Summary
Background
Low-risk limits recommended for alcohol consumption vary substantially across different national guidelines. To define thresholds associated with lowest risk for all-cause mortality and cardiovascular disease, we studied individual-participant data from 599 912 current drinkers without previous cardiovascular disease.
Methods
We did a combined analysis of individual-participant data from three large-scale data sources in 19 high-income countries (the Emerging Risk Factors Collaboration, EPIC-CVD, and the UK Biobank). We characterised dose–response associations and calculated hazard ratios (HRs) per 100 g per week of alcohol (12·5 units per week) across 83 prospective studies, adjusting at least for study or centre, age, sex, smoking, and diabetes. To be eligible for the analysis, participants had to have information recorded about their alcohol consumption amount and status (ie, non-drinker vs current drinker), plus age, sex, history of diabetes and smoking status, at least 1 year of follow-up after baseline, and no baseline history of cardiovascular disease. The main analyses focused on current drinkers, whose baseline alcohol consumption was categorised into eight predefined groups according to the amount in grams consumed per week. We assessed alcohol consumption in relation to all-cause mortality, total cardiovascular disease, and several cardiovascular disease subtypes. We corrected HRs for estimated long-term variability in alcohol consumption using 152 640 serial alcohol assessments obtained some years apart (median interval 5·6 years [5th–95th percentile 1·04–13·5]) from 71 011 participants from 37 studies.
Findings
In the 599 912 current drinkers included in the analysis, we recorded 40 310 deaths and 39 018 incident cardiovascular disease events during 5·4 million person-years of follow-up. For all-cause mortality, we recorded a positive and curvilinear association with the level of alcohol consumption, with the minimum mortality risk around or below 100 g per week. Alcohol consumption was roughly linearly associated with a higher risk of stroke (HR per 100 g per week higher consumption 1·14, 95% CI, 1·10–1·17), coronary disease excluding myocardial infarction (1·06, 1·00–1·11), heart failure (1·09, 1·03–1·15), fatal hypertensive disease (1·24, 1·15–1·33); and fatal aortic aneurysm (1·15, 1·03–1·28). By contrast, increased alcohol consumption was log-linearly associated with a lower risk of myocardial infarction (HR 0·94, 0·91–0·97). In comparison to those who reported drinking >0–≤100 g per week, those who reported drinking >100–≤200 g per week, >200–≤350 g per week, or >350 g per week had lower life expectancy at age 40 years of approximately 6 months, 1–2 years, or 4–5 years, respectively.
Interpretation
In current drinkers of alcohol in high-income countries, the threshold for lowest risk of all-cause mortality was about 100 g/week. For cardiovascular disease subtypes other than myocardial infarction, there were no clear risk thresholds below which lower alcohol consumption stopped being associated with lower disease risk. These data support limits for alcohol consumption that are lower than those recommended in most current guidelines.
Funding
UK Medical Research Council, British Heart Foundation, National Institute for Health Research, European Union Framework 7, and European Research Council.
Introduction
Alcohol consumption guidelines vary substantially across the globe.1, 2 In the USA, for example, an upper limit of 196 g per week (about 11 standard UK glasses of wine or pints of beer per week) is recommended for men, and an upper limit of 98 g per week is recommended for women.1 Similar recommendations apply in Canada and Sweden.2 By contrast, guidelines in Italy, Portugal, and Spain recommend low-risk limits almost 50% higher than these.1, 2 At the other extreme, UK guidelines recommend low-risk limits for men almost half that recommended by US guidelines.1, 2
Such variation in policy might reflect ambiguity about drinking risk thresholds associated with the lowest risk of mortality,3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 as well as uncertainty about the specific consequences of alcohol consumption, including those related to cardiovascular disease subtypes. For example, recent studies have challenged the concept that moderate alcohol consumption is universally associated with lower cardiovascular disease risk,16, 17 but the dose–response associations of alcohol consumption with cardiovascular disease subtypes remain poorly understood. Therefore, to help in the formulation of evidence-based alcohol policy, we analysed individual-participant data from 83 long-term prospective studies in 19 high-income countries. Our aim was to characterise risk thresholds for all-cause mortality and cardiovascular disease subtypes in current drinkers of alcohol.
Methods
Study design, data sources, and participants
We focused our study on current alcohol drinkers for three main reasons. First, alcohol guidelines provide recommendations about low-risk limits only for drinkers (we are unaware of any guidelines that encourage non-drinkers to consume alcohol). Second, a focus on current drinkers should limit potential biases that are difficult to control in observational studies (eg, reverse causality, residual confounding, and unmeasured effect modification) because ex-drinkers include people who might have abstained from alcohol owing to poor health itself,18, 19, 20 as well as those who have changed their habits to achieve a healthier lifestyle. Third, never-drinkers might differ systematically from drinkers in ways that are difficult to measure, but which might be relevant to disease causation.21
We did a combined analysis of individual-participant data from three large-scale data sources available to our consortium, each constituting purpose-designed prospective cohort studies with quantitative information about alcohol consumption (appendix p 21). First, the Emerging Risk Factors Collaboration (ERFC) is a collaboration of prospective cohort studies with information about a variety of risk factors, cardiovascular disease outcomes, and mortality.22 Of the 102 studies in the ERFC with information about alcohol status, 81 contained information about the quantity of consumption. Second, EPIC-CVD, a ten-country case-cohort study nested in the European Prospective Investigation into Cancer and Nutrition (EPIC) prospective cohort study, had quantitative alcohol information from 22 of its 23 contributing centres.23 Third, UK Biobank—a single large prospective study—had cohort-wide data about quantitative alcohol consumption.24 Therefore, our combined analysis included information from a total of 83 prospective studies that each used broadly similar methods to quantify alcohol consumption, record risk factors, and ascertain cause-specific death and cardiovascular disease events. We harmonised records of alcohol consumption across the contributing studies using a conversion of 1 unit=8 g of pure alcohol to a standard scale of grams per week (appendix pp 1–2), enabling a common analytical approach despite variation in the methods used (eg, self-administered vs interview-led questionnaires; food frequency questionnaires vs dietary recall surveys), and in consumption scales over different periods of ascertainment. Details of contributing studies are in appendix pp 3–4, 10–11.
Research in context.
Evidence before this study
We searched for prospective epidemiological studies of alcohol consumption investigating disease risk thresholds published in any language up until March 1, 2017 (with no specified earliest date), in PubMed, Scientific Citation Index Expanded, and Embase using relevant terms (“alcohol”, “mortality”, “survival”, “cardiovascular disease”, “cohort”, and “prospective”). We found many primary reports and literature-based reviews. However, no study had combined the following key features required to achieve reliable estimates of dose–response associations: availability of individual-participant data; quantitative assessment of alcohol consumption levels using validated instruments; periodic re-surveys of alcohol consumption levels; recording of large numbers of deaths (eg, >20 000 deaths); and sufficient detail and power to disaggregate incident cardiovascular disease outcomes into subtypes (eg, >20 000 incident total cardiovascular disease outcomes).
Added value of this study
The current study combined all the key study design features mentioned above, and afforded several additional advantages. First, it reduced the potentially distorting effects of reverse causality by focusing on current drinkers without previous cardiovascular disease who survived at least 12 months of follow-up. Second, it enhanced generalisability by including individual-participant data from 83 prospective studies in 19 different high-income countries. Third, it used a variety of established and emerging risk factors, enabling investigation of potential confounders and mediators.
Implications of all the available evidence
The chief implication of this study for public policy is to support reductions of alcohol consumption limits in existing guidelines, suggesting that the threshold for lowest risk for all-cause mortality is about 100 g per week (about 5–6 standard UK glasses of wine or pints of beer per week). The chief implication for scientific understanding is the strengthening of evidence that the association between alcohol consumption and total cardiovascular disease risk is actually comprised of several distinct and opposite dose–response curves rather than a single J-shaped association.
