Abstract
Despite being a curable disease, tuberculosis (TB) remains a public health problem worldwide mainly due to lengthy treatment, as well as its toxic effects, TB/HIV co-infection and the emergence of resistant Mycobacterium tuberculosis strains. These barriers reinforcing the need for development of new antimicrobial agents, that ideally should reduce the time of treatment and be active against susceptible and resistant strains. Quinones are compounds found in natural sources and among them, the naphthoquinones show antifungal, antiparasitic, and antimycobacterial activity. Thus, we evaluated the potential antimycobacterial activity of six 1,4-naphthoquinones derivatives. We determined the minimum inhibitory concentration (MIC) of the compounds against three M. tuberculosis strains: a pan-susceptible H37Rv (ATCC 27294); one mono-resistant to isoniazid (ATCC 35822); and one mono-resistant to rifampicin (ATCC 35838); the cytotoxicity in the J774A.1 (ATCC TIB-67) macrophage lineage; performed in silico analysis about absorption, distribution, metabolism, and excretion (ADME) and docking sites. All evaluated naphthoquinones were active against the three strains with MIC between 206.6 and 12.5 μM, and the compounds with lower MIC values have also showed low cytotoxicity. Moreover, two naphthoquinones derivatives 5 and 6 probably do not exhibit cross resistance with isoniazid and rifampicin, respectively, and regarding ADME analysis, no compound violated the Lipinski’s rule-of-five. Considering the set of findings in this study, we conclude that these naphthoquinones could be promising scaffolds to develop new therapeutic strategies to TB.
Keywords: Mycobacterium tuberculosis, naphthoquinones, antimicrobials, resistance, tuberculosis
Introduction
According to World Health Organization, tuberculosis (TB) is one of the top 10 causes of death worldwide and, in 2016, there were an estimated the incidence of 10.4 million new cases (WHO, 2017). The main strategies for reducing TB morbidity and mortality rates are related to prophylaxis, early diagnosis and effective treatment of the disease. Bacille Calmette–Guérin (BCG) vaccine has been administered since the beginning of the 20th century in the immunization process, but only protects against the most severe cases of TB (Andersen and Woodworth, 2014; Stefanova, 2014). Despite the technological advances in the diagnostic area and the absence of an effective vaccine, the therapy still is the main tool for TB control.
The first-line therapy regimen for TB is a combination of four drugs: isoniazid (INH), rifampicin (RIF), pyrazinamide and ethambutol, which cure about 85% of patients infected with susceptible strains (WHO, 2017). Other antimicrobials such as kanamycin, amikacin, capreomycin, ethionamide, and levofloxacin are considered second-line drugs. These drugs are also used in the treatment of TB, especially in cases of multidrug-resistant strains (MDR) infection – which are resistant to both INH and RIF – and when there is intolerance to first-line drugs. However, second-line therapy is more expensive, more toxic, and less effective than basic therapy (Ma et al., 2010).
Over the past 15 years, the TB treatment was able to avoid at least 50 million deaths, however, there is still a gap in this regard (WHO, 2017). Although it is a curable disease, there are several factors that hamper the TB control: lengthy treatment, toxic effects, pharmacokinetic interactions with other drugs, TB-HIV co-infection and the emergence of drug resistance (Palomino et al., 2009). Still in this context, the available therapeutic arsenal is insufficient, being ineffective against resistant strains and latent TB (Ma et al., 2010). Thus, new antimicrobial agents with novel mechanisms of action could be effective in the management of TB, reducing cross-resistance, the length of treatment and the adverse effects. It would increase the patient compliancy to the therapy, favoring the cure (Hoagland et al., 2016; Igarashi et al., 2017).
The natural sources have been highlighted in the discovery and development of new antibiotics mainly due to structural diversity of the compounds, therapeutic potential and plethoric mechanisms of action (Bérdy, 2012; Newman and Cragg, 2016). Among the compounds that may be found in a variety of plants, algae, bacteria and fungi, are quinones: aromatic compounds which are classified in anthraquinones, benzoquinones and naphthoquinones, according to their chemical compositions (López-López et al., 2014). The naphthoquinones and derivatives have a naphthalene ring, and some studies have reported its antibacterial, antitumoral, antileishmanial, anthelmintic, and antifungal activity (Coelho et al., 2010; Hazra et al., 2013; Fiorito et al., 2014; Neto et al., 2014; Mata-Santos et al., 2015). Some mechanisms of action have been proposed for naphthoquinones, mainly related to oxidative stress – due to the production of reactive oxygen species (ROS) which induce apoptosis in biological systems (Da Silva et al., 2003) – and to inhibition of DNA gyrase (Karkare et al., 2013).
