Abstract
Cigarette smokers and people exposed to second-hand smoke are at an increased risk for pulmonary viral infections, and yet the mechanism responsible for this heightened susceptibility is not understood. To understand the effect of cigarette smoke on susceptibility to viral infection, we used an air-liquid interface culture system and exposed primary human small airway epithelial cells (SAEC) to whole cigarette smoke, followed by treatment with the viral mimetic polyinosinic polycytidylic acid (poly I:C) or influenza A virus (IAV). We found that prior smoke exposure strongly inhibited production of proinflammatory (interleukin-6 and interleukin-8) and antiviral [interferon-γ-induced protein 10 (IP-10) and interferons] mediators in SAECs in response to poly I:C and IAV infection. Impaired antiviral responses corresponded to increased infection with IAV. This was associated with a decrease in phosphorylation of the key antiviral transcription factor interferon response factor 3 (IRF3). Here, we found that cigarette smoke exposure inhibited activation of Toll-like receptor 3 (TLR3) by impairing TLR3 cleavage, which was required for downstream phosphorylation of IRF3 and production of IP-10. These results identify a novel mechanism by which cigarette smoke exposure impairs antiviral responses in lung epithelial cells, which may contribute to increased susceptibility to respiratory infections.
Keywords: chronic obstructive pulmonary disease, cigarette smoke, lung epithelium, Toll-like receptor 3, viral mimetic polyinosinic polycytidylic acid
INTRODUCTION
Cigarette smokers are at increased risk for contracting respiratory viral infections compared with nonsmokers, and the illnesses that result often have more severe symptoms (1, 17). In those with underlying diseases such as chronic obstructive pulmonary disease (COPD), pulmonary infections have been linked to acute exacerbations, with nearly 50% of exacerbations attributed to a viral infection (4). Acute exacerbations of COPD are a major cause of hospitalization and death associated with the disease and result in 50–75% of the economic costs of COPD (5, 39). Thus, a better understanding of the mechanism of this increased susceptibility to viral infections in smokers is urgently needed. The lung epithelial cells are the first cells to come in contact with inhaled stimuli and are the target of many respiratory viruses. Existing literature on smoke and viral infection focuses on the bronchial epithelium. However, smokers have shortened cilia and defects in mucociliary clearance that allow for viruses and bacteria to reach the lower airways (22, 47). The effect of smoke on epithelial cells of the lower airways is much less studied but remains important in the context of obstructive diseases like COPD, where small airway inflammation contributes significantly to disease pathology (41).
Many studies that investigate the role of cigarette smoke rely on the use of smoke-conditioned media, often termed “smoke extract,” created by drawing mainstream cigarette smoke through culture media (38). Despite the utility of smoke-conditioned media as a tool for exposure of specific cell types that cannot tolerate exposure to whole smoke, many constituents in smoke are poorly soluble and thus are not retained in culture media (45). This, along with issues of consistency in extract preparation between studies, leads to challenges in comparing findings between studies and poor translation to physiological exposure dosing. In contrast, the use of air-liquid interface cultures allows for the exposure of primary epithelial cells to whole cigarette smoke (composed of a mixture of particulates, aerosols, and gaseous components), and the subsequent study of antiviral signaling in epithelial cells thus more closely mimics in vivo exposure.
Cells utilize several pathogen recognition receptors to identify viral insults, including Toll like receptors (TLR), retinoic acid-inducible gene (RIG-I) I like receptors (RLR), and NOD-like receptors (NLR). TLR3 is an endosomal receptor that recognizes double-stranded (ds)RNA, which is an intermediate produced during viral replication and has been implicated in the epithelial immune response to numerous viruses, including influenza A virus (IAV), respiratory syncytial virus (RSV), and rhinovirus (12, 21, 34). Recent work has established that TLR3, like the endosomal TLR7 and TLR9, undergoes proteolytic cleavage in the endosome (7). This cleavage event is important in the activation of downstream antiviral responses (8, 27). The effect of cigarette smoke exposure on TLR3 cleavage has not been studied.
Because smokers are more susceptible to infections, we hypothesized that cigarette smoke impairs appropriate antiviral signaling. Here, we exposed primary human small airway epithelial cells (SAEC) to cigarette smoke at the air-liquid interface and demonstrate for the first time that cigarette smoke disrupts innate immune responses to the viral mimetic polyinosinic polycytidylic acid (poly I:C) by dysregulating the cleavage of TLR3, which is necessary for its activation and downstream antiviral signaling.
METHODS
Cell culture and reagents.
Primary human small airway epithelial cells (SAEC) from three different donors (lot nos. 0000206158, 0000203964, and 0000105938) were purchased from Lonza (Allendale, NJ) and grown in small airway epithelial growth media with supplements as recommended by the supplier. Human embryonic kidney (HEK)-293T cells expressing firefly luciferase (Fluc) under the control of an interferon-sensitive response element (ISRE) promoter have been described previously (28) and were grown in Dulbecco’s modified Eagle’s medium (GIBCO, Carlsbad, CA) supplemented with 10% FBS (Sigma-Aldrich, St. Louis, MO), 2 mM l-glutamine, antibiotic, and antimycotic (GIBCO). High-molecular weight poly I:C was purchased from Invivogen (San Diego, CA). The cathepsin inhibitor z-FA-FMK was purchased from Cayman (Ann Arbor, MI).
Smoke Exposure and poly I:C treatment.
Cells were plated on 1.0-μm polyethylene terephthalate inserts (EMD Millipore, Billerica, MA) and grown submerged in media until confluent. Media were then removed from the apical surface for 24 h to acclimate the cells to the air-liquid interface. The cells were then placed in a closed plexiglass chamber and exposed to either filtered air or mainstream cigarette smoke for 60 min. Mainstream cigarette smoke was generated from a Baumgartner-Jaeger CSM2072i cigarette smoking machine (CH Technologies, Westwood, NJ), using a protocol that smoked two reference cigarettes (3R4F; University of Kentucky) per cycle (1 puff/min of 2-s duration and 35-ml volume) and was diluted with filtered air to achieve total particulate levels between 30 and 80 mg/m3, as measured by gravimetric sampling at the point of exposure (43, 44). Following smoke exposure, media in the basolateral compartment of the wells were replaced to remove residual smoke components that may have dissolved into the media, but smoke particulates that had deposited on the apical surface were not washed off. Unless otherwise noted, cultures were treated immediately following smoke exposure in the apical compartment with 0.5 μg/ml of poly I:C for the indicated times. For inhibitor experiments, cells were cultured with z-FA FMK at the indicated concentrations for 3 days before smoke exposure and throughout poly I:C treatment. Cell viability was assessed using Alamar Blue dye reduction (Thermo Fisher Scientific, Rockford, IL) according to the manufacturer’s protocol.
Viral infection.
Influenza A/Puerto Rico/08/1934 expressing mCherry (PR8 mCherry) has been described previously (29). For in vitro infection, cells were infected at the indicated multiplicity of infection of PR8 mCherry for 1 h in small airway epithelial cell media with no supplements. Cells were then washed with phosphate-buffered saline (PBS), and growth medium with supplements was added. Fluorescent images were taken 24 h postinfection.
