Abstract
A novel series of diarylpyrimidine derivatives, which could simultaneously occupy the classical NNRTIs binding pocket (NNIBP) and the newly reported “NNRTI Adjacent” binding site, were designed, synthesized, and evaluated for their antiviral activities in MT-4 cell cultures. The results demonstrated that six compounds (20, 27 and 31–34) showed excellent activities against wild-type (WT) HIV-1 strain (EC50 = 2.4–3.8 nM), which were more potent than that of ETV (EC50 = 4.0 nM). Furthermore, 20, 27, 33, and 34 showed more potent or equipotent activity against single mutant HIV-1 strains compared to that of ETV. Especially, 20 showed marked antiviral activity, which was 1.5-fold greater against WT and 1.5- to 3-fold greater against L100I, K103N, Y181C, Y188L, and E138K when compared with ETV. In addition, all compounds showed lower toxicity (CC50 = 5.1–149.2 μM) than ETV (CC50 = 2.2 μM). The HIV-1 RT inhibitory assay was further conducted to confirm their binding target. Preliminary structure–activity relationships (SARs), molecular modeling, and calculated physicochemical properties of selected compounds were also discussed comprehensively.
Keywords: HIV-1, AIDS, NNRTIs, drug design, “NNRTI Adjacent” binding site
Attributing to their potent activity, modest toxicity, high specificity, and favorable pharmacokinetic properties, HIV-1 non-nucleoside reverse transcriptase inhibitors (NNRTIs) function as an essential component of highly active antiretroviral therapy (HAART).1,2 Nevirapine (1, NVP), delavirdine (2, DLV), and efavirenz (3, EFV) are first generation NNRTIs approved by U.S. FDA (Figure S1 (i.e., Figure 1 in Supporting Information), but the rapid emergence of drug-resistant compromised their clinical application, among which K103N and Y181C are the two most prevalent NNRTI resistance-associated mutations selected by NVP and EFV.3 Although second generation NNRTIs, etravirine (4, ETV) and rilpivirine (5, RPV), which belong to the diarylpyrimidine (DAPY) family, showed better antiresistance profiles than the first generation NNRTIs, they still suffer from bad pharmacokinetics and cross-drug resistance with long-term clinical therapy.3 Among them, E138K was the most commonly observed resistance mutation in the treatment-emergent of second generation NNRTIs. Therefore, there is an urgent need to exploit novel NNRTI drugs with improved potency against resistance-associated variants.4
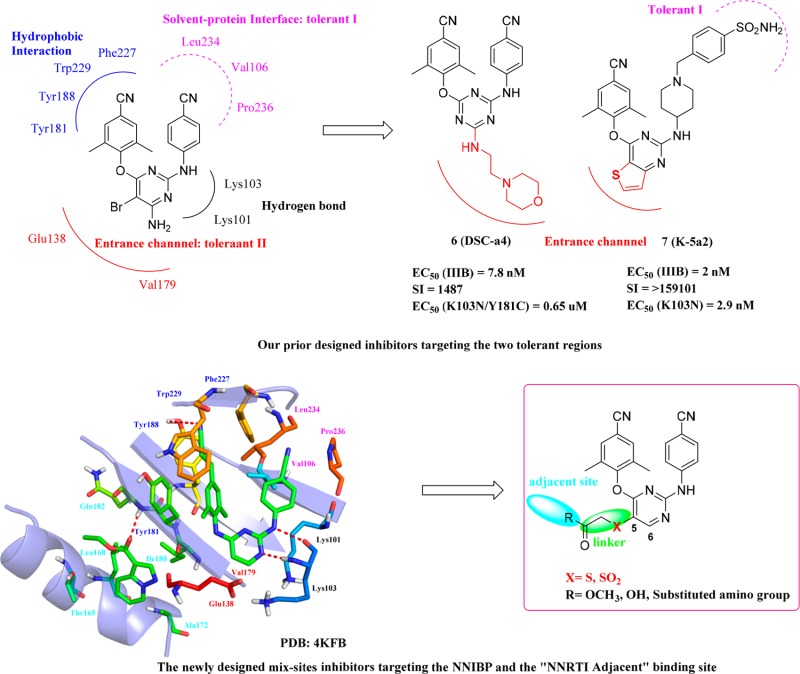
On the basis of the recent X-ray crystallographic studies, solvent-accessible regions such as tolerant region I (the Pro236 hairpin loop) and tolerant region II (the entrance channel, namely, the largely open region in front of Leu100, Lys101, Glu138, and Val179) in the NNIBP are revealed as the two broad regions that could accommodate the newly designed NNRTIs.5,6 Modifications of the DAPY scaffold such as fused heterocycles and/or introducing other groups like piperidine-substituted groups targeting these two tolerant regions have made breakthrough in our lab in recent years (Figure 1).7−10 Notably, series of triazine derivatives and piperidine-substituted thiophene[3,2-d]pyrimidine derivatives were the most remarkable, with a prominent antiviral activity against the clinical prevalent mutations. The most potent compounds DCS-a4 (6) and K-5a2 (7) exhibited EC50 of 7.8 nM and 2 nM against WT HIV-1, respectively.8,11 Encouragingly, 7 showed improved potency against resistance-associated variants.
