Abstract
Key points
The recent development of exogenous ketone supplements allows direct testing of the metabolic effects of elevated blood ketones without the confounding influence of widespread changes experienced with ketogenic diets or prolonged fasting.
In the present study, we determined the effect of (R)‐3‐hydroxybutyl (R)‐3‐hydroxybutyrate ketone monoester on the glycaemic response and insulin sensitivity index during a 2 h oral glucose tolerance test (OGTT) in humans.
The results obtained show that consuming a ketone monoester supplement 30 min prior to an OGTT reduced the glycaemic response and markers of insulin sensitivity without affecting insulin secretion.
The findings of the present study provides evidence that ketone supplements could have therapeutic potential for future application as a glucose‐lowering nutritional supplement.
Abstract
The main objectives of the present study were: (i) to determine whether acute ingestion of ketone monoester (Kme); (R)‐3‐hydroxybutyl (R)‐3‐hydroxybutyrate impacts plasma glucose levels during a standardized oral glucose tolerance test (OGTT) and (ii) to compare changes in insulin concentrations and estimates of insulin sensitivity after acute Kme supplementation. Twenty healthy participants (n = 10 males/females) aged between 18 and 35 years took part in a randomized cross‐over study. After an overnight fast, participants consumed a Kme supplement (ΔG®; TΔS Ltd, UK, Oxford, UK; 0.45 ml kg−1 body weight) or placebo (water) 30 min before completing a 75 g OGTT. Blood samples were collected every 15–30 min over 2.5 h. The participants and study personnel performing the laboratory analyses were blinded to the study condition. Kme acutely raised blood d‐beta‐hydroxybutyrate (β‐OHB) to 3.2 ± 0.6 mm within 30 min with levels remaining elevated throughout the entire OGTT. Compared to placebo, Kme significantly decreased the glucose area under the curve (AUC; −16%, P = 0.001), non‐esterified fatty acid AUC (–44%, P < 0.001) and C‐peptide incremental AUC (P = 0.005), at the same time as improving oral glucose insulin sensitivity index by ∼11% (P = 0.001). In conclusion, a Kme supplement that acutely increased β‐OHB levels up to ∼3 mm attenuated the glycaemic response to an OGTT in healthy humans. The reduction in glycaemic response did not appear to be driven by an increase in insulin secretion, although it was accompanied by improved markers of insulin sensitivity. These results suggest that ketone monoester supplements could have therapeutic potential in the management and prevention of metabolic diseases.
Keywords: ketone bodies, d‐beta‐hydroxybutyrate, glycemic response, non‐esterified fatty acids, insulin sensitivity
Key points
The recent development of exogenous ketone supplements allows direct testing of the metabolic effects of elevated blood ketones without the confounding influence of widespread changes experienced with ketogenic diets or prolonged fasting.
In the present study, we determined the effect of (R)‐3‐hydroxybutyl (R)‐3‐hydroxybutyrate ketone monoester on the glycaemic response and insulin sensitivity index during a 2 h oral glucose tolerance test (OGTT) in humans.
The results obtained show that consuming a ketone monoester supplement 30 min prior to an OGTT reduced the glycaemic response and markers of insulin sensitivity without affecting insulin secretion.
The findings of the present study provides evidence that ketone supplements could have therapeutic potential for future application as a glucose‐lowering nutritional supplement.
Introduction
The ketone bodies, d‐beta‐hydroxybutyrate (β‐OHB) and acetoacetate are produced by the liver under conditions of starvation, very‐low carbohydrate intake and prolonged glycogen‐depleting exercise (Robinson & Williamson, 1980; Balasse & Fery, 1989; Yancy et al. 2004). Several studies, including classical work by Cahill and colleagues (Owen et al. 1969; Cahill, 1976), have demonstrated that β‐OHB, the main ketone body in circulation, can act as an alternative energy substrate for metabolically active tissues such as the brain, heart, kidneys and skeletal muscles. More recently, β‐OHB has been shown to have several cellular signalling functions, including acting as an endogenous histone deacetylase inhibitor, a ligand for cell surface receptors and an inhibitor of the NOD‐like receptor protein 3 inflammasome (Youm et al. 2015). These findings suggest that, in addition to its well‐known role as a fat‐derived energy source, β‐OHB can modify an array of physiological functions.
To date, most studies exploring the direct effects of elevated plasma ketones on markers of metabolic control in human and animal models have employed ketone infusion methods (Balasse & Ooms, 1968; Miles et al. 1981; Müller et al. 1984; Shaw & Wolfe, 1984; Mikkelsen et al. 2015). A consistent finding in studies in which β‐OHB is infused to levels ≥1.0 mm is a reduction in glucose and circulating free fatty acids, which is potentially related to reduced hepatic glucose output and inhibition of adipose tissue lipolysis, respectively. Lowering glucose and free fatty acids could be of potential value for individuals with glucose intolerance and insulin resistance, although infusing β‐OHB is not a practical therapeutic strategy. In recent years, the emergence of exogenous ketone supplements has allowed researchers to investigate the isolated effects of elevated ketones without the presence of metabolic keto‐adaptations or the use of infusions.
Exogenous ketone supplements can be ingested in the form of ketone salts (KS) (e.g. sodium‐potassium β‐OHB) or ketone esters (KE) (available in monoester and diester forms). So far, a limited number of studies have tested the effect of KS and KE on circulating metabolites in rats (Kesl et al. 2016; Ari et al. 2017; de Oliveira Caminhotto et al. 2017). Consistent with the ketone infusion studies mentioned above, exogenous ketone supplements decreased circulating glucose and free fatty acids. The high amount of salt contained in the KS supplement along with potential gastrointestinal distress limit its therapeutic utility and this can be avoided by consuming a ketone monoester (Kme) supplement (Veech, 2004). Kme supplementation using (R)‐3‐hydroxybutyl (R)‐3‐hydroxybutyrate has been shown to provide a safe (Clarke et al. 2012) and novel strategy for rapidly increasing blood β‐OHB to approximately 3 mm within ∼30 min in healthy humans (Cox et al. 2016; Vandoorne et al. 2017). Once ingested, a non‐racemic Kme drink will be metabolized into the d‐isoform of β‐hydroxybutyrate, which is the isoform produced by endogenous ketogenesis (Tate et al. 1971; Desrochers et al. 1995). Therefore, the oral consumption of Kme may be an interesting alternative for increasing β‐OHB and improving metabolic control.