Cytokine measurements.
Supernatant samples were collected from the basolateral compartment, and levels of IL-8, IL-6, and IP-10 were measured by commercial ELISA according to the manufacturer’s instructions (R & D Systems, Minneapolis, MN).
Luciferase assay.
HEK-293T cells expressing mRFP and firefly luciferase under the control of an ISRE promoter have been described previously (28). Supernatant samples, taken from the basolateral compartment, were diluted 1:1 in DMEM supplemented with 10% FBS and added to reporter cells for 24 h. Cells were then washed with PBS, and luciferase activity was measured using the Luciferase Assay System (Promega, Madison, WI).
RNA isolation and PCR.
RNA was harvested using the RNeasy isolation kit (Qiagen), and purity was determined using the 260/280 ratio measured using a Nanodrop instrument (Thermo Fisher Scientific). One microgram of RNA was reverse-transcribed and used for PCR. The primers used were IFNβ forward 5′-AACTGCAACCTTTCGAAGCC-3′, IFNβ reverse 5′-TGTCGCCTACTACCTGTTGTGC-3′, IFNλ forward 5′-GGTGACTTTGGTGCTAGGCT-3′, IFNλ reverse 5′-GGCCTTCTTGAAGCTCGCT-3′, TLR3 forward 5′-CCTGGTTTGTTAATTGGATTAACGA-3′, TLR3 reverse 5′-GAGGTGGAGTGTTGCAAAGGTAGT-3′, IRF3 forward 5′-TACCCGGACCTCCAAGACAC-3′, IRF3 reverse 5′-GGTCCGGCCTACGATGGAA-3′, 18S forward 5′-GGTCGCTCGCTCCTCTCCCA-3′, and 18S reverse 5′-AGGGGCTGACCGGGTTGGTT-3′.
Western blot and antibodies.
Cells were washed with PBS before harvest and lysed in 50 mM Tris, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, and 0.1% SDS (for TLR3) or 50 mM Tris, 150 mM NaCl, 1% NP-40, 50 mM Tris, and 1 mM EDTA (for IRF3). Protein was quantified using the bicinchoninic acid assay, and 15–30 μg of protein was analyzed by Western blot. Monoclonal antibodies for phospho-IRF3 and IRF3 were purchased from Cell Signaling Technology (4947S and 4302S). Monoclonal antibodies for β-tubulin and GAPDH were purchased from Abcam (6046 and 8245). The polyclonal TLR3 antibody was purchased from GeneTex (113022).
Statistical analysis.
Results are reported as means ± SD. Two-way ANOVA with Tukey post hoc correction was performed using GraphPad Prism (La Jolla, CA). Unless otherwise indicated, experiments were reproducible in at least three independent experiments.
RESULTS
Cigarette smoke dampens production of inflammatory mediators.
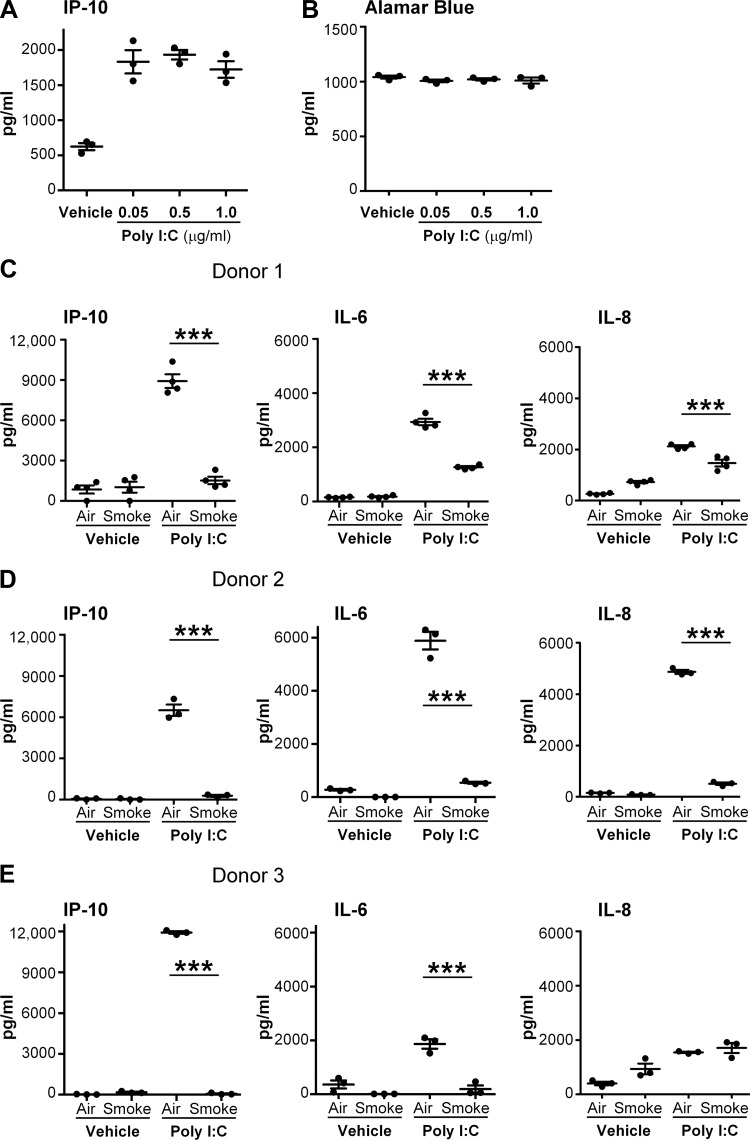
Because poly I:C induces many inflammatory and antiviral molecules, we first determined the effect of cigarette smoke exposure on the production of inflammatory mediators in response to treatment with a viral mimetic. We exposed primary human SAEC to cigarette smoke, followed by poly I:C, and analyzed culture supernatants for the inflammatory markers interleukin 8 (IL-8), interleukin 6 (IL-6), and interferon-γ-induced protein 10 (IP-10), which are known to be produced during viral infection (3, 11). To determine the optimal concentration of poly I:C, a dose-response curve was generated in air-exposed SAEC. Poly I:C at 0.05–1 μg/ml induced robust production of IP-10 without causing overt toxicity (Fig. 1, A and B). A dose of 0.5 μg/ml was used for all future experiments. Cigarette smoke exposure alone was only mildly inflammatory, with no increase in the production of either IP-10 or IL-6 and only modest induction of IL-8 in SAEC from two of the three donors (Fig. 1). Poly I:C treatment in air-exposed cells led to a robust production of the inflammatory mediators IL-8, IL-6, and IP-10 in all three donors (Fig. 1, C and D). However, for each of the donors tested, IL-6 and IP-10 production from poly I:C was strongly suppressed by prior cigarette smoke exposure (Fig. 1, C–E). Interestingly, in two of the three donors (donors 1 and 2), levels of IL-8 were suppressed in smoke-exposed cells, suggesting that IL-8 is regulated differently from IL-6 and IP-10. There was no significant difference in viability between air- and smoke-exposed cells, as measured by alamar blue dye reduction (data not shown).
Fig. 1.
