Abstract
Obesity is a risk factor for asthma and influences airway hyperresponsiveness, which is in part modulated by airway smooth muscle proliferative remodeling. Plasma free fatty acids (FFAs) levels are elevated in obese individuals, and long-chain FFAs act as endogenous ligands for the free fatty acid receptor 1 (FFAR1), which couples to both Gq and Gi proteins. We examined whether stimulation of FFAR1 induces airway smooth muscle cell proliferation through classical MEK/ERK and/or phosphoinositide 3-kinase (PI3K)/Akt signaling pathways. The long-chain FFAs (oleic acid and linoleic acid) and a FFAR1 agonist (GW9508) induced human airway smooth muscle (HASM) cell proliferation, which was inhibited by the MEK inhibitor U0126 and the PI3K inhibitor LY294002. The long-chain FFAs and GW9508 increased phosphorylation of ERK, Akt, and p70S6K in HASM cells and freshly isolated rat airway smooth muscle. Downregulation of FFAR1 in HASM cells by siRNA significantly attenuated oleic acid-induced phosphorylation of ERK and Akt. Oleic acid-induced ERK phosphorylation was blocked by either the Gαi-protein inhibitor pertussis toxin or U0126 and was partially inhibited by either the Gαq-specific inhibitor YM-254890 or the Gβγ signaling inhibitor gallein. Oleic acid significantly inhibited forskolin-stimulated cAMP activity, which was attenuated by pertussis toxin. Akt phosphorylation was inhibited by pertussis toxin, the ras inhibitor manumycin A, the Src inhibitor PP1, or LY294002. Phosphorylation of p70S6K by oleic acid or GW9508 was significantly inhibited by LY294002, U0126, and the mammalian target of rapamycin (mTOR) inhibitor rapamycin. In conclusion, the FFAR1 promoted airway smooth muscle cell proliferation and p70S6K phosphorylation through MEK/ERK and PI3K/Akt signaling pathways.
Keywords: airway remodeling, cell proliferation, FFAR1, free fatty acid, G protein-coupled receptor
INTRODUCTION
Obesity has recently been recognized as one of the major risk factors for asthma (60). In obese individuals, asthma symptoms tends to be more severe (18, 58), and the efficacy of standard medications for asthma is reduced (51). Previous studies have also demonstrated that obesity worsened the key features of asthma including airway hyperresponsiveness, inflammation (10, 44), and airway remodeling (1, 57).
It is generally accepted that airway remodeling is closely related to progression of airway hyperresponsiveness and the severity of asthma (64) and is characterized by increased airway smooth muscle mass within the airway wall that is mainly due to increased smooth muscle cell proliferation (2, 42). There are several signal transduction pathways associated with airway smooth muscle proliferation, i.e., the effects of mitogens are mediated through G protein-coupled receptors (GPCRs), receptor tyrosine kinases, and cytokine receptors (59, 64). Extracellular signal-regulated kinase (ERK) and phosphoinositide 3-kinase (PI3K)/Akt activation appear to be the dominant signal transduction pathways for these receptor-stimulated growth effects in airway smooth muscle cells (9, 64).
High-fat diet increases the risk of asthma (6, 55) and acutely impairs bronchodilator recovery in asthmatic patients (66). In obese individuals, plasma free fatty acid (FFA) levels are elevated because the increased adipose tissue mass releases more FFAs and FFA clearance may be reduced (5). Long chain FFAs such as oleic and linoleic acids act as endogenous ligands for the free fatty acid receptor 1 (FFAR1, also known as GPR40), which couples to both Gq and Gi proteins (29, 61) and the FFAR4 (GPR120, also known as O3FAR1), which couples to Gq (33). The expression of FFAR1 has been well established in pancreatic β cells (8) and contributes to insulin secretion (35), while FFAR4 is expressed in the intestine, adipocytes, macrophages, and the central nervous system and contributes to the secretion of glucagon-like peptide-1, adipocyte differentiation, and anti-inflammatory effects (12, 31, 49). In the airways, FFAR1 has been identified on human bronchial epithelial cells and reported to induce cell proliferation (26). Furthermore, we have recently identified the expression of both FFAR1 and FFAR4 in airway smooth muscle itself, and the activation of FFAR1 induces airway smooth muscle contraction through classical Gq coupling (46). However, it remains unclear whether the activation of the FFAR1 on airway smooth muscle cells leads to cell proliferation. Activation of FFAR1 induces ERK phosphorylation in the breast cancer cell lines stably expressing FFAR1 (62) and in the Flp-In T-REx 293 cell line overexpressing FFAR1 (61). The PI3K/Akt signaling pathway is also involved in oleic acid-stimulated vascular smooth muscle cell proliferation (69). These findings led us to hypothesize that FFA-induced stimulation of FFAR1 on airway smooth muscle cells could promote cell proliferation through ERK- and/or PI3K/Akt-mediated signal transduction.
In the present study, we first questioned whether FFAR ligands promoted airway smooth muscle cell proliferation and whether this involved FFAR1-mediated activation of ERK or Akt pathways. Subsequently, we pharmacologically dissected the signaling intermediates between Gi and Gq proteins and ERK or Akt phosphorylation to elucidate the signaling proteins involved.
METHODS
Materials.
Antibiotic-antimycotic, DMEM/F-12 medium, FBS, and Opti-MEM were obtained from Life Technologies. Gallein, GF109203X, GW9508, and rapamycin were obtained from Tocris Bioscience. Manumycin A was obtained from Cayman Chemical. Pertussis toxin was obtained from Calbiochem. Protease inhibitor cocktail III was obtained from EMD Millipore. YM-254890 was obtained from Wako Pure Chemical Industries. The following antibodies were obtained from Cell Signaling Technology: rabbit anti-phospho ERK1/2 (Thr 202/Tyr 204) polyclonal antibody (No. 9101), rabbit anti-ERK1/2 polyclonal antibody (No. 9102), rabbit anti-phospho c-Raf (Ser 338) monoclonal antibody (No. 9427), rabbit anti-phospho c-Raf (Ser 259) polyclonal antibody (No. 9421), rabbit anti-c-Raf monoclonal antibody (No. 53745), rabbit anti-phospho Akt (Ser 473) monoclonal antibody (No. 4060), rabbit anti-Akt polyclonal antibody (No. 9272), rabbit anti-phospho-p70S6K (Thr 389) polyclonal antibody (No. 9205), rabbit anti-p70S6K polyclonal antibody (No. 9202), rabbit anti-phospho S6 ribosomal protein (Ser 240/244) monoclonal antibody (No. 5364), and rabbit anti-S6 ribosomal protein monoclonal antibody (No. 2217). Secondary horseradish peroxidase-conjugated anti-rabbit IgG (NA934V) was purchased from GE healthcare. HitHunter cAMP XS+ assay kit was purchased from DiscoveRx. The MTT assay kit was purchased from Cayman Chemical. All other chemicals were obtained from Sigma-Aldrich unless otherwise stated.
Cell culture.
Primary cultured human airway smooth muscle (HASM) cells obtained from Lonza (cc-2576 and 00194850) were derived from three subjects (subject 1: 60-yr-old male Caucasian; subject 2: 56-yr-old male Caucasian; and subject 3: 27-yr-old male asthmatic Caucasian). HASM cells were grown in DMEM/F-12 cell culture medium, supplemented with 10% FBS and an antibiotic-antimycotic mix (100 units/ml penicillin G sodium, 100 µg/ml streptomycin sulfate, and 0.25 µg/ml amphotericin B) in an atmosphere of 5% CO2-95% air at 37°C. For cell proliferation assays, HASM cells were incubated with DMEM/F-12 cell culture medium containing 0.5% FBS for 72 h before the assays. Passages 3–8 were used for all experiments.
Isolation of airway smooth muscle from rat trachea.
All studies were approved by the Committee on the Ethics of Animal Experiments in Tohoku University School of Medicine, and they were carried out in accordance with both the Guidelines for Animal Experiments issued by the Tohoku University and The Law (No. 105) and Notification (No. 6) issued by the Japanese government. Adult male Wistar rats (~500 g body wt) were killed by CO2, and the entire trachea was surgically removed, carefully dissected free of adherent connective tissue under a microscope, and immersed in PBS before use.
Cell proliferation assay.
Proliferation of HASM cells was measured using the bromodeoxyuridine (BrdU) cell proliferation ELISA kit (Roche Diagnostics) according to the manufacturer’s instructions. Briefly, after the HASM cells were cultured in black-walled 96-well clear bottom plates at 5,000 cells/well in DMEM/F-12 cell culture medium containing 0.5% FBS for 72 h, the cells were serum-starved for 24 h. The cell culture medium was changed to DMEM/F-12 containing 0.5% FBS, and then incubated with long-chain FFAs (oleic acid or linoleic acid; 10 µM) (DMSO vehicle final concentration of 0.05%) or a selective agonist of FFAR1 (GW9508; 20 µM) (DMSO vehicle final concentration of 0.1%) for 48 h at a final volume of 100 µl/well in the presence or absence of the MEK inhibitor U0126 (5 µM; added to the wells 2 h before the addition of FFAs or GW9508) (DMSO vehicle final concentration of 0.1%) or the PI3K inhibitor LY294002 (3 µM; added to the wells 60 min before the addition of FFAs or GW9508) (DMSO vehicle final concentration of 0.03%). The final concentration of DMSO was set to 0.2% (vol/vol) in every well. The BrdU-labeling solution (10 µl/well, final concentration: 10 µM) was added, and the cells were reincubated for 12 h at 37°C. The medium was aspirated, and the cells were dried for 15 min. The cells were then fixed with FixDenat solution (200 µl/well) and incubated for 30 min at room temperature. After thorough removal of the fixative solution, anti-BrdU-peroxidase working solution (100 µl/well) was added and incubated for 90 min at room temperature. The wells were rinsed three times with washing solution (200 µl/well), and the substrate solution (100 µl/ml) was added and incubated at room temperature for 5 min. The chemiluminescent signals were detected using a multimode microplate reader (Appliskan, Thermo Fisher Scientific).
Cell viability assay.
HASM cell viability after treatment with LY294002 or U0126 was measured using the MTT assay kit (Cayman Chemical) according to the manufacturer’s instructions. Briefly, HASM cells were seeded in a 96-well plate (5000 cells/well) in DMEM/F-12 cell culture medium containing 0.5% FBS for 24 h. Then, the cells were incubated with or without U0126 (5 µM), LY294002 (3 µM), oleic acid (10 µM), linoleic acid (10 µM), or GW9508 (20 µM) for 48 h in a final volume of 100 µl/well. The final concentration of DMSO was set to 0.1% (vol/vol) in every well. Ten microliters of MTT reagent were added to each well and the cells were reincubated for 4 h at 37°C in CO2 incubator. The medium was aspirated, and 100 µl of crystal dissolving solution were added to each well. The absorbance of each sample at 570 nm was measured using a multimode microplate reader (Appliskan; Thermo Fisher Scientific).
Small interfering RNA transfection.
The predesigned small interfering (si)RNA targeting human FFAR1 (Silencer Select Predesigned siRNA No. s194466), human FFAR4 (predesigned to target both FFAR4 transcript variant 1 and variant 2; Silencer Select Predesigned siRNA No. s50347), and a nontargeting siRNA (as negative control; No. 4390843) were obtained from Ambion. HASM cells cultured in DMEM/F-12 growth medium (supplemented with 10% FBS) without antibiotics were grown in six-well plates (seeded in 2.5 ml of the growth medium) until they reached 50% confluence.
Cells were then transfected with the predesigned siRNA targeting human FFAR1, FFAR4, or the nontargeting siRNA using Lipofectamine RNAiMAX (Life Technologies) in serum-free Opti-MEM according to the manufacturer’s instructions. Briefly, Lipofectamine RNAiMAX (Life Technologies) was diluted with Opti-MEM in a 1:50 ratio. Opti-MEM was used to dilute siRNAs at a ratio of 1:100. Diluted siRNA and reagent were mixed in one tube and incubated for 20 min at room temperature to allow the siRNA-Lipofectamine RNAiMAX complexes to form. The siRNA-Lipofectamine RNAiMAX complexes (typically 500 µl/well) were added to the wells and incubated at 37°C in a 5% CO2 incubator for 24 h. The final medium volume was 3 ml, and a final siRNA concentration was 10 nM. At 24 h after transfection, the antibiotic-free medium was replaced with standard growth medium. Once the cells reached near confluence (2–3 days after transfection), the cells were used for subsequent immunoblot analysis.
Immunoblot analysis.
HASM cells with or without siRNA transfection were grown to near confluence in six-well plates and growth arrested for 24 h in serum-free medium. The cells were then stimulated with long-chain FFAs (oleic acid or linoleic acid; 0.5–20 µM) or a selective agonist of FFAR1 (GW9508; 0.5–20 µM) for indicated times (5–120 min). For experiments on rat airway smooth muscle ex vivo tissues, the tissues were incubated with or without either oleic acid (20 µM) or GW9508 (20 µM) for 20 min in PBS at 37°C.
