Abstract
Sphingosine kinase 1 (SphK1) upregulation is associated with pathologic pulmonary vascular remodeling in pulmonary arterial hypertension (PAH), but the mechanisms controlling its expression are undefined. In this study, we sought to characterize the regulation of SphK1 expression by micro-RNAs (miRs). In silico analysis of the SphK1 3′-untranslated region identified several putative miR binding sites, with miR-1-3p (miR-1) being the most highly predicted target. Therefore we further investigated the role of miR-1 in modulating SphK1 expression and characterized its effects on the phenotype of pulmonary artery smooth muscle cells (PASMCs) and the development of experimental pulmonary hypertension in vivo. Our results demonstrate that miR-1 is downregulated by hypoxia in PASMCs and can directly inhibit SphK1 expression. Overexpression of miR-1 in human PASMCs inhibits basal and hypoxia-induced proliferation and migration. Human PASMCs isolated from PAH patients exhibit reduced miR-1 expression. We also demonstrate that miR-1 is downregulated in mouse lung tissues during experimental hypoxia-mediated pulmonary hypertension (HPH), consistent with upregulation of SphK1. Furthermore, administration of miR-1 mimics in vivo prevented the development of HPH in mice and attenuated induction of SphK1 in PASMCs. These data reveal the importance of miR-1 in regulating SphK1 expression during hypoxia in PASMCs. A pivotal role is played by miR-1 in pulmonary vascular remodeling, including PASMC proliferation and migration, and its overexpression protects from the development of HPH in vivo. These studies improve our understanding of the molecular mechanisms underlying the pathogenesis of pulmonary hypertension.
Keywords: hypoxia, micro-RNA-1, pulmonary hypertension, sphingosine kinase 1
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a progressive and fatal disease for which the pathogenic mechanisms are poorly understood and no curative treatments are available (40). PAH is characterized by increases in pulmonary vascular resistance primarily due to uncontrolled pulmonary vascular remodeling, sustained vasoconstriction, or thrombosis in situ (15, 27). Rampant pulmonary artery smooth muscle cell (PASMC) proliferation and apoptosis resistance contribute to increases in pulmonary vascular resistance and pulmonary artery pressure (58), resulting in eventual right ventricular hypertrophy and heart failure, the major cause of death in PAH. Most current PAH therapies are limited to targeting pulmonary vasoconstriction, and patients continue to have a poor long-term prognosis (26). Thus there is an imperative need to understand the mechanisms contributing to pulmonary vascular remodeling to develop new therapies.
The significance of sphingolipid signaling in pulmonary disease has recently emerged. Our group and others have demonstrated that the bioactive lipid sphingosine-1-phosphate (S1P) is involved in the pathogenesis of pulmonary fibrosis and bronchopulmonary dysplasia and in regulation of the vascular endothelial barrier during acute lung injury (14, 18, 22, 41, 43, 48, 50). S1P mediates many important biological functions including cell proliferation, differentiation, motility, and resistance to apoptosis (12), and its synthesis in the lungs and blood is predominantly regulated by sphingosine kinase 1 (SphK1; 17). SphK1, a highly conserved, oncogenic enzyme, has itself been implicated in the promotion of cell proliferation, apoptosis resistance, and angiogenesis (36, 51), and preclinical studies have shown the efficacy of SphK1 inhibition in decreasing tumor size (16, 31). We have recently demonstrated that SphK1 and S1P are elevated in PAH and promote human PASMC (hPASMC) proliferation (7, 52). Additionally, we found that genetic deficiency or pharmacologic inhibition of SphK1 in vivo protects against the development of hypoxia-induced experimental pulmonary hypertension in mice (7). However, the mechanisms involved in the hypoxia-mediated upregulation of SPHK1 expression in PAH and its contribution to hPASMC proliferation are presently not well understood. In this study, we aimed to identify novel molecular mechanisms of hypoxia-induced hPASMC proliferation, focusing on the role of SphK1 expression and its regulation by micro-RNAs (miRs).
As small, noncoding RNAs, miRs play important regulatory roles in animals and plants by base pairing with complementary sequences within mRNA molecules, resulting in cleavage, translational repression, or destabilization (1, 3, 42). The importance of miRs in modulating complex gene expression networks (32) and their contributions to human disease pathogenesis have emerged, including in the field of cardiovascular biology (49). Recent studies have identified several miRs associated with cell-specific phenotypes in PAH and experimental models of pulmonary hypertension (4, 63). A systems-level regulation of micro-RNA networks by miR-130/301 and their targeting of peroxisome proliferator-activated receptor-γ (PPARγ) has been shown to promote pulmonary hypertension by distinct modulation of both PASMCs and pulmonary artery endothelial cells (PAECs; 4). Another recent study demonstrated that both miR-138 and miR-25 downregulate the mitochondrial calcium uniporter complex (MCUC), causing a cancerlike proliferative phenotype in PASMCs in PAH (25). In addition, the bone morphogenetic protein (BMP) signaling pathway, which has been widely shown to modulate vascular remodeling in PAH, was recently found to be controlled by miR-140-5p (46). The regulation of miR expression by hypoxia and their involvement in modulating the phenotype of smooth muscle cells (SMCs) have also been described (6, 8).
Arterial SMCs, unlike cardiac and skeletal myocytes, can switch to a highly proliferative and migratory state under various stimuli, including hypoxia and vascular injury (21). We hypothesized that hypoxia induces downregulation of micro-RNAs targeting SphK1, contributing to enhanced hPASMC proliferation and migration. In this study, we demonstrate that miR-1 is decreased in PASMCs of PAH patients and is downregulated by hypoxia, a major contributor to PAH development, in both cultured PASMCs and in lung tissues from experimental models of PH. In addition, overexpression of miR-1 protected mice from hypoxia-induced pulmonary hypertension (PH) and suppressed the expression of SphK1, a conserved lipid kinase that catalyzes formation of the proproliferative and promigratory lipid S1P.
MATERIALS AND METHODS
Human pulmonary artery smooth muscle cells.
Human pulmonary artery smooth muscle cells (hPASMCs) were isolated from six donors not suitable for lung transplantation and four patients with idiopathic PAH as previously described (2). The smooth muscle phenotype of cultured cells has been authenticated by immunohistochemistry and flow cytometric analysis with antibodies against smooth muscle α-actin and calponin (2). In addition to these cell lines, a male primary hPASMC cell line from Lonza (CC-2581) was used for cell transfection, migration, and proliferation assays at passages 4–8. The miR-1 mimics, inhibitors (antagomirs), or controls (50–100 nmol/l; Qiagen) were transfected using Lipofectamine (Thermo Fisher Scientific) per the manufacturer’s instructions.
Cell proliferation assays.
Cell proliferation was determined using a 5-bromo-2′-deoxyuridine (BrdU) incorporation assay (QIA58; Calbiochem) per the manufacturer’s instructions in a 96-well format. Starting cell densities of 4,000 cells per well were used.
Cell migration assays.
Cell migration was determined using a quantitative Transwell assay as previously described (35). In brief, cell migration across a Transwell membrane with 8-μm pores was assessed following incubation in normoxic or hypoxic (3% O2) conditions. Cells were fixed and stained using Diff-Quik, and the ratio of migrated to total cells was assessed via five random fields per sample. For miR transfection studies, cells were transfected 24 h before transferring to the Transwell insert.
Bioinformatics analysis and miRNA prediction.
In silico analysis for miR target prediction was completed using the microRNA.org target prediction resource (http://34.236.212.39/microrna/home.do), utilizing miRanda sites and miR support vector regression (mirSVR) scoring (5). The target sites predicted using miRanda are scored for likelihood of mRNA downregulation using a regression model trained on sequence and contextual features of the predicted miRNA:mRNA duplex. Using these tools, hsa-miR-1-3p was selected as a miR candidate targeting SPHK1.
Transient transfections and reporter assays.
For all miR transfection studies, miScript miRNA-1 mimics or antagomirs were used (Qiagen) per the manufacturer’s guidelines, with AllStars negative control small interfering RNA (siRNA) used to account for nonsequence effects. The reporter construct containing the firefly luciferase gene fused to the the full-length human SPHK1 3′-untranslated region (UTR) within the pMirTarget vector was ordered from OriGene Technologies. This vector was cotransfected with a control vector expressing a hRluc Renilla luciferase reporter gene and a herpes simplex virus thymidine kinase (HSV-TK) promoter for normalization of transfection efficiency (pGL4.74[hRluc/TK]; Promega). All transfections were performed in triplicate. At passages 4–8, hPASMCs were grown to 75% confluence before transfection with miR mimics (50–100 nmol/l) or antagomirs (100 nmol/l) with controls of the same concentrations using Lipofectamine (Thermo Fisher Scientific) per the manufacturer’s instructions. Cells were harvested for downstream analysis 24–72 h posttransfection. For luciferase reporter assays, cells were transfected concurrently with reporter vectors or controls. Cells were harvested at 24 h, and luciferase activity was measured with the Dual Luciferase Reporter Assay System (Promega) per the manufacturer’s protocol, using a GloMax luminometer (Promega). Transfection efficiency was determined by normalizing firefly luciferase light units to Renilla luciferase light units. Results are representative of at least three independent experiments.