Figure 1.
Our prior work and the newly designed compounds targeting the NNIBP and the “NNRTI Adjacent” binding site.
Recently, an underexploited site named as the “NNRTI Adjacent” binding site (PDB code 4KFB), which is formed by Thr139 (p51), Pro140 (p51), Thr165, Leu168, Lys172, and Ile180, was found as an additional opening tunnel-like pocket. It is located at the p66/p51 interface in the palm subdomain adjacent to Glu138 of p51, separated from the NNIBP by β9 strand. Gratifyingly, the highly conserve residues Pro140, Ile180, and Gln182 were related in pivotal interactions, indicating that it is a promising site for the design of novel DAPY derivatives.12 In this paper, we first attempt to design novel diarylpyrimidine derivatives targeting NNIBP and the “NNRTI Adjacent” binding site simultaneously. The novel target compounds incorporated the privilege skeleton of ETV and the structure characteristics of K-5a2 (Figure 1). The linkers with different length and substituents varying in size and electronic feature were primarily designed based on the chemistry space around the original fragment at NNRTI adjacent site. We also proposed that the privileged aromatic/heterocyclic substituents would improve physicochemical properties.13 Herein, we report the synthesis, biological evaluation, and RT inhibition assay of novel diarylpyrimidine derivatives. Furthermore, preliminary SARs, molecular modeling, and physicochemical properties are also discussed.
The synthetic protocol for 5-substituted diarylpyrimidine derivatives is depicted in Scheme S1.14−17 First, treatment of commercially available 2,4-dichloropyrimidine (8) with 4-hydroxy-3,5-dimethylbenzonitrile (9) afforded intermediate 10. Then 12 was obtained by Buchwald–Hartwig reaction of 10 and 4-aminobenzonitrile (11). With the presence of N-iodosuccinimide (NIS) and CF3COOH, key intermediate 13 was obtained through electrophilic reaction. Subsequently, 13 reacted with methyl thioglycolate or methyl mercaptopropionate to provide important intermediate 14 or 15 via cross-coupling reaction. The oxidative products 16 and 17 were prepared by treating 14 and 15 with m-CPBA. Finally, 14 was hydrolyzed with lithium hydroxide to afford 18 and then yield the target compounds 19–34 by amide condensation reaction. Both analytical and spectral data of the newly synthesized compounds were found in full agreement with the proposed structures.
Antiviral activity was evaluated in MT-4 cell cultures infected with WT HIV-1 strain (IIIB), single mutant strains L100I, K103N, Y181C, Y188L, E138K, and double mutant strains F227L+V106A and K103N+Y181C (RES056). NVP, EFV, ETV, and azidothymidine (AZT) were selected as reference drugs. The values of EC50 (anti-HIV potency), CC50 (cytotoxicity), and SI (selectivity index, CC50/EC50 ratio) are summarized in Tables S1 and S2.
