Abstract
The purine nucleoside adenosine is a present in most body fluids where it regulates a wide variety of physiologic and pharmacologic processes. Adenosine mediates its effects through activating 4 G protein-coupled receptors expressed on the cell membrane: A1, A2A, A2B, and A3. The adenosine receptors are widely distributed in the body, and tissues with high expression include immune tissues, cartilage, bone, heart, and brain. Here we review the source and metabolism of adenosine and the role of adenosine in regulating immunity and cartilage biology.
Graphical Abstract
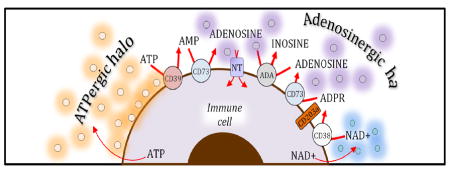
Adenine nucleosides and nucleotides form a halo around cells that regulate immunologic response and cartilage metabolism
Introduction
Adenosine is an endogenous purine nucleoside, a catabolite of ATP, that binds and activates one or more of four transmembrane G-protein-coupled cell surface adenosine receptors (R)s, which are A1R, A2AR, A2BR and A3R (1). Extracellular adenosine can accumulate during inflammation, hypoxia, and associated cellular damage and stress and several studies indicate that it may contribute to innate inflammation (2–4). The accumulation of extracellular adenosine is the result of a multistep process, where ATP is first released from its intracellular pool to the pericellular space, and is then degraded to adenosine by a cascade of cell surface ectonucleotidases, including CD39 (ectonucleoside triphosphate diphosphohydrolase 1 (E-NTPDase1)) and CD73 (5′-ectonucleotidase or Ecto5′NTase) (4–9). Adenosine, the pharmacologic effects of which were first described by Drury and Szent-Gyorgy (10), regulates every organ system in the body, most notably the cardiovascular, nervous, gastrointestinal and immune systems. Indeed, all four of the adenosine receptors are widely expressed throughout the body and different cell types express different combinations of adenosine receptors. In this review we will discuss two newly described regulatory functions of adenosine and adenosine receptors in regulation of Type 2 immunity and chondrocyte homeostasis.
Sources of adenosine
In a cell type and context-dependent manner, several mechanisms for ATP liberation have been proposed, including channel-dependent, cell death, and vesicular release mechanisms (11). ATP release in response to immune activation occurs mostly through Connexin (Cx) and Pannexin (Panx) channels. Cx channels were first described in the 1970s as the principal proteins comprising intercellular gap junctions (12–14). Cx channels are half channels or hemichannels that can readily dock unapposed hemichannels on adjacent cells to form gap junctions. However, they can also remain unapposed and serve as conduits for extracellular ATP liberation. Of the ~25 Cx channels, the ubiquitous Cx43 has been shown to be the major Cx to release ATP (12–14). Panx channels (Panx1, Panx2, and Panx3) are single membrane channels that share several topological and structural features with Cx channels. Panx1 is ubiquitously expressed whereas Panx2 is localized in the bone and Panx3 in cartilage and the central nervous system (12–14). Panx1 channels are the principal Panx channels through which ATP is released (12–14). Macrophages release ATP in response to B. anthracis infection, which is blocked by both pharmacological Cx inhibition and siRNA-mediated Cx43 depletion(15). Eltzschig et al. (16) showed that neutrophils liberate ATP in response to N-formyl-Met-Leu-Phe (fMLP) or leukotriene B4, and that this ATP release is blocked by a peptide inhibitor of Cx43 or in Cx43 knockout (KO) neutrophils. In another study, the fMLP-mediated ATP release by neutrophils was Panx1-dependent (17). T cells release ATP during T cell activation via Panx1 channels (18). In addition to host cells, bacteria or fungi can also release ATP (19, 20). The mechanisms of ATP release during helminth-induced Th2 type immune responses are unknown.
CD39 is the most prominent member of the cell surface E-NTPDase family (11, 21). It has two membrane-spanning domains at its N- and C-termini and its extracellular domain contains five apyrase conserved regions, which are responsible for its catalytic activity. CD39 is widely expressed, whereas the other E-NTPDases are localized to neural tissue or pericytes. CD39 is the major nucleotide-metabolizing enzyme in peripheral blood leukocytes, spleen, lung, and placenta(22), and is expressed on intestinal epithelial cells (23), macrophages (24), neutrophils (16), Tregs (25), Langerhans cells (26), and endothelial cells (27). CD73 is the only surface associated 5′-ectonucleotidase and is anchored to the plasma membrane at the C-terminus by glycosyl-phosphatidylinositol (GPI). CD73 is ubiquitously expressed and the highest expression levels are seen in the gut, brain, kidney, liver, and lung (11, 28). CD73 is readily detected on cells from intestine, blood, spleen, lymph nodes, and bone marrow, and endothelium (28), and within the immune system CD73 is found on the surface of macrophages (29), Treg cells (30), and dendritic cells (31). There is evidence that the expression and function of both CD39 and CD73 are upregulated in the gut following tissue stress and inflammation (23, 32, 33). The regulation of CD39 and CD73 in the gut during a type 2 response is poorly understood.
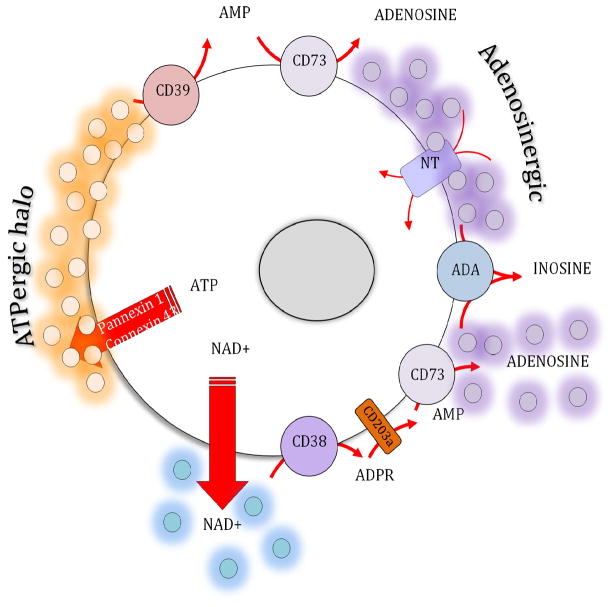
The CD38-CD203a enzyme axis on the cell surface, operating independently or in synergy with the conventional CD39/CD73 pathway, also contributes to the regulation of the adenosine levels (34),(35). CD38 catalyzes the synthesis of cyclic ADP-ribose from nicotinamide adenine dinucleotide (NAD+), and also mediates the hydrolysis of cyclic ADP-ribose to ADP-ribose (ADPR) on the cell surface (36) (see Fig. 1) The pyrophosphatase/phosphodiesterase CD203a can hydrolyze both NAD+ and ADPR to produce AMP, which can then be catabolized to adenosine by CD73 (36, 37) (see Fig 1). In contrast, the adenosine-metabolizing enzyme adenosine deaminase, which is present in plasma and other extracellular fluids at high levels, attenuates extracellular adenosine levels through the conversion of adenosine into inosine (38)(see Fig 1). In addition to regulating immune activation, this enzyme has also been found to play a crucial role in the development of the immune system (38), as illustrated by the severely decreased T and B cell numbers in patients affected by genetic deficiency of adenosine deaminase (39). Adenosine is also taken up by cells via specific nucleoside transporters, which can be inhibited by a number of pharmacologic agents including dipyridamole and ticagrelor (40, 41). Thus, adenosine molecules have a half-life measured in seconds in blood (42) and presumably there is a similarly short half-life in other bodily fluids.
