Abstract
Diphosphoinositol pentakisphosphate (IP7) is critical for the exocytotic capacity of the pancreatic β-cell, but its regulation by the primary instigator of β-cell exocytosis, glucose, is unknown. The high Km for ATP of the IP7-generating enzymes, the inositol hexakisphosphate kinases (IP6K1 and 2) suggests that these enzymes might serve as metabolic sensors in insulin secreting β-cells and act as translators of disrupted metabolism in diabetes. We investigated this hypothesis and now show that glucose stimulation, which increases the ATP/ADP ratio, leads to an early rise in IP7 concentration in β-cells. RNAi mediated knock down of the IP6K1 isoform inhibits both glucose-mediated increase in IP7 and first phase insulin secretion, demonstrating that IP6K1 integrates glucose metabolism and insulin exocytosis. In diabetic mouse islets the deranged ATP/ADP levels under both basal and glucose-stimulated conditions are mirrored in both disrupted IP7 generation and insulin release. Thus the unique metabolic sensing properties of IP6K1 guarantees appropriate concentrations of IP7 and thereby both correct basal insulin secretion and intact first phase insulin release. In addition, our data suggest that a specific cell signaling defect, namely, inappropriate IP7 generation may be an essential convergence point integrating multiple metabolic defects into the commonly observed phenotype in diabetes.
Abbreviations: IP7, di-phosphoinositol pentakisphosphate; IP6K, inositol hexakisphosphate kinase; T2D, type 2 diabetes
Keywords: Inositol hexakisphosphate kinase 1, Diphosphoinositol pentakisphosphate/ IP7, Pancreatic beta cell, Insulin secretion, Type 2 diabetes, ATP/ADP
Graphical abstract
Highlights
-
•
Glucose increases IP7 levels transiently through IP6K1 in pancreatic β-cells.
-
•
IP6K1 decodes glucose-driven increases in ATP/ADP ratio into 1st phase insulin release.
-
•
IP7 production and insulin release mirror perturbed metabolism in diabetic islets.
-
•
IP6K1 acts as a β-cell metabolic sensor under normal and pathological conditions.
1. Introduction
The pancreatic β-cell is an essential player in the control of blood glucose concentration. It converts an increase in blood glucose into metabolic signals that stimulate insulin release and thus regulate whole body glucose homoeostasis. A crucial signal resulting from β-cell glucose metabolism is an increase in the ATP/ADP ratio. A disrupted energy metabolism in pancreatic islets, including higher basal ATP and reduced increases in the ATP/ADP ratio in response to glucose, is associated with type 2 diabetes (T2D), both in rodents and in humans [[1], [2], [3]]. Furthermore, the onset of T2D is strongly linked to a disturbed secretion of insulin from pancreatic β-cells. This includes an elevated basal release of insulin [[4], [5], [6]] and an early selective loss of first phase insulin secretion both in people with pre-diabetes and in T2D patients [4,7]. Recent genome wide association studies suggest a specific defect in the β-cell stimulus-secretion coupling machinery in T2D development [8]. However, a clear connection between the metabolic and secretory defects has not been established. Thus the identification of novel signals that hardwire metabolic changes into exocytosis is of paramount importance in understanding the onset and progression of T2D.
The pancreatic β-cell is an essential player in the control of blood glucose concentration. It converts an increase in blood glucose into metabolic signals that stimulate insulin release and thus regulate whole body glucose homoeostasis. A crucial signal resulting from β-cell glucose metabolism is an increase in the ATP/ADP ratio. A disrupted energy metabolism in pancreatic islets, including higher basal ATP and reduced increases in the ATP/ADP ratio in response to glucose, is associated with type 2 diabetes (T2D), both in rodents and in humans [[1], [2], [3]]. Furthermore, the onset of T2D is strongly linked to a disturbed secretion of insulin from pancreatic β-cells. This includes an elevated basal release of insulin [[4], [5], [6]] and an early selective loss of first phase insulin secretion both in people with pre-diabetes and in T2D patients [4,7]. Recent genome wide association studies suggest a specific defect in the β-cell stimulus-secretion coupling machinery in T2D development [8]. However, a clear connection between the metabolic and secretory defects has not been established. Thus the identification of novel signals that hardwire metabolic changes into exocytosis is of paramount importance in understanding the onset and progression of T2D.
Signaling via derivatives of myo-inositol is central to the β-cell stimulus-secretion coupling [9]. Direct effects on exocytosis can be mediated by inositol hexakisphosphate (IP6) and diphosphoinositol pentakisphosphate (IP7) [9]. IP7 controls the exocytotic capacity of the β-cell [10], but its regulation by the primary stimulus of insulin secretion, glucose, is unknown. In fact, little is known as to how cellular levels of IP7 may be regulated. IP7 is generated by three IP6 kinases (IP6K1–3) [9,[11], [12], [13], [14]], but only types 1 and 2 are expressed in β-cells. Studies on IP6K1 knockout mice [15] or mice treated with TNP [N2-(m-Trifluorobenzyl), N6-(p-nitrobenzyl) purine], a pan-IP6K inhibitor [16], record reduced serum insulin consistent for a role of IP6Ks in in vivo insulin secretion.
IP6Ks have a high Km for ATP [11,12,[17], [18], [19]], (e.g. 1.1 mM for IP6K1 [12]). Since the free concentration of ATP in pancreatic β-cells is around 1 mM [20], glucose-mediated changes in the ATP/ADP ratio might regulate IP7 levels. This idea is supported by the fact that metabolic poisons substantially reduce both ATP/ADP ratio and IP7 concentration [17,21]. Artificial manipulation of ATP/ADP via kinase inhibitors [22] or exogenous addition of this nucleotide [23], also suggest a direct correlation between ATP and IP7. However, no previous studies have investigated the possibility that a naturally occurring, stimulus-dependent increase in ATP/ADP might regulate IP7 levels. Here, we show that it is this bioenergetic sensing by IP6K1 that connects glucose stimulation to insulin secretion. An association study with familial T2D indicated a disrupted IP6K1 gene [24] which offers proof of principal that IP6K1 can play a role in the human disease. However, this is an isolated case. Our current study suggests that even pancreatic β-cells with normal levels of IP6K expression are vulnerable to the defective metabolism in diabetes simply because IP6Ks are hostage to a disrupted ATP/ADP homeostasis common in the disease.
2. Materials and methods
2.1. Reagents
All cell culture reagents were obtained from Life Technologies (Stockholm, Sweden). The rest of the chemicals were purchased from Sigma (Stockholm, Sweden), Merck KGaA (Darmstad, Germany) and VWR (Leyven, Belgium).
2.2. Isolation of human and mouse islets of Langerhans
Regional Ethical Review Boards in Uppsala and Stockholm approved the experiments involving human islets. Human pancreata were obtained within the Nordic Network for Islet Transplantation from deceased donors with total brain infarction after appropriate consent. The islets were isolated and cultured as previously described [25].
The animal studies were approved by the animal ethics committee of Northern Sweden and were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals. The ob/ob (leptin null) mice and lean littermates, originally obtained from Umeå, Sweden, were bred at the Karolinska hospital animal core facility and identified by phenotypic and genotypic analysis [26]. Pancreatic islets were isolated from the pancreata of C57BL/6, lean or ob/ob mice at the age of 12–16 weeks. Mice were shortly anesthetized with isoflurane (Baxter, Kista, Sweden) before cervical dislocation. Ice-cold isolation buffer (HBSS with 0.5% BSA, 100 units per ml penicillin G, 100 mg/ml streptomycin-sulfate and 25 mM HEPES, pH 7.4) containing 1 mg/ml collagenase A or collagenase P (Roche, Stockholm, Sweden) was injected into the pancreas [27]. The pancreata were digested at 37 °C in a water bath. Then, the digested tissue was washed with isolation buffer, the islets were handpicked under a microscope and transferred to culture medium. The isolation of islet from NMRI mice (Bomholtgaard, Ry, Denmark) was performed as previously reported [10].
