Abstract
Nivolumab has been increasingly used for a range of malignancies, and a variety of immune‐related adverse events (irAEs) have been reported with its use. Nivolumab‐induced sialadenitis (inflammation of salivary glands) and xerostomia (dry mouth) have been reported to occur in 0.03% and 0.05% respectively, but there have been no case reports published about these side effects. Sialadenitis is not a life‐threatening irAEs, but xerostomia can become irreversible if not recognized and treated as it can otherwise lower quality of life and result in susceptibility to infection. Therefore, healthcare providers need to know about sialadenitis as one of the irAEs of Nivolumab. Thus, we present the first case of nivolumab‐induced sialadenitis.
A 70‐year old man with pulmonary adenocarcinoma developed sialadenitis 4 months after initiating nivolumab. His serum amylase levels were elevated to 1373 IU/L, and a biopsy of his labial minor salivary glands showed severe lymphocytic inflammation with damage to the glands. His sialadenitis was improved by taking corticosteroids and by ceasing nivolumab.
Keywords: Immune checkpoint inhibitor, immune‐related adverse events, nivolumab, sialadenitis, xerostomia
Introduction
Nivolumab, an anti‐programmed cell death 1 (anti‐PD‐1) immune checkpoint inhibitor (ICI), has been increasingly used for a range of malignancies, and a variety of immune‐related adverse events (irAEs) have been reported. However, no case reports about ICIs‐induced sialadenitis have been reported, and healthcare providers could be unfamiliar with its association with ICIs. We present the first case of nivolumab‐induced sialadenitis.
Case Report
A 70‐year‐old man with a smoking history of 47 pack years was diagnosed with EGFR, ALK, and ROS‐1‐negative stage IIIB (T1aN3M0) adenocarcinoma in July 2015. He had chronic renal failure and combined pulmonary fibrosis and emphysema. He was treated with several lines of cytotoxic agents, including carboplatin‐pemetrexed, carboplatin‐nab‐paclitaxel, carboplatin‐TS‐1, and docetaxel. Nivolumab was started at a dose of 3 mg/kg every 2 weeks as fifth‐line therapy in March 2017. PD‐L1 tumour proportion score was 90%. In May 2017, he presented with a grade 2 nivolumab‐induced rash. On June 15th, blood work interestingly revealed an abrupt elevation of serum amylase levels of 1373 IU/L and pancreatic amylase 66 IU/L (amylase is often checked as part of routine blood work in Japan). On June 15th, clinically, the patient had no pain or swelling in his salivary glands. However, on June 20th, he did indeed develop pain in his right parotid gland, although it resolved within a day. Further blood work showed that not only were anti SS‐A/SS‐B and antinuclear antibodies (ANA) negative but serum IgG4 levels were also within normal range. One week after nivolumab withdrawal, his serum amylase levels were falling into the normal range (Fig. 1), and he began taking nivolumab again. On July 18th, the patient underwent his fourth 18F‐FDG PET/CT as part of routine protocol to rule out metastatic disease and showed increased uptake in his right parotid gland (SUV max 3.5) as well as ascending colon (SUV max 9.7) (Fig. 2A); however, he did not have any symptoms. However, on July 24th, he demonstrated xerostomia and anorexia. When he came to our hospital for a regular check up on July 28th, his serum CRP levels were 15.3 mg/dL, and he was admitted to our hospital for suspected nivolumab‐induced sialadenitis and colitis. On physical examination, blood pressure was 134/70 mmHg, body temperature was 37.3 °C, and heart rate was 100/min. His mouth was dry, but there was no inflammation in his oral cavity. There was no pain or swelling in his salivary glands. Saxon test was positive, demonstrating an abnormally low saliva production. Ultrasonography on his salivary gland showed no abnormal findings. Salivary gland scintigraphy with 99mTc‐per‐ technetate revealed decreased accumulation and secretion of his right parotid gland (Fig. 2B). Biopsy of labial minor salivary glands showed severe lymphocytic inflammation, exfoliation of the epithelium, enlargement of glands with mucous retention, and disappearance of acini (Fig. 2C, D). After undergoing a colonoscopy, he was diagnosed with nivolumab‐induced sialadenitis and grade 2 colitis. Intravenous prednisolone of 50 mg/day (1 mg/kg) and pilocarpine were started. His appetite returned to normal on day 3 of treatment, although his xerostomia did not resolve until day 12, at which time his pilocarpine was discontinued. We changed to prednisolone 40 mg/day, orally, from 50 mg/day, intravenously on day 14. He was discharged on prednisolone 30 mg/day on day 28. Prednisolone was tapered off over 7 weeks. One week after ceasing prednisolone, his colitis returned, and he resumed prednisolone at 25 mg/day with a plan to taper this slowly over 3 months. After ceasing the nivolumab, his lung cancer has not progressed for 6 months without any treatment.
