ongoing since 2004 and has recruited approximately 800 women and 550 men to date (Messerlian in press). Women 18 – 46 years and men 18 – 55 years are eligible to participate and may enroll independently or as a couple. Participants are followed from study entry throughout their fertility care, pregnancy, and labor and delivery. At study entry, study participants complete questionnaires on socio-demographic, lifestyle, and medical and reproductive history, occupational history, diet, and personal care product use. Biospecimen collection includes spot urine and blood samples at baseline enrollment and subsequently when couples undergo treatment with medically assisted reproduction and during each trimester of pregnancy for those achieving conception.
This analysis included male and female participants from the EARTH Study with a singleton infant born between 2005 and 2016 (N=385) and for whom we had measured at least one urinary phenol concentration for the index birth (N=346) (see Figure 1, Participant Flow Chart). Among the 346 singleton infants, at least one urine sample was collected before conception of the index pregnancy for 330 mother-child pairs and 184 father-child pairs and analyzed for paraben concentrations. Measurement of benzophenone-3 and triclosan began in 2012 and preconception urinary concentrations of these phenols were available for 205 mother-child pairs and 101 father-child pairs (see Figure 1 for corresponding prenatal samples). Trained study staff described the study protocol to all participants in detail and answered questions. Participants provided signed informed consent. The study was approved by the Institutional Review Boards of MGH, Harvard T.H. Chan School of Public Health, and the Centers for Disease Control and Prevention (CDC).
Figure 1.
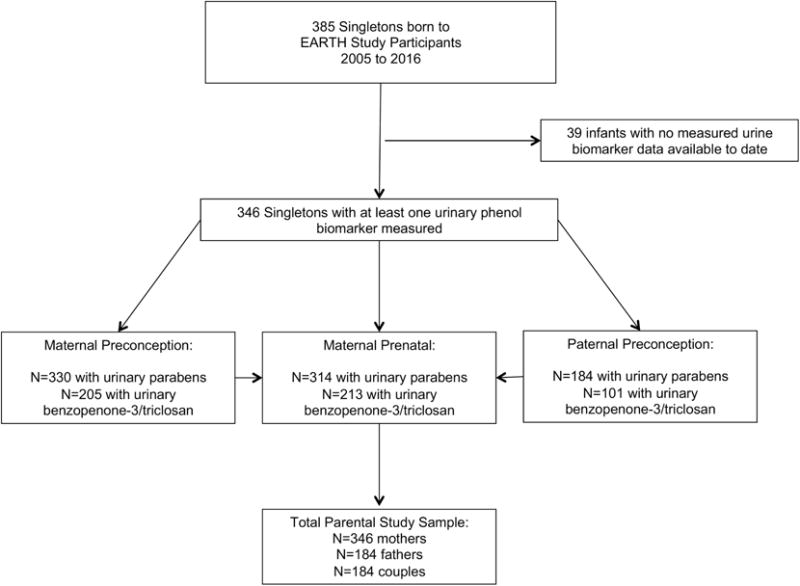
Phenol exposure assessment
At enrollment, men and women provided a single spot urine sample. Women provided up to two additional spot urine samples per fertility treatment cycle: the first sample was obtained during the follicular monitoring phase of the cycle and the second sample was obtained on the day of the fertility procedure (either at time of oocyte retrieval/embryo transfer for in-vitro fertilization [IVF] based treatment or on the day of intrauterine insemination [IUI]). During pregnancy, women also provided one spot urine sample per trimester (median: 6, 21 and 35 weeks gestation). Men provided one additional spot urine sample per treatment cycle on the day when their female partner underwent the fertility procedure (see (Messerlian in press).
Urine was collected in a polypropylene specimen cup and analyzed for specific gravity with a handheld refractometer (National Instrument Company, Inc., Baltimore, MD, USA), divided into aliquots, and frozen for long-term storage at −80 °C. All samples were shipped on dry ice overnight to the CDC (Atlanta, GA, USA) for quantification of urinary phenol concentrations using solid phase extraction coupled with high performance liquid chromatography-isotope dilution tandem mass spectrometry (Silva et al. 2007). The urinary concentrations of the following phenols were measured: benzophenone-3, triclosan, butylparaben, propylparaben, methylparaben, and ethylparaben. The limits of detection (LOD) ranged from 0.1 to 1.0 ng/ml. Concentrations below the LOD were assigned the LOD divided by the square root of two (Hornung 1990). We calculated the molar sum of parabens by dividing each concentration by its molecular weight and then summing:
Birth weight and head circumference outcome assessment
Birth weight in grams (g) and head circumference in centimeters (cm) were abstracted from hospital delivery records by trained study staff. Gestational age was abstracted from delivery records. Gestational age was validated using the American College of Obstetricians and Gynecologists guidelines for births following medically assisted reproduction (ACOG 2014). For IVF-conceived index births, we estimated gestational age as: (outcome date - date of transfer) + 14 + cycle day of transfer. For IUI and non-medically assisted/naturally-conceived pregnancies, we used birth date minus cycle start date confirmed with first trimester ultrasound estimates. Birth weight and head circumference were assessed for implausible values by examining corresponding gestational age and then cross-validation with delivery records by study nurse (corrected for two infants).
Covariates
Demographic characteristics of study participants including age, race, and education were obtained from the enrollment questionnaire. A study nurse measured the height and weight of the male and female participants at baseline entry into the cohort. Body Mass Index (BMI) was calculated as weight (kilograms) divided by height (meters) squared. Smoking status was self-reported at baseline. The treating infertility physician diagnosed the underlying cause of infertility using the Society for Assisted Reproductive Technology definitions. Infant sex, mode of delivery (vaginal vs. caesarian), and date of birth (for season) were abstracted from maternal delivery records by study staff. Clinical information about type of medically assisted reproduction used in the conception cycle of the index birth was abstracted from the electronic medical records by trained study staff. We dichotomized medically assisted reproduction based on type of treatment: all IVF-based procedures (e.g., fresh or frozen IVF protocols, including intracytoplasmic sperm injection) vs. non-IVF based protocols (e.g., IUI with or without ovulation induction/stimulation; ovulation induction/stimulation with timed intercourse, or non-medically assisted/naturally conceived).
Statistical analysis
Urinary phenol concentrations were adjusted for urine dilution by multiplying the concentration by [(SGp-1)/(SGi-1)], where SGi is the specific gravity of the participant’s sample and SGp is the mean specific gravity for all male or all female participants included in the study samples (Pearson et al. 2009). The specific gravity-adjusted phenol concentrations were natural log-transformed to standardize the distribution and reduce the influence of outliers. We estimated mean paternal and maternal preconception phenol exposure by averaging each participant’s log-phenol concentration obtained from study entry and at each treatment cycle up to and including the cycle of the index conception of the singleton birth. We estimated mean maternal prenatal phenol exposure by averaging all trimester-specific log-phenol concentrations obtained from women during the index pregnancy. When only one urine sample was available, the phenol concentration for that single sample was used. We calculated descriptive statistics for phenol concentrations for the three exposure windows as well as the proportion of samples with phenol concentrations below the LOD. We also calculated Pearson correlation coefficients for each log-phenol concentration among couples (paternal vs. maternal).
We estimated associations of paternal and maternal preconception and maternal prenatal log-phenol concentrations with birth outcomes using multivariable linear regression models. We fit a separate model for each phenol biomarker. Beta coefficients and 95% confidence intervals (CIs) represent the difference in birth weight (g) and head circumference (cm) for each log-unit increase in urinary phenol concentration.