Cigarette smoke disrupts the production of proinflammatory mediators in primary human small airway epithelial cells (SAEC) in response to viral mimetic polyinosinic polycytidylic acid (poly I:C). Primary human SAEC were exposed to either air or cigarette smoke for 60 min at the air-liquid interface. Following exposure, the basal medium was changed, and the apical surface was treated with indicated doses of poly I:C. A: interferon-γ-induced protein 10 (IP-10) levels from donor 1 treated with increasing doses of poly I:C. B: Alamar Blue dye reduction assay was performed on cells from donor 1 24 h after treatment with poly I:C. C–E: IP-10, IL-6, and IL-8 were measured in culture supernatants by ELISA from 3 different donors. Bars are means ± SD of triplicate cultures. ***P < 0.001 by 2-way ANOVA with Tukey post hoc correction.
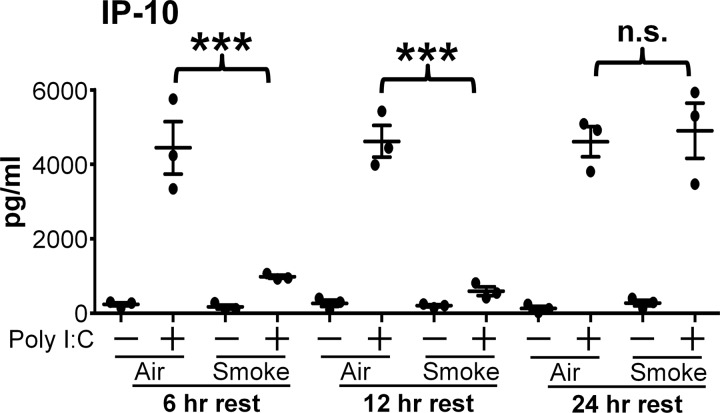
To investigate the time course of the suppressive effects of cigarette smoke, the SAEC were rested for 6 to 24 h after smoke exposure before treatment with poly I:C (Fig. 2). Because of its high production of IP-10, SAEC from donor 1 were used in a time course analysis. Note that in all cases the basal medium was changed immediately after smoke exposure, whereas the apical surface was not disturbed. Smoke-exposed SAEC that were rested for 6 or 12 h still had a significantly reduced production of IP-10 in response to poly I:C. Interestingly, smoke-exposed cells that were rested for 24 h produced IP-10 at levels similar to air-exposed cells following poly I:C treatment (Fig. 2). This suggests that immunosuppression caused by cigarette smoke is transient and that cells can recover the ability to respond to poly I:C treatment between 12 and 24 h after smoke exposure.
Fig. 2.
Immunosuppressive effect of cigarette smoke is transient. SAEC from donor 1 were exposed to air or cigarette smoke for 60 min. The basal medium was changed immediately, but the apical surface was not disturbed. Cells were rested 6–24 h before treatment with poly I:C. Supernatants were harvested 24 h after treatment with poly I:C and analyzed for levels of IP-10 by ELISA. Bars are means ± SD of 1 experiment with triplicate cultures. ***P < 0.001 by 2-way ANOVA with Tukey post hoc correction. NS, not significant.
Cigarette smoke impairs the production of type I and type III interferons.
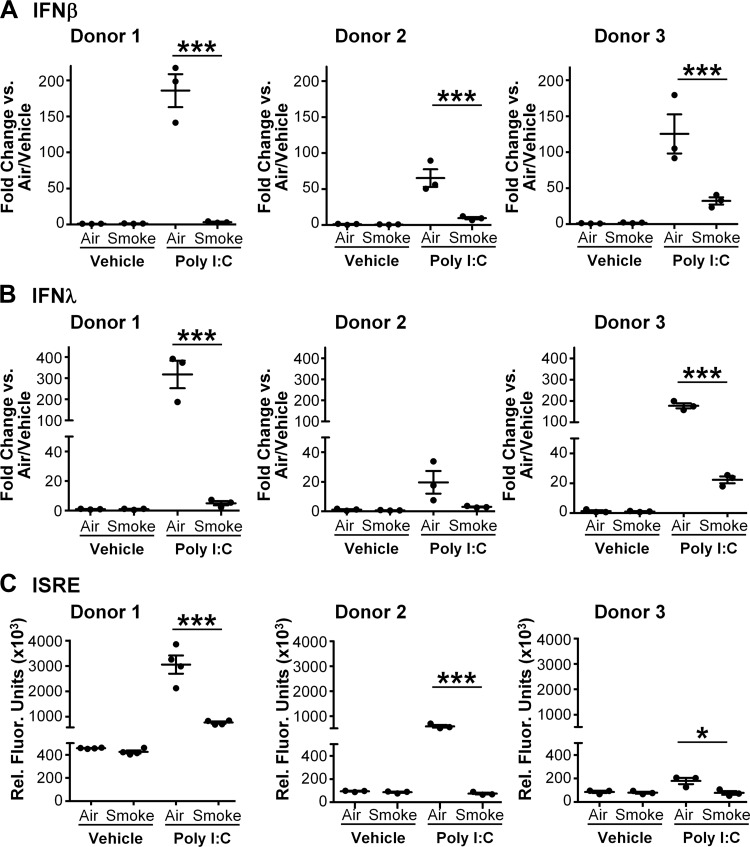
Interferon (IFN) production is crucial to activation of host antiviral responses (33). Type I IFNs, including interferon-α (IFNα) and interferon-β (IFNβ), are important for limiting viral replication and spread. Type III interferons, known as interferon-λ (IFNλ), are also produced early after viral recognition and have been shown to be important in viral infection (19). IP-10 production is also controlled by IFN signaling. Thus, we hypothesized that exposure to whole cigarette smoke would impair the production of type I and type III IFNs. A pilot time course analysis found that levels of IFNβ transcript were increased significantly starting at 6 h after treatment with poly I:C (data not shown). Therefore, we exposed SAEC from three donors to cigarette smoke or air followed by poly I:C, harvested RNA at 6 h, and determined mRNA levels for IFNβ and IFNλ by RT-qPCR. In SAEC from all donors tested, treatment with poly I:C induced mRNA levels of both IFNβ and IFNλ (Fig. 3, A and B). However, prior cigarette smoke exposure strongly inhibited upregulation of both interferons (Fig. 3, A and B).
Fig. 3.
Cigarette smoke disrupts the production of interferons (IFN). SAEC from 3 different donors were exposed to either air or smoke for 60 min as described and then treated with poly I:C. RNA was harvested 6 h after poly I:C treatment, and mRNA levels of IFNβ (A) and IFNλ (B) were measured by quantitative PCR. C: interferon bioactivity was measured in culture supernatants harvested at 24 h post poly I:C treatment using interferon-sensitive reporter element (ISRE) luciferase reporter cells. Bars are means ± SD of triplicate cultures. *P < 0.05, ***P < 0.001 by 2-way ANOVA with Tukey post hoc correction.
To determine the effects of cigarette smoke on IFN protein levels, we utilized a reporter cell line containing a luciferase gene driven by an interferon-sensitive reporter element (ISRE) (28). Supernatants from smoke- or air-exposed SAEC were harvested 24 h after treatment with vehicle or poly I:C and applied to the reporter cell line. Poly I:C alone stimulated production of IFN bioactivity consistent with the RNA results (Fig. 3C). Similar to mRNA levels, cigarette smoke significantly impaired IFN bioactivity by SAEC treated with poly I:C (Fig. 3C).