Figure 1.
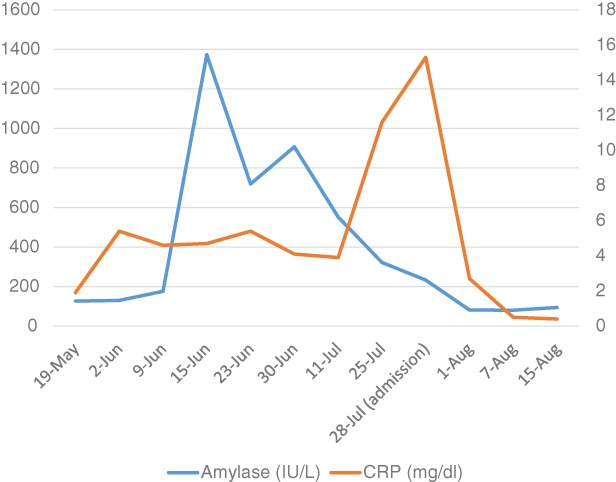
Seriate blood work results of amylase and CRP.
Figure 2.
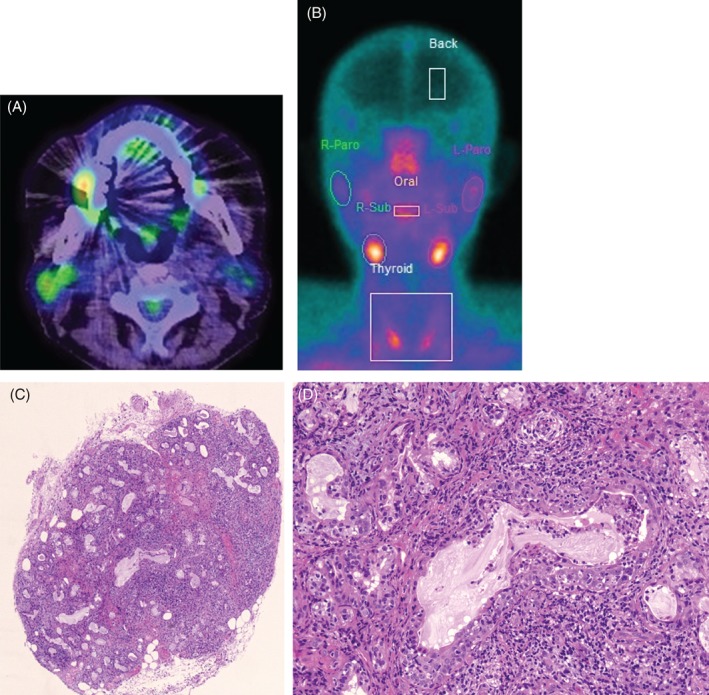
(A) PET showed weak accumulation on the patient’s right parotid gland (arrow). (B) Salivary gland scintigraphy with 99mTc‐per‐ technetate revealed decreased accumulation. (C) Haematoxylin–eosin staining of lower lip tissue showing lymphocytes infiltration and destruction of glands (100×). (D) (400×).
Discussion
Nivolumab‐induced Sialadenitis and xerostomia occurred in 0.03% and 0.05%, respectively, according to the manufacture’s post‐marketing surveillance in Japan 1. As ICIs‐induced sialadenitis is not well known, and xerostomia occurs with several other health conditions, such as diabetes, hypophysitis, or side effects of anti‐cholinergic drugs, ICIs‐induced sialadenitis can be overlooked.
Similar to reported cases in which severe salivary hypofunction suddenly occurred between 2 and 8 months after starting ICIs 2, 3, sialadenitis in our case abruptly occurred 4 months after starting nivolumab. In the reported cases, ICIs were thought to have resulted in the conversion of subclinical Sjogren syndrome (SjS) to a symptomatic sicca syndrome as the autoantibodies ANA, anti‐SSA, or anti‐SSB were already positive prior to initiating ICIs (Table 1). In our case, however, these antibodies were negative, and the salivary gland biopsy revealed severe inflammation and glandular destruction that could be pathologically distinguished from SjS.
Table 1.