Immunoblot analysis.
Lung tissues were perfused with PBS to remove blood and homogenized using radioimmunoprecipitation assay buffer (Sigma) supplemented with protease and phosphatase inhibitor cocktails (Calbiochem). Cells were washed with PBS, and protein was extracted using the same modified radioimmunoprecipitation assay buffer solution. Proteins (10–25 μg) were separated on Mini-Protean TGX precast gels (Bio-Rad), transferred to nitrocellulose membranes, and blocked in 5% nonfat dry milk. Membranes were then probed with primary and secondary antibodies, and bands were visualized by enhanced chemiluminescence (Pierce) per the manufacturer’s instructions. Densitometry was used to quantify protein levels using ImageJ software, and expression levels were normalized to GAPDH or β-actin. The primary antibodies used were SphK1, GAPDH, and horseradish peroxidase-conjugated β-actin (Cell Signaling Technology) with specificity previously determined (7, 59). An anti-rabbit IgG, horseradish peroxidase-linked secondary antibody was used (Cell Signaling Technology).
Lung tissue immunofluorescence microscopy.
Paraffin-embedded mouse lung tissue sections were used for immunostaining following antigen retrieval, using rabbit SphK1 (cat. no. ab71700; Abcam) and cyanine 3 (Cy3)-labeled mouse smooth muscle actin antibodies as previously described (30). The secondary antibody used for SphK1 was Alexa Fluor 488 donkey anti-rabbit IgG (Thermo Fisher Scientific). Mounting media with 4′,6-diamidino-2-phenylindole (DAPI; Life Science) were used to fix the coverslip to a slide. Slides were examined using a Nikon Eclipse E800 fluorescence microscope.
RNA extraction and quantitative real-time PCR analysis.
Total RNA containing miRNA was isolated from hPASMC and lung tissue samples using a miRNeasy kit (Qiagen) and reverse transcribed with the miScript II RT kit (Qiagen). For real-time PCR, miRNA-specific miScript primer assays (Qiagen) were used with RNU6-2 as an internal control. Expression of mRNAs was determined using specific TaqMan primer assays (Applied Biosystems) with GAPDH used as an internal control. Relative changes in mRNA and miRNA expression were calculated using the comparative cycle threshold method.
Measurement of intracellular S1P generation.
S1P levels from hPASMCs were measured using an established method previously described (60). Briefly, total lipid extracts were extracted from cell cultures after exposure to experimental conditions and then subjected to thin-layer chromatography and autoradiography. The areas corresponding to labeled [32P]S1P were excised, and radioactivity was determined by scintillation counting. The data were normalized to total radioactivity in 105 cells.
Terminal deoxynucleotidyl transferase dUTP nick-end labeling assay.
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assays were performed as previously described (28). In brief, hPASMCs at passages 4–8 were grown to 75% confluence before transfection with miR mimics (50–100 nmol/l) with controls of the same concentrations using Lipofectamine (Thermo Fisher Scientific) per the manufacturer’s instructions. TUNEL assays were then performed at 24 h with nuclear DAPI costaining. For quantitative analysis, TUNEL-positive apoptotic cells in ≥5 high-power fields per condition were expressed as percent apoptotic cells compared with controls.
Animal model of hypoxia-mediated pulmonary hypertension.
All experimental protocols using vertebrate animals were reviewed and approved by the University of Illinois at Chicago Animal Care and Use Committee. All experiments were performed in accordance with the approved guidelines and regulations for the use of laboratory animals. In the mouse model of hypoxia-mediated pulmonary hypertension (HPH), 8-wk-old male C57BL/6 mice were exposed to normoxia or hypoxia (10% O2 in a ventilated chamber) for 1 day to 4 wk (n = 3–5 per group). HPH development was assessed by measuring right ventricular systolic pressure via a pressure transducer catheter (Millar) and right ventricular hypertrophy as a weight ratio of the right ventricle divided by the sum of left ventricle and septum [RV/(LV + S)]. Pulmonary artery vessel thickness was calculated as [(external vessel area − internal vessel area)/external vessel area], as previously described (7), using images of lung histological sections (Aperio ImageScope). Lung and heart tissues were collected for further analysis.
For mouse HPH prevention studies, animal-grade mirVana miRNA mimics or negative miRNA control no. 1 (Thermo Fisher) were prepared with Invivofectamine 3.0 reagent (Thermo Fisher) per the manufacturer’s instructions and injected retroorbitally (7.8 mg/kg body wt) weekly for 4 wk starting 1 day before exposure to normoxia or hypoxia (10% O2). HPH development was assessed as described above.
Statistical analysis.
Results are shown as means ± SE from at least three experiments, and statistical significance was calculated with Student’s t-test (*P < 0.05, **P < 0.01, ***P < 0.001 vs. controls) using GraphPad Prism software.
RESULTS
miR-1 targets and regulates the expression of SphK1 in hPASMCs.
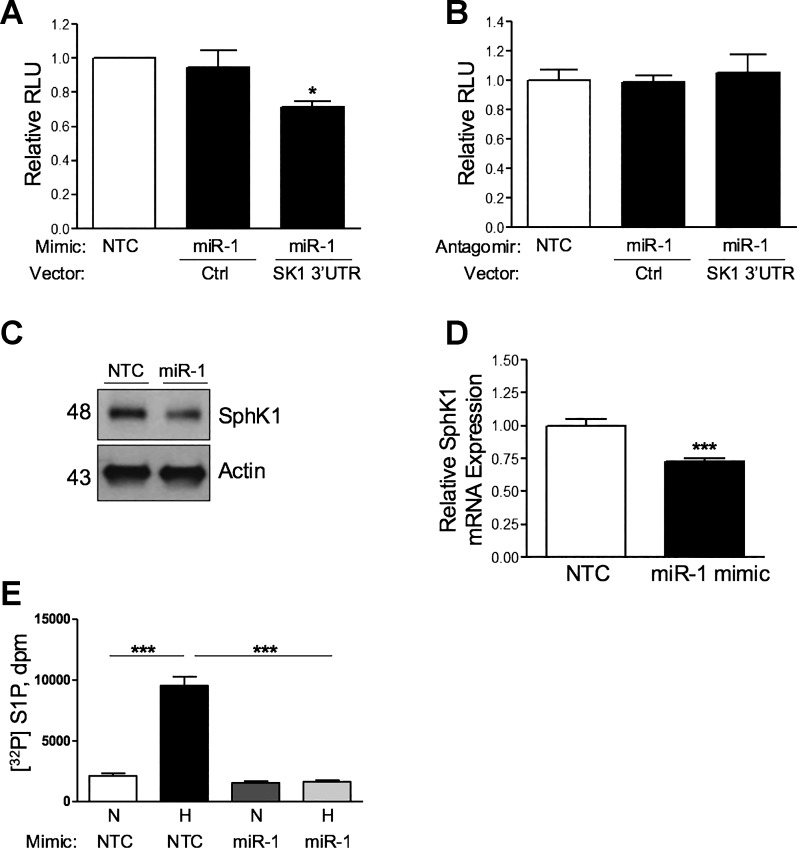
To investigate the potential regulation of SphK1 by miRs, we performed in silico analysis of the human SphK1 3′-UTR to identify predicted miR response elements. This approach identified several putative binding sites, including miR-1-3p (miR-1) as the most highly predicted target of SphK1 with a high probability of downregulation (7-mer-m8 canonical site type, mirSVR score = −0.6270; 5). Suggesting its importance in gene regulation, miR-1 and its SphK1 3′-UTR binding site are highly evolutionarily conserved across species. As bioinformatics analysis identified the putative binding site of miR-1 within the 3′-UTR of SPHK1, we hypothesized that this miR-1 may be a negative regulator of SphK1 expression in hPASMCs. To test this hypothesis, we utilized a luciferase reporter vector containing a sequence-verified clone of the SphK1 3′-UTR designed for micro-RNA target validation. To determine the interaction of miR-1 with the SphK1 3′-UTR, we overexpressed or inhibited miR-1 expression using human miR-1 mimics or antagomirs, respectively, in hPASMCs cotransfected with the SphK1 3′-UTR reporter vector. Overexpression of miR-1 resulted in reduced luciferase activity due to SphK1 3′-UTR binding, while transfection of miR-1 antagomirs had no effect (Fig. 1, A and B). In addition, both protein and mRNA expression levels of SphK1 were significantly decreased in hPASMCs following transfection with the miR-1 mimics (Fig. 1, C and D, respectively), while miR-1 antagomirs did not alter SphK1 expression (data not shown). Transfection of hPASMCs with miR-1 mimics also prevented hypoxia-induced generation of intracellular S1P generation at 48 h but did not alter basal levels of S1P under normoxic conditions (Fig. 1E), demonstrating a mechanistic role of miR-1 in limiting S1P production in hypoxia.
