Abstract
Lumbar interbody fusion involves insertion of a structural graft into an intervertebral disc space to promote bony arthrodesis. It is a well-established surgical strategy for multiple spinal disorders ranging from degenerative conditions to trauma, neoplastic diseases, and deformities requiring correction. Since the inception of lumbar interbody fusion, the most established techniques have been two posterior approaches, the posterior lumbar interbody fusion (PLIF) and the transforaminal lumbar interbody fusion (TLIF). Within the past 15 years, multiple anterolateral approaches to the spine have become widely adopted. These approaches can be performed minimally invasively and spare disruption of the paraspinal muscles and posterior spinal column while enabling wide exposure of the disc space for insertion of interbody grafts much larger than PLIF and TLIF instrumentation. This review highlights three minimally invasive anterolateral approaches: the anterior lumbar interbody fusion (ALIF), the transpsoas lateral lumbar interbody fusion (LLIF), and prepsoas or anterior to the psoas oblique lumbar interbody fusion (OLIF). Relevant topics for discussion and comparison include patient selection, surgical techniques, outcomes, and complications for the three surgical approaches.
Keywords: Anterior lumbar interbody fusion (ALIF), lateral lumbar interbody fusion (LLIF), minimally invasive surgery (MIS), oblique lumbar interbody fusion (OLIF), spinal fusion, extreme lateral interbody fusion (XLIF)
Introduction
Since the initial description of lumbar interbody fusion in 1944 by Briggs and Milligan (1), it has been predominantly a posterior-approach surgery that necessitates variable degrees of paraspinal muscle dissection and posterior bony removal to safely access and prepare the disc space for arthrodesis. Within the past 15 years, significant interest has arisen in minimally invasive anterolateral approaches to the lumbar spine. These approaches obviate the need for disruption of the posterior spinal column, while also allowing wide exposure of the disc space for placement of a large interbody graft, shorter operative times, less blood loss, and indirect decompression of neurological tissue. Three of the most widely used procedures include anterior lumbar interbody fusion (ALIF), transpsoas lateral lumbar interbody fusion (LLIF), and a prepsoas or anterior to the psoas oblique lumbar interbody fusion (OLIF). The primary surgical goal of all three procedures is to implant the largest possible interbody graft within the confines of the surgical exposure to facilitate fusion rates, maximize segmental lordosis, and provide indirect neural decompression by expansion of the bony neuroforamen and distraction of ligamentous stenosis of the central canal. However, ALIF, LLIF, and OLIF differ considerably regarding patient selection, operative planning, surgical execution, and potential risks and complications. This review provides an overview of these three techniques, and highlights the complementary and contrasting aspects among them.
Overview
Approach to the spine
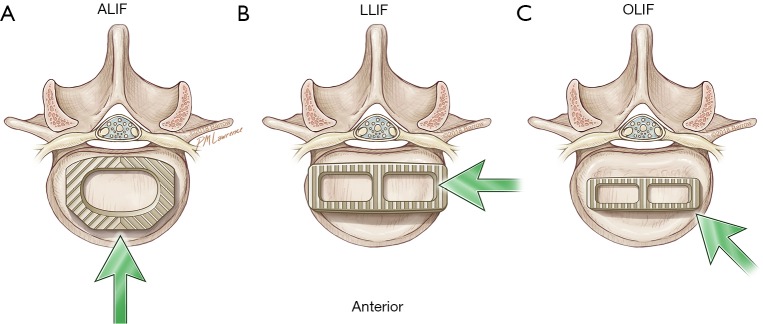
The anterolateral surgical approaches to the lumbar spine are illustrated in Figure 1. An ALIF approach allows a direct midline exposure of the lumbar disc, thereby permitting wide discectomy and placement of a large interbody graft that maximizes coverage of the vertebral body endplate. Typically, L5–S1 is the preferred level of treatment to avoid vascular structures and the iliac bifurcation. In contrast, the LLIF exposes the lateral surface of the disc space by traversing through the psoas and bluntly displacing the lumbar plexus nerves within the muscle. The OLIF accesses the anterolateral surface of the disc space just anterior to the psoas muscle, which is mobilized posteriorly. After preparation of the disc space, the interbody is inserted at an oblique angle and then rotated into a lateral position.
Figure 1.
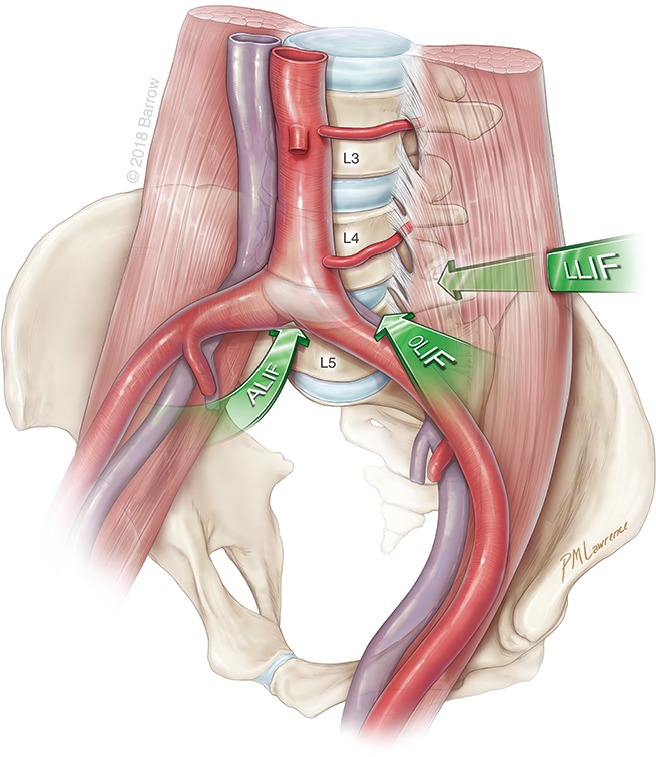
Overview of approaches to the spine. Illustration depicts the three different anterolateral approaches (arrows) to the spine. All three approaches are retroperitoneal. They involve a midline anterior lumbar interbody fusion (ALIF), a lateral lumbar interbody fusion (LLIF), or an oblique lumbar interbody fusion (OLIF). Used with permission from Barrow Neurological Institute, Phoenix, Arizona.