We selected potential confounders a priori based on substantive knowledge using a directed acyclic graph (DAG) (Supplementary Appendix, Figure 1A). Maternal preconception/prenatal covariate models included: maternal age and BMI (continuous), maternal education (<college, college, graduate degree), smoking status (never smoked vs. ever smoked, defined as a current or former smoker), and IVF vs. non-IVF-based treatment. Paternal preconception covariate models included: paternal and maternal age and BMI (continuous), paternal and maternal smoking (ever/never), maternal education (<college, college, graduate degree), and IVF vs. non-IVF-based treatment. We also adjusted for mode of delivery in models for head circumference, and season of birth in our birth weight models given the potential for seasonal variation in the use of personal care products and therefore phenol concentrations (Romano et al. 2017). There is also evidence to suggest that there is seasonal trends in birth weight (Murray et al. 2000).
We further adjusted for phenol concentration co-exposure by partner or prenatal window by additionally including the specific phenol concentration into each individual multivariable model. For example, for paternal preconception exposure, our regression models were: Y[birth weight ]= β0+ β[paternal phenol-concentration] + β[covariates] + β[maternal prenatal phenol-concentration], where phenol represents each of the individual biomarkers assessed separately.
As previous studies have observed sex-specific associations between EDC exposure and size at birth (Philippat et al. 2012; Wolff et al. 2008), we conducted a stratified sensitivity analysis by adding a cross-product term for interaction (phenol concentration*sex). We considered a p-value for the interaction term <0.20 as possible effect-modification by infant sex on the multiplicative scale. In addition, we further adjusted our main paternal covariate-adjusted models for the sum of paternal urinary concentrations of di(2-ethylhexyl) phthalate metabolites (ΣDEHP) based on our previously reported associations with birth weight (Messerlian et al. 2017). Lastly, we conducted a post-hoc sensitivity analysis for our significant paternal benzophenone-3 and birth weight finding whereby we stratified men by normal BMI (<25 kg/m2) and high BMI (≥25 kg/m2) and tested for interaction in order to examine whether results differed by BMI groups. We did this in both continuous models as well as by quartiles in order to fully explore potential confounding by BMI. We performed all statistical analyses using SAS version 9.4 (SAS Institute Inc., Cary, USA).
RESULTS
Study Cohort
The study cohort included 346 mothers and 184 fathers (184 couples) with an average age of 34.8 and 35.7 years at the time of enrollment, respectively. Participants were predominantly Caucasian (women, 86%; men, 89%), and never-smokers (women, 74%; men, 70%). Most women were nulliparous (83%), had college or graduate degrees (94%), 31% presented with a BMI ≥25 kg/m2 and about 33% had a female factor as the primary cause of infertility (Table 1). In men, 68% had a BMI ≥25 kg/m2 and around a third had a male factor infertility diagnosis (Table 1). Among the 346 singletons, 52% were male infants and 56% were conceived after IVF-based treatment. Average birth weight was 3373 g (SD=534) and head circumference was 34.3 cm (SD=2.5), 7.5% of infants were born preterm (<37 weeks, n=26/346), and 3.5% (n=12/346) were low birth weight, however proportions differed by infant sex (Table 2).
Table 1.
Parental characteristics of 346 mothers and 184 fathers participating in the Environment and Reproductive Health (EARTH) Study.
| Parental Characteristic | Mothers N=346 |
Fathers N=184 |
|---|---|---|
| Age (years) | ||
| Mean (SD) | 34.8 (3.9) | 35.7 (4.4) |
| Age>35, n (%) | 145 (42) | 100 (54) |
| Race, n (%) | ||
| White | 298 (86) | 163 (89) |
| Black | 7 (2) | 3 (2) |
| Asian | 28 (8) | 13 (7) |
| Other | 13 (4) | 5 (3) |
| Body Mass Index (BMI) | ||
| Mean (SD) | 24.1 (4.2) | 27.2 (4.4) |
| BMI ≥ 25, n (%) | 107 (31) | 125 (68) |
| Education, n (%) | ||
| < College | 20 (6) | 24 (13) |
| College Graduate | 112 (32) | 49 (27) |
| Graduate Degree | 189 (55) | 74 (40) |
| Missing | 25 (7) | 37 (20) |
| Smoking Status, n (%) | ||
| Never | 256 (74) | 129 (70) |
| Ever (former or current) | 90 (26) | 55 (30) |
| Infertility Diagnosis, n (%) | ||
| Male Factor | 87 (25) | 55 (30) |
| Female Factor | 114 (33) | 56 (30) |
| Unexplained | 145 (42) | 73 (40) |
| Primiparous, n (%) | ||
| Yes | 287 (83) | – |
Table 2.
Birth characteristics of 346 singletons from the Environment and Reproductive Health (EARTH) Study.
| Child Characteristics | All Children N=346 |
Boys n=180 |
Girls n=166 |
|---|---|---|---|
| Male, n (%) | 180 (52) | ||
| Birth Weight (grams) | |||
| Mean (SD) | 3373 (534) | 3448 (532) | 3292 (526) |
| min-max | 1090–5040 | 1310–5040 | 1090–4650 |
| Head Circumference* (cm) | |||
| Mean (SD) | 34.3 (2.5) | 35.0 (2.4) | 33.7 (2.4) |
| min-max | 14.0–54.6 | 29.0–54.6 | 14.0–37.5 |
| Low Birth Weight | |||
| <2500grams, n (%) | 12 (3.5) | 4 (2) | 8 (5) |
| Gestational Age at Birth | |||
| Mean weeks (min-max) | 39.4 (29–42) | 39.4 (32–42) | 39.3 (29–42) |
| Mean days (min-max) | 276 (205–294) | 276 (224–294) | 276 (205–294) |
| Preterm Birth | |||
| <37 weeks, n (%) | 26 (7.5) | 12 (6.7) | 14 (8) |
| Mode of Conception | |||
| IVF | 195 (56) | 103 (57) | 92 (55) |
| Non-IVF or Untreated | 151 (44) | 77 (43) | 74 (45) |
N=227 infants with head circumference measurements.
Phenols exposure
Most participants provided multiple urine samples per exposure window: 78% of men provided 2 or more urine samples (median 2, min-max: 1–10) in the preconception period, and 78% and 85% of women provided 2 or more urine samples in the preconception and prenatal periods, respectively (median 3, min-max: 1–20). The geometric means of the specific gravity–adjusted urinary phenol concentrations are reported in Table 3. Urinary benzophenone-3 geometric mean concentrations were 60.3, 173 and 146 ng/ml in the paternal preconception, maternal preconception and maternal prenatal windows, respectively, while triclosan concentrations were 21.2, 17.6 and 12.2 ng/ml, respectively. Methylparaben and propylparaben geometric mean urinary concentrations were 28.4 and 3.1 ng/ml in the paternal preconception window, respectively; 130 and 26.4 ng/ml in the maternal preconception window; and 95.7 and 17.1 ng/ml in the maternal prenatal window. Butylparaben had the lowest concentrations, ranging from 0.32 to 1.2 ng/ml and had detection frequencies between 20%–50% depending on the window of exposure. The percentage of urine samples with detectable concentrations of phenols ranged from 20% (paternal preconception butylparaben) to 100% (paternal and maternal preconception benzophenone-3) (see Table 3 for all detection limits). Phenol concentrations were moderately correlated among couples and within subject across exposure windows: maternal preconception and prenatal butylparaben had the highest correlation (Pearson’s r = 0.63), while the lowest was found for paternal preconception and maternal prenatal methylparaben (Pearson’s r = 0.08). Paternal and maternal preconception triclosan was also moderately correlated (Pearson’s r = 0.62) (Supplementary Appendix Table 2A). We excluded all ethylparaben and paternal butylparaben from further consideration given the large percentage of samples below the limit of detection (54–80%).