Previous research has demonstrated the antimycobacterial activity of naphthoquinones (Mital et al., 2008; Coelho et al., 2010; Ferreira et al., 2010; Cantos et al., 2012; Uc-Cachón et al., 2014), including against multidrug and extensively drug resistant strains (Dey et al., 2014), bringing attention to the potential of these compounds as a basis for the development of new anti-TB drugs. In this scenario, we aimed to evaluate the antimycobacterial activity of six 1,4-naphthoquinones derivatives against susceptible and drug resistant strains of Mycobacterium tuberculosis.
Materials and Methods
Synthesis
The naphthoquinone 1 is commercial, and the other compounds were synthetized using methods previously described in the literature. Naphthoquinones 2 and 3 were synthesized via cyclization of lapachol and nor-lapachol, respectively (De Moura et al., 2001), while naphthoquinone 4 was synthesized via lawsone alilation in a two steps procedure (Pinto et al., 1997). The amination of 1,4-naphthoquinone with sodium azide resulted in the naphthoquinone 5 (Fieser and Hartwell, 1935) while the amination with aniline resulted in the naphthoquinone 6 (Lisboa et al., 2011) (Table 1). All compounds were solubilized in dimethyl sulfoxide 99.5% (Sigma-Aldrich) at a concentration of 10 mg/ml and stored at 4°C until used.
Table 1.
Characterization of the 1,4-naphthoquinone derivatives.
| Chemical structure | Chemical formula | Nomenclature | |
|---|---|---|---|
| 1 | 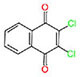 |
C10H4Cl2O2 | 2,3-Dichloronaphthalene- 1,4-dione |
| 2 | 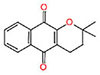 |
C15H14O3 | 2,2-Dimethyl-3,4-dihydro- 2H-benzo[g]chromene-5, 10-dione |
| 3 | 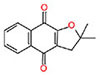 |
C14H12O3 | 2,2-Dimethyl-2,3-dihydronaphtho[2,3-b]furan-4,9-dione |
| 4 | 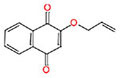 |
C13H10O3 | 2-(Allyloxy)naphthalene-1,4-dione |
| 5 | 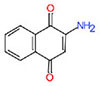 |
C10H7NO2 | 2-Aminonaphthalene-1,4-dione |
| 6 | 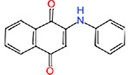 |
C16H11NO2 | 2-(Phenylamino)naphthalene- 1,4-dione |
Strains and Inoculum
The antimicrobial activity of naphthoquinones was evaluated against three strains of M. tuberculosis: a pan-susceptible, H37Rv (ATCC 27294); a mono-resistant to INH (INHR – ATCC 35822) with mutation in the katG gene (S315T); and a mono-resistant to RIF (RMPR – ATCC 35838) with mutation in rpoB gene (H526Y). All strains were cultured in Ogawa-Kudoh, for up to 14 days at 37°C without CO2. The inoculum for each strain was prepared in a glass tube containing beads to break the clumps, in sterile distilled water, according to 1.0 McFarland scale (3 × 108 UFC/mL) (Woods et al., 2011). After this process, it was diluted at a ratio of 1:20 in Middlebrook 7H9 Broth. The tests were performed at the Medical Microbiology Research Center (NUPEMM), at the Federal University of Rio Grande (FURG), under strict conditions required for handling M. tuberculosis.
Resazurin Microtiter Assay (REMA)
The minimum inhibitory concentration (MIC) of the compounds was determined by Resazurin Microtiter Assay (Palomino et al., 2002). Briefly, in a 96-well microplate, 100 μl of 7H9 medium supplemented with 10% OADC (Oleic acid Albumin Dextrose Catalase) was added in the test wells. In the first line was added 100 μl of each compound previously diluted in sterile water; a serial microdilution (1:2) was performed in the x-axis and, after, 100 μl of the last well was discarded. At the end of microdilution was added 100 μL of inoculum in each well and the final range of concentrations evaluated was 200 to 0.8 μg/mL for naphthoquinones, 8 to 0.03 μg/mL for INH and 1.024 to 0.06 μg/mL for RIF. In each plate were added sterility and growth controls. After incubation at 37°C for 7 days was added 30 μL of resazurin 0.02% on the plate and then, 24 h later, the reading of the results was performed. MIC was defined as the lowest concentration of compound capable of inhibiting bacterial growth. All tests were performed in triplicate.
