Abstract
Key points
Contraction of urethral smooth muscle cells (USMCs) contributes to urinary continence. Ca2+ signalling in USMCs was investigated in intact urethral muscles using a genetically encoded Ca2+ sensor, GCaMP3, expressed selectively in USMCs.
USMCs were spontaneously active in situ, firing intracellular Ca2+ waves that were asynchronous at different sites within cells and between adjacent cells.
Spontaneous Ca2+ waves in USMCs were myogenic but enhanced by adrenergic or purinergic agonists and decreased by nitric oxide.
Ca2+ waves arose from inositol trisphosphate type 1 receptors and ryanodine receptors, and Ca2+ influx by store‐operated calcium entry was required to maintain Ca2+ release events.
Ca2+ release and development of Ca2+ waves appear to be the primary source of Ca2+ for excitation–contraction coupling in the mouse urethra, and no evidence was found that voltage‐dependent Ca2+ entry via L‐type or T‐type channels was required for responses to α adrenergic responses.
Abstract
Urethral smooth muscle cells (USMCs) generate myogenic tone and contribute to urinary continence. Currently, little is known about Ca2+ signalling in USMCs in situ, and therefore little is known about the source(s) of Ca2+ required for excitation–contraction coupling. We characterized Ca2+ signalling in USMCs within intact urethral muscles using a genetically encoded Ca2+ sensor, GCaMP3, expressed selectively in USMCs. USMCs fired spontaneous intracellular Ca2+ waves that did not propagate cell‐to‐cell across muscle bundles. Ca2+ waves increased dramatically in response to the α1 adrenoceptor agonist phenylephrine (10 μm) and to ATP (10 μm). Ca2+ waves were inhibited by the nitric oxide donor DEA NONOate (10 μm). Ca2+ influx and release from sarcoplasmic reticulum stores contributed to Ca2+ waves, as Ca2+ free bathing solution and blocking the sarcoplasmic Ca2+‐ATPase abolished activity. Intracellular Ca2+ release involved cooperation between ryanadine receptors and inositol trisphosphate receptors, as tetracaine and ryanodine (100 μm) and xestospongin C (1 μm) reduced Ca2+ waves. Ca2+ waves were insensitive to L‐type Ca2+ channel modulators nifedipine (1 μm), nicardipine (1 μm), isradipine (1 μm) and FPL 64176 (1 μm), and were unaffected by the T‐type Ca2+ channel antagonists NNC‐550396 (1 μm) and TTA‐A2 (1 μm). Ca2+ waves were reduced by the store operated Ca2+ entry blocker SKF 96365 (10 μm) and by an Orai antagonist, GSK‐7975A (1 μm). The latter also reduced urethral contractions induced by phenylephrine, suggesting that Orai can function effectively as a receptor‐operated channel. In conclusion, Ca2+ waves in mouse USMCs are a source of Ca2+ for excitation–contraction coupling in urethral muscles.
Keywords: Ca2+ imaging, urinary continence, lower urinary tract, optogenetics, store‐operated Ca2+ entry
Key points
Contraction of urethral smooth muscle cells (USMCs) contributes to urinary continence. Ca2+ signalling in USMCs was investigated in intact urethral muscles using a genetically encoded Ca2+ sensor, GCaMP3, expressed selectively in USMCs.
USMCs were spontaneously active in situ, firing intracellular Ca2+ waves that were asynchronous at different sites within cells and between adjacent cells.
Spontaneous Ca2+ waves in USMCs were myogenic but enhanced by adrenergic or purinergic agonists and decreased by nitric oxide.
Ca2+ waves arose from inositol trisphosphate type 1 receptors and ryanodine receptors, and Ca2+ influx by store‐operated calcium entry was required to maintain Ca2+ release events.
Ca2+ release and development of Ca2+ waves appear to be the primary source of Ca2+ for excitation–contraction coupling in the mouse urethra, and no evidence was found that voltage‐dependent Ca2+ entry via L‐type or T‐type channels was required for responses to α adrenergic responses.
Introduction
In mammals there is a reciprocal relationship between the contractile state of the bladder and urethra. The bladder suppresses muscle excitability as it fills with urine to accommodate the increase in volume, and during this period the urethra remains contracted to prevent leakage of urine and to maintain urinary continence (Brading, 1999). At the onset of micturition, the urethra relaxes and the bladder contracts to expel the stored urine (Brading, 1999; Hill, 2015).
A major factor closing the urethra during bladder filling is the summed contractions of urethral smooth muscle cells (USMCs), generating tone in a variety of species (Thornbury et al. 1992; Bridgewater et al. 1993; Bradley et al. 2004). Despite abundant evidence that USMCs, which make up the internal urethral sphincter, contribute to urethral closure in mammals (Tanagho & Smith, 1966; Donker et al. 1972; Sogbein et al. 1984; Conte et al. 1991; Bridgewater et al. 1993; Greenland et al. 1996; Janokowski et al. 2006) and the need for new pharmacological targets for the internal urethral sphincter to treat incontinence disorders (Lemack et al. 2007; Delancey et al. 2008; DeLancey, 2010; Staskin et al. 2011; Kedia et al. 2013; Alexandre et al. 2016; Kirschner‐Hermanns et al. 2016; Hokanson et al. 2017), there have been few studies on the control of USMC contractions performed on cells in situ. Isolated USMCs generate current responses and Ca2+ signals to depolarization or agonists, but these isolated cells are rarely rhythmically active (Sergeant et al. 2000; Hollywood et al. 2003; Bradley et al. 2004; Brading, 2006; Kyle, 2014; Drumm et al. 2014c).
In contrast to isolated USMCs, intact urethral tissues display spontaneous electrical activity. Studies on rabbits (Hashitani et al. 1996; Bradley et al. 2004) and guinea‐pigs (Hashitani & Edwards, 1999) showed that intact urethral muscles exhibit spontaneous electrical activity in the form of small amplitude spontaneous transient depolarizations and large amplitude slow waves. Imaging of rabbit urethral smooth muscles (USMs) has confirmed the spontaneous activity of these muscles. Studies by Hashitani et al. (2006) and Hashitani & Suzuki (2007) noted spontaneous Ca2+ oscillations in USMCs in situ, but the characteristics of these Ca2+ signals and their intracellular and extracellular sources were not examined in detail.
While the spontaneous Ca2+ activity of urethral interstitial cells has been examined (Drumm et al. 2014a), the nature of Ca2+ signalling in USMCs is mostly unknown. However, due to the importance of USMCs in generating urethral closure and the lack of effective, non‐surgical treatments for incontinence disorders (Miller et al. 1998; Thaker & Sharma, 2013), there is a need for a better understanding of the mechanisms regulating the contractile activity of these cells and to develop new targets for treatments of incontinence. In this study, we investigated Ca2+ signalling in the mouse urethra using Ca2+ imaging techniques and mice with USMC‐specific expression of the genetically encoded Ca2+ sensor GCaMP3. Imaging with optogenetic approaches provides important benefits over the use of membrane‐permeable Ca2+ dyes (Hashitani et al. 2006; Hashitani & Suzuki, 2007) including reduced photobleaching, use of high power magnification to record the details of subcellular Ca2+ signals, improved signal‐to‐noise characteristics, and loss of contaminating signals from cells other than the cells of interest in complex tissues. In contrast to isolated cells, USMCs in intact muscles were spontaneously active and exhibited intracellular Ca2+ waves. We examined how these spontaneous Ca2+ signals were modulated by neurotransmitters that affect urethral contraction and explored the intracellular and extracellular Ca2+ sources responsible for their generation in situ.
Methods
Ethical approval
All animals used and the protocols carried out in this study were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All procedures were approved by the Institutional Animal Use and Care Committee at the University of Nevada, Reno. We confirm that our experiments conform to the principles and regulations as described by Grundy (2015).
Animals
B6;129S‐Gt(ROSA)26Sortm38(CAG‐GCaMP3)Hze/J (GCaMP3 mice) and male wild type C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Male mice expressing an inducible Cre Recombinase driven by the smooth muscle heavy chain promoter (SmMHC‐Cre mice) were generated by Dr Stefan Offermann (Max‐ Planck‐ Institute, Germany) and gifted to our lab from Dr James Stull of Southwestern University (TX, USA). SmMHC/Cre/eGFP (SMC‐eGFP), mice expressing a fluorescent eGFP molecule in cells expressing smooth muscle heavy chain mice were donated by Dr Michael Kotlikoff of Cornell University (NY, USA).
Tamoxifen preparation and administration
GCaMP3 mice were crossbred with SmMHC‐Cre mice and we refer to the resultant offspring mice as SmMHC‐Cre‐GCaMP3 mice throughout the manuscript. The mice were injected with tamoxifen at ages 6–8 weeks to induce Cre Recombinase and subsequent GCaMP3 expression in USMCs. Tamoxifen (Sigma, St. Louis, MO, USA, T5648; 80 mg) was dissolved in 800 μl of ethanol (Pharmco‐Aaper, 200 proof, absolute, anhydrous) by vortexing for 20 min. Then, 3.2 ml of safflower (generic) was added to create solutions of 20 mg ml−1, which were sonicated for 30 min prior to injection. Mice were injected i.p. with 0.1 ml of tamoxifen solution (2 mg tamoxifen) for three consecutive days. Mice were used 10 days after the first injection and the subsequent expression of GCaMP3 was confirmed by genotyping.
Tissue preparation
Mice were anaesthetized by inhalation of isoflurane (Baxter, Deerfield, IL, USA) and then killed by cervical dislocation before being used for experimentation. The abdomen of the mice was opened, and the bladder and proximal urethra were removed and placed in Krebs–Ringer–bicarbonate solution (KRB). The urethra was opened along a longitudinal incision and the urothelium was carefully removed by sharp dissection.
Calcium imaging
The muscular coat of the urethra was pinned to the base of a 5 ml volume, 60 mm diameter Sylgard‐coated dish. The urethra preparation was continuously perfused with warmed KRB solution at 37°C for an equilibration period of 1 h before experiments were initiated. Following the equilibration period, Ca2+ imaging of circularly orientated USMCs was performed with an Eclipse E600FN microscope (Nikon Inc., Melville, NY, USA) equipped with a 60× 1.0 CFI Fluor lens (Nikon). GCaMP3 was excited at 488 nm. Fluorescence was captured using a FITC HQ series fluorescence filter cube (Nikon). This cube had a 510 nm dichroic with a barrier filter of 535 nm, as previously described (Drumm et al. 2017). The pixel size using this acquisition configuration was 0.225 μm. Image sequences were collected at 33 frames s−1 using a high‐speed EMCCD Camera (Andor iXon X3; ANDOR Technology) and TILLvisION software (T.I.L.L. Photonics GmbH, Grafelfing, Germany). For pharmacological tests, a control period of 30 s was recorded and then KRB containing the appropriate concentration of drug was perfused into the bath for 12–15 min to allow full tissue penetration before another 30 s recording was acquired to ascertain the effects of the drug.
Analysis of Ca2+ transients
Analysis of Ca2+ transients in urethral cells was performed as previously described (Drumm et al. 2014b; Baker et al. 2016). Movies of in situ USMC Ca2+ activity were converted to a stack of TIFF (tagged image file format) images and imported into ImageJ (version1.40, National Institutes of Health, MD, USA, http://rsbweb.nih.gov/ij). Spatio‐temporal (ST) maps of Ca2+ activity in USMCs were generated by drawing a single pixel line along the mid axis of the cell and invoking the ‘reslice’ function in ImageJ; this generated an ST map which was then calibrated for distance and time. The basal Ca2+ fluorescence was acquired from regions of the cell that displayed the most uniform and least intense fluorescence (F 0). The fluorescence value throughout the cell was then divided by the calculated F 0 value to calibrate the ST map for the amplitude of Ca2+ events as F/F 0. Ca2+ transient frequency in USMCs was expressed as the number of events fired per cell per minute (min−1). The amplitude of Ca2+ transients was expressed as ΔF/F 0, the duration of Ca2+ transients was expressed as full duration at half‐maximum amplitude (FDHM) and the spatial spread of Ca2+ transients was expressed as micrometres of cell propagated per Ca2+ transient. Ca2+ wave velocity was calculated by drawing a line along a propagating wave front on an ST map and invoking the ‘Wave Speed’ plugin in ImageJ to produce read‐outs of Ca2+ wave velocity. In experiments where drugs were applied to USMCs, quantification of Ca2+ transient parameters in control and in the presence of a drug would be analysed in three to five representative cells in a field of view (FOV) per animal used in experimentation. A series of time control experiments was performed in which a control period of 20 s of USMC Ca2+ activity was recorded and 15 min later the same FOV was recorded in the absence of any changes in experimental conditions or pharmacological agents for another 20 s. No significant differences were observed in the frequency (P = 0.51), amplitude (P = 0.99), duration (P = 0.96) or spatial spread (P = 0.54) of USMC Ca2+ waves recorded from the same cells in consecutive recordings (data not shown, no. of cells (c) = 12, no. of animals (n) = 5).
Cell isolation and fluorescence‐activated cell sorting (FACS)
USMCs were isolated from SMC‐eGFP+ mice for analysis of gene transcripts. Urethral tissues were obtained as described above in the ‘Tissue preparation’ section. The urothelium was removed and then the remaining muscle segments were cut into fine pieces and incubated in dispersion medium containing per millilitre of Ca2+‐free KRB solution (see ‘Drugs and solutions’ below): 3 mg collagenase (Sigma type 1A), 0.2 mg protease (Sigma type XXIV), 2 mg bovine serum albumin (Sigma) and 2 mg trypsin inhibitor (Sigma) for 10–15 min at 37°C. Tissues were then washed in Ca2+‐free KRB for 10 min to obtain isolated USMCs. USMCs were sorted by FACS (FACSAria II; Becton‐Dickinson) with an excitation laser (488 nm) and emission filter (530/30 nm). USMC sorting was carried out with a 130 μm nozzle at a sheath pressure of 12 p.s.i. and sort rate of 1000–3000 events s−1. Live cells, gated on exclusion of Hoechst 33258 viability indicator (data not shown), were subsequently gated on GFP fluorescence intensity.
RNA extraction and quantitative PCR
Total RNA was isolated from unsorted urethral cells and sorted (purified) USMCs using an illustra RNAspin Mini RNA Isolation Kit (GE Healthcare), and First‐Strand cDNA was synthesized using SuperScript III (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions. The PCR primers used and their GenBank accession numbers are listed in Table 1. PCR was performed using GoTaq DNA Polymerase (Promega, Madison, WI, USA) and PCR products were analysed on 2% agarose gels and visualized by ethidium bromide. Quantitative PCR (qPCR) was performed with the same primers as PCR using SYBR green chemistry on the 7500 HT Real‐time PCR System (Applied Biosystems) and analysed as described previously (Baker et al. 2016; Drumm et al. 2017). Gene expression levels are described in relation to the expression of the housekeeping gene Gapdh ± standard deviation.
Table 1.
Gene primers used in qPCR analysis
| Gene name | Sequence (sense primer on top) | GenBank accession no. |
|---|---|---|
| Gapdh |
|
NM_008084 |
| Pdgfra |
|
NM_011058 |
| Kit |
|
NM_021099 |
| Myh11 |
|
NM_013607 |
| Uchl1 |
|
NM_011670 |
| Trpc1 |
|
NM_011643 |
| Trpc2 |
|
NM_011644 |
| Trpc3 |
|
NM_019510 |
| Trpc4 |
|
NM_016984 |
| Trpc5 |
|
NM_009428 |
| Trpc6 |
|
NM_013838 |
| Tpsab1 |
|
NM_031187 |
| Chrm1 |
|
NM_001112697 |
| Chrm2 |
|
NM_203491 |
| Chrm3 |
|
NM_033269 |
| Chrm4 |
|
NM_007699 |
| Chrm5 |
|
NM_205783 |
| Adra1a |
|
NM_013461 |
| Adra1b |
|
NM_007416 |
| Adra1d |
|
NM_013460 |
| Npr1 |
|
NM_008727 |
| Npr2 |
|
NM_173788 |
| Prkg1a |
|
NM_001013833 |
| Prkg1b |
|
NM_011160 |
| P2ry1 |
|
NM_008772 |
| P2ry2 |
|
NM_008773 |
| P2ry6 |
|
NM_183168 |
| P2ry12 |
|
NM_027571 |
| P2ry13 |
|
NM_028808 |
| P2ry14 |
|
NM_133200 |
| P2rx1 |
|
NM_008771 |
| P2rx2 |
|
NM_153400 |
| P2rx3 |
|
NM_145526 |
| P2rx4 |
|
NM_011026 |
| P2rx5 |
|
NM_033321 |
| P2rx6 |
|
NM_011028 |
| Itpr1 |
|
NM_010585 |
| Itpr2 |
|
NM_019923 |
| Itpr3 |
|
NM_080553 |
| Ryr1 |
|
NM_009109 |
| Ryr2 |
|
NM_023868 |
| Ryr3 |
|
NM_177652 |
| Orai1 |
|
NM_175423 |
| Orai2 |
|
NM_178751 |
| Orai3 |
|
NM_198424 |
Contraction experiments
Ring preparations of mouse urethra (urothelium intact) were attached with tied sutures to a Gould strain gauge and a stable mount and immersed in tissue baths containing 15 ml of oxygenated KRB solution maintained at 37°C. Urethra preparations were initially stretched to 0.25 g of tension followed by a 60 min equilibration period. Viability of tissues was evaluated by their ability to generate reproducible contractile responses to a bolus application of phenylephrine (PE). Contractile data were recorded using AcqKnowledge software and urethral contractions were normalized as a percentage of a bolus PE contraction.