All the synthesized compounds were found to be active against WT HIV-1 with an EC50 of 2.4–216.5 nM and SI between 152 and 15413, with the exception of 17 (EC50 = 1563.9 nM, SI = 9). Notably, 11 compounds (19, 20, 25–27, 29–34) displayed superior activity (EC50 < 10 nM), among which six compounds (20, 27, 31–34) exhibited more potent or equipotent antiviral activity (EC50 = 2.4–3.8 nM) compared to ETV (EC50 = 4.0 nM). The most potent compounds 27 (EC50 = 2.4 nM) and 33 (EC50 = 2.4 nM) exhibited a 1.6-fold greater potency than ETV. Meanwhile, all the compounds showed much lower cytotoxicity (CC50 = 5.1–149.2 μM) than ETV (CC50 = 2.2 μM).
Table S2 showed that compounds with potent activity against WT HIV-1 strain also exhibited potent activity against mutant HIV-1 strains. Among all the target compounds, 20, 27, 33, and 34 demonstrated excellent activity against HIV-1 single mutations L100I, K103N, Y181C, Y188L, and E138K, being more potent or equipotent compared to ETV. Particularly, 20 was the most potent inhibitor and its antiviral efficacy was 1.5- to 3-fold greater against these single mutations than ETV. But for the double mutant strains F227L+V106A and K103N+Y181C, 20 showed less potent activity. It is noteworthy that our newly designed compounds showed an overall and excellent activity for a panel of single mutants and the results are summarized as follows:
(1) In case of the K103N mutant strain, all 13 tested compounds showed lower EC50 (1.4–44.9 nM) and FR (0.4–1.9), being more potent than EFV (EC50 = 81.0 nM, FR = 16.3). Nine compounds (19, 20, 25, 27, 29, 30, and 32–34) provided a single-digit nanomolar activity (EC50 = 1.4–8.3 nM). In particular, 19, 20, 27, and 32–34 displayed prominent inhibitory activity, being 1.3- to 2.3-fold superior to ETV (EC50 = 3.3 nM).
(2) For E138K, all the tested compounds exhibited more potent activity (EC50 = 6.0–157 nM) than NVP. The EC50 values of 10 compounds 19, 20, 25, 27–30, and 32–34 (EC50 = 6.0–34.7 nM) were less than 35 nM, showing more potency than AZT (EC50 = 38.6 nM). Besides, six compounds (20, 27, 30, 32–34) proved to inhibit E138K mutant HIV-1 strain in lower or equal concentration than ETV (EC50 = 16.9 nM).
(3) As for single mutant strains L100I, Y181C, and Y188L, it was noted that compounds 20 and 33 had single-digit nanomolar activity (EC50 = 6.5 and 7.7 nM, respectively) against L100I being slightly more potent compared to AZT (EC50 = 8.3 nM), and compounds 20, 27, and 33 exhibited an EC50 values ranging from 11.1 to 13.8 nM against Y181C, which were somewhat greater than AZT (EC50 = 14.1 nM).
(4) Against the double mutant strains F227L/V106A and Y181C/K103N, the most potent compounds 20, 27, 33, and 34 exhibited a moderate activity (EC50 > 100 nM). It was concluded that the inflexible conformation of compounds caused by bulky groups may account for their moderate activity.
On the basis of the above results, the preliminary SARs could be concluded in terms of the length of the chain and different terminal substitution group. For the former, detailed comparison of 14 (EC50 = 65.9 nM) with 15 (EC50 = 60.2 nM), 16 (EC50 = 68.8 nM) with 17 (EC50 = 1563.9 nM), 19 (EC50 = 6.2 nM) with 20 (EC50 = 2.6 nM), and 27 (EC50 = 2.4 nM) with 31 (EC50 = 3.8 nM) for their activity against WT HIV-1 strain suggested that the length of carbon chain could not govern the antiviral activity proportionally or inversely. It was anticipated that compounds bearing different lengths of carbon chain would present distinct conformations, and so that had distinguished antiviral potency.