To be eligible for the analysis, participants had to have information recorded about their alcohol consumption amount and status (ie, non-drinker vs current drinker), plus age, sex, history of diabetes and smoking status, at least 1 year of follow-up after baseline, and no known baseline history of cardiovascular disease (defined as coronary heart disease, other heart disease, stroke, transient ischaemic attack, peripheral arterial disease, or cardiovascular surgery); appendix p 21. The main analyses focused on current drinkers, whose baseline alcohol consumption was categorised into eight predefined groups according to the amount in grams consumed per week: >0–≤25, >25–≤50, >50–≤75, >75–≤100, >100–≤150, >150–≤250, >250–≤350, and >350 g per week. We assessed alcohol consumption in relation to all-cause mortality, total cardiovascular disease, and the following cardiovascular disease subtypes (defined in appendix p 5): fatal and non-fatal myocardial infarction; fatal and non-fatal coronary disease excluding myocardial infarction; fatal and non-fatal stroke (including ischaemic, haemorrhagic, subarachnoid, and unclassified subtypes of stroke); fatal and non-fatal heart failure; and mortality from other cardiovascular causes, including cardiac dysrhythmia, hypertensive disease, sudden death, and aortic aneurysm.7, 17, 25 In analyses of cardiovascular disease subtypes, participants contributed follow-up time until the first outcome recorded (ie, cardiovascular deaths preceded by non-fatal outcomes were not included). Event times were censored at the end of follow-up or death from non-cardiovascular causes.
Statistical analysis
Hazard ratios (HRs) for alcohol consumption were calculated separately within each study using Cox regression models, stratified by sex and with adjustment for known confounders: age, smoking status (current vs non-current) and history of diabetes. To account for EPIC-CVD's case-cohort design (which was used because lipids and other cardiovascular disease biomarkers were measured only in the case-cohort subset and not the full EPIC cohort), the Cox models for cardiovascular disease events were adapted using Prentice weights and stratified by centre.26 For the four case-control studies nested within prospective cohorts of the ERFC, odds ratios were calculated using, as appropriate, conditional or unconditional logistic regression models, taking into account relevant matching factors. Study-specific estimates were then pooled across studies by random-effects meta-analysis.27 We tested for violation of the proportional hazards assumption by including time interactions with alcohol consumption. To avoid model overfitting, studies with fewer than five incident cases of a particular outcome were excluded from analyses of that particular outcome.
To correct for measurement error and within-person variability in alcohol consumption over time, we estimated long-term average (henceforth, “usual”) alcohol consumption using multi-level regression calibration and information from 152 640 serial assessments in 71 011 individuals from 37 studies. This calculation was achieved either by regressing re-survey measurements (for the repeat alcohol assessments available in the ERFC studies and UK Biobank) or lifetime alcohol consumption measurements (for calculated lifetime alcohol consumption measurements available in EPIC-CVD) on baseline alcohol consumption, adjusted for duration of follow-up and baseline age, sex, smoking status, history of diabetes, other relevant covariate(s), and with random effects for study and re-survey.28, 29 The regression dilution ratio (ie, the calibration slope), which measures the extent of within-person variability,28 was extracted from the calibration model. HRs in this paper relate to usual alcohol consumption levels unless specified otherwise.
We assessed the shapes of associations for all-cause mortality and cardiovascular disease outcomes by calculating study-specific HRs within the predefined groups of baseline alcohol consumption, pooled them by multivariate random-effects meta-analysis, and plotted them against mean usual (and baseline) alcohol consumption within each group. We estimated 95% CIs for each group (including the reference group) that corresponded to the amount of information underlying each group.30, 31 For each major outcome, we determined the best fitting first or second order fractional polynomial32 to describe the association with baseline alcohol consumption (using a 1% significance level as evidence for a second order fractional polynomial over a first order fractional polynomial) using Cox regression models stratified by sex, study, and centre. Further analyses assumed a linear association with alcohol consumption, expressing results per 100 g per week (12·5 units/week) in usual alcohol consumption. To assess the effect of excluding known current drinkers with missing alcohol consumption data, we did a sensitivity analysis using multiple imputation within studies, before combining the data in a meta-analysis. We investigated associations with alcohol type (wine, beer, and spirits), consumption frequency (dichotomised as drinkers who consumed alcohol on ≤2 days per week or those who consumed alcohol on >2 days per week) and episodic heavy drinking (dichotomised as binge drinkers who consumed ≥100 g per drinking occasion or non-binge drinkers who consumed <100 g per drinking occasion).
We used regression calibration methods similar to those described above to estimate and adjust for long-term levels of potential confounding factors or mediators in individuals with available information. HRs were adjusted for usual levels of available potential confounders or mediators, including body-mass index (BMI), systolic blood pressure, high-density-lipoprotein cholesterol (HDL-C), low-density-lipoprotein cholesterol (LDL-C), total cholesterol, fibrinogen, and baseline measures for smoking amount (in pack-years), level of education reached (no schooling or primary education only vs secondary education vs university), occupation (not working vs manual vs office vs other), self-reported physical activity level (inactive vs moderately inactive vs moderately active vs active), self-reported general health (scaled 0–1 where low scores indicate poorer health), self-reported red meat consumption, and self-reported use of anti-hypertensive drugs. We investigated effect modification with formal tests for interaction, using a 0·1% significance threshold to make some allowance for multiple testing. Heterogeneity was investigated by grouping studies according to recorded characteristics and through meta-regression, assessed by the I2 statistic.33 Evidence of small study effects was assessed visually with funnel plots and by Begg and Mazumdar's test34 and Egger's test.35
Methods we used to estimate reductions in life expectancy (years of life lost) are described in the appendix (pp 6–7). Briefly, estimates of cumulative survival from 40 years of age onwards in different categories of baseline alcohol consumption were calculated by applying estimated HRs (specific to age-at-risk) for cause-specific mortality to the detailed mortality component of the US Centers for Disease Control and Prevention's WONDER database,36 which recorded 10 million deaths (from all causes) in more than 305 million individuals in the USA during 2007–10.37, 38 Results were modelled from age 40 years and enabled estimation of years of life lost between light drinkers (defined as those consuming >0–≤100 g/week of alcohol) and pre-defined groups of >100–≤200, >200–≤350, and >350 g per week. This method does not make use of the survival estimates from the modelled data; instead, it makes inferences by estimating age-at-risk specific HRs, which are then combined with external population age-specific mortality rates.39
Analyses used Stata (version 14.2 and 15.1). All p values presented are for 2-sided tests.
Role of the funding source
The funders of the study did not have any role in the study design, data analysis, or reporting of this manuscript. AMW and SK had full access to the combined dataset, and, together with EDA and JD, had responsibility for the decision to submit the manuscript for publication.
Results
Of the 786 787 participants with sufficient information for inclusion in this consortium, 186 875 (19%) reported not drinking at baseline, leaving 599 912 current drinkers without a history of cardiovascular disease at baseline who were eligible for the prespecified principal analysis. The current drinkers were derived from ERFC (247 504 participants), EPIC-CVD (26 036), and the UK Biobank (326 372; table 1). Baseline year of recruitment ranged from 1964 to 2010. The mean age of the participants was 57 years (SD 9). 265 910 (44%) of 599 912 participants were women, and 128 085 (21%) were current smokers (appendix p 12). About 50% reported drinking more than 100 g of alcohol per week, and 8·4% drank more than 350 g per week (table 1). During 5·4 million person-years (median 7·5 years of follow-up [5th–95th percentiles 5·0–18·4]), there were 40 310 deaths from all causes, (including 11 762 vascular and 15 150 neoplastic deaths), and 39 018 first incident cardiovascular disease outcomes, including 12 090 stroke events, 14 539 myocardial infarction events, 7990 coronary disease events excluding myocardial infarction, 2711 heart failure events, and 1121 deaths from other cardiovascular diseases (appendix p 13).