Drugs and solutions
Tissues were perfused with KRB containing (mmol l−1): NaCl, 120.35; KCl, 5.9; NaHCO3, 15.5; NaH2PO4, 1.2; MgCl2, 1.2; CaCl2, 2.5; and glucose, 11.5. KRB was bubbled with a mixture of 97% O2–3% CO2 and warmed to 37 ± 0.2°C. For experiments with 0 mm [Ca2+]o, no CaCl2 was added to the KRB and 0.5 mm EGTA was added. Note that in cell isolation protocols, EGTA was not added to Ca2+ free solutions for cell dispersal. ATP, caffeine, carbachol (CCh), Diethylamine NONOate (DEA NONOate), tetracaine, phenylephrine, cyclopiazonic acid (CPA), nifedipine and nicardipine; Sigma‐Aldrich (St Louis, MO, USA). FPL 64176, isradipine, ryanodine, tetrodotoxin (TTX), thapsigargin and SKF 96365; Tocris Bioscience (Ellisville, MO, USA). NNC‐550396 and TTA‐A2; Alomone Labs (Jerusalem, Israel). GSK‐7975A; Aobious (Gloucester, MA, USA). Xestospongin C; Cayman Chemical (Ann Arbor, MI, USA). All drugs were dissolved in the manufacturer‐recommended solvent and then diluted into KRB solution for use in experiments.
Statistics
Unless otherwise stated in the text, data are represented as mean ± standard error of the mean (SEM). Statistical analyses were performed using Student's t tests or an ANOVA with a multiple comparison Tukey post hoc test as appropriate. In all statistical analyses, P < 0.05 was taken as significant. When describing data, n refers to the number of animals used in that dataset while c refers to the number of cells analysed in that same data set.
Results
Nature of USMC Ca2+ activity
In situ Ca2+ imaging of the urethras of SmMHC‐Cre‐GCaMP3 mice revealed that USMCs within smooth muscle bundles fired spontaneous Ca2+ events that manifested as intracellular Ca2+ waves that arose from discrete initiation sites (Fig. 1 A–C) and propagated in either a uni‐ or bi‐directional fashion (Fig. 1 D–F). Ca2+ waves in USMCs were almost exclusively intracellular, with little to no intercellular propagation within USMC bundles (n = 31). Analysis of USMC intracellular Ca2+ waves was performed by spatio‐temporal (ST) mapping of Ca2+ activity as described in the Methods. An ST map of the Ca2+ waves from the cell highlighted in Fig. 1 is shown in Fig. 2 A, with the corresponding fluorescence trace and 3‐D plots from the ST map in Fig. 2 B–D. Using this analysis (Drumm et al. 2015; Baker et al. 2016) we tabulated USMC Ca2+ waves in terms of their firing frequency, amplitude, duration, spatial spread, velocity and number of sites of initiation for Ca2+ events (i.e. firing sites) per USMC. Across 301 cells from 31 SmMHC‐Cre‐GCaMP3 mice, 5500 Ca2+ events were tabulated, and these data were plotted as frequency histograms in Fig. 2 E (and see also Table 2). In many instances Ca2+ wave velocity could not be accurately measured due to the lack of a uniform, uni‐directional propagating wave front. Thus, the histogram in Fig. 2 Ee represents Ca2+ wave velocities of 726 Ca2+ waves.
Figure 1. Spontaneous intracellular Ca2+ waves in USMCs.
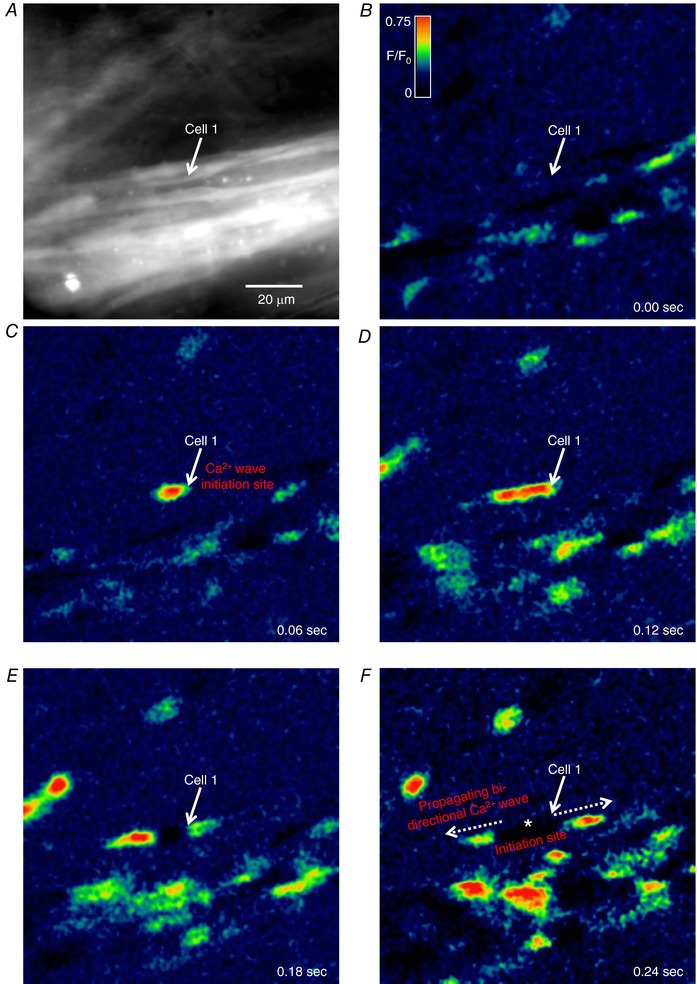
A, representative image of a USMC bundle imaged from a SmMHC‐Cre‐GCaMP3 mouse urethra with a 60× objective. The white arrow highlights a cell of interest. B–F, time‐lapse montage of spontaneous intracellular Ca2+ activity in USMCs in situ with a bi‐directional Ca2+ wave in the highlighted cell emphasized throughout. The initiation site of this Ca2+ wave is indicated in F by the white asterisks. Panels are colour coded as F/F 0 as shown in B.
Figure 2. Characterization of USMC intracellular Ca2+ events.

A, representative spatio‐temporal (ST) map of spontaneous intracellular Ca2+ waves recorded in situ. The ST map is calibrated for amplitude as F/F 0 and colour coded accordingly. B, x–y plot of the Ca2+ activity represented in A. C, 3‐D plot representation of the ST map shown in A. D, 3‐D plot profile of 5 representative Ca2+ waves from the ST map in A. E, summary histograms showing the distribution of values for USMC Ca2+ event frequency (a), amplitude (b), duration (c), spatial spread (d), velocity (e) and number of firing sites (f) (c = 301, n = 31).
Table 2.
Quantification of USMC Ca2+ wave parameters
| Ca2+ wave parameter | Min | Max | Mean | Median | SEM | SD |
|---|---|---|---|---|---|---|
| Frequency (min−1) | 2 | 224 | 51 | 36 | 2.3 | 39.6 |
| Amplitude Δ(F/F 0) | 0.01 | 1.4 | 0.2 | 0.15 | 0.002 | 0.1 |
| FDHM (ms) | 60 | 930 | 226 | 210 | 0.9 | 67.7 |
| Spatial spread (μm) | 0.4 | 117.3 | 25.2 | 18.9 | 0.3 | 19.6 |
| Velocity (μm s−1) | 31 | 601.3 | 108.5 | 88.8 | 2.4 | 65 |
| No. of firing sites per USMC | 1 | 8 | 3.7 | 3 | 0.1 | 1.7 |
As illustrated by the data in Fig. 2 Ef, USMCs often contained multiple firing sites, ranging from a single site to as many as eight (mean 3.7 ± 0.1; Fig. 2 Ef, c = 301, n = 31). Figure 3 A shows an ST map of a USMC firing Ca2+ transients from multiple sites of origin, four of which are indicated by coloured arrows to the left of the ST map and the activity that occurred in these regions is plotted as colour‐coded traces in Fig. 3 B. These traces illustrate that while Ca2+ waves initiated at one firing site can propagate to another region of initiation, there was no apparent relationship between the timing of Ca2+ transient firing at different sites. Instead, Ca2+ transients in USMCs appeared to fire in a stochastic manner with little to no temporal coordination.
Figure 3. Asynchronous firing of USMC intracellular Ca2+ events.

A, representative ST map of USMC Ca2+ activity showing multiple Ca2+ firing sites along the length of the cell, 4 of which are indicated by the black, blue, red and green arrows. B, colour coded plot profiles of the Ca2+ activity at each of the corresponding colour coded Ca2+ firing sites indicated in A. C, image from a FOV of USMCs in situ recorded with a 60× objective showing 2 adjacent cells illustrated by the green and red ROIs. The activity of these adjacent cells is plotted below the image. D, ST maps of cell 1 and cell 2 represented in C which have been uniformly coloured to show all Ca2+ activity in the cell as either red (cell 1) or green (cell 2). These ST maps are then merged at the bottom of the panel.
The stochastic pattern of firing was also apparent between adjacent cells. Figure 3 C shows a FOV with two adjacent highlighted USMCs (as indicated by the green and red regions of interest (ROIs)). The activity of cells 1 and 2 over 30 s is plotted as fluorescence traces below the FOV and shows that there is little or no correlation between Ca2+ transients in the two cells. This was further examined by comparing ST maps from the two cells. Figure 3 D shows ST maps from the highlighted cells where all Ca2+ signals were thresholded to be a uniform red (cell 1) or green colour (cell 2). When these two ST maps were merged, it was apparent that there was little overlap, and using the same analysis in cells from five different animals there was only 14 ± 1.2% overlap of Ca2+ signals in adjacent USMCs. These data indicate that the intracellular activity of one USMC was largely independent of the activity occurring in adjacent USMCs. This asynchronous activity was also observed in relation to urethral contractions. As shown in Movie S1 in the online Supporting information, the firing of intracellular Ca2+ waves in USMCs was associated with small contractions (60× objectives were required to resolve these movements) of individual USMCs in muscle bundles. These contractions, like the intracellular Ca2+ waves occurring asynchronously among cells, did not spread cell‐to‐cell across the muscle bundles. These observations suggest that cellular contractions, occurring independently and asynchronously in many cells within bundles and across many bundles within the tissue, summate to generate tonic contractions of the urethra.
Neuronal control of USMC Ca2+ activity
We next sought to evaluate whether spontaneous Ca2+ waves observed in USMCs are modulated by neural inputs. As shown in Fig. 4 A, tetrodotoxin (TTX; 1 μm) did not alter Ca2+ events in USMCs. TTX did not affect frequency (Fig. 4 Ba, P = 0.76, c = 18, n = 6), amplitude (Fig. 4 Bb, P = 0.06, c = 18, n = 6), duration (Fig. 4 Bc, P = 0.63, c = 18, n = 6), or spatial spread (Fig. 4 Bd, P = 0.36, c = 18, n = 6) of USMC Ca2+ waves, suggesting that the basal level Ca2+ events are myogenic in origin and are not under either basal inhibitory or excitatory neural regulation in urethral muscles in situ.
Figure 4. The effects of TTX and carbachol on USMC Ca2+ transients.
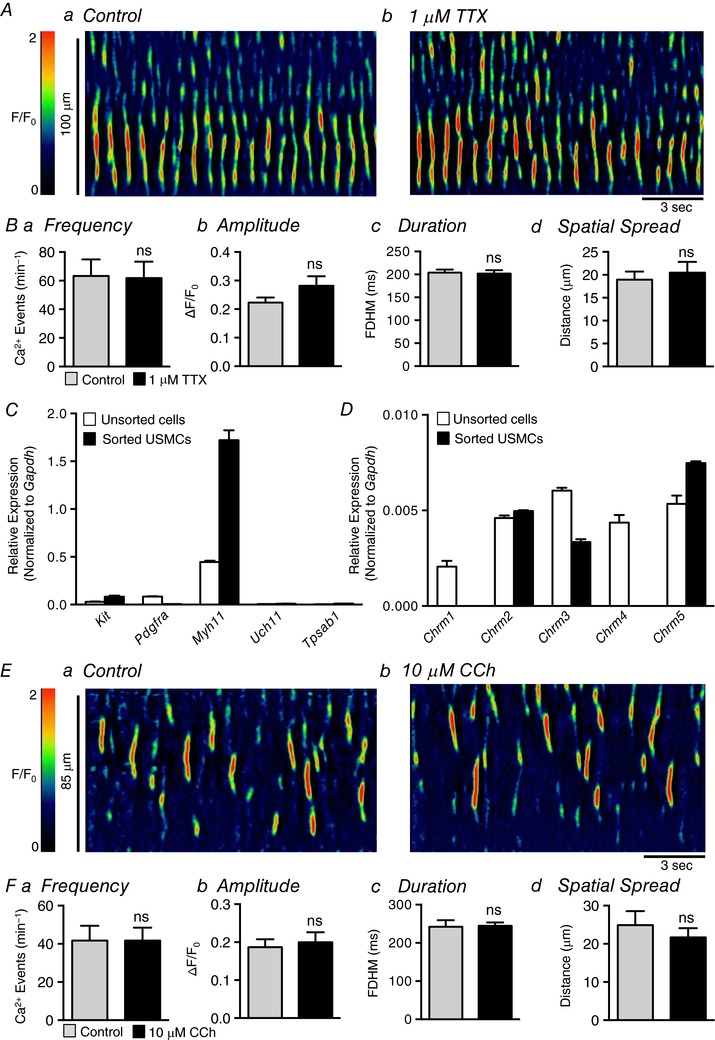
Aa and b, representative ST maps of Ca2+ transient firing within a single USMC recorded in situ during control conditions (a) and after incubation with 1 μm TTX (b). Ba–d, summary data showing the effect of 1 μm TTX on USMC Ca2+ transient frequency (a), amplitude (b), duration (c) and spatial spread (d) (c = 18, n = 6). C, relative expression of cellular identification genes in FACS sorted and unsorted urethral cells from SMC‐eGFP mice determined by qPCR analysis, normalized to Gapdh expression (n = 4). Genes examined are Kit (tyrosine kinase receptor, found in interstitial cells of Cajal), Pdgfra found in PDGFRα+ interstitial cells, Myh11 (smooth muscle myosin), Uch11 (neural marker encoding PGP 9.5) and Tpsab1 (mast cell tryptase). D, relative expression of muscarinic receptor genes in FACS sorted and unsorted urethral cells from SMC‐eGFP mice determined by qPCR analysis, normalized to Gapdh expression (n = 4). Ea and b, representative ST maps of Ca2+ transient firing within a single USMC recorded in situ during control conditions (a) and after incubation with 10 μm CCh (b). Fa–d, summary data showing the effect of 10 μm CCh on USMC Ca2+ transient frequency (a), amplitude (b), duration (c) and spatial spread (d) (c = 13, n = 4). ns, not significant.
We next tested the effects of neurotransmitter agonists on urethral Ca2+ waves. These experiments were performed in conjunction with PCR experiments to detect relative expression of genes encoding receptors and molecules pertinent to USM neurotransmission. Individual USMCs were isolated from SMC‐eGFP+ mice and purified using FACS, as described in the Methods. The sorted cells were evaluated for purity by quantifying the expression of genes encoding cell‐specific markers, such as Kit (interstitial cells of Cajal found in the urethra; McHale et al. 2006), Pdgfra encoding platelet‐derived growth factor receptor α (marker of fibroblast‐like cells in visceral smooth muscles, Kurahashi et al. 2011; Koh et al. 2012; Peri et al. 2015), Myh11 (myosin heavy chain; smooth muscle cells), Uch11 encoding PGP 9.5 (nerve cells) and Tpsab1 (mast cell tryptase). Enriched USMCs isolated from SMC‐eGFP+ mice showed minimal expression of these genes with the exception of the smooth muscle marker Myh11 (Fig. 4 C), and the expression of this gene was enriched in the sorted USMCs, as compared to unsorted cells (1.72 ± 0.1 in sorted USMCs (normalized to Gapdh expression) vs. 0.4 ± 0.01 in unsorted cells, n = 4). Thus, FACS provided a means of determining which neurotransmitter receptor subtypes are specific to USMCs. qPCR showed that USMCs expressed genes encoding muscarinic receptor subtypes for Chrm2 (M2), Chrm3 (M3) and Chrm5 (M5) (Fig. 4 D). However, in spite of muscarinic receptor expression, effects of exogenous carbachol (CCh; 10 μm; Fig. 4 E) on USMC Ca2+ wave frequency (Fig. 4 Fa, P > 0.99, c = 13, n = 4), amplitude (Fig. 4 Fb, P = 0.39, c = 13, n = 4), duration (Fig. 4 Fc, P = 0.91, c = 13, n = 4), or spatial spread (Fig. 4 Fd, P = 0.36, c = 13, n = 4) were not resolved.