Cytotoxicity Assay
The cytotoxicity of the compounds was evaluated on adherent J774A.1 macrophage cell line (ATCC TIB-67). In a 96-well plate, 200 μL of cell suspension (at the concentration of 1 × 105 cells/mL) were cultivated in Dulbecco’s modified Eagle medium (Sigma-Aldrich) supplemented with 10% of fetal bovine serum and maintained for 24 h at 37°C in a humid atmosphere with 5% CO2 (Snewin et al., 1999). After this period, the attached cells were exposed to different concentrations of naphthoquinones (200 to 0.8 μg/mL) and the plate was again incubated overnight. To determinate the concentration of the compound capable of maintaining the viability of 50% of the cells (IC50) were added 30 μL of resazurin 0.01% and after 24 h of incubation the fluorescence was measured by Thermo Plate TP-Reader BioTekTM ELx800TM (Ansar Ahmed et al., 1994).
Selectivity Index (SI)
The SI of the compounds was calculated based on the results of MIC and IC50 of each compound for each strain, according to the following formula: IC50/MIC. SI values equal to or greater than 10 indicate that the compound is pharmacologically safe (Orme, 2001; Pavan et al., 2012).
Theoretical Properties of Absorption, Distribution, Metabolism, and Excretion (ADME)
Characteristics of theoretical absorption, distribution, metabolism, and excretion (ADME) and toxicological effects of the compounds were determined by in silico analysis, using the free software: Molinspiration1, Swiss ADME2 (Daina et al., 2017), and OSIRIS Property Explorer3. According to the Lipinski Rule-of-Five, the following physicochemical parameters were evaluated: molecular weight, logP, H-bond donors, and H-bond acceptors (Lipinski, 2004).
Docking Analysis
Flexible docking simulation was performed by ArgusLab 4.0.1, using Escherichia coli RNA polymerase as a protein template. The structures were from Protein Data Bank (PDB)4 – files 5UAC and 5UAQ. The interaction between proteins (wild-type and mutant H526Y) and the ligands (RMP and compound 6) was evaluated from residues 507 to 533, which comprise the RMP resistance-determining region (RRDR) (Ramaswamy and Musser, 1998). In the docking calculations, it was applied the Ascore as scoring method (Luo et al., 2012).
Results
All naphthoquinones showed inhibitory activity against the three M. tuberculosis strains with MIC ranging between 206.6 and 12.5 μM (Table 2). Besides the naphthoquinones being active against the susceptible strain, the compounds also showed various levels of activity against the resistant strains (Table 2). The compounds 1 and 3 showed, respectively, MIC = 110.6 and 54.8 μM, for all strains evaluated, while naphthoquinones 2 and 4 showed lower inhibitory concentrations against the susceptible strain, compared to the resistant strains. In addition, compounds 1, 2, and 4 exhibited IC50 between 103 and 285 μM, resulting in SI values between 0.07 and 2.8.
Table 2.
Activity of naphthoquinones against three M. tuberculosis strains and IC50 on J774A.1 cells lineage.
| Compound | MIC (μM) |
IC50 (μM) |
SI (IC50/MIC) |
||||
|---|---|---|---|---|---|---|---|
| H37Rv | INHR | RMPR | J774A.1 | H37Rv | INHR | RMPR | |
| 1 | 110.6 | 110.6 | 110.6 | 103 | 0.9 | 0.9 | 0.9 |
| 2 | 103.3 | 206.6 | 206.6 | 285 | 2.8 | 1.4 | 1.4 |
| 3 | 54.8 | 54.8 | 54.8 | >877 | ≥16 | ≥16 | ≥16 |
| 4 | 58.4 | 234 | 234 | 173 | 0.3 | 0.07 | 0.07 |
| 5 | 72.2 | 36.1 | 36.1 | >1156 | ≥16 | ≥32 | ≥32 |
| 6 | 100.4 | 100.4 | 12.5 | >803 | ≥8 | ≥8 | ≥64 |
| INH | 0.438 | 14.6 | ≤0.219 | 3733 | 8523 | 256 | ND |
| RIF | 0.608 | 0.304 | 622.2 | >2489 | ≥4094 | ≥8187 | ≥4 |
MIC, minimum inhibitory concentration; IC50, inhibitory concentration of 50%; SI, Selectivity Index; INHr, ATCC 35822; RMPr, ATCC 35838; ND, not determined.