Table 1.
Study-level and participant-level characteristics of the contributing data sources
| ERFC | EPIC-CVD | UK Biobank | Participants with resurveys of alcohol consumption | ||
|---|---|---|---|---|---|
| Study level characteristics | |||||
| Location | 81 studies in 19 countries | 22 centres in 10 European countries | England, Scotland, and Wales | 37 studies in 15 countries | |
| Years of recruitment | 1964–2008 | 1990–2002 | 2006–10 | 1964–2010 | |
| Year of most recent endpoint follow-up | 2013 | 2009 | 2016 | 2016 | |
| Participant level characteristics | |||||
| Total participants | 356 819 | 30 702 | 358 833 | 89 499 | |
| Known current drinkers at baseline | 247 504 | 26 036 | 326 372 | 71 011 | |
| Weekly baseline alcohol consumption in current drinkers | |||||
| >0–≤25 g per week | 53 418 (22%) | 7906 (30%) | 39 641 (12%) | 12 301 (17% [11 g/week vs 36 g/week]‡) | |
| >25–≤50 g per week | 33 953 (14%) | 3704 (14%) | 39 334 (12%) | 8365 (12% [38 g/week vs 56 g/week]‡) | |
| >50–≤75 g per week | 26 656 (11%) | 2748 (11%) | 42 907 (13%) | 7322 (10% [63 g/week vs 80 g/week]‡) | |
| >75–≤100 g per week | 16 557 (7%) | 2446 (9%) | 36 780 (11%) | 6394 (9% [87 g/week vs 98 g/week]‡) | |
| >100–≤150 g per week | 36 236 (15%) | 2602 (10%) | 55 815 (17%) | 10 051 (14% [126 g/week vs 126 g/week]‡) | |
| >150–≤250 g per week | 31 645 (13%) | 3090 (12%) | 60 025 (18%) | 12 255 (17% [193 g/week vs 173 g/week]‡) | |
| >250–≤350 g per week | 23 607 (10%) | 1744 (7%) | 26 669 (8%) | 6927 (10% [303 g/week vs 248 g/week]‡) | |
| ≥350 g per week | 25 432 (10%) | 1796 (7%) | 25 201 (8%) | 7396 (10% [515 g/week vs 354 g/week]‡) | |
| Baseline characteristics restricted to all current drinkers | |||||
| Alcohol consumption (g/week), median (5th–95th percentiles) | 87·7 (2·2–522·4) | 61·9 (2·6–404·0) | 103·9 (11·8–420·8) | 105·2 (6·0–482·8) | |
| Age (years) at baseline | 57·1 (8·7) | 55·0 (9·2) | 56·5 (8·0) | 55·3 (8·2) | |
| Sex | |||||
| Male | 162 685 (66%) | 13 508 (52%) | 157 809 (48%) | 44 360 (62%) | |
| Female | 84 819 (34%) | 12 528 (48%) | 168 563 (52%) | 26 651 (38%) | |
| Smoking status | |||||
| Not current | 161 037 (65%) | 17 608 (68%) | 293 182 (90%) | 50 930 (72%) | |
| Current | 86 467 (35%) | 8428 (32%) | 33 190 (10%) | 20 081 (28%) | |
| History of diabetes | |||||
| No | 237 685 (96%) | 24 875 (96%) | 315 090 (97%) | 68 159 (96%) | |
| Yes | 9819 (4%) | 1161 (4%) | 11 282 (3%) | 2852 (4%) | |
| BMI, kg/m2 | 26·1 (3·8) | 26·4 (4·1) | 27·0 (4·4) | 26·1 (3·8) | |
| HDL-C, mmol/L | 1·40 (0·41) | 1·40 (0·42) | Not available* | 1·41 (0·41) | |
| Total cholesterol, mmol/L | 5·80 (1·17) | 6·11 (1·16) | Not available* | 5·78 (1·08) | |
| Systolic blood pressure, mm Hg | 136·5 (19·0) | 138·4 (21·3) | 137·9 (18·5) | 134·6 (18·4) | |
| Major outcomes restricted to current drinkers | |||||
| All-cause mortality events | 32 813 | 784† | 6720 | 6912 | |
| All cardiovascular disease | 18 791 | 12 758 | 7469 | 11 597 | |
Data are n, n (%), or mean (SD), unless otherwise indicated. ERFC=Emerging Risk Factors Collaboration. EPIC-CVD=European Prospective Investigation into Cancer and Nutrition—Cardiovascular Disease. BMI=body-mass index. HDL-C=high-density-lipoprotein cholesterol.
At the time of analysis, measurements of HDL-C and total cholesterol were not available in the UK Biobank.
All-cause mortality events from EPIC derive only from the 13 670 participants in the random sub-cohort of EPIC-CVD, rather than from the entire EPIC prospective study.
Mean consumption (g/week) at baseline vs resurvey.
Baseline alcohol consumption varied substantially across studies, was generally lower in more recent calendar periods of recruitment, and was positively skewed (median 96 g/week [5th–95th percentiles 6–448]; appendix p 22). It was weakly and positively correlated with male sex, smoking status and amount, systolic blood pressure, HDL-C level, fibrinogen, and lower socioeconomic status (appendix pp 23–24). 152 640 serial assessments of alcohol consumption were available for 71 011 participants from 37 studies (median interval between baseline and serial measurements 5·6 years [5th–95th percentiles 1·04–13·5]). Participants with serial measurements were younger, had slightly higher baseline alcohol consumption, and were more likely to be men than those without serial measurements (table 1, appendix p 14). The regression dilution ratio for alcohol consumption was 0·50 (95% CI 0·47–0·52), similar to that for systolic blood pressure (0·52, 0·50–0·55) but lower than that for HDL-C concentration (0·74, 0·72–0·76) in a common set of participants.