The role of the sympathetic, adrenergic pathway in modulating USMC Ca2+ waves was also characterized. This is reported to be the primary excitatory neural pathway mediating contractions of USMs, and sympathetic effects have been reported to be due to expression and binding of α1 adrenoceptors (Ek et al. 1977; Andersson et al. 1984; Ito & Kimoto, 1985; Kimoto et al. 1987; Mattiasson et al. 1989; Chen & Brading, 1992; Andersson, 2001; Kedia et al. 2013). Genes encoding all three α1 adrenoceptors were expressed in sorted USMCs (Fig. 5 A). The α1 adrenoceptor agonist phenylephrine (PE; 10 μm) caused dramatic increases in the firing of USMC Ca2+ waves (Fig. 5 B and C). PE nearly doubled the frequency of Ca2+ waves from 46.9 ± 5.7 events min−1 to 88.8 ± 9.6 events min−1 (Fig. 5 Da, P < 0.0001, c = 34, n = 10). PE also increased the duration of Ca2+ events from 225 ± 7.6 ms to 263 ± 11.5 (Fig. 5 Dc, P = 0.0005, c = 34, n = 10) and the spatial spread of Ca2+ events, increasing propagation distance from 21.7 ± 2 μm in control to 36.5 ± 4.9 μm (Fig. 5 Dd, P = 0.0025, c = 34, n = 10). However, PE did not affect Ca2+ wave amplitude significantly (Fig. 5 Db, P = 0.5, c = 34, n = 10). As shown in Movies S2 and S3 in the online Supporting information, PE did not synchronize firing of USMC Ca2+ waves or generate what might be referred to as a global rise in Ca2+. Instead, PE increased the intracellular activity of all the cells in the FOV by increasing the frequency, duration and spatial spread of intracellular Ca2+ waves, and the asynchronous nature of firing of Ca2+ waves was retained in all cells in the FOV.
Figure 5. Phenylephrine increases USMC Ca2+ transient firing.
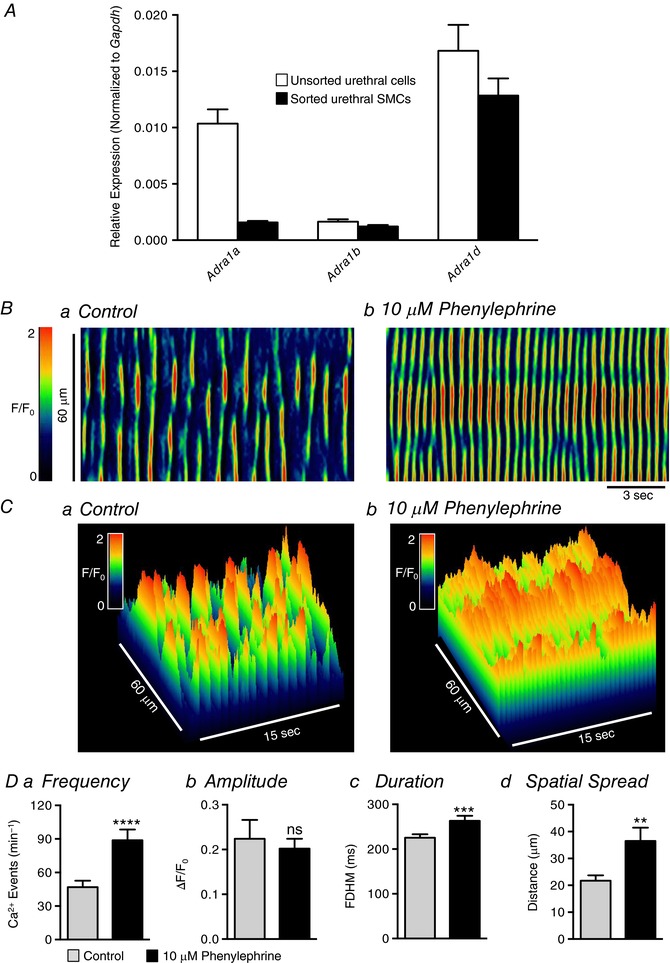
A, relative expression of adrenergic α1 receptor genes in FACS sorted and unsorted urethral cells from SMC‐eGFP mice determined by qPCR analysis, normalized to Gapdh expression (n = 4). Ba and b, representative ST maps of Ca2+ transient firing within a single USMC recorded in situ during control conditions (a) and after incubation with 10 μm phenylephrine (b). C, 3‐D plots of the ST maps shown in A. Da–d, summary data showing the effect of 10 μm phenylephrine on USMC Ca2+ transient frequency (a), amplitude (b), duration (c) and spatial spread (d) (c = 34, n = 10). ns, not significant, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
We also investigated inhibitory neural regulation of USMs, which has been reported to be mediated by the nitric oxide (NO)–guanylate cyclase (GC)–protein kinase G (PKG) pathway (Thornbury et al. 1992; Bridgewater et al. 1993; Dokita et al. 1994; Zygmunt et al. 1995; Waldeck et al. 1998; Costa et al. 2001; Kedia et al. 2013). USMCs expressed genes required for the NO–GC–PKG pathway (Fig. 6 A), and sorted USMCs showed enrichment in transcripts of genes encoding GCα (Npr1; 0.1 ± 0.02 in sorted USMCs compared to 0.01 ± 0.001 in unsorted cells) and GCβ (Npr2; 0.08 ± 0.01 in sorted USMCs compared to 0.04 ± 0.005 in unsorted cells). Application of the NO donor (DEA NONOate; 10 μm) nearly abolished Ca2+ transients in USMCs (Fig. 6 B and C), decreasing USMC firing frequency by 93% (from 55.4 ± 8.1 min−1 in control to 3.7 ± 1.8 min−1 in the presence of DEA NONOate; Fig. 6 Da, P < 0.0001, c = 19, n = 6). DEA NONOate decreased Ca2+ wave amplitude from 0.27 ± 0.06 ΔF/F 0 in control to 0.03 ± 0.01 ΔF/F 0 in DEA NONOate (Fig. 6 Db, P = 0.0012, c = 19, n = 6), Ca2+ event duration was decreased from 213 ± 11.5 ms in control to 58 ± 23.4 ms in the presence of DEA NONOate (Fig. 6 Dc, P < 0.0001, c = 19, n = 6), and the propagation spread of Ca2+ transients was decreased from 34.1 ± 3.9 μm to 5.3 ± 2.3 μm (Fig. 6 Dd, P < 0.0001, c = 19, n = 6).
Figure 6. Nitric oxide decreases USMC Ca2+ transient firing.
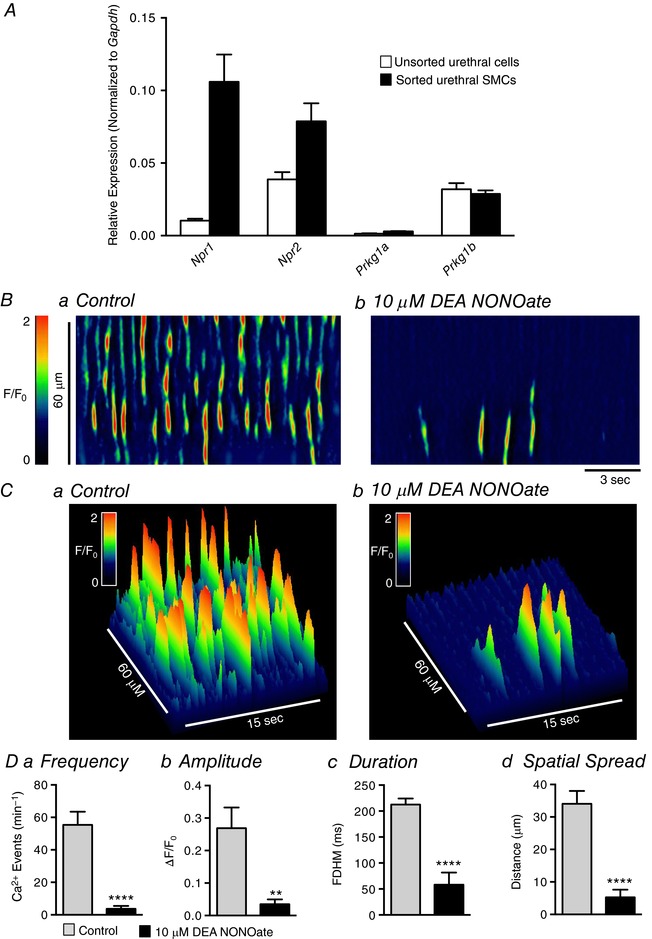
A, relative expression of guanylate cyclase and protein kinase G genes in FACS sorted and unsorted urethral cells from SMC‐eGFP mice determined by qPCR analysis, normalized to Gapdh expression (n = 4). Ba and b, representative ST maps of Ca2+ transient firing within a single USMC recorded in situ during control conditions (a) and after incubation with 10 μm DEA NONOate (b). C, 3‐D plots of the ST maps shown in A. Da–d, summary data showing the effect of 10 μm DEA NONOate on USMC Ca2+ transient frequency (a), amplitude (b), duration (c) and spatial spread (d) (c = 19, n = 6). ** p < 0.01, **** p < 0.0001.
We also explored the actions of purinergic substances on USMC Ca2+ waves. Sorted USMCs showed expression of P2X receptor genes P2rx1–4 and P2rx6 (Fig. 7 A). There was enriched expression in USMCs of P2rx1 (0.01 ± 0.002 sorted USMCs vs. 0.0015 ± 0.0002 unsorted cells), P2rx2 (0.003 ± 0.0004 sorted USMCs vs. 0.0009 ± 0.0004 unsorted cells) and P2rx6 (0.007 ± 0.001 sorted USMCs vs. 0.002 ± 0.0004 unsorted cells). The only P2Y receptor gene resolved in USMCs was P2ry14 (Fig. 7 B; 0.28 ± 0.05 sorted USMCs vs. 0.16 ± 0.018 unsorted cells), while unsorted cells showed expression of genes encoding P2ry1, 2, 6, 12, 13 and 14 (Fig. 7 B).
Figure 7. The effects of purines on USMC Ca2+ transients.
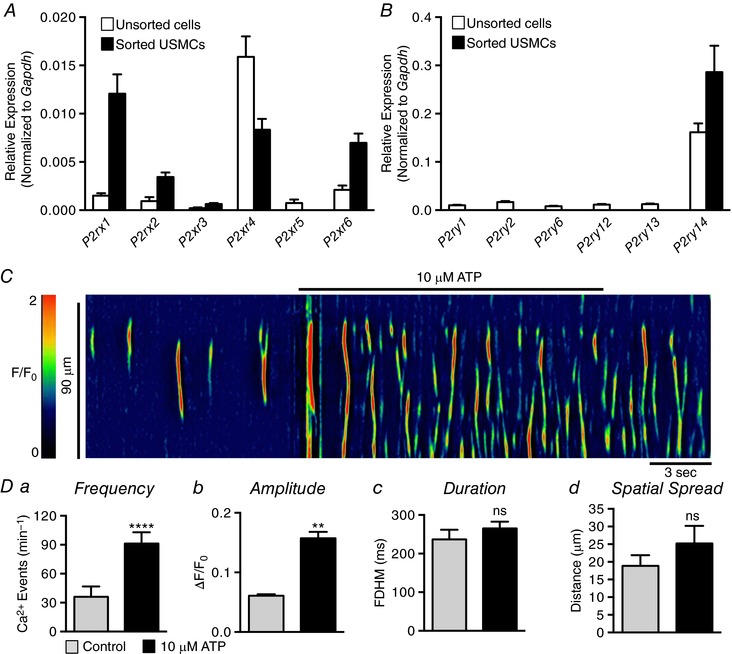
A, relative expression of P2X receptor genes in FACS sorted and unsorted urethral cells from SMC‐eGFP mice determined by qPCR analysis, normalized to Gapdh expression (n = 4). B, relative expression of P2Y receptor genes in FACS sorted and unsorted urethral cells from SMC‐eGFP mice determined by qPCR analysis, normalized to Gapdh expression (n = 4). C, representative ST map showing the effect of 10 μm ATP on Ca2+ transient firing in USMCs. Da–d, summary data showing the effect of 10 μm ATP on USMC Ca2+ transient frequency (a), amplitude (b), duration (c) and spatial spread (d) (c = 5, n = 3). ns, not significant, ** p < 0.01, **** p < 0.0001.
At resting tone, purines such as adenosine triphosphate (ATP) exert excitatory effects on urethral muscles (Callahan & Creed, 1981; Bradley et al. 2010), and we hypothesized, due to expression of several P2X receptor paralogues, that purines would increase Ca2+ events in USMCs similar to PE. We found that ATP exerted excitatory effects on USMC Ca2+ waves (Fig. 7 C). ATP significantly increased USMC Ca2+ wave frequency from 36 ± 10.4 min−1 to 91.2 ± 11.8 min−1 (Fig. 7 Da, P < 0.0001, c = 5, n = 3). ATP also increased the amplitude of Ca2+ waves from 0.06 ± 0.002 ΔF/F 0 to 0.16 ± 0.01 ΔF/F 0 (Fig. 7 Db, P = 0.0012, c = 5, n = 3); however, the duration and spatial spread of Ca2+ transients were not changed significantly by ATP (Fig. 7 Dc and d, P = 0.25, P = 0.24, c = 5, n = 3).
Intracellular sources of Ca2+ contributing to USMC Ca2+ waves
Ca2+ waves in USMCs rely on functional sarcoplasmic reticulum (SR) stores, as sarco(endo)plasmic reticulum Ca2+‐ATPase (SERCA) antagonists thapsigargin (10 μm; Fig. 8 A and B, c = 4, n = 3) or cyclopiazonic acid (CPA; 10 μm; Fig. 8 C and D, c = 15, n = 5) abolished Ca2+ waves in these cells. Ca2+ release from the SR involves the opening of SR Ca2+ channels, such as inositol trisphosphate receptors (IP3Rs), ryanodine receptors (RyRs) or a combination of the two (Berridge et al. 2000). Functional RyRs are tetrameric proteins of which there are three subtypes, RyR1, RyR2 and RyR3 (Amundson & Clapham, 1993), and there are also three IP3R subtypes, IP3R1, IP3R2 and IP3R3 (Berridge et al. 2000). Analysis of transcripts from sorted USMCs and unsorted urethral cells by qPCR showed that the dominant gene encoding SR Ca2+ release channels in urethral cells was Itpr1 (encodes IP3R1), but expression of Itpr2 was also observed (Fig. 8 E). USMCs expressed all three gene paralogues encoding RyRs (Ryr1–3; Fig. 8 F). The data also showed enrichment of Itpr1, Ryr2 and Ryr3 in USMCs in comparison to the unsorted cell population.
Figure 8. Ca2+ transients in USMCs rely on functional SR stores.
