Abstract
Key points
We investigated the contribution of group III/IV muscle afferents to carotid baroreflex resetting during electrically evoked (no central command) and voluntary (requiring central command) isometric knee extension exercise.
Lumbar intrathecal fentanyl was used to attenuate the central projection of μ‐opioid receptor‐sensitive group III/IV leg muscle afferent feedback. Spontaneous carotid baroreflex control was assessed by loading and unloading the carotid baroreceptors with a variable pressure neck chamber.
Group III/IV muscle afferents did not influence spontaneous carotid baroreflex responsiveness at rest or during exercise.
Afferent feedback accounted for at least 50% of the exercise‐induced increase in the carotid baroreflex blood pressure and heart rate operating points, adjustments that are critical for an appropriate cardiovascular response to exercise.
These findings suggest that group III/IV muscle afferent feedback is, independent of central command, critical for the resetting of the carotid baroreflex blood pressure and heart rate operating points, but not for spontaneous baroreflex responsiveness.
Abstract
This study sought to comprehensively investigate the role of metabolically and mechanically sensitive group III/IV muscle afferents in carotid baroreflex responsiveness and resetting during both electrically evoked (EVO, no central command) and voluntary (VOL, requiring central command) isometric single‐leg knee‐extension (15% of maximal voluntary contraction; MVC) exercise. Participants (n = 8) were studied under control conditions (CTRL) and following lumbar intrathecal fentanyl injection (FENT) to inhibit μ‐opioid receptor‐sensitive lower limb muscle afferents. Spontaneous carotid baroreflex control of mean arterial pressure (MAP) and heart rate (HR) were assessed following rapid 5 s pulses of neck pressure (NP, +40 mmHg) or suction (NS, −60 mmHg). Resting MAP (87 ± 10 mmHg) and HR (70 ± 8 bpm) were similar between CTRL and FENT conditions (P > 0.4). In terms of spontaneous carotid baroreflex responsiveness, FENT did not alter the change in MAP or HR responses to NP (+13 ± 5 mmHg, P = 0.85; +9 ± 3 bpm; P = 0.99) or NS (−13 ± 5 mmHg, P = 0.99; −24 ± 11 bpm; P = 0.49) at rest or during either exercise protocol, which were of a remarkably similar magnitude to rest. In contrast, FENT administration reduced the exercise‐induced resetting of the operating point for MAP and HR during both EVO (116 ± 10 mmHg to 100 ± 15 mmHg and 93 ± 14 bpm to 82 ± 10 bpm) and VOL (107 ± 13 mmHg to 100 ± 17 mmHg and 89 ± 10 bpm to 72 ± 10 bpm) exercise bouts. Together, these findings document that group III/IV muscle afferent feedback is critical for the resetting of the carotid baroreflex MAP and HR operating points, independent of exercise‐induced changes in central command, but not for spontaneous carotid baroreflex responsiveness.
Keywords: carotid baroreflex, central command, exercise pressor reflex, metaboreflex
Key points
We investigated the contribution of group III/IV muscle afferents to carotid baroreflex resetting during electrically evoked (no central command) and voluntary (requiring central command) isometric knee extension exercise.
Lumbar intrathecal fentanyl was used to attenuate the central projection of μ‐opioid receptor‐sensitive group III/IV leg muscle afferent feedback. Spontaneous carotid baroreflex control was assessed by loading and unloading the carotid baroreceptors with a variable pressure neck chamber.
Group III/IV muscle afferents did not influence spontaneous carotid baroreflex responsiveness at rest or during exercise.
Afferent feedback accounted for at least 50% of the exercise‐induced increase in the carotid baroreflex blood pressure and heart rate operating points, adjustments that are critical for an appropriate cardiovascular response to exercise.
These findings suggest that group III/IV muscle afferent feedback is, independent of central command, critical for the resetting of the carotid baroreflex blood pressure and heart rate operating points, but not for spontaneous baroreflex responsiveness.
Introduction
Arterial baroreceptors sense and regulate blood pressure at rest and during exercise. However, to operate appropriately at the higher blood pressures associated with physical activity, the carotid baroreflex control of the cardiovascular response needs to be ‘reset’ (Potts et al. 1993; Papelier et al. 1994). This resetting, mediated by both group III/IV muscle afferent feedback (Potts & Mitchell, 1998; Gallagher et al. 2001a; Sala‐Mercado et al. 2010) and neural feedforward mechanisms (central command) (Gallagher et al. 2001b; Ogoh et al. 2002), relocates the carotid baroreflex stimulus–response curve for both mean arterial pressure (MAP) and heart rate (HR) to operate at higher carotid sinus transmural pressures. Despite existing animal data suggesting that both mechanisms are similarly capable of resetting the arterial baroreflex (McIlveen et al. 2001), neural feedback has been, compared with central command, proposed to play a secondary, perhaps modulatory, role in humans (Fadel & Raven, 2012). This postulate is, in part, based on human studies attempting to manipulate either central command or group III/IV‐mediated afferent feedback during or after exercise, but such studies are complicated to design without confounding factors.
Previous experimental approaches in humans intended to individually manipulate either central command or muscle afferent feedback during exercise were faced with the issue of isolating one factor from the other. It was therefore difficult to draw definitive conclusions on the exact role of feedforward and feedback mechanisms. For example, although neuromuscular blocking agents, such as curare, increase central command during exercise, they also cause a predominant activation of type II muscle fibres (Galbo et al. 1987). The preferential recruitment of these fatigue‐prone muscle fibres exacerbates intramuscular metabolic perturbation and, consequently, increases group III/IV muscle afferent feedback. The net effect of such an intervention is exercise characterized by elevated levels of both central command and muscle afferent feedback, a circumstance hindering a clear conclusion regarding the role of central command. Other studies artificially raised feedback from group III/IV muscle afferents during (using medical anti‐shock trousers) (Gallagher et al. 2001a, 2006) or after (using post‐exercise circulatory occlusion; PECO) (Papelier et al. 1997; Fisher et al. 2008) exercise to investigate the role of these sensory neurons in shifting the carotid baroreflex stimulus–response curve. However, when this intervention is implemented during constant‐load exercise, the reduced blood flow with anti‐shock trousers not only raises afferent feedback by exaggerating metabolic perturbation, but also increases peripheral fatigue and, as a consequence, central command. Furthermore, conclusions from PECO studies are limited as this technique engages the reflex at rest, a scenario characterized by different baseline levels of autonomic activity compared to exercise, and predominantly activates metabo‐nociceptors, a subset of metabosensitive afferents not active during normal rhythmic exercise (Light et al. 2008; Jankowski et al. 2013; Pollak et al. 2014; Amann et al. 2015). An additional approach has been to study the role played by muscle afferents on baroreflex resetting by attenuating feedback from these sensory neurons during exercise using epidural anaesthesia (Smith et al. 2003). While this method provided valuable insights, it is important to recognize that local anaesthetics, unlike opioids, cause an increase in central command in order to compensate for the drug‐induced impairment of motoneuron action potential propagation (Smith et al. 2003; Amann et al. 2008). The net effect of using local anaesthetics during exercise is therefore reduced neural feedback, but also elevated central command, a circumstance likely masking the role of muscle afferents in shifting the carotid baroreflex stimulus–response curve.