Further investigation is necessary to explore the metabolic effects of ketone supplementation in humans before Kme can be considered as a therapeutic option. The objectives of the present study were: (i) to determine whether acute ingestion of Kme; (R)‐3‐hydroxybutyl (R)‐3‐hydroxybutyrate impacts plasma glucose levels during a standardized oral glucose tolerance test (OGTT) and (ii) to compare changes in insulin concentrations and estimates of insulin sensitivity after acute Kme supplementation. Accordingly, we conducted a randomized, placebo‐controlled cross‐over experiment in healthy young males and females. As a result of the novelty of Kme and the unknown impacts on the glycaemic response and insulin sensitivity when these supplements are consumed prior to an OGTT, we conducted an initial study in healthy participants aiming to determine the normal physiological responses. We hypothesized that, compared to a placebo, a single dose of Kme taken 30 min prior to a 2 h oral OGTT would reduce the glucose area under the curve (AUC) and improve oral glucose insulin sensitivity (OGIS) index.
Methods
Ethical approval
The present study was approved by the University of British Columbia Clinical Research Ethics Board (ID H16‐01846). The study conformed to the standards set by the Declaration of Helsinki, except for registration in a database. Every participant provided their written informed consent during the initial screening visit.
Research design
The experimental design involved an initial visit for screening and baseline testing and two experimental conditions that each required a 3 h visit to the laboratory. On experimental visits, participants arrived at the laboratory following a ≥10 h overnight fast. According to a randomized cross‐over design, participants consumed either ketone or placebo supplement followed 30 min later by a 75 g glucose drink for a standard 2 h OGTT. The 30 min period was chosen based on previous studies (Cox et al. 2016; Vandoorne et al. 2017) showing that circulating levels of β‐OHB following the ingestion of Kme supplements reached a peak concentration around this time. Participants and study personnel performing laboratory blood sample analyses were blinded to the experimental conditions. Only the researcher preparing the drinks and collecting blood samples was aware of the experimental condition. At least 48 h later, participants returned to the lab following a similar overnight fast and performed the alternate experimental condition.
Participants
Twenty healthy participants (10 males and 10 females) aged between 18 and 35 years were recruited to participate in this study. Exclusion criteria included: (i) currently taking any medications (except for birth control for females); (ii) following a low‐carbohydrate diet or consuming nutritional ketone supplements; (iii) considered competitive athlete engaged in competition or intensive training; and (iv) waist circumference >102 cm for men or >88 cm for women. The baseline characteristics of participants are presented in Table 1.
Table 1.
Characteristics of participants
| Overall | Males | Females | |
|---|---|---|---|
| Number of participants | 20 | 10 | 10 |
| Age (years) | 25.4 ± 4.1 | 25.9 ± 4.2 | 24.8 ± 4.2 |
| Body mass index (kg m–2) | 22.1 ± 2.2 | 23.4 ± 2.2 | 20.9 ± 1.4 |
| Waist circumference (cm) | 70.2 ± 7.2 | 76.1 ± 4.7 | 64.4 ± 3.2 |
| Systolic blood pressure (mmHg) | 116 ± 8 | 120 ± 8 | 112 ± 7 |
| Diastolic blood pressure (mmHg) | 76 ± 8 | 77 ± 9 | 74 ± 6 |
| Fasting glucose (mmol L–1) | 4.5 ± 0.4 | 4.6 ± 0.3 | 4.3 ± 0.3 |
| Fasting insulin (mU L–1) | 3.2 ± 1.3 | 2.8 ± 1.2 | 3.6 ± 1.0 |
| Fasting non‐esterified fatty acids (mmol L–1) | 0.43 ± 0.22 | 0.35 ± 0.13 | 0.52 ± 0.19 |
| 24 h dietary food log | |||
| Total calories (kcal) | 2030 ± 454 | 2177 ± 404 | 1840 ± 472 |
| Fat (%) | 42 ± 12 | 40 ± 14 | 43 ± 9 |
| Protein (%) | 17 ± 5 | 18 ± 3 | 17 ± 6 |
| Carbohydrate (%) | 41 ± 9 | 42 ± 12 | 40 ± 5 |
Data are presented as the mean ± SD.
Glucose, insulin and free fatty acids are reported as the average of fasting measures for both conditions.
Baseline testing
Body weight (kg), height (cm), blood pressure (mmHg) and waist circumference (cm) were measured using standardized methods. Participants were given a 24 h food log to complete on the day prior their first experimental condition. They were also told to avoid exercising and to not consume alcohol for 24 h prior to the experimental trials.
Experimental trials
Participants reported to the laboratory for experimental trials after an overnight fast (≥10 h). The research coordinator confirmed with them that the 24 h food log had been completed, no exercise had been performed and no alcohol had been consumed on the day before. An indwelling i.v. catheter (BD Nexiva, Becton Dickinson Infusion Therapy Systems Inc., Sandy UT, USA) was then inserted into the antecubital vein for repeated blood sampling. At each time point, blood was drawn into 1 × 2 ml of EDTA and 1 × 4 ml serum tubes (BD Vacutainer, Becton Dickinson Infusion Therapy Systems Inc.) for isolation of plasma and serum. Seven i.v. blood draws were performed for each condition. The first collection (−30 min) occurred immediately before the consumption of the ketone or placebo supplement. Participants consumed a Kme supplement in the form of (R)‐3‐hydroxybutyl (R)‐3‐hydroxybutyrate (ΔG®; TΔS Ltd, UK, Oxford, UK; 0.45 ml kg−1 body mass or 482 mg kg−1 body mass) ingested with water and vanilla‐flavoured stevia (SweetLeaf Sweetener®; Wisdom Natural Brands, Gilbert, AZ, USA) in a total volume of 100 ml. Immediately following ingestion of the ketones, participants were given 20 ml of calorie‐free Gatorade (G2) (Gatorade Company, Inc., Chicago, IL, USA) in attempts to remove any remaining flavour of the supplement. In the placebo condition, participants consumed 100 ml of water and vanilla‐flavoured stevia (SweetLeaf) followed by the same 20 ml calorie‐free Gatorade (G2). Participants wore a nose clip when consuming the supplement under both conditions to further mask any flavour. Thirty minutes later, another blood sample was collected (0 min) followed immediately by the consumption of a 75 g oral glucose tolerance test drink (Thermo Scientific, Fisher Scientific Company, Middletown, VA, USA). Another five blood draws occurred at 15, 30, 60, 90 and 120 min after ingestion of the glucose drink. At each time point, β‐OHB was measured in whole blood (Precision Neo, Abbott Laboratories, Witney, UK). After the trial was completed, participants were asked to complete a gastrointestinal distress questionnaire and to guess whether they had the placebo or ketone supplement by answering the question ‘What condition do you think you were in today?’ Before leaving the laboratory, a copy of the 24 h food log was provided to participants who were asked to repeat the exact diet 24 h before the following visit. Subjects were also reminded to not perform exercise or to consume alcohol 24 h prior to the next visit.