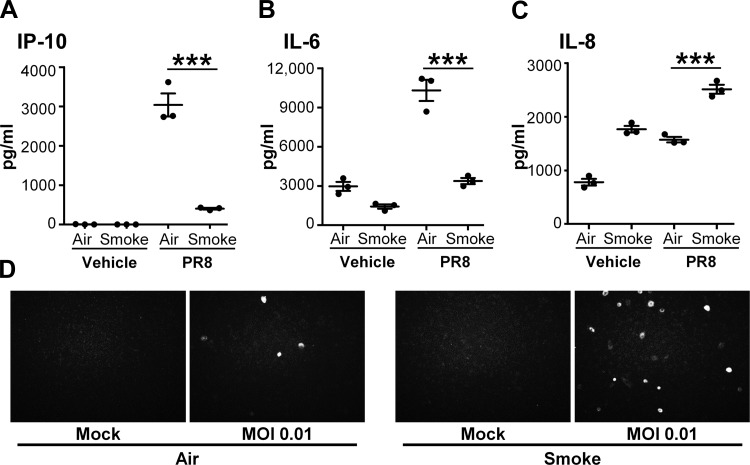
Because cigarette smoke profoundly dampened proinflammatory and antiviral responses to poly I:C, we next investigated whether cigarette smoke similarly affected innate immune responses to IAV. We infected smoke-exposed SAEC with the PR8 mCherry virus (29). We found that, similarly to poly I:C, cigarette smoke exposure resulted in decreased production of IL-6 and IP-10 in response to IAV infection (Fig. 4, A and B). IL-8 levels were increased in smoke-exposed SAEC infected with PR8 mCherry, suggesting that IL-8 production is regulated independently of IL-6 and IP-10 (Fig. 4C). To determine whether this altered innate response impacted viral infectivity, smoke- or air-exposed SAEC were visualized after infection with PR8 mCherry. We found that cigarette smoke-exposed SAEC permitted greater viral replication by the presence of more mCherry-expressing cells (Fig. 4D). Because the smoke-induced inhibition of innate immune responses was similar between poly I:C treatment and IAV infection, further mechanistic studies were performed using only poly I:C due to its specificity for TLR3 signaling.
Fig. 4.
Cigarette smoke impairs epithelial cell response to influenza A virus (IAV). SAEC were exposed to cigarette smoke for 60 min and then infected with WT PR8 (MOI 2). IP-10 (A), IL-6 (B), and IL-8 (C) were measured in culture supernatants. D: air- or smoke-exposed SAEC were infected with Puerto Rico/08/1934 expressing mCherry (PR8 mCherry; MOI 0.01) and imaged 24 h postinfection.
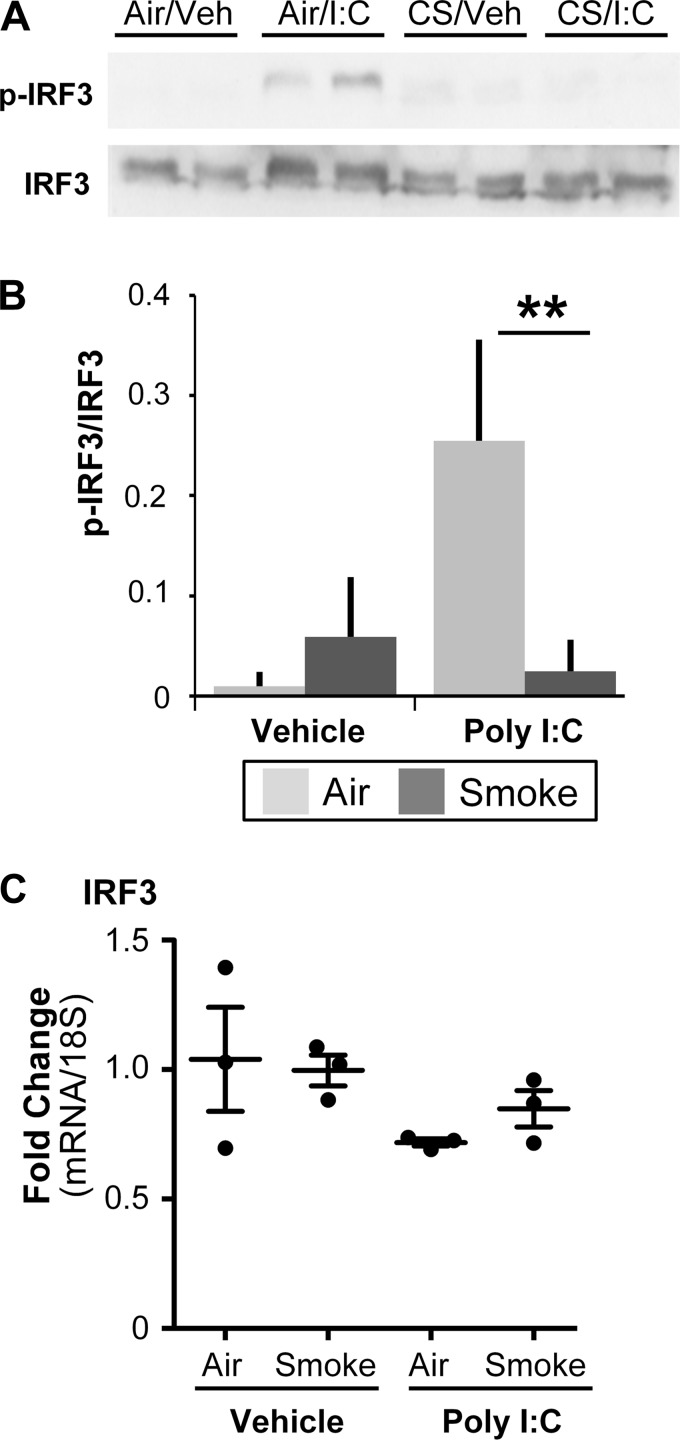
Cigarette Smoke Dampens phosphorylation of IRF3.
Production of IFNs is a tightly regulated process that involves the activation of multiple transcription factors (14). One of these transcription factors, IRF3, is activated during viral infection and is critical to an optimal antiviral response (6). We hypothesized that cigarette smoke would dampen IRF3 activation. In unstimulated cells, IRF3 is located primarily in the cytosol and upon activation is phosphorylated at multiple sites, which allows for dimerization and retention in the nucleus, leading to transcriptional activity (33). Western blot analysis showed that IRF3 phosphorylation at Ser396, which has been shown to be important during viral infection (37), was significantly decreased in smoke-exposed cells following treatment with poly I:C (Fig. 5, A and B). The levels of IRF3 transcript were unaffected by smoke or poly I:C treatment, suggesting that there was little impact of cigarette smoke or poly I:C treatment on total levels of IRF3 (Fig. 5C).
Fig. 5.