2.3. Cell culture
The mouse MIN6m9 cells [28], a gift from Prof. S. Seino, Kobe University Graduate School of Medicine, Japan, were cultured in complete DMEM [29].
2.4. RNA silencing
MIN6m9 and primary islet cells were silenced as previously described [10] using siRNAs from Ambion Inc. (Austin, TX) specific for IP6K1, IP6K2 and non-targeting control (ID = 188560, 71758, 287702, 292211, 4611 and 4613, respectively). The above mentioned siRNAs with Cy3 fluorescent tags at the 5’end of both sense and antisense strands were used in capacitance measurement experiments in primary β-cells so that the transfected cells could be identified.
2.5. Dynamic incubation assays
MIN6m9 cells were seeded in complete DMEM. For experiments involving IP7 measurements, cells were seeded in a DMEM supplemented with 10% dialysed serum (dialyzed using 1000 M.W. cut off membrane, Spectrum labs, The Netherlands), containing 50 μCi/ml [3H] myo-inositol (American radiolabelled chemicals, St. Louis, MO, USA) and labelled for 72–96 h. Throughout this whole study we have used a modified KREBS buffer containing 119 mM NaCl, 4.6 mM KCl, 2 mM or 4 mM CaCl2, 1 mM MgSO4, 0.15 mM Na2HPO4, 0.4 mM KH2PO4, 5 mM NaHCO3, 0.5 mg/ml BSA, 20 mM HEPES pH 7.4. On the day of the experiment, the cells (~83,000 cells/cm2) were pre-incubated, washed and treated with KREBS buffer containing either 10 mM or 0.5 mM glucose for the time indicated in the figures [29]. The perfusates were collected for insulin analysis. Cells were then lysed either with 5% ice-cold trichloroacetic acid (TCA) for inositol phosphates, ATP and ADP extraction or with Mammalian Protein Extraction Reagent, M-PER (Thermofisher Scientific, Stockholm, Sweden) for protein quantification (using Pierce™ BCA protein assay kit, Thermofisher Scientific).
2.6. Static incubation assays
MIN6m9 cells were preincubated for 1 h with modified KREBS buffer containing 0.5 mM glucose. Cells were then stimulated in the same buffer containing 0.5 mM glucose for 3 min by adding different insulin secretogogues such as 10 mM sodium pyruvate, 10 mM l-leucine or 25 mM KCl. After stimulation, the cells were lysed rapidly with ice-cold 5% TCA and the inositol polyphosphates were extracted.
Mouse and human islets were labelled for 72 h with 20 μCi/ml [3H] myo-inositol in a custom-made RPMI 1640 (with a modification in glucose, 11 mM, and inositol, 10 μM) and a CMRL medium supplemented with 10% dialysed serum and 10 μM inositol, respectively. A medium change was carried out after two days. After labelling, the islets (150–200 islets per condition) were pre-incubated in KREBS buffer containing 3 mM glucose for 30 min at 37 °C, then stimulated with 16.7 mM glucose for 3 min, lysed by 5% TCA and the inositol polyphosphates, ATP and ADP were extracted. An aliquot of the conditioned medium was collected for insulin measurements.
2.7. Measurements of insulin concentration
Insulin was analyzed using either the ArcDia 2-photon fluorescence excitation microparticle fluorometry (TPX) assay (ArcDia Group, Turku, Finland) [29], or the AlphaLISA immunoassay kit (Perkin Elmer, Waltham, MA). The assays were performed according to the manufacturer's instructions, using a recombinant human insulin standard to determine the insulin concentration.
2.8. Extraction of inositol phosphates and lipids
EDTA (0.5 M, pH 8.0), was added to acid lysed cells or islets to a final concentration of 2 mM on ice. After 30 min inositol phosphates and lipids were extracted [30]. An aliquot of acid extracted sample was stored separately for ATP/ADP measurements. An aliquot of the lipid extract was dried down and measured by liquid scintillation counting to determine total inositol lipids.
2.9. Separation of inositol phosphates using HPLC
Inositol phosphates were separated by ion-exchange HPLC with a SAX column (250 × 4.6 mm Partisphere 5 μm, Hichrom, Berkshire, England), as previously described [10,31] with a slight modification in buffer B (1.25 M (NH4)2HPO3 in 1 mM EDTA, pH 4.4 with H3PO4) and buffer A (1 mM EDTA). Separated fractions were mixed with Ultima Flo AP Scintillation fluid (Perkin Elmer, The Netherlands) and radioactivity was measured by liquid scintillation counting.
2.10. Measurements of IP6K activity
Pancreatic islets were lysed in a buffer containing 150 mM NaCl, 50 mM Tris, 1% Nonidet P-40, 1 mM EDTA, (pH 7.4) and protease and phosphatase inhibiting cocktail tablets (Roche Diagnostics, Bromma, Sweden). The cell lysate (15 μg) was added to 25 μl assay buffer comprising: 50 mM KCl, 20 mM HEPES pH 7.2 with KOH, 12 mM MgSO4, 10 mM Na2ATP, 1 mM Na2EDTA, 20 mM phosphocreatine, 15.5 Sigma Units/ml phosphocreatine kinase, 10 mM NaF, approximately 20,000 d.p.m. [3H] InsP6, 4 μM InsP6, and 1 protease inhibitor tablet/10 ml. Assays were quenched at various times (20–60 min) with 175 μl assay buffer followed immediately by 40 μl ice-cold 2 M HClO4 plus 0.2 mg/ml InsP6. Samples were neutralized and analyzed by Partsiphere SAX HPLC as previously described [32].
2.11. ATP/ADP measurements
ATP/ADP was measured in acid-extracted cell samples from MIN6m9 cells or islets using the ApoSENSOR kit (Biovison, Milpitas, California), following the manufacturer's instructions.
2.12. Capacitance measurements
Capacitance measurements were performed essentially as previously described [10]. During measurements cells were incubated with 16.7 mM glucose, to mimic the glucose-stimulated state. Electrophysiological recordings were carried out using the perforated patch configuration. Attached cells were subjected to a train of four 500-ms depolarizations (1 Hz) and changes in membrane capacitance were recorded.
2.13. Measurements of [Ca2+]i in MIN6m9 cells
MIN6m9 cells, cultured on coverslips, were loaded with 2 μM Fura-2/AM in a modified KREBS buffer containing 0.5 mM glucose and 2.56 mM CaCl2 for 60 min. The coverslip was mounted as the bottom of an open perifusion chamber maintained at 37 °C and the cells were perifused with the same buffer followed by stimulation with 10 mM glucose. The cells were excited alternatively with 340 and 380 nm from a xenon arc lamp light source (Lambda DG-4, Sutter instrument company) coupled to the microscope (Olympus ix 71) with UPlanSApo 10×/0.4 objective (Olympus). Image analysis as well as control of excitation and detection was accomplished with the MetaFluor software (Molecular Devices). The 16-bit grayscale images with a binning of 1 × 1 were captured every second (exposure time was 150 ms) with a cooled EM-CCD camera (ImagEM X2, Hamamatsu). For analysis, about 30–40 regions of interest (ROIs) were taken into account and [Ca2+]i was evaluated by calculating the ratio of fluorescence signal from 340 and 380 nm after background subtraction (Fura-2 ratio). For each ROI, basal [Ca2+]i (Fura-2 ratio) was measured immediately before glucose stimulation and [Ca2+]i response to glucose (Δ Fura-2 ratio) was calculated by subtracting the basal Fura-2 ratio from the peak [Ca2+]i response.
2.14. FACS analysis of dispersed islet cells
A detailed description of the FACS analysis of dispersed cells from lean or ob/ob islets can be found in the Supplemental data section.
2.15. Statistical analysis
Data are expressed as means ± SEM. The data were statistically analyzed with GraphPad Prism software version 5.0, using the statistical tests described in figure legends and tables.