Table 3.
Distribution of specific gravity normalized urinary phenol biomarker concentrations in 477 paternal preconception, 1370 maternal preconception, and 796 maternal prenatal urine samples from 346 mothers and 184 fathers from the Environment and Reproductive Health (EARTH) Study participants.
| Metabolite | Sample Size (N) |
LOD (ng/ml) |
% Detect | SG-Adjusted GM (GSD)b |
SG-Adjusted Median ng/ml |
IQR 25th – 75th ng/ml |
|---|---|---|---|---|---|---|
| Paternal Preconception | ||||||
|
| ||||||
| Benzophenone-3 | 101 | 0.2 | 100 | 60.3 (7.5) | 62.5 | 28.1 – 118.2 |
| Triclosan | 101 | 1.0 | 72 | 21.2 (3.8) | 23.3 | 4.7 – 81.6 |
| Butylparaben | 184 | 0.1 | 20 | 0.32 (0.03) | 0.22 | 0.12 – 0.53 |
| Propylparaben | 184 | 0.1 | 80 | 3.1 (0.42) | 2.2 | 0.69 – 10.8 |
| Methylparaben | 184 | 1.0 | 98 | 28.4 (2.7) | 24.1 | 10.5 – 61.2 |
| Ethylparaben | 37 | 1.0 | 27 | 1.9 (0.35) | 1.6 | 0.69 – 3.4 |
| ΣParabens1 | – | – | – | 33.9 (0.02) | 28.8 | 11.6 – 77.3 |
|
| ||||||
| Maternal Preconception | ||||||
|
| ||||||
| Benzophenone-3 | 205 | 0.2 | 100 | 173 (18.4) | 150.2 | 63.7 – 451 |
| Triclosan | 205 | 1.0 | 64 | 17.6 (2.2) | 11.9 | 4.7 – 50.8 |
| Butylparaben | 330 | 0.1 | 50 | 1.2 (0.12) | 1.01 | 0.26 – 5.3 |
| Propylparaben | 330 | 0.1 | 95 | 26.4 (2.2) | 32.2 | 10.2 – 77.9 |
| Methylparaben | 330 | 1.0 | 98 | 129.8 (8.6) | 143 | 59.0 – 298 |
| Ethylparaben | 90 | 1.0 | 46 | 6.8 (1.2) | 5.0 | 2.0 – 25.5 |
| ΣParabens | – | – | – | 165 (11.0) | 185 | 79.2 – 387 |
|
| ||||||
| Maternal Prenatal | ||||||
|
| ||||||
| Benzophenone-3 | 213 | 0.2 | 99 | 145.9 (15.4) | 137.6 | 55.0 – 377 |
| Triclosan | 213 | 1.0 | 69 | 12.2 (1.4) | 9.7 | 3.6 – 33.1 |
| Butylparaben | 314 | 0.1 | 45 | 0.73 (0.07) | 0.59 | 0.20 – 2.1 |
| Propylparaben | 314 | 0.1 | 96 | 17.1 (1.6) | 21.7 | 6.0 – 54.1 |
| Methylparaben | 314 | 1.0 | 99 | 95.7 (7.2) | 109 | 37.8 – 247 |
| Ethylparaben | 107 | 1.0 | 36 | 3.0 (0.47) | 2.0 | 0.92 – 4.5 |
| ΣParabens | – | – | – | 119 (9.0) | 137 | 45.5 – 309 |
Abbreviations: Percent detect (% detect); number of urinary samples (N); Limit of Detection (LOD); specific gravity (SG); geometric mean (GM); geometric standard deviation (GSD); interquartile range (IQR); 25th percentile (25th); 75th percentile (75th).
ΣParabens: the molar sum of parabens was estimated by dividing each concentration by its molecular weight and then summing: ΣParabens= [(Butylparaben*(1/194.23)) + (Methylparaben*(1/152.15)) + (Propylparaben*(1/180.20)) + (Ethylparaben*(1/166.18))].
Paternal preconception window
There was a significant positive association between paternal preconception benzophenone-3 and birth weight among all singletons in both unadjusted and adjusted models (Table 4). After adjustment for covariates, each log-unit increase in paternal urinary benzophenone-3 concentration was associated with a 137 g (95% CI: 60, 214) increase in birth weight (Table 4). Additional adjustment for maternal prenatal benzophenone-3 concentration modestly strengthened findings (β= 153 g, 95% CI: 74, 234) (Table 4). In sensitivity analyses, additional adjustment for paternal ΣDEHP concentration did not substantially change these findings (data not shown). Stratification by infant sex revealed larger increases in birth weight among boy infants than girls, although the interaction term was not significant (Supplementary Appendix, Table 2A). When we stratified the total sample by father’s BMI, we observed some differences between men with higher BMI ≥25 kg/m2 (β= 149 g, 95% CI: 66, 232) versus those with BMI<25 kg/m2 (β= 39 g, 95%CI: −143, 220) (p-value for the interaction term=0.27) (data not shown). Additional sensitivity analyses exploring quartiles of benzophenone-3 concentrations in models stratified by BMI revealed positive associations only among overweight or obese men (Supplementary Appendix, Table 3A). The remaining phenols biomarkers examined in the paternal preconception window were not associated with birth weight in the total cohort of singletons or in sex-stratified models (Tables 4 and 2A). We also found limited evidence of associations with paternal phenol exposure and head circumference (Table 5).
Table 4.
Association of natural log-unit increase in paternal preconception, maternal preconception, and maternal prenatal phenol concentrations and birth weight (g) among singletons: unadjusted, covariate-adjusted, and co-exposure-adjusted models.