Consequently, the role of group III/IV muscle afferents in carotid baroreflex resetting and responsiveness during exercise remains unclear due predominantly to confounding factors. To address this issue, we definitively identified the role of group III/IV leg muscle afferents in the carotid baroreflex control of MAP and HR by loading and unloading the carotid baroreceptors with neck pressure and suction during both electrically evoked (EVO, no central command) and voluntary (VOL, requiring central command) exercise, while pharmacologically attenuating afferent feedback from the exercising limb. We hypothesized that group III/IV muscle afferent feedback would be critical for the resetting of the carotid baroreflex MAP and HR operating points, independent of exercise‐induced changes in central command, but not for spontaneous carotid baroreflex responsiveness.
Methods
Ethical approval
Written informed consent was obtained from each participant prior to the beginning of the study. All experimental procedures were approved by the Institutional Review Boards of the University of Utah and the Salt Lake City Veterans Affairs Medical Center, and conducted according to the Declaration of Helsinki for human experimentation, except for registration in a database.
Subjects
Eight recreationally active, healthy men participated in this study (age: 28 ± 5 years; height: 179 ± 3 cm; body mass: 74 ± 7 kg). The subjects were non‐smokers, not medicated and asymptomatic for cardiovascular or respiratory disease, and refrained from vigorous exercise, caffeine and alcohol consumption for 24 h prior to each study visit. The number of participants was determined a priori by statistical power analysis (G*Power, 3.1.9.2) using the following criteria: α error, 0.05; power level (1 – β), 0.8; and effect size, 0.2. The sample size required was n = 6. We intended to increase the power from the minimum level of 0.8 and therefore studied two more subjects (in addition to the required six). The observed power a posteriori was 0.9.
Experimental protocol
For all protocols, the subjects were seated on a custom‐made bench, arms folded across the chest, with a trunk–thigh angle of 120° and a right knee joint angle of 90°. A non‐compliant cuff attached to a calibrated linear strain gauge (MLP 300; Transducer Techniques, Inc., Temecula, CA, USA) was fixed to the subjects’ right ankle, just superior to the malleoli. Subjects performed, for 10 min each, VOL and EVO intermittent (50 s contraction, 10 s relaxation) isometric quadriceps exercise at 15% of their maximal voluntary contraction (MVC) torque. The two exercise bouts were separated by 10 min of passive recovery. This protocol was performed on two separate days, either under control conditions (CTRL) or following lumbar intrathecal fentanyl injection (FENT) impairing feedback from μ‐opioid receptor‐sensitive lower limb muscle afferents. These visits were randomized for order and balanced for condition, but as EVO induces greater intramuscular metabolic perturbation than VOL exercise performed at the same workload (Vanderthommen et al. 2003; McNeil et al. 2006; Vanderthommen & Duchateau, 2007), within visits, VOL always preceded EVO to avoid large carry‐over effects. The two experimental visits were preceded by two familiarization trials where participants were exposed to the VOL and EVO exercise modalities and carotid baroreflex assessment. Additionally, all participants were screened for eligibility to participate in the study based upon the location of the carotid sinus, verified with Doppler ultrasound (Logiq 7, General Electric Medical Systems, Milwaukee, WI, USA), to ensure that the collar fully enclosed the carotid sinuses (Fadel et al. 2003) and the absence of visible plaque deposits. Carotid baroreflex assessment was also performed at rest and during exercise at these familiarization visits to verify that MAP and HR responses were reproducible in both delay and amplitude.
Measurements
Cardiovascular responses
MAP was measured continuously in the radial artery with a Teflon catheter (20 g) connected to a pressure transducer (Transpac IV, ICU Medical, San Clemente, CA, USA) placed at heart level. HR was monitored using a 12‐lead electrocardiogram (16‐bit Micro 1401 mk‐II, Cambridge Electronic Design Ltd, Cambridge, UK). Beat‐by‐beat MAP and HR were recorded at 1 kHz via a personal computer equipped with commercially available software (Spike 2, Cambridge Electronic Design).
Carotid baroreflex assessment
Carotid baroreflex control of MAP and HR was assessed using a neck pressure/neck suction (NP/NS) technique, in which both positive and negative pressure could be applied to the neck through a custom‐made, adjustable collar placed around the anterior two‐thirds of the neck. Computer‐controlled sustained pressures of +40 or −60 mmHg were generated by a variable pressure source and delivered to the neck collar through large‐bore two‐way solenoid valves (model 8215B, Asco, Florham Park, NJ, USA). Between each pressure intervention, the neck chamber pressure was vented to atmospheric pressure. The pressure generated by the neck collar was measured by a transducer (model DP45, Validyne Engineering, Northridge, CA, USA). The computer software gated the pulses of pressure to occur 50 ms after initiation of the R‐wave. This 50 ms delay allowed the artificial NP/NS to coincide with the arterial pressure wave at the carotid sinus. Each pressure pulse was 5 s in duration. At rest, the NP/NS pulse was conducted at an end‐expiratory breath hold to eliminate the confounding effects of respiratory sinus arrhythmia. The total duration of each breath hold was 10 s. During exercise, the need for a breath hold is eliminated (Eckberg et al. 1980) and, therefore, it was not performed during VOL and EVO contractions. Beginning at the third minute of exercise, a 5 s NP/NS pulse was performed 30 s after the initiation of each contraction. One minute was allotted between each NP/NS procedure. NP/NS was performed in quadruplicate and averaged. The carotid baroreflex MAP and HR operating points were determined using the average pre‐stimulus values for MAP and HR (averaged over 5 s), and estimated carotid sinus pressure was calculated as the pre‐stimulus MAP minus the chamber pressure (Fadel et al. 2003). Following NP/NS, peak and time response for MAP or HR were analysed.