3. Results and discussion
3.1. IP7 levels are regulated by prevailing glucose concentrations via IP6K1
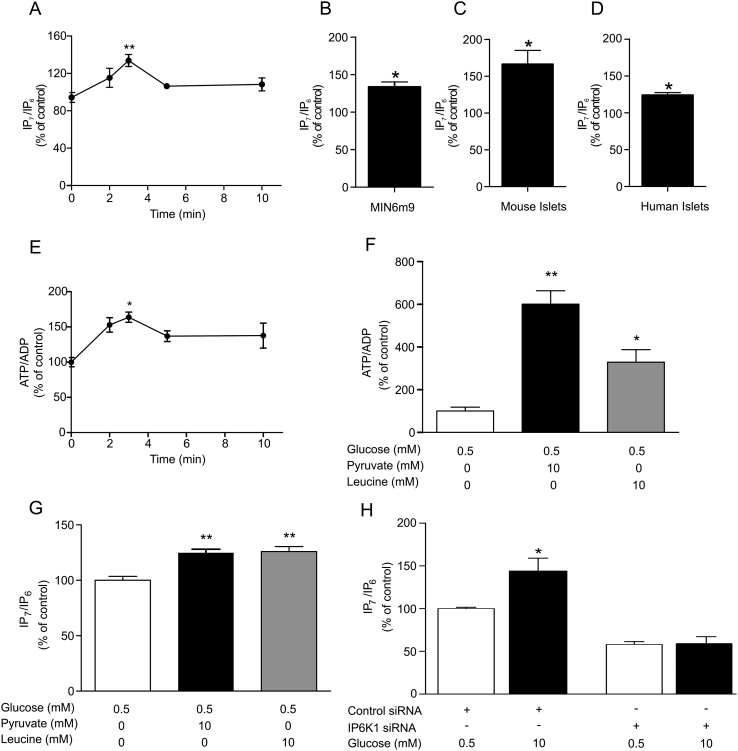
The first goal of this study was to investigate the response of IP7 to glucose. Measurements of IP7 in primary β-cells are challenging due to the amount of material that can be obtained. Thus we intitially used the insulin-secreting mouse β-cell line, MIN6m9. We found that IP7 levels transiently increased following glucose stimulation, peaking at 3 min (Fig. 1A and B). This rise in IP7 levels occurred during the first phase of insulin secretion in these cells which ends at around 5 min [29]. Glucose also transiently induced a 30% increase in IP7 in the hamster HIT-T15 cells [10] (Supplemental Fig. S1A and B). Moreover, glucose elevated IP7 in C57BL/6 mouse islets (Fig. 1C) and in human islets (Fig. 1D), with increases of 66% and 24%, respectively. Thus, our data demonstrate that IP7 is metabolically regulated during first phase insulin secretion in β-cell derived cell lines, as well as in rodent and human islets. In MIN6m9 cells, stimulatory glucose, at 3 min, caused the IP7 rise while inducing a 1.64-fold elevation in the ATP/ADP ratio (Fig. 1E) and an increment in insulin exocytosis (6.28 ± 1.15-fold increase, means ± SEM, n = 3, p < 0.05, 95% confidence interval). The time course for the glucose-mediated change in the ATP/ADP ratio mirrored the time course for the change in IP7 levels (Fig. 1A and E). This is consistent with an intracellular metabolic sequence where increased ATP/ADP leads to increased IP6K activity, which produces IP7, stimulating insulin secretion. To test that this is indeed the case we investigated two alternative insulin secretagogues, pyruvate and leucine [33,34]. In MIN6m9 cells stimulation with either pyruvate (10 mM) or leucine (10 mM) for 3 min increased ATP/ADP (Fig. 1F) and also increased IP7 (Fig. 1G). Apparently, the increase in IP7 reached a maximum at around 30%, irrespective of greater increases in the ATP/ADP ratio mediated by pyruvate and leucine compared to glucose. This is likely to reflect the fact that the IP6K reaches saturation in regards to ATP concentration, given it has a Km of 1.1 mM [12] and the free concentration of ATP in β-cells is around 1 mM [20]. Therefore, doubling the ATP concentration is unlikely to yield further gains in IP7 generation. Stimulation of β-cells with KCl is a method of driving insulin secretion independently of metabolism and thus represents a model of secretion in which we would predict ATP/ADP not to increase [35]. In contrast to pyruvate and leucine, stimulation of MIN6m9 cells with 25 mM KCl, did not increase ATP/ADP (Supplemental Fig. S2A) or IP7 (Supplemental Fig. S2B). In conclusion, we present strong evidence that increases in ATP/ADP are responsible for increasing IP7 in intact cells.
Fig. 1.
Glucose stimulation increases IP7 levels via IP6K1. (A) IP7/IP6 ratios obtained at various time points following 10 mM glucose stimulation of MIN6m9 cells (n = 3–4). There is a significant increase in IP7/IP6 levels between zero and 3 min of glucose stimulation, **p < 0.01, one-way ANOVA followed by Tukey's multiple comparison. (B) The comparison between stimulation with 0.5 and 10 mM glucose for 3 min showed a 34% increase in IP7 levels in MIN6m9 cells (n = 3). (C, D) Exposure to 16.7 mM glucose for 3 min induced an IP7 response in C57BL/6 mouse islets, n = 4 preparations, and human islets, n = 3 donors, *p < 0.05, 95% confidence interval, in panels B-D. (E) ATP/ADP ratios obtained at various time points following 10 mM glucose stimulation of MIN6m9 cells (n = 3–4). There is a significant increase in ATP/ADP levels between zero and 3 min of glucose stimulation, *p < 0.05, one-way ANOVA followed by Tukey's multiple comparison. IP7 is produced by other metabolic fuels that increase ATP/ADP ratio. When MIN6m9 cells were stimulated with either 10 mM pyruvate or 10 mM leucine for 3 min, there was a significant increase in both ATP/ADP ratio, n = 3, (F) and IP7/IP6 levels, n = 4, (G), **p < 0.01, *p < 0.05, one-way ANOVA followed by Tukey's multiple comparison. (H) In MIN6m9 cells, silencing IP6K1 blocked the increase of IP7 induced by 10 mM glucose stimulation for 3 min, n = 3, *p < 0.05, two-way ANOVA (Supplemental Table S1). All bars and plots are means ± SEM.
IP6K1 and 2, i.e. the two IP6K isoforms expressed in β-cells [10], have a high Km for ATP, so theoretically either enzyme could contribute to the glucose-induced increase in IP7. In order to determine the relative role of specific IP6K isoforms we silenced either IP6K1 or IP6K2. Knock down of either isoform was 60–69% [10] (Supplemental Fig. S3A and B). Notably, the glucose-induced rise in IP7 seen in control cells was abolished when IP6K1 was silenced (Fig. 1H). In contrast, the cells were still glucose-responsive after IP6K2 silencing (Supplemental Fig. S4), demonstrating that the glucose-induced elevation in IP7 is mediated by IP6K1. This increase in IP7 is not due to an increase in the levels of IP6K1 protein itself (Supplemental Fig. S3A and C).
Previous in vitro enzyme studies suggested that IP6K1 is more sensitive to changes in ATP/ADP ratio than IP6K2 [17]. Our data show for the first time that IP6K1 can act as a sensor coupling glucose metabolism and changes in the ATP/ADP ratio to IP7 generation in a living cell. An explanation for the strong preference for IP6K1 over IP6K2 in IP7 generation is that some form of cellular compartmentalization exists. We investigated the distribution of IP6Ks in MIN6m9 cells using immunocytochemical techniques. IP6K1 was found to be almost exclusively cytosolic (Supplemental Fig. S5), as has been previously demonstrated in other cells [36]. In contrast, IP6K2 was almost exclusively nuclear in localization (Supplemental Fig. S6), as has been described in several other cell types [9]. Thus there is a separate compartmentalization of IP6K1 and IP6K2 in insulin secreting cells, which could also contribute to the uniqueness of IP6K1’s role in glucose stimulated increases in IP7. Therefore, we propose that the link between glucose, ATP/ADP ratios and IP7 levels cannot be a global cellular phenomenon, since our data suggest it is unlikely to occur in cell types in which IP6K2 is the predominant isoform to be expressed. One such cell type is the human derived HCT116 colon cancer cell line [37], which has been studied extensively for the relationship between bioenergetic status and IP7 [23]. We found that acute glucose stimulation of HCT116 cells did not cause a statistically significant increase in IP7 levels (Supplemental Fig. S7).