Figure 1.
Adenosine metabolism pathways.
Adenosine Receptors
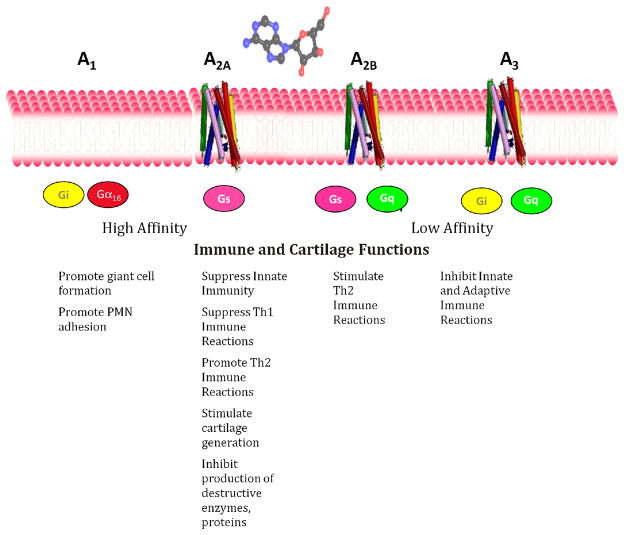
Adenosine in the extracellular space mediates its physiologic and pharmacologic effects via interaction with a family of 4 related 7-transmembrane spanning G protein coupled receptors (GPCRs). These receptors have been named historically in order of their discovery as A1R, A2AR, A2BR and A3R. A1R and A2AR are the most sensitive adenosine receptors and are active in the nanomolar range whereas A2BR and A3R are active in the micromolar range (1). The A1R and A3R signal through Gi-linked proteins and suppress cAMP generation whereas A2AR and A2BR signal through Gs and Golf and activate adenylate cyclase and cAMP-dependent pathways (Figure 2).
Figure 2.
Adenosine receptor signaling pathways.
Adenosine receptors are expressed ubiquitously although different tissues/cell types express different combinations of adenosine receptors. Moreover, in a given tissue specific adenosine receptor expression may be increased or decreased by different external stimuli. A2AR expression increases during many inflammatory reactions as a result of NFkB-mediated stimulation (43–46) and serves as a feedback regulator of inflammation. Moreover, A2AR function increases as a result of inflammatory stimulation; there is diminished desensitization of A2AR after cytokine-mediated stimulation (47).
Regulation of type 2 immunity by adenosine receptors
Based on emerging knowledge on the different effector T-cell and innate lymphoid cell (ILC) lineages, 3 major kinds of cell-mediated effector immunity exist, which are denoted type 1, type 2, and type 3 (48). Type 1 immunity encompasses IFN-γ–secreting group 1 ILCs (ILC1 and natural killer cells), cytotoxic CD8+ T cells, and CD4+ TH1 cells, which protect against intracellular pathogens through recruitment of mononuclear phagocytes. Type 2 immunity, which developed to expulse intestinal and other helminths, consists of ILC2s and TH2 cells which are described in detail below. Type 3 immunity is mediated by ILC3s and TH17 cells producing IL-17, IL-22, or both, which recruit mononuclear phagocytes but also activate neutrophils and induce epithelial antimicrobial responses, therefore mediating protection against extracellular bacteria and fungi.
ARs are widely distributed practically on all types of immune cells, including cells involved in type 1, 2, and 3 immunity (9, 49–56). Whereas the role ARs in regulating type 1and type 3 immunity has been the subject of many recent reviews (57), how ARs regulate type 2 immunity has not been summarized. This is especially important and timely because chronic malnutrition induced by infection with gastrointestinal helminths results in significant morbidity and increased susceptibility to infectious agents. Although primary health care and effective public sanitation can successfully eliminate human gastrointestinal parasitism, immunological intervention may promote control in situations where gastrointestinal parasitism remains endemic and intractable. Immune protection against murine gastrointestinal roundworm infection, manifested by rapid expulsion of nematode larvae during infection, has been shown in many cases to be associated with increases in the type 2 cytokines IL-4, IL-5, IL-9 and IL-13, produced by both innate immune cells and T cells, alternatively activated macrophages (M2) in granulomas, mucosal mastocytosis, eosinophilia, and IgE secretion (58–60). Recent studies suggest that in humans a similar set of cytokines and immune cells are triggered after helminth infection (61). The same immune response (type 2) that orchestrates rapid and effective worm expulsion can also promote tolerance of invading pathogens with recent studies suggesting that the helminth-induced type 2 immune response may have incorporated a wound-healing component as an adaptation to the considerable tissue damage that helminth parasites can cause as they migrate through vital tissues, such as the lung or intestine (62–65). Thus the components of the helminth- induced type 2 immune response may serve multiple functions during infection including effector functions leading to worm expulsion, anti-inflammatory effects, and induction of wound healing.
While type 2 immunity mounted against parasites is crucial for the control of parasitic disease, type 2 immunity can also be triggered by allergens, which can harm the host. IL-4, IL-5, IL-9, and IL-13 are also the major drivers of allergic asthma, and studies in both mice and humans, have shown that they mediate many inflammatory processes, including bronchoconstriction, mucus production, eosinophilia, and immunoglobulin E class switching by B cells (66). In addition to asthma, type 2 immune responses are also central to the development of atopic dermatitis, food allergy, anaphylaxis (67), and a number of fibrotic conditions (68).