In separate experiments, HASM cells were initially pretreated with 10 µM gallein (Gβγ signaling inhibitor; 60 min) (45), 100 nM GF109203X [protein kinase C (PKC) inhibitor; 60 min] (47), 3 µM LY294002 (PI3K inhibitor; 60 min) (9), 3 µM manumycin A (ras inhibitor; 3 h) (63), 100 ng/ml pertussis toxin (Gαi-specific inhibitor; 4 h) (45), 10 µM PP1 (Src inhibitor; 60 min) (52), 1 µM rapamycin [mammalian target of rapamycin (mTOR) inhibitor; 60 min] (32), 5 µM U0126 (MEK inhibitor; 2 h) (9), 5 µM U73122 [phospholipase C (PLC) inhibitor; 30 min] (45), or 1 µM YM-254890 (Gαq-specific inhibitor; 60 min) (34) and then treated for 10 min with 10 µM oleic acid, or 20 µM GW9508. The DMSO vehicle final concentrations were as follows: gallein (0.013%), GF109203X (0.001%), LY294002 (0.03%), manumycin A (0.033%), PP1 (0.02%), rapamycin (0.01%), U0126 (0.1%), YM-254890 (0.01%), oleic acid (0.05% at 10 µM), linoleic acid (0.05% at 10 µM), and GW9508 (0.1% at 20 µM). The final DMSO concentration in wells did not exceed 0.2% in this study. In preliminary studies, we demonstrated that less than 0.2% DMSO alone did not exert any effect on phosphorylation of ERK, Akt, or p70S6K in HASM cells. After treatments, cells were washed twice with ice-cold PBS and lysed in ice-cold RIPA buffer (CST No. 9806; 20 mM Tris·HCl, pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Nonidet P-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, and 1 µg/ml leupeptin) supplemented with 1 mM PMSF and a 1:200 dilution of protease inhibitor cocktail III. For experiments on rat airway smooth muscle ex vivo tissues, the tissue was homogenized (Tekmar Ultra Turrax T25 high-speed homogenizer set at top speed for 30 s) in ice-cold RIPA buffer supplemented with 1 mM PMSF and a 1:200 dilution of protease inhibitor cocktail III. Lysed HASM cells or homogenized rat airway smooth muscle tissue were centrifuged at 15,000 g for 15 min at 4°C, and an aliquot of the supernatant was subjected to protein analysis. The protein concentration of each sample was determined using Pierce BCA reagents (Thermo Fisher Scientific), using BSA as a control. Samples were solubilized by heating to 95°C for 10 min in Laemmli sample buffer (final concentrations: 50 mM Tris·HCl pH 6.8, 2.5% SDS, 6% glycerol, 2.5% 2-mercaptoethanol, and bromophenol blue) before use. Cell lysates containing equal amounts of protein (20 µg) were electrophoresed (7.5% or 10% Mini-Protean TGX precast gel; Bio-Rad) and transferred to PVDF membranes using a Trans-Blot Turbo Transfer System (Bio-Rad) according to the manufacturer’s instruction. The PVDF membranes were blocked for 1 h at room temperature with 5% ECL prime membrane blocking reagent (RPN418; GE Healthcare) in Tris-buffered saline with 0.1% Tween 20 (TBST) and were then probed with antibodies directed against the anti-phospho ERK1/2 (Thr 202/Tyr 204) (rabbit polyclonal, 1:2,000; CST No. 9101), anti-phospho Akt (Ser 473) (rabbit monoclonal, 1:1,000; CST No. 4060), anti-phospho c-Raf (Ser 338) (rabbit monoclonal, 1:1,000; CST No. 9427), anti-phospho c-Raf (Ser 259) (rabbit polyclonal, 1:1,000; CST No. 9421), anti-phospho p70S6K (Thr 389) (rabbit polyclonal, 1:1,000; CST No. 9205), or anti-phospho S6 ribosomal protein (Ser 240/244) (rabbit monoclonal 1:1,000; CST No. 5364) overnight at 4°C. After being washed three times with TBST, membranes were incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary anti-rabbit antibodies (1:5,000; NA934V; GE Healthcare) diluted in 1% membrane blocking reagent in TBST. The signals from the immunoreactive bands were detected by ECL prime (GE Healthcare) according to the manufacturer’s recommendations, and the signal was captured using a chemiluminescent image analyzer (LAS 4000 Mini; GE Healthcare). To confirm equal protein loading, the same PVDF membranes were stripped and reprobed with anti-ERK1/2 (rabbit polyclonal, 1:2,000; CST No. 9102), anti-Akt (rabbit polyclonal, 1:1,000; CST No. 9272), anti-c-Raf (rabbit monoclonal, 1:1,000; CST No. 53745), anti-p70S6K (rabbit polyclonal, 1:1,000; CST No. 9202), or anti-S6 ribosomal protein (rabbit monoclonal 1:1000; CST No. 2217). The band intensities were measured using ImageJ software (National Institues of Health) and are expressed as a ratio of the phosphorylated/total protein.
Cyclic AMP assays.
Cyclic AMP (cAMP) production in primary cultured HASM cells was measured using a HitHunter cAMP XS+ assay kit according to the manufacturer's instructions. Briefly, HASM cells in white-walled 96-well plates were washed twice with warm PBS (37°C). In some experiments, the cells were pretreated for 4 h with pertussis toxin (100 ng/ml) in cell culture medium before being washed with PBS. The cells were incubated for 15 min at 37°C in the absence (basal activity) or presence of 10 µM forskolin ± 10 µM oleic acid. Then, the cAMP XS antibody reagent followed by the mixture of enzyme donor/lysis/chemiluminescence working solution was added to each well. After incubation for 60 min at room temperature, cells were further incubated with the enzyme acceptor reagent for 3 h at room temperature. Luminescence signals were detected using a multimode microplate reader (Appliskan; Thermo Fisher Scientific).
Statistical analysis.
The data were analyzed with two-tailed paired Student’s t-test when comparing means between two groups or repeated-measures ANOVA followed by Bonferroni posttest when comparing multiple groups using GraphPad Prism 6 for Mac OS X (GraphPad Software). Data are presented as means ± SE. P < 0.05 was considered significant.
RESULTS
Long-chain FFAs and GW9508-induced HASM cell proliferation through MEK/ERK and PI3K/Akt pathways.
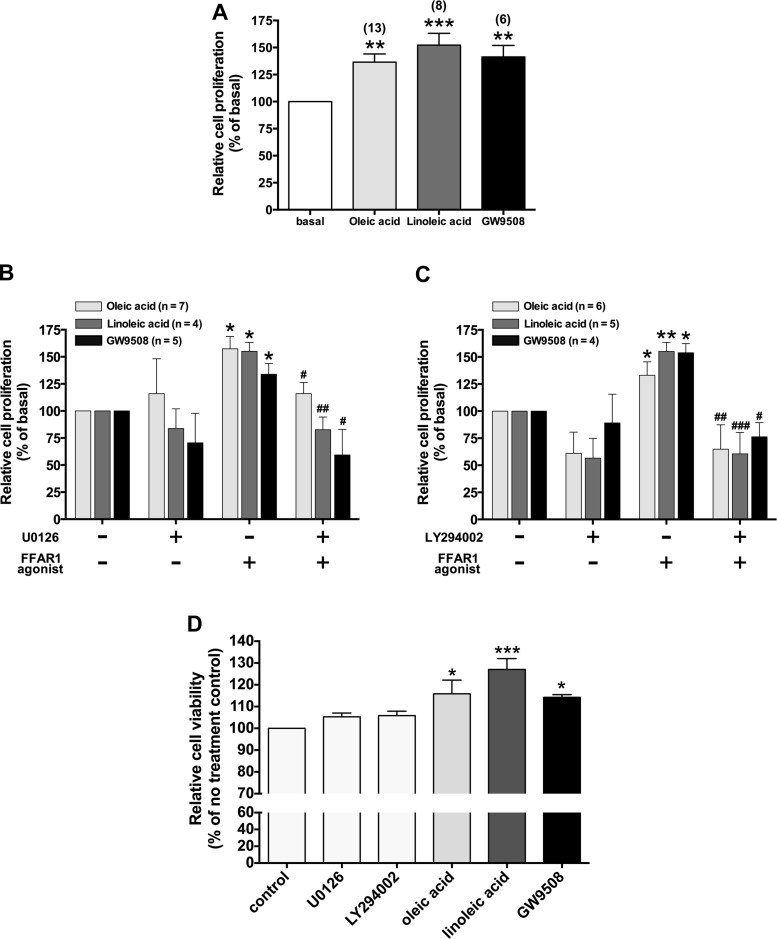
In HASM cells, mitogens act via dual signaling pathways, both ERK- and PI3K-dependent pathways, to control cell growth (9). Oleic acid induces cell proliferation in vascular smooth muscle cells via the PI3K/Akt pathway (69). Oleic acid also induces ERK phosphorylation in breast cancer cells (62). Moreover, in the Flp-In T-REx 293 cell line overexpressing FFAR1, activation of FFAR1 induces ERK phosphorylation (61). Therefore, we first examined HASM cell proliferation following exposure to long-chain FFAs (oleic acid and linoleic acid) or a selective agonist of FFAR1 (GW9508). Treatment of the HASM cells with oleic acid (10 µM), linoleic acid (10 µM), or GW9508 (20 µM) for 48 h significantly induced cell proliferation in HASM cells (oleic acid: 136 ± 7.50% of basal level; P < 0.01, n = 13; linoleic acid: 152 ± 10.9% of basal level; P < 0.001, n = 8; GW9508: 141 ± 10.7% of basal level; P < 0.01, n = 6) (Fig. 1A). We further assessed whether both MEK/ERK- and PI3K/Akt signaling pathways contribute to FFAR1-mediated HASM cell proliferation. Pretreatment of the HASM cells with either a MEK inhibitor U0126 (5 µM; 2 h) or a PI3K inhibitor LY294002 (3 µM; 60 min) markedly inhibited the HASM cell proliferation in response to oleic acid, linoleic acid, and GW9508 (Fig. 1, B and C). HASM cell viability measured with the MTT assay did not show any signs of cytotoxicity in cells treated with U0126 (5 µM), LY294002 (3 µM), oleic acid (10 µM), linoleic acid (10 µM), or GW9508 (20 µM) for 48 h but showed significant increase in absorbance at 570 nm in cells treated with oleic acid, linoleic acid, and GW9508 (Fig. 1D).
Fig. 1.
A: effects of 48-h treatment of the long-chain free fatty acids (oleic acid and linoleic acid) or a selective agonist of FFAR1 (GW9508) on primary cultured human airway smooth muscle (HASM) cells proliferation. Cell proliferation was assayed by the bromodeoxyuridine (BrdU) incorporation assay. Data are shown as percentages of cell proliferation compared with no treatment controls and represent means ± SE. Numbers of experiments are shown in parentheses. B and C: effect of pretreatment of HASM cells with the MEK inhibitor U0126 (5 µM for 2 h) (B) or the phosphoinositide 3-kinase (PI3K) inhibitor LY294002 (3 µM for 60 min) (C) on HASM cells proliferation stimulated by long-chain free fatty acids (oleic acid or linoleic acid; 10 µM each) or a selective agonist of FFAR1 (GW9508; 20 µM) for 48 h. Data are shown as percentages of cell proliferation compared with no treatment controls and represent means ± SE. *P < 0.05; **P < 0.01; ***P < 0.001, compared with no treatment control. #P < 0.05; ##P < 0.01; ###P < 0.001, compared with FFAR1 agonist alone. D: HASM cell viability analysis with MTT assay after 48-h treatment with U0126 (5 µM), LY294002 (3 µM), oleic acid (10 µM), linoleic acid (10 µM), or GW9508 (20 µM); n = 5. Data are shown as percentages of absorbance at 570 nm compared with no treatment control and represent means ± SE. *P < 0.05; ***P < 0.001, compared with no treatment control.
Long-chain FFAs and GW9508 induce phosphorylation of ERK and Akt in HASM cells and rat airway smooth muscle ex vivo tissues.