Cigarette smoke disrupts activation of interferon response factor 3 (IRF3). SAEC were exposed to air or cigarette smoke for 60 min and then treated with poly I:C. Levels of total and phosphorylated IRF3 were measured in lysates 4 h posttreatment with poly I:C. A: representative Western blot with 2 replicate cultures at each condition. B: densitometry of 3 replicate treatments is shown. C: IRF3 mRNA was quantified 6 h posttreatment with poly I:C. **P < 0.01 by 2-way ANOVA with Tukey post hoc correction. Bars are means ± SD of triplicate cultures from 1 donor and are representative of experiments done in 3 strains of SAEC.
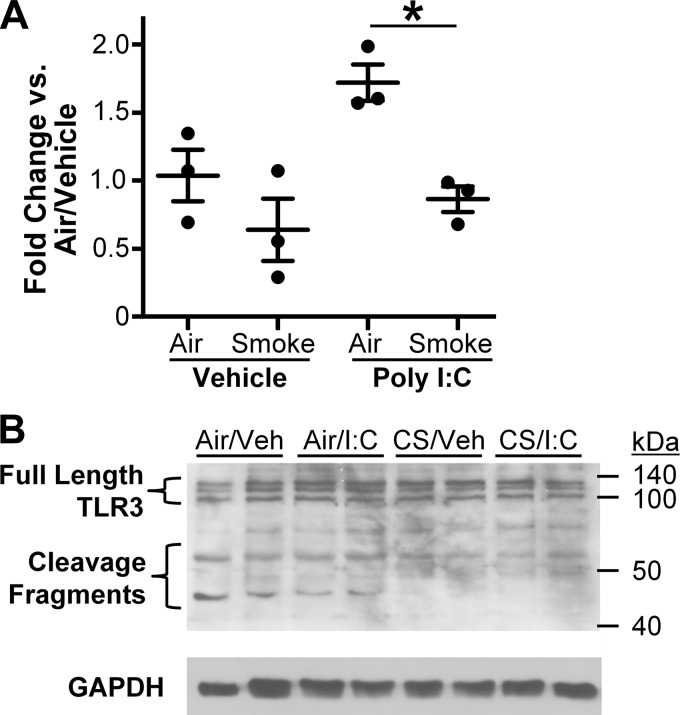
Cigarette smoke alters TLR3 cleavage.
TLR3 has been shown in certain cell types to be upregulated following viral infection (26). To determine whether cigarette smoke altered TLR3 transcription, RNA from air- or smoke-exposed SAEC (from all 3 donors) was harvested at 6 h following poly I:C treatment. TLR3 message levels were unchanged at 2 h (data not shown) and modestly increased 6 h after poly I:C treatment in air-exposed cells, and this increase was inhibited by prior cigarette smoke exposure (Fig. 6A). However, the modest changes in TLR3 message levels do not reflect the strong suppressive effects of cigarette smoke on IFN production or IRF3 phosphorylation. Therefore, we examined the effect of cigarette smoke on TLR3 protein by Western blot. Full-length TLR3 protein is ∼104 kDa in size and is located primarily in the endosomal compartment. Recent reports have shown that endosomal TLRs, including TLR3, TLR7, and TLR9, can be cleaved in the endosome, producing smaller protein fragments (7) that are necessary for efficient downstream antiviral signaling (8). Because of its strong antiviral response, TLR3 protein experiments were performed from donor 1 cells. We exposed SAEC to cigarette smoke and/or poly I:C as above and harvested cell lysates 6 h post poly I:C treatment. Despite modest changes in TLR3 mRNA, expression of full-length TLR3 protein (∼104KD) was unchanged in all treatment groups (Fig. 6B). Interestingly, two prominent bands that correspond to the reported sizes of the COOH-terminal and NH2-terminal TLR3 cleavage products (60 and 45 kDa, respectively) were also detected. The level of TLR3 cleavage fragments were decreased in smoke-exposed SAEC regardless of treatment with polyI:C (Fig. 6B). Treatment with poly I:C in air-exposed cells did not alter the abundance of cleaved TLR3 fragments.
Fig. 6.
Cigarette smoke alters cleavage of TLR3. SAEC were exposed to air or cigarette smoke for 60 min and treated with poly I:C. A: mRNA was harvested at 6 h posttreatment and analyzed for levels of TLR3 transcript by RT-PCR. B: levels of TLR3 protein were detected in cell lysates 6 h after poly I:C treatment. Bars are means ± SD of triplicate cultures and representative of experiments in SAEC from 3 donors. *P < 0.05 by 2-way ANOVA with Tukey post hoc correction.
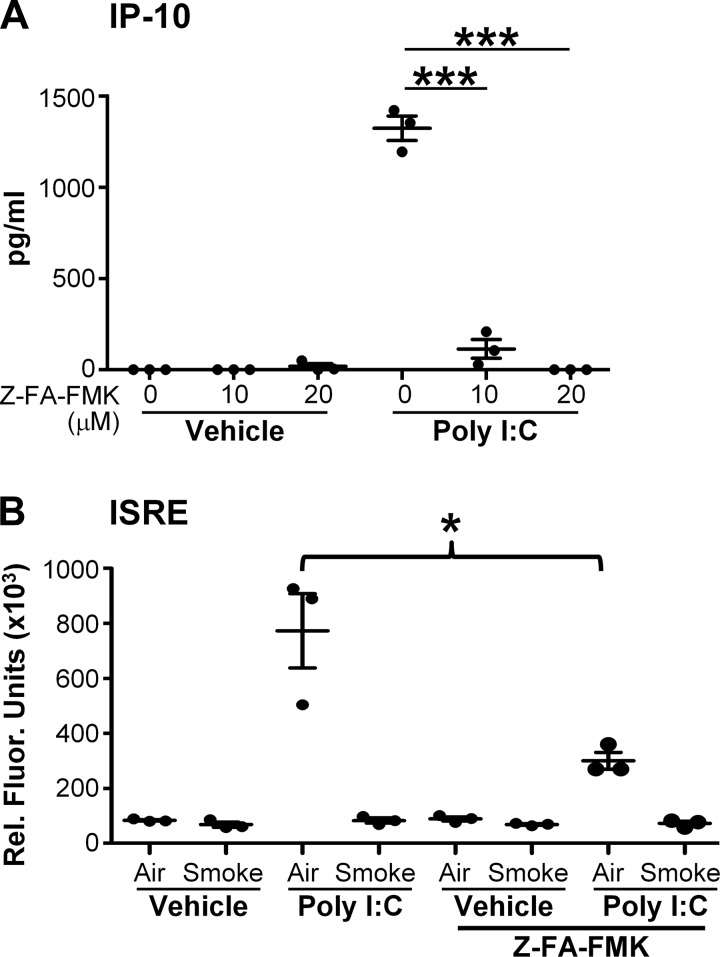
To help determine whether the effects on TLR3 cleavage were responsible for the decrease in inflammatory and antiviral mediators, SAEC were pretreated with z-FA-FMK, which inhibits TLR3 cleavage (8). In SAEC treated with poly I:C alone, Z-FA-FMK strongly suppressed IP-10 production and the upregulation of IFN activity in the conditioned medium (Fig. 7, A and B). This suggests that TLR3 cleavage is required for efficient responses to poly I:C and supports our result that cigarette smoke impairs poly I:C responses by blocking cleavage of TLR3.