Neuromuscular function
Neuromuscular function was assessed using the same custom‐made bench on which the exercise was performed (see ‘Experimental protocol’). A self‐adhesive electrode (3 × 3 cm, Ag–AgCl, Nikomed, Huntingdon Valley, PA, USA) was placed at the stimulation site which resulted in the maximal torque response. The cathode was placed on the femoral triangle and the anode, a 5 × 5 cm self‐adhesive stimulation electrode, was placed on the inferior portion of the gluteus maximus. The positions of these electrodes were marked with indelible ink to ensure a similar stimulation site across visits. A constant current stimulator (Model DS7AH; Digitimer Ltd, Welwyn Garden City, UK) delivered a square wave stimulus (200 μs). To ensure supramaximality during these tests, the stimulation intensity (164 ± 45 mA) was set to 120% of the intensity eliciting the maximal spatial recruitment of the quadriceps. For the evaluation of quadriceps function, potentiated quadriceps twitch torque was measured after each 3 s maximal voluntary contraction (MVC). Three MVCs, separated by 30 s, were performed before and again immediately after (within 10 s) the completion of each exercise trial. Quadriceps twitch torque (Q tw) was evoked by a single electrical stimulation of the femoral nerve 2 s after each MVC. The peak torque for all Q tw and MVC were averaged over the three measurements. Superimposed twitches were delivered during the peak torque of each MVC to calculate voluntary activation (VA) of the quadriceps as follows: VA (%) = (1 – superimposed Q tw/Q tw) × 100 (Merton, 1954). Peripheral quadriceps fatigue was expressed as a percentage reduction in Q tw from pre‐ to post‐exercise.
Voluntary and evoked quadriceps exercise
Both VOL and EVO contractions were performed with the right leg. During VOL, visual feedback was provided in real‐time to accomplish adequate torque. During EVO, the femoral nerve was stimulated electrically (frequency: 40 Hz; pulse width: 200 μs; current: 300 V). The stimulation frequency of 40 Hz was based upon the typical discharge frequency of the quadriceps motoneurons in humans (Pucci et al. 2006; Wust et al. 2008). The stimulation intensity was continuously adjusted (range: 38 ± 16 mA to 62 ± 26 mA) to maintain the target torque output.
Intrathecal fentanyl
Subjects were seated in a flexed position and 1 ml of fentanyl (0.025 mg.ml−1) was delivered intrathecally at the vertebral interspace L3–L4, as previously described (Amann et al. 2009).
Arm cranking test
Any migration of fentanyl beyond the cervical level would complicate the interpretation of our findings. Therefore, the ventilatory response to arm cranking (15 and 30 W for 3 min each; Monark‐Crescent AB, Varberg, Sweden) was assessed before and 10 min after fentanyl injection to evaluate whether a cephalad drug migration to the brain had occurred (Amann et al. 2010). To ensure a similar protocol across test days, the arm cranking test was also performed on the CTRL day.
Statistical analysis
Normality of every dependent variable and sphericity of the variance of the distributions (equal variance) were confirmed using the Kolmogorov–Smirnov test and the Mauchly test, respectively. A Greenhouse–Geisser correction was used when sphericity was violated. Two‐way ANOVA with repeated measures (contraction type × time) was used to test differences over time during exercise on MAP and HR, and before and after exercise on MVC, Q tw, and VA. Two‐way ANOVA with repeated measures (condition × NP/NS measurement) was used to test differences across NP/NS stimuli at rest and during exercise on MAP and HR, baroreflex responsiveness (∆MAP and ∆HR), and time to peak changes in MAP and HR following NP/NS. When a significant difference was found, multiple‐comparison analysis was performed using Tukey's honestly significant difference test. Student's paired t test was used to determine differences across conditions in exercise‐induced increases in MAP with the aim to evaluate the extent of the afferent blockade. Statistical analyses were conducted using Statistica 8.0 (StatSoft Inc, Tulsa, OK, USA). Data presented in the Results section are expressed as the mean ± SD. In cases of multiple non‐significant P values in a single sentence, the lowest P value was reported. Statistical significance was set at P < 0.05.
Results
Fentanyl, resting neuromuscular function and cardiorespiratory response to arm exercise
Intrathecal fentanyl had no effect on resting quadriceps MVC (224 ± 57 vs. 224 ± 60 N m, P = 0.91), VA (91 ± 6 vs. 93 ± 4%, P = 0.14), or Q tw (65 ± 18 vs. 60 ± 17 N m, P = 0.18). Resting (15 ± 3 vs. 17 ± 2 l min−1, P = 0.35) and HR (73 ± 8 vs. 66 ± 9 bpm, P = 0.14) (measured while standing) were similar in CTRL and FENT, respectively. Furthermore, both and HR during arm cranking at 15 W (, 26 ± 4 vs. 26 ± 4 l min−1; HR, 93 ± 6 vs. 88 ± 8 bpm; P > 0.3) and 30 W ( 35 ± 5 vs. 35 ± 7 l min−1; HR, 102 ± 8 vs. 98 ± 9 bpm; P > 0.3) were also similar in CTRL and FENT, respectively.
Exercise‐induced neuromuscular fatigue
As documented in Table 1, quadriceps MVC was reduced following EVO (P < 0.001), but not VOL (P = 0.10). The exercise‐induced reduction in Q tw was greater following EVO compared to VOL (P < 0.001), while VA remained unchanged compared to baseline in both conditions (P > 0.8). Fentanyl had no effect on exercise‐induced changes in MVC, Q tw or VA (P > 0.3).
Table 1.
Effect of voluntary (VOL) and electrically evoked (EVO) quadriceps contractions on peripheral and central fatigue
| VOL | EVO | |||
|---|---|---|---|---|
| CTRL | FENT | CTRL | FENT | |
| MVC (Δ%) | −15 ± 7 | −12 ± 6 | −33 ± 14* | −40 ± 14* |
| Q tw (Δ%) | −19 ± 10 | −23 ± 11 | −47 ± 21* | −59 ± 21* |
| VA (Δ%) | −2 ± 6# | 1 ± 4# | 1 ± 3# | 0 ± 7# |
Data are expressed as a percentage change from baseline. Majority of variables changed significantly from baseline after exercise. Note the similar exercise‐induced changes in neuromuscular function following CTRL and FENT within either exercise modality (P > 0.3). Pre‐exercise mean values for MVC, Q tw, and VA were 224 ± 57 N m, 65 ± 18 N m, and 91 ± 6%, respectively. MVC, maximal isometric voluntary contraction; Q tw, potentiated quadriceps twitch torque; VA, voluntary quadriceps activation. Data are presented as mean ± SD. #Not significantly different from pre‐exercise. * P < 0.05 vs. VOL.
Cardiovascular response to exercise
During EVO, MAP and HR increased by 28 ± 11% and 32 ± 13%, respectively, (P < 0.05) (Fig. 1). Although significant, the exercise‐induced increases in MAP (20 ± 11%) and HR (22 ± 10%) were smaller during VOL compared to EVO (P < 0.05). Fentanyl significantly attenuated the MAP and HR response to both EVO and VOL (Fig. 1).