It is now also clear that type 2 immunity is also important for the maintenance of homeostasis in adipose tissue and liver, and that dysregulation of the various components of type 2 inflammation results in obesity, metabolic syndrome and diabetes (69–71).
As yet though the pathways that initiate the type 2 immune response have only begun to be elucidated. Immune responses to viruses and bacteria are in many cases triggered by recognition of pathogen associated molecular patterns (PAMPS), that in turn bind pattern recognition receptors (PRRs), which can include toll like receptors (TLRs). Interestingly, TLR-signaling is not generally required for helminth-induced type 2 immune responses, although PAMPs binding other ligands have been proposed as contributing to the helminth response (72). It is increasingly clear that endogenously produced danger signals, or DAMPs, elicited following tissue damage may also trigger signaling pathways that promote immune responses (63). A number of potential DAMPs have been proposed including Trefoil factor 2 (TFF2), DNA, and nucleotides (ATP and ADP), all of which may be released into the extracellular space following cellular disruption and there bind cellular receptors that trigger immune responses (73, 74).
Recent studies have suggested that adenosine interacting with A2BR may contribute to components of type 2 immunity and thus can be a DAMP that initiates and promotes type 2 immune responses. In vitro studies have indicated that A2BR signaling can activate mast cells to secrete IL-4 in vitro (75). IL-4 amplifies A2BRs on mast cells indicating the existence of a positive feedback loop consisting of A2BRs and secreted IL-4 in mast cells (76).
A2BRs stimulate dendritic cells to upregulate production of the type 2-skewing cytokine IL-10, and A2BRs skew dendritic cells to promote CD4+ T cell IL-4 production under hypoxia (77, 78). Our studies have shown A2BRs enhance IL-4- or IL-10 mediated alternatively activated (M2) macrophage differentiation (79–82).
In an early in vivo study, A2BR signaling enhanced allergen-induced chronic pulmonary inflammation in an ovalbumin model system (83). Subsequent studies confirmed this proinflammatory role of A2BRs in a cockroach allergen model of asthma (84). Our studies showed that A2BR signaling was required for the development of both innate and adaptive components of the helminth-induced type 2 immune response, including the memory host protective response (85). We further provided intriguing findings suggesting that one mechanism through which A2BR signaling drives type 2 immunity may be through induction of IL-33 expression and downstream upregulation of IL-5 and IL-13 (85). In a recent in vitro study, we found that A2BR signaling down-regulates IL-15 and IL-13 production by group 2 innate lymphoid cells or ILC2s (86), which indicates that the A2BR-mediated enhancement of anti-helminth immunity is independent of ILC2s.
Adenosine also contributes to pulmonary fibrosis by interfering with type 2 inflammation and especially IL-13 (87). In an experimental mouse model of pulmonary fibrosis, A2BRs on alternatively activated macrophages contributed to the progression of fibrosis (88, 89).
The role of other ARs in regulating type 2 immunity is less clear. An in vivo study using adenosine deaminase deficient mice, which have increased systemic adenosine levels, showed that genetic removal of A1Rs was associated with exaggerated production of IL-4 and Il-13 in the lung (90). We demonstrated in an in vitro study that A2ARs suppress the development of T helper 2 lymphocytes (51). In addition, A2AR activation decreases IL-4 production by naïve T cells (91). In agreement with these in vitro results, A2AR activation has anti-inflammatory effects in allergic lung inflammation in vivo (92).
The role of adenosine and adenosine receptors in maintaining chondrocyte and cartilage homeostasis
Depending on the species and the condition, chondrocytes express primarily A2AR and A2BR although in some studies A1R expression has also been documented to play a role in regulating chondrocyte function (93–95). In addition, A3R have been reported to inhibit the development of osteoarthritis by diminishing joint inflammation (96). Direct effects of A2AR and A2BR stimulation on chondrocyte function have been more frequently described (93, 97–100). Interestingly, Mistry and colleagues reported that very high doses of adenosine, achievable only in the presence of inhibitors of enzymes (adenosine deaminase) that degrade adenosine, are toxic to chondrocytes and induce chondrocyte apoptosis (101).
Chondrocytes, the resident cells of cartilage, maintain cartilage by synthesizing new matrix to replace cartilage matrix as it ages. Because cartilage is an avascular tissue chondrocytes obtain their nutrients by diffusion from the synovial fluid. With aging and inflammation there is a growing imbalance between chondrocyte production of matrix and destruction of matrix. We have recently shown that adenosine, derived from ATP released from chondrocytes, plays an important homeostatic role in chondrocyte and cartilage biology and replacement of adenosine in the joint of affected joints prevents progression of osteoarthritis in a rat model of post-traumatic osteoarthritis (97).
Johnson and colleagues first reported that diminished oxidative phosphorylation in chondrocytes leads to diminished ATP levels and diminished matrix replacement (102). In subsequent work this same group reported that chondrocyte ATP depletion leads to the spontaneous development of knee osteoarthritis in a guinea pig model (103). In subsequent work Wang and colleagues reported that human osteoarthritis chondrocytes made less ATP and lower intracellular levels of ATP than unaffected chondrocytes (104). Increasing age and inflammatory cytokines are consistent stimuli for these changes in chondrocyte metabolism (105–107). This process has been termed inflammaging.
Consistent with these observations in aging and osteoarthritic cartilage we have recently reported a novel homeostatic mechanism in cartilage and chondrocytes, disruption of which leads to chondrocyte dysfunction and cartilage loss (97). We discovered that diminished ATP levels, a feature typical of OA chondrocytes, are associated with diminished ATP export into the extracellular space resulting in reduction of extracellular adenosine. Reduction of adenosine in the extracellular space results in less stimulation of A2AR which is required for maintenance of chondrocyte homeostasis. Observations in mice lacking A2AR and ecto-5′ nucleotidase confirm this overall hypothesis; A2AR and ecto-5′ nucleotidase deficient mice develop spontaneous osteoarthritis. Humans lacking ecto-5′ nucleotidase also develop spontaneous osteoarthritis in addition to a number of other abnormalities. Further evidence for the role of adenosine as a homeostatic factor was provided in a rat model of post-traumatic osteoarthritis in which intra-articular injection of liposomal suspensions of adenosine rescue the osteoarthritis phenotype. Whereas much of the effect of exogenous adenosine on suppression of OA activity is likely due to adenosine-mediated suppression of inflammation (cf (108)), in preliminary studies there is clearly a direct anabolic effect of A2AR stimulation on chondrocyte function (aggrecan) and suppression of production of catabolic proteins (MMP13) and markers of chondrocyte hypertrophy (col10a1), consistent with previous reports on the effect of A2AR ligation on chondrocyte production of NO, PGE2 and release of cartilage breakdown products (glycosaminoglycans, (109–111)).