Fig. 1.
Micro-RNA-1 (miR-1) binds to the sphingosine kinase 1 (SphK1) 3′-untranslated region (SK1 3′UTR) and inhibits SphK1 expression. A and B: transfection of human pulmonary artery smooth muscle cells (hPASMCs) with miR-1 mimics (50 nmol/l), but not antagomirs (100 nmol/l), significantly reduces luciferase activity of a SphK1 3′-UTR reporter construct relative to control (Ctrl). Overexpression of miR-1 mimic (50 nmol/l) in hPASMCs significantly decreases SphK1 protein expression (C) and mRNA expression (D) at 48 h. E: transfection of hPASMCs with miR-1 mimics (50 nmol) prevents hypoxia-induced generation of intracellular [32P]sphingosine-1-phosphate ([32P]S1P) generation at 48 h. Here, n ≥ 5 per condition. *P < 0.05, ***P < 0.001 relative to control. NTC, nontargeting control; RLU, relative light units; N, normoxia; H, hypoxia (3% O2); dpm, disintegrations per minute.
Lung expression of miR-1 and SphK1 is dysregulated in the development of hypoxia-mediated pulmonary hypertension.
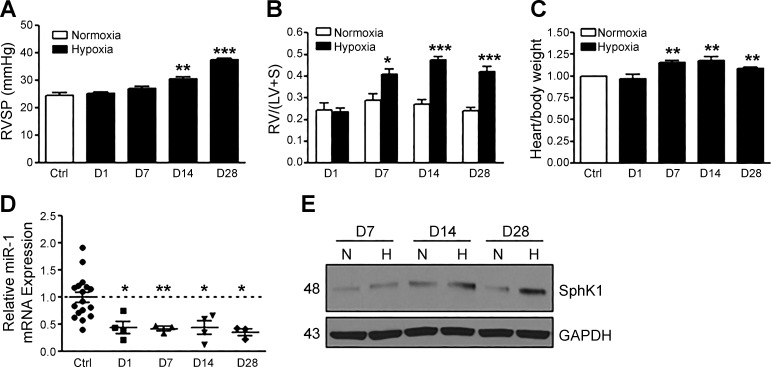
Hypoxia is associated with the pathogenesis of PAH and contributes to pulmonary vascular remodeling. We utilized a mouse model of experimental HPH to investigate the development of disease progression following 1, 7, 14, and 28 days of 10% O2 exposure, assessed by the measurement of right ventricular systolic pressure (RVSP) and right ventricular hypertrophy (RVH). Mice developed significant elevations in RVSP by day 14 and increased RVH and heart weight-to-body weight ratio by day 7, with increased severity of these parameters by day 28 (Fig. 2, A–C). Interestingly, the expression of miR-1 in whole lung was significantly reduced over time following hypoxia exposure (Fig. 2D), and this paralleled an increase in lung SphK1 protein expression (Fig. 2E). Significant elevation in vessel wall thickness of small pulmonary arteries, due to medial hypertrophy of PASMCs, was also observed by day 28 (data not shown), consistent with prior reports (7). These results demonstrate the utility of the HPH mouse model in studying the role of miR-1 expression.
Fig. 2.
Micro-RNA-1 (miR-1) is downregulated in mouse lungs during the progression of experimental hypoxia-mediated pulmonary hypertension (HPH). A and B: HPH development in mice is demonstrated by elevations in right ventricular systolic pressure (RVSP) and right ventricular hypertrophy [measured as a weight ratio of the right ventricle divided by the sum of the left ventricle and septum, RV/(LV + S)] over time [day (D) 1–28 of hypoxia]. C: change in mouse heart weight-to-body weight ratio over the course of HPH development. Expression of miR-1 normalized to RNU6-2 expression is reduced (D) and sphingosine kinase 1 (SphK1) protein expression is increased (E) in mouse lungs during HPH development. Here, n ≥ 5 per condition. *P < 0.05, **P < 0.01, ***P < 0.001 relative to control (Ctrl). N, normoxia; H, hypoxia (3% O2).
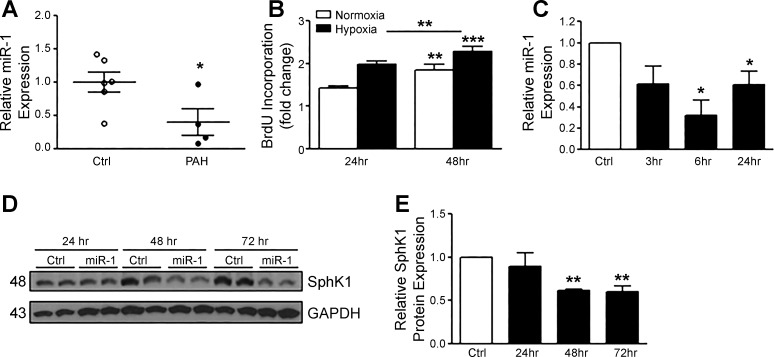
miR-1 expression is reduced in PASMCs from PAH patients and is decreased by hypoxia in hPASMCs.
Since miR-1 was reduced in lungs from HPH mice, we investigated whether PASMCs isolated from patients with PAH had altered levels of miR-1. Expression of miR-1 was significantly reduced in PASMCs from PAH patients vs. controls (Fig. 3A). We then tested whether hypoxia could alter miR-1 expression in hPASMCs. Hypoxia exposure (3% O2) increased the proliferative phenotype of hPASMCs over time (Fig. 3B) and significantly reduced the expression of miR-1 normalized to RNU6-2 expression (Fig. 3C). These data are consistent with our hypothesis that hypoxia may downregulate miR-1, leading to increased SphK1 expression and enhanced cell proliferation. Since overexpression of miR-1 mimics in hPASMCs can reduce SphK1 protein expression under normoxic conditions (Fig. 1C), we tested whether it could also prevent the induction of SphK1 expression by hypoxia. As shown in Fig. 3, D and E, miR-1 overexpression attenuates SphK1 expression under hypoxic conditions in hPASMCs over time, suggesting a significant role of miR-1 in directly targeting the 3′-UTR of SphK1. The transient transfection of miR-1 mimics sustained expression at 72 h, with the most robust expression at 24 h (data not shown).
Fig. 3.
Micro-RNA-1 (miR-1) is decreased in human pulmonary artery smooth muscle cells (hPASMCs) from pulmonary arterial hypertension (PAH) patients, is downregulated by hypoxia, and prevents hypoxia-induced sphingosine kinase 1 (SphK1) expression. A: miR-1 expression normalized to RNU6-2 expression is reduced in hPASMCs isolated from PAH patients (n = 4) vs. controls (Ctrl; n = 6). B: stimulation of hPASMCs with hypoxia induces proliferation (24–48 h, 3% O2). C: hypoxia (3% O2) induces downregulation of miR-1 expression over time in hPASMCs. D and E: miR-1 overexpression in hPASMCs attenuates hypoxia-induced SphK1 protein expression (24–72 h, 50 nmol/l mimics). Here, n ≥ 5 per condition, unless otherwise indicated. *P < 0.05, **P < 0.01, ***P < 0.001 relative to control. BrdU, 5-bromo-2′-deoxyuridine.