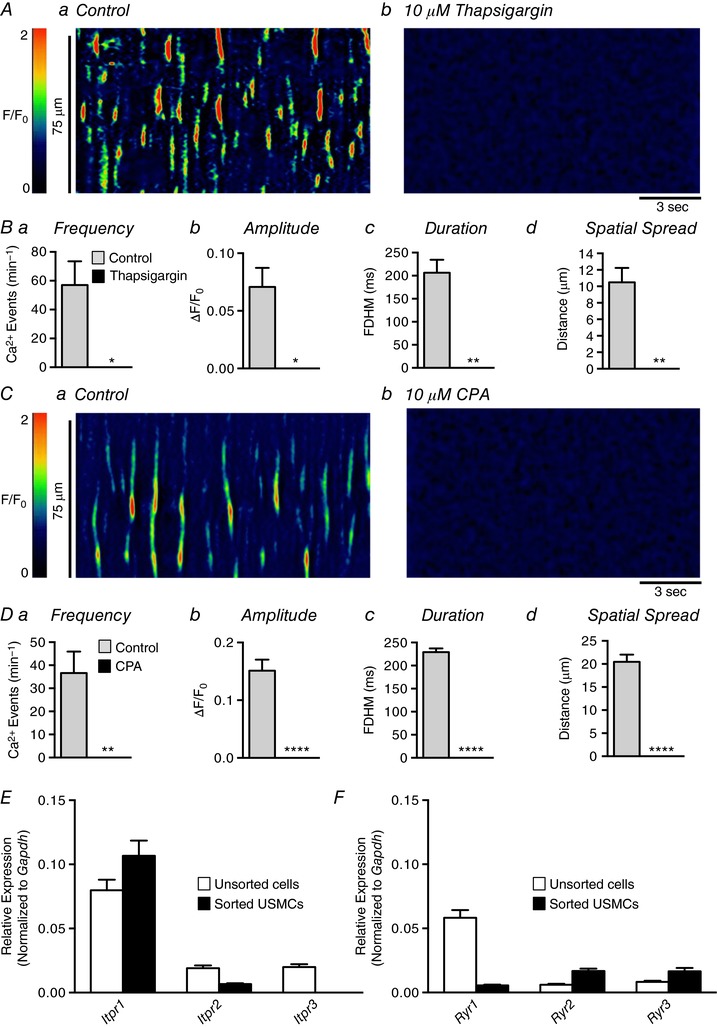
Aa and b, representative ST maps showing the effect of 10 μm thapsigargin on Ca2+ transient firing in USMCs. Ba–d, summary data showing the effect of 10 μm thapsigargin on USMC Ca2+ transient frequency (a), amplitude (b), duration (c) and spatial spread (d) (c = 4, n = 3). Ca and b, representative ST maps showing the effect of 10 μm CPA on Ca2+ transient firing in USMCs. Da–d, summary data showing the effect of 10 μm CPA on USMC Ca2+ transient frequency (a), amplitude (b), duration (c) and spatial spread (d) (c = 13, n = 5). E, relative expression of IP3R genes in FACS sorted and unsorted urethral cells from SMC‐eGFP mice determined by qPCR analysis, normalized to Gapdh expression (n = 4). F, relative expression of RyR genes in FACS sorted and unsorted urethral cells from SMC‐eGFP mice determined by qPCR analysis, normalized to Gapdh expression (n = 4). * p < 0.05, ** p < 0.01, **** p < 0.0001.
Figure 9 A shows that blocking IP3Rs with 100 μm 2‐aminoethyl‐diphenylborinate (2‐APB) reduced Ca2+ wave activity in USMCs and this was quantified in Fig. 9 B. 2‐APB reduced Ca2+ wave frequency from 45.2 ± 8.4 min−1 in control to 2.9 ± 1.3 min−1 in the presence of the IP3R antagonist (Fig. 9 Ba, P < 0.0001, c = 19, n = 6). The amplitude of Ca2+ waves was reduced by 2‐APB from 0.16 ± 0.03 ΔF/F 0 to 0.04 ± 0.015 ΔF/F 0 (Fig. 9 Bb, P = 0.003, c = 19, n = 6). Ca2+ wave duration was reduced from 230 ± 6.2 ms to 66.1 ± 23.5 ms (Fig. 9 Bc, P < 0.0001, c = 19, n = 6) and the spatial spread of Ca2+ waves was reduced from 18.75 ± 1.8 μm to 7 ± 2.8 μm (Fig. 9 Bd, P = 0.0008, c = 19, n = 6). However, it should be noted that non‐specific effects on Ca2+ handling other than blocking IP3Rs have been attributed to 2‐APB (Peppiatt et al. 2003), and therefore we also tested another IP3R antagonist. Xestospongin C (XeC; 1 μm) reduced Ca2+ waves (Fig. 9 Ca and b); Ca2+ wave frequency was reduced from 66.9 ± 10.1 min−1 in control to 37.8 ± 8.8 min−1 (Fig. 9 Da, P < 0.0001, c = 20, n = 5), amplitude was reduced from 0.15 ± 0.02 ΔF/F 0 to 0.08 ± 0.014 ΔF/F 0 (Fig. 9 Db, P = 0.008, c = 20, n = 5), and spatial spread was reduced from 27.8 ± 3.3 μm to 17.6 ± 4 μm (Fig. 9 Dd, P = 0.013, c = 20, n = 5) in the duration of Ca2+ waves in response to XeC were not observed (Fig. 9 Dc, P = 0.75, c = 20, n = 5).
Figure 9. The effect of blocking IP3Rs on USMC Ca2+ waves.
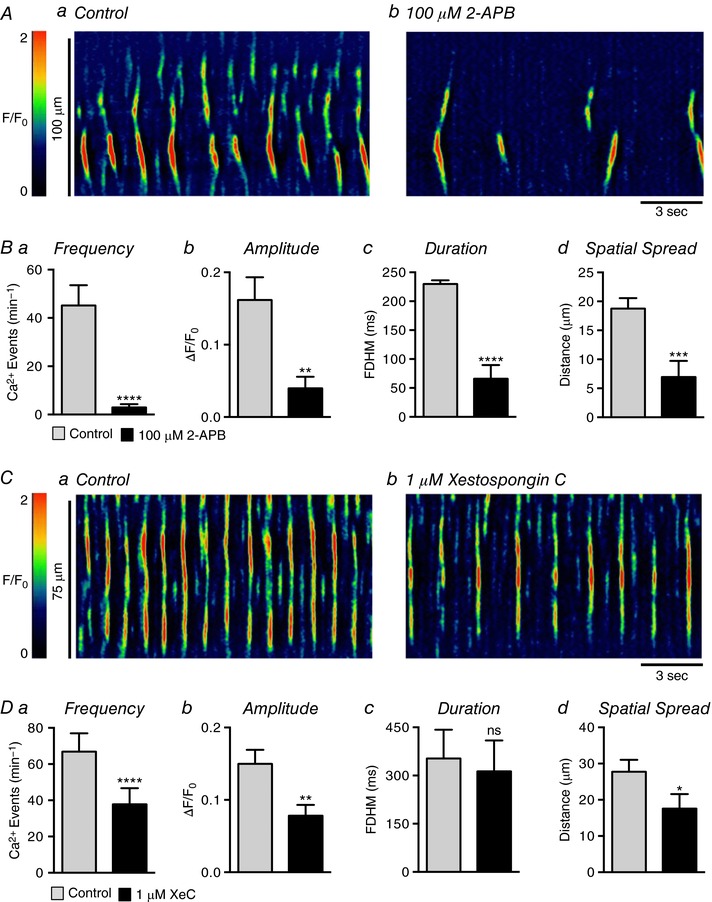
Aa and b, representative ST maps showing the effect of 100 μm 2‐APB on Ca2+ transient firing in USMCs. Ba–d, summary data showing the effect of 100 μm 2‐APB on USMC Ca2+ transient frequency (a), amplitude (b), duration (c) and spatial spread (d) (c = 19, n = 6). Ca and b, representative ST maps showing the effect of 1 μm xestospongin C (XeC) on Ca2+ transient firing in USMCs. Da–d, summary data showing the effect of XeC on USMC Ca2+ transient frequency (a), amplitude (b), duration (c) and spatial spread (d) (c = 20, n = 5). ns, not significant, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
We also studied the effects of blocking RyRs with the compounds tetracaine and ryanodine. The RyR antagonist tetracaine (100 μm) had inhibitory effects on USMC Ca2+ waves similar to those of XeC and 2‐APB (Fig. 10 A). Tetracaine reduced the frequency of Ca2+ waves from 37 ± 6.2 min−1 to 9.5 min−1 (Fig. 10 Ba, P < 0.0001, c = 18, n = 6). Tetracaine reduced the amplitude of Ca2+ waves from 0.14 ± 0.025 ΔF/F 0 to 0.07 ± 0.02 ΔF/F 0 (Fig. 10 Bb, P = 0.008, c = 18, n = 6), the duration from 234 ± 6.5 ms to 130 ± 29.6 ms (Fig. 10 Bc, P = 0.004, c = 18, n = 6), and spatial spread from 19.9 ± 1.6 μm to 9.1 ± 2.4 μm (Fig. 10 Bd, P = 0.005, c = 18, n = 6). Application of ryanodine (100 μm) had similar effects to tetracaine (Fig. 10 C). Ryanodine reduced Ca2+ wave frequency from 40.1 ± 6.6 min−1 to 4.8 ± 2.8 min−1 (Fig. 10 Da, P < 0.0001, c = 14, n = 5). Ryanodine reduced the Ca2+ wave amplitude from 0.09 ± 0.006 ΔF/F 0 to 0.02 ± 0.007 ΔF/F 0 (Fig. 10 Db, P < 0.0001, c = 14, n = 5), the duration from 215.3 ± 7.7 ms to 90.5 ± 34.7 ms (Fig. 10 Dc, P = 0.0025, c = 14, n = 5), and spatial spread from 20.6 ± 2.4 μm to 3.6 ± 1.6 μm (Fig. 10 Dd, P < 0.0001, c = 14, n = 5).
Figure 10. The effect of blocking RyRs on USMC Ca2+ waves.
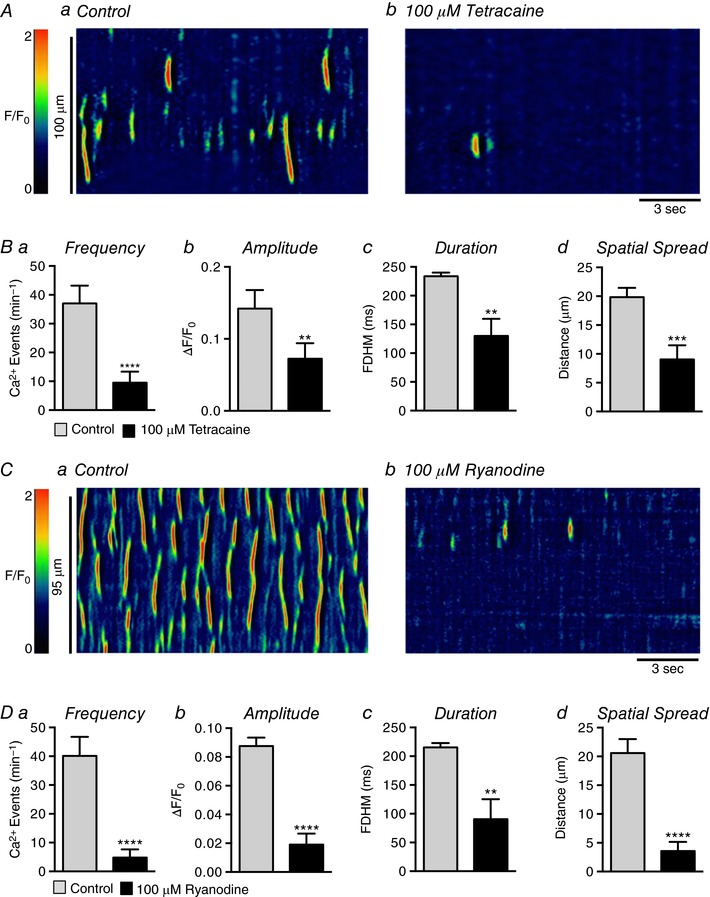
Aa and b, representative ST maps showing the effect of 100 μm tetracaine on Ca2+ transient firing in USMCs. Ba–d, summary data showing the effect of 100 μm tetracaine on USMC Ca2+ transient frequency (a), amplitude (b), duration (c) and spatial spread (d) (c = 18, n = 6). Ca and b, representative ST maps showing the effect of 100 μm ryanodine on Ca2+ transient firing in USMCs. Da–d, summary data showing the effect of 100 μm ryanodine on USMC Ca2+ transient frequency (a), amplitude (b), duration (c) and spatial spread (d) (c = 5, n = 14). * p < 0.01, *** p < 0.001, **** p < 0.0001.
Extracellular sources of Ca2+ contributing to USMC Ca2+ waves
Our findings suggest that Ca2+ waves in USMCs depend upon Ca2+ release from the SR via IP3R1 and RyRs. Nevertheless, Ca2+ influx mechanisms are typically required for maintenance of Ca2+ stores (Berridge & Galione, 1988; Yao & Parker, 1994; Sneyd et al. 1995, 2004). Therefore, we investigated the impact of Ca2+ influx on Ca2+ waves. Removal of [Ca2+]o (Ca2+ free solution also contained 0.5 mm EGTA) caused all Ca2+ transient activity in USMCs to cease within 10 min (Fig. 11 A and B, c = 19, n = 5); however, reducing [Ca2+]o by 50% to 1.25 mm or doubling [Ca2+]o to 5 mm had little effect on Ca2+ waves (Fig. 11 C). The effects of zero [Ca2+]o may have been due to depletion of SR Ca2+ stores resulting from a lack of store refilling after release events. Application of caffeine (10 mm) was used to test this idea. Under control conditions caffeine (10 mm) induced large amplitude Ca2+ transients in USMCs throughout the muscles (0.7 ± 0.1 ΔF/F 0; P = 0.002, c = 7, n = 4; Fig. 11 Da). After incubation in Ca2+ free solution, caffeine (10 mm) induced only ∼5% of maximal Ca2+ released during control conditions (0.03 ± 0.01 ΔF/F 0; Fig. 11 Db and c). Thus, in Ca2+ free conditions, SR luminal Ca2+ appears to decrease to a point where spontaneous Ca2+ release events cease.
Figure 11. Generation of USMC Ca2+ transients requires extracellular Ca2+ influx to replenish SR stores.
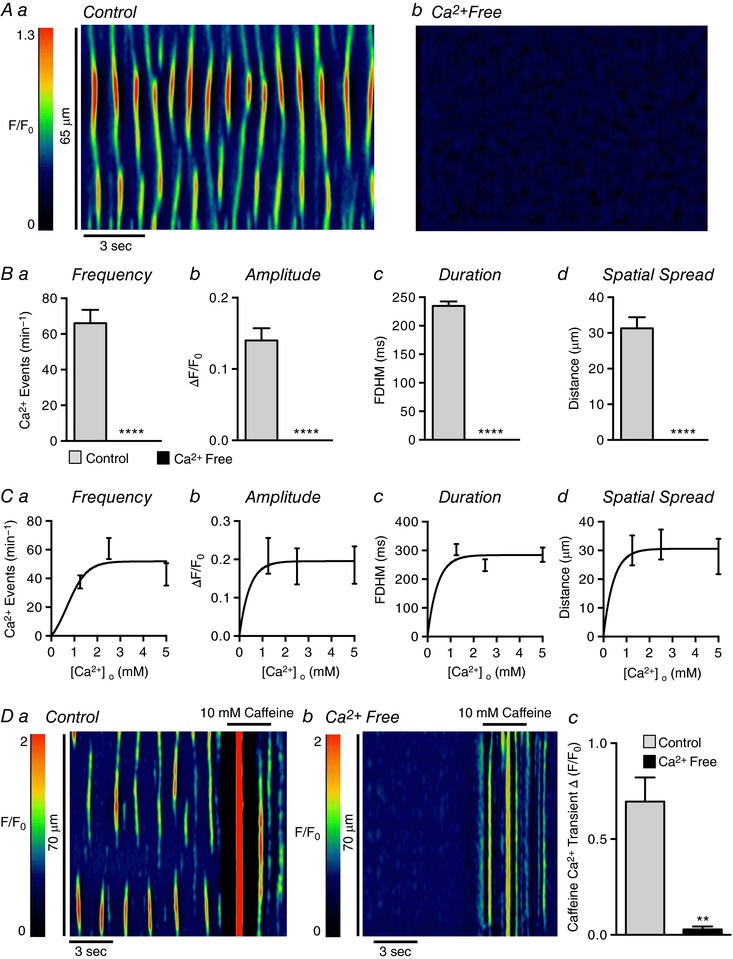
Aa and b, representative ST maps showing the effect of Ca2+ free solution on Ca2+ transient firing in USMCs. Ba–d, summary data showing the effect of Ca2+ free solution on USMC Ca2+ transient frequency (a), amplitude (b), duration (c) and spatial spread (d) (c = 19, n = 5). Ca–d, dose–response graphs showing the effect of lowering or increasing extracellular Ca2+ on USMC Ca2+ transients (c = 7, n = 4). Da and b, representative ST maps showing the effect of Ca2+ free solution on 10 mm caffeine‐induced Ca2+ transients in USMCs. Summary data are shown in Dc (c = 7, n = 4). * p < 0.01, **** p < 0.0001.
