Abstract
Right ventricular (RV) function is the primary prognostic factor for both morbidity and mortality in pulmonary hypertension (PH). RV hypertrophy is initially an adaptive physiological response to increased overload; however, with persistent and/or progressive afterload increase, this response frequently transitions to more pathological maladaptive remodeling. The mechanisms and disease processes underlying this transition are mostly unknown. Angiogenesis has recently emerged as a major modifier of RV adaptation in the setting of pressure overload. A novel paradigm has emerged that suggests that angiogenesis and angiogenic signaling are required for RV adaptation to afterload increases and that impaired and/or insufficient angiogenesis is a major driver of RV decompensation. Here, we summarize our current understanding of the concepts of maladaptive and adaptive RV remodeling, discuss the current literature on angiogenesis in the adapted and failing RV, and identify potential therapeutic approaches targeting angiogenesis in RV failure.
INTRODUCTION
Pulmonary hypertension (PH) is a chronic and progressive disease of the lung vasculature and right heart that is characterized by pulmonary artery (PA) vasoconstriction and remodeling, resulting in increased afterload on the right ventricle (RV) (68, 160, 175, 176). Many common cardiopulmonary diseases, such as chronic obstructive pulmonary disease (COPD), pulmonary fibrosis, sleep disordered breathing, and left heart disease (systolic or diastolic dysfunction as well as valvular heart disease) are complicated by PH and/or RV failure (68, 98, 99, 137, 166). Approximately 70 million patients with these conditions in the US alone are estimated to have PH (27, 71, 95, 102, 103, 128). These patients either have overt RV dysfunction or are at a major risk for developing this condition. In addition, 3.8% of patients with pulmonary embolism develop chronic thromboembolic PH and are therefore at major risk for developing RV failure (121). Last, pulmonary arterial hypertension (PAH), one of the most aggressive types of PH and a condition that arises spontaneously, hereditarily or as a complication of such frequent entities as liver cirrhosis, HIV infection, congenital heart disease, and drug and toxin use, commonly leads to profound and refractory RV failure (148).
Abnormal angiogenic processes in the lung have been recognized as a critical player in PH etiology (123, 184). More recently, the vasculature of the RV has emerged as a critical modulator of RV adaptation to the increased afterload in PH. The objective of this review is to highlight the role of angiogenesis in RV failure. In particular, we 1) briefly introduce the concepts of adaptive and maladaptive remodeling in the RV, 2) discuss our current understanding of angiogenesis in the adapted and failing RV, and 3) identify potential therapeutic approaches targeting angiogenesis in RV failure.
THE CRITICAL ROLE OF RV FUNCTION IN PATIENTS WITH PH
The RV initially compensates for the PH-induced increase in afterload through hypertrophy and increased contractile function (125, 176). However, as PH continues to worsen, the RV’s compensatory mechanisms fail, leading to RV failure and death (175, 176). Consequentially, in many different types of PH, deteriorating RV function is one of the strongest predictors of mortality (11, 35, 54, 70, 130, 169). RV-directed therapies would strengthen the ability of the RV to adapt to the increased afterload, would inhibit maladaptive responses, and would be predicted to improve the functional capacity, quality of life, and longevity of patients with PH (133, 175). However, despite the critical importance of RV function to outcomes in PH, no RV-directed therapies exist. For example, in PAH, current therapies predominantly focus on the pulmonary artery (PA) vasoconstriction component of the disease, indicating a significant treatment gap (17, 45, 90, 152). Furthermore, in PH due to chronic left heart or lung disease, pulmonary vasodilators have been used to decrease RV afterload; however, their use is associated with worse outcomes, such as fluid retention, worsening ventilation-perfusion mismatch, and even death (137, 166). Last, therapies developed for the failing left ventricle (LV) cannot be extrapolated to the RV due to their embryological and anatomic differences (51). This indicates a critical need to develop better therapeutic strategies for patients with PH-induced RV failure.
THE CONCEPT OF ADAPTIVE VS. MALADAPTIVE RV REMODELING
To understand the role of the macro- and microvasculature in the failing RV, it is important to understand how the RV responds to increases in afterload. The specific mechanisms of RV failure development have been elegantly discussed in detail elsewhere (133, 174, 175) and are beyond the scope of this review. Briefly, as RV afterload increases during the development of PH, the RV exhibits compensatory mechanisms that include structural changes, neurohormonal activation, and increased contractility (133, 175) (Fig. 1). On a cellular level, these changes are accompanied by increased angiogenesis, changes in mitochondrial function and substrate utilization, increased production of reactive oxygen species, changes in myosin isoform expression, and altered sarcomere organization (133). These mechanisms allow for a state of adaptive (or compensated) RV hypertrophy (RVH), characterized by a cardiac output that is still sufficient to meet the metabolic demands of the body (175, 176). However, once the RV’s compensatory mechanisms are exhausted, the RV will transition from adaptive to maladaptive (or decompensated) RVH, and RV failure with decreased cardiac output and decreased oxygen delivery develops (175, 176) (Fig. 1). At cellular and molecular levels, maladaptive RVH purportedly is characterized by marked inflammation, oxidative stress, metabolic dysfunction, and impaired calcium handling, with an end result of cell death and profound fibrosis (12, 174, 175). There currently is an unmet need in PH to prevent, delay, or reverse the transition from adaptive to maladaptive RVH (133, 174–176). Impaired angiogenesis may be one of the key processes leading to RV decompensation, and modulating angiogenic signaling may be a novel approach to maintain, prolong, or even reestablish adaptive RVH (14, 124). In the remainder of this review, we will 1) provide a brief overview of angiogenesis, 2) identify potential novel modulators of angiogenic signaling in the RV, and 3) discuss the pros and cons of angiogenic signaling as a target in RV failure.
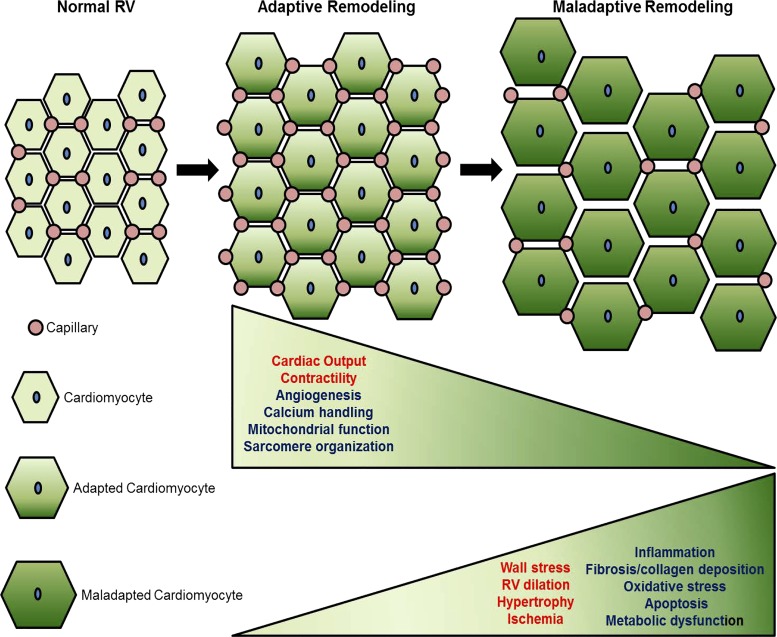
Fig. 1.
Right ventricle (RV) vasculature during transition from adaptive to maladaptive RV remodeling. Current evidence associates RV adaptive remodeling (characterized by maintained RV contractile function) with increased proangiogenic signaling and capillary density, whereas the transition to maladaptive remodeling (characterized by decreased RV contractile function) is marked by RV endothelial cell dysfunction and capillary rarefaction. In addition to capillary rarefaction, the transition from adaptive to maladaptive RV remodeling is characterized by decreased calcium handling, mitochondrial function, and sarcomere organization and increased hypertrophy, ischemia, inflammation, fibrosis, oxidative stress, apoptosis, and metabolic dysfunction. The exact contribution of each of these molecular processes is currently unknown and remains to be determined. Functionally, this process is characterized by worsening cardiac output and increasing RV dilation with increasing wall stress. Functional/structural changes shown in red font; biochemical/molecular changes shown in blue font.
OVERVIEW OF ANGIOGENESIS
To better understand the regulation of angiogenesis in the RV, it is important to understand key concepts of this process. Angiogenesis initiates and directs the proper formation of new, functional blood vessels from existing vessels. It is important to note that angiogenesis is distinct from vasculogenesis; the latter is the de novo formation of vessels through recruitment of circulating hemangioblasts or endothelial progenitor cells (reviewed in Refs. 24, 53, 118, 129). In adult systems, angiogenesis is thought to be the driving mechanism for vessel repair and regeneration. Angiogenesis occurs through two processes: 1) intussusceptive microvascular growth, where new vessels are formed through the splitting of preexisting vessels or 2) sprouting angiogenesis, which consists of endothelial cell (EC) migration, proliferation and tube formation (53). Of these two angiogenic processes, sprouting angiogenesis is the best characterized. Sprouting angiogenesis primarily utilizes two distinct vascular EC phenotypes: tip cells and stalk cells. The tip cell extends, or “sprouts” filopodia and migrates toward a hypoxic or ischemic area. Tip cells induce the migration of the stalk cells into the newly forming vessel branch. Stalk cells are highly proliferative and primarily form the new vessel and lumen. During vessel maturation, excess vessels are pruned in a process known as vessel regression (9, 24, 141). Each vascular endothelial cell (EC) is intrinsically capable of becoming a tip or stalk cell, or to remain as a quiescent EC, or phalanx cell, in the stable vessel (9). Individual ECs actively rotate between each phenotype through rapid induction and repression of a distinct transcriptome (41, 150). This active shuffling between phenotypes involves rapid integration of extracellular pro- and antiangiogenic chemotactic cues from surrounding tissues as well as neighboring vascular ECs (9). The vascular endothelial growth factor (VEGF) family is important for the induction of both cell phenotypes. Not only does VEGF drive tip cell phenotype and migration, it also regulates stalk cell proliferation (10). The ratio of tip cells to stalk cells is crucial for proper formation of new vessels and is tightly regulated by integration of several pathways, including ephrin, notch, bone morphogenetic protein (BMP), Wnt, and VEGF signaling (24, 74). These mechanisms are discussed in greater detail elsewhere (8–10, 24, 112).
When the dynamic regulation of angiogenesis is disrupted or hijacked, it can become a contributing factor to pathogenic processes (24). For example, multiple cancers exploit angiogenic pathways to supply oxygen and nutrients to tumors, driving their growth and fueling inflammatory responses (23, 63, 104). In addition, angiogenesis is relevant to many noncancerous conditions. For example, impaired and/or inappropriate angiogenesis has been linked to multiple cardiovascular diseases such as peripheral vascular disease (33), coronary artery disease (171), and diabetic retinopathy (2, 36).
POTENTIAL RELATIONSHIP BETWEEN THE CARDIAC VASCULATURE AND THE TRANSITION FROM ADAPTIVE TO MALADAPTIVE RVH
Macrovascular Dysfunction in PH
Increased RV afterload results in increased RV wall tension, a process associated with compromised coronary perfusion. This was shown by van Wolferen et al., (170) who demonstrated in patients with PAH that perfusion of the right coronary artery (RCA) was markedly decreased in diastole, whereas this was maintained in control patients. Moreover, increases in RV systolic pressure correlated inversely with decreases in systolic-to-diastolic flow ratio in the RCA. A recent study by Melonche et al. (101) demonstrated that coronary artery remodeling in human and experimental models of PAH was associated with increased RV DNA damage, inflammation, and bromodomain protein-4 (BRD4) overexpression. These data suggest that coronary artery remodeling and flow impairment contribute to a decrease in RV perfusion and clinically relevant mascrovascular impairment in PAH. This phenomenon is likely exacerbated by increased myocardial oxygen demand as a result of increased RV workload.
Microvascular Dysfunction in PH
In addition to RV macrovascular impairment, evidence exists that microvascular alterations occur in the RV as well. Increased RV afterload leads to increased cardiac workload and cardiomyocyte hypertrophy (175, 176). We know from studies of the LV that adequate angiogenesis is critical to maintaining substrate delivery to the hypertrophied myocardium (112, 178). In addition, the endothelium serves as a paracrine “machine” that regulates the function of the surrounding myocardium (108, 175). Capillary dropout and microvascular ischemia therefore have long been postulated to be critical contributors to RV failure development (174). Indeed, analysis of two-dimensional sections in PH models associated with RV failure demonstrates reduced RV vascular density, and this has been identified as a critical mediator of the transition from adaptive to maladaptive RVH (14, 43, 117, 122, 124). Collectively, these studies (summarized in Tables 1 and 2) suggest that the RV microvasculature in PH is dysfunctional and rarefied, rendering it unable to meet the increased substrate demand of the hypertrophied myocardium, thus resulting in clinically relevant RV ischemia (59). A similar paradigm has been proposed in LV failure (112, 147, 178). However, a recent analysis of human RV tissue by a stereological approach noted an increase in total vascular length in PH RVs vs. controls (61). It should be noted, however, that clinical background information about the donor patients was limited. These studies demonstrate that, while there undoubtedly has been progress in the field, controversies exist, and the mechanisms responsible for RV vascular rarefaction and microvascular ischemia remain incompletely understood. The following section reviews the currently known mechanisms and modifiers of angiogenesis in adaptive and maladaptive RV remodeling in more detail.
Table 1.