Figure 1. Mean arterial pressure (MAP) and heart rate (HR) responses to electrically evoked and voluntary quadriceps contractions.
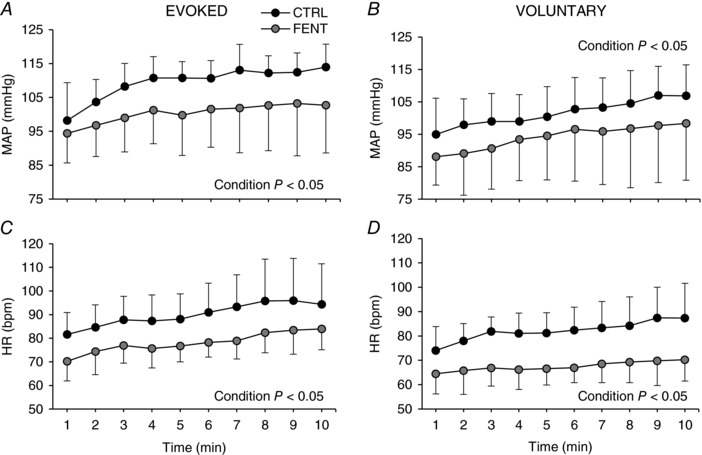
Each data point represents the average over the preceding minute. Data are presented as mean ± SD.
Fentanyl and carotid baroreflex control of MAP and HR at rest
As evidenced by the similar resting MAP (91 ± 11 vs. 86 ± 9 mmHg; P = 0.27) and HR (73 ± 8 vs. 66 ± 9 bpm; P = 0.14) in CTRL and FENT, fentanyl did not alter the baroreceptor MAP and HR operating points at rest. Furthermore, at rest, fentanyl did not alter the MAP response to NP (+13 ± 4 vs. +11 ± 6 mmHg; P = 0.85) or NS (−13 ± 6 vs. −13 ± 5 mmHg; P = 0.99), nor was there any effect on the HR response to NP (+9 ± 3 vs. +9 ± 3 bpm; P = 0.99) or NS (−26 ± 11 vs. −20 ± 9 bpm; P = 0.49). Therefore, as resting data were similar in both conditions, the responses were, for the purpose of clarity, averaged and represented as a single data point in all figures.
Fentanyl and carotid baroreflex control of MAP and HR during EVO
As illustrated in Fig. 2, during EVO and FENT, compared to CTRL, the exercise‐induced increase in MAP was attenuated by ∼58% while HR was attenuated by ∼48%, indicating a reduced shift of the MAP and HR operating points during exercise performed with afferent blockade. Furthermore, while the EVO CTRL resulted in a significant upward and rightward shift of the carotid baroreflex driven MAP and HR response, fentanyl significantly reduced these changes (Fig. 2 A and C). In terms of carotid baroreflex responsiveness, the MAP and HR response to NP or NS were similar during CTRL and FENT exercise (P > 0.6; Fig. 2 B and D). Finally, time to peak change in MAP (6.8 ± 0.8 s, P = 0.85) and HR (3.4 ± 0.5 s, P = 0.88) in response to NP or NS were similar at rest and during exercise and were also not affected by fentanyl.
Figure 2. Carotid baroreflex control of mean arterial pressure (MAP) and heart rate (HR) during electrically evoked quadriceps contractions.
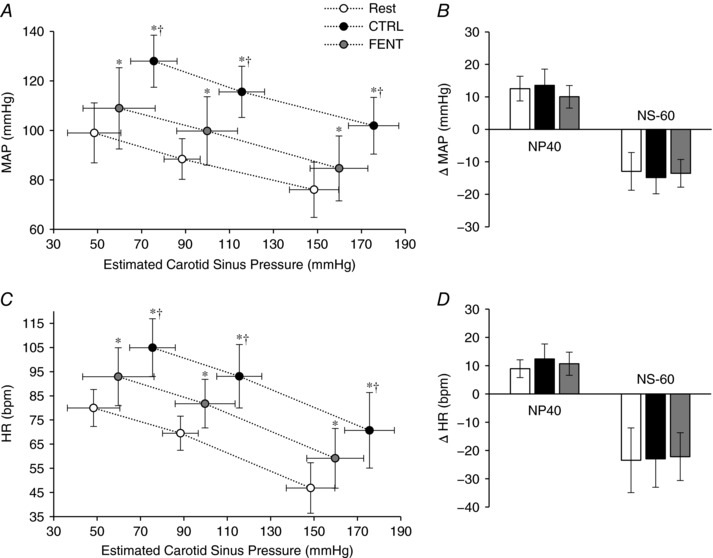
A and C, carotid baroreflex‐mediated response to neck pressure (NP) and neck suction (NS) for MAP (A) and HR (C) at rest and during exercise under control conditions (CTRL) and following lumbar intrathecal fentanyl (FENT) injection. For each condition, the three data points represent (from left to right) the response to NP (+40 mmHg), the operating point and the response to NS (−60 mmHg). B and D, quantification of the carotid baroreflex‐mediated response to NP and NS for MAP (B) and HR (D) expressed as a change from the operating point. Data are presented as mean ± SD. * P < 0.05 vs. Rest. †P < 0.05 vs. FENT.
Fentanyl and carotid baroreflex control of MAP and HR during VOL
As illustrated in Fig. 3, during VOL and FENT, compared to CTRL, the exercise‐induced increase in MAP was attenuated by ∼37% while HR was attenuated by ∼89%, indicating a reduced shift of the MAP and HR operating point during exercise with fentanyl. Furthermore, the VOL CTRL resulted in a significant upward and rightward shift of the carotid baroreflex MAP and HR response (Fig. 3 A and C). Fentanyl significantly reduced the upward and rightward shift of the carotid baroreflex MAP response (Fig. 3 A). Interestingly, while the rightward shift of the carotid baroreflex HR response was significantly reduced during exercise with fentanyl, the upward shift was abolished (P = 0.97) (Fig. 3 C). In terms of carotid baroreflex responsiveness, the MAP and HR responses to NP or NS were similar during CTRL and FENT exercise (P > 0.6; Fig. 3 B and D). Finally, time to peak changes in MAP (6.9 ± 0.8 s, P = 0.25) and HR (3.5 ± 0.6 s, P = 0.77) in response to NP or NS were similar at rest and during exercise and were also not affected by fentanyl. These latencies were similar to those observed during EVO (P > 0.2).
Figure 3. Carotid baroreflex control of mean arterial pressure (MAP) and heart rate (HR) during voluntary quadriceps contractions.