For the INHR strain, compared with the H37Rv, all compounds showed equal or reduced activity, except for compound 5. On the other hand, in comparison with the RMPR strain, the only difference in MIC was related to the compound 6. Interestingly, the compound 6 was the most active against RMPR strain (MIC = 12.5 μM), in a concentration eight times less than the concentration capable of inhibiting the growth of H37Rv and INHR strains (MIC = 110.4 μM).
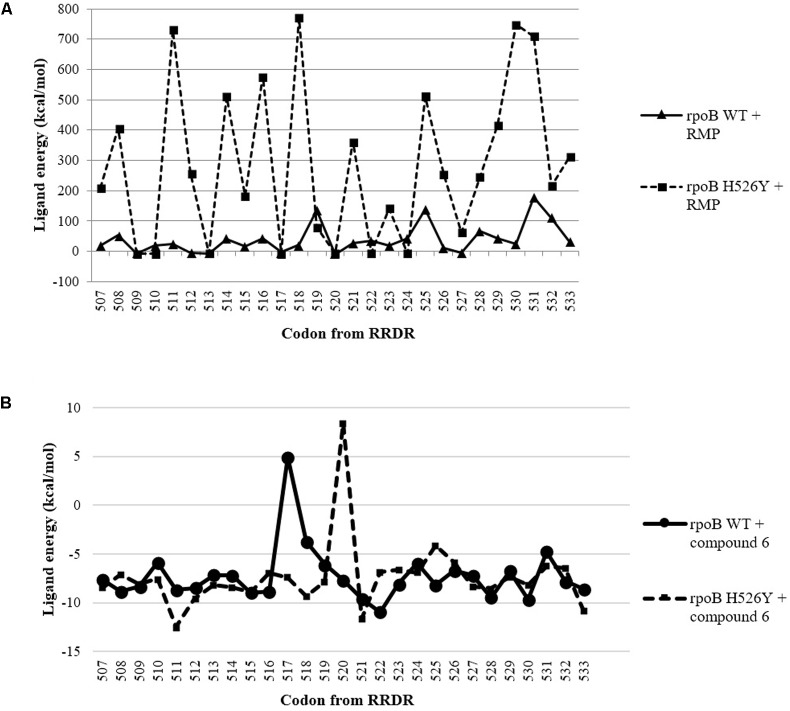
The compound 6 was able to inhibit RMPR strain (MIC = 12.5 μM) at a concentration 50x lower than the drug RMP (MIC = 622.2 uM) and, considering the peculiarity of this strain – which differs from the H37Rv mainly by the mutation in the rpoB gene (H526Y) – we were prompted to explore a possible affinity of this compound with mutant rpoB. By preliminary docking, we observed a reduction in the affinity between RMP and the RRDR site in mutant rpoB target (Figure 1A) while compound 6 showed more negative free energy – strong binding (Silva et al., 2017) – compared to RMP for both wild-type as well as the mutant protein (Figure 1).
FIGURE 1.
Ligand energy between each codon from RRDR of Escherichia coli rpoB gene (WT and H526T) and RMP (A); and compound 6 (B).
Regarding the ADME analysis, all the naphthoquinones evaluated in this study showed high gastrointestinal absorption and are in agreement with the Lipinski’s rule-of-five (Lipinski, 2004): molecular weight ≤ 500, miLogP ≤ 5, H-bond donors ≤ 5, and H-bond acceptors ≤ 10 (Table 3), indicating crucial characteristics for oral bioavailability. In addition, most of the compounds showed none or low toxicity risk related to mutagenicity or tumorigenicity (Table 3).
Table 3.
Absorption, distribution, metabolism, and excretion (ADME) characterization and toxicity risks of naphthoquinones.
| Compound | MW | miLogP | TPSA (Å2) | H-Acceptors | H-Donors | GI absorption | Mutagenic | Tumorigenic |
|---|---|---|---|---|---|---|---|---|
| 1 | 227.04 | 2.88 | 34.14 | 2 | 0 | High | Low | High |
| 2 | 242.27 | 2.95 | 43.38 | 3 | 0 | High | None | None |
| 3 | 228.24 | 2.43 | 43.38 | 3 | 0 | High | None | None |
| 4 | 214.22 | 2.30 | 43.38 | 3 | 0 | High | Low | None |
| 5 | 173.17 | 1.08 | 60.16 | 3 | 2 | High | High | None |
| 6 | 249.26 | 3.59 | 46.17 | 3 | 1 | High | Low | None |
MW, molecular weight; miLogP, octanol–water partition coefficient logP; TPSA, total polar surface area; GI absorption, gastrointestinal absorption.