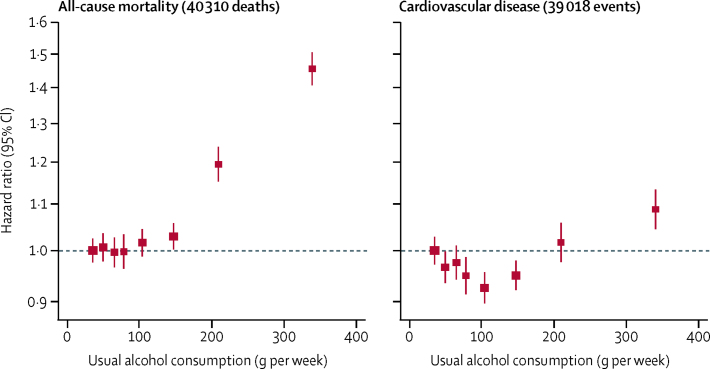
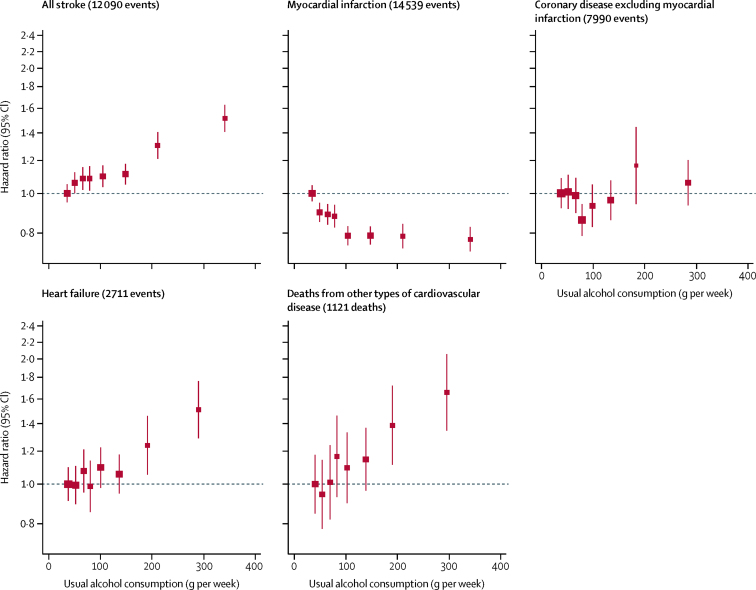
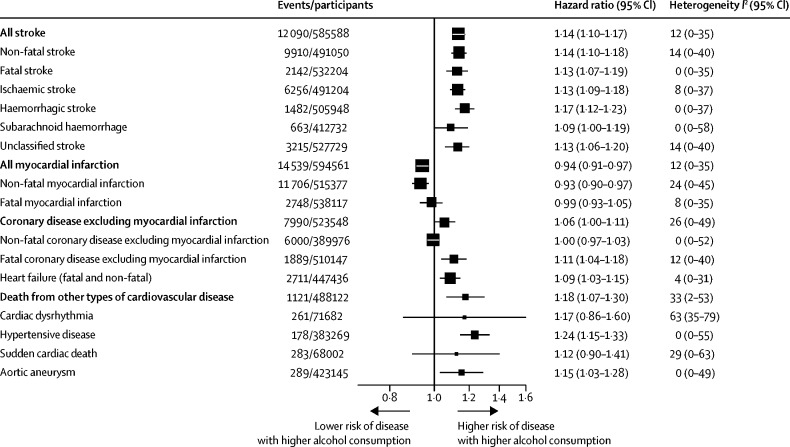
For all-cause mortality, there was a positive and curvilinear association with alcohol consumption, with the lowest risk for those consuming below 100 g per week (figure 1, appendix p 25). Associations were similar for men and women (appendix p 26), but weaker at older ages (appendix p 27). There was a J-shaped association for the aggregate of cardiovascular disease outcomes (figure 1, appendix p 25). However, disaggregation showed two opposing sets of associations (figure 2). After adjustment for age, sex, smoking, and history of diabetes, the amount of alcohol consumed had positive and roughly linear associations with stroke (HR per 100 g/week higher consumption 1·14, 1·10–1·17), coronary disease excluding myocardial infarction (1·06, 1·00–1·11), heart failure (1·09, 1·03–1·15), fatal hypertensive disease (1·24, 1·15–1·33), and fatal aortic aneurysm (1·15, 1·03–1·28; Figure 2, Figure 3). By contrast, there was an inverse and approximately log-linear association with myocardial infarction (0·94, 0·91–0·97; Figure 2, Figure 3). Stroke associations were similar for fatal and non-fatal outcomes (appendix p 28) and across subtypes (appendix p 29). However, for coronary disease excluding myocardial infarction, associations were stronger for fatal than non-fatal outcomes (appendix p 28). For myocardial infarction, inverse associations were possibly more pronounced with non-fatal than fatal outcomes (figure 3, appendix p 28).
Figure 1.
Associations of usual alcohol consumption with all-cause mortality and the aggregate of cardiovascular disease in current drinkers
Cardiovascular disease was defined as an aggregate of myocardial infarction, coronary heart disease, and stroke. Hazard ratios are adjusted for age, smoking, and history of diabetes, and stratified by sex and EPIC centre. The reference category is the lowest baseline alcohol consumption category (between 0 and 25 g/week). HRs are plotted against the mean usual alcohol consumption in each category. Sizes of the boxes are proportional to the inverse of the variance of the log-transformed hazard ratios. Vertical lines represent 95% CIs.
Figure 2.
Associations of usual alcohol consumption with cardiovascular subtypes in alcohol drinkers
Hazard ratios are adjusted for age, smoking, and history of diabetes, and stratified by sex and EPIC centre. The reference category is the lowest baseline alcohol consumption category (between 0 and 25g/week). Hazard ratios are plotted against the mean usual alcohol consumption in each category. Studies with fewer than five events of any outcome were excluded from the analysis of that outcome. Sizes of the boxes are proportional to the inverse of the variance of the log-transformed hazard ratios. Vertical lines represent 95% CIs. Deaths from other cardiovascular disease include the following outcomes: cardiac dysrhythmia, hypertensive disease, sudden death, and aortic aneurysm.
Figure 3.
Hazard ratios for subtypes of cardiovascular outcomes in current drinkers, per 100 g per week higher usual alcohol consumption
Hazard ratios are adjusted for age, smoking, and history of diabetes, and stratified by sex and centre. Studies with fewer than five events of any outcome were excluded from the analysis of that outcome.
With the following notable exceptions, further adjustment for additional covariates did not substantially change HRs (table 2, appendix pp 15, 30). First, adjustment for HDL-C level weakened the inverse association between alcohol consumption and myocardial infarction, but strengthened the positive association between alcohol consumption and both coronary disease and heart failure. Second, adjustment for systolic blood pressure strengthened the inverse association between alcohol consumption and myocardial infarction, but weakened the positive associations between alcohol consumption and all other cardiovascular disease outcomes. Our analysis confirmed the established association of alcohol consumption with cancers of the digestive system, which did not change after additional adjustment for the factors listed above (appendix p 16). Furthermore, additional adjustment for smoking amount abolished the apparent association of alcohol consumption with lung cancer (appendix pp 16), in line with the accepted view that alcohol consumption does not cause lung cancer.40
Table 2.
Hazard ratios for major cardiovascular outcomes in current drinkers, without and with adjustment for usual levels of systolic blood pressure, high-density-lipoprotein cholesterol, or body-mass index
| All stroke | Myocardial infarction | Coronary disease excluding myocardial infarction | Heart failure | Deaths from other types of cardiovascular disease | |
|---|---|---|---|---|---|
| Subset of participants with measurement of systolic blood pressure | |||||
| Cohorts/events | 70/11 297 | 73/13 519 | 46/7789 | 39/2668 | 44/1019 |
| Basic adjustment* | 1·16 (1·11–1·22) | 0·95 (0·91–0·99) | 1·06 (1·00–1·12) | 1·11 (1·04–1·18) | 1·16 (1·06–1·27) |
| Plus adjustment for systolic blood pressure | 1·10 (1·06–1·14) | 0·91 (0·87–0·94) | 1·03 (0·97–1·10) | 1·08 (1·02–1·15) | 1·14 (1·03–1·25) |
| Subset of participants with measurement of high-density-lipoprotein cholesterol | |||||
| Cohorts/events | 56/7982 | 61/9911 | 36/3608 | 29/1886 | 34/690 |
| Basic adjustment* | 1·16 (1·10–1·23) | 0·93 (0·88–0·97) | 1·07 (0·98–1·17) | 1·09 (1·00–1·19) | 1·22 (1·06–1·40) |
| Plus adjustment for high-density-lipoprotein cholesterol | 1·17 (1·11–1·22) | 1·00 (0·96–1·04) | 1·13 (1·05–1·22) | 1·14 (1·01–1·27) | 1·22 (1·08–1·38) |
| Subset of participants with measurement of body-mass index | |||||
| Cohorts/events | 68/11 733 | 71/14 217 | 43/7761 | 36/2566 | 42/1035 |
| Basic adjustment* | 1·15 (1·10–1·19) | 0·95 (0·91–0·98) | 1·06 (1·02–1·12) | 1·12 (1·04–1·20) | 1·16 (1·06–1·27) |
| Plus adjustment for body-mass index | 1·14 (1·10–1·18) | 0·94 (0·91–0·97) | 1·06 (1·01–1·12) | 1·10 (1·03–1·16) | 1·16 (1·06–1·27) |
Data are hazard ratio (95% CI) per 100 g per week higher usual alcohol consumption, unless otherwise indicated. Analyses were restricted to individuals with basic adjustment variables plus the additional variable. Studies with fewer than five events were excluded from the analysis of each outcome.