Studies evaluating the RV vasculature in adaptive RV hypertrophy
| Reference | Adaptive Model(s) | How Was Adaptive Remodeling Defined? | RV Function Measured by |
Main Results |
|---|---|---|---|---|
| Partovian et al. (117) | Rats: Hx (10% , 1–30 days) | DNE | RVSP, RVH, RV function by right heart catheterization | ↑VEGF mRNA at 12 hrs, peaking at 30 days, ↑capillary to myocyte ratio |
| Turek et al. (163) | Rats: Hx (simulated elevation at 3,500 m, 4 wk) | DNE | RVH | ↑Capillary density |
| Lahm et al. (88) | Rats: Hx (10% , 3 wk) | DNE | RVSP, RVH RV function by echocardiograph, right heart catheterization, exercise capacity | ↑Capillary to myocyte ratio |
| Bohuslavovia et al. (16) | Hif1α+/− mice: Hx (12% , 4 wk) | DNE | RVSP, RVH, RV function by right heart catheterization | ↓VEGF |
| Kobayashi et al. (83) | Rats: MCT (60 mg/kg, 3 wk) | DNE | DNE | ↑RV capillarization relative to muscle fibers |
| Sutendra et al. (151) | Rats: MCT (60 mg/kg, 3–4 wk) | RVH, maintained cardiac output and RVSP | RVSP, RVH, RV function by echocardiography, right heart catheterization | ↑HIF1 nuclear localization/activity |
| ↑VEGF-A, ↑SDF1↑RV capillarization | ||||
| Kolb et al. (84) | Mice: Hx (10% , 7 days-3 wk) | RVH, preserved RV function (ie contractility, PA-RV coupling) | RVH | Stereological assessment |
| RV function and hemodynamics by pressure-volume loops | ↑RV length, ↑volume, and ↑surface area at 7 days | |||
| ↑RV EC proliferation | ||||
| ↑VEGFA protein at 3 wk | ||||
| Drake et al. (43) | Rats: Hx (10% , 6 wk) | Preserved cardiac output, TAPSE, preservation/increase of capillaries, absence of fibrosis | DNE | Microarray analysis revealed pathways associated with cell maturation, angiogenesis, and energy metabolism. |
| Rats: PAB (6 wk) | ↑Apelin mRNA and protein in Hx, PAB | |||
| ↑VEGF mRNA in Hx | ||||
| ↑IGF1 mRNA in Hx, PAB | ||||
| Bogaard et al. (14) | Rats: PAB (6 wk) | RVH, preserved cardiac output, TAPSE | RVSP, RVH, RV function by echocardiography, pressure-volume loops, thermodilution | ↑HIF-1 nuclear accumulation |
| Ruiter et al. (131) | Rats: MCT (40 mg/kg, 6 wk) | Preserved cardiac output | RVSP, RVH, RV function by right heart catheterization, endurance testing and echocardiography | ↓Capillarization |
| Piao et al. (122) | Rats: PAB (4 wk) | RVH, preserved function (TAPSE), less severe ischemia | RVSP, RVH RV function by echocardiography, right heart catheterization thermodilution, Langendorff | ↓RV microvasculature |
| Potus et al. (124) | Rats: MCT (60 mg/kg, 2–3 wk) | Rats: RVH, ↑RVSP, slight ↓TAPSE, preserved stroke volume, cardiac output, and RV end-diastolic pressure | Rats: RVSP, RVH, RV function by pressure-volume loops, echocardiograph, endurance testing | Trend for ↑capillary density vs controls |
| ECs isolated from Human compensated RVs | Humans: RVH, absence of LV hypertrophy, preserved TAPSE | Humans: Echocardiography | ↑Tube formation/angiogenic potential mediated through mIR-126 | |
| Handoko et al. (64) | Rats: MCT (40 mg/kg, 6 wk-last 4 wk exercise training) | Preserved cardiac output | RVSP, RVH, RV function by right heart catheterization, endurance testing and echocardiography | Exercise training ↑capillarization |
| Bogaard et al. (13) | Rats: PAB (4 wk + 2 wk TSA or VPA) | RVH, ↑RVSP, preserved cardiac output, TAPSE | RVSP, RVH, RV function by echocardiography, pressure-volume loops | HDAC inhibition ↓RV capillarization, ↓VEGF, ↓Ang1 |
Studies using human tissue are in boldface. Ang1, angiopoietin-1; DNE, did not evaluate adaptive vs. malaptive remodeling; ↑, increase; ↓, decrease; EC, endothelial cell; HIF1, hypoxia-inducible factor 1; Hif1α+/−, Hif1α heterozygous knockout mouse; Hx, Chronic hypoxia; IGF-1, insulin-like growth factor 1; MCT, monocrotaline; PAB, pulmonary artery banding; PABCu2+, pulmonary artery banding + copper-depleted diet; RVH, right ventricular hypertrophy; RVSP, right ventricular systolic pressure; SDF1, stromal derived factor 1; SuHx, Sugen5416 + hypoxia; TAPSE, tricuspid annular plane systolic excursion; TSA, trichostatin A; VEGF, vascular endothelial growth factor; VPA, valproic acid.
Table 2.
Studies evaluating RV vasculature in maladaptive RV hypertrophy
| Reference | Maladaptive Model(s) | How Was Maladaptive Remodeling Defined? | RV Parameters Evaluated | Main Results |
|---|---|---|---|---|
| Partovian et al. (117) | Rats: MCT (60 mg/kg, 1–30 days) | DNE | RVSP, RVH, RV function by right heart catheterization | ↓RV VEGF mRNA |
| Ruiter et al. (131) | Rats: MCT (60 mg/kg, 4 wk) | ↓cardiac output, ↓body weight | RVSP, RVH, RV function by right heart catheterization, endurance testing and echocardiography | ↓RV capillary density |
| Human PAH RVs | ||||
| Piao et al. (122) | Rats: MCT (60 mg/kg, 4 wk) | RVH, more severe ischemia, autonomic remodeling, ↓TAPSE, ↓RV dysfunction compared with PAB | RVSP, RVH RV function by echocardiography, right heart catheterization thermodilution, exercise capacity Contractile function by Langendorff | ↓VEGFA |
| Human PAH and Scleroderma-PAH RVs | ↓RV capillarization | |||
| ↓Coronary blood flow | ||||
| ↓Capillarization in human scleroderma-PAH RVs | ||||
| Sutendra et al. (151) | Rats: MCT (60 mg/kg, 5–6 wk) | ↓RVSP and ↓cardiac output but ↑mean pulmonary artery pressure, continued RV remodeling, development of ascites, weight loss, fluid retention | RVH, RVSP, RV function by right heart catheterization, echocardiograph | Inhibition of HIF1 |
| Activation of p53 | ||||
| ↓VEGFA, ↓SDF1 | ||||
| ↓RV angiogenesis compared with compensated RVs | ||||
| Potus et al. (124) | Rats: MCT (60 mg/kg, 3–4 wk) | Rats-↑RV size, ↑RV end-diastolic pressure increased, ↓TAPSE, ↓stroke volume, and ↓cardiac output | Rats-RVSP, RVH, RV function by pressure-volume loops, echocardiograph, endurance testing Humans-Echocardiography, right heart catheterization | ↓mIR-126 |
| Human decompensated RVs and RV ECs | ↓SPRED1 | |||
| ↓ RV angiogenesis | ||||
| ↓Capillary density | ||||
| Humans-↓TAPSE, RV dilation | Functionally impaired ECs | |||
| Drake et al. (43) | Rats: SuHx | ↓cardiac output, ↓TAPSE, paradoxical movement of interventricular septum, pericardial fluid, ↓capillaries, ↑fibrosis | DNE | ↓Apelin protein |
| Rats: PABCu2+ (6 wk) | ↓VEGF-A, IGF1, apelin and Ang1 mRNA | |||
| Bogaard et al. (14) | Rats: SuHx | RVH, pericardial fluid, paradox movement of septum, RV dilation, ↓TAPSE, ↓cardiac output | RVSP, RVH, RV function by echocardiography, pressure-volume loops, thermodilution | ↓Capillary density |
| ↓VEGFA mRNA and protein | ||||
| Uncoupling of HIF1 and VEGF | ||||
| Bogaard et al. (15) | Rats: SuHx + Carvedilol | RVH, pericardial fluid, paradox movement of septum, RV dilation, ↓TAPSE, ↓cardiac output | RVSP, RVH, RV function by echocardiography, exercise endurance, hemodynamic assessment | Carvedilol treated rats had ↑capillary density |
| Graham et al. (61) | Human PH RVs | DNE | DNE | Stereological assessment of human revealed RVs had ↑capillary length and volume |
| Matori et al. (100) | Rats: MCT (60 mg/kg 30 days +Genistein last 10days) | ↓RV ejection fraction | RVSP, RVH, RV function by right heart catheterization, echocardiograph | ↓ RV capillarization in MCT rats, Genistein treatment ↑capillarization |
| Alzoubi et al. (5) | Rats: SuHx + 5 wk DHEA | ↓TAPSE | RVSP, RVH, RV function by right heart catheterization, echocardiograph | ↓RV capillarization in SuHx rats, treatment with DHEA ↑ RV capillarization |
| ↓Cardiac Index | ||||
| Handoko et al. (64) | Rats: MCT (60 mg/kg, last 4 wk exercise training as able) | ↓cardiac output, | RVSP, RVH, RV function by right heart catheterization, endurance testing and echocardiography | Exercise training ↓capillarization |
| ↓body weight | ||||
| van Albada (168) | Rats: aortocaval shunt + MCT (60 mg/kg + iloprost or aspirin for 30 days) | DNE | Mean pulmonary artery pressure, RVSP, RVH, RV function by right heart catheterization and echocardiograph | MCT ↓capillary to cardiomyocyte ratio |
| Prostacyclin treatment ↑ capillary to cardiomyocyte ratio |
Studies using human tissue are in boldface. Ang1, angiopoietin-1; DNE, did not evaluate adaptive vs. malaptive remodeling; ↑, increase; ↓, decrease; DHEA, dehydroepiandrosterone; EC, endothelial cell; HIF1, hypoxia-inducible factor 1; Hif1α+/−, Hif1α heterozygous knockout mouse; Hx, chronic hypoxia; IGF1, insulin-like growth factor 1; MCT, monocrotaline; PAB, pulmonary artery banding; PABCu2+, pulmonary artery banding + copper depleted diet; RVH, right ventricular hypertrophy; RVSP, right ventricular systolic pressure; SDF1, stromal derived factor 1; SuHx, Sugen5416 + hypoxia; TAPSE, tricuspid annular plane systolic excursion; TSA, trichostatin A; VEGF, vascular endothelial growth factor; VPA, valproic acid.
MECHANISMS AND MODIFIERS OF MICROVASCULAR FUNCTION IN ADAPTIVE AND MALADAPTIVE RV REMODELING
Here, we describe our current understanding of angiogenesis in adaptive and maladaptive RV remodeling. As in the LV, it has been speculated (though not definitively shown) that RV adaptive responses are induced or mediated through increased angiogenesis and that transition to maladaptive remodeling is characterized in part by loss of capillaries. We review the pertinent literature in more detail in the following sections. A summary is outlined in Tables 1 and 2.
Modulators of Microvascular Function of Relevance to the RV
This section discusses molecular mechanisms and pathways that are of interest to the study of angiogenesis in the RV (Fig. 2). We focus on mediators and pathways that either have been identified in the RV already or play a major role in the LV. For comprehensive reviews of the molecular mechanisms driving angiogenesis in health and disease in general, we refer the reader to Refs. 9, 24, 139, 173.
Fig. 2.
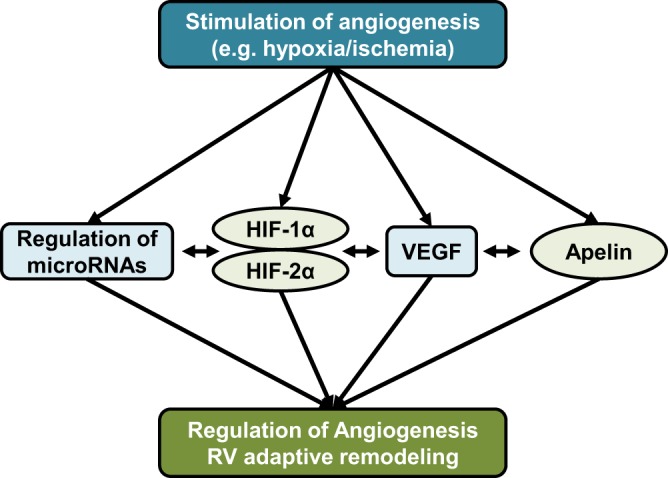
Simplified schematic of currently known pathways regulating angiogenesis in the right ventricle (RV). Proangiogenic factors such as hypoxia or ischemia increase activation and/or abundance of proangiogenic mediators such as hypoxia-inducible factors, VEGF, apelin, and micro-RNAs. Significant cross-talk exists between angiogenic pathways. For example, hypoxia-inducible factors (HIFs) are master regulators that regulate the expression of other regulators to induce angiogenesis during adaptive remodeling of the RV. However, vascular endothelial growth factor (VEGF), apelin, and micro-RNAs are likely also activated independently of HIFs. Decreased or insufficient upregulation of proangiogenic pathways, as well as defects in their downstream signaling pathways are purported to contribute to maladaptive RV remodeling and decompensation.
Hypoxia-Inducible Factors
Hypoxia-inducible factors (HIFs) are major regulators of angiogenesis, vasculogenesis, and EC homeostasis (139). Under homeostatic conditions, quiescent ECs express oxygen sensors prolyl hydroxylase domain proteins 1–3 (PHD1–3), which regulate the expression of HIF-1α and HIF-2α (139). Under hypoxic or ischemic conditions, these sensors become inactive, resulting in the stabilization and accumulation of HIF-1α and HIF-2α. The accumulation of HIFs rapidly regulates the transcriptional expression of a large number of target genes. One purpose of the HIF transcriptome is to stimulate sprouting angiogenesis in an effort to repair or prevent injury by increasing oxygen supply to ischemic or hypoxic tissues (97).
Although the role of HIF-1α and HIF-2α in stimulating angiogenic responses is complex and tightly regulated, in general, HIF-1α promotes vessel sprouting, while HIF-2α mediates vascular homeostasis (50). HIFs are necessary for proper vascular and cardiac development, as demonstrated in mice homozygous for a germline Hif1α-null allele, which is embryonically lethal due to cardiac and vascular defects (85, 132). Furthermore, mice heterozygous for this allele demonstrate impaired vascular development and decreased revascularization of injured or ischemic tissues (22). Cardiomyocyte-specific deletion of Hif1α in mice results in decreased capillary density (69), whereas transgenic mice overexpressing Hif1α in the heart have increased capillary density after myocardial infarction (78), demonstrating the importance of HIF-1α in the response to cardiovascular injury. Similarly, homozygous Hif2α germline null mutations are embryonically lethal and are associated with defects in vascular formation (34, 120, 159). Given the prominent role of HIFs in cardiac development and maintenance of cardiac homeostasis, it is not surprising that several studies have implicated regulators of HIF signaling in heart failure development (107, 134, 183).
In the pulmonary vasculature of PH models, HIF-1α plays a complex role but has been implicated in pathogenic angiogenesis and smooth muscle cell proliferation (reviewed in Refs. 140, 146, 162, 172). Similarly, HIF-2α in ECs has been implicated as a driver of pulmonary vascular remodeling (37, 76, 87). Although studies of HIFs in the LV and pulmonary vasculature have greatly increased our understanding of their roles in these compartments, the role of HIFs in the RV remains largely unexplored, leading to several questions, including (but not limited to) the following. 1) Are HIFs necessary and sufficient to promote angiogenesis in the RV? 2) Is activation of HIFs sufficient to delay the transition to maladaptive remodeling? 3) What is the effect of acute vs. chronic HIF signaling in the RV? While hypoxia-induced angiogenesis suggests a HIF-1α-mediated response in the RV (14, 84, 117, 151, 164), studies directly manipulating the HIF signaling pathway have only begun. Given this pathway’s role in promoting angiogenesis in other ischemic tissues, including the LV and the pulmonary vasculature, it is likely that, at least acutely (84), HIFs are promoting angiogenesis in the RV. However, one should note that HIF-1α also affects RV metabolism (14, 122, 151), thereby regulating RV adaptive and maladaptive responses independently of angiogenesis and demonstrating that this pathway plays a role in regulating RV function that is complex and multifaceted.