Excess adenosine also appears to play a role in chondrocyte and cartilage biology since children lacking adenosine deaminase, in whom plasma adenosine levels increase to the micromolar level (112, 113), develop chondrodysplasia (114). The mechanism for this chondrodysplasia is not well understood and recent studies from mice suggest that although the very high levels of adenosine present in adenosine deaminase-deficient children induce chondrocyte apoptosis (101) these are changes that are not consistent with the cartilage changes observed in children lacking adenosine deaminase.
Summary and Conclusion
Adenosine is a potent regulator of many different physiologic and pharmacologic processes. Adenosine is, for the most part, generated from the extracellular hydrolysis of ATP although it can be transported into the extracellular space directly. While adenosine is present in pharmacologically relevant concentrations in nearly all bodily fluids its concentration can be rapidly increased or decreased. Acting at its receptors adenosine plays an important role in mediating and regulating responses to pathogens and in maintaining homeostasis in the joint. We expect that by capitalizing on our increasing understanding of the role of adenosine in regulating immunity and joint homeostasis, we will be able to use this information to develop new drugs to treat immune and joint diseases. Specifically, as A2BRs are important in initiating and promoting type 2 immunity, A2BR agonists may be used in the future as part of novel vaccine regimens to treat and prevent helminth infections. In disease states, where type 2 immunity is overly activated, such as in asthma, fibrosis, and allergic skin diseases, A2BR antagonists may be beneficial.
The current approaches to medical therapy of osteoarthritis are inadequate; anti-inflammatory drugs (corticosteroids), either injected intra-articularly or by mouth (NSAIDs) dominate the therapeutic approaches to osteoarthritis. Other agents, such as intra-articular injection of hyaluronic acid, are of questionable value as well and joint replacement is an increasingly common option for patients. The development of new therapies targeted to adenosine receptors on chondrocytes could provide a turning point in the treatment of this widespread and debilitating condition.
Acknowledgments
This work was supported by National Institutes of Health grants R01GM066189 (G.H.), R01DK113790 (G.H.), R41GM120838 (G.H.), R01 AR056672 (BNC), R01 AR068593 (BNC), THE NYU-HHC CTSI (1UL1TR001445, BNC) and the Arthritis Foundation (BNC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Hasko G, Sitkovsky MV, Szabo C. Immunomodulatory and neuroprotective effects of inosine. Trends Pharmacol Sci. 2004;25:152–157. doi: 10.1016/j.tips.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annual review of pharmacology and toxicology. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- 5.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn-Schmiedeberg’s archives of pharmacology. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- 6.Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. The Journal of experimental medicine. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonioli L, Pacher P, Vizi ES, Hasko G. CD39 and CD73 in immunity and inflammation. Trends in molecular medicine. 2013;19:355–367. doi: 10.1016/j.molmed.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antonioli L, Blandizzi C, Csoka B, Pacher P, Hasko G. Adenosine signalling in diabetes mellitus-pathophysiology and therapeutic considerations. Nat Rev Endocrinol. 2015;11:228–241. doi: 10.1038/nrendo.2015.10. [DOI] [PubMed] [Google Scholar]
- 9.Antonioli L, Blandizzi C, Pacher P, Hasko G. Immunity, inflammation and cancer: a leading role for adenosine. Nature reviews Cancer. 2013;13:842–857. doi: 10.1038/nrc3613. [DOI] [PubMed] [Google Scholar]
- 10.Drury AN, Szent-Gyorgi A. The physiological activity of adenine compounds with special reference to their action upon the mammalian heart. Journal of Physiology. 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antonioli L, Pacher P, Vizi ES, Hasko G. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013;19:355–367. doi: 10.1016/j.molmed.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lohman AW, Isakson BE. Differentiating connexin hemichannels and pannexin channels in cellular ATP release. FEBS Lett. 2014;588:1379–1388. doi: 10.1016/j.febslet.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Velasquez S, Eugenin EA. Role of Pannexin-1 hemichannels and purinergic receptors in the pathogenesis of human diseases. Frontiers in physiology. 2014;5:96. doi: 10.3389/fphys.2014.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eugenin EA. Role of connexin/pannexin containing channels in infectious diseases. FEBS Lett. 2014;588:1389–1395. doi: 10.1016/j.febslet.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ali SR, Timmer AM, Bilgrami S, Park EJ, Eckmann L, Nizet V, Karin M. Anthrax toxin induces macrophage death by p38 MAPK inhibition but leads to inflammasome activation via ATP leakage. Immunity. 2011;35:34–44. doi: 10.1016/j.immuni.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eltzschig HK, Eckle T, Mager A, Kuper N, Karcher C, Weissmuller T, Boengler K, Schulz R, Robson SC, Colgan SP. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- 17.Bao Y, Chen Y, Ledderose C, Li L, Junger WG. Pannexin 1 channels link chemoattractant receptor signaling to local excitation and global inhibition responses at the front and back of polarized neutrophils. The Journal of biological chemistry. 2013;288:22650–22657. doi: 10.1074/jbc.M113.476283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, Verderio C, Buer J, Scanziani E, Grassi F. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal. 2008;1:ra6. doi: 10.1126/scisignal.1160583. [DOI] [PubMed] [Google Scholar]
- 19.Thammavongsa V, Kern JW, Missiakas DM, Schneewind O. Staphylococcus aureus synthesizes adenosine to escape host immune responses. The Journal of experimental medicine. 2009;206:2417–2427. doi: 10.1084/jem.20090097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russo-Abrahao T, Cosentino-Gomes D, Gomes MT, Alviano DS, Alviano CS, Lopes AH, Meyer-Fernandes JR. Biochemical properties of Candida parapsilosis ecto-5′-nucleotidase and the possible role of adenosine in macrophage interaction. FEMS Microbiol Lett. 2011;317:34–42. doi: 10.1111/j.1574-6968.2011.02216.x. [DOI] [PubMed] [Google Scholar]