| Window/Phenol | Model 1 1 | Model 2 2 | Model 3 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Beta (95% CI) | p-value | N | Beta (95% CI) | p-value | N | Beta (95% CI) | p-value | N | |
|
| |||||||||
| Paternal Preconception | |||||||||
|
| |||||||||
| Benzophenone-3 | 117 (43, 192) | 0.002 | 101 | 137 (60, 214) | 0.0005 | 101 | 153 (74, 234) | 0.0002 | 93 |
| Triclosan | 19 (−35, 72) | 0.49 | 101 | 5 (−46, 55) | 0.85 | 101 | 13 (−50, 77) | 0.68 | 93 |
| Butylparaben4 | – | – | – | – | – | – | – | – | – |
| Propylparaben | −20 (−61, 20) | 0.33 | 184 | −21 (−62, 19) | 0.30 | 184 | −22 (−64, 20) | 0.30 | 171 |
| Methylparaben | −21 (−80, 38) | 0.49 | 184 | −13 (−72, 47) | 0.67 | 184 | −13 (−74, 48) | 0.67 | 171 |
| ΣParabens5 | −24 (−80, 33) | 0.41 | 184 | −19 (−76, 38) | 0.52 | 184 | −20 (−78, 39) | 0.51 | 171 |
|
| |||||||||
| Maternal Preconception | Beta (95% CI) | p-value | N | Beta (95% CI) | p-value | N | Beta (95% CI) | p-value | N |
|
| |||||||||
| Benzophenone-3 | 29 (−17, 75) | 0.22 | 205 | 27 (−18, 72) | 0.23 | 205 | 24 (−40, 88) | 0.46 | 181 |
| Triclosan | −23 (−63, 17) | 0.25 | 205 | −16 (−54, 23) | 0.42 | 205 | 10 (−37, 57) | 0.67 | 181 |
| Butylparaben | −8 (−40, 24) | 0.63 | 330 | 1 (−31, 33) | 0.97 | 330 | 26 (−17, 69) | 0.24 | 298 |
| Propylparaben | −23 (−61, 14) | 0.22 | 330 | −12 (−49, 26) | 0.54 | 330 | −6 (−53, 40) | 0.79 | 298 |
| Methylparaben | −41 (−88, 7) | 0.09 | 330 | −26 (−74, 22) | 0.28 | 330 | −21 (−78, 37) | 0.48 | 298 |
| ΣParabens | −40 (−88, 7) | 0.10 | 330 | −25 (−73, 22) | 0.29 | 330 | −21 (−78, 37) | 0.49 | 298 |
|
| |||||||||
| Maternal Prenatal | Beta (95% CI) | p-value | N | Beta (95% CI) | p-value | N | Beta (95% CI) | p-value | N |
|
| |||||||||
| Benzophenone-3 | −21 (−65, 23) | 0.35 | 213 | −12 (−54, 31) | 0.32 | 213 | −53 (−120, 14) | 0.12 | 93 |
| Triclosan | −41 (−81, −1) | 0.04 | 213 | −38 (−76, 0) | 0.05 | 213 | −48 (−115, 19) | 0.16 | 93 |
| Butylparaben | −27 (−62, 7) | 0.12 | 314 | −25 (−59, 9) | 0.15 | 314 | −15 (−61, 32) | 0.54 | 171 |
| Propylparaben | −33 (−68, 1) | 0.06 | 314 | −32 (−66, 3) | 0.07 | 314 | −24 (−71, 23) | 0.31 | 171 |
| Methylparaben | −33 (−76, 10) | 0.13 | 314 | −29 (−71, 13) | 0.17 | 314 | −28 (−87, 30) | 0.34 | 171 |
| ΣParabens | −35 (−78, 8) | 0.11 | 314 | −31 (−73, 11) | 0.15 | 314 | −30 (−88, 29) | 0.32 | 171 |
Model 1: Unadjusted models.
Paternal Preconception Model 2: adjusted for maternal and paternal age (continuous), maternal and paternal Body Mass Index (continuous), maternal education (<college, college, graduate degree), maternal and paternal smoking (ever/never), IVF-based vs. Non-IVF based treatment, and season (ordinal).
Maternal Preconception and Prenatal Models 2: adjusted for maternal age (continuous), maternal Body Mass Index (continuous), maternal education (<college, college, graduate degree), maternal smoking (ever/never), and IVF-based vs. Non-IVF based treatment, and season (ordinal).
-
-Paternal Preconception model includes respective maternal prenatal phenols concentration;
-
-Maternal Preconception model includes respective maternal prenatal phenols concentration;
-
-Maternal Prenatal model includes respective paternal preconception phenols concentration.
Paternal butylparaben concentrations not analyzed due to the low percentage of urine samples above the limit of detection (20%).
ΣParabens: the molar sum of parabens was estimated by dividing each concentration by its molecular weight and then summing: ΣParabens= [(Butylparaben*(1/194.23)) + (Methylparaben*(1/152.15)) + (Propylparaben*(1/180.20)) + (Ethylparaben*(1/166.18))].
Table 5.
Association of natural log-unit increase in paternal preconception, maternal preconception, and maternal prenatal phenol concentrations and head circumference (cm) among singletons.
| Window/Phenol | Model 11 | Model 2 2 | Model 3 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Beta (95% CI) | p-value | N | Beta (95% CI) | p-value | N | Beta (95% CI) | p-value | N | |
| Paternal Preconception | |||||||||
| Benzophenone-3 | 0.29 (−0.22, 0.80) | 0.26 | 73 | 0.11 (−0.36, 0.76) | 0.64 | 73 | 0.14 (−0.37, 0.66) | 0.59 | 66 |
| Triclosan | 0.19 (−0.15, 0.53) | 0.27 | 73 | 0.12 (−0.18, 0.49) | 0.43 | 73 | 0.03 (−0.40, 0.45) | 0.89 | 66 |
| Butylparaben4 | – | – | – | – | – | – | – | – | – |
| Propylparaben | −0.11 (−0.33, 0.11) | 0.33 | 130 | −0.09 (−0.30, 0.17) | 0.40 | 130 | −0.02 (−0.26, 0.21) | 122 | |
| Methylparaben | −0.11 (−0.44, 0.22) | 0.50 | 130 | −0.03 (−0.35, 0.29) | 0.85 | 130 | 0.08 (−0.26, 0.42) | 0.64 | 122 |
| ΣParabens5 | −0.10 (−0.41, 0.21) | 0.55 | 130 | −0.02 (−0.32, 0.28) | 0.89 | 130 | 0.09 (−0.23, 0.41) | 0.59 | 122 |
| Maternal Preconception | Beta (95% CI) | p-value | N | Beta (95% CI) | p-value | N | |||
| Benzophenone-3 | 0.06 (−0.26, 0.38) | 0.72 | 139 | 0.04 (−0.26, 0.34) | 0.79 | 139 | −0.15 (−0.65, 0.34) | 0.55 | 71 |
| Triclosan | 0.07 (−0.20, 0.34) | 0.62 | 139 | 0.08 (−0.17, 0.33) | 0.53 | 139 | −0.21 (−0.67, 0.35) | 0.36 | 71 |
| Butylparaben | −0.03 (−0.23, 0.16) | 0.73 | 218 | −0.05 (−0.24, 0.14) | 0.86 | 218 | −0.16 (−0.40, 0.08) | 0.18 | 129 |
| Propylparaben | −0.10 (−0.32, 0.12) | 0.36 | 218 | −0.16 (−0.38, 0.06) | 0.15 | 218 | −0.21 (−0.49, 0.06) | 0.13 | 129 |
| Methylparaben | −0.22 (−0.49, 0.05) | 0.12 | 218 | −0.27 (−0.54, 0.0002) | 0.05 | 218 | −0.40 (−0.75, −0.04) | 0.03 | 129 |
| ΣParabens | −0.21 (−0.49, 0.06) | 0.12 | 218 | −0.27 (−0.54, −0.0016) | 0.05 | 218 | −0.40 (−0.75, −0.05) | 0.02 | 129 |
| Maternal Prenatal | Beta (95% CI) | p-value | N | Beta (95% CI) | p-value | N | |||
| Benzophenone-3 | 0.08 (−0.22, 0.39) | 0.73 | 148 | 0.08 (−0.22, 0.38) | 0.60 | 148 | 0.01 (−0.48, 0.50) | 0.97 | 66 |
| Triclosan | 0.25 (−0.004, 0.51) | 0.05 | 148 | 0.22 (−0.03, 0.47) | 0.08 | 148 | 0.22 (−0.21, 0.66) | 0.31 | 66 |
| Butylparaben | 0.05 (−0.15, 0.26) | 0.62 | 208 | 0.02 (−0.18, 0.22) | 0.86 | 208 | −0.05 (−0.31, 0.21) | 0.70 | 122 |
| Propylparaben | −0.06 (−0.27, 0.14) | 0.53 | 208 | −0.13 (−0.33, 0.08) | 0.22 | 208 | −0.20 (−0.45, 0.06) | 0.12 | 122 |
| Methylparaben | −0.06 (−0.31, 0.20) | 0.66 | 208 | −0.11 (−0.36, 0.14) | 0.38 | 208 | −0.24 (−0.55, 0.08) | 0.14 | 122 |
| ΣParabens | −0.07 (−0.32, 0.19) | 0.61 | 208 | −0.13 (−0.38, 0.12) | 0.32 | 208 | −0.25 (−0.56, 0.06) | 0.12 | 122 |
Model 1: Unadjusted models.