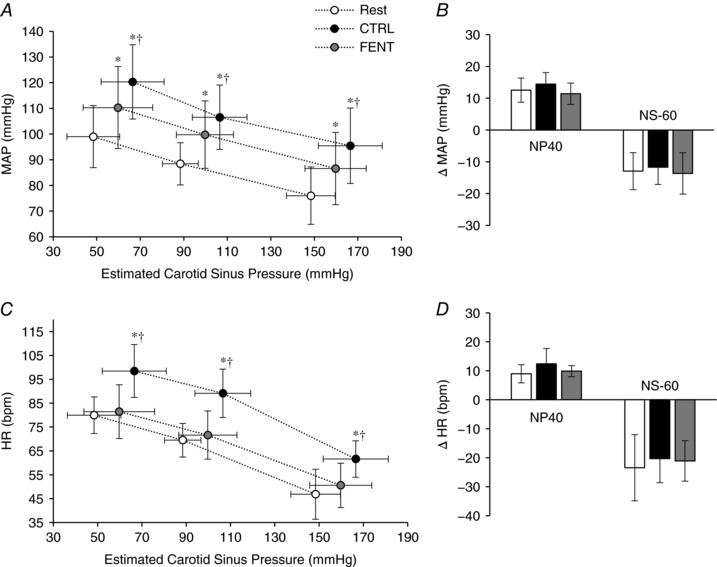
A and C, carotid baroreflex‐mediated response to neck pressure (NP) and neck suction (NS) for MAP (A) and HR (C) at rest and during exercise under control conditions (CTRL) and following lumbar intrathecal fentanyl (FENT) injection. For each condition, the three data points represent (from left to right) the response to NP (+40 mmHg), the operating point, and the response to NS (−60 mmHg). B and D, quantification of the carotid baroreflex‐mediated response to NP and NS for MAP (B) and HR (D) are expressed as a change from the operating point. Data are presented as mean ± SD. * P < 0.05 vs. Rest. †P < 0.05 vs. FENT.
Discussion
This study sought to investigate the role of metabolically and mechanically sensitive group III/IV muscle afferents in carotid baroreflex resetting and responsiveness during exercise. Both EVO and VOL exercise were used to discern the contribution of these sensory neurons in the presence and absence of central command, while spontaneous baroreflex control of MAP and HR was assessed using the NP/NS technique during exercise performed with and without leg muscle afferent feedback. This comprehensive approach, allowing the definitive determination of the role of group III/IV muscle afferents on the carotid baroreflex, yielded several clearly interpretable findings in an area that has been fraught with confounding factors. First, FENT administration reduced the exercise‐induced resetting of the operating point for MAP and HR during both EVO and VOL exercise. In terms of spontaneous carotid baroreflex responsiveness, FENT did not alter the change in MAP or HR evoked by NP or NS at rest or during either exercise paradigm. Together, these findings document that group III/IV muscle afferent feedback is critical for the resetting of the carotid baroreflex MAP and HR operating points, independent of exercise‐induced changes in central command, but not for spontaneous baroreflex responsiveness.
Role of group III/IV muscle afferents in carotid baroreflex control
Attenuation of group III/IV muscle afferent feedback during exercise compromised the typical exercise‐induced resetting of the carotid baroreflex MAP and HR operating points without affecting the capacity of the baroreceptors to respond to hyper‐ and hypotensive stimuli (Figs 2 and 3). The novelty of this study was the use of fentanyl and electrically evoked muscle contractions to definitively identify, independent of the confounding factor of central command, the role of group III/IV muscle afferents in the shift of the baroreflex to operate around a higher MAP and HR during exercise. This is of significance as most previous investigations in humans have, in contrast to the current approach, highlighted the role of group III/IV muscle afferents in baroreflex resetting by exaggerating the intramuscular metabolic milieu via partial or complete circulatory occlusion during or after voluntary exercise (Iellamo et al. 1997; Papelier et al. 1997; Gallagher et al. 2001a, 2006; Fisher et al. 2008; Drew et al. 2008a). Unfortunately, these latter approaches are limited as they not only increase peripheral fatigue, which, inevitably, raises central command at a given work rate, but also alter metaboreceptor firing (Adreani & Kaufman, 1998) and facilitate metabo‐nociceptors, a subset of metabosensitive afferents with little or no activity during normal rhythmic exercise (Light et al. 2008; Jankowski et al. 2013; Pollak et al. 2014; Amann et al. 2015). It is for these reasons that previous studies have concluded that afferent feedback plays a rather minor role in baroreflex resetting (Ogoh et al. 2002; Gallagher et al. 2006). The present findings contradict this notion and support earlier animal studies (McIlveen et al. 2001; Sala‐Mercado et al. 2010) by clearly emphasizing a substantial role for muscle afferent feedback in the exercise‐induced shift of the carotid baroreflex stimulus–response curve for both MAP and HR.
Interestingly, while afferent blockade partially reduced the exercise‐induced upward shift of the operating point for MAP during both contraction modalities, and that for HR during EVO, the shift for HR was completely abolished during VOL exercise (Fig. 3 C). The failure of the operating point for HR to increase from rest is remarkable and suggests a dominant role of muscle afferent feedback in determining the cardiac response and resetting of the baroreflex control of HR during voluntary, low intensity exercise utilizing a small muscle mass. Therefore, central command, which was likely similar during VOL FENT and VOL CTRL (based on the comparable degree of fatigue, Table 1), was unable to, even partially, compensate for the loss of afferent feedback. These findings challenge the hypothesis that feedforward mechanisms dominate the HR response to exercise (Eldridge et al. 1985; Mitchell et al. 1989) and carotid baroreflex resetting during exercise (Fadel & Raven, 2012), at least at low intensities. Additionally, the observed carotid baroreceptor responses during VOL exercise also suggests that the activity of group III/IV muscle afferents is elevated from rest even by relatively low intensity muscle contractions (i.e. 15% MVC) and that this, presumably small, increase in afferent activity is physiologically relevant in humans. This conclusion is indirectly supported by earlier studies on decerebrate cats documenting significant activation of group III and IV muscle afferents even at very low levels (25% of maximal O2 consumption) of dynamic exercise (Adreani et al. 1997).
The consolidated findings from the present investigation largely support an earlier epidural anaesthesia study (partial blockade via local anaesthetics) suggesting that afferent input from skeletal muscle is requisite for baroreflex resetting during exercise (Smith et al. 2003). However, these authors observed a significant role of afferent feedback only on the baroreflex control of MAP, but not of HR. The current results during VOL and EVO exercise confirm the essential role of muscle afferent feedback on resetting the operating point for MAP (Fig. 3 A), but clearly are at odds with the cardiac‐specific findings (Fig. 3 C) from Smith et al. (2003). Although, currently, a definitive explanation for this discrepancy is not apparent, differences in contraction modality (intermittent contractions at 15% MVC in the present study compared to the prior static contraction at 25% MVC) and the likely difference in central command between these studies may play a role.