- 21.Knowles AF. The GDA1_CD39 superfamily: NTPDases with diverse functions. Purinergic Signal. 2011;7:21–45. doi: 10.1007/s11302-010-9214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enjyoji K, Sevigny J, Lin Y, Frenette PS, Christie PD, Esch JS, 2nd, Imai M, Edelberg JM, Rayburn H, Lech M, Beeler DL, Csizmadia E, Wagner DD, Robson SC, Rosenberg RD. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nature medicine. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 23.Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levesque SA, Kukulski F, Enjyoji K, Robson SC, Sevigny J. NTPDase1 governs P2X7-dependent functions in murine macrophages. Eur J Immunol. 2010;40:1473–1485. doi: 10.1002/eji.200939741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. The Journal of experimental medicine. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizumoto N, Kumamoto T, Robson SC, Sevigny J, Matsue H, Enjyoji K, Takashima A. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nature medicine. 2002;8:358–365. doi: 10.1038/nm0402-358. [DOI] [PubMed] [Google Scholar]
- 27.Kaczmarek E, Koziak K, Sevigny J, Siegel JB, Anrather J, Beaudoin AR, Bach FH, Robson SC. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J Biol Chem. 1996;271:33116–33122. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- 28.Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Regateiro FS, Howie D, Nolan KF, Agorogiannis EI, Greaves DR, Cobbold SP, Waldmann H. Generation of anti-inflammatory adenosine byleukocytes is regulated by TGF-beta. Eur J Immunol. 2011;41:2955–2965. doi: 10.1002/eji.201141512. [DOI] [PubMed] [Google Scholar]
- 30.Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J Immunol. 2006;177:6780–6786. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 31.Berchtold S, Ogilvie AL, Bogdan C, Muhl-Zurbes P, Ogilvie A, Schuler G, Steinkasserer A. Human monocyte derived dendritic cells express functional P2X and P2Y receptors as well as ecto-nucleotidases. FEBS Lett. 1999;458:424–428. doi: 10.1016/s0014-5793(99)01197-7. [DOI] [PubMed] [Google Scholar]
- 32.Hart ML, Grenz A, Gorzolla IC, Schittenhelm J, Dalton JH, Eltzschig HK. Hypoxia-inducible factor-1alpha-dependent protection from intestinal ischemia/reperfusion injury involves ecto-5′-nucleotidase (CD73) and the A2B adenosine receptor. J Immunol. 2011;186:4367–4374. doi: 10.4049/jimmunol.0903617. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Hart ML, Henn M, Kohler D, Kloor D, Mittelbronn M, Gorzolla IC, Stahl GL, Eltzschig HK. Role of extracellular nucleotide phosphohydrolysis in intestinal ischemia-reperfusion injury. FASEB J. 2008;22:2784–2797. doi: 10.1096/fj.07-103911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morra M, Zubiaur M, Terhorst C, Sancho J, Malavasi F. CD38 is functionally dependent on the TCR/CD3 complex in human T cells. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1998;12:581–592. doi: 10.1096/fasebj.12.7.581. [DOI] [PubMed] [Google Scholar]
- 35.Bahri R, Bollinger A, Bollinger T, Orinska Z, Bulfone-Paus S. Ectonucleotidase CD38 demarcates regulatory, memory-like CD8+ T cells with IFN-gamma-mediated suppressor activities. PloS one. 2012;7:e45234. doi: 10.1371/journal.pone.0045234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quarona V, Zaccarello G, Chillemi A, Brunetti E, Singh VK, Ferrero E, Funaro A, Horenstein AL, Malavasi F. CD38 and CD157: a long journey from activation markers to multifunctional molecules. Cytometry. Part B, Clinical cytometry. 2013;84:207–217. doi: 10.1002/cyto.b.21092. [DOI] [PubMed] [Google Scholar]
- 37.Horenstein AL, Quarona V, Toscani D, Costa F, Chillemi A, Pistoia V, Giuliani N, Malavasi F. Adenosine Generated in the Bone Marrow Niche Through a CD38-Mediated Pathway Correlates with Progression of Human Myeloma. Molecular medicine. 2016:22. doi: 10.2119/molmed.2016.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antonioli L, Colucci R, La Motta C, Tuccori M, Awwad O, Da Settimo F, Blandizzi C, Fornai M. Adenosine deaminase in the modulation of immune system and its potential as a novel target for treatment of inflammatory disorders. Current drug targets. 2012;13:842–862. doi: 10.2174/138945012800564095. [DOI] [PubMed] [Google Scholar]
- 39.Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L, Scaramuzza S, Andolfi G, Mirolo M, Brigida I, Tabucchi A, Carlucci F, Eibl M, Aker M, Slavin S, Al-Mousa H, Al Ghonaium A, Ferster A, Duppenthaler A, Notarangelo L, Wintergerst U, Buckley RH, Bregni M, Marktel S, Valsecchi MG, Rossi P, Ciceri F, Miniero R, Bordignon C, Roncarolo MG. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. The New England journal of medicine. 2009;360:447–458. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- 40.Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD. The equilibrative nucleoside transporter family, SLC29. Pflugers Archiv: European journal of physiology. 2004;447:735–743. doi: 10.1007/s00424-003-1103-2. [DOI] [PubMed] [Google Scholar]
- 41.Armstrong D, Summers C, Ewart L, Nylander S, Sidaway JE, van Giezen JJ. Characterization of the adenosine pharmacology of ticagrelor reveals therapeutically relevant inhibition of equilibrative nucleoside transporter 1. J Cardiovasc Pharmacol Ther. 2014;19:209–219. doi: 10.1177/1074248413511693. [DOI] [PubMed] [Google Scholar]
- 42.Moser GH, Schrader J, Deussen A. Turnover of adenosine in plasma of human and dog blood. American Journal of Physiology. 1989;256:C799–C806. doi: 10.1152/ajpcell.1989.256.4.C799. [DOI] [PubMed] [Google Scholar]
- 43.Khoa ND, Montesinos MC, Reiss AB, Delano D, Awadallah N, Cronstein BN. Inflammatory cytokines regulate function and expression of adenosine A(2A) receptors in human monocytic THP-1 cells. J Immunol. 2001;167:4026–4032. doi: 10.4049/jimmunol.167.7.4026. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen DK, Montesinos MC, Williams AJ, Kelly M, Cronstein BN. Th1 cytokines regulate adenosine receptors and their downstream signaling elements in human microvascular endothelial cells. Journal of immunology. 2003;171:3991–3998. doi: 10.4049/jimmunol.171.8.3991. [DOI] [PubMed] [Google Scholar]