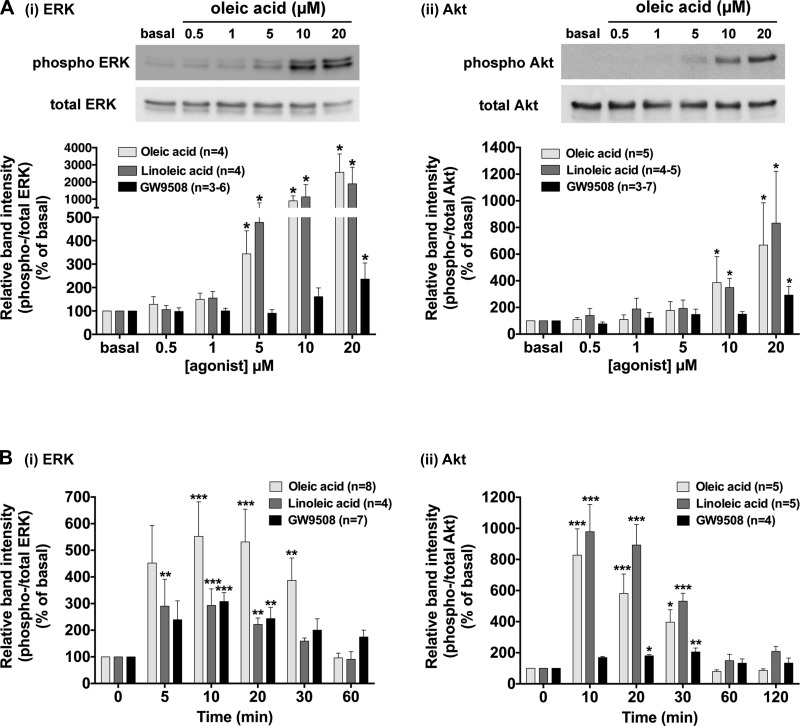
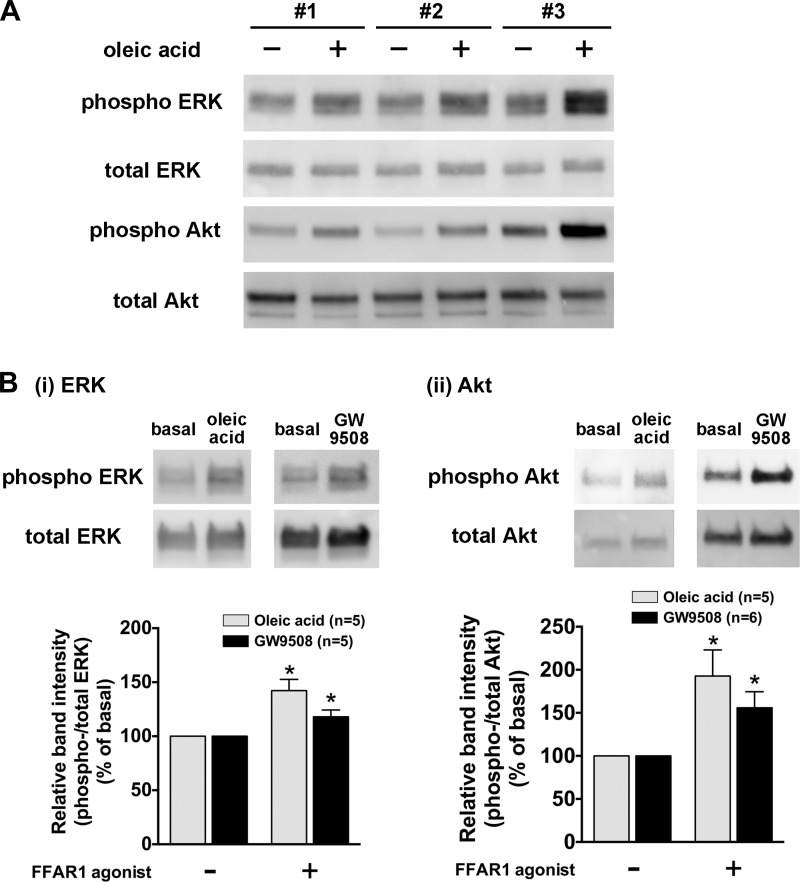
We next examined whether the stimulation of HASM cells or freshly isolated rat airways with long-chain FFAs (oleic acid and linoleic acid) or a selective agonist of FFAR1 (GW9508) phosphorylates ERK or Akt as measured by immunoblotting. These ligands significantly induced phosphorylation of ERK and Akt in HASM cells in a concentration-dependent manner (Fig. 2A). A relatively higher concentration of GW9508 (20 µM) was required to significantly induce the phosphorylation of ERK and Akt in HASM cells compared with the concentrations of oleic acid or linoleic acid required for these phosphorylation events. Phosphorylation of ERK and Akt induced by these ligands was maximal at 10–20 min and then slowly declined to basal levels within 60 min (Fig. 2B). From these data, we chose the concentration of 10 µM of oleic acid and linoleic acid, or 20 µM of GW9508, and duration of treatment of 10 min in the following experiments. The reproducibility of oleic acid-stimulated phosphorylation of ERK and Akt in HASM cells was further confirmed in primary cultured HASM cells derived from three different donors (subject 1: 60-yr-old male Caucasian; subject 2: 56-yr-old male Caucasian; and subject 3: 27-yr-old male asthmatic Caucasian) (Fig. 3A). These results demonstrate that both ERK and Akt are phosphorylated in HASM cells following treatment with the FFAR ligands oleic acid, linoleic acid or GW9508. Furthermore, oleic acid or GW9508 (20 µM for 20 min) significantly induced phosphorylation of ERK and Akt in rat airway smooth muscle ex vivo tissues (Fig. 3B).
Fig. 2.
Effects of long-chain free fatty acids (oleic acid or linoleic acid) or a selective agonist of FFAR1 (GW9508) on the phosphorylation of ERK and Akt in cultured HASM cells. Cells were stimulated with oleic acid, linoleic acid, or GW9508, and subsequently cell lysates were processed to detect phosphorylated (A, top) and total (A, bottom) levels of ERK or Akt by immunoblot. A: concentration-dependent effect of oleic acid, linoleic acid, and GW9508 (0.5–20 µM; 10 min) on the phosphorylation of ERK (i) and Akt (ii) in cultured HASM cells. B: time-course effect of oleic acid (10 µM), linoleic acid (10 µM), or GW9508 (20 µM) on the phosphorylation of ERK (i) and Akt (ii) in cultured HASM cells. Data are shown as a ratio of phosphorylated to total ERK or Akt and expressed relative to basal (i.e., no treatment control) ratios and are expressed as means ± SE. *P < 0.05; **P < 0.01; ***P < 0.001, compared with basal (time 0). Numbers of experiments are shown in parentheses.
Fig. 3.
A: representative immunoblot analysis of oleic acid-stimulated phosphorylation of ERK and Akt in primary cultured HASM cells from 3 different donors (subject 1: 60-yr-old male Caucasian; subject 2: 56-yr-old male Caucasian; and subject 3: 27-yr-old male asthmatic Caucasian). Images are representative of at least 3 independent immunoblot analyses. B: effects of oleic acid or GW9508 on the phosphorylation of ERK (i) and (ii) in rat tracheal smooth muscle. Rat tracheal smooth muscle tissue was stimulated with oleic acid (20 µM) or GW9508 (20 µM) for 20 min, and subsequently the tissue homogenates were processed to detect phosphorylated (top) and total (bottom) levels of ERK or Akt by immunoblot. Phosphorylation of ERK or Akt is presented as a ratio of phosphorylated to total ERK or Akt and expressed relative to basal ratios. Data represent means ± SE. *P < 0.05, compared with basal.
Involvement of G protein-coupled FFAR1 in oleic acid-induced phosphorylation of ERK and Akt.
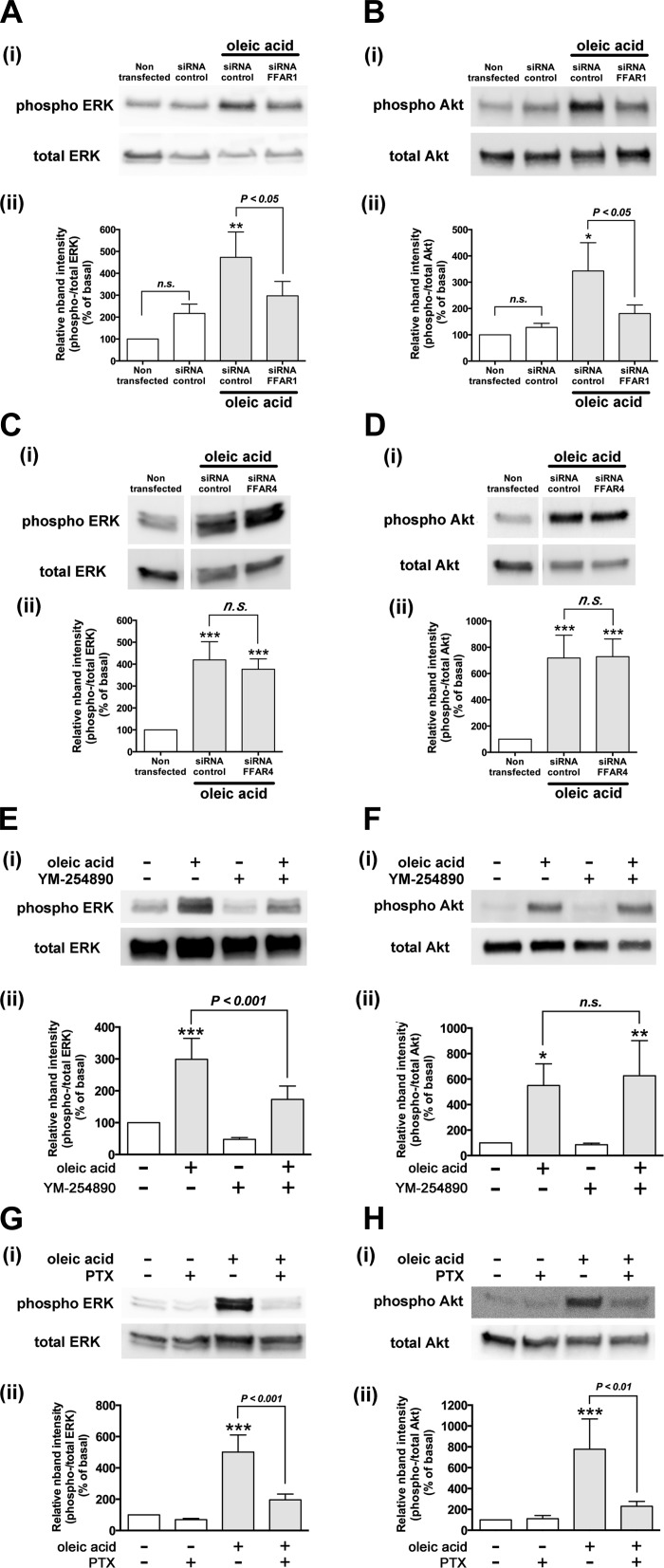
Previous findings have suggested that oleic acid stimulates two subtypes of FFA receptors, FFAR1 and FFAR4 (8, 65), and that its pEC50 value for FFAR1 and FFAR4 is 5.7 and 4.7, respectively (35, 46, 65). We have reported that both FFAR1 and FFAR4 are expressed on HASM cells (46). To confirm oleic acid (10 µM)-induced phosphorylation of ERK and Akt in HASM cells is mediated through FFAR1, HASM cells were treated with human FFAR1- or FFAR4-specific siRNA. The knockdown of FFAR1 and FFAR4 in HASM cells was confirmed by RT-PCR (data not shown). Immunoblot analyses showed that the oleic acid (10 µM)-stimulated phosphorylation of ERK and Akt was significantly decreased in HASM cells transfected with FFAR1-specific siRNA compared with the cells transfected with nontargeting siRNA (ERK; P < 0.05, n = 4, Akt; P < 0.05, n = 3) (Fig. 4, A and B). In contrast, the knockdown of FFAR4 in HASM cells with FFAR4-specific siRNA did not affect oleic acid (10 µM)-stimulated phosphorylation of ERK (n.s., n = 6) or Akt (n.s., n = 8) compared with the cells treated with nontargeting siRNA (Fig. 4, C and D). The treatment of HASM cells with nontargeting siRNA by itself did not significantly change the basal phosphorylation levels of ERK and Akt compared with the nontransfected HASM cells (Fig. 4, A and B). These results demonstrate that FFAR1 but not FFAR4 mediate oleic acid (10 µM)-induced ERK and Akt phosphorylation in HASM cells. Therefore, we selected to use oleic acid (10 µM) as a natural agonist of FFAR1 in the following experiments.
Fig. 4.
A–D: involvement of FFAR1 in oleic acid-induced phosphorylation of ERK and Akt in HASM cells. HASM cells were transfected with either control nontargeting small-interfering RNA (siRNA), FFAR1-specific siRNA, or FFAR4-specific siRNA 3 days before analyses and then stimulated with oleic acid (10 µM, 10 min). The cell lysates were processed to detect phosphorylated (Ai–Hi, top) and total (Ai–Hi, bottom) levels of ERK or Akt by immunoblot. Aii–Hii: graphical analysis. A and B: the effect of downregulation of FFAR1 by siRNA on oleic acid (10 µM, 10 min)-induced phosphorylation of ERK (n = 4) (A) and Akt (n = 3) (B) in HASM cells. *P < 0.05; **P < 0.01, compared with the HASM cells transfected with nontargeting siRNA control without oleic acid stimulation. C and D: the effect of downregulation of FFAR4 by siRNA on oleic acid (10 µM, 10 min)-induced phosphorylation of ERK (n = 6) (C) and Akt (n = 8) (D) in HASM cells. ***P < 0.001, compared with the nontransfected HASM cells without oleic acid stimulation. E–H: effects of inhibitors of either Gαq or Gαi protein on oleic acid-induced phosphorylation of ERK and Akt in HASM cells. HASM cells were pretreated with or without inhibitors and then stimulated with oleic acid (10 µM, 10 min). The cell lysates were processed to detect phosphorylated (top) and total (bottom) levels of ERK or Akt by immunoblot. E and F: effect of Gαq-specific inhibitor YM-254890 (1 µM for 30 min) on oleic acid-induced phosphorylation of ERK (n = 4) (E) or Akt (n = 6) (F) in HASM cells. G and H: effect of Gαi-specific inhibitor pertussis toxin (PTX; 100 ng/ml for 4 h) on oleic acid-induced phosphorylation of ERK (n = 8) (G) or Akt (n = 5) (H) in HASM cells. Phosphorylation of ERK and Akt is presented a ratio of phosphorylated to total ERK or Akt and expressed relative to basal ratios. Data represents means ± SE. *P < 0.05; **P < 0.01; ***P < 0.001, compared with basal. White vertical spaces between the lanes in (C and D) indicate that these lanes were located on the same immunoblot but were not located in neighboring lanes on the original gel and immunoblot image.