For the latter, when we focused on 14 (EC50 = 65.9 nM) and 15 (EC50 = 60.2 nM) compared with their sulfur oxidation products 16 (EC50 = 68.8 nM) and 17 (EC50 = 1563.9 nM), the result suggested that sulfone group has a negative impact on the antiviral activity. Next, comparison of activity of 34 (methoxy group, 3.8 nM), 19 (morpholinyl, 2.6 nM), and 21 (Boc-protected pyrazinyl, 32.6 nM) with that of 22 (Boc-protected pyrazinyl, 88.5 nM), 23 (Boc-protected 3-aminopyrrolyl, 33.9 nM), and 24 (thiomorpholinyl, 38.3 nM) indicated that polar groups are much preferred than groups with lower polarity distinctly. By comparing the activity between 31–33 and 29, 30, it was suggested that compounds with groups in similar size with short linker had similar activity, and small group with short linker (31–33) displayed better activity than those of bulky groups, such as phenol (29) and tetrahydrofuran (30). It was also found that the terminal tert-butyloxycarbonyl group was an inferior group that impeded antiviral potency when comparing 21, 22, and 23 to 28.
In general, these data indicated that modifications at 5-position of pyrimidine ring contribute to improve antiviral activity against WT and a panel of single mutant strains, especially K103N and E138K. The preliminary SARs will support information for future structure modification. In addition, the compounds did not inhibit HIV-2.
To further validate the binding target, all the newly designed compounds were tested for their ability to inhibit recombinant WT HIV-1 RT enzyme. As shown in Table S3, all the tested compounds showed excellent inhibitory activities toward RT with IC50 values ranging from 0.013 to 0.863 μM, being superior to that of NVP (IC50 = 2.32 μM). Meanwhile, eight compounds (14, 27–29, and 31–34) had a better inhibitory activity than EFV (IC50 = 0.03 μM). The IC50 values of the most potent compounds 20, 27, 33, and 34 (IC50 = 0.084, 0.021, 0.023, and 0.026 μM, respectively) were comparable to that of ETV (IC50 = 0.011 μM). It was identified that the results of preliminary SARs for IC50 were consistent with SARs for EC50 discussed above. Furthermore, the line chart was used to observe the consistency between the pEC50 (blue line) and pIC50 (red line) of target compounds in which the compound is sorted according to their pEC50 values in a decreasing order as shown in Figure S3. The value of pIC50 showed a declining trend (purple line) compared with their pEC50, and this indicated the relative coherence between inhibitory against WT HIV-1 strain and HIV-1 RT enzyme. However, the EC50 value of some derivatives such as 14, 15, and 18 were 1.5- to 5-fold lower than their values of IC50, presenting an abnormal deviation, potentially owing to the cellular retention for poor membrane permeability or much higher affinity for the cellular full length enzyme than that of recombinant RT enzyme or metabolism of molecules. Overall these results illustrated that these new compounds displayed high affinity for RT and could specifically target HIV-1 RT and be regarded as typical HIV-1 NNRTIs.
To obtain further insight into the dual-site binding of the newly synthesized compounds to the NNIBP and “NNRTI Adjacent” binding site and rationalize the results of SAR studies, docking experiments were carried out using representative compounds 19, 20, and 27 into WT HIV-1 RT with bound fragment at NNRTI adjacent site (PDB code 4KFB) by using the software SurflexeDock SYBYL-X 2.0. Docking results were visualized with PyMOL (Figure S4).
The binding mode of compound 20 resembled RPV as horseshoe conformation in parent scaffold and the morpholine ring stretched into the NNRTI adjacent site as the fragment located (Figure S4a). Compound 20 maintained the typical double hydrogen bonds with the backbone of Lys101 as described for many other NNRTIs. Besides, the amide group of the linker formed additional double hydrogen bonds with Ile180 and Glu138. Compared with 20, the disappearance of hydrogen bond between 19 and Glu138 may account for its reduced activity compared to 20 (Figure S4b). A close-up view inward the “NNRTI Adjacent” binding site described the morpholine ring stretched into the pocket and occupied the adjacent site through the linker (Figure S4c). As shown in Figure S4d, the detailed docking feature of 27 showed additional hydrogen bonds formed by the NH and C=O of amide with the backbone C=O of Glu138 and NH of Ile180, respectively. Noteworthy, although 20 and 27 had different linker, they both form hydrogen bond with Ile180 and Glu138. It was predicted that different linkers and various terminal groups determine the conformation and then influence the activity. It was also guessed that various conformations and additional binding force played an important role in their excellent activity against HIV-1 mutant strains. To sum up, the molecular modeling analysis explained the theoretical binding mode and potent activity of the designed compounds partially, which was consistent with our original design intention and would assist further structural optimization.