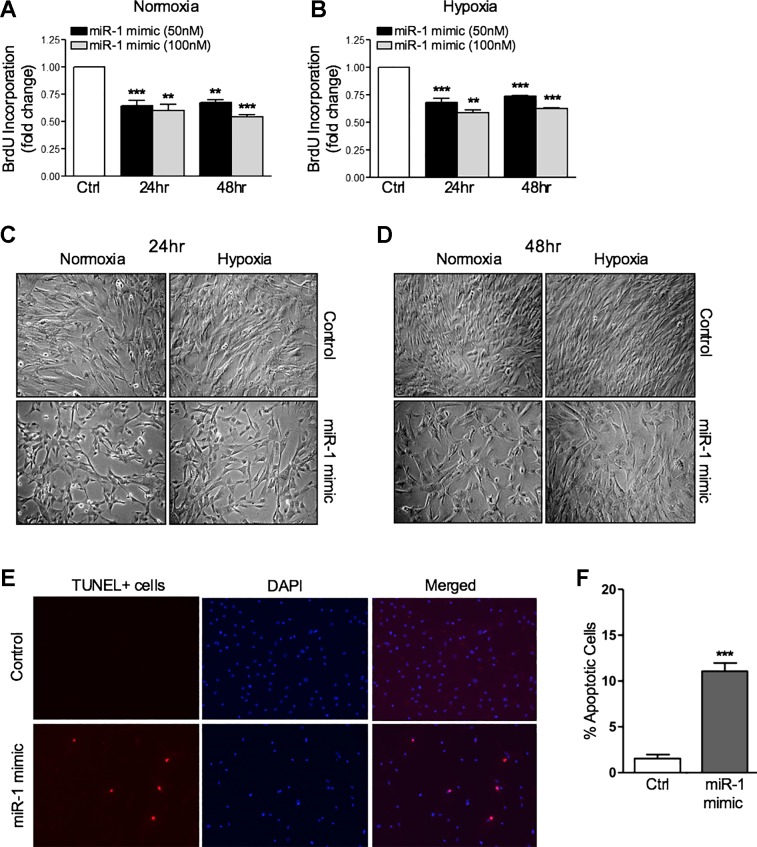
miR-1 regulates the proliferation of hPASMCs.
Because miR-1 is significantly decreased in the hypoxia-induced proliferative state of hPASMCs, we investigated whether altering miR-1 levels with the transfection of miR-1 mimics could alter the proliferative phenotype in these cells. Overexpression of miR-1 mimics at increasing concentrations reduced the proliferative capacity of hPASMCs in both normoxic and hypoxic conditions, respectively (Fig. 4, A and B). Differences in proliferation were also observed by light microscopy, with a substantial reduction in the number of hPASMCs in both normoxia and hypoxia following miR-1 overexpression (Fig. 4, C and D). The proliferative changes induced by miR-1 were not caused by enhanced cytotoxicity, as measured via lactate dehydrogenase activity in cell supernatants (data not shown). TUNEL assays demonstrated an ~11% induction of apoptosis in hPASMC transfection with miR-1 mimics at the highest dose of 100 nmol/l (Fig. 4, E and F). Although this finding may contribute to the antiproliferative effects of miR-1 in hPASMCs, the low level of apoptosis induced by miR-1 mimics is unlikely to account for the significant impact on proliferation observed.
Fig. 4.
Micro-RNA-1 (miR-1) overexpression inhibits human pulmonary artery smooth muscle cell (hPASMC) proliferation in normoxia and hypoxia. Overexpression of miR-1 mimics in hPASMCs (50–100 nmol/l) significantly reduces proliferation in normoxia (A) and hypoxia (3% O2, 24–48 h; B). C and D: representative images of hPASMCs demonstrating miR-1 mimic overexpression in normoxia and hypoxia reduces the rate of proliferation (24 h, ×10 magnification). Representative staining images (E) and quantification of terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay (F) demonstrating induction of apoptosis at low levels by overexpression of miR-1 mimics (100 nmol/l) in hPASMCs (24 h, ×10 magnification). Data are represented as percentage of TUNEL-positive cells of total 4′,6-diamidino-2-phenylindole (DAPI)-positive cells per high-power field per condition (≥5 fields counted). Here, n ≥ 5 per condition. **P < 0.01, ***P < 0.001 relative to control (Ctrl). BrdU, 5-bromo-2′-deoxyuridine.
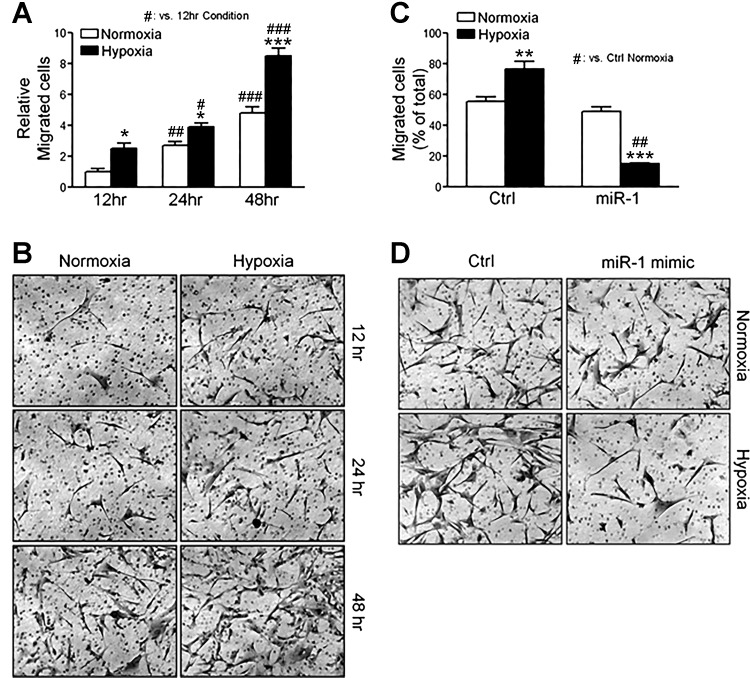
miR-1 regulates the migration of hPASMCs.
Both hPASMC proliferation and migration contribute to pathogenic pulmonary vascular remodeling in PAH. On the basis of the findings that miR-1 overexpression greatly reduced hPASMC proliferation in vitro, we tested whether miR-1 overexpression could also attenuate normoxic or hypoxia-induced hPASMC migration. In a Transwell assay, hPASMCs migrated over time under normoxic conditions, and as expected, migration increased when these cells were exposed to hypoxia for 12–48 h (Fig. 5, A and B). Overexpression of miR-1 inhibited the migration of hPASMCs at normoxia and further reduced the migration under hypoxic conditions (Fig. 5, C and D). This significant reduction in migratory capacity under hypoxic conditions demonstrates the importance of diminished miR-1 expression during this synthetic state of hPASMCs. Thus reduced miR-1 expression in hypoxia can enhance both proliferation and migration in this cell type during in vitro conditions that contribute to pulmonary vascular remodeling.
Fig. 5.
Micro-RNA-1 (miR-1) overexpression inhibits human pulmonary artery smooth muscle cell (hPASMC) migration in hypoxia. Stimulation of hPASMCs with hypoxia induces cell migration in a Transwell assay (12–48 h, 3% O2; A), with representative images (×10 magnification; B). Overexpression of miR-1 mimics in hPASMCs (50 nmol/l) significantly reduces hypoxia-induced cell migration in a Transwell assay (3% O2, 24 h; C), with representative images (×10 magnification; D). Here, n ≥ 5 per condition. *P < 0.05, **P < 0.01, ***P < 0.001 relative to control (Ctrl), unless otherwise indicated. #P < 0.05, ##P < 0.01, ###P < 0.001.
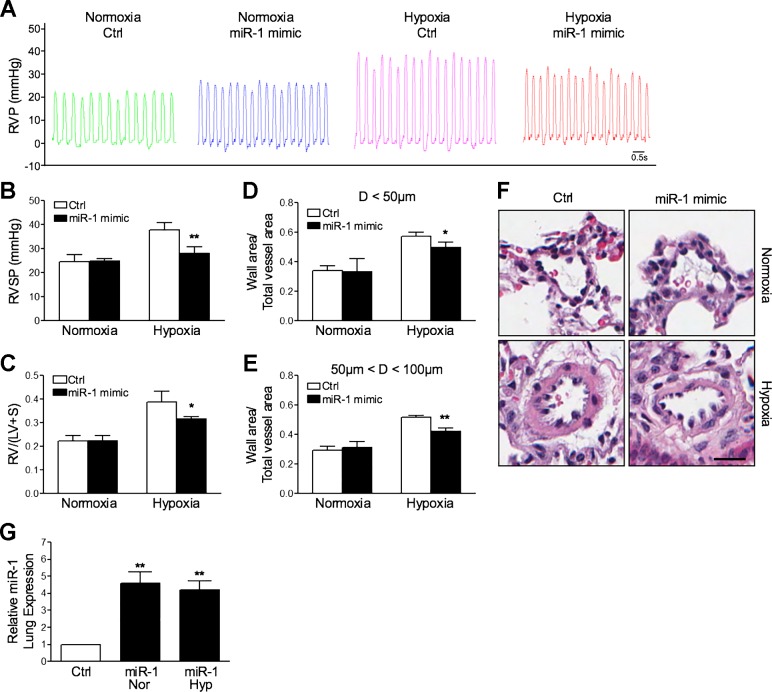
Systemic miR-1 delivery protects from the development of HPH.