Currents attributable to L‐type Ca2+ channels have been reported in human and rabbit USMCs (Hollywood et al. 2003; Bradley et al. 2004), and antagonists of these channels reduced tone in pig urethra (Bridgewater et al. 1993; Brading, 2006) and Ca2+ oscillations in rabbit USMCs (Hashitani & Suzuki, 2007). In the present experiments, nifedipine (1 μm; Fig. 12 A) failed to inhibit the frequency (P = 0.19), amplitude (P = 0.08), duration (P = 0.34) or spatial spread (P = 0.79) of USMC Ca2+ waves (Fig. 12 B, c = 22, n = 6; Fig. 12 A and B). Nicardipine (1 μm) also had no resolvable effect on Ca2+ wave frequency (P = 0.83), amplitude (P = 0.81), duration (P = 0.4) or spatial spread (P = 0.83) (c = 15, n = 5; Fig. 12 C and D). Lack of L‐type Ca2+ channel contribution to USMC Ca2+ waves was also supported by showing that an L‐type Ca2+ agonist, FPL 64176 (1 μm; Fig. 13 A) had no effect on the frequency (P = 0.97), amplitude (P = 0.2), duration (P = 0.19) or spatial spread (P = 0.42) of Ca2+ waves (Fig. 13 B, c = 18, n = 6). In some experiments, instead of perfusing the drug into the bath, FPL 64176 (1 μm) was applied directly via a single bolus. This method of application induced a large Ca2+ transient, lasting 700 ± 98.4 ms with an amplitude of 0.7 ± 0.1 ΔF/F 0 (n = 4; Fig. 13 C). However, the effect was transient, and regular Ca2+ wave activity, similar to control events, developed during the remainder of the recording period (Fig. 13 C, n = 4). We also tested another L‐type channel antagonist that blocks voltage dependent calcium 1.3 (Cav 1.3) as well as Cav1.2 channels, isradipine (Lipscombe et al. 2004). Isradipine (1 μm; Fig. 13 D) also had no significant effects on USMC Ca2+ wave frequency (P = 0.4), amplitude (P = 0.33), duration (P = 0.68) or spatial spread (P = 0.28; Fig. 13 E, c = 14, n = 4).
Figure 12. Spontaneous Ca2+ transients in USMCs are insensitive to L‐type Ca2+ channel blockers.
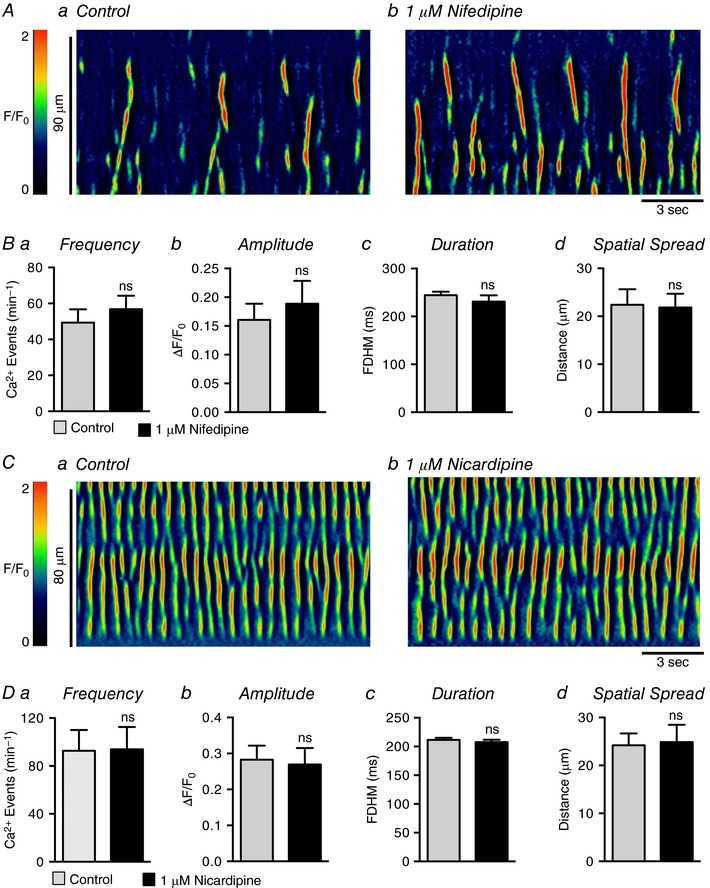
Aa and b, representative ST maps showing the effect of 1 μm nifedipine on Ca2+ transient firing in USMCs. Ba–d, summary data showing the effect of 1 μm nifedipine on USMC Ca2+ transient frequency (a), amplitude (b), duration (c) and spatial spread (d) (c = 22, n = 6). Ca and b, representative ST maps showing the effect of 1 μm nicardipine on Ca2+ transient firing in USMCs. Da–d, summary data showing the effect of 1 μm nicardipine on USMC Ca2+ transient frequency (a), amplitude (b), duration (c) and spatial spread (d) (c = 15, n = 5). ns, not significant.
Figure 13. The effect of FPL 64176 and isradipine on USMC Ca2+ transients.
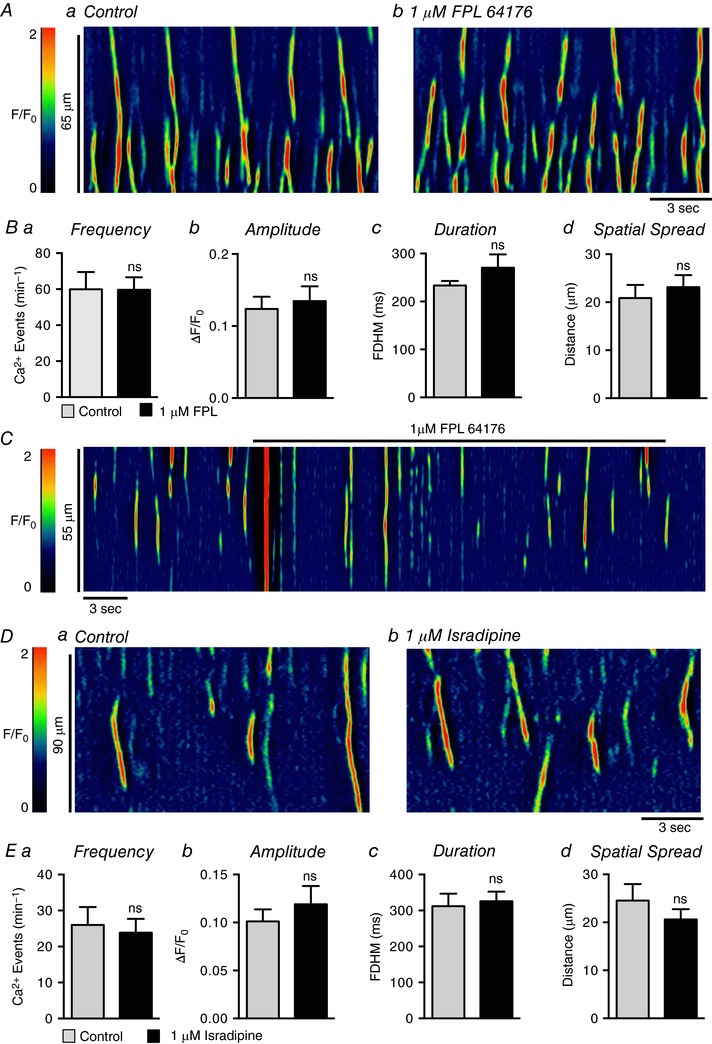
Aa and b, representative ST maps showing the effect of 1 μm FPL 64176 on Ca2+ transient firing in USMCs. Ba–d, summary data showing the effect of 1 μm FPL 64176 on USMC Ca2+ transient frequency (a), amplitude (b), duration (c) and spatial spread (d) (c = 18, n = 6). C, representative ST map showing the effect of rapidly applying 1 μm FPL 64176 to USMC Ca2+ transients. Da and b, representative ST maps showing the effect of 1 μm isradipine on Ca2+ transient firing in USMCs. Ea–d, summary data showing the effect of 1 μm isradipine on USMC Ca2+ transient frequency (a), amplitude (b), duration (c) and spatial spread (d) (c = 14, n = 4). ns, not significant.
We next tested the effects of L‐type Ca2+ channel antagonists on the excitatory responses to PE. As described previously, PE (10 μm) increased USMC Ca2+ waves dramatically (Fig. 14 A and B). In this series of experiments PE increased firing frequency from 38.8 ± 6.9 min−1 to 101 ± 14.8 min−1 (Fig. 14 Ba, P = 0.001, c = 12, n = 4), duration from 251.2 ± 15.8 ms to 322.7 ± 20.2 ms (Fig. 14 Bc, P = 0.01, c = 12, n = 4) and spatial spread from 19.8 ± 3.4 μm to 31.3 ± 4.3 μm (Fig. 14 Bd, P = 0.01, c = 12, n = 4). Nifedipine (1 μm) had no effect on Ca2+ wave frequency (Fig. 14 Ba, P > 0.05, c = 12, n = 4), amplitude (Fig. 14 Bb, P > 0.05, c = 12, n = 4), duration (Fig. 14 Bc, P > 0.05, c = 12, n = 4) or spatial spread (Fig. 14 Bd, P > 0.05, c = 12, n = 4) in response to PE.
Figure 14. PE excitatory response is insensitive to L‐type Ca2+ channel inhibition.
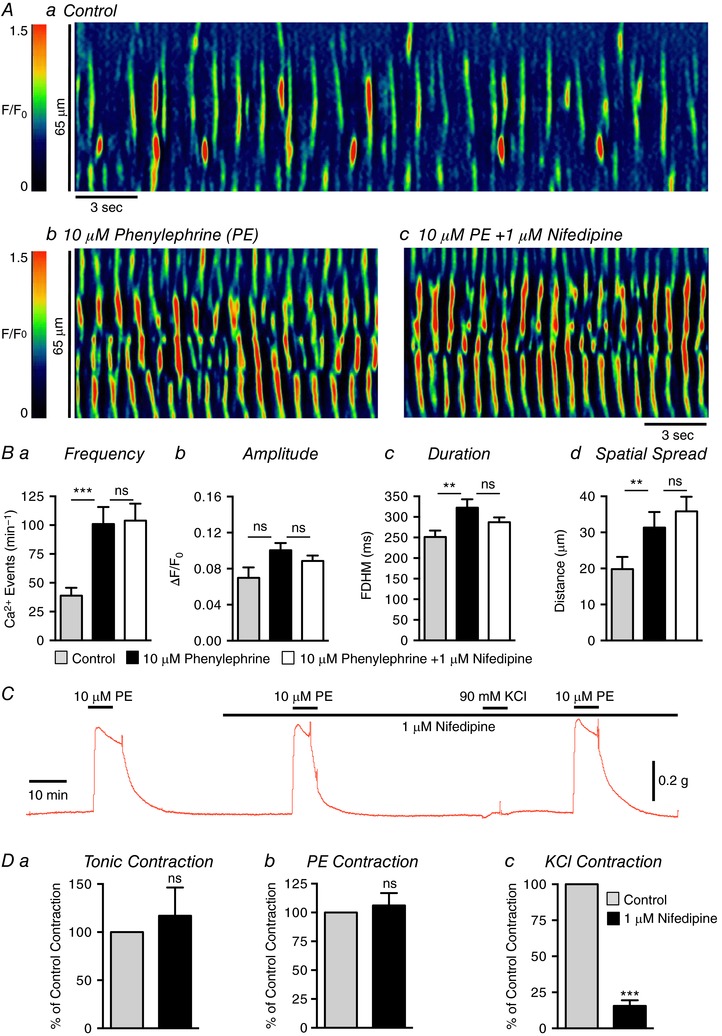
Aa–c, representative ST maps showing Ca2+ transient firing in USMCs during control conditions (a), in the presence of 10 μm phenylephrine (b) and in the presence of 10 μm phenylephrine + 1 μm nifedipine (c). Ba–d, summary data showing the effect of 1 μm nifedipine on the phenylephrine response on USMC Ca2+ transient frequency (a), amplitude (b), duration (c) and spatial spread (d) (c = 12, n = 4). C, representative contractile data showing the effect of 1 μm nifedipine on urethral contractions induced by phenylephrine and 90 mm extracellular K+. D, summary data showing the effect of 1 μm nifedipine on urethral tone (a) and contractions induced by phenylephrine (b) and 90 mm extracellular K+ (c) (n = 6). ns, not significant, ** p < 0.01, *** p < 0.001.
As described above, PE (10 μm) caused large amplitude contractions of USM rings (Fig. 14 C). These effects were reproducible, after a 15 min washout period, with no significant rundown in consecutive PE responses (P = 0.44, n = 6). Contractile responses to PE were not blocked by nifedipine (1 μm). Summary data show that nifedipine had no effect on basal tone (Fig. 14 Da, P = 0.58, n = 6) or contractile responses to PE (Fig. 14 Db, P = 0.59, n = 6). Contractions of USM rings were also enhanced by 90 mm KCl (data not shown), and after 10 min washout periods, these responses were reproducible to subsequent additions of 90 mm KCl (data not shown, P = 0.099, n = 6). Responses to 90 mm KCl, however, were reduced by 84.4% by nifedipine (Fig. 14 Dc, P = 0.002, n = 6). Application of 90 mm KCl to USMCs caused responses similar to FPL 64176 in terms of Ca2+ wave activity (data not shown). After addition of 90 mm KCl, a single global Ca2+ transient occurred and then Ca2+ waves resumed, as before 90 mm KCl was added (n = 5).
Isolated USMCs have also been reported to exhibit T‐type Ca2+ currents (Hollywood et al. 2003; Bradley et al. 2004), and we investigated the possibility that Ca2+ influx occurs through a T‐type Ca2+ conductance. The T‐type Ca2+ channel antagonist, TTA‐A2 (1 μm), did not have inhibitory effects on USMC Ca2+ wave frequency (P = 0.39), amplitude (P = 0.15), duration (P = 0.1) or spatial spread (P = 0.51) (Fig. 15 A and B, c = 16, n = 5). Similarly, NNC‐550396 (1 μm) had no effect on Ca2+ wave frequency (P = 0.4), amplitude (P = 0.23) or spatial spread (P = 0.52) (Fig. 15 C and D, c = 13, n = 4). However, we observed a small but significant increase in Ca2+ wave duration in the presence of NNC‐550396 (Fig. 15 Dc, P = 0.01, c = 13, n = 4).
Figure 15. USMC Ca2+ waves are insensitive to T‐type Ca2+ channel blockers.
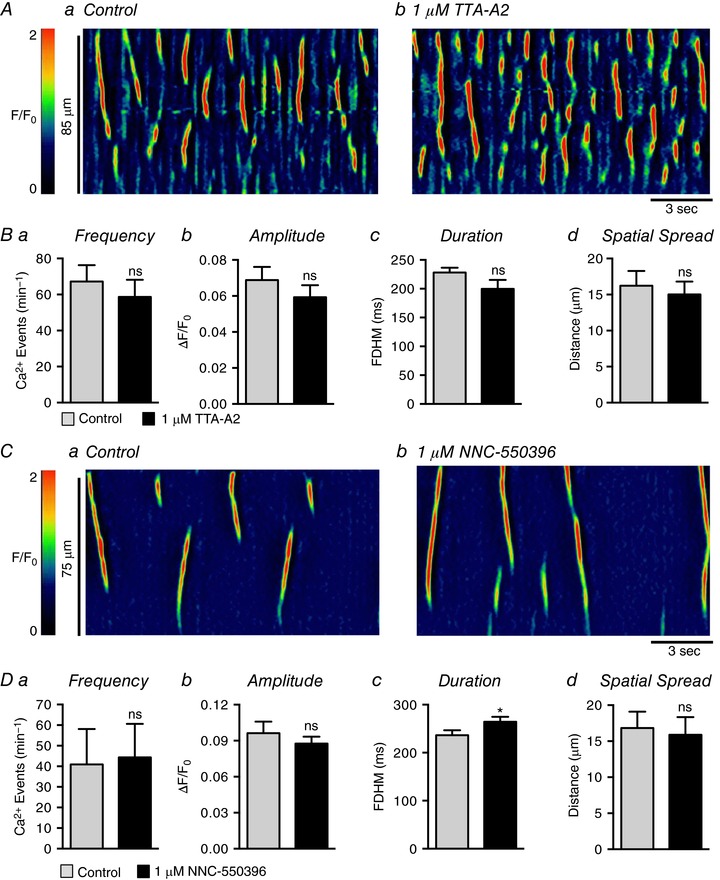
Aa and b , representative ST maps showing the effect of 1 μm TTA‐A2 on Ca2+ transient firing in USMCs. Ba–d, summary data showing the effect of 1 μm TTA‐A2 on USMC Ca2+ transient frequency (a), amplitude (b), duration (c) and spatial spread (d) (c = 16, n = 5). Ca and b, representative ST maps showing the effect of 1 μm NNC‐550396 on Ca2+ transient firing in USMCs. Da–d, summary data showing the effect of 1 μm NNC‐550396 on USMC Ca2+ transient frequency (a), amplitude (b), duration (c) and spatial spread (d) (c = 13, n = 4). ns, not significant, * p < 0.05.