Furthermore, the preliminary physicochemical properties of representative compounds 20, 27, 33, and 34 were examined to evaluate their druglikeness features by utilizing free online molinspiration software (http://www.molinspiration.com/). Lipophilic parameter ligand efficiency (LE) was also calculated.18,19 The results (Table S4) suggested that parameters like molecular weight (MW), hydrogen bond acceptors (nON), hydrogen bond donors (nOHNH), rotatable bonds (nrotb), miLogp, and ligand efficiency (LE) for all tested compounds were consistent with the Lipinski’s “rule of five” and in an acceptable level except for the slight deviation for molecular weight and ligand efficiency of 20. Therefore, it is supposed that these four compounds have the desired physicochemical properties. Besides, the topological polar surface area (tPSA), which is characterized by the absorption and membrane permeability of molecules, showed the four compounds had a value ranging from 123.73 to 136.30 Å2, confirming their advantage for intestinal absorption (<140 Å2) and inability to penetrate the blood–brain barrier, averting the central nervous system toxicity (>60 Å2).20
To the best of our knowledge, we first discovered a new series of dual binding site NNRTIs targeting the NNIBP and the “NNRTI Adjacent” binding site simultaneously. Encouragingly, most of the novel derivatives exhibited significant inhibitory activity toward WT (EC50 < 10 nM) and a panel of single mutant HIV-1 strains in MT-4 cells. Compound 20 proved to be the most potent inhibitor with EC50 of 2.6 nM (WT), 6.5 nM (L100I), 1.4 nM (K103N), 11.6 nM (Y181C), 16.2 nM (Y188L), and 6.0 nM (E138K); it was more potent than ETV against all single mutant strains, though somewhat weaker against double mutant strains F227L+V106A and RES056. In addition, 20 has much lower cytotoxicity (CC50 = 27.2 μM) and higher SI of 10045. In RT inhibition assay, 20 displayed an IC50 of 0.086 μM, which was of the same magnitude as that for ETV. The current results hold great promise for the identification of potential new drug candidates with high potency and desirable druglike properties. Further optimizations focusing on improving the HIV-1 mutant strains inhibitory activity, especially for double mutant strains, are currently in progress and will be reported in due course.
Acknowledgments
We thank K. Erven, K. Uyttersprot, and C. Heens for technical assistance with the HIV assays.
Glossary
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- CC50
50% cytotoxicity concentration
- DAPY
diarylpyrimidine
- NNRTI
non-nucleoside reverse transcriptase inhibitor
- NNIBP
NNRTI binding pocket
- HAART
highly active antiretroviral therapy
- EC50
concentration causing 50% inhibition of antiviral activity
- HIV
human immunodeficiency virus
- RT
reverse transcriptase
- SAR
structure–activity relationship
- SI
selection index
- FR
fold resistance
- WT
wild-type
- NVP
nevirapine
- DLV
delavirdine
- EFV
efavirenz
- ETV
etravirine
- RPV
rilpivirine
- AZT
azidothymidine
- LE
ligand efficiency
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.7b00524.
Financial support from the National Natural Science Foundation of China (NSFC Grants 81273354, 81573347), Key Project of NSFC for International Cooperation (Grant 81420108027), Young Scholars Program of Shandong University (YSPSDU Grant 2016WLJH32), the Fundamental Research Funds of Shandong University (Grant 2017JC006), Key Research and Development Project of Shandong Province (Grant 2017CXGC1401), and KU Leuven (Grant GOA 10/014) is gratefully acknowledged.
The authors declare no competing financial interest.