In this study, by the pharmacokinetic analysis using the Software Swiss ADME, the theoretical inhibitory activity of naphthoquinone derivatives against five cytochrome P450 isoforms was evaluated (CYP450): CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. All compounds showed potential inhibitory activity of CYP1A2 and CYP2C19 isoforms, except for compound 5, which showed interaction only with CYP1A2. In addition, compounds 2 and 6 also showed activity against the 3A4 isoform.
Discussion
We evaluated the antimycobacterial potential of six 1,4-naphthoquinone derivatives: all compounds were active against susceptible and resistant strains of M. tuberculosis, with different MIC values. Mathew et al. (2010) reported the antimycobacterial activity of plumbagin and its derivatives, and Dey et al. (2014) reported the susceptibility of mycobacteria (sensitive and resistant) to naphthoquinones, mainly plumbagin, with MIC between 0.25 and 16 μg/mL. Thus, both highlight the potential of naphthoquinones in the search for new anti-TB drugs. Our research group has developed several studies with a focus on new antimicrobial agents and, although other studies have reported the activity of other naphthoquinone derivatives, including for resistant strains, their mechanism of action is still uncertain (Coelho et al., 2010; Jardim et al., 2015).
Based on the analysis of the structure-activity relationship, the compound 3, which has a tetrahydrofuran radical and anti-trypanosome activity previously described (De Moura et al., 2001), showed MIC = 54.8 μM against the three M. tuberculosis strains, while the compound 2, which contains a tetrahydropyran radical, showed MIC between 103.3 and 206.6 μM. Besides showing a better antimycobacterial activity, the compound 3 has also shown reduced cytotoxicity (IC50 > 877 μM) compared with 2 (IC50 = 285 μM), and both showed none mutagenic or tumorigenic risks (Table 3).
When we analyzed the compounds with nitrogen (5 and 6), it was noticed that phenylamine radical in the compound 6 has decreased the activity for the susceptible and INHR strains, while was able to a threefold increase the activity of this naphthoquinone for RMPR strain, compared with compound 5, which has the amine group (Table 2). The activity of naphthoquinones with nitrogenous radicals also has been described against fungi, gram positive and negative bacteria (Riffel et al., 2002; Rahmoun et al., 2013).
Among the rifampicin-resistant M. tuberculosis, about 35% have the mutation H526Y (Molodtsov et al., 2017) and, considering that compound 6 was active against the RMPR strain, we can infer that there is probably no cross-resistance between this compound and RMP, what was also evidenced in the docking analysis (Molodtsov et al., 2017). Moreover, due to the lower binding energies of the compound 6 when compared to the binding energies of RIF in RRDR, the mutation seems to favor the binding between the naphthoquinone 6 and the RRDR (Figure 1).
Furthermore, among the naphthoquinone derivatives evaluated, the compound 5 was the most active for the INHR strain (MIC = 36.1 μM), which has a mutation in the katG gene (S315T). This result evidences the absence of cross-resistance of this compound with INH, especially in strains that have this mutation.
According to the results of in silico pharmacokinetics, the naphthoquinone derivatives have inhibitory potential in some human CYP450 isoforms. It is known that the electron transfer chain is directly related with activity of enzymes, such as NADH, transferring electrons from lipophilic redox carriers. In this context, the importance of this biosynthetic route as a target in the search for new antimicrobials has been highlighted (Black et al., 2014) and, considering the results obtained in this study, the naphthoquinones could act as other quinones previously described, in the transfer chain of electrons (Heikal et al., 2016).
Conclusion
Considering the potential of quinones as a source of new antimicrobial agents and their biological activities already reported, this set of findings confirm this hypothesis and demonstrate that, naphthoquinones derivatives could be scaffolds for the development of new anti-TB drugs.
Author Contributions
PH, DR, and PdS designed the study. KDM, PC, KDR, TC, and MP contributed with compounds synthesis. PH, LF, and DR performed the experiments. PH, DR, PdS, and KDM analyzed the data and wrote the manuscript. All authors approved the version to be published.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by National Council for Technological and Scientific Development (CNPq) and Coordination for the Improvement of Higher Education Personnel (CAPES).