VEGF
Members of the VEGF family, comprising three main receptors (VEGFR1, VEGFR2, and VEGFR3) and five ligands (VEGF-A, VEGF-B, VEGF-C, VEGF-D, and PIGF), are major regulators of EC function, playing diverse and largely nonredundant roles in vascular homeostasis (23, 48, 173).
VEGF-A is the major ligand associated with stimulating angiogenesis during homeostasis and disease through interaction with its receptor, VEGFR2 (Flk1), leading to the induction of pro-survival and proangiogenic signaling (155, 187). VEGF-B binds primarily to VEGFR1 and prevents angiotensin II–induced cardiac diastolic dysfunction (142) and facilitates fatty acid transport in vascular ECs (62). VEGF-C, which signals through VEGFR2 and VEGFR3, activates endothelial tip cells and is necessary for embryogenesis (165) and is also a key regulator in adult lymphangiogenesis (20). VEGF receptors are critical for VEGF signaling. Germline deletion of Vegfr2 in mouse models is embryonically lethal due to lack of vascular bed formation (143). Interestingly, VEGFR1 (Flt-1) and VEGFR2 exhibit an antagonistic relationship, with VEGFR1 functioning as a decoy receptor to prevent activation of VEGFR2 (49). Along those lines, germline deletion of Flt1 is embryonically lethal due to excessive proliferation of angioblasts (49). Furthermore, a soluble, alternatively spliced variant of VEGFR1, s-Flt1, can repress VEGF signaling by sequestering VEGF, thus demonstrating another mechanism by which the activity of VEGFR2 can be tightly controlled (77).
Interestingly, VEGF-A plasma levels are elevated in patients with severe angioobliterative PAH (44, 116, 138). Plexiform lesions and pulmonary ECs from the lungs of PAH patients also strongly express VEGF-A and VEGFR2 (66, 156). However, whether VEGF-A is necessary for driving pulmonary vascular remodeling has not been elucidated. Like HIF-1α, the role of VEGF signaling in PAH is complex, and contradictory effects have been described (173). These differences are likely due to context- and time-dependent effects. For example, while VEGF signaling in general is thought to be a major contributor to PAH development (31, 156, 161), VEGFR2 blockade with Su5416 is used to induce experimental PH in rodents (32, 110, 156).
In the RV, VEGF-A has frequently, but not always, been reported as upregulated in animal models with adaptive RV remodeling (14, 43, 117, 124, 151) and decreased in animal models with RV failure (14, 15, 122), paralleling the decrease in capillarization shown in RV failure (14, 15, 43, 122, 151). In the rat Su546/hypoxia (SuHx) model of PH, treatment with the adrenergic receptor blocker carvedilol was associated with increased RV VEGF-A expression and RV capillarization (15). Recently, in the same model, it was shown that the antioxidant defense system, heme oxygenase-1, upregulated VEGF-A and increased RV capillarization (14). However, a direct causative link between decreased VEGF expression and capillary rarefaction has not yet been established. On the other hand, it was shown in RVs from patients with decompensated RV function that VEGF-A and VEGFR2 were not decreased compared with compensated RVs (124). Instead, it was shown that the capillary rarefaction observed in the decompensated RVs was due to upregulation of Sprouty-related EVH1 domain-containing protein-1 (SPRED-1), an inhibitor of the VEGF downstream target phospho-ERK1/2 (p42/44 MAPK) (124). This resulted in impaired angiogenic activity in cardiac ECs isolated from decompensated human RVs, which could be chemically and biologically rescued by inhibiting SPRED-1 (124). Together, these observations link VEGF signaling to angiogenesis in the RV. However, further study defining the role of VEGF signaling in adaptive and maladaptive remodeling in the RV is still needed. Furthermore, the role of other VEGF family members (e.g., VEGF-B), has not yet been studied in the RV.
Apelin and Elabela/Toddler
Apelin is a secreted peptide expressed in the vasculature and heart that exerts its effects via the G protein-coupled apelin receptor (APLNR) (6, 79–82, 91). Apelin and APLNR play a critical role in cardiac development (79, 86), angiogenesis (94, 182), pro-survival signaling (4), nitric oxide-dependent vasodilation (186), and inotropic signaling (153). Important for angiogenesis, apelin is expressed in tip and stalk cells, while APLNR is expressed only in stalk cells (94). APLNR is purported to play a role in vasculogenesis and is strongly expressed in circulating endothelial progenitor cells (157). Both hypoxia and VEGF-A induce the expression of APLNR in vascular ECs (94, 145), suggesting significant cross-talk between hypoxia, VEGF signaling, and apelin signaling.
Several lines of evidence suggest a role for apelin in PAH (reviewed in Refs. 6, 80) and cardiovascular disease (reviewed in Refs. 38, 72, 73). For example, apelin-null mice exhibit worsening PH (26), and plasma apelin levels are decreased in PAH patients (26, 55). Furthermore, treatment with APLNR ligand attenuates PH in multiple animal models (4, 46, 47). Finally, hypoxic mice infused with apelin exhibit increased cardiac output (4). Together, these data make a strong case for targeting this pathway therapeutically in cardiopulmonary diseases.
Despite the reported protective effects of apelin in PH, very little is known about its effects in the RV. It was reported in two experimental models of PH of adaptive RV remodeling [chronic hypoxia and pulmonary artery banding (PAB) without RV failure] that RV apelin mRNA and protein expression is increased (43). On the other hand, in models of maladaptive RV remodeling (SuHx, PAB with copper-depleted diet), apelin protein was decreased (43). Our group has recently shown that apelin mRNA is decreased in RVs from male and female SuHx-PH rats and that this decrease was more pronounced in male animals (52). These effects were linked to the female sex steroid 17β-estradiol (E2), as ovariectomy (OVX) decreased the level of apelin in female RVs to the same level found in male RVs, whereas E2 repletion in OVX females restored apelin levels (52). Interestingly, the increase in apelin abundance in intact females and E2-replete OVX females was associated with an increase in RV capillary density (unpublished data), suggesting that E2 and apelin have proangiogenic effects in the RV. Combined, these data suggest a role for apelin in modifying angiogenic responses in the RV. However, the specific mechanisms of apelin-mediated signaling responses in RV vascular homeostasis and adaptive as well as maladaptive remodeling are unknown. This is currently under investigation in our laboratory.
In addition to apelin, Elabela/Toddler is an endogenous ligand of APLNR and is strongly expressed in the pulmonary and cardiac vasculature. For a more in-depth overview of this signaling pathway, we refer the reader to several excellent reviews (28, 29, 180). Recently, exogenous treatment with Elabela/Toddler and apelin were shown to have significant beneficial effects on the pulmonary vasculature and RV function in a monocrotaline rat model of PH, reducing RV systolic pressure, RVH, and pulmonary vascular remodeling (181). PAH patients also exhibited decreased Elabela/Toddler expression in the pulmonary vasculature, LV, and coronary arteries (181). However, as with apelin, the effects of Elabela/Toddler signaling on the RV vasculature remain unknown. Taken together, these data make a compelling case for apelin/APLNR and Elabela/Toddler/APLNR pathways as clinically promising targets in both the pulmonary vasculature and RV in PH.
MicroRNAs
Evidence suggesting the epigenetic control of angiogenesis has been accumulating, particularly through noncoding microRNAs (miRNAs), which induce messenger RNA degradation or block translation (21). Since they target multiple genes rapidly, miRNAs are well positioned to regulate complex processes such as angiogenesis (30, 67, 92, 177). ECs express several miRNAs that are induced by hypoxia or VEGF during adaptive remodeling or acute ischemia (111, 135). Most of those stimulate angiogenesis by repressing angiostatic pathways and stimulating proangiogenic cascades (111). For example, expression of miR-126 is induced by the mechanosensitive transcription factor KLF2A and integrates the mechanosensory stimulus of blood flow (109). Furthermore, this miRNA was shown to play a major role in the transition from adaptive RV remodeling to maladaptive remodeling in PAH (124). Likewise, in monocrotaline PH rats with RV failure, downregulation of miR-208 lead to upregulation and activation of the complex mediator of transcription 13/nuclear receptor corepressor 1 axis and subsequently the inhibition of its target MEF2C (119). Although this mechanism was demonstrated in RV cardiomyocytes, MEF2C is also expressed in ECs, and the targeted deletion of Mef2c in mice is embryonically lethal due to cardiovascular defects and severe vascular abnormalities, making this axis potentially relevant for the RV vasculature (93, 96). Building on the idea that miRNAs are epigenetic regulators of angiogenesis, studies profiling miRNA expression patterns during progressive RV failure identified four RV-specific miRNAs that were upregulated in RV failure: miR-34a, −28, −93, and −148a (126, 127). These miRNAs were associated with DNA repair impediment, oxidant damage, and downregulation of proangiogenic regulatory networks (127, 154). In summary, several miRNAs seem to offer significant pro- or antiangiogenic potential and could rapidly affect adaptive and maladaptive signaling networks in the RV (19, 158). More work is needed to understand the role of miRNAs in RV angiogenesis, adaptive remodeling and failure.
Structural and Molecular Studies of Microvascular Function in Adaptive RVH
Although early studies did not always specifically assess RV function, they provided important information about angiogenic responses in the RV during adaptive remodeling (studies investigating angiogenesis in adaptive RVH are summarized in Table 1). For example, in a chronic rat hypoxia model of PH (10% ), VEGF mRNA expression was potently induced within 12 h after hypoxia exposure in both the RV and LV (117). After prolonged exposure to hypoxia, VEGF mRNA remained elevated in the RV, but not in the LV, peaking at 30 days. This was associated with an increased number of capillaries per RV myocyte in rats exposed to 30 days of hypoxia (117). In another rat model of chronic hypoxia (simulated altitude of 3,500 m for 4 wk), there was an increase of capillary density and decrease of muscle fiber density and fiber-to-capillary ratio in RVs but not in LVs (163). Similarly, our group previously demonstrated increased capillary-to-myocyte ratio in RVs from chronically hypoxic rats (88). Another early study assessed the gene expression profile in chronically hypoxic mouse RVs (12% for 4 wk). To directly test the role of HIF-1α in this model, Bohuslavova et al. (16) analyzed a panel of eleven hypoxia-responsive candidate genes, six of which were HIF-1α target genes. Of those six HIF-1α target genes, two are known regulators of angiogenesis: Vegfa and Flt1. In Hif1a partially deficient hypoxic male mice, the authors found that Vegfa mRNA was the only significantly decreased HIF1-α target gene in the RV (16). This suggests that VEGF is highly dependent on HIF-α mediated induction under chronic hypoxic conditions in the RV. And finally, a study using the rat monocrotaline model of PH (60 mg/kg for 3 wk) demonstrated an increase in RV capillary proliferation relative to muscle fibers (83). Together, these data lead to the observation that early RV adaptive responses are characterized by increased capillarization driven by early hypoxic responses. Whether these responses are driven primarily by VEGF-A and HIF-1α, or if there are also HIF-independent mechanisms at play, is currently not known.
More recent studies revealed that when following the monocrotaline rat model over time (60 mg/kg for 2–6 wk), compensated RVs exhibit increased HIF-1α activity [demonstrated by increased nuclear localization and upregulation of the target protein glucose transporter 1 (Glut1)] as well as increased expression of the proangiogenic regulators VEGF-A and stromal cell-derived factor 1 (SDF1) (151). This was associated with increased RV capillarization, with lectin fluorescence and von Willebrand factor expression peaking at 3 wk after monocrotaline injection. Another study used a stereological analysis approach to determine capillarization in an adaptive mouse model of PH (10% for 3 wk) (84). This study found correlations in RV angiogenesis, RVH, and RV function (assessed by echocardiography and pressure-volume loop measurement). In fact, within 7 days of hypoxia exposure, RV-specific increases in capillary length, surface area, and volume were detected and found to be accompanied by increased RV EC proliferation. Interestingly, whereas continued exposure to hypoxia led to a further increase in hypertrophy, this did not lead to an additional increase in angiogenesis. Finally, while Vegf-a and Vegf-b mRNA were not significantly changed, VEGF-A protein was significantly increased after 3 wk of hypoxia. To better understand the transcriptome that might be mediating RV adaptive responses, Drake et al. (43) performed gene expression microarray analyses in two models of RV adaptive remodeling (chronic hypoxia and PAB). Signaling networks upregulated in adaptive RV remodeling were associated with growth and cellular maintenance, angiogenesis, and energy metabolism. Of particular interest, apelin mRNA and protein were significantly increased in both models, whereas VEGF and insulin-like growth factor 1 (IGF-1) mRNA were significantly increased in at least one model of compensated RVH. Finally, in a PAB model of compensated RVH (6 wk of banding), RV function (evaluated by echocardiography) was preserved, accompanied by a trend for increased Vegf-a mRNA and by significantly upregulated HIF-1α protein and activity (evaluated by nuclear localization) (14). However, neither capillary volume nor density was significantly affected.
However, there are also studies in which RV adaptive remodeling was not associated with increased vascular density or proangiogenic signaling. For example, in a model of stable PH (40 mg/kg monocrotaline; defined as preserved cardiac output assessed by echocardiography), RV remodeling was characterized by decreased capillarization (131). Additionally, in a rat PAB study (PAB for 4 wk, defined as adaptive remodeling by less severe ischemia, and preserved TAPSE), whereas there was a trend for increased VEGF-A, there was a significant decrease RV microvasculature (measured by CD31 staining) and blood flow compared with controls (122).
One published study evaluated vascularization and EC function in human RVs and isolated human RV ECs. That study demonstrated that human ECs isolated from compensated human RVs displayed increased angiogenic potential compared with control human RVs (124). Furthermore, it was shown that antagomir-mediated downregulation of miR-126 decreased angiogenesis and tube formation in compensated human RV ECs.
Taken together, these data indicate an increase in vascularization and proangiogenesis signaling in several models of adaptive RVH. Data are most consistent and most convincing for chronic hypoxia models, whereas data for monocrotaline or PAB models are somewhat more mixed. This may either indicate that hypoxia is a particularly potent angiogenesis stimulator in the RV, or reflect inconsistencies in models and/or experimental approaches [e.g., tightness of band and duration of banding in the PAB model or differences in the metabolization of monocrotaline between rat strains (58, 115)]. Finally, data from human RV tissue and isolated RV ECs suggest that angiogenesis and EC function are maintained in cases of adaptive RV remodeling.
Structural and Molecular Studies of Microvascular Function in Maladaptive RVH
Studies in models of maladaptive RVH generally demonstrate impairments in angiogenesis and microvascular function (summarized in Table 2). However, discrepancies between studies exist. For example, one study using a monocrotaline model of RV failure (60 mg/kg) found RV VEGF mRNA expression decreased by 50% in the RV after 30 days; however, the number of capillaries per RV myocyte was unchanged (117). Conversely, other studies found that monocrotaline (60 mg/kg with a 4-wk experimental period) decreased RV capillary density vs. controls (131), decreased VEGF-A abundance, and RV capillarization compared with controls or PAB rats (122), and reduced coronary blood flow compared with control rats (122). In another study, Sutendra et al. (151) investigated the mechanism of adaptive and maladaptive RV remodeling using the monocrotaline model (60 mg/kg and duration of 5–6 wk). Those authors defined decompensated (maladaptive) remodeling as the point at which RV systolic pressure and cardiac output started decreasing. Interestingly, decompensated RVs were characterized by an inhibition of HIF-1α, activation of p53, and a decrease in angiogenic proteins (VEGF-A, SDF-1) and angiogenesis (evaluated by lectin stain), compared with compensated RVs but, importantly, not controls.