Although L‐type and T‐type Ca2+ channel antagonists did not interfere with spontaneous and PE‐evoked Ca2+ signalling in USMCs, extracellular Ca2+ depletion inhibited Ca2+ waves, suggesting that Ca2+ influx is necessary for maintenance of Ca2+ store function. Therefore, we investigated whether store operated Ca2+ entry (SOCE) might be necessary for the maintenance of Ca2+ waves in these cells. SOCE has been best characterized in non‐excitable cells (Prakriya & Lewis, 2015); however, studies have also shown that SOCE is essential for store refilling and maintaining Ca2+ release in a number of excitable cells (Trebak et al. 2013).
SR Ca2+ store refilling resulting from SOCE occurs either through transient receptor potential cation (TRPC) channels or Orai Ca2+ channels (Trebak & Putney, 2017). USMCs expressed genes for Trpc1, 3, 4 and Trpc6 (Fig. 16 A, n = 4), and higher relative expression of Trpc3 (i.e. 0.014 ± 0.0006 sorted USMCs vs. 0.008 ± 0.00015 unsorted cells), Trpc4 (0.009 ± 0.00007 sorted USMCs vs. 0.0018 ± 0.00005 unsorted urethral cells) and Trpc6 (0.019 ± 0.0006 sorted USMCs vs. 0.0045 ± 0.00016 unsorted urethral cells) was noted (Fig. 16 A, n = 4). USMCs also express all three Orai genes (Fig. 16 B, n = 4).
Figure 16. Store operated Ca2+ entry (SOCE) in USM.
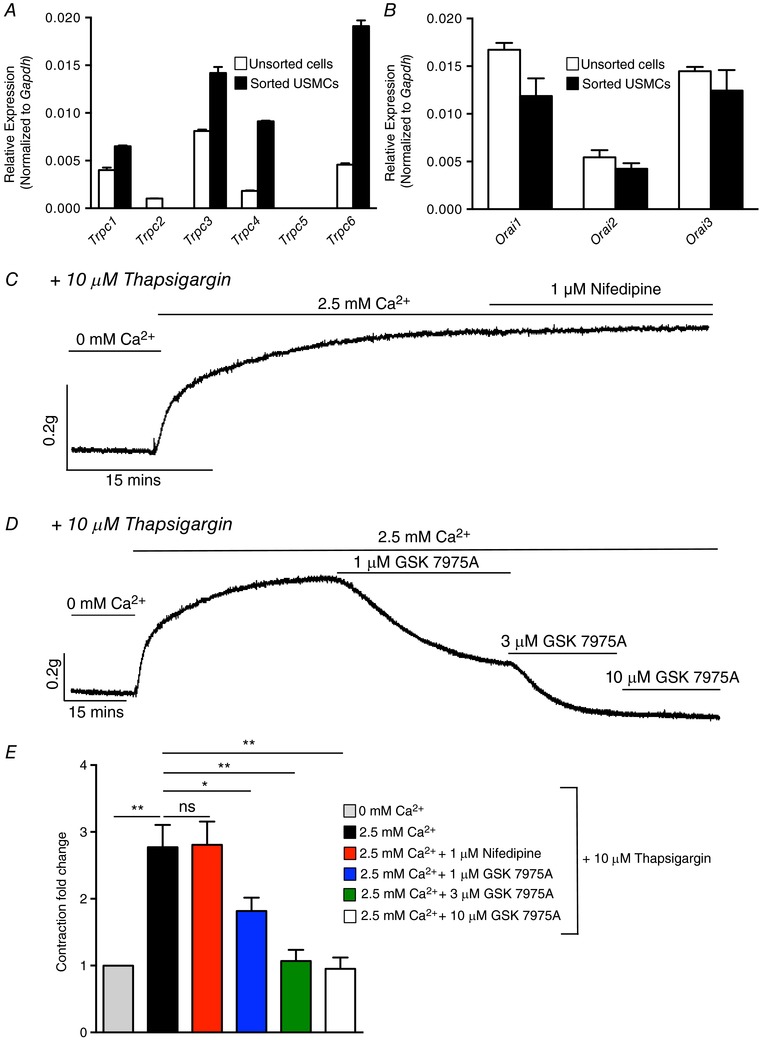
A, relative expression of TRPC genes in FACS sorted and unsorted urethral cells from SMC‐eGFP mice determined by qPCR analysis, normalized to Gapdh expression (n = 4). B, relative expression of Orai channel genes in FACS sorted and unsorted urethral cells from SMC‐eGFP mice determined by qPCR analysis, normalized to Gapdh expression (n = 4). C, representative contractile data showing a protocol to induce SOCE in USM. Basal USM contraction is shown at the start of the trace when intracellular Ca2+ stores were passively depleted by incubating the tissue in 0 mm Ca2+ solution and thapsigargin (10 μm). Reintroduction of [Ca2+]o (2.5 mm) in the continued presence of thapsigargin led to development of a sustained tonic contraction that was insensitive to nifedipine. D, contractile trace showing the effect of GSK 7975A (1–10 μm) on SOCE induced tonic contraction of USM. E, summary data showing the effects of nifedipine and GSK 7975A on the sustained tonic contraction induced in USM using the SOCE protocol described in C and D. Contractions are expressed as fold changes normalized to contractile level in 0 mm Ca2+ + thapsigargin (10 μm; n = 8). ns, not significant, * p < 0.05, ** p < 0.01.
We used contractile experiments to determine whether USM possesses a significant SOCE apparatus, as the expression studies would suggest. In USM ring preparations, SOCE was activated by passive depletion of SR Ca2+ stores by blocking the SERCA pump with thapsigargin (10 μm) while incubating the muscle in 0 mm Ca2+ bathing solution (Fig. 16 C). Upon reintroduction of [Ca2+]o (2.5 mm), in the continued presence of 10 μm thapsigargin, a large sustained tonic contraction developed in USM ((2.7 ± 0.3)‐fold increase, normalized to 0 mm Ca2+ + thapsigargin conditions, P < 0.01, Fig. 16 E, n = 8). This sustained, tonic increase in contraction after reintroduction of Ca2+ is indicative of SOCE (Putney, 1986). The sustained contractile response was not due to activation of L‐type Ca2+ channels, as it was insensitive to nifedipine (1 μm; Fig. 16 C and E, P > 0.05, n = 8). However, the Orai antagonist GSK 7975A (1–10 μm) inhibited the SOCE response (Fig. 16 D). GSK 7975A (1 μm) decreased the contraction activated by SOCE by 32 ± 7% (P < 0.05, n = 8), and it was further reduced by 63 ± 5% and 65 ± 5% by 3 μm (P < 0.01, n = 8) and 10 μm GSK 7975A (P < 0.01, n = 8), respectively (Fig. 16 E).
We then tested the effect of SOCE blockers on Ca2+ waves in USMCs. SKF 96365 (10 μm), a relatively non‐selective SOCE antagonist (Harteneck & Gollasch, 2011) inhibited Ca2+ waves in USMCs (Fig. 17 A), reducing Ca2+ wave frequency from 74.6 ± 12.2 min−1 in control to 14.3 ± 3.9 min−1 (Fig. 17 Ba, P = 0.002, c = 20, n = 5). SKF 96365 also reduced Ca2+ wave amplitude from 0.08 ± 0.006 ΔF/F 0 to 0.04 ± 0.007 ΔF/F 0 (Fig. 17 Bb, P < 0.0001, c = 20, n = 5), duration from 270 ± 14.4 ms to 185 ± 37.2 ms (Fig. 17 Bc, P = 0.018, c = 20, n = 5), and spread from 17.9 ± 1.9 μm in control to 7.2 ± 1.6 μm (Fig. 17 Bd, P < 0.0001, c = 20, n = 5). The Orai antagonist, GSK 7975A (1 μm), also inhibited USMC Ca2+ waves (Fig. 17 C), reducing frequency from 52.5 ± 9.2 min−1 to 23.4 ± 5.2 min−1 (Fig. 17 Da, P < 0.0001, c = 17, n = 6), amplitude from 0.1 ± 0.01 ΔF/F 0 to 0.06 ± 0.01 ΔF/F 0 (Fig. 17 Db, P = 0.011, c = 17, n = 6), duration from 264 ± 12.4 ms in control to 194 ± 22.8 ms in the presence of GSK 7975A (Fig. 17 Dc, P = 0.01, c = 17, n = 6) and spatial spread of Ca2+ waves from 25.2 ± 2.4 to 15.9 ± 2.25 μm (Fig. 17 Dd, P = 0.003, c = 17, n = 6).
Figure 17. SOCE blockers inhibit Ca2+ transients in USMCs.
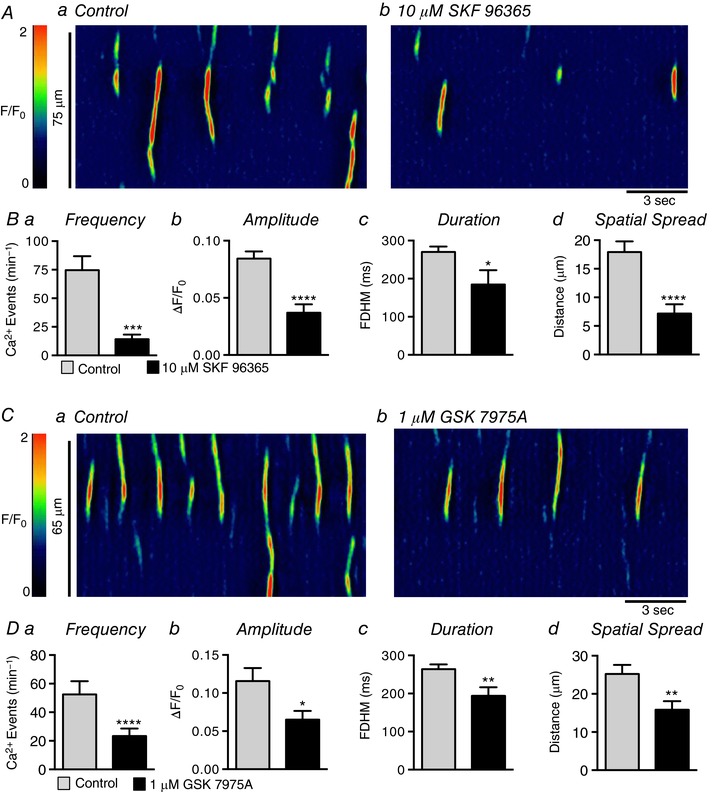
Aa and b , representative ST maps showing the effect of 10 μm SKF 96365 on Ca2+ transient firing in USMCs. Ba–d, summary data showing the effect of 10 μm SKF 96365 on USMC Ca2+ transient frequency (a), amplitude (b), duration (c) and spatial spread (d) (c = 20, n = 5). Ca and b, representative ST maps showing the effect of 1 μm GSK 7975A on Ca2+ transient firing in USMCs. Da–d, summary data showing the effect of 1 μm GSK 7975A on USMC Ca2+ transient frequency (a), amplitude (b), duration (c) and spatial spread (d) (c = 17, n = 6). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Inhibition of Ca2+ waves in USMCs would be predicted to affect responses to PE, so we tested the effects of GSK 7975A on the contractile effects of PE. PE (0.1–30 μm) increased urethral tone (Fig. 18 A); however, when the muscles were treated with GSK 7975A (1 μm), contractions elicited by 10 and 30 μm PE were reduced by 23.3% (P < 0.05) and 25.8% (P < 0.05), respectively (Fig. 18 A and B, n = 6). Increasing the concentration of GSK 7975A caused dose‐dependent inhibition of PE contractions (e.g. 3 μm GSK 7975A reduced the response to 10 μm PE by 32.5%, Fig. 18 B, P < 0.05, n = 6) and 10 μm GSK 7975A reduced the response to 10 μm PE by 62.23% (Fig. 18 B, P < 0.001, n = 6).
Figure 18. SOCE can influence PE induced contractions in USM.

A, representative contractile data showing the effect of 1–10 μm GSK 7975A on urethral contractions induced by increasing concentration of PE (0.1–30 μm). B, summary data showing the effect of 1–10 μm GSK 7975A on urethral contractions induced by increasing concentration of PE (0.1–30 μm, n = 6). * p < 0.05, ** p < 0.01, **** p < 0.0001.
Discussion
In the current study, we characterized spontaneous Ca2+ signalling in mouse USMCs in situ, utilizing a smooth muscle specific, optogenetic Ca2+ sensor, GCaMP3. Using traditional cell permeable Ca2+ indicator dyes, Hashitani and Suzuki (2007) reported the occurrence of spontaneous Ca2+ oscillations in rabbit USMCs in situ, and consistent with our observations, these authors reported that Ca2+ oscillations rarely propagated cell‐to‐cell. Use of GCaMP3 allowed monitoring of subcellular Ca2+ events in USMCs in great detail. We found that Ca2+ waves in murine USMCs were largely confined to single cells and were associated with small asynchronous contractions of USMCs. Ca2+ waves arose from multiple firing sites along the lengths of USMCs, and firing of events was stochastic in nature, with little coordination between Ca2+ firing sites. The activity of adjacent cells within the same bundle was also stochastic, and little or no coordination between USMCs in the same bundle was observed. Robust contraction of USM was elicited by PE, which also increased the frequency, duration and spread of intracellular USMC Ca2+ waves without causing a global rise in [Ca2+]i. Thus, USM contractility can be modulated by influencing the occurrence and characteristics of intracellular Ca2+ waves. Summation of this asynchronous activity appears to be the source of sustained tone in the urethra.
The spontaneous activity of USMCs in situ observed in the current study contrasts with the lack of spontaneous activity observed in isolated USMCs (Sergeant et al. 2000; Hollywood et al. 2003; Bradley et al. 2004; Brading, 2006; Kyle, 2014). Isolated rabbit USMCs display no spontaneous Ca2+ signals, but large amplitude Ca2+ responses were evoked by 60 mm [K+]o (Drumm et al. 2014c). Spontaneous Ca2+ events were ubiquitous in USMCs in situ, and most events were manifest as propagating Ca2+ waves. These contrasting findings between isolated USMCs and cells in situ highlight the need for studies of USMCs in situ to better understand the physiological regulation of urethral contraction.
USMC contraction is thought to have an important myogenic component because urethral tone is resistant to nerve blockade in most mammals (Thornbury et al. 1992; Bridgewater et al. 1993; Brading, 1999). In alignment with this concept, we found that basal Ca2+ wave activity in USMCs was resistant to TTX. While basal Ca2+ waves were myogenic in nature they were modulated by neurotransmitters that are known to influence urethral contraction. We found that isolated murine USMCs express genes encoding muscarinic receptors (M2, M3 and M5). M2 and M3 receptor expression was previously reported in whole muscle preparations of rabbit (Mutoh et al. 1997) and pig urethras (Yamanishi et al. 2002). However, the role of parasympathetic innervation in the urethra is unclear. Some investigators have reported that cholinergic agonists CCh or ACh elicit contractions of urethral smooth muscles (Ek et al. 1977; Sogbein et al. 1984; Mutoh et al. 1997; Yamanishi et al. 2002), but others have argued that blocking cholinergic nerves has no effect on urethral closure pressure (Ulmsten & Andersson, 1977). Urethral smooth muscle contains a sparse population of cholinergic nerves compared to the bladder (Gosling & Dixon, 1975). Our findings with CCh suggested that cholinergic input has little or no direct effect on USMCs and the main excitatory influence on murine USMCs is adrenergic.
We found that mouse USMCs express all three α1 adrenoceptor subtypes (Kimoto et al. 1987; Chen & Brading, 1992; Andersson, 2001) and spontaneous Ca2+ waves were dramatically increased by application of PE, which also led to robust contractions of murine urethral smooth muscles. Conversely, murine USMCs expressed high levels of genes encoding GC and PKG, and Ca2+ waves were decreased by a nitric oxide donor. The inhibitory effects on Ca2+ waves is likely to occur through the NO→GC→PKG pathway, which is known as the main inhibitory pathway exerting relaxation on the internal urethral sphincter during micturition (Thornbury et al. 1992; Bridgewater et al. 1993; Dokita et al. 1994; Zygmunt et al. 1995; Waldeck et al. 1998; Costa et al. 2001).
Purinergic neurotransmission in the urethra is controversial. On pre‐contracted muscles, purines, such as ATP, have been reported to induce relaxation of hamster and pig urethral smooth muscle (Pinna et al. 1998, 2005; Hernandez et al. 2009). However, when applied to basal activity, ATP induces contraction in rabbit urethral smooth muscle (Bradley et al. 2010, 2011). In accordance with the latter findings, our results show that ATP enhanced Ca2+ waves in USMCs and notable contractions of the muscles while imaging. Bradley et al. (2011) suggested the excitatory effects of purines were due to P2X receptors in USMCs, and we found P2rx1–4 and P2rx6 receptor genes expressed in murine USMCs. We did not resolve P2Y receptor genes in USMCs, with the exception of P2ry14. However, P2Y receptor gene expression was observed in the unsorted cell populations, so P2Y expression is likely to occur in cell types other than USMCs. For example, others have reported that non‐contractile interstitial cells from the urethra respond to P2Y agonists (Bradley et al. 2010). ATP and other purinergic mediators released from the urothelium are thought to affect contractions in the bladder (Birder & Andersson, 2013; Merrill et al. 2016); however, at present, it is unknown whether urothelial mediators affect urethral contractility. In the current study, we found that ATP exerted significant excitatory effects on USMC Ca2+ waves, thus it is possible that purines released from urothelium could have paracrine effects on USMCs. Mice with optogenetic sensors expressed in USMCs, as utilized in this study, will serve as valuable tools for assessing the role of urothelium‐derived mediators on urethral excitation–contraction coupling in future studies.