Participants returned to the laboratory (≥10 h fast) at least 48 h later to complete the alternate condition. Adherence to the 24 h diet, exercise and alcohol guidelines was confirmed upon arrival in the laboratory. If no significant deviations were observed, the protocol for the third visit was the same as the previous one, except that participants received the alternate supplement (ketone or placebo). Female participants completed both experimental conditions in the follicular phase (between 3 and 9 days after the beginning of their menstrual cycle).
Blood samples
Upon collection, 10 μl of a dipeptidyl peptidase (DPP‐4) inhibitor (catalogue number DPP4‐010; Millipore, Billerica, MA, USA) was added to the EDTA tube to prevent the degradation of active glucagon‐like peptide‐1 (GLP‐1). The EDTA and serum tubes were centrifuged at 1500 g for 15 min at 4°C. Following centrifugation, plasma and serum samples were stored in a −80°C freezer until assays were performed in a blinded fashion. Blood metabolites were analysed using commercially available kits: serum non‐esterified fatty acids (NEFA) (HR Series; Wako Diagnostics, Mountain View, CA, USA) and serum glucose (glucose hexokinase; Pointe Scientific Inc., Canton, MI, USA) were analysed on a Chemwell 2910 automated analyser (Awareness Technologies, Palm City, FL, USA). Serum C‐peptide (C‐peptide; Meso Scale, Gaithersburg, MD, USA) and plasma GLP‐1 (M/R active GLP‐1, 7–36 amide; Meso Scale) were analysed on a Quickplex SQ 120 (Meso Scale). Serum insulin (human insulin enzyme‐linked immunosorbent assay; Crystal Chem, Elk Grove Village, IL, USA) and serum adiponectin (Rapid Human Adiponectin Immunoassay kit, Antibody and Immunoassay Services; Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong) were analysed on an iMark Microplate Absorbance Reader (Bio‐Rad, Hercules, CA, USA). All assays were run in duplicate. The coefficient of variation for duplicate samples was 4.2% for serum NEFA, 2.9% for serum glucose, 7.8% for serum C‐peptide, 4.1% for plasma GLP‐1, 4.9% for serum insulin and 4.0% for serum adiponectin.
OGIS index
The OGIS index was computed using the model‐based method proposed by Mari et al. (2001).
Dietary food log
The 24 h dietary recall was analysed using Foodmate, version 1.1 (The Nutrition Company, Long Valley, NJ, USA).
Visual analogue scale
After 120 min of each experimental condition, participants were asked to fill out visual analog scales (VAS) (Flint et al. 2000) for five symptoms: nausea, urge to vomit, bloating, belching and cramps. Participants were asked to fill out the VAS based on their global experience and symptoms throughout the 2.5 h experimental condition.
Statistical analysis
Data were analysed using SPSS, version 21 (IBM Corp., Armonk, NY, USA). Normality was assessed using Q–Q plots and Shapiro–Wilk tests within each experimental condition. The 2 h AUC and incremental AUC (iAUC) were calculated using Prism, version 6.0 (GraphPad Software Inc., San Diego, CA, USA) and included time points 0–120 min. AUCs and iAUCs were compared between experimental conditions using a paired Student's t test. VAS differences between conditions were assessed using a Wilcoxon signed rank test. A linear mixed‐effects model including time points −30 to 120 min (condition and time as fixed factors and subject as random factor) was used to determine the treatment effects. Significant interactions were followed up with pre‐planned contrasts by comparing placebo with Kme within each time point using Bonferroni corrections for multiple comparisons. Cohen's d effect size was calculated for all of the significant pre‐planned comparisons. P < 0.05 was considered statistically significant. Data are presented as the mean ± SD, whereas non‐parametric data are presented as the median and range.
A sample size of 20 was calculated a priori to detect a 20% reduction in glucose AUC, which was the predefined primary outcome of interest. A 20% reduction in glucose was based on pilot experiments, which parallels that seen with β‐hydroxybutyrate infusion (Mikkelsen et al. 2015) and acute Kme ingestion (Stubbs et al. 2017b) and was considered clinically relevant based on similar magnitude of reduction with glucose‐lowering medications (Goldstein et al. 2007). Using a mean and SD for glucose AUC from previous data collected in our laboratory (700 ± 160 mm × 120 min), a 20% reduction corresponded to an effect size of 0.875 assuming a moderate correlation of r = 0.5 amongst repeated measures. Using these data with an alpha level of 0.05 and 90% power a sample size of 16 was calculated using G*Power, version 3.1.9.3 (Heinrich‐Heine‐Universität Düsseldorf, Düsseldorf, Germany). To account for 20% dropout or missing blood samples, we aimed to recruit 20 participants.
Results
Baseline characteristics of the participants are presented in Table 1. The β‐OHB, glucose, insulin and C‐peptide responses over time are presented in Fig. 1. A significant condition by time interaction was found for β‐OHB (P < 0.001), with all time points, except −30 min (P = 0.577), being higher after Kme supplementation compared to placebo (all P < 0.001) (Fig. 1 A). Also, significant main effects of time were found for glucose, insulin and C‐peptide (all P < 0.001). There was a main effect of condition observed for glucose (P < 0.001) (Fig. 1 B), with glucose being lower in the Kme condition. No significant effects of condition or condition × time interactions were found for insulin (P = 0.971 and 0.871, respectively) (Fig. 1 C) or C‐peptide (P = 0.078 and 0.489, respectively) (Fig. 1 D).