Footnotes
References
- Andersen P., Woodworth J. S. (2014). Tuberculosis vaccines – rethinking the current paradigm. Trends Immunol. 35 387–395. 10.1016/j.it.2014.04.006 [DOI] [PubMed] [Google Scholar]
- Ansar Ahmed S., Gogal R. M., Walsh J. E. (1994). A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J. Immunol. Methods 170 211–224. 10.1016/0022-1759(94)90396-4 [DOI] [PubMed] [Google Scholar]
- Bérdy J. (2012). Thoughts and facts about antibiotics: where we are now and where we are heading. J. Antibiot. 65 441–441. 10.1038/ja.2012.54 [DOI] [PubMed] [Google Scholar]
- Black P. A., Warren R. M., Louw G. E., Van Helden P. D., Victor T. C., Kana B. D. (2014). Energy metabolism and drug efflux in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 58 2491–2503. 10.1128/AAC.02293-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantos J. B., Coelho T. S., Carneiro P. F., de Moura K. C. G., Pinto M. C. F. R., da Silva Júnior E. N., et al. (2012). Antimicrobial evaluation of quinones and heterocyclic compounds against Mycobacterium marinum, M. kansasii and M. abscessus. Lat. Am. J. Pharm. 31 507–513. [Google Scholar]
- Coelho T. S., Silva R. S. F., Pinto A. V., Pinto M. C. F. R., Scaini C. J., Moura K. C. G., et al. (2010). Activity of β-lapachone derivatives against rifampicin-susceptible and -resistant strains of Mycobacterium tuberculosis. Tuberculosis 90 293–297. 10.1016/j.tube.2010.06.001 [DOI] [PubMed] [Google Scholar]
- Da Silva M. N., Ferreira V. F., De Souza M. C. B. V. (2003). Um panorama atual da química e da farmacologia de naftoquinonas, com ênfase na β-Lapachona e derivados. Quim. Nova 26 407–416. 10.1590/S0100-40422003000300019 [DOI] [Google Scholar]
- Daina A., Michielin O., Zoete V. (2017). SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 7:42717. 10.1038/srep42717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moura K. C. G., Emery F. S., Neves-Pinto C., Pinto M. D. C. F. R., Dantas A. P., Salomão K., et al. (2001). Trypanocidal activity of isolated naphthoquinones from Tabebuia and some heterocyclic derivatives: a review from an interdisciplinary study. J. Braz. Chem. Soc. 12 325–338. 10.1590/S0103-50532001000300003 [DOI] [Google Scholar]
- Dey D., Ray R., Hazra B. (2014). Antitubercular and antibacterial activity of quinonoid natural products against multi-drug resistant clinical isolates. Phyther. Res. 28 1014–1021. 10.1002/ptr.5090 [DOI] [PubMed] [Google Scholar]
- Ferreira S. B., de Carvalho da Silva F., Bezerra F. A. F. M., Lourenço M. C. S., Kaiser C. R., Pinto A. C., et al. (2010). Synthesis of alpha- and beta-pyran naphthoquinones as a new class of antitubercular agents. Arch. Pharm. 343 81–90. 10.1002/ardp.200900162 [DOI] [PubMed] [Google Scholar]
- Fieser L. F., Hartwell J. L. (1935). The reaction of hydrazoic acid with the naphthoquinones. J. Am. Chem. Soc. 57 1482–1484. 10.1021/ja01311a030 [DOI] [Google Scholar]
- Fiorito S., Epifano F., Bruyère C., Mathieu V., Kiss R., Genovese S. (2014). Growth inhibitory activity for cancer cell lines of lapachol and its natural and semi-synthetic derivatives. Bioorg. Med. Chem. Lett. 24 454–457. 10.1016/j.bmcl.2013.12.049 [DOI] [PubMed] [Google Scholar]
- Hazra S., Ghosh S., Das Sarma M., Sharma S., Das M., Saudagar P., et al. (2013). Evaluation of a diospyrin derivative as antileishmanial agent and potential modulator of ornithine decarboxylase of Leishmania donovani. Exp. Parasitol. 135 407–413. 10.1016/j.exppara.2013.07.021 [DOI] [PubMed] [Google Scholar]
- Heikal A., Hards K., Cheung C. Y., Menorca A., Timmer M. S. M., Stocker B. L., et al. (2016). Activation of type II NADH dehydrogenase by quinolinequinones mediates antitubercular cell death. J. Antimicrob. Chemother. 71 2840–2847. 10.1093/jac/dkw244 [DOI] [PubMed] [Google Scholar]