Difference in interbody placement
Unlike posterior interbody techniques, anterolateral approaches to the spine enable a wide exposure of the disc space that is not obscured by the thecal sac and neural elements. Consequently, large interbodies can be placed that greatly exceed the size and dimensions of posterolateral or transforaminal interbodies. The different interbody footprints in relation to the size of the endplate are highlighted in Figure 2. An ALIF exposes the entire width of the disc space from a true anterior-posterior orientation, permitting placement of an interbody that maximizes surface area contact with the vertebral body endplates. An LLIF exposes most of the lateral surface of the disc space and allows insertion of a long, wide interbody that can extend from one diaphysis to the other, securing the strongest bony surface in the endplate. An OLIF first requires insertion of an interbody at an oblique angle. Because the direction of insertion projects the interbody initially into the contralateral posterior direction toward the neuroforamen, the length of the implant is shorter than that for an LLIF and it generally does not extend from diaphysis to diaphysis of the endplate.
Figure 2.
Differences in the footprint of various interbodies. (A) An anterior lumbar interbody fusion (ALIF) fully exposes the anterior span of the disc space and permits insertion of a large interbody graft with a footprint that covers most of the endplate surface area; (B) a lateral lumbar interbody fusion (LLIF) graft is inserted laterally and can be both long and wide, spanning the entire anterior-posterior width of the disc space, including the endplate diaphysis on both sides; (C) an oblique lumbar interbody fusion (OLIF) graft is inserted obliquely in the direction of the contralateral neuroforamen; therefore, its length and width are limited to prevent iatrogenic injury to the nerve root before rotation of the graft into the lateral position. Used with permission from Barrow Neurological Institute, Phoenix, Arizona.
Degree of indirect decompression
All three approaches achieve indirect decompression of neural elements by expanding the bony neuroforamen and reducing ligamentous buckling at the disc space by distraction. Radiographic studies after ALIF show a 67% increase in the foraminal cross-section area (2). For LLIF, a magnetic resonance imaging (MRI)–based study indicated a 24.7% and a 33.1% mean increase in the neuroforaminal and central canal cross-sectional area, respectively (3). Similar to LLIF, OLIF has been found to result in a 30.2% median increase in cross-sectional area of the thecal sac (4) and in a 30.0% average increase in the neuroforamen area (5). Figure 3 illustrates the degree of indirect decompression in a patient after LLIF.
Figure 3.
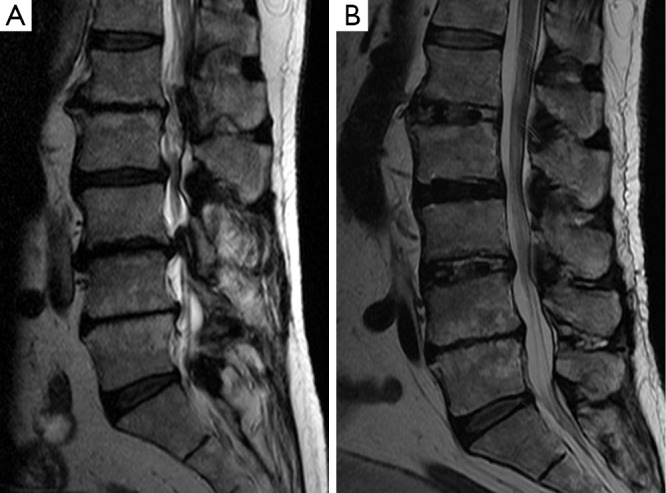
Indirect decompression after lateral lumbar interbody fusion (LLIF). Sagittal T2-weighted magnetic resonance images (A) before and (B) after L1-L3 LLIF for spinal stenosis and coronal deformity in a 69-year-old man with severe back pain and neurogenic claudication. (B) Note expansion of the central canal and visible reduction in nerve root clumping after resolution of the central canal stenosis. Used with permission from Barrow Neurological Institute, Phoenix, Arizona.
Risk of subsidence
Subsidence of interbody grafts results in the potential loss of indirect decompression as bony structures collapse and condense around the interbody. Strategies to prevent subsidence include the use of larger interbodies with more surface area to distribute load across the vertebral endplates or placement of posterior fixation such as pedicle screws to distribute load across the instrumented vertebra. Of the types of interbodies used in the three procedures, the ALIF interbodies are the largest and result in the lowest incidence of subsidence. In a study of 147 patients who underwent stand-alone placement of an ALIF interbody, Rao et al. (6) found that only 10.2% had subsidence without any return of neurological symptoms at the 18-month follow-up. This incidence compares favorably to that for LLIF. Marchi et al. (7) found that at 1-year follow-up, radiographic subsidence occurred in 30% of 61 lumbar levels treated with a stand-alone 18-mm-wide interbody and in 11% of 37 lumbar levels treated with a 22-mm-wide stand-alone interbody. However, Lang et al. (8) found essentially no significant subsidence with a 26-mm-wide stand-alone LLIF interbody across 28 spinal segments analyzed. The overall small length and surface area of OLIF cages compared to ALIF and LLIF counterparts has resulted in almost all published studies reporting incorporation of posterior pedicle screw fixation; no studies have examined the rate of subsidence in stand-alone constructs.
ALIF
Patient selection
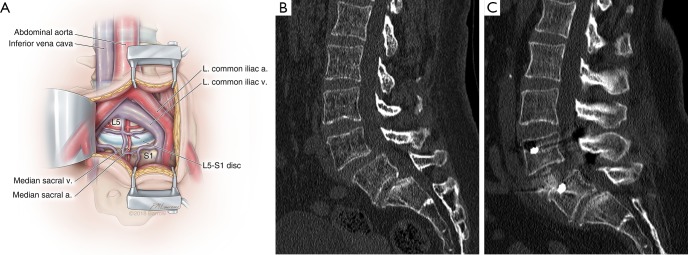
ALIF is suitable for treating patients with degenerative spondylosis causing neurogenic claudication or radiculopathy because it provides indirect decompression of the neuroforamen and the thecal sac by distraction of the adjoining vertebral bodies. A typical ALIF exposure is shown in Figure 4A. Resection of the anterior longitudinal ligament during discectomy allows destabilization of the anterior and middle columns, which can facilitate reduction of spondylolistheses (Figure 4B,C). Furthermore, resection of the anterior longitudinal ligament permits placement of hyper-lordotic implants that can deliver clinically significant segmental and overall lumbar lordosis in spinal deformity patients with sagittal imbalance.