3.2. IP6K1 regulates first phase insulin secretion stimulated by glucose
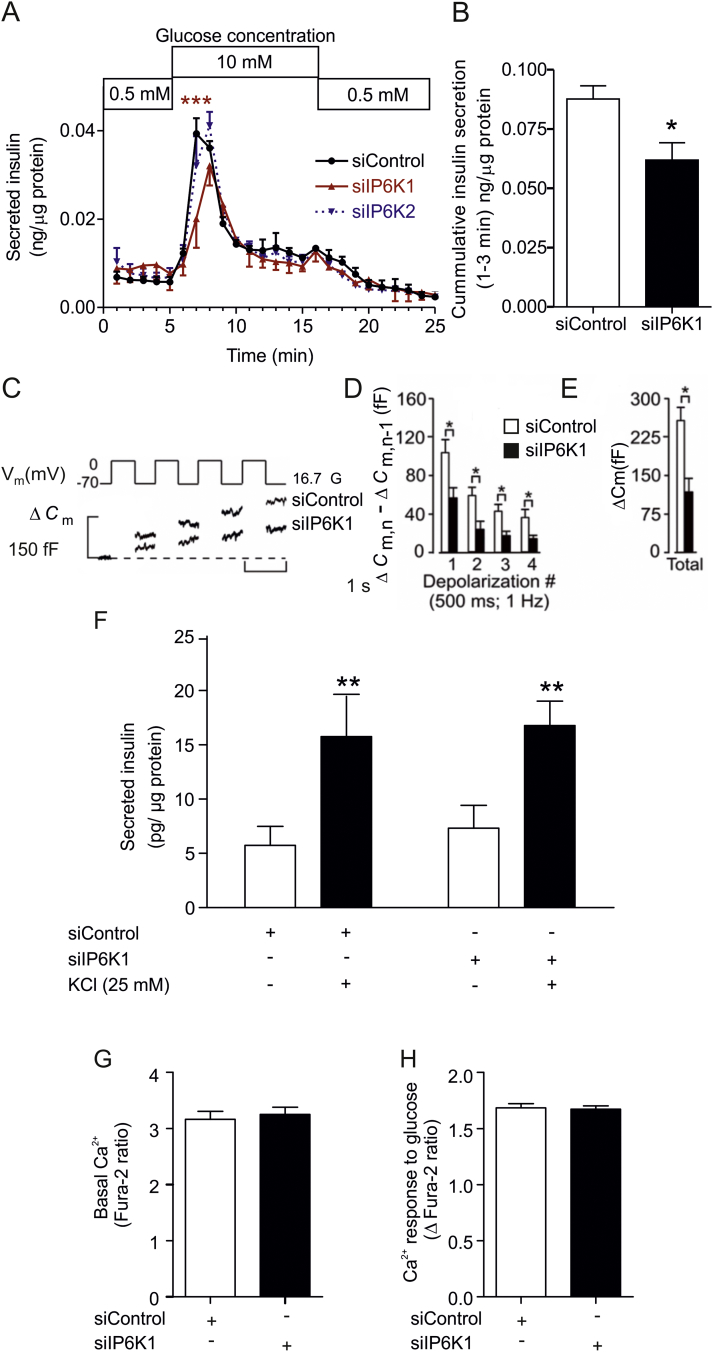
Having established that IP6K1 acts as a “metabolic sensor”, we wanted to evaluate whether knock down of IP6K1 affects specific stages of insulin secretion. We utilized a dynamic insulin release assay to delineate the two phases of glucose-induced insulin secretion in MIN6m9 cells [29]. In these cells first phase insulin secretion peaks between 2 and 3 min after glucose stimulation and the second phase begins after 5 min (Fig. 2A). Silencing IP6K1 led to compromised insulin release, more specifically a reduction in the early rising phase during the first 3 min of glucose stimulation (Fig. 2A and B), whereas it had no effect on second phase insulin secretion. Silencing IP6K2 did not modify exocytosis at any of the time points analyzed (Fig. 2A). Thus, only IP6K1 is required for a proper first phase insulin secretion.
Fig. 2.
IP6K1 regulates first phase of glucose-stimulated exocytosis. (A) Effects of IP6K1 or IP6K2 siRNA on insulin release were studied using dynamic incubation assays in MIN6m9 cells. There was a significant difference in insulin release between control and IP6K1 siRNA after 2 min of glucose stimulation, n = 3, ***p < 0.001, two-way ANOVA (Supplemental Table S1). (B) Insulin secretion data from panel (A) were compiled for the first 3 min, n = 3, *p < 0.05, Student's t-test. (C) Mouse islet cells were transfected with fluorescently tagged siRNA to IP6K1 or a negative control. Fluorescent cells were subjected to a train of four 500-ms depolarizations (1 Hz) using the perforated patch configuration. Increases in cell capacitance (ΔCm) were measured at 16.7 mM glucose (16.7 G) in the extracellular medium. Recordings are representative of 5–8 experiments. (D) Histogram summarizing the average increment in cell capacitance per pulse (ΔCm,n − ΔCm,n−1) during the train of depolarization in cells transfected with either siRNA to IP6K1 or negative control, n = 5–8, *p < 0.05, Student's t-test. (E) Effect of siRNA to IP6K1 on total capacitance increase, n = 5–8, *p < 0.05, Student's t-test. (F) KCl mediated insulin secretion is not mediated via IP6K1. MIN6m9 cells treated with either control siRNA or IP6K1 siRNA were stimulated for 3 min with an addition of 25 mM KCl. There was a significant increase in insulin secretion in both control siRNA and IP6K1 siRNA treated group, n = 3, **p < 0.01, repeated measures two-way ANOVA followed by Bonferroni post-hoc test. (G) Basal [Ca2+]i and (H) glucose (10 mM) induced [Ca2+]i response were measured in control and IP6K1 siRNA-treated MIN6m9 cells (n = 3 preparations each compiled from 30 to 40 ROIs). All bars are means ± SEM. See experimental procedures for [Ca2+]i data analysis.
We have further studied the role of IP6K1 in insulin exocytosis in primary β-cells, using electrophysiology [10]. Cell capacitance measurements showed that exocytosis generated in the presence of stimulatory glucose is curtailed when IP6K1 is silenced (Fig. 2C–E). In our original studies under basal glucose conditions silencing IP6K1 only affected the first depolarizing pulse [10]. In contrast, silencing of IP6K1 reduced exocytosis during the first four depolarizing pulses when the experiments were carried out under stimulatory glucose conditions (Fig. 2C–E). These data suggest that IP6K1, and thus IP7, impacts both immediately- and readily releasable pools [38], which are thought to constitute first-phase insulin release, and potentially reserve pools too. Thus IP6K1 silencing has a much more profound impact on exocytosis in the presence of stimulatory glucose. In order to understand this more pronounced impact it is important to note that in control cells the increase in capacitance observed in the first 4 depolarizing pulses is 3-fold greater in these current experiments (Fig. 2E) than the same pulses employed in our previous experiments carried out under basal glucose conditions (≈87 vs. 260 fF) [10]. This difference suggests that glucose metabolism makes an important contribution to the increased exocytotic capacity. These observations are at least consistent with an increased generation of both ATP and IP7 under glucose-stimulated conditions. KCl is often employed to stimulate insulin secretion independently of glucose metabolism. In our MIN6m9 cell system, under basal glucose conditions, the KCl induced increase in insulin secretion was not significantly altered by IP6K1 silencing (Fig. 2F). These data emphasize the importance of glucose in mediating the full effect of IP6K1 and thus IP7. It is consistent with studies employing TIRF microscopy where glucose has been shown to preferentially mobilize a separate pool of “newcomer” granules which are not liberated by KCl depolarization [39]. We conclude that IP6K1 silencing reduces glucose-stimulated exocytosis in both β-cell derived cell lines and in primary mouse β-cells.