Despite this progress in defining maladaptive RV remodeling, studies have only begun to assess the mechanisms that might be driving the transition from adaptive to maladaptive remodeling. For instance, the previously mentioned study by Potus et al., (124) using a monocrotaline model (60 mg/kg), monitored the development of PH weekly by echocardiography. Between 3 and 4 wk, RV size and RV end-diastolic pressure increased, while TAPSE, stroke volume, and cardiac output significantly decreased. This stage was defined as decompensated RVH. It was observed that in decompensated RVs miR-126 was downregulated, associated with increased expression of SPRED-1, an inhibitor of the VEGF target ERK1/2. The authors concluded that upregulation of SPRED-1 in decompensated RVs inhibits VEGF signaling (despite an increase in VEGF protein), thus inhibiting angiogenesis. Interestingly, treatment with a miR-126 mimic increased angiogenesis in vitro and increased vascular density in vivo.
A study using different rat models of RV failure [SuHx (Su5416 injection followed by 4 wk of 10% O2 and 2 wk of 21% O2) or PAB with dietary copper depletion to inhibit HIF-1α] noted capillary rarefaction, associated with decreased mRNA of the angiogenic factors VEGF-A, IGF-1, apelin, and angiopoietin-1 compared with adaptive models (chronic hypoxia and PAB). Interestingly, apelin and IGF-1 protein expression, but not VEGF-A or angiopoietin-1, were decreased compared with animals with adaptive RV remodeling (43). This study defined decompensation by reduced cardiac output, echo measurements of decreased RV function (e.g., TAPSE), septal shift, presence of pericardial effusion, as well as loss of capillaries and presence of RV fibrosis. Similarly, our group found capillary numbers were decreased in the SuHx rat model vs. controls (Fig. 3). Subsequent studies found that SuHx-RVs exhibited decreased capillary density as well as VEGF-A mRNA and protein, whereas HIF-1α stabilization and nuclear accumulation remained intact, suggesting an uncoupling of VEGF-A and HIF-1α (14). This study also found that the RV vasculature was morphologically heterogeneous, with vessels being narrow and pruned in some areas and dilated and irregularly shaped in other areas. Interestingly, stimulation of heme oxygenase-1 with dietary supplementation with protandim prevented capillary loss, recoupled VEFG-A and HIF-1α, and preserved RV function. Finally, in another study, upregulation of VEGF-A and reduction in capillary rarefaction were achieved in SuHx rats after treatment with the β-adrenergic receptor antagonist carvedilol, accompanied by improved RV function (15).
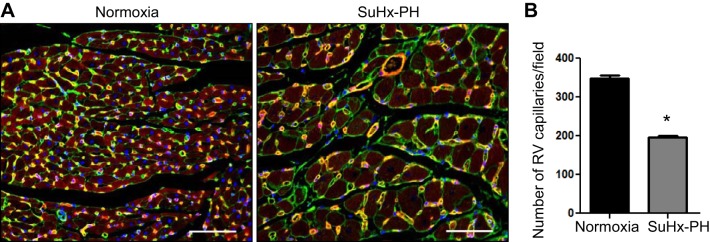
Fig. 3.
RV vascular rarefaction in Sugen5416 + Hypoxia- pulmonary hypertension (SuHx-PH) rats. A: immunofluorescence overlay of WGA (wheat germ agglutinin, green, cardiomyocytes), Lectin Griffonia simplicifolia (red, endothelial cells) and DAPI (blue, nuclei) stains in female normoxic control or SuHx-PH rats. B: capillaries were quantified from 4 fields per animal; n = 4 animals /group. Images were taken at ×20 magnification; scale bars, 50 µm. Data are expressed as means ± SE *P < 0.05 vs. normoxic control. Note that this analysis used traditional 2-D analysis similar to previous studies in the field.
Studies using human RV tissues are rare (boldface text in Tables 1 and 2). Initial studies in human PAH RVs demonstrated that the myocardial tissue of 10 PAH patients exhibited decreased capillary density compared with RV tissue from patients with myocardial infarction (131). Another study reported RV myocyte hypertrophy and capillary rarefaction in patients with scleroderma-associated PAH (122). Potus et al. studied RV free wall tissues from humans with normal RVs, compensated RVH, and PAH patients with decompensated RV failure and found that patients with decompensated RV failure exhibited decreased capillary density, functionally impaired ECs (defined as inability to form networks in vitro), and decreased miR-126 expression compared with controls and compensated RVs (124). Although these studies suggest a decrease in vascular density in human RV failure, a recent study utilizing stereological approaches in human RV tissue from PAH patients (n = 4), found that RV volume and length were actually increased in PAH vs. control (n = 3) tissue (61). These findings are important, as they are the first to use unbiased stereological approaches in human RV; however, it should be noted that clinical parameters from these patients were not reported, ischemia tissue was not detected in the RVs, and the sample size was limited, making it challenging to determine whether these RVs were decompensated at the time of patient death.
Taken together, the majority of these studies demonstrate that vascular density decreased and angiogenesis is impaired in maladaptive RVH. The current body of evidence suggests that proangiogenic mediators such as VEGF or miR-126 are either decreased or insufficiently increased, or are inhibited in the setting of maladaptive RVH. However, we have only started to identify the drivers of these processes. Furthermore, not all studies demonstrate these findings. Differences in techniques (e.g., two-dimensional analysis vs. unbiased stereology), control groups, animal models used (see Table 3 for an overview), animal strains, disease stage, and RV region (e.g., apex vs. outflow tract) may explain such discrepancies in results. Clearly, further study of the role of angiogenesis in adaptive and maladaptive RV remodeling while paying meticulous attention to these factors is needed.
Table 3.
Commonly used animal models to assess RV hypertrophy and failure
| Hypoxia | PA Banding | Monocrotaline | Sugen/Hypoxia | |
|---|---|---|---|---|
| Method used | Exposure to 10% (or ½ atmosphere) for 3–5 wks | Suture or clip around main PA | Subcutaneous injection of alkaloid monocrotaline (40–60 mg/kg, 2–6 wk) | Subcutaneous injection of VEGFR2 antagonist Su5416 + hypoxia (2–4 wks) + room air (≥2 wks) |
| Type of RV remodeling | Adaptive | Adaptive or maladaptive (depending on tightness of band and duration of banding) | In general, maladaptive (may be adaptive in early stages) | Maladaptive |
| Cardiac output | Maintained or slightly decreased | Maintained or decreased (dependent on tightness of band and duration of banding) | Decreased | Decreased |
| Effect of model on RV ECs | Strong induction of EC proliferation and chronic hypoxia responses | Increased shear-stress signaling responses due to changes in RV load | Strong EC toxin, initial injection likely induces robust EC apoptosis | VEGFR2 inhibition, likely preventing initial pro-angiogenic response to hypoxia |
| RV vascular effects | Capillary proliferation | Capillary volume and density maintained or slightly decreased | Vascular inflammation, decreased capillary density | Decreased capillary volume and density |
| Pulmonary vascular effects | Yes | No | Yes | Yes |
| Strains used | Rats, Mice | Rats | Rats | Rats |
| Factors affecting disease phenotype | Sex, age, animal strain, degree and duration of hypoxia | Sex, age, animal strain, tightness and duration of banding | Sex, age, animal strain, MCT dose and duration of model | Sex, age, animal strain, Su5416 vendor, duration of model |
| Human condition modeled | Chronic hypoxia, high altitude, some aspects of chronic lung disease | RV afterload increase without pulmonary vascular injury (e.g., pulmonary stenosis) | Inflammatory PAH with RV dysfunction | PAH resulting from EC injury |
Animal models of RV hypertrophy and failure from References (1, 3, 14, 15, 42, 56–58, 60, 65, 89, 113, 114, 136, 144, 149, 156, 179). MCT, monocrotaline; PA, pulmonary artery; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension; RV, right ventricle; RVSP, right ventricular systolic pressure; VEGFR2, vascular endothelial growth factor receptor 2; PH development is more pronounced in younger animals.
POTENTIAL ROLE OF PROANGIOGENIC THERAPEUTIC INTERVENTIONS FOR THE FAILING RV
Although the role of angiogenesis in adaptive vs. maladaptive remodeling of the RV is not yet fully understood, preliminary evidence exists that regulation of angiogenesis may be a potential therapeutic intervention to attenuate RV failure. Treatment strategies shown to be associated with increased RV vascularization and/or increased proangiogenic mediator expression include carvedilol (associated with increased VEGF-A protein and RV vascularization) (15), the soy phytoestrogen genistein (associated with increased RV vascularization) (100), steroid dehydroepiandrosterone (DHEA; associated with prevention of capillary rarefaction) (5), exercise training (associated with increased RV capillarization in a model of stable PH but not progressive PH) (64), protandim (associated with prevention of capillary loss through regulation of heme oxygenase-1) (14) or prostacyclin therapy (associated with an increase in capillary-to-cardiomyocyte ratio in a rat model of flow-associated PH) (168). However, since these strategies were pursued in PH models where they also attenuated pulmonary vascular remodeling, it is unclear whether the beneficial effects on the RV vasculature were due to a direct effect on the RV or merely a consequence of RV afterload reduction.
As mentioned above, treatment with miR-126 mimic restored RV vascularization and RV function in a rat monocrotaline model of PH (124). Since these effects occurred without any significant changes in the pulmonary vasculature, and since miR-126 enhances the angiogenic potential of ECs isolated from human decompensated RVs, it is likely that miR-126 exerts direct protective effects on the RV vasculature.
A variety of proangiogenic interventions have been shown to be beneficial in LV failure (reviewed in Refs. 112, 178). However, it is unknown whether such strategies would also be beneficial in the failing RV. It remains to be determined whether proangiogenic strategies are able to delay the progression from adaptive to maladaptive RVH. Another important question is how therapeutic approaches aimed at enhancing angiogenesis in the RV would affect the pulmonary vasculature in PAH. This is an important point, since dysregulated and possibly exaggerated angiogenesis has been linked to PAH pathogenesis (39, 167, 173).
KNOWLEDGE GAPS AND PATHWAYS FORWARD
Despite recent progress, several knowledge gaps remain. A summary of the current knowledge gaps in RV angiogenesis is provided in Table 4. Most of our knowledge regarding cardiomyocyte hypertrophy, remodeling, and angiogenesis comes from models of LV failure (112). We currently do not yet know if angiogenesis is necessary or sufficient for myocyte hypertrophy in the RV, as it is in the LV (112). Expanding on this, we currently do not know for sure if decreased angiogenesis is contributing to maladaptive remodeling. Recent studies have begun to link impaired angiogenesis to RV decompensation (14, 43, 124), but a clear causal relationship has yet to be established. To address this knowledge gap, we propose a move from studies using loss of RV capillaries as a descriptive characteristic of RV failure to mechanistic studies with the goal of altering angiogenesis to determine its role in the progression to RV failure.
Table 4.
Key knowledge gaps in the assessment of angiogenesis in the RV
| What are the molecular regulators of RV angiogenesis (proangiogenic and angiostatic)? |
| Is angiogenesis necessary and sufficient to induce RV cardiomyocyte hypertrophy? |
| Does decreased angiogenesis contribute to maladaptive remodeling in the RV? |
| How should we define adaptive remodeling in the RV? |
| How should we define maladaptive remodeling in the RV? |
| What methodological approach should we use to measure RV function vs failure? |
| What is the most accurate methodological approach to quantifying RV vascularization? |
| Are we introducing unintentional sampling bias into our traditional approaches to capillary density? |
| Does the use of different RV endothelial cell markers (CD31, von Willebrand factor, or lectin) between researchers introduce bias to our quantification of capillaries? |
| How do interventions targeting the pulmonary vasculature affect the vasculature of the RV? How do interventions targeting the RV vasculature affect the pulmonary vasculature? Can these compartments be targeted separately? |
| How can we maximize results from the study of RV angiogenesis in vitro? What can we learn from studies of RV cardiomyocyte-endothelial cell interactions on a chip or from iPSC-cardiomyocytes from PH patients? |
| How can we maximize the collection of human RV tissue from well phenotyped patients and controls? |
| How can we best monitor RV vascular function in clinical studies? |
RV, right ventricle.
A better characterization of the molecular regulators of RV angiogenesis is needed. Studies have only begun to identify such mechanisms, and further mechanistic studies are needed to identify upstream regulators and downstream targets responsible for regulating beneficial and detrimental angiogenic processes in the RV in health and disease. Mechanistic studies, including both unbiased screens for angiogenic and angiostatic mediators and hypothesis-driven assessment of specific regulatory candidates during multiple stages of RV remodeling will advance the field. Identification of plasma or imaging biomarkers reflecting angiogenic signaling in the RV would be of great value.
A particular problem in the field is that the RV functional stage is not fully characterized in many studies and that the terms “adaptive” and “maladaptive” are used differently by different investigators, making comparisons between studies challenging. A uniform definition of adaptive and maladaptive RVH is needed. This inconsistency is further confounded by differences in animal models and animal strains (Table 3). A “best” animal model of RV failure probably does not exist. Rather, the model used should be a function of the experimental question asked. For example, SuHx-PH shares features of human PAH and RV failure, but interventions aimed at modulating angiogenesis in the RV may also affect the pulmonary vasculature (and thus RV afterload). PAB avoids this problem, but the RV remodeling in this model is not always maladaptive. Therefore, whenever possible, results should be corroborated in more than one animal model. Finally, thorough RV phenotyping in preclinical (via echo and pressure-volume loop assessment) and clinical (via serial echos or MRI throughout the patient’s disease progression as well as right heart catheterization) studies would be a step forward.
Building on the challenge of incomplete RV phenotyping, another important knowledge gap is the absence of RV vascular assessment in preclinical and clinical interventions targeting the pulmonary vasculature. This is important, since RV function and RV vascular health may be negatively affected by interventions targeting the pulmonary vasculature in PAH. An example is the use of use of histone deacetylase (HDAC) inhibitors in PAH. Several HDAC inhibitors have been shown to be beneficial for the pulmonary vasculature in models of PAH (18, 25, 185). On the other hand, the HDAC inhibitors trichostatin A and valproic acid exerted negative effects on the RV vasculature (13). Specifically, in a 4-wk PAB rat model, animals treated with either agent for 2 wk exhibited decreased RV capillarization as well as decreased VEGF-A and angiopoietin-1 expression (13). Other studies of HDAC inhibition in experimental PH did not evaluate RV capillarization (18, 25, 40, 185). Careful evaluation of any potential cardiotoxic effects of HDAC inhibitors is therefore warranted before use in human PH patients. However, it should be noted that effects of HDAC inhibitors are highly class specific and that results from one class cannot be extrapolated to other classes or more selective HDAC inhibitors. Similarly, endothelin-1 blockade in PAH may be associated with negative effects on RV inotropic signaling (106). Future studies will need to evaluate whether and how the pulmonary vasculature can be safely targeted in PAH without negatively affecting the RV vasculature (and vice versa). Experts have suggested that RV function should be closely monitored in clinical trials of PAH drugs (7).