Reported cases of xerostomia after use of immune checkpoint inhibitors.
| Reference | Age | Gender | Type of malignancy | Cancer therapy | Time to onset of xerostomia after initiating ICI (months) | Treatment | irAE response to treatment | Antibodies results |
|---|---|---|---|---|---|---|---|---|
| 2 | 61 | M | NSCLC | Anti‐PD‐1 | 2 | Pilocarpine | Improved | ANA titre 320, SS‐A, SS‐B negative |
| 2 | 57 | M | Melanoma | Anti‐PD‐1, Anti‐CTLA‐4 | 2 | Prednisone 1 mg/kg/day cevimeline | No improvement | ANA titre 320, SS‐A, SS‐B negative |
| 2 | 74 | M | Melanoma | Anti‐CTLA‐4 | 8 | Prednisone 1 mg/kg/day | Improved | ANA titre 80, SS‐A, SS‐B negative |
| 2 | 74 | F | Melanoma | Anti‐PD‐1 | 8 | Prednisone 40 mg/day | Improved | ANA, SS‐A negative SS‐B positive |
| 3 | 56 | F | Renal carcinoma | Anti‐PD‐1, Anti‐CTLA‐4 | 3 | ICI withdrawn, symptomatic treatment | Improved | ANA titre 1280, SS‐A, SS‐B positive |
| 3 | 58 | F | Squamous cell carcinoma of the cervix | Anti‐PD‐1 | 3 | Symptomatic treatment | Improved | ANA titre 160, SS‐A positive |
| 3 | 60 | M | Melanoma | Anti‐PD‐1, BRAF and MEK inhibitors | 2 | ICI withdrawn, prednisone | Improved | ANA titre 640, SS‐A negative before ICI use but positive after irAE occurred |
| Our case | 70 | M | NSCLC | Anti‐PD‐1 | 4 | ICI withdrawn, prednisolone 1 mg/kg/day, pilocarpine | Improved | ANA, SS‐A, SS‐B negative |
In the past reports of ICIs‐induced irAEs such as colitis myocarditis, or pneumonitis, biopsies demonstrate dominant infiltration of CD8+ T cells at injured tissues 4, 5. We performed immunostaining of tissues from our patient’s salivary gland using CD4 and CD8 antibodies, but CD8+ T cell did not show remarkable dominance in number or particular distribution around injured glands. It might be because the patient’s sialadenitis was already at the chronic inflammatory stage when his lip biopsy was performed.
In the past cases of ICIs‐induced sialadenitis, some patients recovered without taking corticosteroids, but one patient did not recover even after taking prednisone 1 mg/kg (Table 1). There is no grading scale for determining the severity of sialadenitis due to irAEs nor is there a guideline‐recommended treatment. We used prednisolone 1 mg/kg considering our patient coincidently had grade 2 colitis. More cases are needed to define optimal treatment of ICIs‐induced sialadenitis.
Sialadenitis is not a life‐threatening irAE, but xerostomia can become chronic and irreversible. As a result, it can significantly lower patients’ quality of life and make them not only susceptible to infection but also put them at risk of other health concerns. Therefore, healthcare providers should be keenly aware of the potential ICIs‐induced sialadenitis when examining the patients. If patients on ICIs demonstrate xerostomia or elevated salivary amylase level, corticosteroids should be considered as well as stopping ICIs. The fact that our patient had elevated serum amylase level 6 weeks prior to onset of the symptom raises the important question of whether or not to check serum amylase levels periodically for all patients taking ICIs.
Disclosure Statement
Appropriate written informed consent was obtained for the publication of this case report and accompanying images.
Acknowledgments
The authors appreciate Arakawa Hiroki M.D. (Department of Dermatology, Tokyo Saiseikai Central Hospital), Nakazawa Atsushi M.D. and Ogawa Ayumu M.D. (Department of Gastroenteology, Tokyo Saiseikai Central Hospital), Ohba Hiroiku, D.D.S. (Department of Dentistry, Tokyo Saiseikai Central Hospital), and Takebayashi Akiko M.D. (Department of Otolaryngology, Tokyo Saiseikai Central Hospital) for their assistance. We also thank Ms. Masami Yoshihara (the medical interpreter and the medical coordinator, Tokyo Saiseikai Central Hospital) for the critical reading of our manuscript.
Takahashi, S , Chieko, X , Sakai, T , Hirose, S , Nakamura, M . Nivolumab‐induced sialadenitis. Respirology Case Reports. 2018;e00322. https://doi.org/10.1002/rcr2.322
Associate Editor: Jonathan Williamson
References
- 1. Ono Pharmaceutical Co. Ltd . A post‐marketing use‐results survey (all‐case surveillance). 4 July 2014 through 30 July 2017.
- 2. Cappelli LC, Gutierreez AK, Baer AN, et al. 2017. Inflammatory arthritis and sicca syndrome induced novolmab and ipilimumab. Ann. Rheum. Dis. 76(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Le Burel S, Champiat S, Routier E, et al. 2018. Onset of connective tissue disease following anti‐PD1/PD‐L1 cancer immunotherapy. Ann. Rheum. Dis. 77(3):468–470. [DOI] [PubMed] [Google Scholar]
- 4. Läubli H, Balmelli C, Bossard M, et al. 2015. Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. J. Immunother. Cancer 3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kimura T, Fukushima S, Miyashita A, et al. 2016. Myasthenic crisis and polymyositis induced by one dose of novolumab. Cancer Sci. 107:1055–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]