Supplementary Material
References
- Zhan P.; Pannecouque C.; De Clercq E.; Liu X. Anti-HIV drug discovery and development: current innovations and future trends. J. Med. Chem. 2016, 59, 2849–2878. 10.1021/acs.jmedchem.5b00497. [DOI] [PubMed] [Google Scholar]
- Zhan P.; Chen X.; Li D.; Fang Z.; De Clercq E.; Liu X. HIV-1 NNRTIs: structural diversity, pharmacophore similarity, and implications for drug design. Med. Res. Rev. 2013, 33, E1–E72. 10.1002/med.20241. [DOI] [PubMed] [Google Scholar]
- Kinch M. S.; Patridge E. An analysis of FDA-approved drugs for infectious disease: HIV/AIDS drugs. Drug Discovery Today 2014, 19, 1510–1513. 10.1016/j.drudis.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Li D.; Zhan P.; De Clercq E.; Liu X. Strategies for the design of HIV-1 Non-Nucleoside Reverse Transcriptase Inhibitors: lessons from the development of seven representative paradigms. J. Med. Chem. 2012, 55, 3595–3613. 10.1021/jm200990c. [DOI] [PubMed] [Google Scholar]
- Zhan P.; Liu X.; Li Z.; Pannecouque C.; De Clercq E. Design strategies of novel NNRTIs to overcome drug resistance. Curr. Med. Chem. 2009, 16, 3903–3917. 10.2174/092986709789178019. [DOI] [PubMed] [Google Scholar]
- Ekkati A. R.; Bollini M.; Domaoal R. A.; Spasov K. A.; Anderson K. S.; Jorgensen W. L. Discovery of dimeric inhibitors by extension into the entrance channel of HIV-1 reverse transcriptase. Bioorg. Med. Chem. Lett. 2012, 22, 1565–1568. 10.1016/j.bmcl.2011.12.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.; Chen W.; Kang D.; Lu X.; Li X.; Liu Z.; Huang B.; Daelemans D.; Pannecouque C.; De Clercq E.; Zhan P.; Liu X. Design, synthesis and anti-HIV evaluation of novel diarylpyridine derivatives targeting the entrance channel of NNRTI binding pocket. Eur. J. Med. Chem. 2016, 109, 294–304. 10.1016/j.ejmech.2015.11.039. [DOI] [PubMed] [Google Scholar]
- Chen X.; Meng Q.; Qiu L.; Zhan P.; Liu H.; De Clercq E.; Pannecouque C.; Liu X. Design, synthesis and anti-HIV evaluation of novel triazine derivatives targeting the entrance channel of the NNRTI binding pocket. Chem. Biol. Drug Des. 2015, 86, 122–128. 10.1111/cbdd.12471. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Chen W.; Zhan P.; De Clercq E.; Pannecouque C.; Liu X. Design, synthesis and anti-HIV evaluation of novel diarylnicotinamide derivatives (DANAs) targeting the entrance channel of the NNRTI binding pocket through structure-guided molecular hybridization. Eur. J. Med. Chem. 2014, 87, 52–62. 10.1016/j.ejmech.2014.09.054. [DOI] [PubMed] [Google Scholar]
- Huang B.; Liang X.; Li C.; Chen W.; Liu T.; Li X.; Sun Y.; Fu L.; Liu H.; De Clercq E.; Pannecouque C.; Zhan P.; Liu X. Fused heterocycles bearing bridgehead nitrogen as potent HIV-1 NNRTIs. Part 4: design, synthesis and biological evaluation of novel imidazo[1,2-a]pyrazines. Eur. J. Med. Chem. 2015, 93, 330–337. 10.1016/j.ejmech.2015.02.022. [DOI] [PubMed] [Google Scholar]
- Kang D.; Fang Z.; Li Z.; Huang B.; Zhang H.; Lu X.; Xu H.; Zhou Z.; Ding X.; Daelemans D.; De Clercq E.; Pannecouque C.; Zhan P.; Liu X. Design, synthesis, and evaluation of thiophene[3,2-d]pyrimidine derivatives as HIV-1 Non-nucleoside reverse transcriptase inhibitors with significantly improved drug resistance profiles. J. Med. Chem. 2016, 59, 7991–8007. 10.1021/acs.jmedchem.6b00738. [DOI] [PubMed] [Google Scholar]