Basic adjustment includes age, smoking, and history of diabetes, and stratification by sex and centre.
When including never-drinkers and ex-drinkers, we reproduced previously reported U-shaped associations of alcohol consumption with total cardiovascular disease and all-cause mortality (appendix p 31). However, we observed notable differences in baseline characteristics between never drinkers and current drinkers (eg, in relation to sex, ethnicity, smoking, and diabetes status; appendix p 12), supporting the validity of focusing on current drinkers in our main analysis. We recorded similar findings to those reported above in sensitivity analyses that involved the following approaches: used multiple imputation rather than complete-case analysis (appendix p 32); used fractional polynomials (appendix p 34); used a fixed-effect meta-analysis (appendix p 35); included studies that recorded fewer than five events for a particular outcome (appendix p 36); provided separate analyses of men and women (appendix p 17, appendix p 26); omitted outcomes recorded in the initial 5 years of follow-up (appendix p 18); excluded participants with diabetes or other known chronic diseases at baseline (appendix p 18); and restricted the analyses to studies that recorded both non-fatal and fatal endpoints (appendix p 37). Associations of baseline alcohol consumption with all-cause mortality were stronger in drinkers of beer or spirits than of wine, and in those drinking less frequently (when consuming the same weekly amount), including binge drinkers (appendix p 38). However, people showing these behaviours had higher baseline levels of smoking and other indicators of lower socioeconomic status, suggesting the potential for confounding effects (appendix pp 19–20). For cardiovascular disease subtypes, HRs tended to be higher in beer and spirit drinkers than in wine drinkers, but not significantly so in direct comparisons involving a common set of participants (appendix p 39).
We noted little heterogeneity in the studies contributing results for stroke (I2=12%), myocardial infarction (I2=12%), coronary disease excluding myocardial infarction (I2=26%), heart failure (I2=4%) or deaths from other types of cardiovascular disease (I2=33%; figure 3). HRs for the cardiovascular disease outcomes we studied were broadly similar for different geographical regions, decade of study enrolment, by data source (ie, ERFC, EPIC-CVD, and UK Biobank), and alcohol assessment method (appendix pp 40–42). HRs for the cardiovascular disease outcomes were generally higher at younger ages, but did not vary substantially by sex, history of diabetes, proatherogenic lipids, BMI, smoking status, or other individual-level characteristics (appendix pp 43–45). There was no evidence of small study effects (appendix p 46). Our data showed no evidence of violation of the proportional hazards assumption.
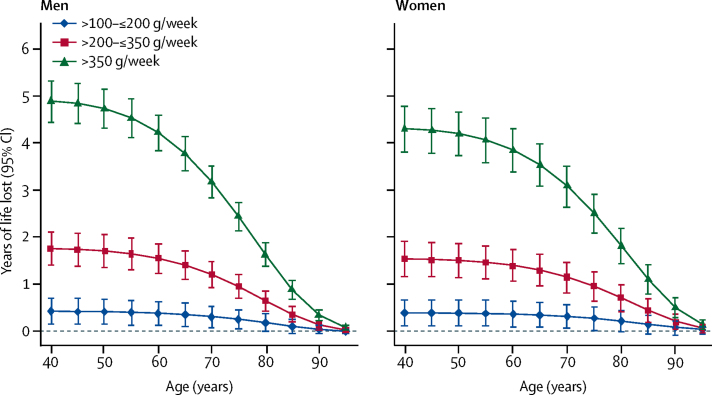
In comparison to those who reported drinking >0–≤100 g (mean usual 56 g) alcohol per week, those who reported drinking >100–≤200 g (mean usual 123 g) per week, >200–≤350 g (mean usual 208 g) per week or >350 g (mean usual 367 g) per week had shorter life expectancy at age 40 years of approximately 6 months, 1–2 years, or 4–5 years respectively (figure 4). Similarly, men who reported consuming above the UK upper limit of 112 g per week had a shorter life expectancy at age 40 years of 1·6 years (95% CI 1·3–1·8), and men who reported drinking above the US upper limit of 196 g per week had a shorter life expectancy at age 40 years of 2·7 years (2·4–3·1) compared with men who reported drinking below these respective upper limits. Thus, men who reported drinking less than 100 g alcohol per week had about a 1–2 years longer life expectancy at age 40 years than those who reported drinking 196 g per week (appendix p 47). Women who reported drinking above either the UK threshold (112 g per week) or US threshold (98 g per week) had about 1·3 (1·1–1·5) years shorter life expectancy at age 40 years compared with women who reported drinking below these thresholds (appendix p 47). About 20% of the alcohol-related survival difference for men (and slightly less for women) was attributed to excess death from cardiovascular disease (appendix p 47). Similar findings to those for the US population were observed when modelling was based on EU mortality rates (data not shown).
Figure 4.
Estimated future years of life lost by extent of reported baseline alcohol consumption compared with those who reported consuming >0–≤100 g per week
The estimates of cumulative survival from 40 years of age onwards in the alcohol-drinking groups were calculated by applying hazard ratios (specific to age at risk) for all-cause mortality associated with categorised baseline alcohol consumption to US death rates at the age of 40 years or older. Mean usual levels of alcohol consumption within each baseline alcohol consumption category were 56, 123, 208 and 367 g per week, respectively, for the groups >0–≤100 g per week, >100–≤200 g per week, >200–≤350 g per week, and >350 g per week.
Discussion
The main finding of this analysis was that the threshold for lowest risk for all-cause mortality was about 100 g per week. For men, we estimated that long-term reduction of alcohol consumption from 196 g per week (the upper limit recommended in US guidelines) to 100 g per week or below was associated with about 1–2 years of longer life expectancy at age 40 years. Exploratory analyses suggested that drinkers of beer or spirits, as well as binge drinkers, had the highest risk for all-cause mortality.
Our study has highlighted the complex and diverse potential mechanisms by which alcohol consumption may exert cardiovascular effects.41, 42 It has shown that the association between alcohol consumption and total cardiovascular disease risk comprises several distinct and opposite dose–response curves, rather than a single J-shaped association. In particular, whereas higher alcohol consumption was roughly linearly associated with a higher risk of all stroke subtypes, coronary disease excluding myocardial infarction, heart failure, and several less common cardiovascular disease subtypes, it was approximately log-linearly associated with a lower risk of myocardial infarction. Our results are concordant with recent observational data and Mendelian randomisation studies.16, 43, 44, 45, 46
Our results contribute toward understanding of the basis for these directionally divergent cardiovascular disease associations. For example, our data have suggested that elevated systolic blood pressure could mediate alcohol consumption's positive association with stroke and coronary disease excluding myocardial infarction.44, 47, 48 By contrast, pathways related to HDL-C (but not necessarily HDL-C itself49, 50, 51, 52) could mediate alcohol consumption's inverse association with myocardial infarction. Both blood pressure and HDL-C are known to increase in response to alcohol consumption.50 They have contrasting associations with cardiovascular disease outcomes: the inverse association of HDL-C with cardiovascular disease is substantially stronger for coronary disease than stroke,53, 54 whereas the positive association of systolic blood with cardiovascular disease is considerably stronger for stroke than coronary disease.55 However, we did not find convincing evidence that other known risk factors were important mediators or confounders.