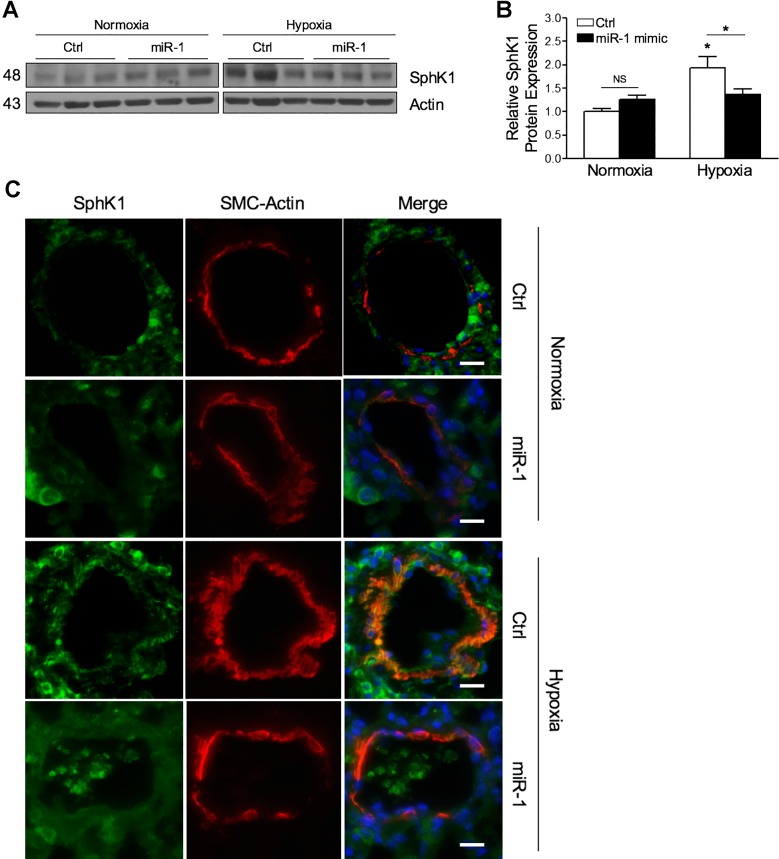
To test whether increasing expression of miR-1 in vivo could prevent HPH development, we delivered either nontargeting or miR-1 mimics via retroorbital injection to mice weekly throughout their 4-wk hypoxia course. Compared with controls, mice receiving miR-1 mimics were protected against the development of HPH, with significant attenuation of RVSP elevation and RVH (Fig. 6, A–C). Hypoxia-induced pulmonary vascular remodeling was also reduced with miR-1 mimic treatment (Fig. 6, D–F). No changes were observed in any of these parameters under normoxic conditions with miR-1 administration. Systemic administration of miR-1 mimics prevented the reduction in miR-1 expression induced by hypoxia, which resulted in a fourfold to fivefold increase in expression in the lung (Fig. 6G). To test whether increased miR-1 expression altered SphK1 expression in this model, we measured SphK1 protein in lung tissues. Overexpression of miR-1 had no significant effect on SphK1 under normoxic conditions but reduced SphK1 expression induction with hypoxia exposure (Fig. 7, A and B). To test whether SphK1 expression was altered in mouse PASMCs receiving miR-1 mimics, we measured both SphK1 and α-smooth muscle actin (α-SMA), a marker for smooth muscle cells, by immunofluorescence imaging in histological lung sections. SphK1 expression colocalized with α-SMA and was significantly reduced in mice receiving miR-1 mimics in both normoxic and hypoxic conditions (Fig. 7C). These results indicate that overexpression of miR-1 mimics reduces the development of experimental HPH and, together with the in vitro data presented in this study, suggest that altered expression of SphK1 expression by miR-1 may contribute to disease pathogenesis.
Fig. 6.
Micro-RNA-1 (miR-1) overexpression in vivo prevents the development of hypoxia-mediated pulmonary hypertension (HPH) in mice. Compared with controls (Ctrl), mice in the HPH model receiving systemic miR-1 mimics (7.8 mg/kg) develop less severe right ventricular systolic pressure (RVSP) elevation (A and B), right ventricular hypertrophy [calculated as a weight ratio of the right ventricle divided by the sum of left ventricle and septum, RV/(LV + S); C], and pulmonary vascular remodeling in arteries with diameters D < 50 µM and 50–100 µM, respectively (D and E). F: representative histological images of small pulmonary arteries in normoxia and hypoxia, with and without treatment of systemic miR-1 mimics. Scale bar = 20 µm. G: systemic administration of miR-1 mimics (7.8 mg/kg) in mice resulted in a fourfold to fivefold increase in lung expression in both normoxic (Nor) and hypoxic (Hyp) conditions, normalized to RNU6-2 expression (24 h following final injection). Here, n ≥ 5 per condition. *P < 0.05, **P < 0.01 relative to control. RVP, right ventricular pressure.
Fig. 7.
Micro-RNA-1 (miR-1) overexpression in vivo reduces induction of sphingosine kinase 1 (SphK1) in the lungs and pulmonary arteries. Representative Western blotting in whole lung tissues from mice with hypoxia-mediated pulmonary hypertension (HPH) demonstrates attenuation of SphK1 expression following systemic miR-1 mimic administration (7.8 mg/kg; A), with data quantification relative to actin expression (B). C: immunofluorescence staining demonstrates that hypoxia-induced SphK1 protein expression in small pulmonary arteries is reduced in miR-1 mimic-treated mice compared with controls (Ctrl). Scale bars = 20 µm. *P < 0.05 relative to control. NS, not significant; SMC, smooth muscle cell.
DISCUSSION
PAH is a devastating lung disease for which curative treatments are unavailable and patient morbidity and mortality remain high (26, 40). Despite active research in uncovering the pathogenic mechanisms resulting in this disease, therapies targeting the prevention or reversal of pulmonary vascular remodeling and subsequent elevations in pulmonary arterial pressure have not been successful. The present study provides evidence of the role of miR-1, a muscle-specific micro-RNA, and its posttranscriptional regulation of SphK1 in the pathogenesis of PAH and highlights its potential as a novel therapeutic target.
In the present study, using an in silico approach, we identify miR-1 as a potential regulator of SphK1 expression. We found that miR-1 is highly downregulated by hypoxia in hPASMCs, as well as in the lungs of mice throughout the progression of experimental HPH, coincident with increased SphK1 expression. Expression of miR-1 was also significantly reduced in PASMCs isolated from patients with PAH. Hypoxia-mediated decreases in miR-1 expression were associated with enhanced hPASMC proliferation and migration in vitro, and overexpression of miR-1 inhibited these effects. Additionally, we provide in vivo evidence that miR-1 overexpression prevents the development of experimental HPH and reduces the overexpression of SphK1 in PASMCs in this model. Although there are likely multiple mRNA targets of miR-1, the regulation of SphK1 expression is consistent with our findings that this kinase is highly influential in promoting PASMC proliferation and the development of experimental pulmonary hypertension (7). These experiments describe a novel role of miR-1 in regulating the intricate molecular mechanisms underlying pathogenic pulmonary vascular remodeling in PAH.
Our previous work has demonstrated that elevated levels of both S1P and SphK1 in the lung are associated with PAH (7). These studies showed that both genetic knockout of SphK1 in mice and pharmacologic SphK1 inhibition in rats could prevent the development of hypoxia-mediated PH. Inhibiting the S1P receptor 2 (S1PR2) in mice also prevented PH development, and mice heterozygous for S1P lyase, which catabolizes S1P, were more susceptible to PH (7). Together, these studies define the role of SphK1-S1P signaling in PAH. Here we identified miR-1 as a negative regulator of SphK1 expression. Under basal conditions, normal miR-1 expression limits SphK1 expression, whereas in hypoxia the reduction in miR-1 allows for SphK1 upregulation at both mRNA and protein levels.
Unrestrained PASMC proliferation and migration, as well are hypoxic vasoconstriction, are major contributors to pulmonary vascular remodeling in PAH, and upregulation of the SphK1-S1P signaling axis has been implicated in these pathogenic processes (7, 11, 53). We have previously demonstrated that both overexpression of SphK1 and stimulation with S1P promote the proliferative phenotype of hPASMCs. Several studies have also reported that miRNAs can regulate cell proliferation and migration associated with vascular remodeling by several mechanisms, including targeting of ion channels, mitochondrial function, and BMP receptor 2 (BMPR2) signaling pathways (19). Here, we report that miR-1 is downregulated in PASMCs isolated from PAH patients and overexpression of miR-1 can reduce hypoxia-induced SphK1 expression and attenuate PASMC proliferation and migration. These studies indicate the critical role of miR-1 in regulating pulmonary vascular remodeling in PAH. The relevance of these findings also extends beyond PAH, as SphK1 and S1P are well-known mediators of cell proliferation, apoptosis resistance, and angiogenesis in myriad cell types, including cancer cells (36, 51).