Figure 4.
Anterior lumbar interbody fusion (ALIF) and reduction of spondylolisthesis. (A) Illustration of a mini-open ALIF approach, in which the peritoneum is reflected laterally to expose the retroperitoneal vessels and spine. At L5-S1, the working corridor is typically between the iliac vessels below their bifurcation. This allows a full anterior-posterior exposure of the disc space in its entirety. At L4–5 and higher lumbar levels, the approach is limited by how much mobilization of the vascular structures can be achieved. a., artery; L. left; v., vein; (B) preoperative sagittal computed tomogram (CT) of a 53-year-old woman with intractable back pain demonstrates a grade I spondylolisthesis at L4–5 that was demonstrated to be mobilized on flexion-extension imaging; (C) postoperative CT of the same patient after a minimally invasive L4–5 ALIF and placement of posterior percutaneous pedicle screws demonstrates full reduction of the spondylolisthesis and clinically significant restoration of disc height. Used with permission from Barrow Neurological Institute, Phoenix, Arizona.
Contraindications for ALIF include major previous abdominal or retroperitoneal surgeries, severe peripheral vascular disease, and transitional anatomy at the vertebral body, which may make the disc space angle inaccessible. Typically, ALIF is performed most safely at the L5-S1 level because of the large working corridor between the iliac vessels. Higher lumbar vertebra up to L2–3 can be reached, but oftentimes their exposure is limited because of the need for extensive vascular mobilization of the aorta and iliac vessels, as well as because of impediments created by retroperitoneal viscera such as the pancreas and kidneys.
Surgical approach
Anterior approaches to the lumbar spine first emerged in the 1930s for the treatment of spondylolisthesis by Capener (9) and for Pott’s disease by Ito et al. (10). Since then, various surgical techniques, including open or laparoscopic transperitoneal approaches and retroperitoneal exposures, have been developed. Since the late 1990s, the preferred surgical approach that has become the most widely used is a mini-open retroperitoneal technique popularized by Brau et al. (11). The patient is positioned supine on a radiolucent operating table, and a mini-transverse Pfannenstiel incision is planned that is based on the levels being treated. For L5–S1, the incision is typically located two-thirds of the distance between the umbilicus and the pubic symphysis. For L4–5, the incision is made just below the umbilicus. Higher lumbar levels and surgeries targeting multiple levels can use a paramedian vertical incision.
After the incision is made, the anterior rectus sheath is incised and the rectus muscle is mobilized. The posterior rectus sheath or transversalis fascia is incised and dissected free from the underlying peritoneum. Blunt dissection is used to carefully peel and reflect the peritoneum off the ventral retroperitoneum in a lateral to medial direction, thereby exposing the ventral spine and the associated major vasculature. At L5–S1, a working corridor can be achieved between the iliac vessels below the aortic and inferior vena cava bifurcation (Figure 4A). For higher levels, vascular mobilization of the aorta and iliac vessels is required. After exposure, discectomy and instrumentation are performed. Closure is performed with reapproximation of the anterior rectus sheath and cutaneous layers. Generally, the posterior sheath does not contribute significant structural integrity to the closure and does not need to be reapproximated unless it demonstrates substantial thickness.
Outcomes
Contemporary studies within the past 15 years have shown that ALIF fusion rates exceed 90%. Rao et al. (12) reported on a prospective cohort of 125 consecutive ALIF patients with a mean follow-up of 20 months. They documented a 94.4% radiographic fusion rate for stand-alone implants and statistically significant improvement in patient reports of pain outcomes [visual analog scale (VAS) pain score for back pain, 7.2 preoperative to 2.8 postoperative, P<0.001] for multiple indications that include spondylosis, spondylolisthesis, and adjacent segment disease. Lee et al. (13) reported on 73 patients who underwent ALIF and posterior percutaneous pedicle screws for treatment of isthmic spondylolisthesis and found a fusion rate of 97.3% with the addition of posterior fixation. Regarding neural decompression, a radiographic study by Rao et al. (2) demonstrated a statistically significant 67% (P<0.01) increase in the cross-sectional area of the neuroforamen at the interbody level due to distraction of the vertebral bodies.
Complications
Complications from ALIF occur predominantly while obtaining intra-abdominal access and exposure. Devastating complications related to injury of the large bowel, pancreas, and other viscera have been reported (14), but these are rare, particularly in modern case series with newer instrumentation, a standardized retroperitoneal approach, and careful selection of patients with no history of intra-abdominal surgery. The most relevant contemporary complications relate to vascular injury and retrograde ejaculation. Vessel injury, particularly to the iliac veins, is a major concern during the surgical approach and discectomy. In general, small venous lacerations less than 5 mm long can be treated with pressure and hemostatic agents. Larger tears and arterial injuries require more involved repair using sutures. Brau et al. (15) reported a vascular injury rate of 1.9% in 1,315 consecutive cases, with 76% of the injuries being venous. Quraishi et al. (16) reported a 4.6% incidence of vascular injury in 304 patients that required a vascular surgeon to assist with repair. Hamdan et al. (17) reported a 1.9% incidence of major vascular injury in 480 patients that required major repair efforts. Thus, the overall risk of vascular injury appears to approximate 3% across large case series.
Retrograde ejaculation is a risk for men undergoing any intra-abdominal surgery because of the possibility of injury to the superior hypogastric plexus that lies beneath the peritoneum, ventral to the aorta and left common iliac vein. Reports in the surgical literature consistently indicate that the risk of retrograde ejaculation is approximately 2% with a retroperitoneal exposure and 10-fold higher with transperitoneal approaches. Sasso et al. (18) documented a 1.7% incidence of retrograde ejaculation in 116 ALIF patients undergoing a retroperitoneal exposure and a 13.3% incidence in 30 patients undergoing a laparoscopic approach. Similarly, Escobar et al. (19) found a 2% and 25% incidence of retrograde ejaculation in a cohort of patients undergoing open retroperitoneal versus laparoscopic transperitoneal exposure for ALIF. Gentle dissection and en-bloc reflection of the peritoneum during exposure of the ventral spine may reduce the risk of injury to the plexus, but such injury is often unavoidable and should be thoroughly discussed with male patients during the informed consent process prior to surgery.