FFAR1 is a G protein-coupled receptor, which couples to both Gαq and Gαi proteins (8, 22, 61, 68). To examine whether the Gαq and Gαi proteins were involved in the signaling cascade of FFAR1-mediated phosphorylation of ERK and Akt, HASM cells were pretreated with the Gαq-specific inhibitor YM-254890 (1 µM; 30 min) or the Gi-protein inhibitor pertussis toxin (100 ng/ml; 4 h). Oleic acid (10 µM)-stimulated ERK phosphorylation was significantly inhibited by pretreatment with YM-254890 (P < 0.001 compared with oleic acid alone, n = 4; Fig. 4E), while Akt phosphorylation was not affected (n.s., n = 6; Fig. 4F). In contrast, pertussis toxin abolished the oleic acid-stimulated phosphorylation of both ERK (P < 0.001, n = 8; Fig. 4G) and Akt (P < 0.001, n = 5; Fig. 4H). These results demonstrate that oleic acid-FFAR1 couples through Gi to both ERK and Akt phosphorylation and couples through Gq to ERK but not Akt phosphorylation in HASM cells.
Effects of inhibitors of Gβγ subunits, PLC, PKC, and Src kinase on oleic acid-induced phosphorylation of ERK in HASM cells.
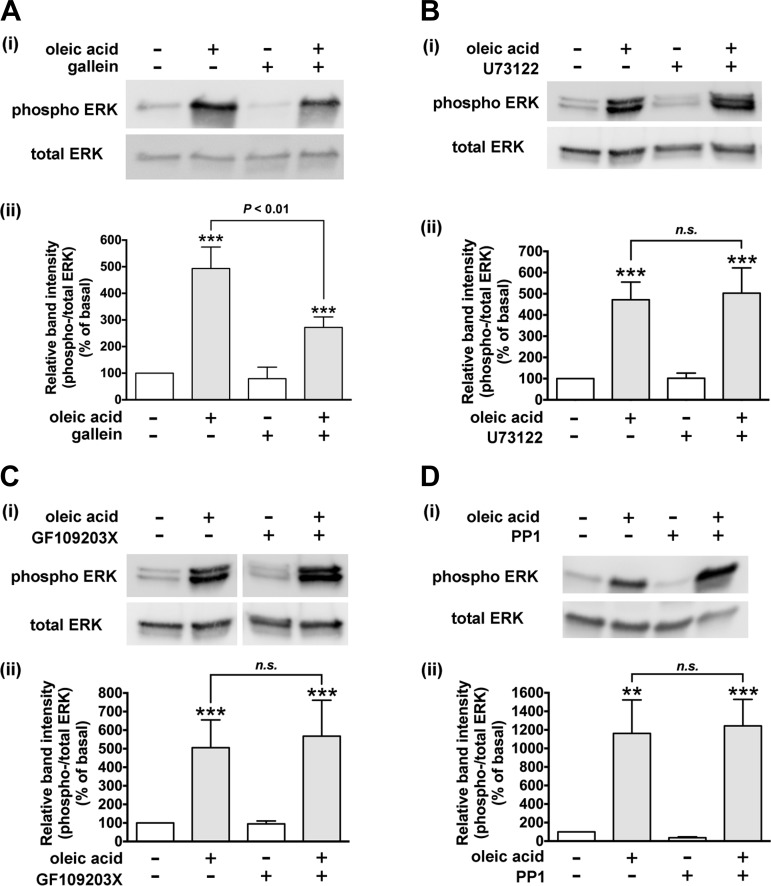
Gβγ subunits dissociated from Gi or Gq stimulate ERK signaling (23). To examine the involvement of Gβγ subunits in FFAR1-mediated ERK phosphorylation, we measured oleic acid-stimulated phosphorylation of ERK in the presence or absence of the Gβγ signaling inhibitor gallein. Pretreatment of the HASM cells with gallein (10 µM; 60 min) significantly inhibited the oleic acid (10 µM; 10 min)-stimulated ERK phosphorylation (P < 0.01, n = 8; Fig. 5A). These results demonstrate that Gβγ signaling is involved in oleic acid-FFAR1-mediated ERK phosphorylation; however, these results do not distinguish from which heterotrimeric G protein the Gβγ subunits originate.
Fig. 5.
Effect of inhibitors of Gβγ subunits, phospholipase C-β (PLC-β), protein kinase C (PKC), or Src on oleic acid-induced phosphorylation of ERK in HASM cells. Cells were pretreated with Gβγ signaling inhibitor gallein (10 µM for 60 min; n = 8) (A), phospholipase C-β inhibitor U73122 (5 µM for 30 min; n = 7) (B), PKC inhibitor GF109203X (100 nM for 60 min; n = 4) (C), or Src inhibitor PP1 (10 µM for 60 min; n = 5) (D) before treatment with oleic acid (10 µM) for 10 min. The cell lysates were processed to detect phosphorylated (Ai–Di, top) and total (Ai–Di, bottom) levels of ERK by immunoblot. Aii–Dii: graphical analysis. Phosphorylation of ERK is presented as a ratio of phosphorylated to total ERK and expressed relative to basal ratios. Data represent means ± SE. **P < 0.01; ***P < 0.001, compared with basal. White vertical spaces between the lanes in C indicate that these lanes were located on the same immunoblot but were not located in neighboring lanes on the original gel and immunoblot image.
Gβγ subunits dissociated from Gi proteins can also cross activate PLC-β (21, 45), which is classically known to be activated by Gαq and Gβγ subunits dissociated from Gq. The PLC-β-mediated activation of ERK can occur by at least two signaling pathways: 1) the PLC-β/PKC/c-Raf/MEK/ERK pathway: activation of PLC-β induces PKC-mediated activation of c-Raf, which leads to ERK phosphorylation (23); or 2) the PLC-β/IP3/Ca2+/Pyk2/Src/ras/c-Raf/MEK/ERK pathway: activation of PLC-β leads to inositol 1,4,5-triphosphate (IP3)-mediated increases in intracellular Ca2+ (45), and Ca2+-calmodulin-mediated activation of Pyk2 kinase. The Pyk2 kinase activates Src, which could lead to activation of ras and subsequently phosphorylation of ERK (23, 39). Therefore, we examined whether PLC-β, PKC, and Src were involved in the FFAR1-mediated phosphorylation of ERK. However, oleic acid-stimulated phosphorylation of ERK was not inhibited by pretreatment of HASM cells with either the PLC-β inhibitor U73122 (5 µM, 30 min) (n.s., n = 7), the PKC inhibitor GF109203X (100 nM, 60 min) (n.s., n = 4), or the Src inhibitor PP1 (10 µM, 60 min) (n.s., n = 5) (Fig. 5, B, C, and D). These results demonstrate that PLC-β, PKC, and Src are not involved in the FFAR1-mediated phosphorylation of ERK.
Oleic acid-induced cAMP activity and c-Raf phosphorylation in HASM cells.
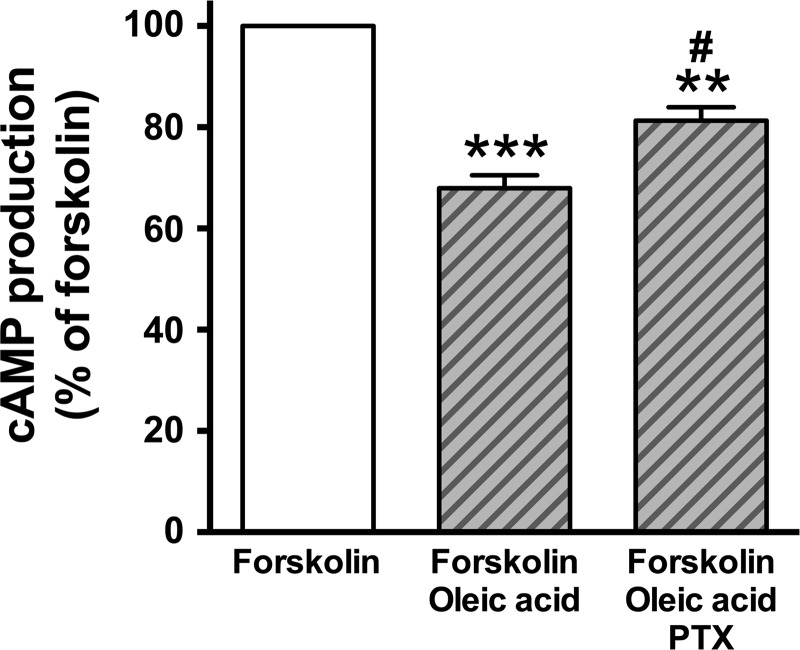
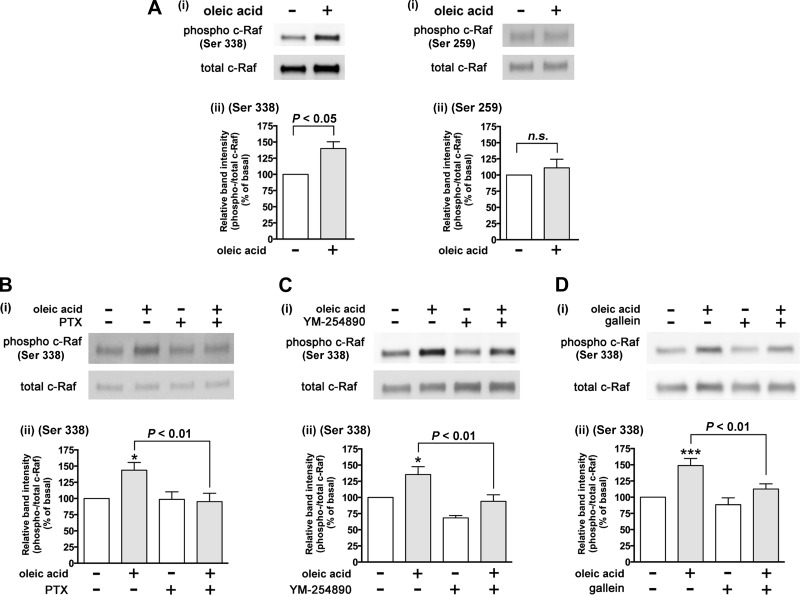
One known signaling pathway that links Gi to ERK is via a Gi effect on PKA. The stimulation of Gi-coupled receptors leads to inhibition of adenylyl cyclase activity resulting in a reduction in cellular cAMP production (4, 47). Gi coupled receptor-dependent activation of ERK involves an inhibitory effect on cAMP levels, which leads to the decreased PKA activity. PKA-mediated phosphorylation of c-Raf Ser 259 leads to inhibition of phosphorylation of c-Raf at Ser 338 (16, 17). In contrast, when PKA activity is decreased, the inhibitory effect of PKA-mediated phosphorylation of c-Raf on Ser 259 phosphorylation is relieved, thus potentiating the phosphorylation of c-Raf at Ser 338, which subsequently phosphorylates ERK (Gi/adenylyl cyclase/cAMP/PKA/c-Raf/ERK pathway) (13, 16, 17, 23, 27, 54). To examine whether oleic acid inhibits cAMP production in cultured HASM cells through Gi-coupled FFAR1, we measured cAMP activity in the presence or absence of the oleic acid in HASM cells. Oleic acid (10 µM) significantly inhibited the forskolin-stimulated cAMP activity (P < 0.001, n = 6), which was significantly attenuated by pretreatment with pertussis toxin (100 ng/ml; 4 h) (P < 0.05, n = 6) (Fig. 6). Moreover, oleic acid did not induce phosphorylation of c-Raf at Ser 259 (n.s., n = 5), which allowed for the phosphorylation c-Raf at Ser 338 (P < 0.05, n = 6) (Fig. 7A). These results are consistent with oleic acid-FFAR1 coupling through Gi to inhibit adenylyl cyclase/cAMP/PKA to release PKA inhibition of c-Raf as one of the cellular mechanism for Gi-coupled FFAR1 increasing c-Raf/ERK phosphorylation in HASM cells.
Fig. 6.
Effect of oleic acid (10 µM) on forskolin (10 µM)-stimulated cAMP activity in HASM cells. Cells were pretreated with pertussis toxin (PTX; 100 ng/ml) for 4 h before simultaneous treatment with oleic acid and forskolin for 15 min. n = 6. Data represent means ± SE. **P < 0.01; ***P < 0.001, compared with forskolin alone. #P < 0.05, compared with forskolin + oleic acid.
Fig. 7.