- Hoagland D. T., Liu J., Lee R. B., Lee R. E. (2016). New agents for the treatment of drug-resistant Mycobacterium tuberculosis. Adv. Drug Deliv. Rev. 102 55–72. 10.1016/j.addr.2016.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi M., Ishizaki Y., Takahashi Y. (2017). New antituberculous drugs derived from natural products: current perspectives and issues in antituberculous drug development. J. Antibiot. 10.1038/ja.2017.126 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Jardim G. A. M., Cruz E. H. G., Valença W. O., Resende J. M., Rodrigues B. L., Ramos D. F., et al. (2015). On the search for potential antimycobacterial drugs: synthesis of naphthoquinoidal, phenazinic and 1,2,3-triazolic compounds and evaluation against Mycobacterium tuberculosis. J. Braz. Chem. Soc. 26 1013–1027. 10.5935/0103-5053.20150067 [DOI] [Google Scholar]
- Karkare S., Chung T. T. H., Collin F., Mitchenall L. A., McKay A. R., Greive S. J., et al. (2013). The naphthoquinone diospyrin is an inhibitor of DNA gyrase with a novel mechanism of action. J. Biol. Chem. 288 5149–5156. 10.1074/jbc.M112.419069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski C. A. (2004). Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov. Today Technol. 1 337–341. 10.1016/j.ddtec.2004.11.007 [DOI] [PubMed] [Google Scholar]
- Lisboa C. D. S., Santos V. G., Vaz B. G., De Lucas N. C., Eberlin M. N., Garden S. J. (2011). C-H functionalization of 1,4-naphthoquinone by oxidative coupling with anilines in the presence of a catalytic quantity of copper(II) acetate. J. Org. Chem. 76 5264–5273. 10.1021/jo200354u [DOI] [PubMed] [Google Scholar]
- López-López L. I., Nery-Flores D. S., Silva-Belmares Y. S., Sáenz-Galindo A. (2014). Naphthoquinones: biological properties and synthesis of lawsone and derivatives — A structured review. Vitae 21 248–258. [Google Scholar]
- Luo H. J., Wang J. Z., Zhou Y., Zou K. (2012). Docking study on trametenolic acid B as a α-glucosidase inhibitor. Med. Chem. Res. 21 2141–2144. 10.1007/s00044-011-9741-y 23742858 [DOI] [Google Scholar]
- Ma Z., Lienhardt C., McIlleron H., Nunn A. J., Wang X. (2010). Global tuberculosis drug development pipeline: the need and the reality. Lancet 375 2100–2109. 10.1016/S0140-6736(10)60359-9 [DOI] [PubMed] [Google Scholar]
- Mata-Santos T., Pinto N. F., Mata-Santos H. A., De Moura K. G., Carneiro P. F., Carvalho T. D. S., et al. (2015). Anthelmintic activity of lapachol, β-lapachone and its derivatives against Toxocara canis larvae. Rev. Inst. Med. Trop. Sao Paulo 57 197–204. 10.1590/S0036-46652015000300003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R., Kruthiventi A. K., Prasad J. V., Kumar S. P., Srinu G., Chatterji D. (2010). Inhibition of mycobacterial growth by plumbagin derivatives. Chem. Biol. Drug Des. 76 34–42. 10.1111/j.1747-0285.2010.00987.x [DOI] [PubMed] [Google Scholar]
- Mital A., Negi V. S., Ramachandran U. (2008). Synthesis and biological evaluation of substituted naphthoquinone derivatives as potent antimycobacterial agents. ARKIVOC 2008 176–192. 10.3998/ark.5550190.0009.f17 [DOI] [PubMed] [Google Scholar]
- Molodtsov V., Scharf N. T., Stefan M. A., Garcia G. A., Murakami K. S. (2017). Structural basis for rifamycin resistance of bacterial RNA polymerase by the three most clinically important RpoB mutations found in Mycobacterium tuberculosis. Mol. Microbiol. 103 1034–1045. 10.1111/mmi.13606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto J. B. A., Da Silva C. R., Neta M. A. S., Campos R. S., Siebra J. T., Silva R. A. C., et al. (2014). Antifungal activity of naphthoquinoidal compounds in vitro against fluconazole-resistant strains of different Candida species: a special emphasis on mechanisms of action on Candida tropicalis. PLoS One 9:e93698. 10.1371/journal.pone.0093698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman D. J., Cragg G. M. (2016). Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 79 629–661. 10.1021/acs.jnatprod.5b01055 [DOI] [PubMed] [Google Scholar]