Fig. 7.
Inhibition of TLR3 cleavage dampens innate immune responses. SAEC were treated with vehicle or the indicated concentration of the cathepsin inhibitor z-FA-FMK before treatment with poly I:C. A: IP-10 levels were determined in the culture supernatant. B: ISRE luciferase activity was measured in the supernatants from SAEC pretreated with 10uM z-FA-FMK. Bars are means ± SD of triplicate cultures and representative of experiments in SAEC from 3 donors. ***P < 0.001, *P < 0.05 by 2-way ANOVA with Tukey post hoc correction.
DISCUSSION
Epidemiologically, smokers are at increased risk of respiratory infections, and their infections tend to be more severe, with increased morbidity and risk of hospitalization relative to nonsmokers (1, 17, 18). This is especially true in the case of chronic lung diseases such as COPD and asthma (24). Recent work in animal models and in human studies has suggested that chronic smoking dysregulates B and T cell function and impairs adaptive immune responses to pathogens (23, 40). The effects of smoke on early innate responses in lung epithelial cells, the first target for most respiratory infections, are less well studied. Previous reports have investigated the effect cigarette smoke exposure has on epithelial cells of the upper airway. For example, cigarette smoke extract suppresses IFN production and IRF3 activation in bronchial epithelial cells (3), whereas nasal epithelial cells from smokers have impaired upregulation of IFNα and IRF7 following viral infection (16). Here, we studied primary human SAEC exposed to smoke at the air-liquid interface, as defects in upper airway mucociliary clearance, seen in smokers, leave the lower airways susceptible to viral infection. We report that cigarette smoke exposure dampens the innate antiviral responses of SAEC exposed to poly I:C, a dsRNA viral mimetic.
Cigarette smoke by itself was only mildly proinflammatory in primary human SAEC, with only around a twofold induction of IL-8 in two of the three strains tested (Fig. 1). Although this seems counterintuitive, given the strong association between smoking and lung disease, it is consistent with other studies that have found that cigarette smoke extract is strongly inflammatory only in the human A549 adenocarcinoma cell line but not in primary lung epithelial cells (13, 15, 46). This data is also consistent with recent data from our laboratory showing a twofold increase in IL-8 production in primary SAEC exposed to dung biomass smoke (25). Variability between donors in the amount of inflammation and smoke-induced suppression of inflammatory responses is likely due to individual variation in susceptibility to cigarette smoke. Evidence suggests that other lung cell types, including fibroblasts and resident macrophages, respond in a strong proinflammatory way to cigarette smoke (15).
To investigate the effect of cigarette smoke on epithelial innate immune responses, we exposed primary human SAEC to cigarette smoke, followed by poly I:C treatment. Poly I:C alone is strongly proinflammatory, inducing proinflammatory cytokines like IL-8 and IL-6 and antiviral response genes like IFNβ, IFNλ, and IP-10, acting through cleavage of TLR3 (Figs. 1–6). However, when SAEC were exposed to cigarette smoke before poly I:C treatment, proinflammatory and antiviral responses were profoundly suppressed, with decreased upregulation of IL-6 and decreased transcription and production of antiviral type I and type III interferons (Figs. 1–3). Similar results were obtained by infecting SAEC with IAV following smoke exposure. IAV infection stimulated production of IP-10, IL-6, and IL-8. Production of IL-6 and IP-10 was profoundly suppressed by prior smoke exposure. Consistent with this result, smoke-exposed SAECs also exhibited increased viral replication (Fig. 4).
Because IFR3 is a key transcription factor responsible for induction of IFN, we demonstrated that cigarette smoke exposure blocks IRF3 phosphorylation (Fig. 5). These results are similar to earlier studies using submerged cultures with cigarette smoke extract (3, 31). Importantly, we looked upstream of IRF3 activation and are the first to show that although cigarette smoke had little impact on TLR3 mRNA or full-length TLR3, cigarette smoke prevented TLR3 posttranslational processing, blocking cleavage that is necessary for interferon production (Figs. 6 and 7).
TLR3 is a well-described pathogen recognition receptor that is involved in the host response to viral infection. Whereas poly I:C is the prototypic TLR3 agonist, dsRNA is a product of viral replication and a possible source of TLR3 activation (36). Although poly I:C can increase TLR3 transcription (26, 42), we observed increased IFN transcription in air-exposed cells as early as 6 h after poly I:C treatment, well before noticeable changes in full-length TLR3 protein (Figs. 3 and 6). And we did not observe changes in TLR3 mRNA at 2 h post-poly I:C treatment (data not shown). These results suggested that if suppression of antiviral responses by cigarette smoke acted through TLR3, it was due to posttranslational modification.
Recent work has focused on elucidating the mechanism of activation of endosomal TLRs by cleavage. TLR3 was recently shown to be subject to cleavage when transfected into mouse cells (7). Studies in human renal pigmented epithelial cells determined that TLR3 is cleaved by cathepsin B and H (8). Poly I:C must enter the endosome and be cleaved to transmit a signal, as blocking endosome formation or acidification inhibits TLR3 cleavage and downstream signaling (8, 32). Here, we show that inhibition of cathepsins with Z-FA-FMK was able to mimic the smoke-induced impairment of innate immune responses to polyI:C in air-exposed cells (Fig. 7). The effect of environmental stimuli on TLR3 cleavage has to our knowledge not been investigated. Our work provides the first clue that environmental stimuli can affect TLR processing. Interestingly, we found that the smoke-induced suppression of IP-10 was restored in smoke-exposed cells that had been rested for 24 h (Fig. 2). One explanation is that resting the cells allows for the natural turnover of damaged cellular proteins, thus allowing a restoration of proper antiviral responsiveness.
Exogenous poly I:C is the prototypical agonist for TLR3 and is a highly used tool for studying antiviral defenses. Like viral infection, poly I:C treatment induces IFNs through the activation of IRF3. Although RNA viruses can be recognized by cytosolic receptors, including melanoma differentiation-associated gene (MDA5) and RIG-I, TLR3 is still important for proper immune responses (20, 35). For example, viral RNA from IAV infection is recognized through RIG-I; however, TLR3-knockout mice have impaired inflammatory responses to IAV infection, leading to an increase in viral burden (20, 21). TLR3 likely plays a role in detecting dsRNA released from dead infected cells. Although we show here that TLR3 signaling is impaired, the effect of smoke on RIG-I and MDA signaling is not well characterized but will be the subject of future work.
The innate immune response is important for effective control of viral infection. Signals produced early after viral infection help to establish an antiviral state to limit viral spread. Mice with defects in innate immune signaling pathways and IFN responses have impaired clearance of viral infection (2, 9, 20). Cigarette smoke-exposed mice also have been shown to have increased viral or bacterial burdens following infection (10, 23, 30). However, it is curious that cigarette smoke exposure impaired generation of IL-6 by SAEC, whereas animal studies have shown that smoke-exposed mice have increased inflammation, including IL-6 production, following infection with IAV (10, 30). We hypothesize that defective early innate responses, such as those demonstrated here, contribute to delayed adaptive responses, delayed clearance of the pathogens, and ultimately to increased disease pathology. Thus restoration of effective and timely control of innate immune responses is an important area for therapeutic intervention in those exposed to cigarette smoke. Here, we identify cleavage of TLR3 as a new mechanism of action of cigarette smoke. Targeting TLR3 cleavage could be a new therapeutic target to restore proper antiviral signals in those exposed to cigarette smoke.