Figure 1. β‐OHB, glucose, insulin and C‐peptide responses following a single dose of Kme supplement or placebo.
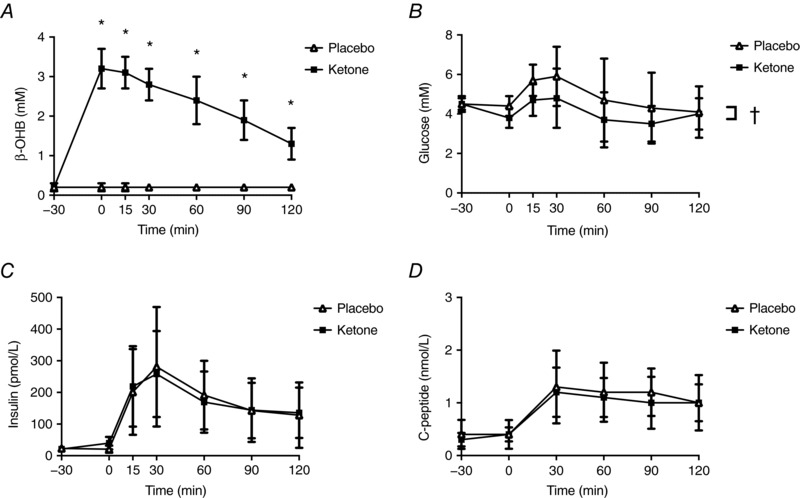
Supplements were consumed in the fasted state followed 30 min later by a 75 g OGTT. A, β‐OHB, B, glucose. C, insulin. D, C‐peptide. * P < 0.001 vs. placebo within time point, Bonferroni adjusted post hoc. †P < 0.001, significant main effect of condition.
AUCs for β‐OHB, glucose, insulin and C‐peptide are presented in Figs 2 and 3. The Kme supplement significantly increased β‐OHB AUC compared to placebo (1104%, P < 0.001, d = 8.9) (Fig. 2 A). Compared to placebo, the Kme supplement significantly decreased glucose AUC (−17%, P < 0.001, d = 1.1) (Fig. 2 B) and glucose iAUC (−40%, P = 0.016, d = 0.7) (Fig. 3 A). No differences were observed between Kme and placebo for the insulin AUC (−3%, P = 0.710, d = 0.1) (Fig. 2 C), insulin iAUC (−15%, P = 0.087, d = 0.4) (Fig. 3 B) or C‐peptide AUC (−9%, P = 0.151, d = 0.4) (Fig. 2 D), whereas C‐peptide iAUC showed a significant decrease in the Kme condition (−21%, P = 0.005, d = 0.9) (Fig. 3 C). The OGIS index improved by ∼11% in the Kme condition (P < 0.001, d = 1.0) (Fig. 4).
Figure 2. Two hour AUC following a single dose of Kme supplement or placebo.
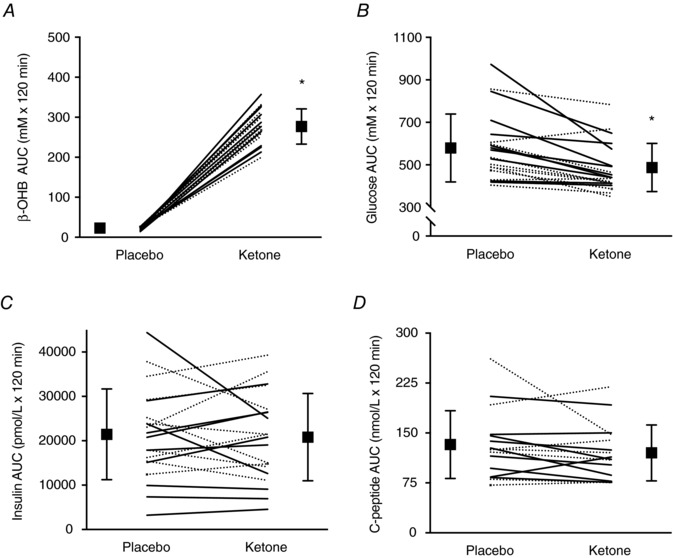
Supplements were consumed in the fasted state followed 30 min later by a 75 g OGTT. A, β‐OHB AUC. B, glucose AUC. C, insulin AUC. D, C‐peptide AUC. Continuous lines represent individual male participants and dashed lines represent individual female participants. * P = 0.001 vs. placebo.
Figure 3. Two‐hour iAUC following a single dose of Kme supplement or placebo.
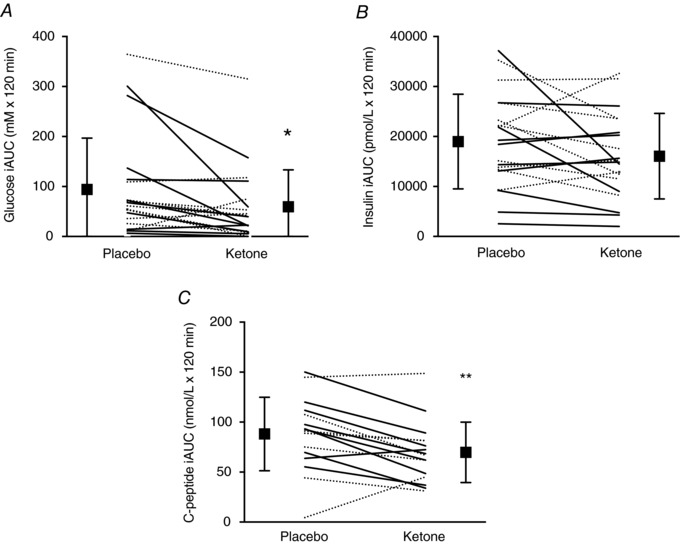
Supplements were consumed in the fasted state followed 30 min later by a 75 g OGTT. A, glucose iAUC. B, insulin iAUC. C, C‐peptide iAUC. Continuous lines represent individual male participants and dashed lines represent individual female participants. ** P < 0.01 vs. placebo, * P < 0.05 vs. placebo.