Paternal Preconception Model 2: adjusted for maternal and paternal age (continuous), maternal and paternal Body Mass Index (continuous), maternal education (<college, college, graduate degree), maternal and paternal smoking (ever/never), IVF-based vs. Non-IVF based treatment, and mode of delivery (vaginal vs. c-section).
Maternal Preconception and Prenatal Model 2: adjusted for maternal age (continuous), maternal Body Mass Index (continuous), maternal education (<college, college, graduate degree), maternal smoking (ever/never), IVF-based vs. Non-IVF based treatment, and mode of delivery (vaginal vs. c-section).
-
-Paternal Preconception model includes respective maternal prenatal phenols concentration;
-
-Maternal Preconception model includes respective maternal prenatal phenols concentration;
-
-Maternal Prenatal model includes respective paternal preconception phenols concentration.
Paternal butylparaben concentrations not analyzed due to the low percentage of urine samples above the limit of detection (20%).
ΣParabens: the molar sum of parabens was estimated by dividing each concentration by its molecular weight and then summing: ΣParabens= [(Butylparaben*(1/194.23)) + (Methylparaben*(1/152.15)) + (Propylparaben*(1/180.20)) + (Ethylparaben*(1/166.18))].
Maternal preconception window
Few associations were found between maternal preconception urinary phenol biomarkers and birth weight in unadjusted or covariate-adjusted models (Tables 4); nor did we observe any sex specific differences (Supplementary Appendix, Table 2A). We did observe a decrease in head circumference for each log-unit increase in methylparaben concentrations (β= −0.27 cm, 95% CI: −0.54, 0, Table 5, Model 2). Additional adjustment for maternal prenatal methylparaben concentrations strengthened this association (β= −0.40 cm, 95% CI: −0.75, −0.04, Table 5, Model 3). Similar results were observed with the sum of the paraben concentrations (Table 5, Model 2 and 3).
Maternal prenatal window
Maternal prenatal triclosan concentrations were associated with birth weight decrement. In covariate-adjusted models, each log-unit increase in urinary triclosan concentration during pregnancy was associated with a 38 g (95% CI: −76, 0) decrease in birth weight (Table 4, Model 2). Additional adjustment for paternal triclosan concentrations strengthened the association, however, this finding was no longer significant (Table 4, Model 3). In sex-stratified analysis, prenatal propylparaben concentration showed a sexually-dimorphic pattern: boys had a 67 g (95% CI: −133, −2) decrease in birth weight compared with only a 2 g (95% CI: −62, 58) decrease among girls (p-value for interaction, 0.15) (Supplementary Appendix, Table 2A). None of the maternal prenatal phenol concentrations examined was associated with head circumference (Tables 5).
DISCUSSION
In this prospective preconception cohort of women and men from a fertility center, we found that paternal preconception urinary concentrations of benzophenone-3 were positively associated with birth weight. However, this result appears stronger among men with higher BMI than normal BMI. While we observed no associations with birth weight in relation to the maternal preconception window of exposure, there appeared to be a small decrease in head circumference associated with higher concentrations of parabens, most notably for methylparaben. We also observed a decrease in birth weigh in relation higher prenatal triclosan concentrations as well a decrease in birth weight among boys in relation to higher propylparaben concentrations in pregnancy. Overall, adjusting for partner’s exposure or for exposure in the prenatal window did not consistently change our results, although there was a general tendency toward attenuation for several phenols examined. Maternal prenatal models whereby we additionally adjusted for paternal preconception phenol concentrations were restricted to a smaller subset of couples and therefore a smaller sample size, which could have added to the variability in observed results from these models.
Our results show potential evidence of associations of some paternal and maternal phenol concentrations and birth size. However, our results should be interpreted cautiously given this is the first study to report some of these findings in this modest-sized subfertile cohort, we performed multiple comparisons, and results should be replicated in larger datasets. Our paternal benzophenone-3 finding, which was stronger among men with a higher BMI, may be due to confounding by body surface area resulting in greater amount of benzophenone-3 product use among larger men who would have constitutionally larger babies irrespective of exposure (Klebanoff et al. 1998). However, we cannot fully rule out a potential association of paternal benzophenone-3 exposure with birth weight given that even among men with higher BMI we found a positive p-trend across quartiles.
The urinary concentrations of phenols measured in our study were within the ranges reported for the US general population (CDC 2015). Biomonitoring studies have demonstrated that human exposure to these phenols is ubiquitous. Around 99%, 96% and 75% of the US population presents detectable urinary concentrations of parabens, benzophenone-3, and triclosan, respectively (Calafat et al. 2008a; Calafat et al. 2008b; Calafat et al. 2010). In general, exposure to these phenols correlates with higher incomes and higher use of personal care products. Females therefore show consistently higher urinary concentrations than males (Calafat et al. 2010; Han et al. 2016), in agreement with the observed distribution of phenols in paternal and maternal windows of exposure in our study sample. As differences in exposure patterns among couples might interact to influence study outcomes, and fathers and mothers often share some lifestyle and personal care products, we additionally adjusted for phenol concentration co-exposure by partner or prenatal window. Although, the between couple (or exposure window) correlations were low to moderate in our data and we observed only a limited impact of this additional adjustment.
Although the experimental literature regarding the endocrine effects of benzophenone-3 is rather limited (Kinnberg et al. 2015), both estrogenic and anti-androgenic mechanisms of action have been reported for benzophenone-3 and its metabolites (Watanabe et al. 2015). However, mechanisms are likely complex, with anti-estrogenic activity also reported (Kim and Choi 2014). Furthermore, benzophenone-3 driven reproductive impairments have been reported in zebra fish and other aquatic experimental animals (Ghazipura et al. 2017; Kinnberg et al. 2015). Among other phenols, triclosan has been shown to be estrogenic in vitro (Huang et al. 2014) as well as activate the aryl hydrocarbon receptor (AhR), induce reactive oxygen species (Szychowski et al. 2016), and exert anti-androgenic actions affecting testosterone biosynthesis in rats (Kumar et al. 2008). Currently, the eco-toxicological impact of these chemicals has been in the spotlight based on some evidence that benzophenone-3 might affect aquatic life and bioaccumulate in marine organisms, potentially entering the food chain (Sanchez-Quiles and Tovar-Sanchez 2015), while triclosan, apart from its toxicity to aquatic organisms, has been shown to undergo phototransformation into potentially more toxic and persistent compounds such as chlorinated dibenzodioxins (Bedoux et al. 2012; Buth et al. 2010; Kim and Choi 2014). Meanwhile, parabens show potential estrogenic activity with evidence of inducing diverse reproductive effects in vivo (Golden et al. 2005).
In humans, prenatal phenol exposure has been associated with offspring birth size (Etzel et al. 2017; Geer et al. 2017; Philippat et al. 2012; Wolff et al. 2008). Philippat and colleagues reported increased birth weight in response to higher urinary benzophenone-3 concentrations in pregnancy (Philippat et al. 2012). Although this study was limited to only examining boy infants, results are similar in direction to our paternal benzophenone-3 models. In contrast, our maternal prenatal benzophenone-3 models were not associated with birth weight. Moreover, Wolff and colleagues found that higher prenatal urinary benzophenone-3 concentrations were associated with decreased birth weight among girls but with increased birth weight in boys (Wolff et al. 2008). Therefore, prior associations between prenatal benzophenone-3 exposure and higher birth weight appear to be in the same direction as our paternal preconception finding. However, additional research would help increase our understanding and elucidate if these associations are attributable to confounding with parental anthropometric factors, or to benzophenone-3 itself.