Shared features of cancer development with the unrestricted vascular cell proliferation, apoptosis resistance, and glycolytic shifts of PAH have recently been appreciated (20, 44). In these studies, a “pseudohypoxic environment” is proposed where glycolysis is predominant and hypoxia-inducible factor 1α is activated under normoxia (13, 47). These observations are consistent with our findings of reduced miR-1 expression in PASMCs isolated from PAH patients in normoxic conditions. Importantly, inhibiting the oncogenic properties of SphK1 has shown efficacy in preclinical studies to decrease tumor growth (16, 31), and therefore induction of miR-1 to limit SphK1 expression may be beneficial. Recent reports have also associated downregulation of miR-1 with cancer development in various cell types (38, 39, 55, 56) via its modulation of cell proliferation, migration, and invasion. These studies suggest the ubiquitous role of miR-1 in the regulation of cell proliferation and are consistent with our findings that miR-1 is antiproliferative in PASMCs.
In SMCs, miR-1 has also been reported to regulate gene expression. It is an important modulator of cardiac and skeletal muscle proliferation, with excess expression leading to a reduced pool of proliferating ventricular cardiomyocytes in vivo (9, 61, 62). Expression of miR-1 can be induced by myocardin, a transcriptional activator of serum response factor (SRF), to inhibit cell proliferation and contractility in human vascular SMCs (8, 29), potentially through regulation of proviral integration site for Moloney murine leukemia virus-1 (Pim-1), a recently identified biomarker in PAH (45). Downregulation of miR-1 has also been observed in vascular SMCs isolated from spontaneously hypertensive rats and may regulate proliferation by targeting insulin-like growth factor I (IGF-I; 37), a growth factor involved in neonatal HPH (57). In addition, genetic knockout of miR-1 in mice results in uniform lethality before weaning due to cardiac dysfunction, and hearts from these mice exhibit gene expression characteristics more similar to vascular smooth muscle (24). This suggests that the influence of miR-1 on the proliferation and migration of PASMCs likely functions through multiple parallel mechanisms to achieve the resulting cellular phenotype.
Micro-RNAs have shown promise as potential therapeutic targets and biomarkers for many diseases, including PAH. Interestingly, a recent study exploring the role of circulating miRNAs in human PH demonstrated reduced expression of miR-1 in the buffy coat of patients with both moderate and severe PH (54). Here, we have demonstrated that miR-1 is strongly downregulated early in and throughout the course of experimental hypoxia-induced PH in the lungs and that this downregulation is retained in PASMCs from PAH patients. This suggests that miR-1 plays a role in early disease pathogenesis rather than having an end-stage disease effect. Overexpression of miR-1 can reduce the pulmonary vascular remodeling phenotype both in vitro and in vivo; therefore identifying mechanisms to elevate or sustain miR-1 expression in PAH may be beneficial.
There are several limitations to the present study that warrant further investigation. The ability of miR-1 to decrease SphK1 levels in PASMCs is one mechanism through which it regulates vascular remodeling, but other direct targets of miR-1 involved in PAH development may contribute to our findings here and would be important to identify in future studies. Table 1 provides a list of the most highly predicted mRNA targets of miR-1. This list includes two genes, brain-derived neurotrophic factor (BDNF) and gap junction α-1 protein/connexin 43 (GJA1/Cx43), that have been previously associated with PASMC pathobiology (10, 23, 33). Another potential limitation is that miR-1 overexpression induces apoptosis in hPASMCs, though the low level of induction even at high mimic concentrations is unlikely to account for the significant impact on proliferation we observed. Furthermore, in addition to unrestrained PASMC proliferation and apoptosis resistance, pulmonary vascular remodeling involves neointimal formation due to PAEC dysfunction and proliferation (34, 58). The studies described here did not investigate the role of the SphK1-S1P axis on the phenotype of PAECs; this would provide valuable insight into disease mechanisms and deserves exploration in future studies. Finally, though we have used idiopathic PAH samples in this study and demonstrated a role of miR-1 under normoxic conditions in PASMCs, several of our experiments utilize hypoxia as a model of disease, which more classically parallels group 3 PH. As discussed above, we believe that hypoxia and normoxic activation of hypoxia-related signaling pathways are fundamental to the development of PAH and that our studies therefore offer important insights into this group of PH as well.
Table 1.
Predicted mRNA targets of miR-1 in human cells
| Gene Symbol | Gene Description | mirSVR Score |
|---|---|---|
| ZNF280C | Zinc finger protein 280C | −3.21 |
| BDNF | Brain-derived neurotrophic factor | −3.19 |
| GJA1 | Gap junction α-1 protein [connexin 43 (Cx43)] | −3.03 |
| KIF2A | Kinesin family member 2A | −2.42 |
| SLC44A1 | Solute carrier family 44 member 1 | −2.29 |
| FAM104B | Family with sequence similarity 104 member B | −2.19 |
| YWHAQ | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein-θ | −2.19 |
| HACD3 | 3-Hydroxyacyl-CoA dehydratase 3 | −2.11 |
| MEX3C | Mex-3 RNA-binding family member C | −2.07 |
| GLCCI1 | Glucocorticoid-induced 1 | −2.07 |
| MGC70870 | COOH-terminal binding protein-2 pseudogene | −1.93 |
| RP2 | RP2, ARL3 GTPase-activating protein | −1.92 |
| UST | Uronyl 2-sulfotransferase | −1.89 |
| CDK14 | Cyclin-dependent kinase 14 | −1.85 |
| CHSY1 | Chondroitin sulfate synthase 1 | −1.85 |
Data were collected from microRNA.org (http://34.236.212.39/microrna/home.do); miR-1, micro-RNA-1; mirSVR, miR support vector regression; ARL3, ADP-ribosylation factor-like protein-3; RP2, retinitis pigmentosa protein-2.
Together, these novel data demonstrate that miR-1 can target SphK1 to regulate molecular mechanisms in PH with effects on PASMCs and pulmonary arterial remodeling. The activation of miR-1 in the pulmonary vasculature may therefore provide therapeutic benefit in PH, a group of diseases in significant need of new treatments.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute (NHLBI) Grants R01-HL-127342, R01-HL-111656, and R01-HL-133951 (to R. F. Machado) and P01-HL-98050 and R01-HL-127342 (to V. Natarajan) and P01-HL-103453, R01-HL-115008, and R37-HL-060917 (to S. Comhair); a Pulmonary Hypertension Association grant (to J. Chen); American Heart Association Predoctoral Fellowship Grant 15PRE2190004 (to J. R. Sysol); NLHBI National Research Service Award F30 Fellowship FHL128034A (to J. R. Sysol); University of Illinois at Chicago (UIC) Center for Clinical and Translational Science Pre-doctoral Education for Clinical and Translational Scientists Award (to J. R. Sysol); and UIC Biomedical Deiss Fund Award (to J. R. Sysol).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.R.S., V.N., and R.F.M. conceived and designed research; J.R.S., J.C., S.S., S.Z., and S.A.C. performed experiments; J.R.S., J.C., S.S., S.Z., and R.F.M. analyzed data; J.R.S., J.C., S.S., V.N., and R.F.M. interpreted results of experiments; J.R.S. and S.Z. prepared figures; J.R.S. drafted manuscript; R.F.M. edited and revised manuscript; J.R.S., J.C., S.S., S.A.C., V.N., and R.F.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Serpil C. Erzurum and the Lung Biology Tissue and Cell Core of the Department of Pathobiology at the Cleveland Clinic for generously providing human PAH and control PASMC lines used for these studies.