One important point of consideration is that RV angiogenesis has been evaluated in multiple animal models of PH using traditional two-dimensional approaches to quantify capillary counts. This approach can lead to underestimation of effective capillary length and surface area and unintentional sampling bias and has led to inconsistent findings (75, 105). On the other hand, data reporting capillary rarefaction in the failing RV obtained from several laboratories are consistent and are in line with data reporting vascular rarefaction in LV hypertrophy. Further studies are needed to define the “best” way of assessing RV vascularization in tissues. Such studies should be accompanied by the development of noninvasive imaging markers of RV vascularization (e.g., via CT, MRI, or PET) that could be used in animal studies as well as clinical studies.
Last, studies employing human RV tissue are rare. More investigations using clinically well-characterized human RV tissues, in particular from patients with adaptive remodeling, are clearly needed and would advance the field. Additionally, the development of new technologies such as the “heart on a chip” (to study RV EC-cardiomyocyte interactions) and cardiomyocytes or ECs differentiated from human PH-induced pluripotent stem cells (to screen drugs and determine cytotoxicity) may help overcome limitations of RV tissue rarity and allow for greater molecular and biochemical characterization of the cell types comprising the RV.
CONCLUSION
RV failure is a major determinant of morbidity and mortality in various types of PH. An important clinical need exists to identify the mechanisms that lead to RV failure development in PH and that facilitate the transition from adaptive to maladaptive RV remodeling. Angiogenesis has emerged as a clinically relevant modulator of RV failure development. The current body of evidence suggests that impaired angiogenesis and vascular rarefaction are major drivers of RV decompensation. However, we are only beginning to understand the underlying mechanisms leading to EC dysfunction and vascular rarefaction in the failing RV. Further studies are needed to identify regulators and downstream targets of angiogenic modulators in the RV. Ultimately, such investigations may lead to the development of novel therapeutic approaches that maintain or restore vascular health in the pressure-overloaded RV, thus enhancing RV function and improving patient outcomes.
GRANTS
A. L. Frump was supported by the American Heart Association (AHA) 17POST33670365 Postdoctoral fellowship. Dr. Bonnet was supported by the Canadian Institutes of Health Research (CIHR) and the Heart and Stroke Foundation of Canada and holds a research chair on vascular biology financed by the Canadian Institutes of Health Research. V. A. de Jesus Perez was supported by the National Institutes of Health (NIH) R01 HL-134776 and K08 HL-105884-01, and a Pulmonary Hypertension Association K08 Supplement Award. T Lahm was supported by Department of Veterans Affairs Merit Review Award 2 I01 BX0020-05, AHA 17GRNT33690017, and NIH 1R56 HL-134736-01A1.
DISCLOSURES
T. Lahm served as consultant on the Actelion sceintific review panel, on the speaker bureau for Bayer, and received research reagents from Eli Lilly.
AUTHOR CONTRIBUTIONS
A.L.F., S.B., V.A.d.J.P., and T.L. conceived and designed research; A.L.F. interpreted results of experiments; A.L.F. prepared figures; A.L.F., S.B., V.A.d.J.P., and T.L. drafted manuscript; A.L.F., S.B., V.A.d.J.P., and T.L. edited and revised manuscript; A.L.F., S.B., V.A.d.J.P., and T.L. approved final version of manuscript.
REFERENCES
- 1.Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation 121: 2747–2754, 2010. doi: 10.1161/CIRCULATIONAHA.109.927681. [DOI] [PubMed] [Google Scholar]
- 2.Adamis AP, Miller JW, Bernal M-T, D’Amico DJ, Folkman J, Yeo T-K, Yeo K-T. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol 118: 445–450, 1994. doi: 10.1016/S0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- 3.Akhavein F, St-Michel EJ, Seifert E, Rohlicek CV. Decreased left ventricular function, myocarditis, and coronary arteriolar medial thickening following monocrotaline administration in adult rats. J Appl Physiol (1985) 103: 287–295, 2007. doi: 10.1152/japplphysiol.01509.2005. [DOI] [PubMed] [Google Scholar]
- 4.Alastalo TP, Li M, Pde Jesus Perez V, Pham D, Sawada H, Wang JK, Koskenvuo M, Wang L, Freeman BA, Chang HY, Rabinovitch M. Disruption of PPARγ/β-catenin-mediated regulation of apelin impairs BMP-induced mouse and human pulmonary arterial EC survival. J Clin Invest 121: 3735–3746, 2011. doi: 10.1172/JCI43382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alzoubi A, Toba M, Abe K, O’Neill KD, Rocic P, Fagan KA, McMurtry IF, Oka M. Dehydroepiandrosterone restores right ventricular structure and function in rats with severe pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 304: H1708–H1718, 2013. doi: 10.1152/ajpheart.00746.2012. [DOI] [PubMed] [Google Scholar]
- 6.Andersen CU, Hilberg O, Mellemkjær S, Nielsen-Kudsk JE, Simonsen U. Apelin and pulmonary hypertension. Pulm Circ 1: 334–346, 2011. doi: 10.4103/2045-8932.87299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Archer SL. Riociguat for pulmonary hypertension—a glass half full. N Engl J Med 369: 386–388, 2013. doi: 10.1056/NEJMe1306684. [DOI] [PubMed] [Google Scholar]
- 8.Aspalter IM, Gordon E, Dubrac A, Ragab A, Narloch J, Vizán P, Geudens I, Collins RT, Franco CA, Abrahams CL, Thurston G, Fruttiger M, Rosewell I, Eichmann A, Gerhardt H. Alk1 and Alk5 inhibition by Nrp1 controls vascular sprouting downstream of Notch. Nat Commun 6: 7264, 2015. doi: 10.1038/ncomms8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beets K, Huylebroeck D, Moya IM, Umans L, Zwijsen A. Robustness in angiogenesis: notch and BMP shaping waves. Trends Genet 29: 140–149, 2013. doi: 10.1016/j.tig.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Blanco R, Gerhardt H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb Perspect Med 3: a006569, 2013. doi: 10.1101/cshperspect.a006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boerrigter BG, Bogaard HJ, Trip P, Groepenhoff H, Rietema H, Holverda S, Boonstra A, Postmus PE, Westerhof N, Vonk-Noordegraaf A. Ventilatory and cardiocirculatory exercise profiles in COPD: the role of pulmonary hypertension. Chest 142: 1166–1174, 2012. doi: 10.1378/chest.11-2798. [DOI] [PubMed] [Google Scholar]
- 12.Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest 135: 794–804, 2009. doi: 10.1378/chest.08-0492. [DOI] [PubMed] [Google Scholar]
- 13.Bogaard HJ, Mizuno S, Hussaini AA, Toldo S, Abbate A, Kraskauskas D, Kasper M, Natarajan R, Voelkel NF. Suppression of histone deacetylases worsens right ventricular dysfunction after pulmonary artery banding in rats. Am J Respir Crit Care Med 183: 1402–1410, 2011. doi: 10.1164/rccm.201007-1106OC. [DOI] [PubMed] [Google Scholar]
- 14.Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, Ockaili R, McCord JM, Voelkel NF. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation 120: 1951–1960, 2009. doi: 10.1161/CIRCULATIONAHA.109.883843. [DOI] [PubMed] [Google Scholar]
- 15.Bogaard HJ, Natarajan R, Mizuno S, Abbate A, Chang PJ, Chau VQ, Hoke NN, Kraskauskas D, Kasper M, Salloum FN, Voelkel NF. Adrenergic receptor blockade reverses right heart remodeling and dysfunction in pulmonary hypertensive rats. Am J Respir Crit Care Med 182: 652–660, 2010. doi: 10.1164/rccm.201003-0335OC. [DOI] [PubMed] [Google Scholar]
- 16.Bohuslavová R, Kolář F, Kuthanová L, Neckář J, Tichopád A, Pavlinkova G. Gene expression profiling of sex differences in HIF1-dependent adaptive cardiac responses to chronic hypoxia. J Appl Physiol (1985) 109: 1195–1202, 2010. doi: 10.1152/japplphysiol.00366.2010. [DOI] [PubMed] [Google Scholar]
- 17.Bonnet S, Provencher S, Guignabert C, Perros F, Boucherat O, Schermuly RT, Hassoun PM, Rabinovitch M, Nicolls MR, Humbert M. Translating Research into Improved Patient Care in Pulmonary Arterial Hypertension. Am J Respir Crit Care Med 195: 583–595, 2017. doi: 10.1164/rccm.201607-1515PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boucherat O, Chabot S, Paulin R, Trinh I, Bourgeois A, Potus F, Lampron M-C, Lambert C, Breuils-Bonnet S, Nadeau V, Paradis R, Goncharova EA, Provencher S, Bonnet S. HDAC6: a novel histone deacetylase implicated in pulmonary arterial hypertension. Sci Rep 7: 4546, 2017. doi: 10.1038/s41598-017-04874-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boucherat O, Potus F, Bonnet S. microRNA and Pulmonary Hypertension. Adv Exp Med Biol 888: 237–252, 2015. doi: 10.1007/978-3-319-22671-2_12. [DOI] [PubMed] [Google Scholar]
- 20.Bry M, Kivelä R, Holopainen T, Anisimov A, Tammela T, Soronen J, Silvola J, Saraste A, Jeltsch M, Korpisalo P, Carmeliet P, Lemström KB, Shibuya M, Ylä-Herttuala S, Alhonen L, Mervaala E, Andersson LC, Knuuti J, Alitalo K. Vascular endothelial growth factor-B acts as a coronary growth factor in transgenic rats without inducing angiogenesis, vascular leak, or inflammation. Circulation 122: 1725–1733, 2010. doi: 10.1161/CIRCULATIONAHA.110.957332. [DOI] [PubMed] [Google Scholar]
- 21.Buysschaert I, Schmidt T, Roncal C, Carmeliet P, Lambrechts D. Genetics, epigenetics and pharmaco-(epi)genomics in angiogenesis. J Cell Mol Med 12, 6B: 2533–2551, 2008. doi: 10.1111/j.1582-4934.2008.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai Z, Zhong H, Bosch-Marce M, Fox-Talbot K, Wang L, Wei C, Trush MA, Semenza GL. Complete loss of ischaemic preconditioning-induced cardioprotection in mice with partial deficiency of HIF-1 alpha. Cardiovasc Res 77: 463–470, 2007. doi: 10.1093/cvr/cvm035. [DOI] [PubMed] [Google Scholar]
- 23.Carmeliet P. Angiogenesis in health and disease. Nat Med 9: 653–660, 2003. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 24.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature 473: 298–307, 2011. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavasin MA, Demos-Davies K, Horn TR, Walker LA, Lemon DD, Birdsey N, Weiser-Evans MC, Harral J, Irwin DC, Anwar A, Yeager ME, Li M, Watson PA, Nemenoff RA, Buttrick PM, Stenmark KR, McKinsey TA. Selective class I histone deacetylase inhibition suppresses hypoxia-induced cardiopulmonary remodeling through an antiproliferative mechanism. Circ Res 110: 739–748, 2012. doi: 10.1161/CIRCRESAHA.111.258426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandra SM, Razavi H, Kim J, Agrawal R, Kundu RK, de Jesus Perez V, Zamanian RT, Quertermous T, Chun HJ. Disruption of the apelin-APJ system worsens hypoxia-induced pulmonary hypertension. Arterioscler Thromb Vasc Biol 31: 814–820, 2011. doi: 10.1161/ATVBAHA.110.219980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaouat A, Weitzenblum E, Krieger J, Oswald M, Kessler R. Pulmonary hemodynamics in the obstructive sleep apnea syndrome. Results in 220 consecutive patients. Chest 109: 380–386, 1996. doi: 10.1378/chest.109.2.380. [DOI] [PubMed] [Google Scholar]
- 28.Chapman NA, Dupré DJ, Rainey JK. The apelin receptor: physiology, pathology, cell signalling, and ligand modulation of a peptide-activated class A GPCR. Biochem Cell Biol 92: 431–440, 2014. doi: 10.1139/bcb-2014-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaves-Almagro C, Castan-Laurell I, Dray C, Knauf C, Valet P, Masri B. Apelin receptors: From signaling to antidiabetic strategy. Eur J Pharmacol 763, Pt B: 149–159, 2015. doi: 10.1016/j.ejphar.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Chistiakov DA, Orekhov AN, Bobryshev YV. The role of miR-126 in embryonic angiogenesis, adult vascular homeostasis, and vascular repair and its alterations in atherosclerotic disease. J Mol Cell Cardiol 97: 47–55, 2016. doi: 10.1016/j.yjmcc.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Cho YJ, Han JY, Lee SG, Jeon BT, Choi WS, Hwang YS, Roh GS, Lee JD. Temporal changes of angiopoietins and Tie2 expression in rat lungs after monocrotaline-induced pulmonary hypertension. Comp Med 59: 350–356, 2009. [PMC free article] [PubMed] [Google Scholar]
- 32.Ciuclan L, Bonneau O, Hussey M, Duggan N, Holmes AM, Good R, Stringer R, Jones P, Morrell NW, Jarai G, Walker C, Westwick J, Thomas M. A novel murine model of severe pulmonary arterial hypertension. Am J Respir Crit Care Med 184: 1171–1182, 2011. doi: 10.1164/rccm.201103-0412OC. [DOI] [PubMed] [Google Scholar]
- 33.Collinson DJ, Donnelly R. Therapeutic angiogenesis in peripheral arterial disease: can biotechnology produce an effective collateral circulation? Eur J Vasc Endovasc Surg 28: 9–23, 2004. doi: 10.1016/j.ejvs.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 34.Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, Nemery B, Dewerchin M, Van Veldhoven P, Plate K, Moons L, Collen D, Carmeliet P. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med 8: 702–710, 2002. doi: 10.1038/nm721. [DOI] [PubMed] [Google Scholar]
- 35.Cottin V, Le Pavec J, Prévot G, Mal H, Humbert M, Simonneau G, Cordier JF. Pulmonary hypertension in patients with combined pulmonary fibrosis and emphysema syndrome. Eur Respir J 35: 105–111, 2010. doi: 10.1183/09031936.00038709. [DOI] [PubMed] [Google Scholar]
- 36.Crawford TN, Alfaro DV III, Kerrison JB, Jablon EP. Diabetic retinopathy and angiogenesis. Curr Diabetes Rev 5: 8–13, 2009. doi: 10.2174/157339909787314149. [DOI] [PubMed] [Google Scholar]