Our methyl- and propylparaben findings are somewhat consistent with a recent study by Geer and colleagues showing inverse associations between maternal urinary butyl- and propylparaben concentrations and birth size (Geer et al. 2017). Our most consistent finding, however, is the observed association between triclosan exposure in pregnancy and a non-sex specific decrease in birth weight. Post-hoc analyses across quartiles of prenatal triclosan concentrations supported a positive linear trend. Compared to the lowest quartile, the difference in birth weight in quartile 2, 3, and 4 was: −32 g (95% CI: −216, 151); −135 g (95%CI: −319, 48); −181 g (95% CI: −362, −1) with a p-value for trend across quartiles of 0.03. Similar associations have been reported in relation to urinary triclosan concentrations measured in pregnancy and birth size (Etzel et al. 2017; Philippat et al. 2014). While these prior studies also reported decreases in head circumference in relation to tricolsan exposure in pregnancy they did not adjust for mode of delivery (vaginal vs. caesarian). Our prenatal triclosan concentrations were also associated with head circumference, however, this association was attenuated and no longer significant after we adjusted for mode of delivery (data not shown).
While most epidemiological studies have assessed exposure to EDCs during the prenatal period, little is currently known about the developmental effects of EDCs when exposure is assessed prior to conception. Emerging research suggests that the preconception period may represent a sensitive and largely unexplored critical window of exposure (Braun et al. 2017). Experimental research suggests that both male and female preconception exposure to EDCs can lead to multigenerational developmental effects that are thought to be driven by epigenetic dysregulation in germ cells (including different patterns of DNA methylation, histone modification and non-coding RNAs) (Chen et al. 2016; Kumar et al. 2013; Rando 2012; Robinson et al. 2012; Xin et al. 2015).
A major strength of the EARTH Study is its prospective, preconception design. Studying a subfertile population from a large academic fertility setting enabled us to examine three critical windows of exposure, including fathers’ exposure during the preconception period. If subfertile couples are more sensitive to the adverse effects of EDCs in preconception and pregnancy our findings may not be as generalizable to men and women from the overall population without fertility concern. Nevertheless, infertile couples represent an important vulnerable sub-population given the growing number of babies born using IVF-based treatment, estimated to be 1.6% of all births or >68,000 births annually in the USA, with even higher proportions in certain European nations. The fraction of births using non-IVF based treatment, such as IUI, is even higher at ~4.6% (~191,000 births), totaling >250,000 births per year in the USA (Dyer et al. 2016; Schieve et al. 2009; Sunderam et al. 2017; Zegers-Hochschild et al. 2014).
Our sex-stratified analyses were limited by fairly small subgroups and these results should be interpreted cautiously, although they point to a possible dimorphic effect on birth weight in relation to maternal prenatal propylparaben exposure. Indeed, others have previously reported effects by child sex in relation to phenol exposure and birth weight (Philippat et al. 2012; Wolff et al. 2008). Currently there is a strong interest in understanding sex-specific effects of EDCs during pregnancy, which could be driven by differences in androgen/estrogen balance (Liu et al. 2016), thyroid function (Aker et al. 2016), dimorphic interaction with the placenta (Rosenfeld 2015), differential xeno-metabolism, and/or differential epigenetic or gene-environment interactions (Al-Qaraghouli and Fang 2017; Gabory et al. 2009).
While long-term exposure assessment of non-persistent EDCs is difficult given their short elimination half-lives and the episodic nature of their exposure, another major strength of our study was that we had multiple urine samples for each of the three exposure windows for the vast majority of participants. We were therefore able to partially account for the within-person variability in urinary concentrations of these biomarkers by using the average concentration from multiple urine samples provided in the preconception and prenatal exposure periods. We cannot, however, rule out some degree of exposure misclassification given the limitations inherent in measuring exposure in spot urine samples, which represents a small snapshot of an individual’s overall exposure. While the EARTH Study design enabled us to comprehensively assess multiple paternal and maternal preconception and prenatal urine samples, allowing us to adjust for and carefully examine each exposure window in relation to birth weight, we acknowledge that in order to do so numerous comparisons were undertaken, which could have resulted in false positive associations occurring by chance. Lastly, future research should focus on examining mixtures of non-persistent EDCs among men and women to better understand the totality of exposure among individuals and its potential relationship with birth outcomes.
CONCLUSION
Our study provides preliminary evidence that paternal preconception benzophenone-3 exposure may be associated with increased birth weight, however this result may be due to differences among heavier men. This study highlights the need to go beyond the current paradigm focused solely on mothers and take into account fathers’ environmental exposures in perinatal and pediatric health outcomes. Our results also suggest that higher maternal triclosan and possibly propylparaben exposure during pregnancy may be related to lower infant birth weight. Overall, our observed associations were compound-, parent- and window-specific, reflecting the complexity of environment and health effects. Although our findings should be interpreted with caution in light of inherent study limitations, these results highlight the potential relevance of prospective fathers and pregnant mothers’ exposure to phenols on birth outcomes.
Supplementary Material
Highlights.
Pregnancy concentrations of some phenols have been associated with infant size at birth.
There is limited data on the effect of paternal and maternal preconception exposure.
Some paternal and maternal phenol concentrations were associated with birth size, however, results may be due to residual confounding or be particular to the cohort under study.
Acknowledgments
The authors gratefully acknowledge Xiaoyun Ye, Xiaoliu Zhou, and Tao Jia (CDC, Atlanta, GA) for measuring the urinary concentrations of phenol biomarkers.