Figure 4. Oral glucose insulin sensitivity index following a single dose of Kme supplement or placebo.
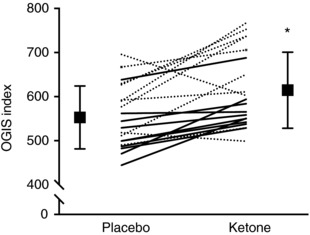
Supplements were consumed in the fasted state followed 30 min later by a 75 g OGTT. Continuous lines represent individual male participants and dashed lines represent individual female participants. * P = 0.001 vs. placebo.
A condition × time interaction was observed for serum NEFA (P < 0.001) (Fig. 5 A). Pre‐planned contrasts comparing the two conditions within each time point revealed significant differences at 0 min (P < 0.001, d = 1.7), 15 min (P < 0.001, d = 1.9), 30 min (P < 0.001, d = 1.4), 60 min (P = 0.001, d = 0.8), 90 min (P = 0.004, d = 0.8) and 120 min (P = 0.005, d = 1.0). NEFA AUC was also decreased by ∼44% after Kme compared to placebo (P < 0.001, d = 1.8) (Fig. 5 B). A significant condition × time interaction was seen for GLP‐1 (P = 0.002), with GLP‐1 being lower at time 30 in the Kme condition (P < 0.001, d = 1.4) (Fig. 6 A). There were no significant effects of condition, time, or condition × time interactions for adiponectin (all P > 0.239). Gastrointestinal symptoms were generally low to non‐existent, with medians of zero for all symptoms except nausea, where the median was 1 (out of 100 mm) in the ketone condition (Table 2). Wilcoxon signed rank tests revealed no significant differences between conditions for symptoms of nausea, urge to vomit, bloating, belching and cramps (all P > 0.05) (Table 2).
Figure 5. NEFA following a single dose of Kme supplement or placebo.
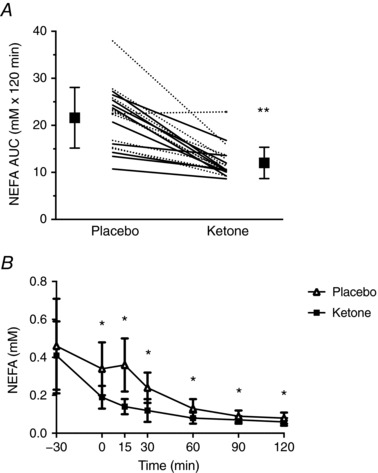
Supplements were consumed in the fasted state followed 30 min later by a 75 g OGTT. A, NEFA response over time. B, 2 h AUC. Continuous lines represent individual male participants and dashed lines represent individual female participants. ** P < 0.001 vs. placebo. * P < 0.01 vs. placebo within time point, Bonferroni adjusted post hoc.
Figure 6. GLP‐1 and adiponectin responses following a single dose of Kme supplement or placebo.
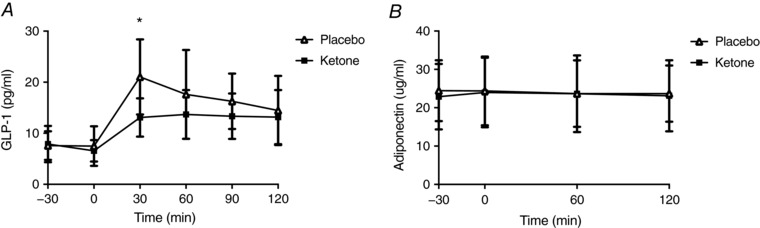
Supplements were consumed in the fasted state followed 30 min later by a 75 g OGTT. A, GLP‐1. B, adiponectin. * P < 0.01 vs. placebo within time point, Bonferroni adjusted post hoc.
Table 2.
Gastrointestinal symptoms assessed at the end of the Kme supplement and placebo experimental trials
| Nausea | Urge to vomit | Bloating | Belching | Cramps | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range | Median | Range | Median | Range | Median | Range | Median | Range | Median | |
| Placebo | 0‐15 | 0 | 0–4 | 0 | 0–9 | 0 | 0–40 | 0 | 0–10 | 0 |
| Ketone | 0‐40 | 1 | 0–22 | 0 | 0–33 | 0 | 0–32 | 0 | 0–8 | 0 |
| Wilcoxon signed‐rank tests | ||||||||||
| P value | 0.06 | 0.09 | 0.60 | 0.92 | 0.89 | |||||
| Effect sizes | ||||||||||
| Cohen's d | 0.5 | 0.6 | 0.3 | 0.1 | 0.1 | |||||
Results are expressed in millimetres (0–100).
Discussion
The present study aimed to determine whether a single dose of Kme consumed 30 min before a 2 h OGTT could attenuate the glycaemic response in young healthy individuals. The results obtained demonstrate that, in individuals with fasting glucose levels within the normal range (4.0–6.0 mm) (Cheng, 2013), 2 h total and incremental glucose AUCs are significantly lowered by a single dose of Kme supplement compared to a placebo. This reduction in glycaemic response following the ingestion of Kme was accompanied by a decrease in circulating NEFA levels and an improvement in the OGIS index, a marker of insulin sensitivity. The decrease in C‐peptide iAUC under the Kme condition also supports the notion that exogenous ketone supplementation may lower glucose via improved insulin sensitivity.