- Bauman J. D.; Patel D.; Dharia C.; Fromer M. W.; Ahmed S.; Frenkel Y.; Vijayan R. S.; Eck J. T.; Ho W. C.; Das K.; Shatkin A. J.; Arnold E. Detecting allosteric sites of HIV-1 reverse transcriptase by X-ray crystallographic fragment screening. J. Med. Chem. 2013, 56, 2738–2746. 10.1021/jm301271j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSimone R. W.; Currie K. S.; Mitchell S. A.; Darrow J. W.; Pippin D. A. Privileged structures: applications in drug discovery. Comb. Chem. High Throughput Screening 2004, 7, 473–493. 10.2174/1386207043328544. [DOI] [PubMed] [Google Scholar]
- Janssen P. A.; Lewi P. J.; Arnold E.; Daeyaert F.; de Jonge M.; Heeres J.; Koymans L.; Vinkers M.; Guillemont J.; Pasquier E.; Kukla M.; Ludovici D.; Andries K.; de Béthune M. P.; Pauwels R.; Das K.; Clark A. D. Jr.; Frenkel Y. V.; Hughes S. H.; Medaer B.; De Knaep F.; Bohets H.; De Clerck F.; Lampo A.; Williams P.; Stoffels P. In search of a novel anti-HIV drug: multidisciplinary coordination in the discovery of 4-[[4-[[4-[(1E)-2-cyanoethenyl]-2,6-dimethylphenyl]amino]-2-pyrimidinyl]amino]benzonitrile (R278474, rilpivirine). J. Med. Chem. 2005, 48, 1901–1909. 10.1021/jm040840e. [DOI] [PubMed] [Google Scholar]
- Taldone T.; Kang Y.; Patel H. J.; Patel M. R.; Patel P. D.; Rodina A.; Patel Y.; Gozman A.; Maharaj R.; Clement C. C.; et al. Heat shock protein 70 inhibitors. 2. 2,5′-Thiodipyrimidines, 5-(phenylthio)pyrimidines, 2-(pyridin-3-ylthio)pyrimidines, and 3-(phenylthio)pyridines as reversible binders to an allosteric site on heat shock protein 70. J. Med. Chem. 2014, 57, 1208–1224. 10.1021/jm401552y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D.; Fang Z.; Huang B.; Lu X.; Zhang H.; Xu H.; Huo Z.; Zhou Z.; Yu Z.; Meng Q.; et al. Structure-based optimization of thiophene[3,2-d]pyrimidine derivatives as potent HIV-1 non-nucleoside reverse transcriptase inhibitors with improved potency against resistance-associated variants. J. Med. Chem. 2017, 60, 4424–4443. 10.1021/acs.jmedchem.7b00332. [DOI] [PubMed] [Google Scholar]
- Sugawara K.; Kawabata N.. Preparation of oxime compounds as glucokinase activators. Patent Appl. JP2010018526, filed Jan 28, 2010, pending.
- Hopkins A. L.; Keserü G. M.; Leeson P. D.; Rees D. C.; Reynolds C. H. The role of ligand efficiency metrics in drug discovery. Nat. Rev. Drug Discovery 2014, 13, 105–121. 10.1038/nrd4163. [DOI] [PubMed] [Google Scholar]
- Huang B.; Li C.; Chen W.; Liu T.; Yu M.; Fu L.; Sun Y.; Liu H.; De Clercq E.; Pannecouque C.; Balzarini J.; Zhan P.; Liu X. Fused heterocycles bearing bridgehead nitrogen as potent HIV-1 NNRTIs. Part 3: optimization of [1,2,4]triazolo[1,5-a]pyrimidine core via structure-based and physicochemical property-driven approaches. Eur. J. Med. Chem. 2015, 92, 754–765. 10.1016/j.ejmech.2015.01.042. [DOI] [PubMed] [Google Scholar]
- Clark D. E.; Pickett S. D. Computational methods for the prediction of ‘drug-likeness’. Drug Discovery Today 2000, 5, 49–58. 10.1016/S1359-6446(99)01451-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.