The spontaneous response to NP/NS in the current study was unchanged with intrathecal fentanyl (Figs 2 and 3, panels B and D) suggesting that group III/IV muscle afferent input may not play a role in the sensitivity of the carotid baroreflex control of MAP and HR. While this contrasts with earlier animal work documenting that muscle afferent feedback decreases spontaneous HR baroreflex sensitivity in exercising dogs (Sala‐Mercado et al. 2010), our findings agree with most, but not all (Drew et al. 2008b), human studies which have used a full stimulus–response curve (i.e. multiple levels of NP and NS) and suggest no influence of these sensory neurons on baroreflex gain (Raymond et al. 2000; Gallagher et al. 2001a; Smith et al. 2003). However, since these findings/conclusions are specific to the carotid baroreflex, the modified Oxford technique (Ebert & Cowley, 1992), which perturbs blood pressure across a wide range engaging both carotid and aortic baroreceptors, might have offered a better estimation of the role of muscle afferents in arterial baroreflex sensitivity. Due to time constraints dictated by the restricted time of action for fentanyl (∼1 h), we were not able to incorporate this technique and were limited to quadruplicate measurements made at two levels of NP and NS representing the upper and lower ends of the curve (Keller et al. 2003). Finally, since NP and NS evoked similar pressor and cardioacceleratory responses at rest and during FENT and CTRL exercise, it might be concluded that the interaction between the group III/IV‐mediated cardiovascular reflex, i.e. the so‐called exercise pressor reflex (Mitchell et al. 1983), and the carotid baroreflex in spontaneous control of MAP and HR is simply additive. Nonetheless, the current study cannot sufficiently address the exact nature of the interaction (additive, occlusive or facilitative) between these two reflexes, which is intimately dependent on the haemodynamic parameter being investigated (Kaur et al. 2016).
It is important to note that this study was only performed on men and, given existing sex differences in blood pressure regulation (Joyner et al. 2016), the conclusions may not be generalizable to women.
Voluntary versus electrically evoked contractions: critical considerations
Although it is tempting to address the role of central command in carotid baroreflex control by comparing the cardiovascular responses during EVO and VOL exercise, a critical difference between the two protocols precludes this approach. Specifically, despite the similar work performed during both trials, the exercise‐induced decrease in twitch torque was greater during the EVO trial (Table 1), which may be a consequence of a differing intramuscular metabolic perturbation (Blain et al. 2016; Broxterman et al. 2017) between exercise modalities. Indeed, a previous 31P NMRS study documented a greater accumulation of hydrogen ions and inorganic phosphate during evoked compared to voluntary exercise at the same workload (Vanderthommen et al. 2003). Consequently, the metabolic stimuli and, therefore, group III/IV muscle afferent feedback were presumably greater during EVO compared to VOL exercise, and likely account for the greater cardiovascular response during EVO (Fig. 1). The, presumably, different degrees of afferent feedback between EVO and VOL trials not only prevent distinct conclusions on the actual role of central command in the cardiovascular response and baroreflex control during exercise, but also preclude a direct comparison of the significance of muscle afferents within each of the two trials. Regardless, we consider our approach of including both protocols as not only comprehensive but also complimentary. Indeed, while the results based on the VOL contractions largely confirm previous findings obtained during ‘normal’ exercise, the experiments based on electrically evoked contractions provide new insight on the actual importance and the isolated role of group III/IV muscle afferents in baroreflex control.
The specificity and efficacy of lumbar intrathecal fentanyl
The similar cardio‐ventilatory responses to arm cranking performed with and without lumbar fentanyl suggest that any potential cephalad migration of the drug was limited to the thoracic level, likely below T2. This provides confidence for the interpretation of our findings as the confounding effects associated with the binding of fentanyl on brain receptors can be excluded (Amann et al. 2010). Despite the fact that the site of action of fentanyl was confined to dorsal horn neurons below T2, it is unknown whether inhibition of presynaptic substance P (Jessell & Iversen, 1977) and glutamate (Kangrga & Randic, 1991) release and/or postsynaptic receptors ultimately accounts for the afferent blockade.
Although the exact distribution is currently unknown, group III/IV muscle afferents contain μ‐ and δ‐opioid receptors (Yaksh & Reddy, 1981; Yaksh & Noueihed, 1985), both of which contribute to the reflex‐mediated increase in blood pressure during exercise (Pomeroy et al. 1986; Hill & Kaufman, 1990) (i.e. the so‐called exercise pressor reflex; Mitchell et al. 1983). Consequently, as the opioid peptide fentanyl is a selective μ receptor agonist, the chosen approach only blocked a portion of all group III/IV muscle afferents. Preliminary findings from a recent mouse study suggest that ∼40% of dorsal root ganglia innervating metabosensitive group III/IV muscle afferents have μ receptors (Zhang et al. 2016), while the percentage of mechanosensitive group III/IV afferents is completely unknown. Regardless, as the discharge frequency of each of these fibres increases largely in proportion to their specific stimuli (Kaufman et al. 1983), it is more important to know what fraction of the central projection mediated by group III/IV muscle afferents is blocked by fentanyl. Previous findings in humans and animals provide the basis for speculation on this issue. Specifically, complete afferent blockade during electrically evoked leg exercise in humans via lumbar epidural bupivacaine completely abolishes the pressor response observed with intact afferent feedback (Strange et al. 1993). Similarly, the normally occurring pressor response to electrically evoked muscle contractions in cats is eliminated after the dorsal roots are cut (McCloskey & Mitchell, 1972). In contrast, intrathecal [d‐Ala2, N‐MePhe4, Gly‐ol]‐enkephalin (DAMGO), a μ‐opioid receptor agonist, only attenuates the pressor response to electrically evoked triceps surae contractions in anaesthetized cats by approximately 55% (Hill & Kaufman, 1990). Furthermore, the MAP response to electrically evoked quadriceps contractions in the current study was about 58% lower during the exercise performed with afferent blockade compared to control conditions (Fig. 4). Collectively, these findings suggest that fentanyl may eliminate approximately half of the central projection mediated by group III/IV muscle afferents and caution that the observed physiological consequences likely underestimate the role of these sensory neurons in regulating cardiovascular and fatigue responses in exercising humans.
Figure 4. Effect of fentanyl on the pressor response to electrically evoked quadriceps contractions.
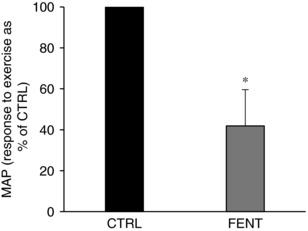
Calculations were performed on MAP at the operating point between the 4th and the 10th minute (i.e. ‘steady state’). Data are presented as mean ± SD. * P < 0.05 vs. CTRL.