During the writing up of the present study, the first data in humans reporting a glucose‐lowering effect of Kme supplements in the resting state were reported (Stubbs et al. 2017b). This recent publication demonstrated that a Kme supplement ingested following the consumption of a standard meal decreased glucose levels from 5.5 to 4.7 mm over a 4 h study. This decrease of ∼15% is of similar magnitude as the 16% decrease in glucose AUC observed in the present study. Our study adds to these findings and extends the glucose‐lowering effect to Kme consumption prior to the ingestion of glucose, suggesting that pre‐meal supplementation with exogenous ketones could reduce the glycaemic response. The hypoglycaemic action of ketone bodies has been shown in β‐OHB infusion studies in both humans and animals (Balasse & Ooms, 1968; Miles et al. 1981; Müller et al. 1984; Shaw & Wolfe, 1984; Mikkelsen et al. 2015). Among these previous studies, Miles et al. (1981) and Mikkelsen et al. (2015) showed that β‐OHB, infused to levels of ∼2.0 mm, significantly decreased circulating glucose and suppressed endogenous glucose production by ∼20% in healthy males. The glucose‐lowering action of ketones may be the result of a direct effect on hepatic glucose production because it has been shown to occur in the absence of changes in insulin or glucagon (Balasse & Ooms, 1968; Binkiewicz et al. 1974; Sherwin et al. 1975). However, ketone body infusion has also been shown in some studies to stimulate pancreatic β‐cell insulin secretion (Madison et al. 1964; Balasse et al. 1970; Jenkins et al. 1970). Previous studies using Kme supplement have observed a two‐fold increase in insulin levels during a post‐exercise hyperglycaemic clamp (Holdsworth et al. 2017) and a small increase in the fasting state (Stubbs et al. 2017, 2018), whereas no change was seen following a post‐exercise high‐dose protein‐carbohydrate drink (Vandoorne et al. 2017). The potential insulinogenic action of ketone bodies remains a matter of debate, although the results of the present study clearly indicate that, if Kme stimulates insulin secretion, this effect is not additive to the stimulus of a standardized 75 g glucose drink. By contrast to a potential increase in insulin secretion following the use of exogenous ketones, we observed a decrease in C‐peptide iAUC during the OGTT. Miles et al. (1981) reported an increase in C‐peptide levels following the infusion of β‐OHB in fasted humans. The discrepancy between their report and the findings of the present study might be explained by our study involving the ingestion of exogenous ketones followed by glucose instead of isolated infusion of ketones in the basal state. The decrease in C‐peptide was accompanied by a decrease in incretin hormone GLP‐1 in the Kme condition. This observation is in agreement with Stubbs et al. (2018) who reported a decrease in GLP‐1 following a Kme supplement in the fasting state. GLP‐1 is produced by the gut in response to food (or carbohydrate) ingestion and acts to potentiate insulin secretion (Seino et al. 2010). Thus, a decrease in GLP‐1 might be linked to the observed decrease in C‐peptide iAUC following the Kme supplement. Additional studies are needed to determine the underlying mechanisms linking Kme ingestion to reduced glycaemic response, including a further exploration of the effects on insulin sensitivity, insulin secretion and GLP‐1 involvement.
As shown in Figs 2 and 3, not all participants responded the same way to the KME treatment in terms of glycaemic or insulin responses. This is typical for human physiology experiments and the present study was not designed to attempt to quantify individual responses or predictors, although this may be an interesting future direction for exogenous ketone research. The present study was not able to directly test the underlying mechanisms responsible for the reduced glycaemic response after Kme ingestion. However, based on the observations of several previous studies performed in both human and animal models (Mebane & Madison, 1964; Miles et al. 1981; Mikkelsen et al. 2015), it could be speculated that the reduced glycaemic response is mainly attributable to β‐OHB‐mediated reduction in hepatic glucose output. Studies using tracer techniques along with Kme supplementation would be necessary to validate these assumptions.
Although starvation and a low‐carbohydrate ketogenic diet increase the release and utilization of NEFA (Schwarz et al. 1995; Cahill, 2006; Volek et al. 2008), β‐OHB can directly inhibit lipolysis via agonism of the nicotinic acid receptor GPR109A (also known as HM74A, PUMA‐G) on adipocytes (Taggart et al. 2005). In line with the results of the present study, several studies involving ketone infusion have reported a significant decrease in NEFA levels under both fasting and fed conditions (Balasse & Ooms, 1968; Miles et al. 1981; Mikkelsen et al. 2015; Stubbs et al. 2017b). Noteworthy, the Kme supplement in the present study was able to immediately decrease NEFA in the fasted state (–30 vs. 0 min time points) and further decrease NEFA levels on top of the anti‐lipolytic effect of glucose ingestion (time points 15–120 min). Because acutely decreasing circulating NEFA is associated with improved insulin sensitivity and/or glucose tolerance, it is possible that the improvement in insulin sensitivity in the Kme condition could be the result of the decrease in circulating NEFA (Saloranta et al. 1993; Santomauro et al. 1999; Cusi et al. 2007). The absence of change in adiponectin levels following Kme is in accordance with a previous study using KS and suggests that this protein was not involved in the insulin sensitivity improvement process (de Oliveira Caminhotto et al. 2017). Adiponectin is an adipokine that is associated with improved insulin sensitivity (Yadav et al. 2013) and its secretion can be stimulated via the nicotinic acid receptor (Plaisance et al. 2009).
Some limitations of the present study should be acknowledged. First, the present study was conducted with healthy young individuals and so the results may not apply to clinical populations with metabolic impairments. Given that Kme is so new, we aimed to investigate the response in healthy individuals to reduce the confounding influence of insulin resistance, β‐cell dysfunction and medications, all of which could confound interpretation of OGTT results. Along this line, more studies are needed to determine the possible impact of Kme supplementation in individuals with impaired glucose tolerance. Second, the use of the OGIS index limits mechanistic interpretation of our findings compared to a more direct assessment of insulin sensitivity such as a hyperinsulinaemic‐euglycaemic clamp or stable isotope glucose tracers. However, the OGIS correlates well with clamp‐derived measures in healthy individuals and, if Kme is to be used therapeutically to lower glucose, we consider it important to demonstrate efficacy in reducing glycaemic response because, ultimately, individuals consume carbohydrates, which contributes to hyperglycaemia in real‐life. Finally, the present study could not rule out potential effects of the Kme on the digestion and absorption of the glucose drink. Accordingly, future studies could explore gastric emptying aiming to better understand the mechanisms behind the reduced glycaemic response following the Kme supplement.
In conclusion, a Kme supplement that acutely increased β‐OHB levels up to ∼3 mm attenuated the glycaemic response to an OGTT in healthy humans. The reduction in glycaemic response along with improved markers of insulin sensitivity was not driven by increased insulin secretion but could be related to a β‐OHB‐mediated reduction in hepatic glucose output. These acute effects on a reduced glycaemic response and insulin sensitivity suggest that ketone monoester supplements could have therapeutic potential in the management and prevention of metabolic disease.