Effects of oleic acid on phosphorylation of c-Raf in cultured HASM cells. Cells were stimulated with oleic acid, and subsequently cell lysates were processed to detect phosphorylated and total levels of c-Raf by immunoblot. For A–D: i: immunoblot analysis; ii: graphical analysis. A: effects of oleic acid (10 µM) on the phosphorylation of c-Raf at either Ser 338 (n = 6) or Ser 259 (n = 5) in HASM cells. B–D: effects of inhibitors of either Gαi or Gαq protein or Gβγ subunits on oleic acid-induced phosphorylation of c-Raf at Ser 338 in HASM cells. Cells were pretreated with the Gαi-specific inhibitor pertussis toxin (PTX; 100 ng/ml for 4 h; n = 5; B), Gαq-specific inhibitor YM-254890 (1 µM for 30 min; n = 8; C), or Gβγ signaling inhibitor gallein (10 µM for 30 min; n = 7; D) before treatment with oleic acid (10 µM) for 10 min. Phosphorylation of c-Raf is presented as a ratio of phosphorylated to total c-Raf and then normalized to basal levels. Data represent means ± SE. *P < 0.05; ***P < 0.001, compared with basal.
We further examined whether Gαi and Gαq protein and Gβγ subunits were involved in the FFAR1-mediated phosphorylation of c-Raf, an upstream molecule of MEK/ERK pathway. Oleic acid (10 µM; 10 min)-stimulated c-Raf Ser 338 phosphorylation was significantly inhibited by the Gi-protein inhibitor pertussis toxin (100 ng/ml; 4 h) (P < 0.01, n = 5), the Gαq-specific inhibitor YM-254890 (1 µM; 30 min) (P < 0.01, n = 8), and the Gβγ signaling inhibitor gallein (10 µM; 60 min) (P < 0.01, n = 7) (Fig. 7, B, C, and D). Collectively, these results suggest that Gi and Gq protein and Gβγ subunits dissociated from FFAR1 activate the c-Raf/ERK signaling pathway, which is not mediated via PLC-β, PKC, and Src.
Effects of inhibitors of Gβγ subunits, Src, and ras on oleic acid-induced phosphorylation of Akt in HASM cells.
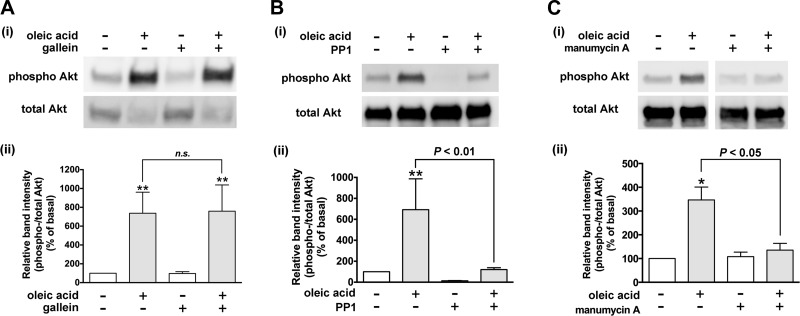
Akt is activated in response to Gi-coupled receptors through activation of PI3K, which can be activated by Gβγ subunits or ras (37, 48). In addition, the Gαi protein is also able to activate Akt through Src (11). Therefore, we examined whether the Gβγ subunits, Src, or ras is involved in oleic acid-stimulated Akt phosphorylation in HASM cells. Pretreatment of HASM cells with the Gβγ signaling inhibitor gallein (10 µM; 60 min) did not affect the oleic acid (10 µM; 10 min)-induced Akt phosphorylation in HASM cells (n.s., n = 7) (Fig. 8A). In contrast, pretreatment of the cells with the ras inhibitor manumycin A (3 µM; 3 h) or the Src inhibitor PP1 (10 µM; 60 min) completely blocked oleic acid-induced Akt phosphorylation (P < 0.01, n = 8 and P < 0.05, n = 3, respectively) (Fig. 8, B and C). These results demonstrate that the Gi activation of Akt does not involve Gβγ subunits but proceeds through ras and Src.
Fig. 8.
Effects of inhibitors of Gβγ subunits, ras, or Src on oleic acid-induced phosphorylation of Akt in HASM cells. Cells were pretreated with the Gβγ signaling inhibitor gallein (10 µM for 60 min; n = 7) (A), Src inhibitor PP1 (10 µM for 60 min; n = 8) (B), or ras inhibitor manumycin A (C) (3 µM for 3 h; n = 3) before treatment with oleic acid (10 µM) for 10 min. The cell lysates were processed to detect phosphorylated (Ai–Ci, top) and total (Ai–Ci, bottom) levels of Akt by immunoblot. Aii–Cii: graphical analysis. Phosphorylation of Akt is presented a ratio of phosphorylated to total Akt and expressed relative to basal ratios. Data represent means ± SE. *P < 0.05; **P < 0.01, compared with basal. White vertical spaces between the lanes in C indicate that these lanes were located on the same immunoblot but were not located in neighboring lanes on the original gel and immunoblot image.
Effects of inhibitors of MEK and PI3K on oleic acid-induced phosphorylation of ERK and Akt in HASM cells.
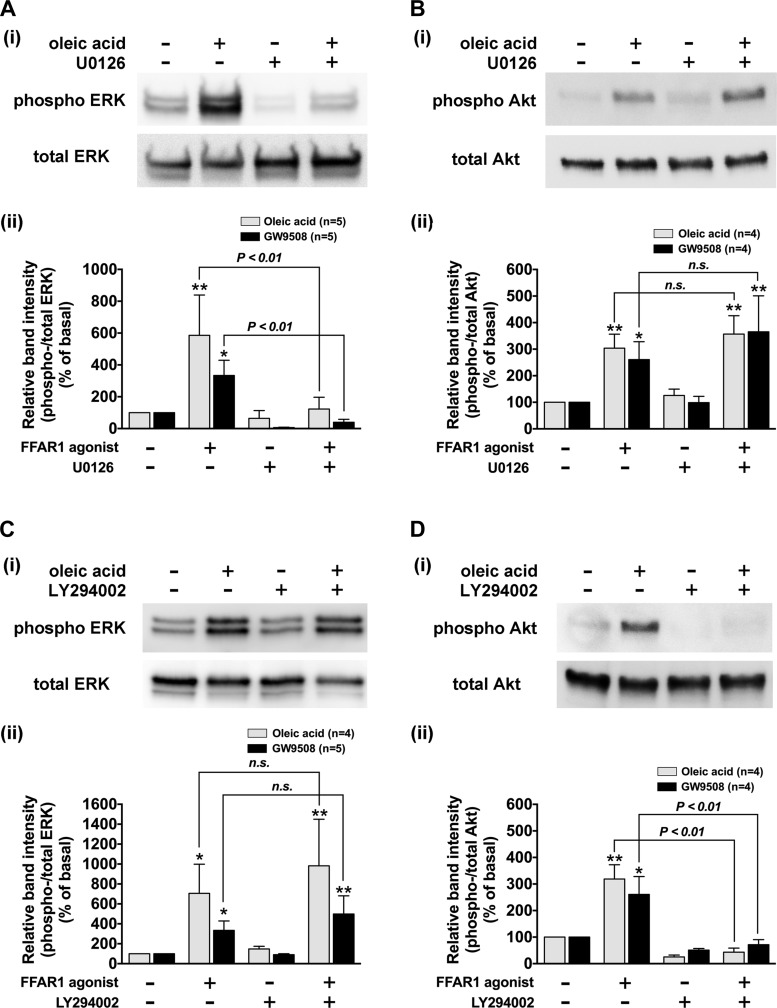
To confirm that the stimulation of FFAR1 by oleic acid (10 µM; 10 min) or GW9508 (20 µM; 10 min) phosphorylates ERK and Akt through MEK and PI3K, respectively, HASM cells were pretreated with the MEK inhibitor U0126 (5 µM; 2 h) or the PI3K inhibitor LY294002 (3 µM; 60 min). The FFAR1 agonist-stimulated ERK phosphorylation was significantly inhibited by U0126 (P < 0.01, n = 5; Fig. 9A), while Akt phosphorylation was not affected (n.s., n = 4; Fig. 9B). In contrast, the pretreatment of the HASM cells with LY294002 did not affect the FFAR1 agonist-stimulated ERK phosphorylation (n.s., n = 4–5; Fig. 9C) but significantly inhibited Akt phosphorylation (P < 0.01, n = 4; Fig. 9D). These results confirm that FFAR1 agonist-induced phosphorylation of ERK proceeds through MEK and that FFAR1 agonist-induced phosphorylation of Akt proceeds through PI3K.
Fig. 9.
Effects of inhibitors of MEK or phosphoinositide 3-kinase (PI3K) on FFAR1-mediated phosphorylation of ERK and Akt in HASM cells. Cells were pretreated with or without inhibitors, and then stimulated with oleic acid (10 µM, 10 min) or GW9508 (20 µM, 10 min). The cell lysates were processed to detect phosphorylated (Ai–Di, top) and total (Ai–Di, bottom) levels of ERK or Akt by immunoblot. Aii–Dii: graphical analysis. A and B: effect of MEK inhibitor U0126 (5 µM for 2 h) on oleic acid- or GW9508-induced phosphorylation of ERK (A) or Akt in HASM cells (B). C and D: effect of the PI3K inhibitor LY294002 (3 µM for 60 min) on oleic acid- or GW9508-induced phosphorylation of ERK (C) or Akt (D) in HASM cells. Phosphorylation of ERK and Akt are presented as a ratio of phosphorylated to total ERK or Akt and expressed relative to basal ratios. Data represent means ± SE. *P < 0.05; **P < 0.01, compared with basal. Numbers of experiments are shown in parentheses.
Effects of inhibitors of PI3K, MEK, and mTOR on FFAR1-mediated phosphorylation of p70S6K and S6 ribosomal protein in HASM cells.
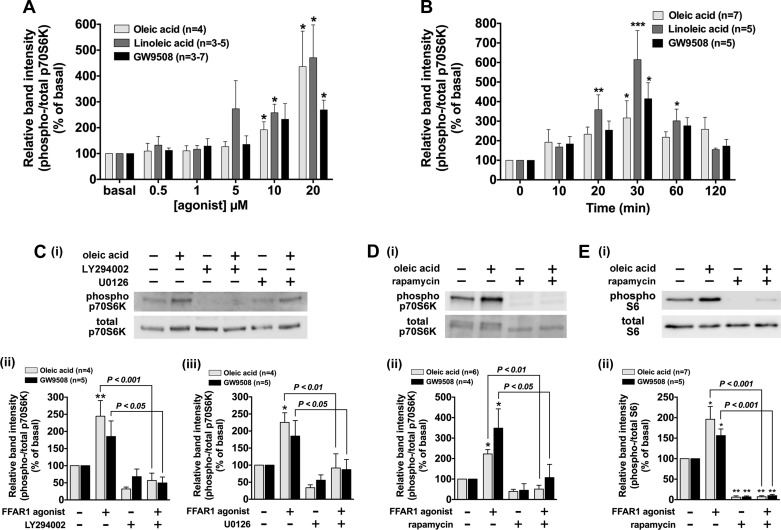
Activation of Akt induces phosphorylation of p70S6K which promotes hyperplasia and hypertrophy of HASM cells (3, 70). Thus we examined whether p70S6K was phosphorylated in response to FFAR1 activation with oleic acid, linoleic acid, and GW9508. These FFAR1 ligands induced p70S6K phosphorylation in a concentration-dependent manner (Fig. 10A). Phosphorylation of p70S6K reached a maximum at 30 min and returned to the basal levels within 120 min (Fig. 10B). Pretreatment of HASM cells with the PI3K inhibitor LY294002 (3 µM; 60 min) abolished p70S6K phosphorylation induced by oleic acid (P < 0.001, n = 4) and GW9508 (P < 0.05, n = 5) (Fig. 10C). Since previous findings have suggested that stimulation of the PI3K/Akt pathway induces p70S6K phosphorylation via mTORC1 (28, 40, 43), we examined the effect of the mTOR inhibitor rapamycin on FFAR1-stimulated p70S6K phosphorylation. Pretreatment of HASM cells with rapamycin (1 µM; 60 min) abolished p70S6K phosphorylation induced by oleic acid (P < 0.01, n = 6) or GW9508 (P < 0.05, n = 4) (Fig. 10D). In addition, the ERK pathway has been reported to cross activate PI3K-Akt-mTORC1 signaling pathway by regulating mTOR (43). Therefore, we examined whether the pretreatment of HASM cells with the MEK inhibitor U0126 (5 µM; 2 h) attenuated the phosphorylation of p70S6K. U0126 significantly inhibited the FFAR1 agonist-stimulated p70S6K phosphorylation induced by oleic acid (P < 0.01, n = 4) or GW9508 (P < 0.05, n = 5) (Fig. 10C). These results demonstrate that FFAR1 agonists induce phosphorylation of p70S6K through PI3K-Akt-mTOR signaling and that ERK can cross talk to phosphorylate p70S6K.
Fig. 10.