Our data suggest Ca2+ waves in USMCs arose from the release of Ca2+ from intracellular stores. Hashitani et al. (2006) reported previously that Ca2+ oscillations in rabbit USMCs were not consistently blocked by SERCA pump inhibitors, but we found that the SERCA pump inhibitors thapsigargin and CPA abolished Ca2+ transients in mouse USMCs in all experiments. The SR channels responsible for Ca2+ release appeared to involve both IP3Rs and RyRs, as antagonists of both receptors blocked Ca2+ waves. Itpr1 was the dominant Ca2+ release channel gene expressed by USMCs, but the inhibitory effects of tetracaine and ryanodine in addition to the expression of Ryr1–3 genes suggest the possibility that clusters of RyRs may initiate or amplify Ca2+ release from IP3R1. Interaction between IP3Rs and RyRs to generate intracellular Ca2+ waves has been reported in other smooth muscle types. In rat portal vein myocytes, Ca2+ waves could be induced by both release of caged IP3 or by applications of caffeine suggesting the involvement of IP3Rs and RyRs in the generation of Ca2+ waves (Boittin et al. 1998, 1999). In rat gastric myocytes CCh induced whole cell Ca2+ events that were inhibited by IP3R or RyR blockers (White & McGeown, 2002). One type of SR Ca2+ channel might act as an ‘initiator’ or an ‘amplifier’ of a primary Ca2+ transient. In pressurized arteries from murine cremaster muscles, Ca2+ waves were blocked by inhibiting IP3Rs with 2‐APB, but initiating Ca2+ sparks remained. Tetracaine blocked both events suggesting that RyRs were the initiating spark and Ca2+ release from IP3Rs amplified the sparks into propagating waves (Westcott et al. 2012). A similar situation occurs in rabbit urethral interstitial cells where RyRs also initiate waves in rabbit urethral interstitial cells, which is then amplified to a propagating wave by additional Ca2+ release from IP3Rs (Drumm et al. 2015). Our data also suggest cooperation between RyRs and IP3Rs in USMCs; further work will be needed to clarify if an ‘initiator’ and ‘amplifier’ relationship exists between the two receptor types.
Studies on isolated USMCs have shown that these cells possess Ca2+ conductances, such as L‐ and T‐type Ca2+ channels (Hollywood et al. 2003; Bradley et al. 2004). Using membrane permeable dyes, Hashitani and Suzuki (2007) reported that nicardipine reduced the amplitude of Ca2+ oscillations in rabbit USMCs. In murine USMCs, neither dihydropyridines nor the L‐type Ca2+ channel agonist FPL 64176 had any apparent effect on Ca2+ signalling. Our findings suggesting no role for L‐type Ca2+ channels in urethral Ca2+ waves are not without precedent. Larsson et al. (1984) found that nifedipine failed to abolish contractions of rabbit urethra induced by noradrenaline (NA), suggesting that other Ca2+ influx or release pathways are important in this tissue. Nifedipine also failed to decrease urethral closure pressure in cats (Mawby et al. 1991). Nifedipine also failed to inhibit contractions of sheep urethra induced by NA and was 1000 times less effective at blocking NA induced contractions than those elicited by 124 mm K+ (Garcia‐Pascual et al. 1991). Nifedipine had no effect on human urethral tone in vitro or in vivo (Forman et al. 1978). T‐type Ca2+ currents have also been reported in USMCs (Hollywood et al. 2003; Bradley et al. 2004; Fry & Jabr, 2014). However, specific T‐type Ca2+ channel blockers that potently block T‐type currents (Huang et al. 2004; Kraus et al. 2010; Drumm et al. 2017) had no effect on Ca2+ waves in USMCs.
The reliance on Ca2+ influx to sustain Ca2+ waves and the lack of effects of L‐type and T‐type Ca2+ conductances led us to hypothesize that Ca2+ stores in USMCs may be refilled by SOCE. The phenomenon of SOCE was first described in non‐excitable cells (Putney, 1986), but it also occurs in excitable muscle cells (Gibson et al. 1998; Quinn et al. 2004; Bradley et al. 2005; Trebak et al. 2013; Prakriya & Lewis, 2015). SOCE depends upon the Orai Ca2+ channels (Trebak & Putney, 2017), and these channels are gated by interactions with an SR luminal Ca2+ sensor (STIM) (Liou et al. 2005; Feske et al. 2006; Zhang et al. 2006; Soboloff et al. 2012). USMCs expressed transcripts for all three Orai channel paralogues, and SKF 96365, a poorly selective Orai antagonist, and GSK 7975A, a more selective antagonist, inhibited Ca2+ waves in USMCs. Taken together, these data suggest that SOCE is an important mechanism for sustaining spontaneous Ca2+ activity in USMCs, and this Ca2+ transport system also has an important role in sustaining urethral contractions. Our data suggest that Orai channels, possibly due to expression of Orai1 and Orai3 (Fig. 16 B; Mignen et al. 2008), fill the role of a receptor‐operated channel, supplying the Ca2+ required for responses to PE.
A similar situation has recently been reported in airway smooth muscle, where Ca2+ oscillations can be induced by agonists such as methacholine (MCh) (Boie et al. 2017). MCh‐induced Ca2+ oscillations were relatively insensitive to high concentrations of nifedipine, although nifedipine could reduce Ca2+ oscillations induced by high external K+. Despite the lack of effect of blocking L‐type Ca2+ channels, the Orai1 blocker GSK 7975A blocked MCh‐induced Ca2+ oscillations in these airway smooth muscle cells (Boie et al. 2017), similar to the effects of GSK 7975A on basal USMC Ca2+ waves and responses to PE in the current study. The observations led the authors of the study on airway muscles to conclude that Ca2+ entry due to SOCE, and not via L‐type Ca2+ channels, contributes to the control of airway smooth muscle tone.
In many smooth muscle cells Ca2+ transients are not coupled directly to contractions but have seemingly paradoxical effects by regulating membrane potential in such a manner as to restrain contractility. For example, Ca2+ sparks are linked to activation of large‐conductance Ca2+‐activated K+ (BK) channels and tend to cause membrane hyperpolarization or at least to moderate depolarization (Nelson et al. 1995; Jagger et al. 2000). Hyperpolarization tends to reduce activation of voltage‐dependent Ca2+ channels and restrains the development of contractions. In other smooth muscles Ca2+ release leads to activation of Ca2+‐activated Cl− conductances, resulting in inward current and depolarization (ZhuGe et al. 1998; Bootman et al. 2001). The open probability of voltage‐dependent Ca2+ channels increases in response to depolarization, so Ca2+ transients in these cells are indirectly linked to contraction. Our results suggest that Ca2+ waves in USMCs are directly responsible for excitation–contraction coupling, constituting a pure example of pharmaco‐mechanical coupling (Somlyo et al. 1999).
In conclusion, we characterized spontaneous and evoked Ca2+ signalling in mouse USMCs in situ using cell specific expression of GCaMP3. Ca2+ waves in USMCs appear to be a source of Ca2+ for the activation of the contractile apparatus instead of an indirect mechanism for regulating contraction via activation of Ca2+‐dependent conductances in the plasma membrane. Ca2+ waves were greatly stimulated by excitatory neurotransmitters and inhibited by NO, the major inhibitory neurotransmitter. Responses to the major sympathetic neurotransmitter, working through α1‐adrenoceptors, did not depend upon depolarization‐induced Ca2+ influx through activation of L‐type Ca2+ channels, which are expressed in USMCs, because dihydropyridines did not affect contractile responses to PE. Generation of Ca2+ waves in USMCs depends upon release of Ca2+ from the SR mediated by both IP3Rs and RyRs. A Ca2+ entry mechanism is apparent in the regulation of Ca2+ waves, however, because Ca2+‐free external solution and SOCE antagonists inhibited Ca2+ waves. Our results also provide evidence that Orai channels provide a receptor‐operated Ca2+ entry mechanism required to cause excitation–contraction coupling in response to PE. This study of Ca2+ signalling in USMCs in situ provides new insights into the physiological mechanisms responsible for contractions in urethral muscles.
Additional information
Competing interests
None declared.
Author contributions
B.T.D., G.P.S. and K.M.S. contributed to the conception and design of the experiments. B.T.D., B.E.R., C.A.C., S.A.B., G.P.S., M.A.H., K.D.T. and K.M.S. contributed to the collection, analysis and interpretation of data. All experiments were performed at University of Nevada, Reno School of Medicine. B.T.D., B.E.R., C.A.C., S.A.B., G.P.S., M.A.H., K.D.T. and K.M.S. contributed to drafting the article and/or revising it critically for important intellectual content. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This project was supported by exploratory funding from the University of Nevada and from P01 DK41315 from the NIDDK that supported Core laboratories providing molecular and immunohistochemical support. USMCs were sorted by the FACS/flow cytometry shared resource lab that is supported by a Phase III COBRE award (P30‐GM110767). B.T.D. and S.A.B. received salary support from R01 DK‐091336 and K.M.S. received salary support from P01 DK41315.
Supporting information
Disclaimer: Supporting information has been peer‐reviewed but not copyedited.
Movie S1. Spontaneous intracellular Ca2+ waves in USMC are associated with USMC contractions.
Movie S2. Spontaneous Ca2+ waves in USMC in situ.
Movie S3. Phenylephrine increases Ca2+ waves in USMC.
Acknowledgements
The authors wish to thank Lauren Peri for conducting the molecular expression studies, Byoung Koh for collecting cells by FACs, Kathleen Keef for assistance with contractile experiments and Nancy Horowitz for the maintenance and breeding of mice.
Biography
Bernard Drumm received his PhD in Physiology from Dundalk Institute of Technology, Ireland in 2013. His research investigates how Ca2+ signalling impacts the functions of visceral smooth muscle organs. His doctoral work, studying Ca2+ wave propagation in urethral cells was followed by a postdoctoral position at the University of Nevada, Reno (UNR), where he studied the regulation of pacemaker and neuroeffector mechanisms in gastrointestinal interstitial cells of Cajal. Dr Drumm is currently a Research Assistant Professor at UNR, where he uses optogenetic approaches to investigate intestinal motility and lower urinary tract smooth muscle contractility.

Edited by: Kim Barrett & Kathleen Morgan
References
- Alexandre EC, Kiguti LR, Calmasini FB, Silva FH, da Silva KP, Ferreira R, Ribeiro CA, Mónica FZ, Pupo AS & Antunes E (2016). Mirabegron relaxes urethral smooth muscle by a dual mechanism involving β3‐adrenoceptor activation and α1‐adrenoceptor blockade. Br J Pharmacol 173, 415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amundson J & Clapham D (1993). Calcium waves. Curr Biol 3, 375–382. [DOI] [PubMed] [Google Scholar]
- Andersson KE (2001). Neurotransmission and drug effects in urethral smooth muscle. Scand J Urol Nephrol Suppl 207, 26–34. [DOI] [PubMed] [Google Scholar]
- Andersson KE, Larsson B & Sjögren C (1984). Characterisation of the α‐adrenoceptors in the female rabbit urethra. Br J Pharmacol 81, 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SA, Drumm BT, Saur D, Hennig GW, Ward SM & Sanders KM (2016). Spontaneous Ca2+ transients in interstitial cells of Cajal located within the deep muscular plexus of the murine small intestine. J Physiol 594, 3317–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ & Galione A (1988). Cytosolic calcium oscillators. FASEB J 2, 3074–3082. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P & Bootman MD (2000). The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1, 11–21. [DOI] [PubMed] [Google Scholar]
- Birder L & Andersson KE (2013). Urothelial signaling. Physiol Rev 93, 653–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boie S, Chen J, Sanderson MJ & Sneyd J (2017). The relative contributions of store‐operated and voltage‐gated Ca2+ channels to the control of Ca2+ oscillations in airway smooth muscle. J Physiol 595, 3129–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boittin FX, Coussin F, Macrez N, Mironneau C & Mironneau J (1998). Inositol 1,4,5‐triphosphate and ryanodine sensitive Ca2+ release channel‐dependent Ca2+ signalling in rat portal vein myocytes. Cell Calcium 23, 303–311. [DOI] [PubMed] [Google Scholar]
- Boittin FX, Macrez N, Halet G & Mironneau J (1999). Norepinephrine‐induced Ca2+ waves depend on InsP3 and ryanodine receptor activation in vascular myocytes. Am J Physiol 277, C139–C151. [DOI] [PubMed] [Google Scholar]
- Bootman MD, Lipp P & Berridge MJ (2001). The organization and functions of local Ca2+ signals. J Cell Sci 114, 2213–2222. [DOI] [PubMed] [Google Scholar]
- Brading AF (1999). The physiology of the mammalian urinary outflow tract. Exp Physiol 84, 215–221. [DOI] [PubMed] [Google Scholar]
- Brading AF (2006). Spontaneous activity of lower urinary tract smooth muscles: correlation between ion channels and function. J Physiol 570, 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley E, Hollywood MA, McHale NG, Thornbury KD & Sergeant GP (2005). Pacemaker activity in urethral interstitial cells is not dependent on capacitative calcium entry. Am J Physiol Cell Physiol 289, C625–C632. [DOI] [PubMed] [Google Scholar]
- Bradley E, Kadima S, Drumm B, Hollywood MA, Thornbury KD, McHale NG & Sergeant GP (2010). Novel excitatory effects of adenosine triphosphate on contractile and pacemaker activity in rabbit urethral smooth muscle. J Urol 183, 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley E, Kadima S, Kyle B, Hollywood MA, Thornbury KD, McHale NG & Sergeant GP (2011). P2X receptor currents in smooth muscle cells contribute to nerve mediated contractions of rabbit urethral smooth muscle. J Urol 186, 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley JE, Anderson UA, Woolsey SM, Thornbury KD, McHale NG & Hollywood MA (2004). Characterisation of T‐type calcium current and its contributions to electrical activity in rabbit urethra. Am J Physiol Cell Physiol 286, C1078–C1088. [DOI] [PubMed] [Google Scholar]
- Bridgewater M, MacNeil HF & Brading AF (1993). Regulation of tone in pig urethral smooth muscle. J Urol 150, 223–228. [DOI] [PubMed] [Google Scholar]
- Callahan SM & Creed KE (1981). Electrical and mechanical activity of the isolated lower urinary tract of the guinea‐pig. Br J Pharmacol 74, 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HI & Brading AF (1992). The contribution of α‐adrenoreceptors to neurally‐mediated contractions of the rabbit urethral smooth muscle. J Pharmacol 106, 302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte B, Maggi A, Parlani M, Lopez G, Manzini S & Giachetti A (1991). Simultaneous recordings of vesical and urethral pressure in urethane‐anesthetized rats: effects of neuromuscular blocking agents on the activity of the external urethral sphincter. J Pharmacol Methods 26, 161–171. [DOI] [PubMed] [Google Scholar]
- Costa G, Labadía A, Triguéro D, Jimenez E & García‐Pascual Á (2001). Nitrergic relaxation in urethral smooth muscle: involvement of potassium channels and alternative redox forms of NO. Naunyn‐Schmiedebergs Arch Pharmacol 364, 516–523. [DOI] [PubMed] [Google Scholar]
- DeLancey JO (2010). Why do women have stress urinary incontinence? Neurourol Urodyn 29, Suppl. 1, S13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLancey JO, Trowbridge ER, Miller JM, Morgan DM, Guire K, Fenner DE, Weadock WJ & Ashton‐Miller JA (2008). Stress urinary incontinence: relative importance of urethral support and urethral closure pressure. J Urol 179, 2286–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokita S, Smith S, Nishimoto T, Wheeler M & Weiss R (1994). Involvement of nitric oxide and cyclic GMP in rabbit urethral relaxation. Eur J Pharmacol 269, 269–275. [DOI] [PubMed] [Google Scholar]
- Donker PJ, Ivanovici F & Noach EL (1972). Analyses of the urethral pressure profile by means of electromyography and the administration of drugs. Br J Urol 44, 180–193. [DOI] [PubMed] [Google Scholar]