Our study's access to individual-participant data avoided limitations of previous literature-based reviews.56 To limit reverse causality, our study focused on current drinkers without baseline cardiovascular disease and omitted the initial period of follow-up. To limit confounding, our study adjusted for a variety of risk factors. To correct for misclassification in alcohol consumption and covariates, our study also used extensive information on serial assessments. Our results were robust to a variety of sensitivity analyses. Generalisability of the findings was enhanced by inclusion of data from 83 prospective studies based in many different high-income countries recruited between 1964 and 2010. Although alcohol consumption levels declined during this period, HRs were similar over calendar time.
Nevertheless, our study has some potential limitations. Self-reported alcohol consumption data are prone to bias and are challenging to harmonise across studies conducted over different time periods that used varying instruments and methods to record such data.20, 57 We did not, however, identify major differences in results across studies that used differing alcohol measurement instruments. Despite our study's access to extensive serial alcohol re-surveys from mid-life, our study could not investigate alcohol consumption during the entire life course. Misclassification in outcomes would have diluted dose-response associations, suggesting that true underlying associations of alcohol consumption with cardiovascular disease subtypes are stronger and more divergent than we observed. Because we did not generally have access to additional alcohol-related adverse outcomes (eg, non-fatal liver disease, injuries, or psychiatric comorbidities), we probably under-estimated potential benefits associated with lowering alcohol consumption. Because some individuals who reduced, but did not cease, alcohol consumption due to health complications were probably included in our analysis, we cannot exclude the effects of reverse causation (especially since some contributing studies did not record baseline chronic disease other than cardiovascular disease). Therefore, alternative study designs including randomised trials58 are needed, to control more completely for residual biases (including those related to studying ex-drinkers and never-drinkers).
In conclusion, our study shows that among current drinkers, the threshold for lowest risk of all-cause mortality was about 100 g per week. For cardiovascular disease subtypes other than myocardial infarction, there were no clear thresholds below which lower alcohol consumption stopped being associated with a lower disease risk. These data support adoption of lower limits of alcohol consumption than are recommended in most current guidelines.
Acknowledgments
Acknowledgments
The study's coordinating centre (Emerging Risk Factors Collaboration and EPIC-CVD Coordinating Centres, Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK) has been underpinned by grants from the UK Medical Research Council (G0800270 and MR/L003120/1), British Heart Foundation (SP/09/002, RG/08/014 and RG13/13/30194), National Institute for Health Research (through the National Institute for Health Research Cambridge Biomedical Research Centre), European Commission Framework 7 (through the EPIC-CVD award; HEALTH-F2-2012-279233), and the European Research Council (through an Advanced Investigator Award to JD; 268834). JD holds a BHF Professorship and NIHR Senior Investigator Award. A study website Funding for the EPIC-InterAct project was provided by the EU FP6 programme (grant number LSHM_CT_2006_037197). A study website includes a list that investigators have provided of funding agencies that have supported individual EPIC centres. A study website includes a list that investigators have provided of funding agencies that have supported individual cohorts of the ERFC contributing to the present consortium. This research has been conducted using the UK Biobank resource (application 21886). We thank Nicola Kerrison and Stephen Sharp (both from the University of Cambridge MRC Epidemiology Unit, Cambridge, UK) for the former's data management in the EPIC-InterAct subcohort and the latter's statistical input into development of the EPIC-CVD's analytical guidelines.
Contributors
All the authors contributed to data collection, and to the design, analysis, interpretation, and re-drafting of this report. AMW and SK had full access to the combined data and did the statistical analysis. AMW, EDA, and JD drafted the manuscript and had responsibility for submission of the manuscript for publication.
Data management team
Thomas Bolton, Catherine Perry, Sarah Spackman, and Matthew Walker.
Coordinating centre
Thomas Bolton, Stephen Burgess, Adam S Butterworth, Emanuele Di Angelantonio, Stephen Kaptoge, Lisa Pennells, Catherine Perry, David Stevens, Sarah Spackman, Simon G Thompson, Matthew Walker, Angela M Wood, and John Danesh (principal investigator).
Declaration of interests
ASB reports grants from European Commission Framework 7 (HEALTH-F2-2012-279233), the European Research Council (268834), the British Heart Foundation (SP/09/002 and RG/08/014 and RG13/13/30194), the UK Medical Research Council (G0800270 and MR/L003120/1), from National Institute for Health Research (through the NIHR Cambridge Biomedical Research Centre), during the conduct of the study; and grants from Merck, Biogen, Bioverativ, Novartis, and Pfizer, outside the submitted work. BMP reports that he serves on the DSMB of a clinical trial funded by Zoll LifeCor and on the Steering Committee of the Yale Open Data Access Project funded by Johnson & Johnson. MD reports grants from Japan Society for the Promotion of Science, during the conduct of the study. EDA reports grants from European Commission Framework 7, the European Research Council, the British Heart Foundation, the UK Medical Research Council, and the National Institute for Health Research, during the conduct of the study; and grants from NHS Blood and Transplant, outside the submitted work. EB reports grants from the National Health and Medical Research Council of Australia, during the conduct of the study. HMK reports a research agreement (through Yale) from Johnson & Johnson (Janssen) and Medtronic to develop methods of clinical trial data sharing; personal fees from UnitedHealth, IBM Watson, Element Science, and Aetna; a personal health information platform from Hugo; grants from the FDA and Medtronic; and contracts from Centers for Medicare & Medicaid Services to develop and maintain measures that are publicly reported, outside the submitted work. JD reports grants from the UK Medical Research Council, the British Heart Foundation, the UK National Institute of Health Research, and the European Commision, during the conduct of the study; personal fees and non-financial support from Merck Sharp and Dohme UK Atherosclerosis, personal fees and non-financial support from Novartis Cardiovascular and Metabolic Advisory Board, grants from the British Heart Foundation, European Research Council, Merck, the National Institute of Health Research, NHS Blood and Transplant, Novartis, Pfizer, the UK Medical Research Council, the Wellcome Trust, and AstraZeneca, and personal fees and non-financial support from Pfizer Population Research Advisory Panel, outside the submitted work. ML reports grants from National Institutes of Health, during the conduct of the study; grants from National Kidney Foundation, outside the submitted work; and Funding from the National Institutes of Health, Grant 5U10AA025286, to Johns Hopkins University. MS reports grants from the UK Medical Research Council, the British Heart Foundation, the National Institute for Health Research, European Commission Framework 7, and the European Research Council, during the conduct of the study. NvS reports grants from the Netherlands Ministry of Health Welfare and Sports, Directorate of Long-Term Care, during the conduct of the study. OHF reports grants from Nestle and Metagenics, outside the submitted work. PJN reports grants from the NIH, during the conduct of the study. SGT reports grants from the UK Medical Research Council and the British Heart Foundation, during the conduct of the study. SKi reports grants from FFG COMET program: “Research Center of Excellence in Vascular Ageing—Tyrol, VASCage” (K-Project No. 843536) funded by the BMVIT, BMWFW, Wirtschaftsagentur Wien and Standortagentur Tirol, outside the submitted work. SKa reports grants from the UK Medical Research Council and the British Heart Foundation, during the conduct of the study. WK reports personal fees from AstraZeneca, Novartis, Pfizer, The Medicines Company, GSK, DalCor, Sanofi, Berlin-Chemie, Kowa, and Amgen; grants and non-financial support from Roche Diagnostics, Beckmann, Singulex, and Abbott, outside the submitted work. The other authors declare no competing interests.