Effects of oleic acid, linoleic acid, or GW9508 on phosphorylation of p70S6K and S6 ribosomal protein in cultured HASM cells. Cells were stimulated with oleic acid, linoleic acid, or GW9508, and subsequently cell lysates were processed to detect phosphorylated and total levels of p70S6K or S6 ribosomal protein by immunoblot. For A–E: i: immunoblot analysis; ii: graphical analysis A: concentration-dependent effect of oleic acid, linoleic acid, and GW9508 (0.5–0 µM; 10 min) on the p70S6K phosphorylation in cultured HASM cells. B: time-course effect of oleic acid (10 µM), linoleic acid (10 µM), and GW9508 (20 µM) on the p70S6K phosphorylation in cultured HASM cells. C: effect of the PI3K inhibitor LY294002 (3 µM for 60 min) or the MEK inhibitor U0126 (5 µM for 2 h) on oleic acid (10 µM; 10 min)- or GW9508 (20 µM; 10 min)-induced p70S6K phosphorylation in HASM cells. D: effect of the mammalian target of rapamycin (mTOR) inhibitor rapamycin (1 µM for 60 min) on oleic acid- or GW9508-induced phosphorylation of p70S6K in HASM cells. E: effect of rapamycin (1 µM for 60 min) on oleic acid- or GW9508-induced phosphorylation of S6 ribosomal protein in HASM cells. Phosphorylation of p70S6K and S6 ribosomal protein are presented as a ratio of phosphorylated to total p70S6K or S6 and expressed relative to basal ratios. Data are shown as percentages of basal cell phosphorylation and are expressed as means ± SE. *P < 0.05; **P < 0.01; ***P < 0.001, compared with basal (time 0). Numbers of experiments are shown in parentheses.
p70S6K subsequently phosphorylates the S6 ribosomal protein (15). Thus we sought to determine whether stimulation of FFAR1 induced phosphorylation of the S6 ribosomal protein in HASM cells. Stimulation of FFAR1 with oleic acid (10 µM) or GW9508 (20 µM) phosphorylated S6 ribosomal protein in HASM cells, and their phosphorylation was completely blocked by the pretreatment with rapamycin (oleic acid; P < 0.01, n = 7, GW9508; P < 0.01, n = 5) (Fig. 10E).
DISCUSSION
The present study is the first to demonstrate that long-chain FFAs and GW9508 (a selective agonist of FFAR1) induce cell proliferation and phosphorylate ERK, Akt, and p70S6K in HASM cells in a concentration-dependent manner. MEK/ERK signaling is mediated by FFAR1 that is coupled to Gαi proteins, which induces the inhibition of cAMP activity. In addition, Gαq proteins and Gβγ subunits are involved in FFAR1-stimulated ERK phosphorylation. In contrast, the PI3K/Akt signaling is solely mediated by Gαi protein coupled to FFAR1, and ras and Src are involved in the Akt phosphorylation. Both MEK/ERK and PI3K/Akt pathways induce FFAR1-mediated p70S6K phosphorylation and HASM cell proliferation. Phosphorylation of p70S6K further phosphorylates S6 ribosomal protein.
To date, little is known regarding FFA-induced mitogenic signaling in HASM cells. Previous studies in other cell types have reported that oleic acid induces cell proliferation through MEK/ERK-dependent and/or PI3K/Akt-dependent pathways in human breast cancer cells, rat vascular smooth muscle cells, and bovine neutrophils and mammary epithelial cells (30, 62, 67, 69). In the present study, we demonstrated that long-chain FFAs or GW9508 induce the cell proliferation through both PI3K- and MEK-mediated pathways and induce the phosphorylation of ERK, Akt, and p70S6K in HASM cells in a concentration-dependent manner. The concentration of long-chain FFAs and GW9508, which is required to significantly induce cell proliferation and phosphorylation of these kinases in HASM cells was 5–20 µM. It has been reported that although total plasma concentrations of FFAs in humans varies from 90 to 2,500 µM, >99% of FFAs are bound to serum albumin and only a small fraction of FFAs is free in plasma (53). In HASM cells, the pEC50 value of FFAR1-agonist-induced intracellular Ca2+ concentration mobilization was 5–6 (46). These findings suggest that physiologically relevant concentrations of long-chain FFAs could induce cell proliferation and induce phosphorylation of ERK, Akt, and p70S6K in HASM cells. The phosphorylation of ERK and Akt was rapid, reaching a maximal level within 10–20 min of exposure to the ligand, and transient, returning to basal levels within 60 min. These kinetic properties are similar to the previous report that long-chain FFAs and GW9508 phosphorylate ERK within 5–30 min in the Flp-In T-Rex 293 cell line overexpressing FFAR1 and bovine neutrophils stably expressing FFAR1 (30, 61). Activation of GPCRs results in transient and/or sustained responses in ERK phosphorylation. Transient ERK activation involves G protein-dependent pathways while sustained ERK activation involves G protein-independent pathways such as those involving β-arrestin (19). These findings indicate that FFAR1-mediated transient ERK phosphorylation is solely mediated through G protein-dependent pathways. This idea is further supported by our findings that the pretreatment the HASM cells with the inhibitors of Gαq, Gαi, and Gβγ significantly inhibited oleic acid-induced transient ERK phosphorylation. In HASM cells, Gi or Gq protein-coupled receptor agonists such as histamine (agonist of histamine H1 receptor that couples to Gq) or carbachol (agonist of M2/M3 muscarinic receptors, which couple to Gi/Gq) induced transient ERK activation, which returned to basal levels of phosphorylation within 60 min (50). These findings agree with the FFAR1-mediated ERK phosphorylation in the present study.
A previous study in vascular smooth muscle cells demonstrated that Akt phosphorylation induced by oleic acid was also rapid and transient (5–60 min) (69), which agrees with the results of the present study. In the present study, the onset and the time of peak phosphorylation of p70S6K induced by long-chain FFAs or GW9508 was relatively slower than the phosphorylation of ERK and Akt. Moreover, 48 h-treatment of HASM cells with long-chain FFAs or GW9508 induced cell proliferation, which was still abrogated by inhibitors of MEK or PI3K. These findings suggest that although the FFAR1-stimulated phosphorylation of ERK and Akt was transient, these early phase activations of ERK and Akt could contribute to the subsequent phosphorylation of p70S6K and cell proliferation in HASM cells.
We further demonstrated that oleic acid or GW9508 significantly increased phosphorylation of ERK and Akt in rat airway smooth muscle ex vivo tissues. This finding suggests that phosphorylation of ERK and Akt, which we demonstrated in the cell based studies is a component of the mitogenic effect of FFAR1 agonists.
Previous findings have suggested that long-chain FFAs stimulate two subtypes of long-chain free fatty acid receptors, FFAR1 and FFAR4 (8, 65). We have reported that both FFAR1 and FFAR4 are expressed on HASM cells (46). These findings suggest that long-chain FFAs could activate both FFAR1 and FFAR4 expressed on HASM cells. However, the potency and selectivity of long-chain FFAs on FFAR1 and FFAR4 vary. Oleic acid has a higher affinity for FFAR1 compared with FFAR4 (pEC50 for FFAR1 and FFAR4 is 5.7 and 4.7, respectively) (35, 46, 65). In the present study, we confirmed that oleic acid (10 µM) induced phosphorylation of both ERK and Akt via FFAR1 activation as opposed to FFAR4 via siRNA knockdown strategies. In contrast, the potency of other long-chain FFAs such as linoleic acid or α-linolenic acid on FFAR1 has been reported to be similar to their potency on FFAR4 (linoleic acid; pEC50 for FFAR1 and FFAR4 is 6.0 and 5.9, α-linolenic acid; pEC50 for FFAR1 and FFAR4 is 5.9 and 6.2, respectively) (7, 46). In the present study, linoleic acid also induced phosphorylation of ERK, Akt, and p70S6K and cell proliferation in HASM cells. However, due to its lower selectivity, linoleic acid could induce phosphorylation of ERK, Akt, and p70S6K and cell proliferation through FFAR4 as well as FFAR1. Further studies are required to examine the role of FFAR4 on HASM cell proliferation.
Human FFAR1 is classically coupled to both Gαq and/or Gαi proteins (8, 35, 61, 68). We have previously reported in HASM cells that FFAR1-stimulated intracellular Ca2+ concentration increases are solely mediated via the Gαq protein in HASM cells (46). However, in the present study, oleic acid-induced c-Raf/ERK phosphorylation was abolished by the Gαi-specific inhibitor pertussis toxin and partially inhibited by Gαq-specific inhibitor YM-254890, suggesting that oleic acid-induced ERK phosphorylation was primarily mediated via Gαi protein coupling to FFAR1 while the contribution of Gαq protein stimulation by FFAR1 is less. Furthermore, Akt phosphorylation induced by oleic acid was not inhibited by YM-254890 but abolished by pertussis toxin, suggesting that Akt phosphorylation is solely mediated via the Gαi protein coupled to FFAR1. G protein-coupled receptors frequently exhibit biased agonism resulting in the coupling to two or more G proteins, which is dictated by the cellular context. This results in the initiation of signaling networks rather than a linear sequence of intracellular signaling cascades (56). These findings indicate that stimulation of FFAR1 in HASM cells induces multiple downstream intracellular signaling cascades through both Gαq and Gαi proteins.
Activation of the MEK/ERK pathway contributes to cell proliferation in airway smooth muscle (38, 50). The Gi-coupled receptor classically induces ERK phosphorylation through several intracellular signaling pathways. One possible mechanism involves its inhibitory effect on adenylyl cyclase, which leads to a decrease in cAMP levels and PKA activity. PKA can phosphorylated c-Raf at Ser 259, which results in inhibition of phosphorylation of c-Raf at Ser 338 (16, 17). In contrast, a Gαi-mediated decrease in cAMP levels and subsequent decrease in PKA activity relieve the inhibitory effect of PKA on c-Raf by Ser 259 phosphorylation, thus allowing for the potentiation of the phosphorylation of c-Raf at Ser 338, which subsequently phosphorylates ERK (Gi/adenylyl cyclase/cAMP/PKA/c-Raf/ERK pathway) (13, 16, 17, 23, 27, 54). In the present study, oleic acid significantly inhibited forskolin-stimulated cAMP production in HASM cells in a pertussis toxin-sensitive manner, and oleic acid did not induce the phosphorylation of c-Raf at Ser 259 but allowed for phosphorylation at Ser 338. These findings are consistent with decreased PKA activity preventing c-Raf Ser 259 phosphorylation, resulting in enhanced phosphorylation of c-Raf on Ser 338 resulting in enhanced activity of the c-Raf/MEK/ERK signaling pathway (Gi/adenylyl cyclase/cAMP/PKA/c-Raf/ERK pathway). It was reported that Raf S259 mutants are constitutively hyperphosphorylated on Ser 338 (16, 17), suggesting that the lack of c-Raf Ser 259 phosphorylation would allow for increased phosphorylation of c-Raf at Ser 338. Taken together, oleic acid-induced inhibition of cAMP production would be one of the key components of the oleic acid-induced phosphorylation of c-Raf/ERK through Gαi protein in HASM cells. Another Gi-mediated ERK signaling pathway involves dissociated Gβγ subunits from the Gi protein (14). In the present study, the Gβγ signaling inhibitor gallein attenuated oleic acid-induced c-Raf/ERK phosphorylation, suggesting involvement of Gβγ subunits in c-Raf/ERK phosphorylation. Both dissociated Gβγ subunits and Gαq proteins activate PLC-β (21, 45), which in turn induce PKC-mediated activation of c-Raf, an upstream molecule of ERK (23) (PLC-β/PKC/c-Raf/MEK/ERK pathway). In addition, PLC-β leads to an IP3-mediated increase in intracellular Ca2+ (45), and Ca2+-calmodulin-mediated activation of Pyk2 kinase. The Pyk2 kinase activates Src, which leads to activated ras and subsequently phosphorylates ERK (23, 39) (PLC-β/IP3/Ca2+/Pyk2/Src/ras/c-Raf/MEK/ERK pathway). However, in the present study, the oleic acid-stimulated phosphorylation of ERK was not affected by the presence of either the PLC-β inhibitor U73122, the PKC inhibitor GF109203X, or the Src kinase inhibitor PP1. These results suggest that although Gβγ subunits and the Gαq protein are involved in FFAR1-mediated ERK phosphorylation, the ERK signaling cascade is not mediated by the PLC-β/PKC/c-Raf/MEK/ERK pathway nor the PLC-β/IP3/Ca2+/Pyk2/Src/ras/c-Raf/MEK/ERK pathway. However, the downstream signaling cascade of Gβγ subunits and Gαq that mediate FFAR1-stimulated ERK phosphorylation is still unclear, and requires further study.
Activation of the PI3K/Akt pathway contributes to cell proliferation and hypertrophy in airway smooth muscle (40). It is well documented that Akt is activated in response to Gi-coupled receptors and Gβγ subunits activate Akt through direct activation of PI3K (37, 48). However, contrary to these findings, the present study demonstrates that oleic acid-induced phosphorylation of Akt was not inhibited by the Gβγ signaling inhibitor gallein. Ciccarelli et al. (11) showed similar results, where Gi-coupled β2 adrenergic receptor-mediated Akt phosphorylation in vascular endothelial cells was not inhibited by blockade of Gβγ and suggested that Gαi is the key mediator of this signaling. We have reported that Gαi activates ras in HASM cells (20), and previous studies have shown that Gi-coupled receptors regulate the nonreceptor tyrosine kinase Src, which acts as an intermediate between Gαi and ras/PI3K (11, 41, 70). These findings were consistent with our results that inhibitors of ras (manumycin A), Src (PP1), or PI3K (LY294002) abolished oleic acid-stimulated Akt phosphorylation. Collectively, the results of the present study demonstrate that FFAR1-mediated Akt phosphorylation is mediated through the Gαi/ras/Src/PI3K/Akt signaling pathway.