We tested whether silencing IP6K1 impacted the main driving force of exocytosis, namely increases in cytoplasmic free Ca2+concentration ([Ca2+]i). Silencing IP6K1 in MIN6m9 cells had no effect on either basal or glucose stimulated [Ca2+]i (Fig. 2G and H). This demonstrates that IP6K1 does not operate upstream of [Ca2+]i. In these experiments the glucose-driven changes in [Ca2+]i are a direct reflection of ATP-sensitive K+ (KATP) channel activity. Like IP6K1, the KATP channel also senses elevations in the ATP/ADP ratio and couples these to increases in exocytosis. Since [Ca2+]i was unaffected when IP6K1 was silenced, but insulin release was still compromised, we can deduce that decreasing IP6K1 activity impairs exocytosis without affecting KATP channel activity. Our observations are consistent with reports on KATP channel-independent, glucose-stimulated insulin secretion [40].
3.3. Glucose-driven IP7 responses are compromised in the islets of diabetic ob/ob mice
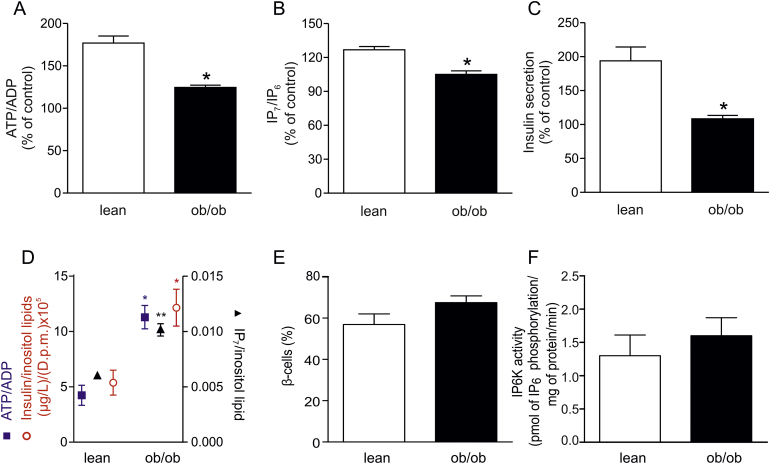
In order to further validate the role of IP6K as a metabolic sensor, we have studied its significance to the production of IP7 in a model of disrupted metabolism and diabetes, namely the ob/ob mouse. We envisaged that IP6Ks translate metabolic defects into an elevated basal secretion and a disturbed first phase insulin release also observed in T2D. Our colony of ob/ob mice enters a strongly diabetic phase at an early age of 3 months, with a fasting blood glucose of 8–9 mM and a defective glucose tolerance [26]. We measured ATP/ADP ratios in response to glucose in islets from lean and ob/ob mice. Fig. 3A shows that there was a 77% increase in the ATP/ADP ratio in lean islets, whereas there was only a 24.5% increase in ob/ob islets, in agreement with earlier studies [1,2]. We expected that this disrupted ATP/ADP response should impact on IP6K function, hindering the increased generation of IP7 and thus resulting in deficient exocytosis. IP7 was enhanced by 27% in lean islets stimulated with glucose, whereas there was no effect on IP7 in the diabetic islets (Fig. 3B). In addition, there was a 90% increase in glucose-stimulated insulin secretion in lean islets whereas in ob/ob islets increased release of insulin was impaired (Fig. 3C). As anticipated from published data [1], basal ATP/ADP was elevated in ob/ob islets compared to lean controls (Fig. 3D). Interestingly, basal IP7 and insulin secretion were also proportionally higher (Fig. 3D). The elevated basal levels of IP7 are not caused by a change in proportion of β-cells in the ob/ob islets vs. the lean islets from mice at this age (Fig. 3E). This is in contrast to islets from older ob/ob animals, which have higher IP7 levels due to a higher β-cell proportion [10], 60 vs. 90%, respectively. Neither are the elevated levels of IP7 likely to be driven by an increased IP6K activity in ob/ob islets (Fig. 3F). These data from diabetic islets under basal conditions reinforce the view both that IP6K activity accurately mirrors the metabolic state of the cells and that the disturbed energy metabolism in diabetes can exert its influence on insulin secretion via IP7. Thus the metabolic sensing of IP6K1 becomes a possible contributor to two features of the disease phenotype, namely, increased basal secretion and reduced first phase insulin secretion.
Fig. 3.
Glucose-stimulated ATP/ADP, IP7 and insulin release are compromised in islets from diabetic ob/ob mice. (A) ATP/ADP, (B) IP7 and (C) insulin secretion in response to stimulation with 16.7 mM glucose for 3 min, in pancreatic islets obtained from diabetic ob/ob mice and age-matched lean male mice. Data are presented as a percentage of unstimulated control. (D) Comparison of basal levels of ATP/ADP, IP7 and insulin secretion in lean vs. ob/ob islets. Using data derived from experiments depicted in (A–C) we normalized all the data to inositol lipids to demonstrate the relationship between the basal levels of ATP/ADP, IP7 and insulin secretion in islets from either lean or ob/ob mice. There is a close association between the levels of these three parameters. All bars and plots are means ± SEM, from 3 to 4 independent experiments, *p < 0.05, **p < 0.01, Student's t-test. (E) Proportion of β-cells in islets isolated from ob/ob and lean mice at the age of 3 months. The analysis of insulin expressing cells by FACS shows that the relative proportion of β-cells over the total islet cells is not significantly different (Student's t-test) between ob/ob (n = 5) and lean (n = 4) islet preparations. Data are expressed as means ± SEM. (F) Initial rates of IP6K activity of cell lysates from lean (n = 3) and ob/ob (n = 4) islet preparations, means ± SEM. The activity is not significantly different (Student's t-test).
In diabetes one can think of two alternative explanations for the defective production of IP7. One of these alternatives is a malfunctioning IP6K1, the other, as we suggest above, is impaired ATP/ADP generation. Although there is an isolated familial case of human diabetes where IP6K1 gene disruption is associated with T2D [24], this phenotype was not detected in the majority of patients examined [24]. Thus in most cases of T2D available evidence supports the idea that IP6K1 function is normal and that it is hostage to the disturbed energy metabolism in the disease. Strengthening this view, IP6K1 knockout in a mouse does not lead to diabetes [15,41], although plasma insulin is reduced [15]. In contrast, in diabetic ob/ob mouse islets, both in previously reported experiments [1,2] and our current work (Fig. 3), there is increased basal ATP/ADP and this ATP/ADP changes little following glucose stimulation. Furthermore, in our ob/ob islet experiments we detect no significant changes in IP6K activity in lysates from control verses ob/ob islets when normal levels of ATP are supplied (Fig. 3F). More importantly for human disease, the disturbed ATP/ADP phenotype has been observed in islets from several different patients with completely different familial backgrounds [3]. Thus the idea that IP6K1 knockout may be a diabetes treatment, because of its positive effect on insulin-sensitivity [16,41], is probably not an optimal approach as far as the β-cell is concerned. A better strategy, for the β-cell, would be to establish the basis of the metabolic defect(s) that underscore the disrupted ATP/ADP ratios. Therapeutic intervention in those metabolic defects could restore β-cell sensitivity to glucose that will naturally correct IP7 signaling mechanisms in insulin secretion.