Additional information
Competing interests
TΔS Ltd provided the ketone ester, ΔG®. The intellectual property covering the uses of ketones and ketone esters is owned by BTG Ltd, the University of Oxford, the National Institutes of Health and TΔS Ltd. Should royalties ever accrue from these patents, Professor Kieran Clarke, as co‐inventor, will receive a share of the royalties under the terms proscribed by Oxford University. Professor Kieran Clarke is a director of TΔS Ltd., a company spun out of the University of Oxford to develop and commercialize products based on the science of ketone bodies in human nutrition.
Author contributions
ÉM‐C, JPL and KC conceived and designed the experiments. ÉM‐C, HN and HR performed the experiments. ÉM‐C, JPL, HN and HR analysed the data. ÉM‐C and JPL wrote the paper with input from all authors. All authors read and approved the final manuscript submitted for publication, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Acknowledgements
JPL is supported by a Canadian Institutes of Health Research (CIHR) New Investigator Salary Award (MSH‐141980) and a Michael Smith Foundation for Health Research (MSFHR) Scholar Award (16890). This study was funded by a Natural Sciences and Engineering Research Council (NSERC) of Canada Discovery Grant (Grant number 435807).
Biography
Étienne Myette‐Côté is a PhD candidate in the School of Health and Exercise Sciences at the University of British‐Columbia in Kelowna, BC, Canada. His research is designed to investigate the effects of dietary macronutrient modulation on metabolic health, vascular function and inflammatory status in individuals with type 2 diabetes. Currently, he is investigating the impact of exogenous ketone supplementation on exercise performance and a broad range of health outcomes, including glucose control and insulin secretion. His future aspirations include lecturing at the University level and researching methods to better combine lifestyle interventions and glucose‐lowering medications.

Linked articles This article is highlighted by a Perspective by Egan. To read this Perspective, visit https://doi.org/10.1113/JP275938.
Edited by: Kim Barrett & Bettina Mittendorfer
This is an Editor's Choice article from the 15 April 2018 issue.
References
- Ari C, Kovács Z, Juhasz G, Murdun C, Goldhagen CR, Koutnik AP, Poff AM, Kesl SL & D'Agostino DP (2017). Exogenous ketone supplements reduce anxiety‐related behavior in Sprague‐Dawley and Wistar Albino Glaxo/Rijswijk rats. Front Mol Neurosci 9, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasse E & Ooms H (1968). Changes in the concentrations of glucose, free fatty acids, insulin and ketone bodies in the blood during sodium‐hydroxybutyrate infusions in man. Diabetologia 4, 133–135. [DOI] [PubMed] [Google Scholar]
- Balasse E, Ooms H & Lambilliotte J (1970). Evidence for a stimulatory effect of ketone bodies on insulin secretion in man. Horm Metab Res 2, 371–372. [DOI] [PubMed] [Google Scholar]
- Balasse EO & Fery F (1989). Ketone body production and disposal: effects of fasting, diabetes, and exercise. Diabetes Metab Rev 5, 247–270. [DOI] [PubMed] [Google Scholar]
- Binkiewicz A, Sadeghi‐Nejad A, Hochman H, Loridan L & Senior B (1974). An effect of ketones on the concentrations of glucose and of free fatty acids in man independent of the release of insulin. J Pediatr 84, 226–231. [DOI] [PubMed] [Google Scholar]
- Cahill GF Jr (1976). Starvation in man. Clin Endocrinol Metab 5, 397–415. [DOI] [PubMed] [Google Scholar]
- Cahill GF Jr (2006). Fuel metabolism in starvation. Annu Rev Nutr 26, 1–22. [DOI] [PubMed] [Google Scholar]
- Cheng A (2013). Canadian diabetes association clinical practice guidelines expert committee: Canadian diabetes association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 37, S1–S212. [DOI] [PubMed] [Google Scholar]
- Clarke K, Tchabanenko K, Pawlosky R, Carter E, King MT, Musa‐Veloso K, Ho M, Roberts A, Robertson J & VanItallie TB (2012). Kinetics, safety and tolerability of (R)‐3‐hydroxybutyl (R)‐3‐hydroxybutyrate in healthy adult subjects. Regul Toxicol Pharmacol 63, 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox PJ, Kirk T, Ashmore T, Willerton K, Evans R, Smith A, Murray AJ, Stubbs B, West J & McLure SW (2016). Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab 24, 256–268. [DOI] [PubMed] [Google Scholar]
- Cusi K, Kashyap S, Gastaldelli A, Bajaj M & Cersosimo E (2007). Effects on insulin secretion and insulin action of a 48‐h reduction of plasma free fatty acids with acipimox in nondiabetic subjects genetically predisposed to type 2 diabetes. Am J Physiol Endocriol Metab 292, E1775–E1781. [DOI] [PubMed] [Google Scholar]
- de Oliveira Caminhotto R, Komino ACM, de Fatima Silva F, Andreotti S, Sertié RAL, Reis GB & Lima FB (2017). Oral β‐hydroxybutyrate increases ketonemia, decreases visceral adipocyte volume and improves serum lipid profile in Wistar rats. Nutr Metab 14, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrochers S, Dubreuil P, Brunet J, Jette M, David F, Landau BR & Brunengraber H (1995). Metabolism of (R, S)‐1,3‐butanediol acetoacetate esters, potential parenteral and enteral nutrients in conscious pigs. Am J Physiol Endocriol Metab 268, E660–E667. [DOI] [PubMed] [Google Scholar]
- Flint A, Raben A, Blundell J & Astrup A (2000). Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes 24, 38. [DOI] [PubMed] [Google Scholar]
- Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J & Williams‐Herman DE (2007). Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase‐4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care 30, 1979–1987. [DOI] [PubMed] [Google Scholar]
- Holdsworth DA, Cox PJ, Kirk T, Stradling H, Impey SG & Clarke K (2017). A ketone ester drink increases postexercise muscle glycogen synthesis in humans. Med Sci Sports Exerc 49, 1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins D, Hunter W & Goff D (1970). Ketone bodies and evidence for increased insulin secretion. Nature 227, 384–385. [DOI] [PubMed] [Google Scholar]