Funding: Work supported by grants ES R01 009718 from the National Institute of Environmental Health Sciences (NIEHS). CM was supported by a post-doctoral fellowship award from the Canadian Institutes of Health Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Financial Interests: The authors declare they have no actual or potential competing interests.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
References
- ACOG. Method for estimating due date. Committee opinion no. 611 Obstetrics & Gynecology. 2014;124:863–866. doi: 10.1097/01.AOG.0000454932.15177.be. [DOI] [PubMed] [Google Scholar]
- Aker AM, Watkins DJ, Johns LE, Ferguson KK, Soldin OP, Anzalota Del Toro LV, et al. Phenols and parabens in relation to reproductive and thyroid hormones in pregnant women. Environ Res. 2016;151:30–37. doi: 10.1016/j.envres.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Qaraghouli M, Fang YMV. Effect of fetal sex on maternal and obstetric outcomes. Front Pediatr. 2017;5:144. doi: 10.3389/fped.2017.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JL, Olsen LW, Sorensen TI. Weight at birth and all-cause mortality in adulthood. Epidemiology. 2008;19:197–203. doi: 10.1097/EDE.0b013e31816339c6. [DOI] [PubMed] [Google Scholar]
- Basso O, Wilcox AJ, Weinberg CR. Birth weight and mortality: Causality or confounding? Am J Epidemiol. 2006;164:303–311. doi: 10.1093/aje/kwj237. [DOI] [PubMed] [Google Scholar]
- Bedoux G, Roig B, Thomas O, Dupont V, Le Bot B. Occurrence and toxicity of antimicrobial triclosan and by-products in the environment. Environ Sci Pollut Res Int. 2012;19:1044–1065. doi: 10.1007/s11356-011-0632-z. [DOI] [PubMed] [Google Scholar]
- Braun JM, Messerlian C, H R. Fathers matter: Why it’s time to consider the impact of paternal environmental exposures on children’s health. Current Epidemiology Reports. 2017 doi: 10.1007/s40471-017-0098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buth JM, Steen PO, Sueper C, Blumentritt D, Vikesland PJ, Arnold WA, et al. Dioxin photoproducts of triclosan and its chlorinated derivatives in sediment cores. Environ Sci Technol. 2010;44:4545–4551. doi: 10.1021/es1001105. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Wong LY, Ye X, Reidy JA, Needham LL. Concentrations of the sunscreen agent benzophenone-3 in residents of the united states: National health and nutrition examination survey 2003–2004. Environ Health Perspect. 2008a;116:893–897. doi: 10.1289/ehp.11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Urinary concentrations of triclosan in the u.S. Population: 2003–2004. Environ Health Perspect. 2008b;116:303–307. doi: 10.1289/ehp.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Bishop AM, Needham LL. Urinary concentrations of four parabens in the u.S. Population: Nhanes 2005–2006. Environ Health Perspect. 2010;118:679–685. doi: 10.1289/ehp.0901560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins K, Devaskar SU. Fetal origins of adult disease. Curr Probl Pediatr Adolesc Health Care. 2011;41:158–176. doi: 10.1016/j.cppeds.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Fourth report on human exposure to environmental chemicals, updated tables. Atlanta: Ga, us: Department of Health and Human Services, Centers for Disease Control and Prevention; 2015. Feb, 2015. http://wwwcdcgov/biomonitoring/pdf/FourthReport_UpdatedTables_Feb2015pdf. [Google Scholar]
- Chen Q, Yan W, Duan E. Epigenetic inheritance of acquired traits through sperm rnas and sperm rna modifications. Nat Rev Genet. 2016;17:733–743. doi: 10.1038/nrg.2016.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong JL, Hunt RW, Anderson PJ, Howard K, Thompson DK, Wang HX, et al. Head growth in preterm infants: Correlation with magnetic resonance imaging and neurodevelopmental outcome. Pediatrics. 2008;121:e1534–1540. doi: 10.1542/peds.2007-2671. [DOI] [PubMed] [Google Scholar]
- Dann AB, Hontela A. Triclosan: Environmental exposure, toxicity and mechanisms of action. J Appl Toxicol. 2011;31:285–311. doi: 10.1002/jat.1660. [DOI] [PubMed] [Google Scholar]
- Dyer S, Chambers GM, de Mouzon J, Nygren KG, Zegers-Hochschild F, Mansour R, et al. International committee for monitoring assisted reproductive technologies world report: Assisted reproductive technology 2008, 2009 and 2010. Hum Reprod. 2016;31:1588–1609. doi: 10.1093/humrep/dew082. [DOI] [PubMed] [Google Scholar]
- Etzel TM, Calafat AM, Ye X, Chen A, Lanphear BP, Savitz DA, et al. Urinary triclosan concentrations during pregnancy and birth outcomes. Environ Res. 2017;156:505–511. doi: 10.1016/j.envres.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federal-Register. 2016. 50 u.S.C. 4601 et seq.; 50 u.S.C. 1701 et seq.; e.O. 13026, 61 fr 58767, 3 cfr, 1996 comp., p. 228; e.O. 13222, 66 fr 44025,3 cfr, 2001 comp., p. 783; notice of august 4, 2016, 81 fr 52587, august 8, 2016.
- Ferguson KK, Meeker JD, Cantonwine DE, Chen YH, Mukherjee B, McElrath TF. Urinary phthalate metabolite and bisphenol a associations with ultrasound and delivery indices of fetal growth. Environ Int. 2016 doi: 10.1016/j.envint.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Colacino JA, Lewis RC, Meeker JD. Personal care product use among adults in nhanes: Associations between urinary phthalate metabolites and phenols and use of mouthwash and sunscreen. J Expo Sci Environ Epidemiol. 2017;27:326–332. doi: 10.1038/jes.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen H, Nielsen JK, Morck TA, Hansen PW, Jensen JF, Nielsen O, et al. Urinary excretion of phthalate metabolites, phenols and parabens in rural and urban danish mother-child pairs. Int J Hyg Environ Health. 2013;216:772–783. doi: 10.1016/j.ijheh.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Gabory A, Attig L, Junien C. Sexual dimorphism in environmental epigenetic programming. Mol Cell Endocrinol. 2009;304:8–18. doi: 10.1016/j.mce.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Geer LA, Pycke BF, Waxenbaum J, Sherer DM, Abulafia O, Halden RU. Association of birth outcomes with fetal exposure to parabens, triclosan and triclocarban in an immigrant population in brooklyn, new york. J Hazard Mater. 2017;323:177–183. doi: 10.1016/j.jhazmat.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazipura M, McGowan R, Arslan A, Hossain T. Exposure to benzophenone-3 and reproductive toxicity: A systematic review of human and animal studies. Reprod Toxicol. 2017;73:175–183. doi: 10.1016/j.reprotox.2017.08.015. [DOI] [PubMed] [Google Scholar]
- Giulivo M, Lopez de Alda M, Capri E, Barcelo D. Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. A review. Environ Res. 2016;151:251–264. doi: 10.1016/j.envres.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Golden R, Gandy J, Vollmer G. A review of the endocrine activity of parabens and implications for potential risks to human health. Crit Rev Toxicol. 2005;35:435–458. doi: 10.1080/10408440490920104. [DOI] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. Edc-2: The endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015;36:E1–E150. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidry VT, Longnecker MP, Aase H, Eggesbo M, Zeiner P, Reichborn-Kjennerud T, et al. Measurement of total and free urinary phenol and paraben concentrations over the course of pregnancy: Assessing reliability and contamination of specimens in the norwegian mother and child cohort study. Environ Health Perspect. 2015;123:705–711. doi: 10.1289/ehp.1408325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Lim YH, Hong YC. Ten-year trends in urinary concentrations of triclosan and benzophenone-3 in the general u.S. Population from 2003 to 2012. Environ Pollut. 2016;208:803–810. doi: 10.1016/j.envpol.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Hornung RW, R LD. Estimation of average concentration in the presence of nondetectable values. Applied Occupational and Environmental Hygiene. 1990;5:46–51. [Google Scholar]
- Huang H, Du G, Zhang W, Hu J, Wu D, Song L, et al. The in vitro estrogenic activities of triclosan and triclocarban. J Appl Toxicol. 2014;34:1060–1067. doi: 10.1002/jat.3012. [DOI] [PubMed] [Google Scholar]