REFERENCES
- 1.Ambros V. The functions of animal microRNAs. Nature 431: 350–355, 2004. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 2.Aytekin M, Comhair SA, de la Motte C, Bandyopadhyay SK, Farver CF, Hascall VC, Erzurum SC, Dweik RA. High levels of hyaluronan in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 295: L789–L799, 2008. doi: 10.1152/ajplung.90306.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297, 2004. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 4.Bertero T, Lu Y, Annis S, Hale A, Bhat B, Saggar R, Saggar R, Wallace WD, Ross DJ, Vargas SO, Graham BB, Kumar R, Black SM, Fratz S, Fineman JR, West JD, Haley KJ, Waxman AB, Chau BN, Cottrill KA, Chan SY. Systems-level regulation of microRNA networks by miR-130/301 promotes pulmonary hypertension. J Clin Invest 124: 3514–3528, 2014. doi: 10.1172/JCI74773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betel D, Koppal A, Agius P, Sander C, Leslie C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol 11: R90, 2010. doi: 10.1186/gb-2010-11-8-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bienertova-Vasku J, Novak J, Vasku A. MicroRNAs in pulmonary arterial hypertension: pathogenesis, diagnosis and treatment. J Am Soc Hypertens 9: 221–234, 2015. doi: 10.1016/j.jash.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Tang H, Sysol JR, Moreno-Vinasco L, Shioura KM, Chen T, Gorshkova I, Wang L, Huang LS, Usatyuk PV, Sammani S, Zhou G, Raj JU, Garcia JG, Berdyshev E, Yuan JX, Natarajan V, Machado RF. The sphingosine kinase 1/sphingosine-1-phosphate pathway in pulmonary arterial hypertension. Am J Respir Crit Care Med 190: 1032–1043, 2014. doi: 10.1164/rccm.201401-0121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Yin H, Jiang Y, Radhakrishnan SK, Huang ZP, Li J, Shi Z, Kilsdonk EP, Gui Y, Wang DZ, Zheng XL. Induction of microRNA-1 by myocardin in smooth muscle cells inhibits cell proliferation. Arterioscler Thromb Vasc Biol 31: 368–375, 2011. doi: 10.1161/ATVBAHA.110.218149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet 38: 228–233, 2006. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen M, Liu Y, Yi D, Wei L, Li Y, Zhang L. Tanshinone IIA promotes pulmonary artery smooth muscle cell apoptosis in vitro by inhibiting the JAK2/STAT3 signaling pathway. Cell Physiol Biochem 33: 1130–1138, 2014. doi: 10.1159/000358682. [DOI] [PubMed] [Google Scholar]
- 11.Chowdhury A, Sarkar J, Chakraborti T, Chakraborti S. Role of Spm-Cer-S1P signalling pathway in MMP-2 mediated U46619-induced proliferation of pulmonary artery smooth muscle cells: protective role of epigallocatechin-3-gallate. Cell Biochem Funct 33: 463–477, 2015. doi: 10.1002/cbf.3136. [DOI] [PubMed] [Google Scholar]
- 12.Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 381: 800–803, 1996. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 13.Dromparis P, Paulin R, Sutendra G, Qi AC, Bonnet S, Michelakis ED. Uncoupling protein 2 deficiency mimics the effects of hypoxia and endoplasmic reticulum stress on mitochondria and triggers pseudohypoxic pulmonary vascular remodeling and pulmonary hypertension. Circ Res 113: 126–136, 2013. doi: 10.1161/CIRCRESAHA.112.300699. [DOI] [PubMed] [Google Scholar]
- 14.Dudek SM, Jacobson JR, Chiang ET, Birukov KG, Wang P, Zhan X, Garcia JG. Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J Biol Chem 279: 24692–24700, 2004. doi: 10.1074/jbc.M313969200. [DOI] [PubMed] [Google Scholar]
- 15.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med 351: 1655–1665, 2004. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- 16.French KJ, Upson JJ, Keller SN, Zhuang Y, Yun JK, Smith CD. Antitumor activity of sphingosine kinase inhibitors. J Pharmacol Exp Ther 318: 596–603, 2006. doi: 10.1124/jpet.106.101345. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda Y, Kihara A, Igarashi Y. Distribution of sphingosine kinase activity in mouse tissues: contribution of SPHK1. Biochem Biophys Res Commun 309: 155–160, 2003. doi: 10.1016/S0006-291X(03)01551-1. [DOI] [PubMed] [Google Scholar]
- 18.Gorshkova I, Zhou T, Mathew B, Jacobson JR, Takekoshi D, Bhattacharya P, Smith B, Aydogan B, Weichselbaum RR, Natarajan V, Garcia JG, Berdyshev EV. Inhibition of serine palmitoyltransferase delays the onset of radiation-induced pulmonary fibrosis through the negative regulation of sphingosine kinase-1 expression. J Lipid Res 53: 1553–1568, 2012. doi: 10.1194/jlr.M026039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grant JS, White K, MacLean MR, Baker AH. MicroRNAs in pulmonary arterial remodeling. Cell Mol Life Sci 70: 4479–4494, 2013. doi: 10.1007/s00018-013-1382-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guignabert C, Tu L, Le Hiress M, Ricard N, Sattler C, Seferian A, Huertas A, Humbert M, Montani D. Pathogenesis of pulmonary arterial hypertension: lessons from cancer. Eur Respir Rev 22: 543–551, 2013. doi: 10.1183/09059180.00007513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hao H, Gabbiani G, Bochaton-Piallat ML. Arterial smooth muscle cell heterogeneity: implications for atherosclerosis and restenosis development. Arterioscler Thromb Vasc Biol 23: 1510–1520, 2003. doi: 10.1161/01.ATV.0000090130.85752.ED. [DOI] [PubMed] [Google Scholar]
- 22.Harijith A, Pendyala S, Reddy NM, Bai T, Usatyuk PV, Berdyshev E, Gorshkova I, Huang LS, Mohan V, Garzon S, Kanteti P, Reddy SP, Raj JU, Natarajan V. Sphingosine kinase 1 deficiency confers protection against hyperoxia-induced bronchopulmonary dysplasia in a murine model: role of S1P signaling and Nox proteins. Am J Pathol 183: 1169–1182, 2013. doi: 10.1016/j.ajpath.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartman W, Helan M, Smelter D, Sathish V, Thompson M, Pabelick CM, Johnson B, Prakash YS. Role of hypoxia-induced brain derived neurotrophic factor in human pulmonary artery smooth muscle. PLoS One 10: e0129489, 2015. doi: 10.1371/journal.pone.0129489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heidersbach A, Saxby C, Carver-Moore K, Huang Y, Ang YS, de Jong PJ, Ivey KN, Srivastava D. microRNA-1 regulates sarcomere formation and suppresses smooth muscle gene expression in the mammalian heart. eLife 2: e01323, 2013. doi: 10.7554/eLife.01323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong Z, Chen KH, DasGupta A, Potus F, Dunham-Snary K, Bonnet S, Tian L, Fu J, Breuils-Bonnet S, Provencher S, Wu D, Mewburn J, Ormiston ML, Archer SL. MicroRNA-138 and microRNA-25 down-regulate mitochondrial calcium uniporter, causing the pulmonary arterial hypertension cancer phenotype. Am J Respir Crit Care Med 195: 515–529, 2017. doi: 10.1164/rccm.201604-0814OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaïci A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Cottin V, Degano B, Jaïs X, Montani D, Souza R, Simonneau G. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 122: 156–163, 2010. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- 27.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med 351: 1425–1436, 2004. doi: 10.1056/NEJMra040291. [DOI] [PubMed] [Google Scholar]
- 28.Jalali S, Ramanathan GK, Parthasarathy PT, Aljubran S, Galam L, Yunus A, Garcia S, Cox RR Jr, Lockey RF, Kolliputi N. Mir-206 regulates pulmonary artery smooth muscle cell proliferation and differentiation. PLoS One 7: e46808, 2012. doi: 10.1371/journal.pone.0046808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang Y, Yin H, Zheng XL. MicroRNA-1 inhibits myocardin-induced contractility of human vascular smooth muscle cells. J Cell Physiol 225: 506–511, 2010. doi: 10.1002/jcp.22230. [DOI] [PubMed] [Google Scholar]
- 30.John AE, Wilson MR, Habgood A, Porte J, Tatler AL, Stavrou A, Miele G, Jolly L, Knox AJ, Takata M, Offermanns S, Jenkins RG. Loss of epithelial Gq and G11 signaling inhibits TGFβ production but promotes IL-33-mediated macrophage polarization and emphysema. Sci Signal 9: ra104, 2016. doi: 10.1126/scisignal.aad5568. [DOI] [PubMed] [Google Scholar]