A question, that was not the main focus of the present study, is how IP7 is acting at the molecular level. Earlier work indicated a non-catalytic role for IP6K1 in promoting neuronal exocytosis by binding GRAB, a GEF for Rab3A [42]. In contrast, again in neuronal systems, IP7 has been suggested to be an inhibitor of exocytosis via synaptotagmin 1 [43]. However, this form of synaptotagmin is not present in primary β-cells [44] and there are also clear molecular differences between synaptic vesicle fusion and the fusion of large dense core vesicles that constitute insulin secretion [38,39]. More promising are recent publications showing IP7 and IP6K1 regulating various aspects of the cytoskeletal network [[45], [46], [47], [48]]. A role on the cytoskeleton is consistent with the idea that IP6K1 may preferentially affect newcomer granules [39], whose transport to the plasma membrane will be mediated by cytoskeletal elements. Thus future work should be directed towards the role of these IP6K1- related cytoskeletal elements and insulin secretion.
Our new observations complement a growing body of literature that suggests that IP6Ks are fundamental players in the regulation of cellular metabolism [48]. This includes roles in ATP generation [49], phosphate homeostasis [50,51] and polyphosphate regulation [51,52].
4. Conclusions
We have confirmed our hypothesis that in pancreatic β-cells IP6K1 detects changes in metabolism and translates them into insulin secretion. More importantly, we have discovered a key missing link between glucose metabolism, insulin exocytosis and the early secretory defects observed in T2D (Fig. 4). Our study is directly relevant to human diabetes, since impaired ATP generation in response to nutrients, in association with mitochondrial dysfunction, has been found in islets from patients with T2D [3,4]. The regulation of IP6K1 activity is likely to be a vulnerable point in early disease development. T2D is a multifactorial, polygenetic disease, and one unresolved mystery is how this large variety of risk factors and defects lead to a common phenotype, namely elevated basal insulin secretion and reduction of first phase insulin secretion. We now identify IP6K1 as a viable candidate able to integrate established metabolic defects with the universal phenotype expressed in T2D patients.
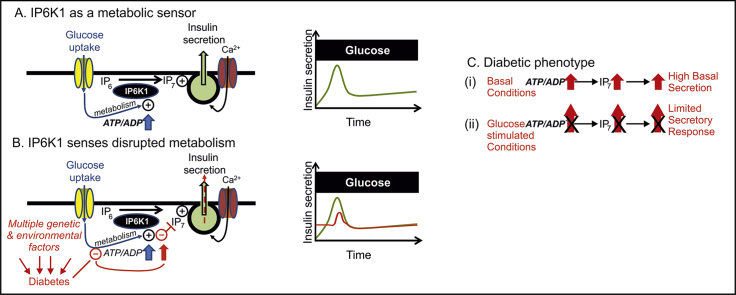
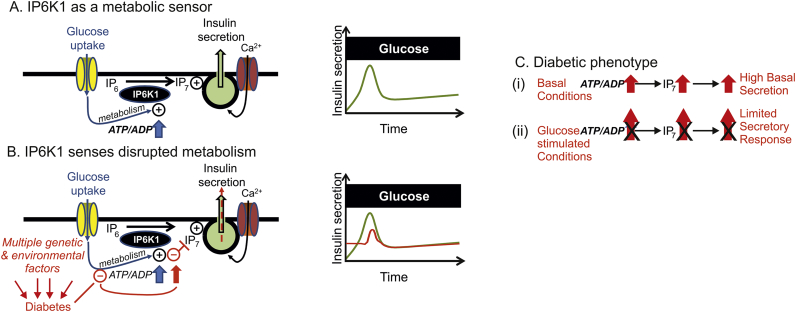
Fig. 4.
Scheme depicting the proposed role of IP6K1 as a metabolic sensor in both physiology and pathophysiology. (A) Glucose regulates IP6K1 mediated production of IP7 via the ATP/ADP ratio and contributes to first phase insulin secretion (right graph). (B) Red text and drawing indicate how multiple genetic and environmental factors may converge on cellular metabolism to disrupt ATP/ADP regulation, thus disturbing IP7 production and leading to increased basal secretion and reduced first phase insulin secretion. (C) Two aspects of the diabetic phenotype, increased basal insulin secretion and reduced responsiveness to glucose stimulation could be underscored by the transmission of defective metabolism into disturbed IP7 production. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Author contributions
C.J.B. and P-.O.B. conceived the project. C.J.B., P-.O.B., J.O.M., S.H.R., S.S.R., J.K., J.G. and E.D. designed experiments. S.S.R., J.K., G-.C.G., J.G., K. T.d.S., S.B.S., C.G., S.S.F., C.I., M.K., J.O.M., E.D., E.L.N. and C.J.B. carried out experiments. S.S.R., J.K., G-.C.G., J.G., S.B.S., M.K., E.D. and C.J.B. analyzed data. C.J.B. and P-.O.B. wrote the paper with input from all authors.
Acknowledgments
Acknowledgments
We thank Dr. A. Ramos, Dr. I.B. Leibiger and F. Tessaro for some practical help. This study was supported by the Swedish Research Council, the Novo Nordisk Foundation, Karolinska Institutet, the Swedish Diabetes Association, The Family Knut and Alice Wallenberg Foundation, Diabetes Research and Wellness Foundation, Swedish Foundation for Strategic Research, Berth von Kantzow's Foundation, The Skandia Insurance Company Ltd., Strategic Research Programme in Diabetes at Karolinska Institutet, ERC-2013-AdG 338936-BetaImage, the Stichting af Jochnick Foundation, the Family Erling-Persson Foundation, grants 2010/02272-0,2014/05214-1 and 2017/11540-7 from FAPESP, grant 301617/2016-3 from CNPq (PQ-1D), grant from STINT and CAPES. Human islets were provided through the JDRF award 31-2008-416 (ECIT Islet for Basic Research program). This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Conflict of interest declaration
P.-O.B. is CEO of the biotech company Biocrine AB, and C.J.B. and M.K. are consultants with this company.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cellsig.2018.03.001.
Contributor Information
Christopher J. Barker, Email: chris.barker@ki.se.
Per-Olof Berggren, Email: per-olof.berggren@ki.se.