- 37.Dai Z, Li M, Wharton J, Zhu MM, Zhao YY. Prolyl-4 Hydroxylase 2 (PHD2) Deficiency in Endothelial Cells and Hematopoietic Cells Induces Obliterative Vascular Remodeling and Severe Pulmonary Arterial Hypertension in Mice and Humans Through Hypoxia-Inducible Factor-2α. Circulation 133: 2447–2458, 2016. doi: 10.1161/CIRCULATIONAHA.116.021494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dalzell JR, Rocchiccioli JP, Weir RA, Jackson CE, Padmanabhan N, Gardner RS, Petrie MC, McMurray JJ. The Emerging Potential of the Apelin-APJ System in Heart Failure. J Card Fail 21: 489–498, 2015. doi: 10.1016/j.cardfail.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 39.de Jesus Perez VA. Molecular pathogenesis and current pathology of pulmonary hypertension. Heart Fail Rev 21: 239–257, 2016. doi: 10.1007/s10741-015-9519-2. [DOI] [PubMed] [Google Scholar]
- 40.De Raaf MA, Hussaini AA, Gomez-Arroyo J, Kraskaukas D, Farkas D, Happé C, Voelkel NF, Bogaard HJ. Histone deacetylase inhibition with trichostatin A does not reverse severe angioproliferative pulmonary hypertension in rats (2013 Grover Conference series). Pulm Circ 4: 237–243, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.del Toro R, Prahst C, Mathivet T, Siegfried G, Kaminker JS, Larrivee B, Breant C, Duarte A, Takakura N, Fukamizu A, Penninger J, Eichmann A. Identification and functional analysis of endothelial tip cell-enriched genes. Blood 116: 4025–4033, 2010. doi: 10.1182/blood-2010-02-270819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dias CA, Assad RS, Caneo LF, Abduch MC, Aiello VD, Dias AR, Marcial MB, Oliveira SA. Reversible pulmonary trunk banding. II. An experimental model for rapid pulmonary ventricular hypertrophy. J Thorac Cardiovasc Surg 124: 999–1006, 2002. doi: 10.1067/mtc.2002.124234. [DOI] [PubMed] [Google Scholar]
- 43.Drake JI, Bogaard HJ, Mizuno S, Clifton B, Xie B, Gao Y, Dumur CI, Fawcett P, Voelkel NF, Natarajan R. Molecular signature of a right heart failure program in chronic severe pulmonary hypertension. Am J Respir Cell Mol Biol 45: 1239–1247, 2011. doi: 10.1165/rcmb.2010-0412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eddahibi S, Humbert M, Sediame S, Chouaid C, Partovian C, Maître B, Teiger E, Rideau D, Simonneau G, Sitbon O, Adnot S. Imbalance between platelet vascular endothelial growth factor and platelet-derived growth factor in pulmonary hypertension. Effect of prostacyclin therapy. Am J Respir Crit Care Med 162: 1493–1499, 2000. doi: 10.1164/ajrccm.162.4.2003124. [DOI] [PubMed] [Google Scholar]
- 45.Erzurum S, Rounds SI, Stevens T, Aldred M, Aliotta J, Archer SL, Asosingh K, Balaban R, Bauer N, Bhattacharya J, Bogaard H, Choudhary G, Dorn GW II, Dweik R, Fagan K, Fallon M, Finkel T, Geraci M, Gladwin MT, Hassoun PM, Humbert M, Kaminski N, Kawut SM, Loscalzo J, McDonald D, McMurtry IF, Newman J, Nicolls M, Rabinovitch M, Shizuru J, Oka M, Polgar P, Rodman D, Schumacker P, Stenmark K, Tuder R, Voelkel N, Sullivan E, Weinshilboum R, Yoder MC, Zhao Y, Gail D, Moore TM. Strategic plan for lung vascular research: An NHLBI-ORDR Workshop Report. Am J Respir Crit Care Med 182: 1554–1562, 2010. doi: 10.1164/rccm.201006-0869WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Falcão-Pires I, Gonçalves N, Henriques-Coelho T, Moreira-Gonçalves D, Roncon-Albuquerque R Jr, Leite-Moreira AF. Apelin decreases myocardial injury and improves right ventricular function in monocrotaline-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol 296: H2007–H2014, 2009. doi: 10.1152/ajpheart.00089.2009. [DOI] [PubMed] [Google Scholar]
- 47.Fan XF, Wang Q, Mao SZ, Hu LG, Hong L, Tian LX, Gao YQ, Gong YS. [Protective and therapeutic effect of apelin on chronic hypoxic pulmonary hypertension in rats]. Zhongguo Ying Yong Sheng Li Xue Za Zhi 26: 9–12, 2010. [PubMed] [Google Scholar]
- 48.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 9: 669–676, 2003. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 49.Fong GH, Zhang L, Bryce DM, Peng J. Increased hemangioblast commitment, not vascular disorganization, is the primary defect in flt-1 knock-out mice. Development 126: 3015–3025, 1999. [DOI] [PubMed] [Google Scholar]
- 50.Fraisl P, Mazzone M, Schmidt T, Carmeliet P. Regulation of angiogenesis by oxygen and metabolism. Dev Cell 16: 167–179, 2009. doi: 10.1016/j.devcel.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 51.Friedberg MK, Redington AN. Right versus left ventricular failure: differences, similarities, and interactions. Circulation 129: 1033–1044, 2014. doi: 10.1161/CIRCULATIONAHA.113.001375. [DOI] [PubMed] [Google Scholar]
- 52.Frump AL, Goss KN, Vayl A, Albrecht M, Fisher A, Tursunova R, Fierst J, Whitson J, Cucci AR, Brown MB, Lahm T. Estradiol improves right ventricular function in rats with severe angioproliferative pulmonary hypertension: effects of endogenous and exogenous sex hormones. Am J Physiol Lung Cell Mol Physiol 308: L873–L890, 2015. doi: 10.1152/ajplung.00006.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geudens I, Gerhardt H. Coordinating cell behaviour during blood vessel formation. Development 138: 4569–4583, 2011. doi: 10.1242/dev.062323. [DOI] [PubMed] [Google Scholar]
- 54.Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol 37: 183–188, 2001. doi: 10.1016/S0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 55.Goetze JP, Rehfeld JF, Carlsen J, Videbaek R, Andersen CB, Boesgaard S, Friis-Hansen L. Apelin: a new plasma marker of cardiopulmonary disease. Regul Pept 133: 134–138, 2006. doi: 10.1016/j.regpep.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 56.Gomez-Arroyo J, Mizuno S, Szczepanek K, Van Tassell B, Natarajan R, dos Remedios CG, Drake JI, Farkas L, Kraskauskas D, Wijesinghe DS, Chalfant CE, Bigbee J, Abbate A, Lesnefsky EJ, Bogaard HJ, Voelkel NF. Metabolic gene remodeling and mitochondrial dysfunction in failing right ventricular hypertrophy secondary to pulmonary arterial hypertension. Circ Heart Fail 6: 136–144, 2013. doi: 10.1161/CIRCHEARTFAILURE.111.966127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gomez-Arroyo J, Saleem SJ, Mizuno S, Syed AA, Bogaard HJ, Abbate A, Taraseviciene-Stewart L, Sung Y, Kraskauskas D, Farkas D, Conrad DH, Nicolls MR, Voelkel NF. A brief overview of mouse models of pulmonary arterial hypertension: problems and prospects. Am J Physiol Lung Cell Mol Physiol 302: L977–L991, 2012. doi: 10.1152/ajplung.00362.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gomez-Arroyo JG, Farkas L, Alhussaini AA, Farkas D, Kraskauskas D, Voelkel NF, Bogaard HJ. The monocrotaline model of pulmonary hypertension in perspective. Am J Physiol Lung Cell Mol Physiol 302: L363–L369, 2012. doi: 10.1152/ajplung.00212.2011. [DOI] [PubMed] [Google Scholar]
- 59.Gómez A, Bialostozky D, Zajarias A, Santos E, Palomar A, Martínez ML, Sandoval J. Right ventricular ischemia in patients with primary pulmonary hypertension. J Am Coll Cardiol 38: 1137–1142, 2001. doi: 10.1016/S0735-1097(01)01496-6. [DOI] [PubMed] [Google Scholar]
- 60.Gomez A, Mink S. Interaction between effects of hypoxia and hypercapnia on altering left ventricular relaxation and chamber stiffness in dogs. Am Rev Respir Dis 146: 313–320, 1992. doi: 10.1164/ajrccm/146.2.313. [DOI] [PubMed] [Google Scholar]
- 61.Graham BB, Koyanagi D, Kandasamy B, Tuder RM. Right ventricle vasculature in human pulmonary hypertension assessed by stereology. Am J Respir Crit Care Med 196: 1075–1077, 2017. doi: 10.1164/rccm.201702-0425LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hagberg C, Mehlem A, Falkevall A, Muhl L, Eriksson U. Endothelial fatty acid transport: role of vascular endothelial growth factor B. Physiology (Bethesda) 28: 125–134, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 100: 57–70, 2000. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 64.Handoko ML, de Man FS, Happé CM, Schalij I, Musters RJ, Westerhof N, Postmus PE, Paulus WJ, van der Laarse WJ, Vonk-Noordegraaf A. Opposite effects of training in rats with stable and progressive pulmonary hypertension. Circulation 120: 42–49, 2009. doi: 10.1161/CIRCULATIONAHA.108.829713. [DOI] [PubMed] [Google Scholar]
- 65.Hessel MH, Steendijk P, den Adel B, Schutte CI, van der Laarse A. Characterization of right ventricular function after monocrotaline-induced pulmonary hypertension in the intact rat. Am J Physiol Heart Circ Physiol 291: H2424–H2430, 2006. doi: 10.1152/ajpheart.00369.2006. [DOI] [PubMed] [Google Scholar]
- 66.Hirose S, Hosoda Y, Furuya S, Otsuki T, Ikeda E. Expression of vascular endothelial growth factor and its receptors correlates closely with formation of the plexiform lesion in human pulmonary hypertension. Pathol Int 50: 472–479, 2000. doi: 10.1046/j.1440-1827.2000.01068.x. [DOI] [PubMed] [Google Scholar]
- 67.Hoelscher SC, Doppler SA, Dreßen M, Lahm H, Lange R, Krane M. MicroRNAs: pleiotropic players in congenital heart disease and regeneration. J Thorac Dis 9, Suppl 1: S64–S81, 2017. doi: 10.21037/jtd.2017.03.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoeper MM, Humbert M, Souza R, Idrees M, Kawut SM, Sliwa-Hahnle K, Jing ZC, Gibbs JS. A global view of pulmonary hypertension. Lancet Respir Med 4: 306–322, 2016. doi: 10.1016/S2213-2600(15)00543-3. [DOI] [PubMed] [Google Scholar]
- 69.Huang Y, Hickey RP, Yeh JL, Liu D, Dadak A, Young LH, Johnson RS, Giordano FJ. Cardiac myocyte-specific HIF-1alpha deletion alters vascularization, energy availability, calcium flux, and contractility in the normoxic heart. FASEB J 18: 1138–1140, 2004. [DOI] [PubMed] [Google Scholar]
- 70.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaïci A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Cottin V, Degano B, Jaïs X, Montani D, Souza R, Simonneau G. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 122: 156–163, 2010. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- 71.Hyduk A, Croft JB, Ayala C, Zheng K, Zheng ZJ, Mensah GA. Pulmonary hypertension surveillance–United States, 1980-2002. MMWR Surveill Summ 54: 1–28, 2005. [PubMed] [Google Scholar]
- 72.Iwanaga Y, Kihara Y, Takenaka H, Kita T. Down-regulation of cardiac apelin system in hypertrophied and failing hearts: Possible role of angiotensin II-angiotensin type 1 receptor system. J Mol Cell Cardiol 41: 798–806, 2006. doi: 10.1016/j.yjmcc.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 73.Japp AG, Newby DE. The apelin-APJ system in heart failure: pathophysiologic relevance and therapeutic potential. Biochem Pharmacol 75: 1882–1892, 2008. doi: 10.1016/j.bcp.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 74.Jin Y, Kaluza D, Jakobsson L. VEGF, Notch and TGFβ/BMPs in regulation of sprouting angiogenesis and vascular patterning. Biochem Soc Trans 42: 1576–1583, 2014. doi: 10.1042/BST20140231. [DOI] [PubMed] [Google Scholar]
- 75.Kaneko N, Matsuda R, Toda M, Shimamoto K. Three-dimensional reconstruction of the human capillary network and the intramyocardial micronecrosis. Am J Physiol Heart Circ Physiol 300: H754–H761, 2011. doi: 10.1152/ajpheart.00486.2010. [DOI] [PubMed] [Google Scholar]
- 76.Kapitsinou PP, Rajendran G, Astleford L, Michael M, Schonfeld MP, Fields T, Shay S, French JL, West J, Haase VH. The endothelial prolyl-4-hydroxylase domain 2/hypoxia-inducible factor 2 axis regulates pulmonary artery pressure in mice. Mol Cell Biol 36: 1584–1594, 2016. doi: 10.1128/MCB.01055-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA 90: 10705–10709, 1993. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kido M, Du L, Sullivan CC, Li X, Deutsch R, Jamieson SW, Thistlethwaite PA. Hypoxia-inducible factor 1-alpha reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. J Am Coll Cardiol 46: 2116–2124, 2005. doi: 10.1016/j.jacc.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 79.Kidoya H, Takakura N. Biology of the apelin-APJ axis in vascular formation. J Biochem 152: 125–131, 2012. doi: 10.1093/jb/mvs071. [DOI] [PubMed] [Google Scholar]
- 80.Kim J. Apelin-APJ signaling: a potential therapeutic target for pulmonary arterial hypertension. Mol Cells 37: 196–201, 2014. doi: 10.14348/molcells.2014.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kleinz MJ, Davenport AP. Immunocytochemical localization of the endogenous vasoactive peptide apelin to human vascular and endocardial endothelial cells. Regul Pept 118: 119–125, 2004. doi: 10.1016/j.regpep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 82.Kleinz MJ, Skepper JN, Davenport AP. Immunocytochemical localisation of the apelin receptor, APJ, to human cardiomyocytes, vascular smooth muscle and endothelial cells. Regul Pept 126: 233–240, 2005. doi: 10.1016/j.regpep.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 83.Kobayashi H, Yoshimura Y, Suzuki H, Hosoda Y. Regional difference of capillary-to-fiber ratio in the heart of monocrotaline-treated rats. Basic Res Cardiol 89: 118–127, 1994. [DOI] [PubMed] [Google Scholar]
- 84.Kolb TM, Peabody J, Baddoura P, Fallica J, Mock JR, Singer BD, D’Alessio FR, Damarla M, Damico RL, Hassoun PM. Right ventricular angiogenesis is an early adaptive response to chronic hypoxia-induced pulmonary hypertension. Microcirculation 22: 724–736, 2015. doi: 10.1111/micc.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kotch LE, Iyer NV, Laughner E, Semenza GL. Defective vascularization of HIF-1alpha-null embryos is not associated with VEGF deficiency but with mesenchymal cell death. Dev Biol 209: 254–267, 1999. doi: 10.1006/dbio.1999.9253. [DOI] [PubMed] [Google Scholar]