- Drumm BT, Hennig GW, Battersby MJ, Cunningham EK, Sung TS, Ward SM, Sanders KM & Baker SA (2017). Clustering of Ca2+ transients in interstitial cells of Cajal defines slow wave duration. J Gen Physiol 149, 703–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumm BT, Koh SD, Andersson KE & Ward SM (2014a). Calcium signalling in Cajal‐like interstitial cells of the lower urinary tract. Nat Rev Urol 11, 555–564. [DOI] [PubMed] [Google Scholar]
- Drumm BT, Large RJ, Hollywood MA, Thornbury KD, Baker SA, Harvey BJ, McHale NG & Sergeant GP (2015). The role of Ca2+ influx in spontaneous Ca2+ wave propagation in interstitial cells of Cajal from the rabbit urethra. J Physiol 593, 3333–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumm BT, Sergeant GP, Hollywood MA, Thornbury KD, McHale NG & Harvey BJ (2014b). The role of cAMP dependent protein kinase in modulating spontaneous intracellular Ca2+ waves in interstitial cells of Cajal from the rabbit urethra. Cell Calcium 56, 181–187. [DOI] [PubMed] [Google Scholar]
- Drumm BT, Sergeant GP, Hollywood MA, Thornbury KD, Matsuda TT, Baba A, Harvey BJ & McHale NG (2014c). The effect of high [K+]o on spontaneous Ca2+ waves in freshly isolated interstitial cells of Cajal from the rabbit urethra. Physiol Rep 2, e00203 https://doi.org/10.1002/phy2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek A, Alm P, Andersson KE & Persson CG (1977). Adrenoceptor and cholinoceptor mediated responses of the isolated human urethra. Scand J Urol Nephrol 11, 97–102. [DOI] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M & Rao A (2006). A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441, 179–185. [DOI] [PubMed] [Google Scholar]
- Forman A, Andersson KE, Henriksson L, Rud T & Ulmsten U (1978). Effects of nifedipine on the smooth muscle of the human urinary tract in vitro and in vivo . Acta Pharmacol Toxicol (Copenh) 43, 111–118. [DOI] [PubMed] [Google Scholar]
- Fry CH & Jabr RI (2014). T‐type Ca2+ channels and the urinary and male genital tracts. Pflugers Arch 466, 781–789. [DOI] [PubMed] [Google Scholar]
- Garcia‐Pascual A, Costa G, Garcia‐Sacristan A & Andersson KE (1991). Calcium dependence of contractile activation of isolated sheep urethra. II: responses to exogenous noradrenaline. Pharmacol Toxicol 69, 270–275. [DOI] [PubMed] [Google Scholar]
- Gibson A, McFadzean I, Wallace P & Wayman CP (1998). Capacitative Ca2+ entry and the regulation of smooth muscle tone. Trends Pharmacol Sci 197, 266–269. [DOI] [PubMed] [Google Scholar]
- Gosling JA & Dixon JS (1975). The structure and innervation of smooth muscle in the wall of the bladder neck and proximal urethra. Br J Urol 47, 549–558. [DOI] [PubMed] [Google Scholar]
- Greenland JE, Dass N & Brading AF (1996). Intrinsic urethral closure mechanisms in the female pig. Scand J Urol Nephrol 179, 75–80. [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology . J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harteneck C & Gollasch M (2011). Pharmacological modulation of diacylglycerol‐sensitive TRPC3/6/7 channels. Curr Pharm Biotechnol 12, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H & Edwards FR (1999). Spontaneous and neurally activated depolarizations in smooth muscle cells of the guinea‐pig urethra. J Physiol 514, 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H & Suzuki H (2007). Properties of spontaneous Ca2+ transients recorded from interstitial cells of Cajal‐like cells of the rabbit urethra in situ . J Physiol 583, 505–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Van Helden DF & Suzuki H (1996). Properties of spontaneous depolarizations in circular smooth muscle cells of rabbit urethra. Br J Pharmacol 118, 1627–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Yanai Y, Kohri K & Suzuki H (2006). Heterogeneous CPA sensitivity of spontaneous excitation in smooth muscle of the rabbit urethra. Br J Pharmacol 148, 340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez M, Knight GE, Wildman SS & Burnstock G (2009). Role of ATP and related purines in inhibitory neurotransmission to the pig urinary bladder neck. Br J Pharmacol 157, 1463–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill WG (2015). Control of urinary drainage and voiding. Clin J Am Soc Nephrol 10, 480–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokanson JA, Langdale CL, Sridhar A & Grill WM (2017). OAB without an overactive bladder in the acute prostaglandin E2 rat model. Am J Physiol Renal Physiol 313, F1169–F1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollywood MA, Woolsey S, Walsh IK, Keane PF, McHale NG & Thornbury KD (2003). T‐ and L‐type Ca2+ currents in freshly dispersed smooth muscle cells from the human proximal urethra. J Physiol 550, 753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, L , Keyser, BM , Tagmose TM, Hansen JB, Taylor JT, Zhuang H, Zhang M, Ragsdale DS & Li M (2004). NNC 55‐0396 [(1S,2S)‐2‐(2‐(N‐[(3‐benzimidazol‐2‐yl)propyl]‐N‐methylamino)ethyl)‐6‐fluoro‐1,2,3,4‐tetrahydro‐1‐isopropyl‐2‐naphtyl cyclopropanecarboxylate dihydrochloride]: a new selective inhibitor of T‐type calcium channels. J Pharmacol Exp Ther 309, 193–199. [DOI] [PubMed] [Google Scholar]
- Ito Y & Kimoto Y (1985). The neural and non‐neural mechanisms involved in urethral activity in rabbits. J Physiol 367, 57–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger JH, Porter VA, Lederer WJ & Nelson MT (2000). Calcium sparks in smooth muscle. Am J Physiol Cell Physiol 278, 235–256. [DOI] [PubMed] [Google Scholar]
- Jankowski RJ, Prantil RL, Chancellor MB, de Groat WC, Huard J & Vorp DA (2006). Biomechanical characterization of the urethral musculature. Am J Physiol Renal Physiol 290, 1127–1134. [DOI] [PubMed] [Google Scholar]
- Kedia GT, Oelke M, Sohn M, Kuczyk MA & Uckert S (2013). Pharmacologic characterization of human male urethral smooth muscle: an in vitro approach. Urology 82, 1451.e13–1451.e19. [DOI] [PubMed] [Google Scholar]
- Kimoto Y, Nozaki M & Itoh T (1987). Actions of the alpha‐1 adrenoceptor blocker bunazosin on the norepinephrine‐induced contraction of smooth muscles in the rabbit proximal urethra. J Pharmacol Exp Ther 241, 1017–1022. [PubMed] [Google Scholar]
- Kirschner‐Hermanns R, Anding R, Rosier P, Birder L, Andersson KE & Djurhuus JC (2016). Fundamentals and clinical perspective of urethral sphincter instability as a contributing factor in patients with lower urinary tract dysfunction – ICI‐RS 2014. Neurourol Urodyn 35, 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh BH, Roy R, Hollywood MA, Thornbury KD, McHale NG, Sergeant GP, Hatton WJ, Ward SM, Sanders KM & Koh SD (2012). Platelet‐derived growth factor receptor‐α cells in mouse urinary bladder: a new class of interstitial cells. J Cell Mol Med 16, 691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus RL, Li Y, Gregan Y, Gotter AL, Uebele VN, Fox SV, Doran SM, Barrow JC, Yang ZQ, Reger TS, Koblan KS & Renger JJ (2010). In vitro characterization of T‐type calcium channel antagonist TTA‐A2 and in vivo effects on arousal in mice. J Pharmacol Exp Ther 335, 409–417. [DOI] [PubMed] [Google Scholar]
- Kurahashi M, Zheng H, Dwyer L, Ward SM, Koh SD & Sanders KM (2011). A functional role for the ‘fibroblast‐like cells’ in gastrointestinal smooth muscles. J Physiol 589, 697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle BD (2014). Ion channels of the mammalian urethra. Channels (Austin) 8, 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson B, Hogestatt ED, Mattiasson A & Andersson KE (1984). Differential effects of nifedipine, verapamil and diltiazem on noradrenaline‐induced contractions, adrenergic transmitter release and alpha‐adrenoceptor binding in the female rabbit urethra. Naunyn Schmiedebergs Arch Pharmacol 326, 14–21. [DOI] [PubMed] [Google Scholar]
- Lemack GE, Amundsen C, Badlani G, Griebling T, Peters K, Rodriguez L, Singla A, Smith C, Sullivan M & Te A (2007). Highlights from the combined Society of Urodynamics and Female Urology and International Society of Pelvic Neuromodulation annual meeting. J Urol 177, 691–695. [DOI] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr & Meyer T (2005). STIM is a Ca2+ sensor essential for Ca2+‐store‐depletion‐triggered Ca2+ influx. Curr Biol 15, 1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscombe D, Helton TD & Xu W (2004). L‐type calcium channels: the low down. J Neurophysiol 92, 2633–2641. [DOI] [PubMed] [Google Scholar]
- McHale NG, Hollywood MA, Sergeant GP, Shafei M, Thornbury KD & Ward SM (2006). Organisation and function of ICC in the urinary tract. J Physiol 576, 689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiasson A, Andersson KE, Andersson PO, Larsson B, Sjögren C & Uvelius B (1989). Nerve‐mediated functions in the circular and longitudinal muscle layers of the proximal female rabbit urethra. J Urol 143, 155–160. [DOI] [PubMed] [Google Scholar]
- Mawby DI, Meric S, Crichlow EC & Papich MG (1991). Pharmacological relaxation of the urethra in male cats: a study of the effects of phenoxybenzamine, diazepam, nifedipine and xylazine. Can J Vet Res 55, 28–32. [PMC free article] [PubMed] [Google Scholar]
- Merrill L, Gonzalez EJ, Girard BM & Vizzard MA (2016). Receptors, channels, and signalling in the urothelial sensory system in the bladder. Nat Rev Urol 13, 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignen O, Thompson JL & Shuttleworth TJ (2008). Both Orai1 and Orai3 are essential components of the arachidonate‐regulated Ca2+‐selective (ARC) channels. J Physiol 586, 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JM, Ashton‐Miller JA & DeLancey JO (1998). A pelvic muscle precontraction can reduce cough related urine loss in selected women with mild SUI. J Am Geriatr Soc 46, 870–874. [DOI] [PubMed] [Google Scholar]
- Mutoh S, Latifpour J, Saito M & Weiss RM (1997). Evidence for the presence of regional differences in the subtype specificity of muscarinic receptors in rabbit lower urinary tract. J Urol 157, 717–721. [PubMed] [Google Scholar]
- Nelson MT, Cheng M, Santana LF, Bonev AD, Knot HJ & Lederer WJ (1995). Relaxation of arterial smooth muscle by calcium sparks. Science 270, 633–637. [DOI] [PubMed] [Google Scholar]
- Peppiatt CM, Collins TJ, Mackenzie L, Conway SJ, Holmes AB, Bootman MD, Berridge MJ, Seo JT & Roderick HL (2003). 2‐Aminoethoxydiphenyl borate (2‐APB) antagonises inositol 1,4,5‐trisphosphate‐induced calcium release, inhibits calcium pumps and has a use‐dependent and slowly reversible action on store‐operated calcium entry channels. Cell Calcium 34, 97–108. [DOI] [PubMed] [Google Scholar]
- Peri LE, Koh BH, Ward GK, Bayguinov Y, Hwang SJ, Gould TW, Mullan CJ, Sanders KM & Ward SM (2015). A novel class of interstitial cells in the mouse and monkey female reproductive tracts. Biol Reprod 92, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna C, Glass R, Knight GE, Bolego C, Puglisi L & Burnstock G (2005). Purine‐ and pyrimidine‐induced response and P2Y receptor characterization in the hamseter proximal urethra. Br J Pharmacol 144, 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna C, Puglisi L & Burnstock G (1998). ATP and vasoactive intestinal polypeptide relaxant responses in hamster isolate proximal urethra. Br J Pharmacol 124, 1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M & Lewis RS (2015). Store‐operated calcium channels. Physiol Rev 95, 1383–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW (1986). A model for receptor‐regulated calcium entry. Cell Calcium 7, 1–12. [DOI] [PubMed] [Google Scholar]
- Quinn T, Molloy M, Smyth A & Baird AW (2004). Capacitative calcium entry in guinea pig gallbladder smooth muscle in vitro. Life Sci 74, 1659–1669. [DOI] [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McCloskey KD, Thornbury KD & McHale NG (2000). Specialised pacemaking cells in the rabbit urethra. J Physiol 526, 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneyd J, Keizer JE & Sanderson MJ (1995). Mechanisms of calcium oscillations and waves: a quantitative analysis. FASEB J 9, 1463–1472. [DOI] [PubMed] [Google Scholar]
- Sneyd J, Tsaneava‐Atanasova K, Yule DI, Thompson JL & Shuttleworth TJ (2004). Control of calcium oscillations by membrane fluxes. Proc Natl Acad Sci USA 101, 1392–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soboloff J, Rothberg BS, Madesh M & Gill DL (2012). STIM proteins: dynamic calcium signal transducers. Nat Rev Mol Cell Biol 13, 549–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogbein SK, Downie JW & Awad SA (1984). Urethral response during bladder contraction induced by subcutaneous bethanechol chloride elicitation of a sympathetic reflex urethral constriction. J Urol 131, 791–795. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Wu X, Walker LA & Somlyo AV (1999). Pharmacomechanical coupling: the role of calcium, G‐proteins, kinases and phosphatases. Rev Physiol Biochem Pharmacol 134, 201–234. [DOI] [PubMed] [Google Scholar]
- Staskin D, Tubaro A, Norton PA & Ashton‐Miller JA (2011). Mechanisms of continence and surgical care in female and male SUI: surgical research initiatives. Neurourol Urodyn 30, 704–707. [DOI] [PubMed] [Google Scholar]
- Thaker H & Sharma AK (2013). Regenerative medicine based applications to combat stress urinary incontinence. World J Stem Cells 5, 112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebak M & Putney JW Jr (2017). ORAI calcium channels. Physiology (Bethesda) 32, 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanagho EA & Smith DR (1966). The anatomy and function of the bladder neck. Br J Urol 38, 54–71. [DOI] [PubMed] [Google Scholar]
- Thornbury KD, Hollywood MA & McHale NG (1992). Mediation by nitric oxide of neurogenic relaxation of the urinary bladder neck muscle in sheep. J Physiol 451, 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebak M, Zhang W, Ruhle B, Henkel MM, Gonzalez‐Cobos JC, Motiani RK, Stolwijk JA, Newton RL & Zhang X (2013). What role for store‐operated Ca2+ entry in muscle? Microcirculation 20, 330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmsten U & Andersson KE (1977). The effects of emperone on intravesical and intra‐urethral pressures in women with urgency incontinence. Scand J Urol Nephrol 11, 103–109. [DOI] [PubMed] [Google Scholar]
- Waldeck K, Ny L, Persson K & Andersson KE (1998). Mediators and mechanisms of relaxation in rabbit urethral smooth muscle. Br J Pharmacol 123, 617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westcott EB, Goodwin EL, Segal SS & Jackson WF (2012). Function and expression of ryanodine receptors and inositol 1,4,5‐trisphosphate receptors in smooth muscle cells of murine feed arteries and arterioles. J Physiol 590, 1849–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C & McGeown JG (2002). Carbachol triggers RyR‐dependent Ca2+ release via activation of IP3 receptors in isolated rat gastric myocytes. J Physiol 542, 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi T, Chapple CR, Yasuda K, Yoshida K & Chess‐Williams R (2002). The role of M2 muscarinic receptor subtypes mediating contraction of the circular and longitudinal smooth muscle of the pig proximal urethra. J Urol 168, 308–314. [PubMed] [Google Scholar]
- Yao Y & Parker I (1994). Ca2+ influx modulation of temporal and spatial patterns of inositol trisphosphate‐mediated Ca2+ liberation in Xenopus oocytes. J Physiol 476, 17–28. [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA & Cahalan MD (2006). Genome‐wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release‐activated Ca2+ channel activity. Proc Natl Acad Sci USA 103, 9357–9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZhuGe R, Sims S, Tuft RA, Fogarty KE & Walsh JV Jr (1998). Ca2+ sparks activate K+ and Cl− channels, resulting in spontaneous transient currents in guinea‐pig tracheal myocytes. J Physiol 513, 711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PK, Zygmunt PM, Högestätt E & Andersson KE (1995). NANC neurotransmission in lamina propria of the rabbit urethra: regulation by different subsets of calcium channels. Br J Pharmacol 115, 1020–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclaimer: Supporting information has been peer‐reviewed but not copyedited.
Movie S1. Spontaneous intracellular Ca2+ waves in USMC are associated with USMC contractions.
Movie S2. Spontaneous Ca2+ waves in USMC in situ.
Movie S3. Phenylephrine increases Ca2+ waves in USMC.