Appendix A. Supplementary data
Supplementary material
References
- 1.Saleh M.C., Wheeler M.B., Chan C.B. Endogenous islet uncoupling protein-2 expression and loss of glucose homeostasis in ob/ob mice. J. Endocrinol. 2006;190(3):659–667. doi: 10.1677/joe.1.06715. [DOI] [PubMed] [Google Scholar]
- 2.Yoshihara E., Fujimoto S., Inagaki N., Okawa K., Masaki S., Yodoi J., Masutani H. Disruption of TBP-2 ameliorates insulin sensitivity and secretion without affecting obesity. Nat. Commun. 2010;1:127. doi: 10.1038/ncomms1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anello M., Lupi R., Spampinato D., Piro S., Masini M., Boggi U., Del P.S., Rabuazzo A.M., Purrello F., Marchetti P. Functional and morphological alterations of mitochondria in pancreatic beta cells from type 2 diabetic patients. Diabetologia. 2005;48(2):282–289. doi: 10.1007/s00125-004-1627-9. [DOI] [PubMed] [Google Scholar]
- 4.Ahren B. Type 2 diabetes, insulin secretion and beta-cell mass. Curr. Mol. Med. 2005;5(3):275–286. doi: 10.2174/1566524053766004. [DOI] [PubMed] [Google Scholar]
- 5.Weyer C., Hanson R.L., Tataranni P.A., Bogardus C., Pratley R.E. A high fasting plasma insulin concentration predicts type 2 diabetes independent of insulin resistance: evidence for a pathogenic role of relative hyperinsulinemia. Diabetes. 2000;49(12):2094–2101. doi: 10.2337/diabetes.49.12.2094. [DOI] [PubMed] [Google Scholar]
- 6.Dankner R., Chetrit A., Shanik M.H., Raz I., Roth J. Basal-state hyperinsulinemia in healthy normoglycemic adults is predictive of type 2 diabetes over a 24-year follow-up: a preliminary report. Diabetes Care. 2009;32(8):1464–1466. doi: 10.2337/dc09-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerasi E., Luft R. The plasma insulin response to glucose infusion in healthy subjects and in diabetes mellitus. Acta Endocrinol. 1967;55(2):278–304. doi: 10.1530/acta.0.0550278. [DOI] [PubMed] [Google Scholar]
- 8.Rosengren A.H., Braun M., Mahdi T., Andersson S.A., Travers M.E., Shigeto M., Zhang E., Almgren P., Ladenvall C., Axelsson A.S., Edlund A., Pedersen M.G., Jonsson A., Ramracheya R., Tang Y., Walker J.N., Barrett A., Johnson P.R., Lyssenko V., McCarthy M.I., Groop L., Salehi A., Gloyn A.L., Renstrom E., Rorsman P., Eliasson L. Reduced insulin exocytosis in human pancreatic beta-cells with gene variants linked to type 2 diabetes. Diabetes. 2012;61(7):1726–1733. doi: 10.2337/db11-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barker C.J., Berggren P.O. New horizons in cellular regulation by inositol polyphosphates: insights from the pancreatic beta-cell. Pharmacol. Rev. 2013;65(2):641–669. doi: 10.1124/pr.112.006775. [DOI] [PubMed] [Google Scholar]
- 10.Illies C., Gromada J., Fiume R., Leibiger B., Yu J., Juhl K., Yang S.N., Barma D.K., Falck J.R., Saiardi A., Barker C.J., Berggren P.O. Requirement of inositol pyrophosphates for full exocytotic capacity in pancreatic beta cells. Science. 2007;318(5854):1299–1302. doi: 10.1126/science.1146824. [DOI] [PubMed] [Google Scholar]
- 11.Saiardi A. Cell signalling by inositol pyrophosphates. Subcell. Biochem. 2012;59:413–443. doi: 10.1007/978-94-007-3015-1_14. [DOI] [PubMed] [Google Scholar]
- 12.Shears S.B. Diphosphoinositol polyphosphates: metabolic messengers? Mol. Pharmacol. 2009;76(2):236–252. doi: 10.1124/mol.109.055897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakraborty A., Kim S., Snyder S.H. Inositol pyrophosphates as mammalian cell signals. Sci. Signal. 2011;4(188):re1. doi: 10.1126/scisignal.2001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wundenberg T., Mayr G.W. Synthesis and biological actions of diphosphoinositol phosphates (inositol pyrophosphates), regulators of cell homeostasis. Biol. Chem. 2012;393(9):979–998. doi: 10.1515/hsz-2012-0133. [DOI] [PubMed] [Google Scholar]
- 15.Bhandari R., Juluri K.R., Resnick A.C., Snyder S.H. Gene deletion of inositol hexakisphosphate kinase 1 reveals inositol pyrophosphate regulation of insulin secretion, growth, and spermiogenesis. Proc. Natl. Acad. Sci. U. S. A. 2008;105(7):2349–2353. doi: 10.1073/pnas.0712227105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghoshal S., Zhu Q., Asteian A., Lin H., Xu H., Ernst G., Barrow J.C., Xu B., Cameron M.D., Kamenecka T.M. TNP [N2-(m-Trifluorobenzyl), N6-(p-nitrobenzyl) purine] ameliorates diet induced obesity and insulin resistance via inhibition of the IP6K1 pathway. Mol. Metab. 2016;5(10):903–917. doi: 10.1016/j.molmet.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wundenberg T., Grabinski N., Lin H., Mayr G.W. Discovery of InsP6-kinases as InsP6-dephosphorylating enzymes provides a new mechanism of cytosolic InsP6 degradation driven by the cellular ATP/ADP ratio. Biochem. J. 2014;462(1):173–184. doi: 10.1042/BJ20130992. [DOI] [PubMed] [Google Scholar]
- 18.Voglmaier S.M., Bembenek M.E., Kaplin A.I., Dorman G., Olszewski J.D., Prestwich G.D., Snyder S.H. Purified inositol hexakisphosphate kinase is an ATP synthase: diphosphoinositol pentakisphosphate as a high-energy phosphate donor. Proc. Natl. Acad. Sci. U. S. A. 1996;93(9):4305–4310. doi: 10.1073/pnas.93.9.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saiardi A., Erdjument-Bromage H., Snowman A.M., Tempst P., Snyder S.H. Synthesis of diphosphoinositol pentakisphosphate by a newly identified family of higher inositol polyphosphate kinases. Curr. Biol. 1999;9(22):1323–1326. doi: 10.1016/s0960-9822(00)80055-x. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy H.J., Pouli A.E., Ainscow E.K., Jouaville L.S., Rizzuto R., Rutter G.A. Glucose generates sub-plasma membrane ATP microdomains in single islet beta-cells. Potential role for strategically located mitochondria. J. Biol. Chem. 1999;274(19):13281–13291. doi: 10.1074/jbc.274.19.13281. [DOI] [PubMed] [Google Scholar]
- 21.Nagel A., Barker C.J., Berggren P.O., Illies C. Diphosphosinositol polyphosphates and energy metabolism: assay for ATP/ADP ratio. Methods Mol. Biol. 2010;645:123–131. doi: 10.1007/978-1-60327-175-2_8. [DOI] [PubMed] [Google Scholar]
- 22.Rajasekaran S.S., Illies C., Shears S.B., Wang H., Ayala T.S., Martins J.O., Darè E., Berggren P.O., Barker C.J. Protein kinase- and lipase inhibitors of inositide metabolism deplete IP7 indirectly in pancreatic beta-cells: off-target effects on cellular bioenergetics and direct effects on IP6K activity. Cell. Signal. 2018;42:127–133. doi: 10.1016/j.cellsig.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu C., Nguyen H.N., Ganini D., Chen Z., Jessen H.J., Gu Z., Wang H., Shears S.B. KO of 5-InsP7 kinase activity transforms the HCT116 colon cancer cell line into a hypermetabolic, growth-inhibited phenotype. Proc. Natl. Acad. Sci. U. S. A. 2017;114(45):11968–11973. doi: 10.1073/pnas.1702370114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamimura J., Wakui K., Kadowaki H., Watanabe Y., Miyake K., Harada N., Sakamoto M., Kinoshita A., Yoshiura K., Ohta T., Kishino T., Ishikawa M., Kasuga M., Fukushima Y., Niikawa N., Matsumoto N. The IHPK1 gene is disrupted at the 3p21.31 breakpoint of t(3;9) in a family with type 2 diabetes mellitus. J. Hum. Genet. 2004;49(7):360–365. doi: 10.1007/s10038-004-0158-z. [DOI] [PubMed] [Google Scholar]
- 25.Goto M., Eich T.M., Felldin M., Foss A., Kallen R., Salmela K., Tibell A., Tufveson G., Fujimori K., Engkvist M., Korsgren O. Refinement of the automated method for human islet isolation and presentation of a closed system for in vitro islet culture. Transplantation. 2004;78(9):1367–1375. doi: 10.1097/01.tp.0000140882.53773.dc. [DOI] [PubMed] [Google Scholar]