LLIF
Patient selection
LLIF is suitable for the treatment of a broad array of pathologic conditions, from degenerative spondylosis with instability to trauma, scoliosis, and spondylolisthesis. Typically, the spine levels from T12–L5 can be accessed laterally. The L5–S1 interspace is generally inaccessible from a direct lateral approach because of impedance by the iliac crests, the iliac vasculature that approaches the ventrolateral aspect of the vertebral body, and the anterior course of the lumbar nervous plexus, which increases the risk of traction injury from minimally invasive retractors.
Contraindications to LLIF include previous extensive retroperitoneal surgery (e.g., renal surgery) and transitional anatomy when targeting L4–5 because a sacralized L5 may contain variant psoas anatomy and an anteriorly displaced lumbar plexus. Interbody subsidence is a considerable risk for treatment failure (20); therefore, osteoporosis (T-score ≤−2.5) is a relative contraindication for surgery. Patients with osteopenia (T-score −1.0 to −2.5) may require surgical intervention with wider implants or posterior pedicle screw fixation to decrease the risk of subsidence (8,21).
Surgical technique
First described in 2001 by Pimenta et al. (22), the LLIF involves exposure of the lateral aspect of the lumbar spine by a retroperitoneal corridor through the psoas muscle (Figure 5A). Initial surgical approaches were performed with visualization of the psoas dissection. However, modern systems, such as the eXtreme Lateral Interbody Fusion (XLIF) (NuVasive, Inc., San Diego, CA, USA) and the direct lateral interbody fusion (DLIF) (Medtronic, Memphis, TN, USA) have adapted minimally invasive retractors to allow atraumatic blunt dissection through the psoas with subsequent direct visualization of the lateral disc space. Although the general surgical principles and work flows are similar in all LLIF instrumentation systems, a major difference is the use of a directional triggered–electromyography (EMG) neuromonitoring system with the XLIF (NuVasive, Inc.) platform that we will describe in greater detail below.
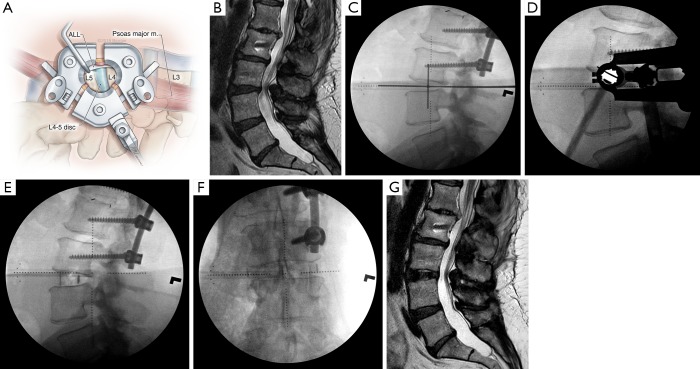
Figure 5.
Overview of lateral lumbar interbody fusion (LLIF). (A) Illustration of the LLIF operative field through a minimally invasive retractor allows visualization of most of the lateral length of the disc space, as well as the anterior longitudinal ligament (ALL), which typically is kept intact except when anterior column realignment is necessary. m., muscle; (B) preoperative sagittal T2-weighted magnetic resonance image (MRI) of a 57-year-old woman with a previous L1-L3 fusion and distal adjacent segment instability at L3-4, with resultant severe central canal stenosis causing intractable neurogenic claudication; (C) intraoperative lateral fluoroscopy with a cross hair is used to mark the incision on the patient’s right flank. The center of the incision is approximately one-third of the anterior-posterior (AP) length of the disc space. At L4–5, it is centered on one-half of the AP length; (D) intraoperative lateral fluoroscopy shows the docking location of the minimally invasive lateral retractor; (E) lateral and (F) AP radiographs taken immediately after placement of an LLIF interbody cage demonstrate distraction of the disc space and increased segmental lordosis. A long interbody is placed, spanning the entire wide of the disc space from each diaphysis; (G) postoperative MRI demonstrates restoration of the L3–4-disc height and indirect decompression of the central canal. Used with permission from Barrow Neurological Institute, Phoenix, Arizona.
Extensive preoperative imaging is critical for optimal LLIF surgical planning (Figure 5B). Axial MRI or computed tomography (CT) is necessary to ensure that intra-abdominal blood vessels are not vulnerable to injury in the path of lateral access and discectomy. An anterior-posterior radiograph or CT should be obtained to evaluate whether the iliac crest will obscure the disc space, which may require additional flexion of the torso during positioning.
The patient is positioned in the lateral decubitus position on a radiolucent operating table with the approach side facing up. The hips and knees are flexed to relax the psoas muscle as much as possible, and the iliac crest is positioned at the level of the table break to allow flexion at the torso. A roll is placed below the axilla to decompress the brachial plexus and under the down-facing iliac crest to promote torso flexion. Fluoroscopy is then performed to aid in positioning the patient so that perfect anterior-posterior and lateral radiographs are obtained without having to adjust the rotation or tilt angle of the fluoroscope. After suitable positioning, the patient is secured to the table with tape, and fluoroscopy is used to plan a transverse flank incision in line with the disc space (Figure 5C). The incision is centered on the posterior one-third of the disc space except at L4–5, where it is centered on the middle of the disc space.
After the incision is made, electrocautery is used to expose and divide the external oblique fascia. The muscle layers (external and internal oblique and transversus abdominis) are then bluntly dissected with two tonsil hemostats until the transversalis fascia is encountered. The blunt tip of the hemostat is then used to gently puncture the transversalis fascia to allow entry into the retroperitoneal space. Blunt finger dissection is used to mobilize the peritoneum anteriorly off the psoas muscle, which can be palpated deep. The first muscle dilator is then inserted into the top of the psoas fascia with the surgeon’s finger guiding it anteriorly to prevent injury to the peritoneum.