- Orme I. (2001). Search for new drugs for treatment of tuberculosis. Antimicrob. Agents Chemother. 45 1943–1946. 10.1128/AAC.45.7.1943-1946.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomino J.-C., Martin A., Camacho M., Guerra H., Swings J., Portaels F. (2002). Resazurin microtiter assay plate: simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 46 2720–2722. 10.1128/AAC.46.8.2720-2722.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomino J. C., Ramos D. F., Almeida P., Silva D. (2009). New anti-tuberculosis drugs: strategies, sources and new molecules. Curr. Med. Chem. 16 1898–1904. 10.2174/092986709788186066 [DOI] [PubMed] [Google Scholar]
- Pavan F. R., Sato D. N., Leite C. Q. F. (2012). “An approach to the search for new drugs against tuberculosis,” in Understanding Tuberculosis – New Approaches to Fighting against Drug Resistance, ed. Cardona P.-J. (London: IntechOpen; ), 137–146. [Google Scholar]
- Pinto A. V., Pinto C. N., Pinto Mdo C., Rita R. S., Pezzella C. A., et al. (1997). Trypanocidal activity of synthetic heterocyclic derivatives of active quinones from Tabebuia sp. Arzneimittelforschung 47 74–79. [PubMed] [Google Scholar]
- Rahmoun N. M., Boucherit-Atmani Z., Benabdallah M., Boucherit K., Villemin D., Choukchou-Braham N. (2013). Antimicrobial activities of the henna extract and some synthetic naphthoquinones derivatives. Am. J. Med. Biol. Res. 1 16–22. 10.12691/ajmbr-1-1-3 [DOI] [Google Scholar]
- Ramaswamy S., Musser J. M. (1998). Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis: 1998 update. Tuber. Lung Dis. 79 3–29. 10.1054/tuld.1998.0002 [DOI] [PubMed] [Google Scholar]
- Riffel A., Medina L. F., Stefani V., Santos R. C., Bizani D., Brandelli A. (2002). In vitro antimicrobial activity of a new series of 1,4-naphthoquinones. Braz. J. Med. Biol. Res. 35 811–818. 10.1590/S0100-879X2002000700008 [DOI] [PubMed] [Google Scholar]
- Silva L., Carrion L. L., von Groll A., Costa S. S., Junqueira E., Ramos D. F., et al. (2017). In vitro and in silico analysis of the efficiency of tetrahydropyridines as drug efflux inhibitors in Escherichia coli. Int. J. Antimicrob. Agents 49 308–314. 10.1016/j.ijantimicag.2016.11.024 [DOI] [PubMed] [Google Scholar]
- Snewin V. A., Gares M. P., Gaora P. Ó., Hasan Z., Brown I. N., Young D. B. (1999). Assessment of immunity to mycobacterial infection with luciferase reporter constructs. Infect. Immun. 67 4586–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova T. (2014). Quality control and safety assessment of BCG vaccines in the post-genomic era. Biotechnol. Biotechnol. Equip. 28 387–391. 10.1080/13102818.2014.927200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uc-Cachón A. H., Borges-Argáez R., Said-Fernández S., Vargas-Villarreal J., González-Salazar F., Méndez-González M., et al. (2014). Naphthoquinones isolated from Diospyros anisandra exhibit potent activity against pan-resistant first-line drugs Mycobacterium tuberculosis strains. Pulm. Pharmacol. Ther. 27 114–120. 10.1016/j.pupt.2013.08.001 [DOI] [PubMed] [Google Scholar]
- WHO (2017). WHO Global Tuberculosis Report 2017. Geneva: World Health Organization. [Google Scholar]
- Woods G. L., Brown-Elliott B. A., Conville P. S., Desmond E. P., Hall G. S., Lin G. (2011). Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; approved standard-second edition. Clin. Lab. Stand. Inst. 26 1–61. 10.1306/E4FD4657-1732-11D7-8645000102C1865D [DOI] [PubMed] [Google Scholar]