In the present study, FFAR1-mediated ERK phosphorylation was completely blocked by the MEK inhibitor U0126 in HASM cells, while Akt phosphorylation was not inhibited nor potentiated by the inhibitor. In contrast, FFAR1-mediated Akt phosphorylation was completely blocked by the PI3K inhibitor LY294002 in HASM cells, while ERK phosphorylation was not inhibited nor potentiated by the inhibitor. Furthermore, the blockade of either MEK or PI3K completely inhibited the HASM cell proliferation followed by stimulation of FFAR1. These results suggest that both MEK/ERK and PI3K/Akt pathways independently induce FFAR1-mediated cell proliferation in HASM cells. Interestingly, the present study demonstrates that FFAR1-stimulated phosphorylation of p70S6K, which is the downstream molecule of the PI3K/Akt/mTORC1 pathway, was abrogated by the MEK inhibitor. Since ERK has been shown to cross activate mTORC1 (43), these results suggest that the ERK signaling pathway-induced p70S6K phosphorylation was likely mediated via mTORC1 and converges on the PI3K/Akt/mTOR signaling pathway to activate p70S6K. p70S6K is a highly regulated kinase and its activation promotes HASM cell hyperplasia (3), which is a key feature of airway remodeling. The S6 ribosomal protein is one of the downstream phosphorylation targets of p70S6K and is responsible for airway smooth muscle cell proliferation (24). In the present study, our results showed that stimulation of FFAR1 induced phosphorylation of the S6 ribosomal protein, which was abolished by rapamycin. These findings would support the supposition that activation of FFAR1 contributes to airway smooth muscle cell proliferation through mTOR/p70S6K/S6 ribosomal protein.
A limitation of this study is that we did not examine the interaction of FFAR1 receptor activation with receptor tyrosine kinase activation and their effects on airway smooth muscle cell growth. A variety of agonists that activate Gi- or Gq-coupled receptors have the potential to augment receptor tyrosine kinase-induced airway smooth muscle growth (25). Our results have shown that Src, which integrates signaling from G protein-coupled receptors and receptor tyrosine kinases (36), mediates FFAR1-induced Akt phosphorylation in HASM cells. Further studies are warranted to elucidate the interaction of FFAR1- and tyrosine kinase receptor-mediated mitogenic signaling in airway smooth muscle.
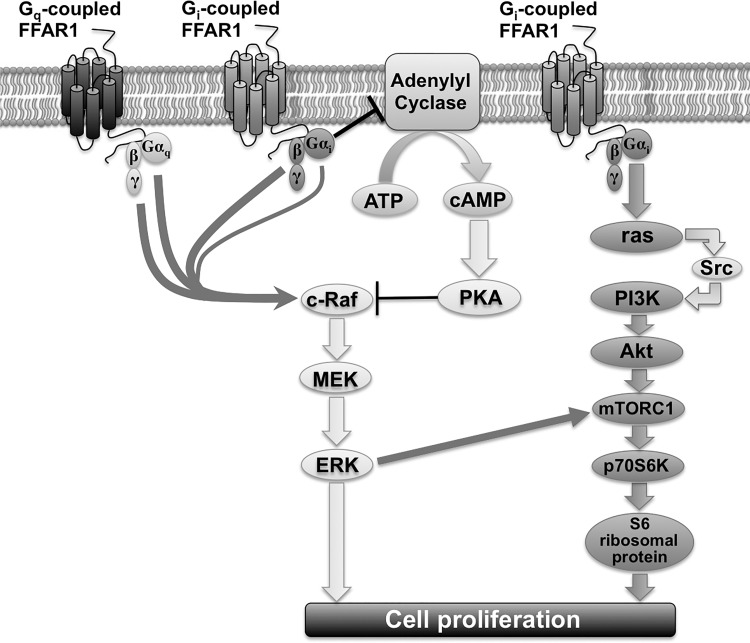
In conclusion, our results reveal that the activation of the FFAR1 promoted p70S6K phosphorylation and airway smooth muscle cell proliferation through both MEK/ERK and PI3K/Akt signaling pathways. The MEK/ERK signaling pathway was primarily mediated through Gαi-mediated inhibition of cAMP, while the PI3K/Akt signaling pathway is mediated through Gαi/ras/Src signaling (Fig. 11). These results suggest that FFAR1 on airway smooth muscle could contribute to the cellular proliferative response to plasma FFAs and could be an important regulator of airway remodeling especially in obese individuals.
Fig. 11.
Schematic drawing of FFAR1-stimulated signaling cascade of ERK and Akt in HASM cells. Gi-coupled FFAR1 inhibits adenylyl cyclase activity, which inhibits cAMP production. Reduced cAMP production leads to decreased PKA activity. The decreased PKA activity in turn relieves the inhibitory effect of PKA on c-Raf, thus potentiating the c-Raf/MEK/ERK signaling. Both the Gαq subunit and Gβγ subunit, which is dissociated from both Gi and Gq following FFAR1 activation, contribute to ERK phosphorylation, although the signaling pathway does not involve PLC-β, PKC, or Src. Gi-coupled FFAR1 also activates the PI3K/Akt pathway, which is mediated through Gαi/ras/Src signaling. The MEK/ERK pathway directly induces cell proliferation, and the PI3K/Akt pathway induces p70S6K phosphorylation via mTORC1. The MEK/ERK pathway also cross activates mTORC1 and induces phosphorylation of p70S6K. p70S6K phosphorylates S6 ribosomal protein and leads to HASM cell proliferation.
GRANTS
This work was supported by the National Institutes of Health Grants GM-065281 and HL-122340 (to C. W. Emala) and Grant-in-Aid from the Japan Society for the Promotion of Science (24689072 and 26560376; to K. Mizuta).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.W.E. and K.M. conceived and designed research; A.M., N.M., S.S., and K.M. performed experiments; A.M., S.S., E.M., C.W.E., and K.M. analyzed data; A.M., S.S., C.W.E., and K.M. interpreted results of experiments; A.M., S.S., and K.M. prepared figures; N.M., C.W.E., and K.M. drafted manuscript. A.M., N.M., S.S., E.M., C.W.E., and K.M. edited and revised manuscript; A.M., N.M., S.S., E.M., C.W.E., and K.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Koji Takiguchi and Takeru Kondo for technical assistance.
REFERENCES
- 1.Bates JH, Dixon AE. Potential role of the airway wall in the asthma of obesity. J Appl Physiol (1985) 118: 36–41, 2015. doi: 10.1152/japplphysiol.00684.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergeron C, Al-Ramli W, Hamid Q. Remodeling in asthma. Proc Am Thorac Soc 6: 301–305, 2009. doi: 10.1513/pats.200808-089RM. [DOI] [PubMed] [Google Scholar]
- 3.Billington CK, Kong KC, Bhattacharyya R, Wedegaertner PB, Panettieri RA Jr, Chan TO, Penn RB. Cooperative regulation of p70S6 kinase by receptor tyrosine kinases and G protein-coupled receptors augments airway smooth muscle growth. Biochemistry 44: 14595–14605, 2005. doi: 10.1021/bi0510734. [DOI] [PubMed] [Google Scholar]
- 4.Birnbaumer L, Abramowitz J, Brown AM. Receptor-effector coupling by G proteins. Biochim Biophys Acta 1031: 163–224, 1990. doi: 10.1016/0304-4157(90)90007-Y. [DOI] [PubMed] [Google Scholar]
- 5.Björntorp P, Bergman H, Varnauskas E. Plasma free fatty acid turnover rate in obesity. Acta Med Scand 185: 351–356, 1969. doi: 10.1111/j.0954-6820.1969.tb07347.x. [DOI] [PubMed] [Google Scholar]
- 6.Black PN, Sharpe S. Dietary fat and asthma: is there a connection? Eur Respir J 10: 6–12, 1997. doi: 10.1183/09031936.97.10010006. [DOI] [PubMed] [Google Scholar]
- 7.Briscoe CP, Peat AJ, McKeown SC, Corbett DF, Goetz AS, Littleton TR, McCoy DC, Kenakin TP, Andrews JL, Ammala C, Fornwald JA, Ignar DM, Jenkinson S. Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: identification of agonist and antagonist small molecules. Br J Pharmacol 148: 619–628, 2006. doi: 10.1038/sj.bjp.0706770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, Murdock PR, Sauls HR Jr, Shabon U, Spinage LD, Strum JC, Szekeres PG, Tan KB, Way JM, Ignar DM, Wilson S, Muir AI. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem 278: 11303–11311, 2003. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- 9.Burgess JK, Lee JH, Ge Q, Ramsay EE, Poniris MH, Parmentier J, Roth M, Johnson PR, Hunt NH, Black JL, Ammit AJ. Dual ERK and phosphatidylinositol 3-kinase pathways control airway smooth muscle proliferation: differences in asthma. J Cell Physiol 216: 673–679, 2008. doi: 10.1002/jcp.21450. [DOI] [PubMed] [Google Scholar]
- 10.Calixto MC, Lintomen L, Schenka A, Saad MJ, Zanesco A, Antunes E. Obesity enhances eosinophilic inflammation in a murine model of allergic asthma. Br J Pharmacol 159: 617–625, 2010. doi: 10.1111/j.1476-5381.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciccarelli M, Cipolletta E, Santulli G, Campanile A, Pumiglia K, Cervero P, Pastore L, Astone D, Trimarco B, Iaccarino G. Endothelial β2 adrenergic signaling to AKT: role of Gi and SRC. Cell Signal 19: 1949–1955, 2007. doi: 10.1016/j.cellsig.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Cintra DE, Ropelle ER, Moraes JC, Pauli JR, Morari J, Souza CT, Grimaldi R, Stahl M, Carvalheira JB, Saad MJ, Velloso LA. Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity. PLoS One 7: e30571, 2012. doi: 10.1371/journal.pone.0030571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook SJ, McCormick F. Inhibition by cAMP of Ras-dependent activation of Raf. Science 262: 1069–1072, 1993. doi: 10.1126/science.7694367. [DOI] [PubMed] [Google Scholar]
- 14.Crespo P, Gutkind JS. Activation of MAPKs by G protein-coupled receptors. Methods Mol Biol 250: 203–210, 2004. doi: 10.1385/1-59259-671-1:203. [DOI] [PubMed] [Google Scholar]
- 15.Deng H, Hershenson MB, Lei J, Bitar KN, Fingar DC, Solway J, Bentley JK. p70 Ribosomal S6 kinase is required for airway smooth muscle cell size enlargement but not increased contractile protein expression. Am J Respir Cell Mol Biol 42: 744–752, 2010. doi: 10.1165/rcmb.2009-0037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhillon AS, Meikle S, Yazici Z, Eulitz M, Kolch W. Regulation of Raf-1 activation and signalling by dephosphorylation. EMBO J 21: 64–71, 2002. doi: 10.1093/emboj/21.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhillon AS, Pollock C, Steen H, Shaw PE, Mischak H, Kolch W. Cyclic AMP-dependent kinase regulates Raf-1 kinase mainly by phosphorylation of serine 259. Mol Cell Biol 22: 3237–3246, 2002. doi: 10.1128/MCB.22.10.3237-3246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dixon AE, Holguin F, Sood A, Salome CM, Pratley RE, Beuther DA, Celedón JC, Shore SA; American Thoracic Society Ad Hoc Subcommittee on Obesity and Lung Disease . An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc 7: 325–335, 2010. doi: 10.1513/pats.200903-013ST. [DOI] [PubMed] [Google Scholar]
- 19.Eishingdrelo H, Kongsamut S. Minireview: targeting GPCR activated ERK pathways for drug discovery. Curr Chem Genomics Transl Med 7: 9–15, 2013. doi: 10.2174/2213988501307010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emala CW, Liu F, Hirshman CA. Giα but not Gqα is linked to activation of p21(ras) in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 276: L564–L570, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Ethier MF, Madison JM. Adenosine A1 receptors mediate mobilization of calcium in human bronchial smooth muscle cells. Am J Respir Cell Mol Biol 35: 496–502, 2006. doi: 10.1165/rcmb.2005-0290OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujiwara K, Maekawa F, Yada T. Oleic acid interacts with GPR40 to induce Ca2+ signaling in rat islet β-cells: mediation by PLC and L-type Ca2+ channel and link to insulin release. Am J Physiol Endocrinol Metab 289: E670–E677, 2005. doi: 10.1152/ajpendo.00035.2005. [DOI] [PubMed] [Google Scholar]
- 23.Goldsmith ZG, Dhanasekaran DN. G protein regulation of MAPK networks. Oncogene 26: 3122–3142, 2007. doi: 10.1038/sj.onc.1210407. [DOI] [PubMed] [Google Scholar]
- 24.Goncharova EA, Lim PN, Chisolm A, Fogle HW 3rd, Taylor JH, Goncharov DA, Eszterhas A, Panettieri RA Jr, Krymskaya VP. Interferons modulate mitogen-induced protein synthesis in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 299: L25–L35, 2010. doi: 10.1152/ajplung.00228.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gosens R, Roscioni SS, Dekkers BG, Pera T, Schmidt M, Schaafsma D, Zaagsma J, Meurs H. Pharmacology of airway smooth muscle proliferation. Eur J Pharmacol 585: 385–397, 2008. doi: 10.1016/j.ejphar.2008.01.055. [DOI] [PubMed] [Google Scholar]
- 26.Gras D, Chanez P, Urbach V, Vachier I, Godard P, Bonnans C. Thiazolidinediones induce proliferation of human bronchial epithelial cells through the GPR40 receptor. Am J Physiol Lung Cell Mol Physiol 296: L970–L978, 2009. doi: 10.1152/ajplung.90219.2008. [DOI] [PubMed] [Google Scholar]