- 26.Ilegems E., Dicker A., Speier S., Sharma A., Bahow A., Edlund P.K., Leibiger I.B., Berggren P.O. Reporter islets in the eye reveal the plasticity of the endocrine pancreas. Proc. Natl. Acad. Sci. U. S. A. 2013;110(51):20581–20586. doi: 10.1073/pnas.1313696110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Köhler M., Darè E., Ali M.Y., Rajasekaran S.S., Moede T., Leibiger B., Leibiger I.B., Tibell A., Juntti-Berggren L., Berggren P.O. One-step purification of functional human and rat pancreatic alpha cells, Integr. Biol. (Camb.) 2012;4(2):209–219. doi: 10.1039/c2ib00125j. [DOI] [PubMed] [Google Scholar]
- 28.Minami K., Yano H., Miki T., Nagashima K., Wang C.Z., Tanaka H., Miyazaki J.I., Seino S. Insulin secretion and differential gene expression in glucose-responsive and -unresponsive MIN6 sublines. Am. J. Physiol. Endocrinol. Metab. 2000;279(4):E773–E781. doi: 10.1152/ajpendo.2000.279.4.E773. [DOI] [PubMed] [Google Scholar]
- 29.Leibiger B., Moede T., Uhles S., Barker C.J., Creveaux M., Domin J., Berggren P.O., Leibiger I.B. Insulin-feedback via PI3K-C2alpha activated PKBalpha/Akt1 is required for glucose-stimulated insulin secretion. FASEB J. 2010;24(6):1824–1837. doi: 10.1096/fj.09-148072. [DOI] [PubMed] [Google Scholar]
- 30.Barker C.J., Illies C., Berggren P.O. HPLC separation of inositol polyphosphates. Methods Mol. Biol. 2010;645:21–46. doi: 10.1007/978-1-60327-175-2_2. [DOI] [PubMed] [Google Scholar]
- 31.Larsson O., Barker C.J., Sjoholm A., Carlqvist H., Michell R.H., Bertorello A., Nilsson T., Honkanen R.E., Mayr G.W., Zwiller J., Berggren P.O. Inhibition of phosphatases and increased Ca2+ channel activity by inositol hexakisphosphate. Science. 1997;278(5337):471–474. doi: 10.1126/science.278.5337.471. [DOI] [PubMed] [Google Scholar]
- 32.Safrany S.T., Ingram S.W., Cartwright J.L., Falck J.R., McLennan A.G., Barnes L.D., Shears S.B. The diadenosine hexaphosphate hydrolases from Schizosaccharomyces pombe and Saccharomyces cerevisiae are homologues of the human diphosphoinositol polyphosphate phosphohydrolase. Overlapping substrate specificities in a MutT-type protein. J. Biol. Chem. 1999;274(31):21735–21740. doi: 10.1074/jbc.274.31.21735. [DOI] [PubMed] [Google Scholar]
- 33.Yang J., Chi Y., Burkhardt B.R., Guan Y., Wolf B.A. Leucine metabolism in regulation of insulin secretion from pancreatic beta cells. Nutr. Rev. 2010;68(5):270–279. doi: 10.1111/j.1753-4887.2010.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gohring I., Sharoyko V.V., Malmgren S., Andersson L.E., Spegel P., Nicholls D.G., Mulder H. Chronic high glucose and pyruvate levels differentially affect mitochondrial bioenergetics and fuel-stimulated insulin secretion from clonal INS-1 832/13 cells. J. Biol. Chem. 2014;289(6):3786–3798. doi: 10.1074/jbc.M113.507335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hatlapatka K., Willenborg M., Rustenbeck I. Plasma membrane depolarization as a determinant of the first phase of insulin secretion. Am. J. Physiol. Endocrinol. Metab. 2009;297(2):E315–E322. doi: 10.1152/ajpendo.90981.2008. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh S., Shukla D., Suman K., Lakshmi B.J., Manorama R., Kumar S., Bhandari R. Inositol hexakisphosphate kinase 1 maintains hemostasis in mice by regulating platelet polyphosphate levels. Blood. 2013;122(8):1478–1486. doi: 10.1182/blood-2013-01-481549. [DOI] [PubMed] [Google Scholar]
- 37.Koldobskiy M.A., Chakraborty A., Werner J.K., Jr., Snowman A.M., Juluri K.R., Vandiver M.S., Kim S., Heletz S., Snyder S.H. p53-mediated apoptosis requires inositol hexakisphosphate kinase-2. Proc. Natl. Acad. Sci. U. S. A. 2010;107(49):20947–20951. doi: 10.1073/pnas.1015671107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rorsman P., Renstrom E. Insulin granule dynamics in pancreatic beta cells. Diabetologia. 2003;46(8):1029–1045. doi: 10.1007/s00125-003-1153-1. [DOI] [PubMed] [Google Scholar]
- 39.Seino S., Shibasaki T., Minami K. Dynamics of insulin secretion and the clinical implications for obesity and diabetes. J. Clin. Invest. 2011;121(6):2118–2125. doi: 10.1172/JCI45680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henquin J.C. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49(11):1751–1760. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- 41.Chakraborty A., Koldobskiy M.A., Bello N.T., Maxwell M., Potter J.J., Juluri K.R., Maag D., Kim S., Huang A.S., Dailey M.J., Saleh M., Snowman A.M., Moran T.H., Mezey E., Snyder S.H. Inositol pyrophosphates inhibit Akt signaling, thereby regulating insulin sensitivity and weight gain. Cell. 2010;143(6):897–910. doi: 10.1016/j.cell.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo H.R., Saiardi A., Nagata E., Ye K., Yu H., Jung T.S., Luo X., Jain S., Sawa A., Snyder S.H. GRAB: a physiologic guanine nucleotide exchange factor for Rab3A, which interacts with inositol hexakisphosphate kinase. Neuron. 2001;31(3):439–451. doi: 10.1016/s0896-6273(01)00384-1. [DOI] [PubMed] [Google Scholar]
- 43.Lee T.S., Lee J.Y., Kyung J.W., Yang Y., Park S.J., Lee S., Pavlovic I., Kong B., Jho Y.S., Jessen H.J., Kweon D.H., Shin Y.K., Kim S.H., Yoon T.Y., Kim S. Inositol pyrophosphates inhibit synaptotagmin-dependent exocytosis. Proc. Natl. Acad. Sci. U. S. A. 2016;113(29):8314–8319. doi: 10.1073/pnas.1521600113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gauthier B.R., Wollheim C.B. Synaptotagmins bind calcium to release insulin. Am. J. Physiol. Endocrinol. Metab. 2008;295(6):E1279–E1286. doi: 10.1152/ajpendo.90568.2008. [DOI] [PubMed] [Google Scholar]
- 45.Fu C., Xu J., Cheng W., Rojas T., Chin A.C., Snowman A.M., Harraz M.M., Snyder S.H. Neuronal migration is mediated by inositol hexakisphosphate kinase 1 via alpha-actinin and focal adhesion kinase. Proc. Natl. Acad. Sci. U. S. A. 2017;114(8):2036–2041. doi: 10.1073/pnas.1700165114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chanduri M., Rai A., Malla A.B., Wu M., Fiedler D., Mallik R., Bhandari R. Inositol hexakisphosphate kinase 1 (IP6K1) activity is required for cytoplasmic dynein-driven transport. Biochem. J. 2016;473(19):3031–3047. doi: 10.1042/BCJ20160610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jadav R.S., Kumar D., Buwa N., Ganguli S., Thampatty S.R., Balasubramanian N., Bhandari R. Deletion of inositol hexakisphosphate kinase 1 (IP6K1) reduces cell migration and invasion, conferring protection from aerodigestive tract carcinoma in mice. Cell. Signal. 2016;28(8):1124–1136. doi: 10.1016/j.cellsig.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chakraborty A. The inositol pyrophosphate pathway in health and diseases. Biol. Rev. Camb. Philos. Soc. 2017 doi: 10.1111/brv.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szijgyarto Z., Garedew A., Azevedo C., Saiardi A. Influence of inositol pyrophosphates on cellular energy dynamics. Science. 2011;334(6057):802–805. doi: 10.1126/science.1211908. [DOI] [PubMed] [Google Scholar]
- 50.Wild R., Gerasimaite R., Jung J.Y., Truffault V., Pavlovic I., Schmidt A., Saiardi A., Jessen H.J., Poirier Y., Hothorn M., Mayer A. Control of eukaryotic phosphate homeostasis by inositol polyphosphate sensor domains. Science. 2016;352(6288):986–990. doi: 10.1126/science.aad9858. [DOI] [PubMed] [Google Scholar]
- 51.Azevedo C., Saiardi A. Eukaryotic phosphate homeostasis: the inositol pyrophosphate perspective. Trends Biochem. Sci. 2017;42(3):219–231. doi: 10.1016/j.tibs.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 52.Lonetti A., Szijgyarto Z., Bosch D., Loss O., Azevedo C., Saiardi A. Identification of an evolutionarily conserved family of inorganic polyphosphate endopolyphosphatases. J. Biol. Chem. 2011;286(37):31966–31974. doi: 10.1074/jbc.M111.266320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material