- 45.Bshesh K, Zhao B, Spight D, Biaggioni I, Feokistov I, Denenberg A, Wong HR, Shanley TP. The A2A receptor mediates an endogenous regulatory pathway of cytokine expression in THP-1 cells. Journal of Leukocyte Biology. 2002;72:1027–1036. [PubMed] [Google Scholar]
- 46.Morello S, Ito K, Yamamura S, Lee KY, Jazrawi E, Desouza P, Barnes P, Cicala C, Adcock IM. IL-1beta and TNF-{alpha} Regulation of the Adenosine Receptor (A2A) Expression: Differential Requirement for NF-{kappa}B Binding to the Proximal Promoter. Journal of immunology. 2006;177:7173–7183. doi: 10.4049/jimmunol.177.10.7173. [DOI] [PubMed] [Google Scholar]
- 47.Khoa ND, Postow M, Danielsson J, Cronstein BN. Tumor necrosis factor-alpha prevents desensitization of Galphas-coupled receptors by regulating GRK2 association with the plasma membrane. Molecular pharmacology. 2006;69:1311–1319. doi: 10.1124/mol.105.016857. [DOI] [PubMed] [Google Scholar]
- 48.Annunziato F, Romagnani C, Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol. 2015;135:626–635. doi: 10.1016/j.jaci.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Hasko G, Szabo C, Nemeth ZH, Kvetan V, Pastores SM, Vizi ES. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol. 1996;157:4634–4640. [PubMed] [Google Scholar]
- 50.Hasko G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, Marton A, Szabo C. Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2000;14:2065–2074. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 51.Csoka B, Himer L, Selmeczy Z, Vizi ES, Pacher P, Ledent C, Deitch EA, Spolarics Z, Nemeth ZH, Hasko G. Adenosine A2A receptor activation inhibits T helper 1 and T helper 2 cell development and effector function. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2008;22:3491–3499. doi: 10.1096/fj.08-107458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cronstein BN, Sitkovsky M. Adenosine and adenosine receptors in the pathogenesis and treatment of rheumatic diseases. Nat Rev Rheumatol. 2017;13:41–51. doi: 10.1038/nrrheum.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med. 2012;367:2322–2333. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hatfield SM, Sitkovsky M. A2A adenosine receptor antagonists to weaken the hypoxia-HIF-1alpha driven immunosuppression and improve immunotherapies of cancer. Curr Opin Pharmacol. 2016;29:90–96. doi: 10.1016/j.coph.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sitkovsky M, Ohta A. Targeting the hypoxia-adenosinergic signaling pathway to improve the adoptive immunotherapy of cancer. J Mol Med (Berl) 2013;91:147–155. doi: 10.1007/s00109-013-1001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sitkovsky MV, Hatfield S, Abbott R, Belikoff B, Lukashev D, Ohta A. Hostile, hypoxia-A2-adenosinergic tumor biology as the next barrier to overcome for tumor immunologists. Cancer Immunol Res. 2014;2:598–605. doi: 10.1158/2326-6066.CIR-14-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sitkovsky MV. T regulatory cells: hypoxia-adenosinergic suppression and re-direction of the immune response. Trends in immunology. 2009;30:102–108. doi: 10.1016/j.it.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 58.Anthony RM, Rutitzky LI, Urban JF, Jr, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patel N, Kreider T, Urban JF, Jr, Gause WC. Characterisation of effector mechanisms at the host:parasite interface during the immune response to tissue-dwelling intestinal nematode parasites. Int J Parasitol. 2009;39:13–21. doi: 10.1016/j.ijpara.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nature reviews Immunology. 2011;11:375–388. doi: 10.1038/nri2992. [DOI] [PubMed] [Google Scholar]
- 61.Gaze S, McSorley HJ, Daveson J, Jones D, Bethony JM, Oliveira LM, Speare R, McCarthy JS, Engwerda CR, Croese J, Loukas A. Characterising the mucosal and systemic immune responses to experimental human hookworm infection. PLoS pathogens. 2012;8:e1002520. doi: 10.1371/journal.ppat.1002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, Millman A, Van Rooijen N, Urban JF, Jr, Wynn TA, Gause WC. An essential role for T(H)2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med. 2012;18:260–266. doi: 10.1038/nm.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gause WC, Wynn TA, Allen JE. Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat Rev Immunol. 2013 doi: 10.1038/nri3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herbert DR, Orekov T, Roloson A, Ilies M, Perkins C, O’Brien W, Cederbaum S, Christianson DW, Zimmermann N, Rothenberg ME, Finkelman FD. Arginase I suppresses IL-12/IL-23p40-driven intestinal inflammation during acute schistosomiasis. J Immunol. 2010;184:6438–6446. doi: 10.4049/jimmunol.0902009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Allen JE, Wynn TA. Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS pathogens. 2011;7:e1002003. doi: 10.1371/journal.ppat.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sy CB, Siracusa MC. The Therapeutic Potential of Targeting Cytokine Alarmins to Treat Allergic Airway Inflammation. Front Physiol. 2016;7:214. doi: 10.3389/fphys.2016.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hammad H, Lambrecht BN. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity. 2015;43:29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 68.Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol. 2015;15:271–282. doi: 10.1038/nri3831. [DOI] [PubMed] [Google Scholar]
- 69.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. The Journal of experimental medicine. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang HE, Locksley RM. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, Locksley RM. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wills-Karp M, Rani R, Dienger K, Lewkowich I, Fox JG, Perkins C, Lewis L, Finkelman FD, Smith DE, Bryce PJ, Kurt-Jones EA, Wang TC, Sivaprasad U, Hershey GK, Herbert DR. Trefoil factor 2 rapidly induces interleukin 33 to promote type 2 immunity during allergic asthma and hookworm infection. J Exp Med. 2012;209:607–622. doi: 10.1084/jem.20110079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ryzhov S, Goldstein AE, Matafonov A, Zeng D, Biaggioni I, Feoktistov I. Adenosine-activated mast cells induce IgE synthesis by B lymphocytes: an A2B-mediated process involving Th2 cytokines IL-4 and IL-13 with implications for asthma. Journal of immunology. 2004;172:7726–7733. doi: 10.4049/jimmunol.172.12.7726. [DOI] [PubMed] [Google Scholar]