Lateral fluoroscopy is performed to verify the dilator position over the disc space. The dilator is then advanced bluntly through the psoas muscle and docked onto the spine. Directional EMG monitoring obtained through the dilator is used to monitor muscle responses in a 360-degree fashion. The goal is to have lower threshold responses posterior to the dilator, thereby ensuring that the retractor will be anterior to the femoral nerve and lumbar plexus. If EMG findings are not worrisome, a guidewire is inserted into the disc space and sequential dilators are inserted over the initial dilator with EMG monitoring repeated with each dilation. The lateral access retractor is inserted over the final dilator, and a docking shim is inserted through the posterior blade of the retractor into the disc space to secure the retractor in place (Figure 5D). Discectomy and preparation of the endplate are then performed, and the widest interbody device that can be placed safely is inserted (Figure 5E,F,G).
Outcomes
Fusion rates for LLIF are high. Berjano et al. (23) reported a 97.4% fusion rate in 78 LLIF levels assessed with CT imaging at a mean follow-up of 34.5 months. Rodgers et al. (24) similarly reported a 97% fusion rate across 63 patients assessed by CT imaging at 12 months after surgery. For spondylolisthesis, LLIF has been demonstrated to be extremely effective in reducing the degree of slippage of both grade I and grade II lesions with clinically significant improvement in clinical pain scores (24,25). When used for spinal deformity, LLIF can provide substantial segmental coronal and sagittal Cobb angle correction (26,27).
Complications
Neurological injury to the lumbar plexus is the greatest risk from LLIF, and it has the potential to result in sensory and motor deficits. Unfortunately, reports of neurological complications in the surgical literature are inconsistent regarding the anatomical source, the severity of injury, and details about which LLIF technique was used, and what neuromonitoring, if any, was available. As a result, the incidence of thigh paresthesia and numbness after LLIF has been reported to range widely from 0.7% to 30%, and the incidence of motor weakness has ranged from 3.4% to 23.7% (23,28-30). However, when neurological symptoms occur, most are temporary, with 90% resolving spontaneously within a year after surgery (29). Notably, in the largest consecutive case series of 600 LLIF patients all treated with the XLIF (NuVasive, Inc.) platform under directional neuromonitoring, Rodgers et al. (28) reported a 0.7% incidence of transient neurological injury.
Abdominal wall paresis with a resultant “pseudohernia” has been reported as a rare complication of LLIF likely due to iatrogenic injury to the nerves innervating the abdominal wall muscles during the initial exposure for LLIF (31). For that reason, we recommend using only blunt dissection through the abdominal wall musculature rather than electrocautery or sharp dissection. Catastrophic bowel injury (32) and great vessel injury have also been reported, although rarely, with a reported incidence of vascular complications of 0.56% in a large patient series by Kueper et al. (33). Bowel injury can occur with inadequate release and anterior reflection of the peritoneum, making injury possible as the initial dilator and guidewire are passed through the psoas muscle. Vessel injury occurs most commonly during discectomy when an instrument passes too far anteriorly across the contralateral annulus, injuring nearby vessels.
OLIF
Patient selection
The OLIF procedure has indications similar to those of the LLIF with regard to treating degenerative, traumatic, and scoliotic conditions. Limitations involve the initial posteromedial trajectory of the interbody, which has a small risk of displacing additional disc or ligamentous material in the direction of the central canal or the contralateral neuroforamen, making the procedure relatively contraindicated in patients with high-grade central canal stenosis. Furthermore, patients with spondylolisthesis more severe than Meyerding grade I are unsuitable for OLIF because they do not have enough “overlap” of the two vertebral endplates in an oblique trajectory at the disc space to accommodate support for the interbody (34). In terms of anatomical accessibility, an OLIF can target L1 to S1 and is not blocked by the iliac crests for the lower lumbar levels because of the anterior oblique trajectory to the spine that can traverse under the anterior slope of the crest.
Surgical procedure
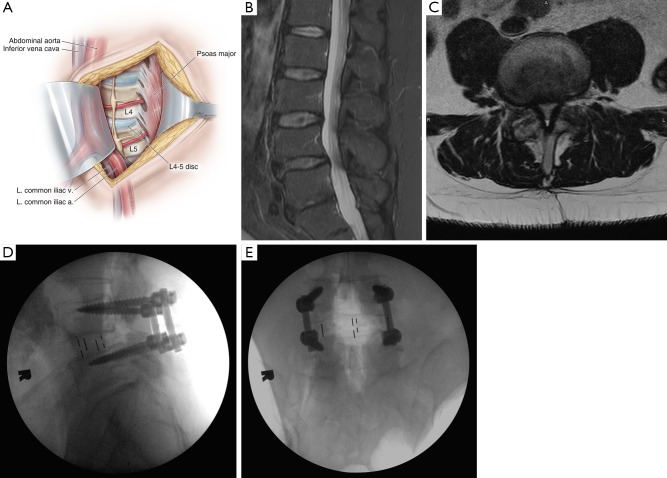
The first description of an OLIF approach was published in 1997 by Mayer (35), but the official name and acronym were not coined until 2012 by Silvestre et al. (36). Patient positioning and setup for OLIF are similar to those for LLIF. Key differences are the lack of any need for neurological monitoring with OLIF and the substitution of intraoperative CT with stereotactic navigation for OLIF rather than the fluoroscopic imaging used for LLIF (37). With the patient in a lateral decubitus position, the projected disc space is marked along the patient’s flank, and a transverse incision is planned, centered on the anterior margin of the disc space. Skin incision and division of the external oblique muscle fascia are performed with electrocautery, followed by blunt dissection through the abdominal wall muscles until the transversalis fascia is exposed and bluntly traversed to enter the retroperitoneal space. The peritoneum is mobilized anteriorly, and the psoas muscle is identified with blunt dissection of its anterolateral attachments off the disc space. The oblique working corridor is established by anterior retraction of the peritoneum and vascular structures concurrently with posterior retraction of the psoas muscle (Figure 6A). The retraction of the psoas muscle from its anterior margin makes the lumbar plexus completely posterior to the retractor, precluding the need for neuromonitoring. Discectomy is performed and an interbody is inserted into the disc space (Figure 6B,C,D,E).
Figure 6.