Conclusion
Utilizing a comprehensive approach to determine the role of group III/IV muscle afferent feedback in carotid baroreflex control, it was documented that these afferents are critical for the resetting of the carotid baroreflex MAP and HR operating points, independent of exercise‐induced changes in central command. However, group III/IV muscle afferent feedback does not play a role in carotid baroreflex responsiveness during exercise.
Additional information
Competing interests
The authors have no competing interests.
Author contributions
This work was conducted at the Salt Lake City Department of Veterans Affairs Medical Centre. T.J.H. and M.A. conceived and designed the study. All authors executed the study. T.J.H. analysed the data. T.J.H. and M.A. interpreted the data and prepared the manuscript. All authors edited, revised and approved the final version of the manuscript. All authors agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by the National Institutes of Health (HL‐103786 and HL‐116579 to M.A.) and the Department of Veterans Affairs (E1572P to M.A.).
Acknowledgements
The authors wish to thank all the subjects who took part in this study.
Biography
Thomas J. Hureau received his PhD in exercise physiology at the University of Nice Sophia Antipolis (France) in 2015. He recently completed a postdoctoral fellowship at the University of Utah Vascular Research Laboratory (USA) under the supervision of Markus Amann. He is currently an Assistant Professor at the University of Strasbourg (France). Using an integrative physiology approach, his research is presently focused on the role of group III/IV muscle afferent feedback in the regulation of cardiovascular responses and the development of neuromuscular fatigue during exercise in humans.

Edited by: Michael Hogan & John Osborn
References
- Adreani CM, Hill JM & Kaufman MP (1997). Responses of group III and IV muscle afferents to dynamic exercise. J Appl Physiol 82, 1811–1817. [DOI] [PubMed] [Google Scholar]
- Adreani CM & Kaufman MP (1998). Effect of arterial occlusion on responses of group III and IV afferents to dynamic exercise. J Appl Physiol 84, 1827–1833. [DOI] [PubMed] [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF & Dempsey JA (2010). Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol 109, 966–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Proctor LT, Sebranek JJ, Eldridge MW, Pegelow DF & Dempsey JA (2008). Somatosensory feedback from the limbs exerts inhibitory influences on central neural drive during whole body endurance exercise. J Appl Physiol 105, 1714–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Proctor LT, Sebranek JJ, Pegelow DF & Dempsey JA (2009). Opioid‐mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol 587, 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Sidhu SK, Weavil JC, Mangum TS & Venturelli M (2015). Autonomic responses to exercise: group III/IV muscle afferents and fatigue. Auton Neurosci 188, 19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blain GM, Mangum TS, Sidhu SK, Weavil JC, Hureau TJ, Jessop JE, Bledsoe AD, Richardson RS & Amann M (2016). Group III/IV muscle afferents limit the intramuscular metabolic perturbation during whole body exercise in humans. J Physiol 594, 5303–5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxterman RM, Layec G, Hureau TJ, Amann M & Richardson RS (2017). Skeletal muscle bioenergetics during all‐out exercise: mechanistic insight into the oxygen uptake slow component and neuromuscular fatigue. J Appl Physiol 122, 1208–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew RC, Bell MP & White MJ (2008a). Modulation of spontaneous baroreflex control of heart rate and indexes of vagal tone by passive calf muscle stretch during graded metaboreflex activation in humans. J Appl Physiol 104, 716–723. [DOI] [PubMed] [Google Scholar]
- Drew RC, McIntyre DB, Ring C & White MJ (2008b). Local metabolite accumulation augments passive muscle stretch‐induced modulation of carotid‐cardiac but not carotid‐vasomotor baroreflex sensitivity in man. Exp Physiol 93, 1044–1057. [DOI] [PubMed] [Google Scholar]
- Ebert TJ & Cowley AW Jr (1992). Baroreflex modulation of sympathetic outflow during physiological increases of vasopressin in humans. Am J Physiol 262, H1372–H1378. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Kifle YT & Roberts VL (1980). Phase relationship between normal human respiration and baroreflex responsiveness. J Physiol 304, 489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE, Kiley JP & Waldrop TG (1985). Stimulation by central command of locomotion, respiration and circulation during exercise. Respir Physiol 59, 313–337. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Ogoh S, Keller DM & Raven PB (2003). Recent insights into carotid baroreflex function in humans using the variable pressure neck chamber. Exp Physiol 88, 671–680. [DOI] [PubMed] [Google Scholar]
- Fadel PJ & Raven PB (2012). Human investigations into the arterial and cardiopulmonary baroreflexes during exercise. Exp Physiol 97, 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JP, Young CN & Fadel PJ (2008). Effect of muscle metaboreflex activation on carotid‐cardiac baroreflex function in humans. Am J Physiol Heart Circ Physiol 294, H2296–H2304. [DOI] [PubMed] [Google Scholar]
- Galbo H, Kjaer M & Secher NH (1987). Cardiovascular, ventilatory and catecholamine responses to maximal dynamic exercise in partially curarized man. J Physiol 389, 557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher KM, Fadel PJ, Smith SA, Stromstad M, Ide K, Secher NH & Raven PB (2006). The interaction of central command and the exercise pressor reflex in mediating baroreflex resetting during exercise in humans. Exp Physiol 91, 79–87. [DOI] [PubMed] [Google Scholar]
- Gallagher KM, Fadel PJ, Stromstad M, Ide K, Smith SA, Querry RG, Raven PB & Secher NH (2001a). Effects of exercise pressor reflex activation on carotid baroreflex function during exercise in humans. J Physiol 533, 871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher KM, Fadel PJ, Stromstad M, Ide K, Smith SA, Querry RG, Raven PB & Secher NH (2001b). Effects of partial neuromuscular blockade on carotid baroreflex function during exercise in humans. J Physiol 533, 861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JM & Kaufman MP (1990). Attenuation of reflex pressor and ventilatory responses to static muscular contraction by intrathecal opioids. J Appl Physiol 68, 2466–2472. [DOI] [PubMed] [Google Scholar]
- Iellamo F, Legramante JM, Raimondi G & Peruzzi G (1997). Baroreflex control of sinus node during dynamic exercise in humans: effects of central command and muscle reflexes. Am J Physiol 272, H1157–H1164. [DOI] [PubMed] [Google Scholar]