- Kesl SL, Poff AM, Ward NP, Fiorelli TN, Ari C, Van Putten AJ, Sherwood JW, Arnold P & D'Agostino DP (2016). Effects of exogenous ketone supplementation on blood ketone, glucose, triglyceride, and lipoprotein levels in Sprague–Dawley rats. Nutr Metab 13, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison LL, Mebane D, Unger RH & Lochner A (1964). The hypoglycemic action of ketones. II. Evidence for a stimulatory feedback of ketones on the pancreatic beta cells. J Clin Invest 43, 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari A, Pacini G, Murphy E, Ludvik B & Nolan JJ (2001). A model‐based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care 24, 539–548. [DOI] [PubMed] [Google Scholar]
- Mebane D & Madison LL (1964). Hypoglycemic action of ketones. I. Effects of ketones on hepatic glucose output and peripheral glucose utilization. J Lab Clin Med 63, 177–192. [PubMed] [Google Scholar]
- Mikkelsen KH, Seifert T, Secher NH, Grøndal T & van Hall G (2015). Systemic, cerebral and skeletal muscle ketone body and energy metabolism during acute hyper‐D‐β‐hydroxybutyratemia in post‐absorptive healthy males. J Clin Endocrinol Metab 100, 636–643. [DOI] [PubMed] [Google Scholar]
- Miles JM, Haymond MW & Gerich JE (1981). Suppression of glucose production and stimulation of insulin secretion by physiological concentrations of ketone bodies in man. J Clin Endocrinol Metab 52, 34–37. [DOI] [PubMed] [Google Scholar]
- Müller MJ, Paschen U & Seitz HJ (1984). Effect of ketone bodies on glucose production and utilization in the miniature pig. J Clin Invest 74, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen OE, Felig P, Morgan AP, Wahren J & Cahill GF Jr (1969). Liver and kidney metabolism during prolonged starvation. J Clin Invest 48, 574–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaisance EP, Lukasova M, Offermanns S, Zhang Y, Cao G & Judd RL (2009). Niacin stimulates adiponectin secretion through the GPR109A receptor. Am J Physiol Endocriol Metab 296, E549–E558. [DOI] [PubMed] [Google Scholar]
- Robinson AM & Williamson DH (1980). Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev 60, 143–187. [DOI] [PubMed] [Google Scholar]
- Saloranta C, Groop L, Ekstrand A, Franssila‐Kallunki A, Eriksson J & Taskinen MR (1993). Different acute and chronic effects of acipimox treatment on glucose and lipid metabolism in patients with type 2 diabetes. Diabet Med 10, 950–957. [DOI] [PubMed] [Google Scholar]
- Santomauro A, Boden G, Silva M, Rocha DM, Santos RF, Ursich M, Strassmann PG & Wajchenberg BL (1999). Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes 48, 1836–1841. [DOI] [PubMed] [Google Scholar]
- Schwarz J‐M, Neese RA, Turner S, Dare D & Hellerstein MK (1995). Short‐term alterations in carbohydrate energy intake in humans. Striking effects on hepatic glucose production, de novo lipogenesis, lipolysis, and whole‐body fuel selection. J Clin Invest 96, 2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino Y, Fukushima M & Yabe D (2010). GIP and GLP‐1, the two incretin hormones: similarities and differences. J Diabetes Investig 1, 8–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J & Wolfe RR (1984). Influence of beta‐hydroxybutyrate infusion on glucose and free fatty acid metabolism in dogs. Am J Physiol Endocriol Metab 247, E756–E764. [DOI] [PubMed] [Google Scholar]
- Sherwin R, Hendler R & Felig P (1975). Effect of ketone infusions on amino acid and nitrogen metabolism in man. J Clin Invest 55, 1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs BJ, Cox PJ, Evans RD, Santer P, Miller JJ, Faull OK, Magor‐Elliott S, Hiyama S, Stirling M & Clarke K (2017). On the metabolism of exogenous ketones in humans. Front Physiol 8, 848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs BJ, Cox PJ, Evans RD, Cyranka M, Clarke K & de Wet H (2018). A ketone ester drink lowers human ghrelin and appetite. Obesity 26, 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart AK, Kero J, Gan X, Cai T‐Q, Cheng K, Ippolito M, Ren N, Kaplan R, Wu K & Wu T‐J (2005). (D)‐β‐hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA‐G. J Biol Chem 280, 26649–26652. [DOI] [PubMed] [Google Scholar]
- Tate RL, Mehlman MA & Tobin RB (1971). Metabolic fate of 1, 3‐butanediol in the rat: conversion to β‐hydroxybutyrate. J Nutr 101, 1719–1726. [DOI] [PubMed] [Google Scholar]
- Vandoorne T, De Smet S, Ramaekers M, Van Thienen R, De Bock K, Clarke K & Hespel P (2017). Intake of a ketone ester drink during recovery from exercise promotes mTORC1 signaling but not glycogen resynthesis in human muscle. Front Physiol 8, 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veech RL (2004). The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids 70, 309–319. [DOI] [PubMed] [Google Scholar]
- Volek JS, Fernandez ML, Feinman RD & Phinney SD (2008). Dietary carbohydrate restriction induces a unique metabolic state positively affecting atherogenic dyslipidemia, fatty acid partitioning, and metabolic syndrome. Prog Lipid Res 47, 307–318. [DOI] [PubMed] [Google Scholar]
- Yadav A, Kataria MA, Saini V & Yadav A (2013). Role of leptin and adiponectin in insulin resistance. Clin Chim Acta 417, 80–84. [DOI] [PubMed] [Google Scholar]
- Yancy WS Jr, Olsen MK, Guyton JR, Bakst RP & Westman EC (2004). A low‐carbohydrate, ketogenic diet versus a low‐fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med 140, 769–777. [DOI] [PubMed] [Google Scholar]
- Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D'Agostino D, Planavsky N, Lupfer C, Kanneganti TD, Kang S, Horvath TL, Fahmy TM, Crawford PA, Biragyn A, Alnemri E & Dixit VD (2015). The ketone metabolite beta‐hydroxybutyrate blocks NLRP3 inflammasome‐mediated inflammatory disease. Nat Med 21, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]