Overview of an oblique lumbar interbody fusion (OLIF). (A) Illustration of an OLIF exposure, with the medial portion of the psoas major muscle mobilized and retracted posteriorly to expose more of the disc space annulus. The great vessels anterior to the psoas are protected by another retractor. The anterolateral portion of the disc space is then exposed, allowing preparation and insertion of an interbody at an oblique angle that is then rotated to rest laterally; (B) lateral and (C) axial perioperative T2-weighted magnetic resonance images of a 57-year-old man with progressive back pain and neurogenic claudication demonstrate severe L4–5 central canal stenosis and grade I spondylolisthesis; (D) lateral and (E) anterior-posterior radiographs taken during intraoperative placement of the OLIF interbody with percutaneous posterior pedicle screws show good placement of the interbody and reduction of the spondylolisthesis. Note that the width of the interbody does not touch the lateral diaphyses of the disc space. Used with permission from Barrow Neurological Institute, Phoenix, Arizona. a., artery; L. left; m., muscle; v., vein.
Outcomes
Early clinical studies have shown good clinical outcomes when OLIF is used to treat patients with back pain and radiculopathy caused by spondylosis, and statistically significant decreases have been reported in VAS scores and Oswestry Disability Index scores (36,38-40). Ohtori et al. (38) evaluated 12 patients treated with OLIF to correct kyphoscoliosis and found reductions in back pain VAS scores 1 year after surgery (preoperative mean VAS 9.5±3.5, postoperative mean VAS 2.3±1.7, P=0.005). Similarly, in 35 patients with spinal stenosis and neurogenic claudication, Ohtori et al. (39) found significant 1-year postoperative reductions in leg pain scores (preoperative mean VAS 8.2±2.7, postoperative mean VAS 1.5±0.80, P=0.005). Fusion rates have been described in only a few reports but appear comparable to those for ALIF and LLIF. Kim et al. (41) reported a 12-month fusion rate of 92.9% in 29 OLIF patients with posterior pedicle screw fixation assessed with CT-imaging. Lin et al. (42) evaluated 52 patients undergoing stand-alone OLIF without posterior instrumentation and reported a fusion rate of 81.9% at more than 12 months after surgery assessed by CT. Hynes (43) reported a 96% 6-month fusion rate assessed by CT in 279 levels, but it is unknown how many had posterior fixation.
Complications
The most common complication associated with OLIF is transient thigh numbness and hip flexion weakness likely due to retraction of the psoas muscle and associated sensory nerves. In his 186-patient cohort, Hynes (43) encountered thigh numbness in 16% and weakness of the hips and quads in 6.5%, both resolving in 95% of patients by 3 months. Silvestre et al. (36) reported a 2.2% incidence of transient incisional pain and 1.7% incidence of lower-extremity sympathetic chain disruption symptoms after OLIF in 179 patients. In their 28-patient cohort, Fujibayashi et al. (4) reported that 7.1% of patients experienced transient leg weakness and 21.4% experienced transient numbness. DiGiorgio et al. (37) reported a 6.1% incidence of transient thigh numbness in their 49-patient cohort.
Another complication of OLIF is vascular injury, mainly involving venous structures anterior to the psoas at a rate similar to that for ALIF. Hynes (43) reported a vascular injury rate of 1.1% in 186 patients. Silvestre et al. (36) reported a venous injury rate of 1.7% in 176 patients. Ohtori et al. (38) reported a 2.8% incidence of segmental artery injury in their 35-patient cohort. Other infrequently reported complications include injury to the sympathetic trunk, resulting in temperature discrepancies to the lower extremities, and postoperative ileus due to greater anterior retraction on the peritoneum (44).
Summary of key points
ALIF, LLIF, and OLIF are powerful techniques to achieve spinal arthrodesis by delivering interbody grafts with larger footprints compared with those of traditional TLIF implants. These procedures can be performed in a minimally invasive fashion with little blood loss.
ALIF is safest at the L5–S1 interspace but the risk of vascular injury increases when targeting proximal lumbar segments because of the increased manipulation of the great vessels necessary to expose the anterior surface of the lumbar spine.
LLIF requires neuromonitoring to ensure safe traversal of the psoas muscle and positioning of the retractor with respect to the lumbar plexus.
OLIF does not require neuromonitoring because of its pre-psoas approach, but the technique appears to have a higher risk of vascular injury, and long-term fusion and subsidence rates have not been reported.
Conclusions
ALIF, LLIF, and OLIF have unique surgical applications, executions, and associated complications (Table 1). All 3 procedures have similarly high fusion rates. ALIF is generally limited to treating L4-S1, confers the greatest degree of indirect decompression, and has the lowest risk of subsidence, but risk factors include vascular injury and thus ALIF generally requires an approach surgeon. LLIF with long, wide interbody grafts can be used to treat most of the lumbar levels from L1 to L5 with a low risk of subsidence, but it requires directional neuromonitoring to minimize neurological complications. OLIF can be used to treat all lumbar levels from L1 to S1 without the need for neuromonitoring, but it has a higher risk of vascular injury than LLIF. Thus, OLIF requires concurrent posterior fixation because of the presumably higher risk of subsidence due to the smaller interbody grafts limited by the discectomy corridor. The optimal approach depends heavily on the individual structural constraints of the pathology, the anatomy of the individual patient, and the familiarity of the surgeon with available options.
Table 1. Summary of procedure characteristics.
| Procedure | Targets | Limitations | Size of interbody | Risk of subsidence | Logistical requirements | Risks |
|---|---|---|---|---|---|---|
| ALIF | L4–S1, can reach L2–3 with extensive vascular dissection | Previous abdominal surgery | Largest | Low; stand-alone |
Frequently requires an approach surgeon | 3% vascular injury; 2% retrograde ejaculation |
| LLIF | T12–L5 | Previous retroperitoneal surgery; transitional anatomy |
Large | Low; with wider stand-alone implants |
Neuromonitoring | 0.7–30% transient neurological symptoms |
| OLIF | L1–S1 | Grade 2 or higher spondylolisthesis; high-grade central canal stenosis |
Medium | High; without pedicle screws | L5–S1 may require additional training or an approach surgeon | 6.1–21.4% transient neurological symptoms; 1.6% vascular injury |
ALIF, anterior lumbar interbody fusion; LLIF, lateral lumbar interbody fusion; OLIF, oblique lumbar interbody fusion.