- Kim S, Choi K. Occurrences, toxicities, and ecological risks of benzophenone-3, a common component of organic sunscreen products: A mini-review. Environ Int. 2014;70:143–157. doi: 10.1016/j.envint.2014.05.015. [DOI] [PubMed] [Google Scholar]
- Kinnberg KL, Petersen GI, Albrektsen M, Minghlani M, Awad SM, Holbech BF, et al. Endocrine-disrupting effect of the ultraviolet filter benzophenone-3 in zebrafish, danio rerio. Environ Toxicol Chem. 2015;34:2833–2840. doi: 10.1002/etc.3129. [DOI] [PubMed] [Google Scholar]
- Klebanoff MA, Mednick BR, Schulsinger C, Secher NJ, Shiono PH. Father’s effect on infant birth weight. Am J Obstet Gynecol. 1998;178:1022–1026. doi: 10.1016/s0002-9378(98)70542-3. [DOI] [PubMed] [Google Scholar]
- Kramer MS. Determinants of low birth weight: Methodological assessment and meta-analysis. Bull World Health Organ. 1987;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Kumar K, Jain S, Hassan T, Dada R. Novel insights into the genetic and epigenetic paternal contribution to the human embryo. Clinics (Sao Paulo) 2013;68(Suppl 1):5–14. doi: 10.6061/clinics/2013(Sup01)02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Balomajumder C, Roy P. Disruption of lh-induced testosterone biosynthesis in testicular leydig cells by triclosan: Probable mechanism of action. Toxicology. 2008;250:124–131. doi: 10.1016/j.tox.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Lenters V, Portengen L, Rignell-Hydbom A, Jonsson BA, Lindh CH, Piersma AH, et al. Prenatal phthalate, perfluoroalkyl acid, and organochlorine exposures and term birth weight in three birth cohorts: Multi-pollutant models based on elastic net regression. Environ Health Perspect. 2016;124:365–372. doi: 10.1289/ehp.1408933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C, Kannan K. Widespread occurrence of benzophenone-type uv light filters in personal care products from china and the united states: An assessment of human exposure. Environ Sci Technol. 2014;48:4103–4109. doi: 10.1021/es405450n. [DOI] [PubMed] [Google Scholar]
- Liu C, Xu X, Zhang Y, Li W, Huo X. Associations between maternal phenolic exposure and cord sex hormones in male newborns. Hum Reprod. 2016;31:648–656. doi: 10.1093/humrep/dev327. [DOI] [PubMed] [Google Scholar]
- Mathews TJ, Driscoll AK. Trends in infant mortality in the united states, 2005-2014. NCHS Data Brief. 2017:1–8. [PubMed] [Google Scholar]
- Messerlian C, Braun JM, Minguez-Alarcon L, Williams PL, Ford JB, Mustieles V, et al. Paternal and maternal urinary phthalate metabolite concentrations and birth weight of singletons conceived by subfertile couples. Environ Int. 2017;107:55–64. doi: 10.1016/j.envint.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerlian C, Williams PL, Ford JB, Chavarro J, Mínguez-Alarcón L, Dadd R, Braun JM, Gaskins AJ, Meeker JD, James-Todd T, Chiu Y-H, Nassan FL, Souter I, Petrozza J, Keller M, Toth T, Calafat AM, Hauser R. The environment and reproductive health (earth) study: A prospective preconception cohort. Human Reproduction Open. doi: 10.1093/hropen/hoy001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray LJ, O’Reilly DP, Betts N, Patterson CC, Davey Smith G, Evans AE. Season and outdoor ambient temperature: Effects on birth weight. Obstet Gynecol. 2000;96:689–695. doi: 10.1016/s0029-7844(00)01022-x. [DOI] [PubMed] [Google Scholar]
- Mustieles V, Perez-Lobato R, Olea N, Fernandez MF. Bisphenol a: Human exposure and neurobehavior. Neurotoxicology. 2015;49:174–184. doi: 10.1016/j.neuro.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Pearson MA, Lu C, Schmotzer BJ, Waller LA, Riederer AM. Evaluation of physiological measures for correcting variation in urinary output: Implications for assessing environmental chemical exposure in children. J Expo Sci Environ Epidemiol. 2009;19:336–342. doi: 10.1038/jes.2008.48. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Anderson AW, Ehrenkranz R, Staib LH, Tageldin M, Colson E, et al. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics. 2003;111:939–948. doi: 10.1542/peds.111.5.939. [DOI] [PubMed] [Google Scholar]
- Philippat C, Mortamais M, Chevrier C, Petit C, Calafat AM, Ye X, et al. Exposure to phthalates and phenols during pregnancy and offspring size at birth. Environ Health Perspect. 2012;120:464–470. doi: 10.1289/ehp.1103634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C, Botton J, Calafat AM, Ye X, Charles MA, Slama R. Prenatal exposure to phenols and growth in boys. Epidemiology. 2014;25:625–635. doi: 10.1097/EDE.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando OJ. Daddy issues: Paternal effects on phenotype. Cell. 2012;151:702–708. doi: 10.1016/j.cell.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson L, Gallos ID, Conner SJ, Rajkhowa M, Miller D, Lewis S, et al. The effect of sperm DNA fragmentation on miscarriage rates: A systematic review and meta-analysis. Hum Reprod. 2012;27:2908–2917. doi: 10.1093/humrep/des261. [DOI] [PubMed] [Google Scholar]
- Romano ME, Kalloo G, Etzel T, Braun JM. Re: Seasonal variation in exposure to endocrine-disrupting chemicals. Epidemiology. 2017;28:e42–e43. doi: 10.1097/EDE.0000000000000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CS. Sex-specific placental responses in fetal development. Endocrinology. 2015;156:3422–3434. doi: 10.1210/en.2015-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Quiles D, Tovar-Sanchez A. Are sunscreens a new environmental risk associated with coastal tourism? Environ Int. 2015;83:158–170. doi: 10.1016/j.envint.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Schieve LA, Devine O, Boyle CA, Petrini JR, Warner L. Estimation of the contribution of non-assisted reproductive technology ovulation stimulation fertility treatments to us singleton and multiple births. Am J Epidemiol. 2009;170:1396–1407. doi: 10.1093/aje/kwp281. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL, Jr, Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;860:106–112. doi: 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Sunderam S, Kissin DM, Crawford SB, Folger SG, Jamieson DJ, Warner L, et al. Assisted reproductive technology surveillance - united states, 2014. MMWR Surveill Summ. 2017;66:1–24. doi: 10.15585/mmwr.ss6606a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szychowski KA, Wnuk A, Kajta M, Wojtowicz AK. Triclosan activates aryl hydrocarbon receptor (ahr)-dependent apoptosis and affects cyp1a1 and cyp1b1 expression in mouse neocortical neurons. Environ Res. 2016;151:106–114. doi: 10.1016/j.envres.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Valero De Bernabe J, Soriano T, Albaladejo R, Juarranz M, Calle ME, Martinez D, et al. Risk factors for low birth weight: A review. Eur J Obstet Gynecol Reprod Biol. 2004;116:3–15. doi: 10.1016/j.ejogrb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Kojima H, Takeuchi S, Uramaru N, Sanoh S, Sugihara K, et al. Metabolism of uv-filter benzophenone-3 by rat and human liver microsomes and its effect on endocrine-disrupting activity. Toxicol Appl Pharmacol. 2015;282:119–128. doi: 10.1016/j.taap.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ. On the importance–and the unimportance–of birthweight. Int J Epidemiol. 2001;30:1233–1241. doi: 10.1093/ije/30.6.1233. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, et al. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect. 2008;116:1092–1097. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Carlson A, Schwartz JM, Giudice LC. Proceedings of the summit on environmental challenges to reproductive health and fertility: Executive summary. Fertil Steril. 2008;89:e1–e20. doi: 10.1016/j.fertnstert.2008.01.065. [DOI] [PubMed] [Google Scholar]
- Xin F, Susiarjo M, Bartolomei MS. Multigenerational and transgenerational effects of endocrine disrupting chemicals: A role for altered epigenetic regulation? Semin Cell Dev Biol. 2015;43:66–75. doi: 10.1016/j.semcdb.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Mansour R, Ishihara O, Adamson GD, de Mouzon J, Nygren KG, et al. International committee for monitoring assisted reproductive technology: World report on assisted reproductive technology, 2005. Fertil Steril. 2014;101:366–378. doi: 10.1016/j.fertnstert.2013.10.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