- 76.Hua X, Chason KD, Patel JY, Naselsky WC, Tilley SL. IL-4 amplifies the pro-inflammatory effect of adenosine in human mast cells by changing expression levels of adenosine receptors. PLoS One. 2011;6:e24947. doi: 10.1371/journal.pone.0024947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang M, Ma C, Liu S, Shao Q, Gao W, Song B, Sun J, Xie Q, Zhang Y, Feng A, Liu Y, Hu W, Qu X. HIF-dependent induction of adenosine receptor A2b skews human dendritic cells to a Th2-stimulating phenotype under hypoxia. Immunology and cell biology. 2010;88:165–171. doi: 10.1038/icb.2009.77. [DOI] [PubMed] [Google Scholar]
- 78.Novitskiy SV, Ryzhov S, Zaynagetdinov R, Goldstein AE, Huang Y, Tikhomirov OY, Blackburn MR, Biaggioni I, Carbone DP, Feoktistov I, Dikov MM. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood. 2008;112:1822–1831. doi: 10.1182/blood-2008-02-136325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Csoka B, Selmeczy Z, Koscso B, Nemeth ZH, Pacher P, Murray PJ, Kepka-Lenhart D, Morris SM, Jr, Gause WC, Leibovich SJ, Hasko G. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2012;26:376–386. doi: 10.1096/fj.11-190934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koscso B, Csoka B, Kokai E, Nemeth ZH, Pacher P, Virag L, Leibovich SJ, Hasko G. Adenosine augments IL-10-induced STAT3 signaling in M2c macrophages. Journal of leukocyte biology. 2013;94:1309–1315. doi: 10.1189/jlb.0113043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Csoka B, Koscso B, Toro G, Kokai E, Virag L, Nemeth ZH, Pacher P, Bai P, Hasko G. A2B adenosine receptors prevent insulin resistance by inhibiting adipose tissue inflammation via maintaining alternative macrophage activation. Diabetes. 2014;63:850–866. doi: 10.2337/db13-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ferrante CJ, Pinhal-Enfield G, Elson G, Cronstein BN, Hasko G, Outram S, Leibovich SJ. The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Ralpha) signaling. Inflammation. 2013;36:921–931. doi: 10.1007/s10753-013-9621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zaynagetdinov R, Ryzhov S, Goldstein AE, Yin H, Novitskiy SV, Goleniewska K, Polosukhin VV, Newcomb DC, Mitchell D, Morschl E, Zhou Y, Blackburn MR, Peebles RS, Jr, Biaggioni I, Feoktistov I. Attenuation of chronic pulmonary inflammation in A2B adenosine receptor knockout mice. Am J Respir Cell Mol Biol. 2010;42:564–571. doi: 10.1165/rcmb.2008-0391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Belikoff BG, Vaickus LJ, Sitkovsky M, Remick DG. A2B adenosine receptor expression by myeloid cells is proinflammatory in murine allergic-airway inflammation. J Immunol. 2012;189:3707–3713. doi: 10.4049/jimmunol.1201207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Patel N, Wu W, Mishra PK, Chen F, Millman A, Csoka B, Koscso B, Eltzschig HK, Hasko G, Gause WC. A2B adenosine receptor induces protective antihelminth type 2 immune responses. Cell Host Microbe. 2014;15:339–350. doi: 10.1016/j.chom.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 86.Csoka B, Nemeth ZH, Duerr CU, Fritz JH, Pacher P, Hasko G. Adenosine receptors differentially regulate type 2 cytokine production by IL-33-activated bone marrow cells, ILC2s, and macrophages. FASEB J. 2017 doi: 10.1096/fj.201700770R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blackburn MR, Lee CG, Young HW, Zhu Z, Chunn JL, Kang MJ, Banerjee SK, Elias JA. Adenosine mediates IL-13-induced inflammation and remodeling in the lung and interacts in an IL-13-adenosine amplification pathway. The Journal of clinical investigation. 2003;112:332–344. doi: 10.1172/JCI16815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Philip K, Mills TW, Davies J, Chen NY, Karmouty-Quintana H, Luo F, Molina JG, Amione-Guerra J, Sinha N, Guha A, Eltzschig HK, Blackburn MR. HIF1A up-regulates the ADORA2B receptor on alternatively activated macrophages and contributes to pulmonary fibrosis. FASEB J. 2017 doi: 10.1096/fj.201700219R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karmouty-Quintana H, Philip K, Acero LF, Chen NY, Weng T, Molina JG, Luo F, Davies J, Le NB, Bunge I, Volcik KA, Le TT, Johnston RA, Xia Y, Eltzschig HK, Blackburn MR. Deletion of ADORA2B from myeloid cells dampens lung fibrosis and pulmonary hypertension. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2015;29:50–60. doi: 10.1096/fj.14-260182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun CX, Young HW, Molina JG, Volmer JB, Schnermann J, Blackburn MR. A protective role for the A1 adenosine receptor in adenosine-dependent pulmonary injury. The Journal of clinical investigation. 2005;115:35–43. doi: 10.1172/JCI22656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Romio M, Reinbeck B, Bongardt S, Huls S, Burghoff S, Schrader J. Extracellular purine metabolism and signaling of CD73-derived adenosine in murine Treg and Teff cells. Am J Physiol Cell Physiol. 2011;301:C530–539. doi: 10.1152/ajpcell.00385.2010. [DOI] [PubMed] [Google Scholar]