Acknowledgements
The authors thank the staff of Neuroscience Publications at Barrow Neurological Institute for assistance with manuscript preparation.
Footnotes
Conflicts of Interest: Dr. Uribe is a consultant for NuVasive, Inc. The other authors have no conflicts of interest to declare.
References
- 1.Briggs H, Milligan PR. Chip fusion of the low back following exploration of the spinal canal. JBJS 1944;26:125-30. [Google Scholar]
- 2.Rao PJ, Maharaj MM, Phan K, et al. Indirect foraminal decompression after anterior lumbar interbody fusion: a prospective radiographic study using a new pedicle-to-pedicle technique. Spine J 2015;15:817-24. 10.1016/j.spinee.2014.12.019 [DOI] [PubMed] [Google Scholar]
- 3.Oliveira L, Marchi L, Coutinho E, et al. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine (Phila Pa 1976) 2010;35:S331-7. 10.1097/BRS.0b013e3182022db0 [DOI] [PubMed] [Google Scholar]
- 4.Fujibayashi S, Hynes RA, Otsuki B, et al. Effect of indirect neural decompression through oblique lateral interbody fusion for degenerative lumbar disease. Spine 2015;40:E175-E82. 10.1097/BRS.0000000000000703 [DOI] [PubMed] [Google Scholar]
- 5.Sato J, Ohtori S, Orita S, et al. Radiographic evaluation of indirect decompression of mini-open anterior retroperitoneal lumbar interbody fusion: oblique lateral interbody fusion for degenerated lumbar spondylolisthesis. Eur Spine J 2017;26:671-8. 10.1007/s00586-015-4170-0 [DOI] [PubMed] [Google Scholar]
- 6.Rao PJ, Phan K, Giang G, et al. Subsidence following anterior lumbar interbody fusion (ALIF): a prospective study. J Spine Surg 2017;3:168-75. 10.21037/jss.2017.05.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchi L, Abdala N, Oliveira L, et al. Radiographic and clinical evaluation of cage subsidence after stand-alone lateral interbody fusion. J Neurosurg Spine 2013;19:110-8. 10.3171/2013.4.SPINE12319 [DOI] [PubMed] [Google Scholar]
- 8.Lang G, Navarro-Ramirez R, Gandevia L, et al. Elimination of subsidence with 26-mm-wide cages in extreme lateral interbody fusion. World Neurosurg 2017;104:644-52. 10.1016/j.wneu.2017.05.035 [DOI] [PubMed] [Google Scholar]
- 9.Spondylolisthesis Capener N. Br J Surg 1932;19:374-86. 10.1002/bjs.1800197505 [DOI] [Google Scholar]
- 10.Ito H, Tsuchiya J, Asami G. A new radical operation for Pott’s disease: report of ten cases. JBJS 1934;16:499-515. [Google Scholar]
- 11.Brau SA. Mini-open approach to the spine for anterior lumbar interbody fusion: description of the procedure, results and complications. Spine J 2002;2:216-23. 10.1016/S1529-9430(02)00184-5 [DOI] [PubMed] [Google Scholar]
- 12.Rao PJ, Loganathan A, Yeung V, et al. Outcomes of anterior lumbar interbody fusion surgery based on indication: a prospective study. Neurosurgery 2015;76:7-23; discussion 23-4. 10.1227/NEU.0000000000000561 [DOI] [PubMed] [Google Scholar]
- 13.Lee SH, Choi WG, Lim SR, et al. Minimally invasive anterior lumbar interbody fusion followed by percutaneous pedicle screw fixation for isthmic spondylolisthesis. Spine J 2004;4:644-9. 10.1016/j.spinee.2004.04.012 [DOI] [PubMed] [Google Scholar]
- 14.Rajaraman V, Vingan R, Roth P, et al. Visceral and vascular complications resulting from anterior lumbar interbody fusion. J Neurosurg 1999;91:60-4. [DOI] [PubMed] [Google Scholar]
- 15.Brau SA, Delamarter RB, Schiffman ML, et al. Vascular injury during anterior lumbar surgery. Spine J 2004;4:409-12. 10.1016/j.spinee.2003.12.003 [DOI] [PubMed] [Google Scholar]
- 16.Quraishi NA, Konig M, Booker SJ, et al. Access related complications in anterior lumbar surgery performed by spinal surgeons. Eur Spine J 2013;22 Suppl 1:S16-20. 10.1007/s00586-012-2616-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamdan AD, Malek JY, Schermerhorn ML, et al. Vascular injury during anterior exposure of the spine. J Vasc Surg 2008;48:650-4. 10.1016/j.jvs.2008.04.028 [DOI] [PubMed] [Google Scholar]
- 18.Sasso RC, Kenneth Burkus J, LeHuec JC. Retrograde ejaculation after anterior lumbar interbody fusion: transperitoneal versus retroperitoneal exposure. Spine (Phila Pa 1976) 2003;28:1023-6. 10.1097/01.BRS.0000062965.47779.EB [DOI] [PubMed] [Google Scholar]
- 19.Escobar E, Transfeldt E, Garvey T, et al. Video-assisted versus open anterior lumbar spine fusion surgery: a comparison of four techniques and complications in 135 patients. Spine (Phila Pa 1976) 2003;28:729-32. 10.1097/01.BRS.0000051912.04345.96 [DOI] [PubMed] [Google Scholar]
- 20.Tempel ZJ, McDowell MM, Panczykowski DM, et al. Graft subsidence as a predictor of revision surgery following stand-alone lateral lumbar interbody fusion. J Neurosurg Spine 2018;28:50-6. 10.3171/2017.5.SPINE16427 [DOI] [PubMed] [Google Scholar]
- 21.Le TV, Baaj AA, Dakwar E, et al. Subsidence of polyetheretherketone intervertebral cages in minimally invasive lateral retroperitoneal transpsoas lumbar interbody fusion. Spine 2012;37:1268-73. 10.1097/BRS.0b013e3182458b2f [DOI] [PubMed] [Google Scholar]
- 22.Pimenta L. Lateral endoscopic transpsoas retroperitoneal approach for lumbar spine surgery. VIII Brazilian Spine Society Meeting, Belo Horizonte, Minas Gerais, Brazil; May, 2001. [Google Scholar]