- Jankowski MP, Rau KK, Ekmann KM, Anderson CE & Koerber HR (2013). Comprehensive phenotyping of group III and IV muscle afferents in mouse. J Neurophysiol 109, 2374–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell TM & Iversen LL (1977). Opiate analgesics inhibit substance P release from rat trigeminal nucleus. Nature 268, 549–551. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Wallin BG & Charkoudian N (2016). Sex differences and blood pressure regulation in humans. Exp Physiol 101, 349–355. [DOI] [PubMed] [Google Scholar]
- Kangrga I & Randic M (1991). Outflow of endogenous aspartate and glutamate from the rat spinal dorsal horn in vitro by activation of low‐ and high‐threshold primary afferent fibers. Modulation by mu‐opioids. Brain Res 553, 347–352. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH & Mitchell JH (1983). Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55, 105–112. [DOI] [PubMed] [Google Scholar]
- Kaur J, Alvarez A, Hanna HW, Krishnan AC, Senador D, Machado TM, Altamimi YH, Lovelace AT, Dombrowski MD, Spranger MD & O'Leary DS (2016). Interaction between the muscle metaboreflex and the arterial baroreflex in control of arterial pressure and skeletal muscle blood flow. Am J Physiol Heart Circ Physiol 311, H1268–H1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller DM, Wasmund WL, Wray DW, Ogoh S, Fadel PJ, Smith ML & Raven PB (2003). Carotid baroreflex control of leg vascular conductance at rest and during exercise. J Appl Physiol 94, 542–548. [DOI] [PubMed] [Google Scholar]
- Light AR, Hughen RW, Zhang J, Rainier J, Liu Z & Lee J (2008). Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol 100, 1184–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey DI & Mitchell JH (1972). Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224, 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlveen SA, Hayes SG & Kaufman MP (2001). Both central command and exercise pressor reflex reset carotid sinus baroreflex. Am J Physiol Heart Circ Physiol 280, H1454–H1463. [DOI] [PubMed] [Google Scholar]
- McNeil CJ, Murray BJ & Rice CL (2006). Differential changes in muscle oxygenation between voluntary and stimulated isometric fatigue of human dorsiflexors. J Appl Physiol 100, 890–895. [DOI] [PubMed] [Google Scholar]
- Merton PA (1954). Voluntary strength and fatigue. J Physiol 123, 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP & Iwamoto GA (1983). The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45, 229–242. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Reeves DR Jr, Rogers HB & Secher NH (1989). Epidural anaesthesia and cardiovascular responses to static exercise in man. J Physiol 417, 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoh S, Wasmund WL, Keller DM, O‐Yurvati A, Gallagher KM, Mitchell JH & Raven PB (2002). Role of central command in carotid baroreflex resetting in humans during static exercise. J Physiol 543, 349–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papelier Y, Escourrou P, Gauthier JP & Rowell LB (1994). Carotid baroreflex control of blood pressure and heart rate in men during dynamic exercise. J Appl Physiol 77, 502–506. [DOI] [PubMed] [Google Scholar]
- Papelier Y, Escourrou P, Helloco F & Rowell LB (1997). Muscle chemoreflex alters carotid sinus baroreflex response in humans. J Appl Physiol 82, 577–583. [DOI] [PubMed] [Google Scholar]
- Pollak KA, Swenson JD, Vanhaitsma TA, Hughen RW, Jo D, White AT, Light KC, Schweinhardt P, Amann M & Light AR (2014). Exogenously applied muscle metabolites synergistically evoke sensations of muscle fatigue and pain in human subjects. Exp Physiol 99, 368–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeroy G, Ardell JL & Wurster RD (1986). Spinal opiate modulation of cardiovascular reflexes in the exercising dog. Brain Res 381, 385–389. [DOI] [PubMed] [Google Scholar]
- Potts JT & Mitchell JH (1998). Rapid resetting of carotid baroreceptor reflex by afferent input from skeletal muscle receptors. Am J Physiol 275, H2000–H2008. [DOI] [PubMed] [Google Scholar]
- Potts JT, Shi XR & Raven PB (1993). Carotid baroreflex responsiveness during dynamic exercise in humans. Am J Physiol 265, H1928–H1938. [DOI] [PubMed] [Google Scholar]
- Pucci AR, Griffin L & Cafarelli E (2006). Maximal motor unit firing rates during isometric resistance training in men. Exp Physiol 91, 171–178. [DOI] [PubMed] [Google Scholar]
- Raymond J, Davis GM, van Der Plas MN, Groeller H & Simcox S (2000). Carotid baroreflex control of heart rate and blood pressure during ES leg cycling in paraplegics. J Appl Physiol 88, 957–965. [DOI] [PubMed] [Google Scholar]
- Sala‐Mercado JA, Ichinose M, Coutsos M, Li Z, Fano D, Ichinose T, Dawe EJ & O'Leary DS (2010). Progressive muscle metaboreflex activation gradually decreases spontaneous heart rate baroreflex sensitivity during dynamic exercise. Am J Physiol Heart Circ Physiol 298, H594–H600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Querry RG, Fadel PJ, Gallagher KM, Stromstad M, Ide K, Raven PB & Secher NH (2003). Partial blockade of skeletal muscle somatosensory afferents attenuates baroreflex resetting during exercise in humans. J Physiol 551, 1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange S, Secher NH, Pawelczyk JA, Karpakka J, Christensen NJ, Mitchell JH & Saltin B (1993). Neural control of cardiovascular responses and of ventilation during dynamic exercise in man. J Physiol 470, 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderthommen M & Duchateau J (2007). Electrical stimulation as a modality to improve performance of the neuromuscular system. Exerc Sport Sci Rev 35, 180–185. [DOI] [PubMed] [Google Scholar]
- Vanderthommen M, Duteil S, Wary C, Raynaud JS, Leroy‐Willig A, Crielaard JM & Carlier PG (2003). A comparison of voluntary and electrically induced contractions by interleaved 1H‐ and 31P‐NMRS in humans. J Appl Physiol 94, 1012–1024. [DOI] [PubMed] [Google Scholar]
- Wust RC, Morse CI, de Haan A, Jones DA & Degens H (2008). Sex differences in contractile properties and fatigue resistance of human skeletal muscle. Exp Physiol 93, 843–850. [DOI] [PubMed] [Google Scholar]
- Yaksh TL & Noueihed R (1985). The physiology and pharmacology of spinal opiates. Annu Rev Pharmacol Toxicol 25, 433–462. [DOI] [PubMed] [Google Scholar]
- Yaksh TL & Reddy SV (1981). Studies in the primate on the analgetic effects associated with intrathecal actions of opiates, alpha‐adrenergic agonists and baclofen. Anesthesiology 54, 451–467. [DOI] [PubMed] [Google Scholar]
- Zhang J, Light AR, Hughen RW, Huang H, Tang W, Jensen JC, Low SA & Scherrer G (2016). Mu and delta opioid receptors on muscle innervating group III/IV afferent neurons modulate muscle fatigue and ache. Neuroscience 2016 Abstracts. Society for Neuroscience, Washington, DC.