- 86.Kuba K, Zhang L, Imai Y, Arab S, Chen M, Maekawa Y, Leschnik M, Leibbrandt A, Markovic M, Schwaighofer J, Beetz N, Musialek R, Neely GG, Komnenovic V, Kolm U, Metzler B, Ricci R, Hara H, Meixner A, Nghiem M, Chen X, Dawood F, Wong KM, Sarao R, Cukerman E, Kimura A, Hein L, Thalhammer J, Liu PP, Penninger JM. Impaired heart contractility in Apelin gene-deficient mice associated with aging and pressure overload. Circ Res 101: e32–e42, 2008. doi: 10.1161/CIRCRESAHA.107.158659. [DOI] [PubMed] [Google Scholar]
- 87.Labrousse-Arias D, Castillo-González R, Rogers NM, Torres-Capelli M, Barreira B, Aragonés J, Cogolludo Á, Isenberg JS, Calzada MJ. HIF-2α-mediated induction of pulmonary thrombospondin-1 contributes to hypoxia-driven vascular remodelling and vasoconstriction. Cardiovasc Res 109: 115–130, 2016. doi: 10.1093/cvr/cvv243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lahm T, Albrecht M, Fisher AJ, Selej M, Patel NG, Brown JA, Justice MJ, Brown MB, Van Demark M, Trulock KM, Dieudonne D, Reddy JG, Presson RG, Petrache I. 17β-Estradiol attenuates hypoxic pulmonary hypertension via estrogen receptor-mediated effects. Am J Respir Crit Care Med 185: 965–980, 2012. doi: 10.1164/rccm.201107-1293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lahm T, Tuder RM, Petrache I. Progress in solving the sex hormone paradox in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 307: L7–L26, 2014. doi: 10.1152/ajplung.00337.2013. [DOI] [PubMed] [Google Scholar]
- 90.Lajoie AC, Lauzière G, Lega J-C, Lacasse Y, Martin S, Simard S, Bonnet S, Provencher S. Combination therapy versus monotherapy for pulmonary arterial hypertension: a meta-analysis. Lancet Respir Med 4: 291–305, 2016. doi: 10.1016/S2213-2600(16)00027-8. [DOI] [PubMed] [Google Scholar]
- 91.Lee DK, Cheng R, Nguyen T, Fan T, Kariyawasam AP, Liu Y, Osmond DH, George SR, O’Dowd BF. Characterization of apelin, the ligand for the APJ receptor. J Neurochem 74: 34–41, 2000. doi: 10.1046/j.1471-4159.2000.0740034.x. [DOI] [PubMed] [Google Scholar]
- 92.Lima J Jr, Batty JA, Sinclair H, Kunadian V. MicroRNAs in ischemic heart disease: from pathophysiology to potential clinical applications. Cardiol Rev 25: 117–125, 2017. doi: 10.1097/CRD.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 93.Lin Q, Lu J, Yanagisawa H, Webb R, Lyons GE, Richardson JA, Olson EN. Requirement of the MADS-box transcription factor MEF2C for vascular development. Development 125: 4565–4574, 1998. [DOI] [PubMed] [Google Scholar]
- 94.Liu Q, Hu T, He L, Huang X, Tian X, Zhang H, He L, Pu W, Zhang L, Sun H, Fang J, Yu Y, Duan S, Hu C, Hui L, Zhang H, Quertermous T, Xu Q, Red-Horse K, Wythe JD, Zhou B. Genetic targeting of sprouting angiogenesis using Apln-CreER. Nat Commun 6: 6020, 2015. doi: 10.1038/ncomms7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Executive summary: heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation 121: 948–954, 2010. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 96.Maiti D, Xu Z, Duh EJ. Vascular endothelial growth factor induces MEF2C and MEF2-dependent activity in endothelial cells. Invest Ophthalmol Vis Sci 49: 3640–3648, 2008. doi: 10.1167/iovs.08-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell 40: 294–309, 2010. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maron BA, Choudhary G, Khan UA, Jankowich MD, McChesney H, Ferrazzani SJ, Gaddam S, Sharma S, Opotowsky AR, Bhatt DL, Rocco TP, Aragam JR. Clinical profile and underdiagnosis of pulmonary hypertension in US veteran patients. Circ Heart Fail 6: 906–912, 2013. doi: 10.1161/CIRCHEARTFAILURE.112.000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maron BA, Hess E, Maddox TM, Opotowsky AR, Tedford RJ, Lahm T, Joynt KE, Kass DJ, Stephens T, Stanislawski MA, Swenson ER, Goldstein RH, Leopold JA, Zamanian RT, Elwing JM, Plomondon ME, Grunwald GK, Barón AE, Rumsfeld JS, Choudhary G. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program. Circulation 133: 1240–1248, 2016. doi: 10.1161/CIRCULATIONAHA.115.020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Matori H, Umar S, Nadadur RD, Sharma S, Partow-Navid R, Afkhami M, Amjedi M, Eghbali M. Genistein, a soy phytoestrogen, reverses severe pulmonary hypertension and prevents right heart failure in rats. Hypertension 60: 425–430, 2012. doi: 10.1161/HYPERTENSIONAHA.112.191445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meloche J, Lampron MC, Nadeau V, Maltais M, Potus F, Lambert C, Tremblay E, Vitry G, Breuils-Bonnet S, Boucherat O, Charbonneau E, Provencher S, Paulin R, Bonnet S. Implication of inflammation and epigenetic readers in coronary artery remodeling in patients with pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol 37: 1513–1523, 2017. doi: 10.1161/ATVBAHA.117.309156. [DOI] [PubMed] [Google Scholar]
- 102.Mohammed SF, Hussain I, AbouEzzeddine OF, Takahama H, Kwon SH, Forfia P, Roger VL, Redfield MM. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation 130: 2310–2320, 2014. doi: 10.1161/CIRCULATIONAHA.113.008461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Morrison DA, Adcock K, Collins CM, Goldman S, Caldwell JH, Schwarz MI. Right ventricular dysfunction and the exercise limitation of chronic obstructive pulmonary disease. J Am Coll Cardiol 9: 1219–1229, 1987. doi: 10.1016/S0735-1097(87)80459-X. [DOI] [PubMed] [Google Scholar]
- 104.Moschetta M, Mishima Y, Sahin I, Manier S, Glavey S, Vacca A, Roccaro AM, Ghobrial IM. Role of endothelial progenitor cells in cancer progression. Biochim Biophys Acta 1846: 26–39, 2014. [DOI] [PubMed] [Google Scholar]
- 105.Mühlfeld C. Quantitative morphology of the vascularisation of organs: A stereological approach illustrated using the cardiac circulation. Ann Anat 196: 12–19, 2014. doi: 10.1016/j.aanat.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 106.Nagendran J, Sutendra G, Paterson I, Champion HC, Webster L, Chiu B, Haromy A, Rebeyka IM, Ross DB, Michelakis ED. Endothelin axis is upregulated in human and rat right ventricular hypertrophy. Circ Res 112: 347–354, 2013. doi: 10.1161/CIRCRESAHA.111.300448. [DOI] [PubMed] [Google Scholar]
- 107.Naito AT, Okada S, Minamino T, Iwanaga K, Liu ML, Sumida T, Nomura S, Sahara N, Mizoroki T, Takashima A, Akazawa H, Nagai T, Shiojima I, Komuro I. Promotion of CHIP-mediated p53 degradation protects the heart from ischemic injury. Circ Res 106: 1692–1702, 2010. doi: 10.1161/CIRCRESAHA.109.214346. [DOI] [PubMed] [Google Scholar]
- 108.Narmoneva DA, Vukmirovic R, Davis ME, Kamm RD, Lee RT. Endothelial cells promote cardiac myocyte survival and spatial reorganization: implications for cardiac regeneration. Circulation 110: 962–968, 2004. doi: 10.1161/01.CIR.0000140667.37070.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nicoli S, Standley C, Walker P, Hurlstone A, Fogarty KE, Lawson ND. MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature 464: 1196–1200, 2010. doi: 10.1038/nature08889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nicolls MR, Mizuno S, Taraseviciene-Stewart L, Farkas L, Drake JI, Al Husseini A, Gomez-Arroyo JG, Voelkel NF, Bogaard HJ. New models of pulmonary hypertension based on VEGF receptor blockade-induced endothelial cell apoptosis. Pulm Circ 2: 434–442, 2012. doi: 10.4103/2045-8932.105031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ohtani K, Dimmeler S. Control of cardiovascular differentiation by microRNAs. Basic Res Cardiol 106: 5–11, 2011. doi: 10.1007/s00395-010-0139-7. [DOI] [PubMed] [Google Scholar]
- 112.Oka T, Akazawa H, Naito AT, Komuro I. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res 114: 565–571, 2014. doi: 10.1161/CIRCRESAHA.114.300507. [DOI] [PubMed] [Google Scholar]
- 113.Okada K, Tanaka Y, Bernstein M, Zhang W, Patterson GA, Botney MD. Pulmonary hemodynamics modify the rat pulmonary artery response to injury. A neointimal model of pulmonary hypertension. Am J Pathol 151: 1019–1025, 1997. [PMC free article] [PubMed] [Google Scholar]
- 114.Ostádal B, Urbanová D, Ressl J, Procházka J, Pelouch V, Widimský J. Changes of the right and left ventricles in rats exposed to intermittent high altitude hypoxia. Cor Vasa 23: 111–120, 1981. [PubMed] [Google Scholar]
- 115.Pan LC, Wilson DW, Segall HJ. Strain differences in the response of Fischer 344 and Sprague-Dawley rats to monocrotaline induced pulmonary vascular disease. Toxicology 79: 21–35, 1993. doi: 10.1016/0300-483X(93)90203-5. [DOI] [PubMed] [Google Scholar]
- 116.Papaioannou AI, Zakynthinos E, Kostikas K, Kiropoulos T, Koutsokera A, Ziogas A, Koutroumpas A, Sakkas L, Gourgoulianis KI, Daniil ZD. Serum VEGF levels are related to the presence of pulmonary arterial hypertension in systemic sclerosis. BMC Pulm Med 9: 18, 2009. doi: 10.1186/1471-2466-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Partovian C, Adnot S, Eddahibi S, Teiger E, Levame M, Dreyfus P, Raffestin B, Frelin C. Heart and lung VEGF mRNA expression in rats with monocrotaline- or hypoxia-induced pulmonary hypertension. Am J Physiol 275: H1948–H1956, 1998. [DOI] [PubMed] [Google Scholar]
- 118.Patan S. Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. J Neurooncol 50: 1–15, 2000. doi: 10.1023/A:1006493130855. [DOI] [PubMed] [Google Scholar]
- 119.Paulin R, Sutendra G, Gurtu V, Dromparis P, Haromy A, Provencher S, Bonnet S, Michelakis ED. A miR-208-Mef2 axis drives the decompensation of right ventricular function in pulmonary hypertension. Circ Res 116: 56–69, 2015. doi: 10.1161/CIRCRESAHA.115.303910. [DOI] [PubMed] [Google Scholar]
- 120.Peng J, Zhang L, Drysdale L, Fong GH. The transcription factor EPAS-1/hypoxia-inducible factor 2alpha plays an important role in vascular remodeling. Proc Natl Acad Sci USA 97: 8386–8391, 2000. doi: 10.1073/pnas.140087397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pengo V, Lensing AW, Prins MH, Marchiori A, Davidson BL, Tiozzo F, Albanese P, Biasiolo A, Pegoraro C, Iliceto S, Prandoni P; Thromboembolic Pulmonary Hypertension Study Group . Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 350: 2257–2264, 2004. doi: 10.1056/NEJMoa032274. [DOI] [PubMed] [Google Scholar]
- 122.Piao L, Fang YH, Parikh K, Ryan JJ, Toth PT, Archer SL. Cardiac glutaminolysis: a maladaptive cancer metabolism pathway in the right ventricle in pulmonary hypertension. J Mol Med (Berl) 91: 1185–1197, 2013. doi: 10.1007/s00109-013-1064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Potus F, Malenfant S, Graydon C, Mainguy V, Tremblay È, Breuils-Bonnet S, Ribeiro F, Porlier A, Maltais F, Bonnet S, Provencher S. Impaired angiogenesis and peripheral muscle microcirculation loss contribute to exercise intolerance in pulmonary arterial hypertension. Am J Respir Crit Care Med 190: 318–328, 2014. [DOI] [PubMed] [Google Scholar]
- 124.Potus F, Ruffenach G, Dahou A, Thebault C, Breuils-Bonnet S, Tremblay È, Nadeau V, Paradis R, Graydon C, Wong R, Johnson I, Paulin R, Lajoie AC, Perron J, Charbonneau E, Joubert P, Pibarot P, Michelakis ED, Provencher S, Bonnet S. Downregulation of MicroRNA-126 Contributes to the Failing Right Ventricle in Pulmonary Arterial Hypertension. Circulation 132: 932–943, 2015. doi: 10.1161/CIRCULATIONAHA.115.016382. [DOI] [PubMed] [Google Scholar]
- 125.Rain S, Handoko ML, Trip P, Gan CT, Westerhof N, Stienen GJ, Paulus WJ, Ottenheijm CA, Marcus JT, Dorfmüller P, Guignabert C, Humbert M, Macdonald P, Dos Remedios C, Postmus PE, Saripalli C, Hidalgo CG, Granzier HL, Vonk-Noordegraaf A, van der Velden J, de Man FS. Right ventricular diastolic impairment in patients with pulmonary arterial hypertension. Circulation 128: 2016–2025, 2013. doi: 10.1161/CIRCULATIONAHA.113.001873. [DOI] [PubMed] [Google Scholar]
- 126.Reddy S, Bernstein D. Molecular Mechanisms of Right Ventricular Failure. Circulation 132: 1734–1742, 2015. doi: 10.1161/CIRCULATIONAHA.114.012975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Reddy S, Zhao M, Hu DQ, Fajardo G, Hu S, Ghosh Z, Rajagopalan V, Wu JC, Bernstein D. Dynamic microRNA expression during the transition from right ventricular hypertrophy to failure. Physiol Genomics 44: 562–575, 2012. doi: 10.1152/physiolgenomics.00163.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 289: 194–202, 2003. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 129.Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol 11: 73–91, 1995. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 130.Rivera-Lebron BN, Forfia PR, Kreider M, Lee JC, Holmes JH, Kawut SM. Echocardiographic and hemodynamic predictors of mortality in idiopathic pulmonary fibrosis. Chest 144: 564–570, 2013. doi: 10.1378/chest.12-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ruiter G, Ying Wong Y, de Man FS, Louis Handoko M, Jaspers RT, Postmus PE, Westerhof N, Niessen HW, van der Laarse WJ, Vonk-Noordegraaf A. Right ventricular oxygen supply parameters are decreased in human and experimental pulmonary hypertension. J Heart Lung Transplant 32: 231–240, 2013. doi: 10.1016/j.healun.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 132.Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J 17: 3005–3015, 1998. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ryan JJ, Archer SL. The right ventricle in pulmonary arterial hypertension: disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circ Res 115: 176–188, 2014. doi: 10.1161/CIRCRESAHA.113.301129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, Shimizu I, Asahara T, Hamada H, Tomita S, Molkentin JD, Zou Y, Komuro I. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature 446: 444–448, 2007. doi: 10.1038/nature05602. [DOI] [PubMed] [Google Scholar]