Contributor Information
Angela M Wood, Email: amw79@medschl.cam.ac.uk.
John Danesh, Email: jd292@medschl.cam.ac.uk.
Emerging Risk Factors Collaboration/EPIC-CVD/UK Biobank Alcohol Study Group:
Angela M Wood, Stephen Kaptoge, Adam Butterworth, Peter Willeit, Samantha Warnakula, Thomas Bolton, Ellie Paige, Dirk S Paul, Michael Sweeting, Stephen Burgess, Steven Bell, William Astle, David Stevens, Albert Koulman, Randi M Selmer, Monique Verschuren, Shinichi Sato, Inger Njølstad, Mark Woodward, Salomaa Veikko, Børge G Nordestgaard, Bu B Yeap, Astrid Flecther, Olle Melander, Lewis H Kuller, Beverley Balkau, Michael Marmot, Wolfgang Koenig, Edoardo Casiglia, Cyrus Cooper, Volker Arndt, Oscar H Franco, Patrik Wennberg, John Gallacher, Agustín Gómez de la Cámara, Henry Völzke, Christina C Dahm, Caroline E Dale, Manuela Bergmann, Carlos Crespo, Yvonne T van der Schouw, Rudolf Kaaks, Leon A Simons, Pagona Lagiou, Josje D Schoufour, Jolanda M.A Boer, Timothy J Key, Beatriz Rodriguez, Conchi Moreno-Iribas, Karina W Davidson, James O Taylor, Carlotta Sacerdote, Robert B Wallace, J. Ramon Quiros, Eric B Rimm, Rosario Tumino, Dan G Blazer III, Allan Linneberg, Makoto Daimon, Salvatore Panico, Barbara Howard, Guri Skeie, Veikko Salomaa, Timo Strandberg, Elisabete Weiderpass, Paul J Nietert, Bruce M Psaty, Daan Kromhout, Elena Salamanca-Fernandez, Stefan Kiechl, Harlan M Krumholz, Sara Grioni, Domenico Palli, José M Huerta, Jackie Price, Johan Sundström, Larraitz Arriola, Hisatomi Arima, Ruth C Travis, Demosthenes B Panagiotakos, Anna Karakatsani, Antonia Trichopoulou, Tilman Kühn, Diederick E Grobbee, Elizabeth Barrett-Connor, Natasja van Schoor, Heiner Boeing, Kim Overvad, Jussi Kauhanen, Nick Wareham, Claudia Langenberg, Nita Forouhi, Maria Wennberg, Jean-Pierre Després, Mary Cushman, Jackie A Cooper, Carlos J Rodriguez, Masaru Sakurai, Jonathan E Shaw, Matthew Knuiman, Trudy Voortman, Christa Meisinger, Anne Tjønneland, Hermann Brenner, Luigi Palmieri, Jean-Pierre Dallongeville, Eric J Brunner, Gerd Assmann, Maurizio Trevisan, Richard F Gillumn, Ian Ford Ford, Naveed Sattar, Mariana Lazo, Simon Thompson, Pietro Ferrari, David A Leon, George Davey Smith, Richard Peto, Rod Jackson, Emily Banks, Emanuele Di Angelantonio, and John Danesh
Supplementary Material
References
- 1.Department of Health Alcohol Guidelines Review: Report from the guidelines development group to the UK Chief Medical Officers. 2016. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/545739/GDG_report-Jan2016.pdf (accessed Feb 5, 2018).
- 2.Kalinowski A, Humphreys K. Governmental standard drink definitions and low-risk alcohol consumption guidelines in 37 countries. Addiction. 2016;111:1293–1298. doi: 10.1111/add.13341. [DOI] [PubMed] [Google Scholar]
- 3.Stampfer MJ, Colditz GA, Willett WC, Speizer FE, Hennekens CH. A prospective study of moderate alcohol consumption and the risk of coronary disease and stroke in women. N Engl J Med. 1988;319:267–273. doi: 10.1056/NEJM198808043190503. [DOI] [PubMed] [Google Scholar]
- 4.Boffetta P, Garfinkel L. Alcohol drinking and mortality among men enrolled in an American Cancer Society prospective study. Epidemiology. 1990;1:342–348. doi: 10.1097/00001648-199009000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Thun MJ, Peto R, Lopez AD. Alcohol consumption and mortality among middle-aged and elderly U.S. adults. N Engl J Med. 1997;337:1705–1714. doi: 10.1056/NEJM199712113372401. [DOI] [PubMed] [Google Scholar]
- 6.Mukamal K, Conigrave K, Mittleman M. Roles of drinking pattern and type of alcohol consumed in coronary heart disease in men. N Engl J Med. 2003;348:109–118. doi: 10.1056/NEJMoa022095. [DOI] [PubMed] [Google Scholar]
- 7.Corrao G, Bagnardi V, Zambon A, La Vecchia C. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev Med. 2004;38:613–619. doi: 10.1016/j.ypmed.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 8.Hvidtfeldt UA, Tolstrup JS, Jakobsen MU. Alcohol intake and risk of coronary heart disease in younger, middle-aged, and older adults. Circulation. 2010;121:1589–1597. doi: 10.1161/CIRCULATIONAHA.109.887513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patra J, Taylor B, Irving H. Alcohol consumption and the risk of morbidity and mortality for different stroke types—a systematic review and meta-analysis. BMC Public Health. 2010;10:258. doi: 10.1186/1471-2458-10-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roerecke M, Rehm J. The cardioprotective association of average alcohol consumption and ischaemic heart disease: a systematic review and meta-analysis. Addiction. 2012;107:1246–1260. doi: 10.1111/j.1360-0443.2012.03780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergmann MM, Rehm J, Klipstein-Grobusch K. The association of pattern of lifetime alcohol use and cause of death in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Int J Epidemiol. 2013;42:1772–1790. doi: 10.1093/ije/dyt154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leong DP, Smyth A, Teo KK. Patterns of alcohol consumption and myocardial infarction risk: observations from 52 countries in the INTERHEART case-control study. Circulation. 2014;130:390–398. doi: 10.1161/CIRCULATIONAHA.113.007627. [DOI] [PubMed] [Google Scholar]
- 14.Ferrari P, Licaj I, Muller DC. Lifetime alcohol use and overall and cause-specific mortality in the European Prospective Investigation into Cancer and nutrition (EPIC) study. BMJ Open. 2014;4:e005245. doi: 10.1136/bmjopen-2014-005245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xi B, Veeranki SP, Zhao M, Ma C, Yan Y, Mi J. Relationship of alcohol consumption to all-cause, cardiovascular, and cancer-related mortality in U.S. adults. J Am Coll Cardiol. 2017;70:913–922. doi: 10.1016/j.jacc.2017.06.054. [DOI] [PubMed] [Google Scholar]
- 16.Smyth A, Teo KK, Rangarajan S. Alcohol consumption and cardiovascular disease, cancer, injury, admission to hospital, and mortality: a prospective cohort study. Lancet. 2015;386:1945–1954. doi: 10.1016/S0140-6736(15)00235-4. [DOI] [PubMed] [Google Scholar]
- 17.Bell S, Daskalopoulou M, Rapsomaniki E. Association between clinically recorded alcohol consumption and initial presentation of 12 cardiovascular diseases: population based cohort study using linked health records. BMJ. 2017;356:j909. doi: 10.1136/bmj.j909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson R, Broad J, Connor J, Wells S. Alcohol and ischaemic heart disease: probably no free lunch. Lancet. 2005;366:1911–1912. doi: 10.1016/S0140-6736(05)67770-7. [DOI] [PubMed] [Google Scholar]
- 19.Knott CS, Coombs N, Stamatakis E, Biddulph JP. All cause mortality and the case for age specific alcohol consumption guidelines: pooled analyses of up to 10 population based cohorts. BMJ. 2015;350:h384. doi: 10.1136/bmj.h384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emberson JR, Bennett DA. Effect of alcohol on risk of coronary heart disease and stroke: causality, bias, or a bit of both? Vasc Health Risk Manag. 2006;2:239–249. doi: 10.2147/vhrm.2006.2.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng Fat L, Cable N, Marmot MG, Shelton N. Persistent long-standing illness and non-drinking over time, implications for the use of lifetime abstainers as a control group. J Epidemiol Community Health. 2014;68:71–77. doi: 10.1136/jech-2013-202576. [DOI] [PubMed] [Google Scholar]