- 23.Berjano P, Langella F, Damilano M, et al. Fusion rate following extreme lateral lumbar interbody fusion. Eur Spine J 2015;24 Suppl 3:369-71. 10.1007/s00586-015-3929-7 [DOI] [PubMed] [Google Scholar]
- 24.Rodgers WB, Lehmen JA, Gerber EJ, et al. Grade 2 spondylolisthesis at L4-5 treated by XLIF: safety and midterm results in the “worst case scenario”. ScientificWorldJournal 2012;2012:356712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmadian A, Verma S, Mundis GM, Jr, et al. Minimally invasive lateral retroperitoneal transpsoas interbody fusion for L4-5 spondylolisthesis: clinical outcomes. J Neurosurg Spine 2013;19:314-20. 10.3171/2013.6.SPINE1340 [DOI] [PubMed] [Google Scholar]
- 26.Acosta FL, Liu J, Slimack N, et al. Changes in coronal and sagittal plane alignment following minimally invasive direct lateral interbody fusion for the treatment of degenerative lumbar disease in adults: a radiographic study. J Neurosurg Spine 2011;15:92-6. 10.3171/2011.3.SPINE10425 [DOI] [PubMed] [Google Scholar]
- 27.Sharma AK, Kepler CK, Girardi FP, et al. Lateral lumbar interbody fusion: clinical and radiographic outcomes at 1 year: a preliminary report. J Spinal Disord Tech 2011;24:242-50. [DOI] [PubMed] [Google Scholar]
- 28.Rodgers WB, Gerber EJ, Patterson J. Intraoperative and early postoperative complications in extreme lateral interbody fusion: an analysis of 600 cases. Spine (Phila Pa 1976) 2011;36:26-32. 10.1097/BRS.0b013e3181e1040a [DOI] [PubMed] [Google Scholar]
- 29.Cummock MD, Vanni S, Levi AD, et al. An analysis of postoperative thigh symptoms after minimally invasive transpsoas lumbar interbody fusion. J Neurosurg Spine 2011;15:11-8. 10.3171/2011.2.SPINE10374 [DOI] [PubMed] [Google Scholar]
- 30.Knight RQ, Schwaegler P, Hanscom D, et al. Direct lateral lumbar interbody fusion for degenerative conditions: early complication profile. J Spinal Disord Tech 2009;22:34-7. 10.1097/BSD.0b013e3181679b8a [DOI] [PubMed] [Google Scholar]
- 31.Dakwar E, Le TV, Baaj AA, et al. Abdominal wall paresis as a complication of minimally invasive lateral transpsoas interbody fusion. Neurosurg Focus 2011;31:E18. 10.3171/2011.7.FOCUS11164 [DOI] [PubMed] [Google Scholar]
- 32.Balsano M, Carlucci S, Ose M, et al. A case report of a rare complication of bowel perforation in extreme lateral interbody fusion. Eur Spine J 2015;24 Suppl 3:405-8. 10.1007/s00586-015-3881-6 [DOI] [PubMed] [Google Scholar]
- 33.Kueper J, Fantini GA, Walker BR, et al. Incidence of vascular complications during lateral lumbar interbody fusion: an examination of the mini-open access technique. Eur Spine J 2015;24:800-9. 10.1007/s00586-015-3796-2 [DOI] [PubMed] [Google Scholar]
- 34.Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 2015;1:2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mayer HM. A new microsurgical technique for minimally invasive anterior lumbar interbody fusion. Spine 1997;22:691-9. 10.1097/00007632-199703150-00023 [DOI] [PubMed] [Google Scholar]
- 36.Silvestre C, Mac-Thiong JM, Hilmi R, et al. Complications and morbidities of mini-open anterior retroperitoneal lumbar interbody fusion: oblique lumbar interbody fusion in 179 patients. Asian Spine J 2012;6:89-97. 10.4184/asj.2012.6.2.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DiGiorgio AM, Edwards CS, Virk MS, et al. Stereotactic navigation for the prepsoas oblique lateral lumbar interbody fusion: technical note and case series. Neurosurgical Focus 2017;43:E14. 10.3171/2017.5.FOCUS17168 [DOI] [PubMed] [Google Scholar]
- 38.Ohtori S, Mannoji C, Orita S, et al. Mini-open anterior retroperitoneal lumbar interbody fusion: oblique lateral interbody fusion for degenerated lumbar spinal kyphoscoliosis. Asian Spine J 2015;9:565-72. 10.4184/asj.2015.9.4.565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohtori S, Orita S, Yamauchi K, et al. Mini-open anterior retroperitoneal lumbar interbody fusion: oblique lateral interbody fusion for lumbar spinal degeneration disease. Yonsei Med J 2015;56:1051-9. 10.3349/ymj.2015.56.4.1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zairi F, Sunna TP, Westwick HJ, et al. Mini-open oblique lumbar interbody fusion (OLIF) approach for multi-level discectomy and fusion involving L5-S1: Preliminary experience. Orthop Traumatol Surg Res 2017;103:295-9. 10.1016/j.otsr.2016.11.016 [DOI] [PubMed] [Google Scholar]
- 41.Kim JS, Choi WS, Sung JH. 314 minimally invasive oblique lateral interbody fusion for L4-5: clinical outcomes and perioperative complications. Neurosurgery 2016;63 Suppl 1:190-1. 10.1227/01.neu.0000489803.65103.8427399512 [DOI] [Google Scholar]
- 42.Lin JF, Iundusi R, Tarantino U, et al. Intravertebral plate and cage system via lateral trajectory for lumbar interbody fusion—a novel fixation device. Spine J 2010;10:S86 10.1016/j.spinee.2010.07.231 [DOI] [Google Scholar]
- 43.Hynes RA. Oblique lateral interbody fusion (OLIF) technique and complications in 457 levels L1 to S1. International Society for the Advancement of Spine Surgery Conference, Miami Beach, Florida; April 30-May 2, 2014. [Google Scholar]
- 44.Li JX, Phan K, Mobbs R. Oblique lumbar interbody fusion: technical aspects, operative outcomes, and complications. World Neurosurg 2017;98:113-23. 10.1016/j.wneu.2016.10.074 [DOI] [PubMed] [Google Scholar]