- 135.Santoro MM, Nicoli S. miRNAs in endothelial cell signaling: the endomiRNAs. Exp Cell Res 319: 1324–1330, 2013. doi: 10.1016/j.yexcr.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schäfer S, Ellinghaus P, Janssen W, Kramer F, Lustig K, Milting H, Kast R, Klein M. Chronic inhibition of phosphodiesterase 5 does not prevent pressure-overload-induced right-ventricular remodelling. Cardiovasc Res 82: 30–39, 2009. doi: 10.1093/cvr/cvp002. [DOI] [PubMed] [Google Scholar]
- 137.Seeger W, Adir Y, Barberà JA, Champion H, Coghlan JG, Cottin V, De Marco T, Galiè N, Ghio S, Gibbs S, Martinez FJ, Semigran MJ, Simonneau G, Wells AU, Vachiéry JL. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol 62, Suppl: D109–D116, 2013. doi: 10.1016/j.jacc.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 138.Selimovic N, Bergh CH, Andersson B, Sakiniene E, Carlsten H, Rundqvist B. Growth factors and interleukin-6 across the lung circulation in pulmonary hypertension. Eur Respir J 34: 662–668, 2009. doi: 10.1183/09031936.00174908. [DOI] [PubMed] [Google Scholar]
- 139.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell 148: 399–408, 2012. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Semenza GL. Pulmonary vascular responses to chronic hypoxia mediated by hypoxia-inducible factor 1. Proc Am Thorac Soc 2: 68–70, 2005. doi: 10.1513/pats.200404-029MS. [DOI] [PubMed] [Google Scholar]
- 141.Senger DR, Davis GE. Angiogenesis. Cold Spring Harb Perspect Biol 3: a005090, 2011. doi: 10.1101/cshperspect.a005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Serpi R, Tolonen AM, Huusko J, Rysä J, Tenhunen O, Ylä-Herttuala S, Ruskoaho H. Vascular endothelial growth factor-B gene transfer prevents angiotensin II-induced diastolic dysfunction via proliferation and capillary dilatation in rats. Cardiovasc Res 89: 204–213, 2011. doi: 10.1093/cvr/cvq267. [DOI] [PubMed] [Google Scholar]
- 143.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376: 62–66, 1995. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 144.Sharma S, Taegtmeyer H, Adrogue J, Razeghi P, Sen S, Ngumbela K, Essop MF. Dynamic changes of gene expression in hypoxia-induced right ventricular hypertrophy. Am J Physiol Heart Circ Physiol 286: H1185–H1192, 2004. doi: 10.1152/ajpheart.00916.2003. [DOI] [PubMed] [Google Scholar]
- 145.Sheikh AY, Chun HJ, Glassford AJ, Kundu RK, Kutschka I, Ardigo D, Hendry SL, Wagner RA, Chen MM, Ali ZA, Yue P, Huynh DT, Connolly AJ, Pelletier MP, Tsao PS, Robbins RC, Quertermous T. In vivo genetic profiling and cellular localization of apelin reveals a hypoxia-sensitive, endothelial-centered pathway activated in ischemic heart failure. Am J Physiol Heart Circ Physiol 294: H88–H98, 2008. doi: 10.1152/ajpheart.00935.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shimoda LA, Semenza GL. HIF and the lung: role of hypoxia-inducible factors in pulmonary development and disease. Am J Respir Crit Care Med 183: 152–156, 2011. doi: 10.1164/rccm.201009-1393PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest 115: 2108–2118, 2005. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 62, Suppl: D34–D41, 2013. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 149.Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol 297: L1013–L1032, 2009. doi: 10.1152/ajplung.00217.2009. [DOI] [PubMed] [Google Scholar]
- 150.Strasser GA, Kaminker JS, Tessier-Lavigne M. Microarray analysis of retinal endothelial tip cells identifies CXCR4 as a mediator of tip cell morphology and branching. Blood 115: 5102–5110, 2010. doi: 10.1182/blood-2009-07-230284. [DOI] [PubMed] [Google Scholar]
- 151.Sutendra G, Dromparis P, Paulin R, Zervopoulos S, Haromy A, Nagendran J, Michelakis ED. A metabolic remodeling in right ventricular hypertrophy is associated with decreased angiogenesis and a transition from a compensated to a decompensated state in pulmonary hypertension. J Mol Med (Berl) 91: 1315–1327, 2013. doi: 10.1007/s00109-013-1059-4. [DOI] [PubMed] [Google Scholar]
- 152.Sutendra G, Michelakis ED. Pulmonary arterial hypertension: challenges in translational research and a vision for change. Sci Transl Med 5: 208sr5, 2013. doi: 10.1126/scitranslmed.3005428. [DOI] [PubMed] [Google Scholar]
- 153.Szokodi I, Tavi P, Földes G, Voutilainen-Myllylä S, Ilves M, Tokola H, Pikkarainen S, Piuhola J, Rysä J, Tóth M, Ruskoaho H. Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circ Res 91: 434–440, 2002. doi: 10.1161/01.RES.0000033522.37861.69. [DOI] [PubMed] [Google Scholar]
- 154.Tabuchi T, Satoh M, Itoh T, Nakamura M. MicroRNA-34a regulates the longevity-associated protein SIRT1 in coronary artery disease: effect of statins on SIRT1 and microRNA-34a expression. Clin Sci (Lond) 123: 161–171, 2012. doi: 10.1042/CS20110563. [DOI] [PubMed] [Google Scholar]
- 155.Takahashi T, Ueno H, Shibuya M. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene 18: 2221–2230, 1999. doi: 10.1038/sj.onc.1202527. [DOI] [PubMed] [Google Scholar]
- 156.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 15: 427–438, 2001. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- 157.Tempel D, de Boer M, van Deel ED, Haasdijk RA, Duncker DJ, Cheng C, Schulte-Merker S, Duckers HJ. Apelin enhances cardiac neovascularization after myocardial infarction by recruiting aplnr+ circulating cells. Circ Res 111: 585–598, 2012. doi: 10.1161/CIRCRESAHA.111.262097. [DOI] [PubMed] [Google Scholar]
- 158.Thum T, Batkai S. MicroRNAs in right ventricular (dys)function (2013 Grover Conference series). Pulm Circ 4: 185–190, 2014. doi: 10.1086/675981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev 12: 3320–3324, 1998. doi: 10.1101/gad.12.21.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tuder RM, Archer SL, Dorfmüller P, Erzurum SC, Guignabert C, Michelakis E, Rabinovitch M, Schermuly R, Stenmark KR, Morrell NW. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J Am Coll Cardiol 62, Suppl: D4–D12, 2013. doi: 10.1016/j.jacc.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tuder RM, Flook BE, Voelkel NF. Increased gene expression for VEGF and the VEGF receptors KDR/Flk and Flt in lungs exposed to acute or to chronic hypoxia. Modulation of gene expression by nitric oxide. J Clin Invest 95: 1798–1807, 1995. doi: 10.1172/JCI117858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tuder RM, Yun JH, Bhunia A, Fijalkowska I. Hypoxia and chronic lung disease. J Mol Med (Berl) 85: 1317–1324, 2007. doi: 10.1007/s00109-007-0280-4. [DOI] [PubMed] [Google Scholar]
- 163.Turek Z, Grandtner M, Kreuzer F. Cardiac hypertrophy, capillary and muscle fiber density, muscle fiber diameter, capillary radius and diffusion distance in the myocardium of growing rats adapted to a simulated altitude of 3500 m. Pflugers Arch 335: 19–28, 1972. doi: 10.1007/BF00586932. [DOI] [PubMed] [Google Scholar]
- 164.Turek Z, Hoofd LJ, Ringnalda BE, Rakusan K. Myocardial capillarity of rats exposed to simulated high altitude. Adv Exp Med Biol 191: 249–255, 1985. doi: 10.1007/978-1-4684-3291-6_25. [DOI] [PubMed] [Google Scholar]
- 165.Tvorogov D, Anisimov A, Zheng W, Leppänen VM, Tammela T, Laurinavicius S, Holnthoner W, Heloterä H, Holopainen T, Jeltsch M, Kalkkinen N, Lankinen H, Ojala PM, Alitalo K. Effective suppression of vascular network formation by combination of antibodies blocking VEGFR ligand binding and receptor dimerization. Cancer Cell 18: 630–640, 2010. doi: 10.1016/j.ccr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 166.Vachiéry JL, Adir Y, Barberà JA, Champion H, Coghlan JG, Cottin V, De Marco T, Galiè N, Ghio S, Gibbs JS, Martinez F, Semigran M, Simonneau G, Wells A, Seeger W. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol 62, Suppl: D100–D108, 2013. doi: 10.1016/j.jacc.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 167.Vaillancourt M, Ruffenach G, Meloche J, Bonnet S. Adaptation and remodelling of the pulmonary circulation in pulmonary hypertension. Can J Cardiol 31: 407–415, 2015. doi: 10.1016/j.cjca.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 168.van Albada ME, Berger RM, Niggebrugge M, van Veghel R, Cromme-Dijkhuis AH, Schoemaker RG. Prostacyclin therapy increases right ventricular capillarisation in a model for flow-associated pulmonary hypertension. Eur J Pharmacol 549: 107–116, 2006. doi: 10.1016/j.ejphar.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 169.van de Veerdonk MC, Kind T, Marcus JT, Mauritz GJ, Heymans MW, Bogaard HJ, Boonstra A, Marques KM, Westerhof N, Vonk-Noordegraaf A. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol 58: 2511–2519, 2011. doi: 10.1016/j.jacc.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 170.van Wolferen SA, Marcus JT, Westerhof N, Spreeuwenberg MD, Marques KM, Bronzwaer JG, Henkens IR, Gan CT, Boonstra A, Postmus PE, Vonk-Noordegraaf A. Right coronary artery flow impairment in patients with pulmonary hypertension. Eur Heart J 29: 120–127, 2007. doi: 10.1093/eurheartj/ehm567. [DOI] [PubMed] [Google Scholar]
- 171.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 89: e1–e7, 2001. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 172.Veith C, Schermuly RT, Brandes RP, Weissmann N. Molecular mechanisms of hypoxia-inducible factor-induced pulmonary arterial smooth muscle cell alterations in pulmonary hypertension. J Physiol 594: 1167–1177, 2016. doi: 10.1113/JP270689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Voelkel NF, Gomez-Arroyo J. The role of vascular endothelial growth factor in pulmonary arterial hypertension. The angiogenesis paradox. Am J Respir Cell Mol Biol 51: 474–484, 2014. doi: 10.1165/rcmb.2014-0045TR. [DOI] [PubMed] [Google Scholar]
- 174.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB; National Heart, Lung, and Blood Institute Working Group on Cellular and Molecular Mechanisms of Right Heart Failure . Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 114: 1883–1891, 2006. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 175.Vonk-Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, Naeije R, Newman J, Oudiz RJ, Provencher S, Torbicki A, Voelkel NF, Hassoun PM. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol 62, Suppl: D22–D33, 2013. doi: 10.1016/j.jacc.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 176.Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. J Am Coll Cardiol 69: 236–243, 2017. doi: 10.1016/j.jacc.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 178.Wang H, Cai J. The role of microRNAs in heart failure. Biochim Biophys Acta 1863: 2019–2030, 2017. doi: 10.1016/j.bbadis.2016.11.034. [DOI] [PubMed] [Google Scholar]
- 179.Ware JA, Simons M. Angiogenesis in ischemic heart disease. Nat Med 3: 158–164, 1997. doi: 10.1038/nm0297-158. [DOI] [PubMed] [Google Scholar]
- 180.Werchan PM, Summer WR, Gerdes AM, McDonough KH. Right ventricular performance after monocrotaline-induced pulmonary hypertension. Am J Physiol 256: H1328–H1336, 1989. [DOI] [PubMed] [Google Scholar]
- 181.Yang P, Maguire JJ, Davenport AP. Apelin, Elabela/Toddler, and biased agonists as novel therapeutic agents in the cardiovascular system. Trends Pharmacol Sci 36: 560–567, 2015. doi: 10.1016/j.tips.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yang P, Read C, Kuc RE, Buonincontri G, Southwood M, Torella R, Upton PD, Crosby A, Sawiak SJ, Carpenter TA, Glen RC, Morrell NW, Maguire JJ, Davenport AP. Elabela/Toddler is an endogenous agonist of the apelin APJ receptor in the adult cardiovascular system, and exogenous administration of the peptide compensates for the downregulation of its expression in pulmonary arterial hypertension. Circulation 135: 1160–1173, 2017. doi: 10.1161/CIRCULATIONAHA.116.023218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yang X, Zhu W, Zhang P, Chen K, Zhao L, Li J, Wei M, Liu M. Apelin-13 stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in myocardial microvascular endothelial cells. Mol Med Rep 9: 1590–1596, 2014. doi: 10.3892/mmr.2014.1984. [DOI] [PubMed] [Google Scholar]
- 184.Yoshida M, Shiojima I, Ikeda H, Komuro I. Chronic doxorubicin cardiotoxicity is mediated by oxidative DNA damage-ATM-p53-apoptosis pathway and attenuated by pitavastatin through the inhibition of Rac1 activity. J Mol Cell Cardiol 47: 698–705, 2009. doi: 10.1016/j.yjmcc.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 185.Yuan K, Orcholski ME, Panaroni C, Shuffle EM, Huang NF, Jiang X, Tian W, Vladar EK, Wang L, Nicolls MR, Wu JY, de Jesus Perez VA. Activation of the Wnt/planar cell polarity pathway is required for pericyte recruitment during pulmonary angiogenesis. Am J Pathol 185: 69–84, 2015. doi: 10.1016/j.ajpath.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhao L, Chen CN, Hajji N, Oliver E, Cotroneo E, Wharton J, Wang D, Li M, McKinsey TA, Stenmark KR, Wilkins MR. Histone deacetylation inhibition in pulmonary hypertension: therapeutic potential of valproic acid and suberoylanilide hydroxamic acid. Circulation 126: 455–467, 2012. doi: 10.1161/CIRCULATIONAHA.112.103176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhong JC, Yu XY, Huang Y, Yung LM, Lau CW, Lin SG. Apelin modulates aortic vascular tone via endothelial nitric oxide synthase phosphorylation pathway in diabetic mice. Cardiovasc Res 74: 388–395, 2007. doi: 10.1016/j.cardiores.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 188.Zhuang G, Yu K, Jiang Z, Chung A, Yao J, Ha C, Toy K, Soriano R, Haley B, Blackwood E, Sampath D, Bais C, Lill JR, Ferrara N. Phosphoproteomic analysis implicates the mTORC2-FoxO1 axis in VEGF signaling and feedback activation of receptor tyrosine kinases. Sci Signal 6: ra25, 2013. doi: 10.1126/scisignal.2003572. [DOI] [PubMed] [Google Scholar]