- 22.Danesh J, Erqou S, Walker M. The Emerging Risk Factors Collaboration: Analysis of individual data on lipid, inflammatory and other markers in over 1.1 million participants in 104 prospective studies of cardiovascular diseases. Eur J Epidemiol. 2007;22:839–869. doi: 10.1007/s10654-007-9165-7. [DOI] [PubMed] [Google Scholar]
- 23.Danesh J, Saracci R, Berglund G. EPIC-Heart: The cardiovascular component of a prospective study of nutritional, lifestyle and biological factors in 520,000 middle-aged participants from 10 European countries. Eur J Epidemiol. 2007;22:129–141. doi: 10.1007/s10654-006-9096-8. [DOI] [PubMed] [Google Scholar]
- 24.Sudlow C, Gallacher J, Allen N. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conen D. Alcohol consumption and incident cardiovascular disease: not just one unifying hypothesis. Eur Heart J. 2015;36:897–898. doi: 10.1093/eurheartj/ehv021. [DOI] [PubMed] [Google Scholar]
- 26.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73:1–11. [Google Scholar]
- 27.Thompson S, Kaptoge S, White I. Statistical methods for the time-to-event analysis of individual participant data from multiple epidemiological studies. Int J Epidemiol. 2010;39:1345–1359. doi: 10.1093/ije/dyq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fibrinogen Studies Collaboration. Wood AM, White I, Thompson SG, Lewington S, Danesh J. Regression dilution methods for meta-analysis: assessing long-term variability in plasma fibrinogen among 27,247 adults in 15 prospective studies. Int J Epidemiol. 2006;35:1570–1578. doi: 10.1093/ije/dyl233. [DOI] [PubMed] [Google Scholar]
- 29.Wood AM, Thompson SG, Kostis JB. Correcting for multivariate measurement error by regression calibration in meta-analyses of epidemiological studies. Stat Med. 2009;28:1067–1092. doi: 10.1002/sim.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Easton DF, Peto J, Babiker AG. Floating absolute risk: an alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Stat Med. 1991;10:1025–1035. doi: 10.1002/sim.4780100703. [DOI] [PubMed] [Google Scholar]
- 31.Plummer M. Improved estimates of floating absolute risk. Stat Med. 2004;23:93–104. doi: 10.1002/sim.1485. [DOI] [PubMed] [Google Scholar]
- 32.Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol. 1999;28:964–974. doi: 10.1093/ije/28.5.964. [DOI] [PubMed] [Google Scholar]
- 33.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 34.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088. [PubMed] [Google Scholar]
- 35.Egger M, Davey Smith G, Schneider M. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. National Center for Health Statistics Underlying cause of death 1999–2015 on CDC WONDER Online Database (released Dec, 2016) https://wonder.cdc.gov/ucd-icd10.html (accessed Feb 5, 2018).
- 37.WHO . World Health Organization; Geneva, Switzerland: 2007. WHO Statistical Information System (WHOSIS) [Google Scholar]
- 38.UN Population Division . United Nations; New York, NY: 2005. World Population Prospects. [Google Scholar]
- 39.Emerging Risk Factors Collaboration. Di Angelantonio E, Kaptoge S. Association of cardiometabolic multimorbidity with mortality. JAMA. 2015;314:52–60. doi: 10.1001/jama.2015.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Personal habits and indoor combustions. Volume 100 E. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum. 2012;100(Pt E):1–538. [PMC free article] [PubMed] [Google Scholar]
- 41.Lazo M, Chen Y, McEvoy JW. Alcohol consumption and cardiac biomarkers: The atherosclerosis risk in communities (ARIC) study. Clin Chem. 2016;62:1202–1210. doi: 10.1373/clinchem.2016.255778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kiechl S, Willeit J. Complex association between alcohol consumption and myocardial infarction: always good for a new paradox. Circulation. 2014;130:383–386. doi: 10.1161/CIRCULATIONAHA.114.011036. [DOI] [PubMed] [Google Scholar]
- 43.Holmes MV, Dale CE, Zuccolo L. Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ. 2014;349:g4164. doi: 10.1136/bmj.g4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho Y, Shin S-Y, Won S, Relton CL, Davey Smith G, Shin M-J. Alcohol intake and cardiovascular risk factors: a Mendelian randomisation study. Sci Rep. 2015;5:18422. doi: 10.1038/srep18422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Au Yeung SL, Jiang C, Cheng KK. Moderate alcohol use and cardiovascular disease from Mendelian randomization. PLoS One. 2013;8:e68054. doi: 10.1371/journal.pone.0068054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yun KE, Chang Y, Yun S-C. Alcohol and coronary artery calcification: an investigation using alcohol flushing as an instrumental variable. Int J Epidemiol. 2017;46:950–962. doi: 10.1093/ije/dyw237. [DOI] [PubMed] [Google Scholar]
- 47.Taylor AE, Lu F, Carslake D. Exploring causal associations of alcohol with cardiovascular and metabolic risk factors in a Chinese population using Mendelian randomization analysis. Sci Rep. 2015;5:14005. doi: 10.1038/srep14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen L, Smith GD, Harbord RM, Lewis SJ. Alcohol intake and blood pressure: a systematic review implementing a Mendelian randomization approach. PLoS Med. 2008;5:e52. doi: 10.1371/journal.pmed.0050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zanoni P, Khetarpal SA, Larach DB. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science. 2016;351:1166–1171. doi: 10.1126/science.aad3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ. 2011;342:d636. doi: 10.1136/bmj.d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Voight BF, Peloso GM, Orho-Melander M. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.HPS3/TIMI55-REVEAL Collaborative Group Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377:1217–1227. doi: 10.1056/NEJMoa1706444. [DOI] [PubMed] [Google Scholar]
- 53.The Emerging Risk Factors Collaboration. Di Angelantonio E, Sarwar N. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woodward M, Barzi F, Feigin V. Associations between high-density lipoprotein cholesterol and both stroke and coronary heart disease in the Asia Pacific region. Eur Heart J. 2007;28:2653–2660. doi: 10.1093/eurheartj/ehm427. [DOI] [PubMed] [Google Scholar]
- 55.MacMahon S, Peto R, Collins R. Blood pressure, stroke, and coronary heart disease. Part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765–774. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 56.Ahmed I, Sutton AJ, Riley RD. Assessment of publication bias, selection bias, and unavailable data in meta-analyses using individual participant data: a database survey. BMJ. 2012;344:d7762. doi: 10.1136/bmj.d7762. [DOI] [PubMed] [Google Scholar]
- 57.Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: state of the science and challenges for research. Addiction. 2003;98:1–12. doi: 10.1046/j.1359-6357.2003.00586.x. [DOI] [PubMed] [Google Scholar]
- 58.Mukamal KJ, Clowry CM, Murray MM. Moderate alcohol consumption and chronic disease: the case for a long-term trial. Alcohol Clin Exp Res. 2016;40:2283–2291. doi: 10.1111/acer.13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.