- 92.da Rocha Lapa F, de Oliveira AP, Accetturi BG, de Oliveira Martins I, Domingos HV, de Almeida Cabrini D, de Lima WT, Santos AR. Anti-inflammatory effects of inosine in allergic lung inflammation in mice: evidence for the participation of adenosine A2A and A 3 receptors. Purinergic Signal. 2013;9:325–336. doi: 10.1007/s11302-013-9351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Varani K, De Mattei M, Vincenzi F, Gessi S, Merighi S, Pellati A, Ongaro A, Caruso A, Cadossi R, Borea PA. Characterization of adenosine receptors in bovine chondrocytes and fibroblast-like synoviocytes exposed to low frequency low energy pulsed electromagnetic fields. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2008;16:292–304. doi: 10.1016/j.joca.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 94.Koolpe M, Pearson D, Benton HP. Expression of both P1 and P2 purine receptor genes by human articular chondrocytes and profile of ligand-mediated prostaglandin E2 release. Arthritis and rheumatism. 1999;42:258–267. doi: 10.1002/1529-0131(199902)42:2<258::AID-ANR7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 95.Choi H, Choi Y, Kim J, Bae J, Roh J. Longitudinal bone growth is impaired by direct involvement of caffeine with chondrocyte differentiation in the growth plate. Journal of anatomy. 2017;230:117–127. doi: 10.1111/joa.12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bar-Yehuda S, Rath-Wolfson L, Del Valle L, Ochaion A, Cohen S, Patoka R, Zozulya G, Barer F, Atar E, Pina-Oviedo S, Perez-Liz G, Castel D, Fishman P. Induction of an antiinflammatory effect and prevention of cartilage damage in rat knee osteoarthritis by CF101 treatment. Arthritis and rheumatism. 2009;60:3061–3071. doi: 10.1002/art.24817. [DOI] [PubMed] [Google Scholar]
- 97.Corciulo C, Lendhey M, Wilder T, Schoen H, Cornelissen AS, Chang G, Kennedy OD, Cronstein BN. Endogenous adenosine maintains cartilage homeostasis and exogenous adenosine inhibits osteoarthritis progression. Nat Commun. 2017;8:15019. doi: 10.1038/ncomms15019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fini M, Pagani S, Giavaresi G, De Mattei M, Ongaro A, Varani K, Vincenzi F, Massari L, Cadossi M. Functional tissue engineering in articular cartilage repair: is there a role for electromagnetic biophysical stimulation? Tissue engineering. Part B, Reviews. 2013;19:353–367. doi: 10.1089/ten.TEB.2012.0501. [DOI] [PubMed] [Google Scholar]
- 99.Ongaro A, Varani K, Masieri FF, Pellati A, Massari L, Cadossi R, Vincenzi F, Borea PA, Fini M, Caruso A, De Mattei M. Electromagnetic fields (EMFs) and adenosine receptors modulate prostaglandin E(2) and cytokine release in human osteoarthritic synovial fibroblasts. Journal of cellular physiology. 2012;227:2461–2469. doi: 10.1002/jcp.22981. [DOI] [PubMed] [Google Scholar]
- 100.Vincenzi F, Targa M, Corciulo C, Gessi S, Merighi S, Setti S, Cadossi R, Goldring MB, Borea PA, Varani K. Pulsed electromagnetic fields increased the anti-inflammatory effect of A(2)A and A(3) adenosine receptors in human T/C-28a2 chondrocytes and hFOB 1.19 osteoblasts. PloS one. 2013;8:e65561. doi: 10.1371/journal.pone.0065561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mistry D, Chambers MG, Mason RM. The role of adenosine in chondrocyte death in murine osteoarthritis and in a murine chondrocyte cell line. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2006;14:486–495. doi: 10.1016/j.joca.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 102.Johnson K, Jung A, Murphy A, Andreyev A, Dykens J, Terkeltaub R. Mitochondrial oxidative phosphorylation is a downstream regulator of nitric oxide effects on chondrocyte matrix synthesis and mineralization. Arthritis and rheumatism. 2000;43:1560–1570. doi: 10.1002/1529-0131(200007)43:7<1560::AID-ANR21>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 103.Johnson K, Svensson CI, Etten DV, Ghosh SS, Murphy AN, Powell HC, Terkeltaub R. Mediation of spontaneous knee osteoarthritis by progressive chondrocyte ATP depletion in Hartley guinea pigs. Arthritis and rheumatism. 2004;50:1216–1225. doi: 10.1002/art.20149. [DOI] [PubMed] [Google Scholar]
- 104.Wang Y, Zhao X, Lotz M, Terkeltaub R, Liu-Bryan R. Mitochondrial biogenesis is impaired in osteoarthritis chondrocytes but reversible via peroxisome proliferator-activated receptor gamma coactivator 1alpha. Arthritis & rheumatology. 2015;67:2141–2153. doi: 10.1002/art.39182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!) Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2013;21:16–21. doi: 10.1016/j.joca.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 106.Loeser RF, Collins JA, Diekman BO. Ageing and the pathogenesis of osteoarthritis. Nature reviews Rheumatology. 2016;12:412–420. doi: 10.1038/nrrheum.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sellam J, Berenbaum F. Is osteoarthritis a metabolic disease? Joint, bone, spine: revue du rhumatisme. 2013;80:568–573. doi: 10.1016/j.jbspin.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 108.Hasko G, Cronstein B. Regulation of inflammation by adenosine. Frontiers in immunology. 2013;4:85. doi: 10.3389/fimmu.2013.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tesch AM, MacDonald MH, Kollias-Baker C, Benton HP. Endogenously produced adenosine regulates articular cartilage matrix homeostasis: enzymatic depletion of adenosine stimulates matrix degradation. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2004;12:349–359. doi: 10.1016/j.joca.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 110.Tesch AM, MacDonald MH, Kollias-Baker C, Benton HP. Effects of an adenosine kinase inhibitor and an adenosine deaminase inhibitor on accumulation of extracellular adenosine by equine articular chondrocytes. Am J Vet Res. 2002;63:1512–1519. doi: 10.2460/ajvr.2002.63.1512. [DOI] [PubMed] [Google Scholar]
- 111.Tesch AM, MacDonald MH, Kollias-Baker C, Benton HP. Chondrocytes respond to adenosine via A(2)receptors and activity is potentiated by an adenosine deaminase inhibitor and a phosphodiesterase inhibitor. Osteoarthritis Cartilage. 2002;10:34–43. doi: 10.1053/joca.2001.0479. [DOI] [PubMed] [Google Scholar]
- 112.Hirschhorn R, Roegner V, Rubinstein A, Papageorgiou P. Plasma deoxyadenosine, adenosine, and erythrocyte deoxyATP are elevated at birth in an adenosine deaminase-deficient child. The Journal of clinical investigation. 1980;65:768–771. doi: 10.1172/JCI109725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hirschhorn R, Roegner-Maniscalco V, Kuritsky L, Rosen FS. Bone marrow transplantation only partially restores purine metabolites to normal in adenosine deaminase-deficient patients. Journal of Clinical Investigation. 1981;68:1387–1393. doi: 10.1172/JCI110389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cederbaum SD, Kaitila I, Rimoin DL, Stiehm ER. The chondro-osseous dysplasia of adenosine deaminase deficiency with severe combined immunodeficiency. J Pediatr. 1976;89:737–742. doi: 10.1016/s0022-3476(76)80793-7. [DOI] [PubMed] [Google Scholar]