GRANTS
This work was funded in part by National Institutes of Health (NIH) Grants R01-HL-120908, T32-HL-066988, and P30-ES-001247, the C. Jane Davis and C. Robert Davis Professorship, and the Wright Family Professorship. The project described in this publication was supported by University of Rochester CTSA award no. UL1TR002001 from the National Center for Advancing Translational Sciences of the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.F.D., C.E.M., A.N., T.H.T., L.M.-S., R.P.P., and P.J.S. conceived and designed research; P.F.D., C.E.M., and A.N. performed experiments; P.F.D. analyzed data; P.F.D., C.E.M., A.N., T.H.T., L.M.-S., R.P.P., and P.J.S. interpreted results of experiments; P.F.D. and T.H.T. prepared figures; P.F.D. drafted manuscript; P.F.D., C.E.M., A.N., T.H.T., L.M.-S., R.P.P., and P.J.S. edited and revised manuscript; P.F.D., C.E.M., A.N., T.H.T., L.M.-S., R.P.P., and P.J.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Stephen Pollock for help in creating the figures.
REFERENCES
- 1.Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med 164: 2206–2216, 2004. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- 2.Arimori Y, Nakamura R, Yamada H, Shibata K, Maeda N, Kase T, Yoshikai Y. Type I interferon limits influenza virus-induced acute lung injury by regulation of excessive inflammation in mice. Antiviral Res 99: 230–237, 2013. doi: 10.1016/j.antiviral.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Bauer CM, Dewitte-Orr SJ, Hornby KR, Zavitz CC, Lichty BD, Stämpfli MR, Mossman KL. Cigarette smoke suppresses type I interferon-mediated antiviral immunity in lung fibroblast and epithelial cells. J Interferon Cytokine Res 28: 167–179, 2008. doi: 10.1089/jir.2007.0054. [DOI] [PubMed] [Google Scholar]
- 4.Celli BR, Barnes PJ. Exacerbations of chronic obstructive pulmonary disease. Eur Respir J 29: 1224–1238, 2007. doi: 10.1183/09031936.00109906. [DOI] [PubMed] [Google Scholar]
- 5.Celli BR, MacNee W, Agusti A, Anzueto A, Berg B, Buist AS, Calverley PMA, Chavannes N, Dillard T, Fahy B, Fein A, Heffner J, Lareau S, Meek P, Martinez F, McNicholas W, Muris J, Austegard E, Pauwels R, Rennard S, Rossi A, Siafakas N, Tiep B, Vestbo J, Wouters E, ZuWallack R. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 23: 932–946, 2004. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 6.Doyle S, Vaidya S, O’Connell R, Dadgostar H, Dempsey P, Wu T, Rao G, Sun R, Haberland M, Modlin R, Cheng G. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity 17: 251–263, 2002. doi: 10.1016/S1074-7613(02)00390-4. [DOI] [PubMed] [Google Scholar]
- 7.Ewald SE, Engel A, Lee J, Wang M, Bogyo M, Barton GM. Nucleic acid recognition by Toll-like receptors is coupled to stepwise processing by cathepsins and asparagine endopeptidase. J Exp Med 208: 643–651, 2011. doi: 10.1084/jem.20100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Cattaneo A, Gobert FX, Müller M, Toscano F, Flores M, Lescure A, Del Nery E, Benaroch P. Cleavage of Toll-like receptor 3 by cathepsins B and H is essential for signaling. Proc Natl Acad Sci USA 109: 9053–9058, 2012. doi: 10.1073/pnas.1115091109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García-Sastre A, Egorov A, Matassov D, Brandt S, Levy DE, Durbin JE, Palese P, Muster T. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 252: 324–330, 1998. doi: 10.1006/viro.1998.9508. [DOI] [PubMed] [Google Scholar]
- 10.Gualano RC, Hansen MJ, Vlahos R, Jones JE, Park-Jones RA, Deliyannis G, Turner SJ, Duca KA, Anderson GP. Cigarette smoke worsens lung inflammation and impairs resolution of influenza infection in mice. Respir Res 9: 53, 2008. doi: 10.1186/1465-9921-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayden FG, Fritz R, Lobo MC, Alvord W, Strober W, Straus SE. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J Clin Invest 101: 643–649, 1998. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hewson CA, Jardine A, Edwards MR, Laza-Stanca V, Johnston SL. Toll-like receptor 3 is induced by and mediates antiviral activity against rhinovirus infection of human bronchial epithelial cells. J Virol 79: 12273–12279, 2005. doi: 10.1128/JVI.79.19.12273-12279.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holownia A, Wielgat P, Rysiak E, Braszko JJ. Intracellular and Extracellular Cytokines in A549 Cells and THP1 Cells Exposed to Cigarette Smoke. Adv Exp Med Biol 910: 39–45, 2016. doi: 10.1007/5584_2016_214. [DOI] [PubMed] [Google Scholar]
- 14.Honda K, Takaoka A, Taniguchi T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity 25: 349–360, 2006. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Hsiao HM, Sapinoro RE, Thatcher TH, Croasdell A, Levy EP, Fulton RA, Olsen KC, Pollock SJ, Serhan CN, Phipps RP, Sime PJ. A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation. PLoS One 8: e58258, 2013. doi: 10.1371/journal.pone.0058258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaspers I, Horvath KM, Zhang W, Brighton LE, Carson JL, Noah TL. Reduced expression of IRF7 in nasal epithelial cells from smokers after infection with influenza. Am J Respir Cell Mol Biol 43: 368–375, 2010. doi: 10.1165/rcmb.2009-0254OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kark JD, Lebiush M. Smoking and epidemic influenza-like illness in female military recruits: a brief survey. Am J Public Health 71: 530–532, 1981. doi: 10.2105/AJPH.71.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kark JD, Lebiush M, Rannon L. Cigarette smoking as a risk factor for epidemic a(h1n1) influenza in young men. N Engl J Med 307: 1042–1046, 1982. doi: 10.1056/NEJM198210213071702. [DOI] [PubMed] [Google Scholar]
- 19.Kim S, Kim MJ, Kim CH, Kang JW, Shin HK, Kim DY, Won TB, Han DH, Rhee CS, Yoon JH, Kim HJ. The Superiority of IFN-λ as a Therapeutic Candidate to Control Acute Influenza Viral Lung Infection. Am J Respir Cell Mol Biol 56: 202–212, 2017. [DOI] [PubMed] [Google Scholar]
- 20.Le Goffic R, Balloy V, Lagranderie M, Alexopoulou L, Escriou N, Flavell R, Chignard M, Si-Tahar M. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog 2: e53, 2006. doi: 10.1371/journal.ppat.0020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Goffic R, Pothlichet J, Vitour D, Fujita T, Meurs E, Chignard M, Si-Tahar M. Cutting Edge: Influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J Immunol 178: 3368–3372, 2007. doi: 10.4049/jimmunol.178.6.3368. [DOI] [PubMed] [Google Scholar]