- 31.Kapitonov D, Allegood JC, Mitchell C, Hait NC, Almenara JA, Adams JK, Zipkin RE, Dent P, Kordula T, Milstien S, Spiegel S. Targeting sphingosine kinase 1 inhibits Akt signaling, induces apoptosis, and suppresses growth of human glioblastoma cells and xenografts. Cancer Res 69: 6915–6923, 2009. doi: 10.1158/0008-5472.CAN-09-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim VN, Nam JW. Genomics of microRNA. Trends Genet 22: 165–173, 2006. doi: 10.1016/j.tig.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Kwapiszewska G, Chwalek K, Marsh LM, Wygrecka M, Wilhelm J, Best J, Egemnazarov B, Weisel FC, Osswald SL, Schermuly RT, Olschewski A, Seeger W, Weissmann N, Eickelberg O, Fink L. BDNF/TrkB signaling augments smooth muscle cell proliferation in pulmonary hypertension. Am J Pathol 181: 2018–2029, 2012. doi: 10.1016/j.ajpath.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 34.Lai YC, Potoka KC, Champion HC, Mora AL, Gladwin MT. Pulmonary arterial hypertension: the clinical syndrome. Circ Res 115: 115–130, 2014. doi: 10.1161/CIRCRESAHA.115.301146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leggett K, Maylor J, Undem C, Lai N, Lu W, Schweitzer K, King LS, Myers AC, Sylvester JT, Sidhaye V, Shimoda LA. Hypoxia-induced migration in pulmonary arterial smooth muscle cells requires calcium-dependent upregulation of aquaporin 1. Am J Physiol Lung Cell Mol Physiol 303: L343–L353, 2012. doi: 10.1152/ajplung.00130.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Limaye V, Li X, Hahn C, Xia P, Berndt MC, Vadas MA, Gamble JR. Sphingosine kinase-1 enhances endothelial cell survival through a PECAM-1-dependent activation of PI-3K/Akt and regulation of Bcl-2 family members. Blood 105: 3169–3177, 2005. doi: 10.1182/blood-2004-02-0452. [DOI] [PubMed] [Google Scholar]
- 37.Liu K, Ying Z, Qi X, Shi Y, Tang Q. MicroRNA-1 regulates the proliferation of vascular smooth muscle cells by targeting insulin-like growth factor 1. Int J Mol Med 36: 817–824, 2015. doi: 10.3892/ijmm.2015.2277. [DOI] [PubMed] [Google Scholar]
- 38.Liu YN, Yin J, Barrett B, Sheppard-Tillman H, Li D, Casey OM, Fang L, Hynes PG, Ameri AH, Kelly K. Loss of androgen-regulated microRNA 1 activates SRC and promotes prostate cancer bone metastasis. Mol Cell Biol 35: 1940–1951, 2015. doi: 10.1128/MCB.00008-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mataki H, Enokida H, Chiyomaru T, Mizuno K, Matsushita R, Goto Y, Nishikawa R, Higashimoto I, Samukawa T, Nakagawa M, Inoue H, Seki N. Downregulation of the microRNA-1/133a cluster enhances cancer cell migration and invasion in lung-squamous cell carcinoma via regulation of Coronin1C. J Hum Genet 60: 53–61, 2015. doi: 10.1038/jhg.2014.111. [DOI] [PubMed] [Google Scholar]
- 40.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J; American College of Cardiology Foundation Task Force on Expert Consensus Documents; American Heart Association; American College of Chest Physicians; American Thoracic Society, Inc; Pulmonary Hypertension Association . ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 53: 1573–1619, 2009. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Ni X, Epshtein Y, Chen W, Zhou T, Xie L, Garcia JG, Jacobson JR. Interaction of integrin β4 with S1P receptors in S1P- and HGF-induced endothelial barrier enhancement. J Cell Biochem 115: 1187–1195, 2014. doi: 10.1002/jcb.24770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park JH, Shin C. MicroRNA-directed cleavage of targets: mechanism and experimental approaches. BMB Rep 47: 417–423, 2014. doi: 10.5483/BMBRep.2014.47.8.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med 169: 1245–1251, 2004. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- 44.Rai PR, Cool CD, King JA, Stevens T, Burns N, Winn RA, Kasper M, Voelkel NF. The cancer paradigm of severe pulmonary arterial hypertension. Am J Respir Crit Care Med 178: 558–564, 2008. doi: 10.1164/rccm.200709-1369PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Renard S, Paulin R, Breuils-Bonnet S, Simard S, Pibarot P, Bonnet S, Provencher S. Pim-1: a new biomarker in pulmonary arterial hypertension. Pulm Circ 3: 74–81, 2013. doi: 10.4103/2045-8932.109917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rothman AM, Arnold ND, Pickworth JA, Iremonger J, Ciuclan L, Allen RM, Guth-Gundel S, Southwood M, Morrell NW, Thomas M, Francis SE, Rowlands DJ, Lawrie A. MicroRNA-140-5p and SMURF1 regulate pulmonary arterial hypertension. J Clin Invest 126: 2495–2508, 2016. doi: 10.1172/JCI83361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryan J, Dasgupta A, Huston J, Chen KH, Archer SL. Mitochondrial dynamics in pulmonary arterial hypertension. J Mol Med (Berl) 93: 229–242, 2015. doi: 10.1007/s00109-015-1263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shea BS, Tager AM. Role of the lysophospholipid mediators lysophosphatidic acid and sphingosine 1-phosphate in lung fibrosis. Proc Am Thorac Soc 9: 102–110, 2012. doi: 10.1513/pats.201201-005AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature 469: 336–342, 2011. doi: 10.1038/nature09783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sobel K, Menyhart K, Killer N, Renault B, Bauer Y, Studer R, Steiner B, Bolli MH, Nayler O, Gatfield J. Sphingosine 1-phosphate (S1P) receptor agonists mediate pro-fibrotic responses in normal human lung fibroblasts via S1P2 and S1P3 receptors and Smad-independent signaling. J Biol Chem 288: 14839–14851, 2013. doi: 10.1074/jbc.M112.426726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strub GM, Maceyka M, Hait NC, Milstien S, Spiegel S. Extracellular and intracellular actions of sphingosine-1-phosphate. Adv Exp Med Biol 688: 141–155, 2010. doi: 10.1007/978-1-4419-6741-1_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sysol JR, Natarajan V, Machado RF. PDGF induces SphK1 expression via Egr-1 to promote pulmonary artery smooth muscle cell proliferation. Am J Physiol Cell Physiol 310: C983–C992, 2016. doi: 10.1152/ajpcell.00059.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tabeling C, Yu H, Wang L, Ranke H, Goldenberg NM, Zabini D, Noe E, Krauszman A, Gutbier B, Yin J, Schaefer M, Arenz C, Hocke AC, Suttorp N, Proia RL, Witzenrath M, Kuebler WM. CFTR and sphingolipids mediate hypoxic pulmonary vasoconstriction. Proc Natl Acad Sci USA 112: E1614–E1623, 2015. doi: 10.1073/pnas.1421190112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei C, Henderson H, Spradley C, Li L, Kim IK, Kumar S, Hong N, Arroliga AC, Gupta S. Circulating miRNAs as potential marker for pulmonary hypertension. PLoS One 8: e64396, 2013. doi: 10.1371/journal.pone.0064396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiao H, Zeng J, Li H, Chen K, Yu G, Hu J, Tang K, Zhou H, Huang Q, Li A, Li Y, Ye Z, Wang J, Xu H. MiR-1 downregulation correlates with poor survival in clear cell renal cell carcinoma where it interferes with cell cycle regulation and metastasis. Oncotarget 6: 13201–13215, 2015. doi: 10.18632/oncotarget.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu X, Wu X, Jiang Q, Sun Y, Liu H, Chen R, Wu S. Downregulation of microRNA-1 and microRNA-145 contributes synergistically to the development of colon cancer. Int J Mol Med 36: 1630–1638, 2015. doi: 10.3892/ijmm.2015.2364. [DOI] [PubMed] [Google Scholar]
- 57.Yang Q, Sun M, Ramchandran R, Raj JU. IGF-1 signaling in neonatal hypoxia-induced pulmonary hypertension: role of epigenetic regulation. Vascul Pharmacol 73: 20–31, 2015. doi: 10.1016/j.vph.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuan JX, Rubin LJ. Pathogenesis of pulmonary arterial hypertension: the need for multiple hits. Circulation 111: 534–538, 2005. doi: 10.1161/01.CIR.0000156326.48823.55. [DOI] [PubMed] [Google Scholar]
- 59.Zhang W, Mottillo EP, Zhao J, Gartung A, VanHecke GC, Lee JF, Maddipati KR, Xu H, Ahn YH, Proia RL, Granneman JG, Lee MJ. Adipocyte lipolysis-stimulated interleukin-6 production requires sphingosine kinase 1 activity. J Biol Chem 289: 32178–32185, 2014. doi: 10.1074/jbc.M114.601096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao Y, Kalari SK, Usatyuk PV, Gorshkova I, He D, Watkins T, Brindley DN, Sun C, Bittman R, Garcia JG, Berdyshev EV, Natarajan V. Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: role of lipid phosphate phosphatase-1 and sphingosine kinase 1. J Biol Chem 282: 14165–14177, 2007. doi: 10.1074/jbc.M701279200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ, Srivastava D. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell 129: 303–317, 2007. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 62.Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature 436: 214–220, 2005. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 63.Zhou G, Chen T, Raj JU. MicroRNAs in pulmonary arterial hypertension. Am J Respir Cell Mol Biol 52: 139–151, 2015. doi: 10.1165/rcmb.2014-0166TR. [DOI] [PMC free article] [PubMed] [Google Scholar]