- 27.Häfner S, Adler HS, Mischak H, Janosch P, Heidecker G, Wolfman A, Pippig S, Lohse M, Ueffing M, Kolch W. Mechanism of inhibition of Raf-1 by protein kinase A. Mol Cell Biol 14: 6696–6703, 1994. doi: 10.1128/MCB.14.10.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halayko AJ, Kartha S, Stelmack GL, McConville J, Tam J, Camoretti-Mercado B, Forsythe SM, Hershenson MB, Solway J. Phophatidylinositol-3 kinase/mammalian target of rapamycin/p70S6K regulates contractile protein accumulation in airway myocyte differentiation. Am J Respir Cell Mol Biol 31: 266–275, 2004. doi: 10.1165/rcmb.2003-0272OC. [DOI] [PubMed] [Google Scholar]
- 29.Hardy S, St-Onge GG, Joly E, Langelier Y, Prentki M. Oleate promotes the proliferation of breast cancer cells via the G protein-coupled receptor GPR40. J Biol Chem 280: 13285–13291, 2005. doi: 10.1074/jbc.M410922200. [DOI] [PubMed] [Google Scholar]
- 30.Hidalgo MA, Nahuelpan C, Manosalva C, Jara E, Carretta MD, Conejeros I, Loaiza A, Chihuailaf R, Burgos RA. Oleic acid induces intracellular calcium mobilization, MAPK phosphorylation, superoxide production and granule release in bovine neutrophils. Biochem Biophys Res Commun 409: 280–286, 2011. doi: 10.1016/j.bbrc.2011.04.144. [DOI] [PubMed] [Google Scholar]
- 31.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 11: 90–94, 2005. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 32.Hsu CK, Lin CC, Hsiao LD, Yang CM. Mevastatin ameliorates sphingosine 1-phosphate-induced COX-2/PGE2-dependent cell migration via FoxO1 and CREB phosphorylation and translocation. Br J Pharmacol 172: 5360–5376, 2015. doi: 10.1111/bph.13326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hudson BD, Shimpukade B, Mackenzie AE, Butcher AJ, Pediani JD, Christiansen E, Heathcote H, Tobin AB, Ulven T, Milligan G. The pharmacology of TUG-891, a potent and selective agonist of the free fatty acid receptor 4 (FFA4/GPR120), demonstrates both potential opportunity and possible challenges to therapeutic agonism. Mol Pharmacol 84: 710–725, 2013. doi: 10.1124/mol.113.087783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ichimonji I, Tomura H, Mogi C, Sato K, Aoki H, Hisada T, Dobashi K, Ishizuka T, Mori M, Okajima F. Extracellular acidification stimulates IL-6 production and Ca2+ mobilization through proton-sensing OGR1 receptors in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 299: L567–L577, 2010. doi: 10.1152/ajplung.00415.2009. [DOI] [PubMed] [Google Scholar]
- 35.Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 422: 173–176, 2003. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- 36.Krymskaya VP, Goncharova EA, Ammit AJ, Lim PN, Goncharov DA, Eszterhas A, Panettieri RA Jr. Src is necessary and sufficient for human airway smooth muscle cell proliferation and migration. FASEB J 19: 428–430, 2005. doi: 10.1096/fj.04-2869fje. [DOI] [PubMed] [Google Scholar]
- 37.Leblais V, Jo SH, Chakir K, Maltsev V, Zheng M, Crow MT, Wang W, Lakatta EG, Xiao RP. Phosphatidylinositol 3-kinase offsets cAMP-mediated positive inotropic effect via inhibiting Ca2+ influx in cardiomyocytes. Circ Res 95: 1183–1190, 2004. doi: 10.1161/01.RES.0000150049.74539.8a. [DOI] [PubMed] [Google Scholar]
- 38.Lee JH, Johnson PR, Roth M, Hunt NH, Black JL. ERK activation and mitogenesis in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 280: L1019–L1029, 2001. doi: 10.1152/ajplung.2001.280.5.L1019. [DOI] [PubMed] [Google Scholar]
- 39.Lei J, Ingbar DH. Src kinase integrates PI3K/Akt and MAPK/ERK1/2 pathways in T3-induced Na-K-ATPase activity in adult rat alveolar cells. Am J Physiol Lung Cell Mol Physiol 301: L765–L771, 2011. doi: 10.1152/ajplung.00151.2011. [DOI] [PubMed] [Google Scholar]
- 40.Ma L, Brown M, Kogut P, Serban K, Li X, McConville J, Chen B, Bentley JK, Hershenson MB, Dulin N, Solway J, Camoretti-Mercado B. Akt activation induces hypertrophy without contractile phenotypic maturation in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 300: L701–L709, 2011. doi: 10.1152/ajplung.00119.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma YC, Huang J, Ali S, Lowry W, Huang XY. Src tyrosine kinase is a novel direct effector of G proteins. Cell 102: 635–646, 2000. doi: 10.1016/S0092-8674(00)00086-6. [DOI] [PubMed] [Google Scholar]
- 42.Mauad T, Bel EH, Sterk PJ. Asthma therapy and airway remodeling. J Allergy Clin Immunol 120: 997–1009, 2007. doi: 10.1016/j.jaci.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 43.Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci 36: 320–328, 2011. doi: 10.1016/j.tibs.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Misso NL, Petrovic N, Grove C, Celenza A, Brooks-Wildhaber J, Thompson PJ. Plasma phospholipase A2 activity in patients with asthma: association with body mass index and cholesterol concentration. Thorax 63: 21–26, 2008. doi: 10.1136/thx.2006.074112. [DOI] [PubMed] [Google Scholar]
- 45.Mizuta K, Mizuta F, Xu D, Masaki E, Panettieri RA Jr, Emala CW. Gi-coupled γ-aminobutyric acid-B receptors cross-regulate phospholipase C and calcium in airway smooth muscle. Am J Respir Cell Mol Biol 45: 1232–1238, 2011. doi: 10.1165/rcmb.2011-0088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mizuta K, Zhang Y, Mizuta F, Hoshijima H, Shiga T, Masaki E, Emala CW Sr. Novel identification of the free fatty acid receptor FFAR1 that promotes contraction in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 309: L970–L982, 2015. doi: 10.1152/ajplung.00041.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mizuta K, Zhang Y, Xu D, Masaki E, Panettieri RA Jr, Emala CW. The dopamine D2 receptor is expressed and sensitizes adenylyl cyclase activity in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 302: L316–L324, 2012. doi: 10.1152/ajplung.00130.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.New DC, Wu K, Kwok AW, Wong YH. G protein-coupled receptor-induced Akt activity in cellular proliferation and apoptosis. FEBS J 274: 6025–6036, 2007. doi: 10.1111/j.1742-4658.2007.06116.x. [DOI] [PubMed] [Google Scholar]
- 49.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142: 687–698, 2010. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Orsini MJ, Krymskaya VP, Eszterhas AJ, Benovic JL, Panettieri RA Jr, Penn RB. MAPK superfamily activation in human airway smooth muscle: mitogenesis requires prolonged p42/p44 activation. Am J Physiol Lung Cell Mol Physiol 277: L479–L488, 1999. [DOI] [PubMed] [Google Scholar]
- 51.Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J 27: 495–503, 2006. doi: 10.1183/09031936.06.00077205. [DOI] [PubMed] [Google Scholar]
- 52.Poh YC, Na S, Chowdhury F, Ouyang M, Wang Y, Wang N. Rapid activation of Rac GTPase in living cells by force is independent of Src. PLoS One 4: e7886, 2009. doi: 10.1371/journal.pone.0007886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Potter BJ, Sorrentino D, Berk PD. Mechanisms of cellular uptake of free fatty acids. Annu Rev Nutr 9: 253–270, 1989. doi: 10.1146/annurev.nu.09.070189.001345. [DOI] [PubMed] [Google Scholar]
- 54.Radhika V, Dhanasekaran N. Transforming G proteins. Oncogene 20: 1607–1614, 2001. doi: 10.1038/sj.onc.1204274. [DOI] [PubMed] [Google Scholar]
- 55.Rodríguez-Rodríguez E, Perea JM, Jiménez AI, Rodríguez-Rodríguez P, López-Sobaler AM, Ortega RM. Fat intake and asthma in Spanish schoolchildren. Eur J Clin Nutr 64: 1065–1071, 2010. doi: 10.1038/ejcn.2010.127. [DOI] [PubMed] [Google Scholar]
- 56.Rozengurt E. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol 213: 589–602, 2007. doi: 10.1002/jcp.21246. [DOI] [PubMed] [Google Scholar]
- 57.Saraiva SA, Silva AL, Xisto DG, Abreu SC, Silva JD, Silva PL, Teixeira TP, Parra ER, Carvalho AL, Annoni R, Mauad T, Capelozzi VL, Silva PM, Martins MA, Rocco PR. Impact of obesity on airway and lung parenchyma remodeling in experimental chronic allergic asthma. Respir Physiol Neurobiol 177: 141–148, 2011. doi: 10.1016/j.resp.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 58.Schatz M, Hsu JW, Zeiger RS, Chen W, Dorenbaum A, Chipps BE, Haselkorn T. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. J Allergy Clin Immunol 133: 1549–1556, 2014. doi: 10.1016/j.jaci.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 59.Shifren A, Witt C, Christie C, Castro M. Mechanisms of remodeling in asthmatic airways. J Allergy (Cairo) 2012: 316049, 2012. doi: 10.1155/2012/316049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shore SA, Johnston RA. Obesity and asthma. Pharmacol Ther 110: 83–102, 2006. doi: 10.1016/j.pharmthera.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 61.Smith NJ, Stoddart LA, Devine NM, Jenkins L, Milligan G. The action and mode of binding of thiazolidinedione ligands at free fatty acid receptor 1. J Biol Chem 284: 17527–17539, 2009. doi: 10.1074/jbc.M109.012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soto-Guzman A, Robledo T, Lopez-Perez M, Salazar EP. Oleic acid induces ERK1/2 activation and AP-1 DNA binding activity through a mechanism involving Src kinase and EGFR transactivation in breast cancer cells. Mol Cell Endocrinol 294: 81–91, 2008. doi: 10.1016/j.mce.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 63.Stapleton CM, Joo JH, Kim YS, Liao G, Panettieri RA Jr, Jetten AM. Induction of ANGPTL4 expression in human airway smooth muscle cells by PMA through activation of PKC and MAPK pathways. Exp Cell Res 316: 507–516, 2010. doi: 10.1016/j.yexcr.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tagaya E, Tamaoki J. Mechanisms of airway remodeling in asthma. Allergol Int 56: 331–340, 2007. doi: 10.2332/allergolint.R-07-152. [DOI] [PubMed] [Google Scholar]
- 65.Watson SJ, Brown AJ, Holliday ND. Differential signaling by splice variants of the human free fatty acid receptor GPR120. Mol Pharmacol 81: 631–642, 2012. doi: 10.1124/mol.111.077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wood LG, Garg ML, Gibson PG. A high-fat challenge increases airway inflammation and impairs bronchodilator recovery in asthma. J Allergy Clin Immunol 127: 1133–1140, 2011. doi: 10.1016/j.jaci.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 67.Yonezawa T, Haga S, Kobayashi Y, Katoh K, Obara Y. Unsaturated fatty acids promote proliferation via ERK1/2 and Akt pathway in bovine mammary epithelial cells. Biochem Biophys Res Commun 367: 729–735, 2008. doi: 10.1016/j.bbrc.2007.12.190. [DOI] [PubMed] [Google Scholar]
- 68.Yonezawa T, Katoh K, Obara Y. Existence of GPR40 functioning in a human breast cancer cell line, MCF-7. Biochem Biophys Res Commun 314: 805–809, 2004. doi: 10.1016/j.bbrc.2003.12.175. [DOI] [PubMed] [Google Scholar]
- 69.Yun MR, Lee JY, Park HS, Heo HJ, Park JY, Bae SS, Hong KW, Sung SM, Kim CD. Oleic acid enhances vascular smooth muscle cell proliferation via phosphatidylinositol 3-kinase/Akt signaling pathway. Pharmacol Res 54: 97–102, 2006. doi: 10.1016/j.phrs.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 70.Zamani A, Qu Z. Serotonin activates angiogenic phosphorylation signaling in human endothelial cells. FEBS Lett 586: 2360–2365, 2012. doi: 10.1016/j.febslet.2012.05.047. [DOI] [PubMed] [Google Scholar]