- 22.Leopold PL, O’Mahony MJ, Lian XJ, Tilley AE, Harvey BG, Crystal RG. Smoking is associated with shortened airway cilia. PLoS One 4: e8157, 2009. doi: 10.1371/journal.pone.0008157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lugade AA, Bogner PN, Thatcher TH, Sime PJ, Phipps RP, Thanavala Y. Cigarette smoke exposure exacerbates lung inflammation and compromises immunity to bacterial infection. J Immunol 192: 5226–5235, 2014. doi: 10.4049/jimmunol.1302584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mallia P, Johnston SL. How viral infections cause exacerbation of airway diseases. Chest 130: 1203–1210, 2006. doi: 10.1378/chest.130.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCarthy CE, Duffney PF, Gelein R, Thatcher TH, Elder A, Phipps RP, Sime PJ. Dung biomass smoke activates inflammatory signaling pathways in human small airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 311: L1222–L1233, 2016. doi: 10.1152/ajplung.00183.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miettinen M, Sareneva T, Julkunen I, Matikainen S. IFNs activate toll-like receptor gene expression in viral infections. Genes Immun 2: 349–355, 2001. doi: 10.1038/sj.gene.6363791. [DOI] [PubMed] [Google Scholar]
- 27.Murakami Y, Fukui R, Motoi Y, Kanno A, Shibata T, Tanimura N, Saitoh S, Miyake K. Roles of the cleaved N-terminal TLR3 fragment and cell surface TLR3 in double-stranded RNA sensing. J Immunol 193: 5208–5217, 2014. doi: 10.4049/jimmunol.1400386. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen DN, Kim P, Martínez-Sobrido L, Beitzel B, García-Sastre A, Langer R, Anderson DG. A novel high-throughput cell-based method for integrated quantification of type I interferons and in vitro screening of immunostimulatory RNA drug delivery. Biotechnol Bioeng 103: 664–675, 2009. doi: 10.1002/bit.22312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nogales A, Baker SF, Martínez-Sobrido L. Replication-competent influenza A viruses expressing a red fluorescent protein. Virology 476: 206–216, 2015. doi: 10.1016/j.virol.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oostwoud LC, Gunasinghe P, Seow HJ, Ye JM, Selemidis S, Bozinovski S, Vlahos R. Apocynin and ebselen reduce influenza A virus-induced lung inflammation in cigarette smoke-exposed mice. Sci Rep 6: 20983, 2016. doi: 10.1038/srep20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Proud D, Hudy MH, Wiehler S, Zaheer RS, Amin MA, Pelikan JB, Tacon CE, Tonsaker TO, Walker BL, Kooi C, Traves SL, Leigh R. Cigarette smoke modulates expression of human rhinovirus-induced airway epithelial host defense genes. PLoS One 7: e40762, 2012. doi: 10.1371/journal.pone.0040762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qi R, Singh D, Kao CC. Proteolytic processing regulates Toll-like receptor 3 stability and endosomal localization. J Biol Chem 287: 32617–32629, 2012. doi: 10.1074/jbc.M112.387803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Randall RE, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol 89: 1–47, 2008. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- 34.Rudd BD, Burstein E, Duckett CS, Li X, Lukacs NW. Differential role for TLR3 in respiratory syncytial virus-induced chemokine expression. J Virol 79: 3350–3357, 2005. doi: 10.1128/JVI.79.6.3350-3357.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudd BD, Smit JJ, Flavell RA, Alexopoulou L, Schaller MA, Gruber A, Berlin AA, Lukacs NW. Deletion of TLR3 alters the pulmonary immune environment and mucus production during respiratory syncytial virus infection. J Immunol 176: 1937–1942, 2006. doi: 10.4049/jimmunol.176.3.1937. [DOI] [PubMed] [Google Scholar]
- 36.Sen GC, Sarkar SN. Transcriptional signaling by double-stranded RNA: role of TLR3. Cytokine Growth Factor Rev 16: 1–14, 2005. doi: 10.1016/j.cytogfr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Servant MJ, Grandvaux N, tenOever BR, Duguay D, Lin R, Hiscott J. Identification of the minimal phosphoacceptor site required for in vivo activation of interferon regulatory factor 3 in response to virus and double-stranded RNA. J Biol Chem 278: 9441–9447, 2003. doi: 10.1074/jbc.M209851200. [DOI] [PubMed] [Google Scholar]
- 38.Shapiro SD. Smoke gets in your cells. Am J Respir Cell Mol Biol 31: 481–482, 2004. doi: 10.1165/rcmb.F285. [DOI] [PubMed] [Google Scholar]
- 39.Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 60: 925–931, 2005. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stämpfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol 9: 377–384, 2009. doi: 10.1038/nri2530. [DOI] [PubMed] [Google Scholar]
- 41.Sturton G, Persson C, Barnes PJ. Small airways: an important but neglected target in the treatment of obstructive airway diseases. Trends Pharmacol Sci 29: 340–345, 2008. doi: 10.1016/j.tips.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 42.Tanabe M, Kurita-Taniguchi M, Takeuchi K, Takeda M, Ayata M, Ogura H, Matsumoto M, Seya T. Mechanism of up-regulation of human Toll-like receptor 3 secondary to infection of measles virus-attenuated strains. Biochem Biophys Res Commun 311: 39–48, 2003. doi: 10.1016/j.bbrc.2003.09.159. [DOI] [PubMed] [Google Scholar]
- 43.Thatcher TH, Maggirwar SB, Baglole CJ, Lakatos HF, Gasiewicz TA, Phipps RP, Sime PJ. Aryl hydrocarbon receptor-deficient mice develop heightened inflammatory responses to cigarette smoke and endotoxin associated with rapid loss of the nuclear factor-kappaB component RelB. Am J Pathol 170: 855–864, 2007. doi: 10.2353/ajpath.2007.060391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thatcher TH, McHugh NA, Egan RW, Chapman RW, Hey JA, Turner CK, Redonnet MR, Seweryniak KE, Sime PJ, Phipps RP. Role of CXCR2 in cigarette smoke-induced lung inflammation. Am J Physiol Lung Cell Mol Physiol 289: L322–L328, 2005. doi: 10.1152/ajplung.00039.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thorne D, Adamson J. A review of in vitro cigarette smoke exposure systems. Exp Toxicol Pathol 65: 1183–1193, 2013. doi: 10.1016/j.etp.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 46.Witherden IR, Vanden Bon EJ, Goldstraw P, Ratcliffe C, Pastorino U, Tetley TD. Primary human alveolar type II epithelial cell chemokine release: effects of cigarette smoke and neutrophil elastase. Am J Respir Cell Mol Biol 30: 500–509, 2004. doi: 10.1165/rcmb.4890. [DOI] [PubMed] [Google Scholar]
- 47.Xavier RF, Ramos D, Ito JT, Rodrigues FM, Bertolini GN, Macchione M, de Toledo AC, Ramos EM. Effects of cigarette smoking intensity on the mucociliary clearance of active smokers. Respiration 86: 479–485, 2013. doi: 10.1159/000348398. [DOI] [PubMed] [Google Scholar]