Abstract
AIM
To clarify the diagnostic performance of endocytoscopy for differentiation between neoplastic and non-neoplastic colorectal diminutive polyps.
METHODS
Patients who underwent endocytoscopy between October and December 2016 at Sano Hospital were prospectively recruited. When diminutive polyps (≤ 5 mm) were detected, the lesions were evaluated by endocytoscopy after being stained with 0.05% crystal violet and 1% methylene blue. The diminutive polyps were classified into five categories (EC 1a, 1b, 2, 3a, and 3b). Endoscopists were asked to take a biopsy from any lesion diagnosed as EC1b (indicator of hyperplastic polyp) or EC2 (indicator of adenoma). We have assessed the diagnostic performance of endocytoscopy for EC2 and EC1b lesions by comparison with the histopathology of the biopsy specimen.
RESULTS
A total of 39 patients with 63 diminutive polyps were analyzed. All polyps were evaluated by endocytoscopy. The mean polyp size was 3.3 ± 0.9 mm. Among the 63 diminutive polyps, 60 were flat and 3 were pedunculated. The mean time required for EC observation, including the time for staining with crystal violet and methylene blue, was 3.0 ± 1.9 min. Histopathologic evaluation showed that 13 polyps were hyperplastic and 50 were adenomas. The sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of EC2 for adenoma compared with EC1b for hyperplastic polyp were 98.0%, 92.3%, 96.8%, 98.0% and 92.3%, respectively. There were only two cases of disagreement between the endoscopic diagnosis made by endocytoscopy and the corresponding histopathological diagnosis.
CONCLUSION
Endocytoscopy showed a high diagnostic performance for differentiating between neoplastic and non-neoplastic colorectal diminutive polyps, and therefore has the potential to be used for “real-time histopathology”.
Keywords: Endocytoscopy, Diagnostic performance, Diminutive polyp, Endocytoscopic classification, Real-time histopathology
Core tip: Single-Charge Coupled Device integrated type endocytoscopy (OLYMPUS, Japan) is going to be newly launched in 2018. Endocytoscopy with approximately 500-fold magnification capability allows us to observe both structural and cellular atypia in vivo, and is expected to be used as an optical biopsy. In our prospective study, we aimed to clarify the diagnostic performance of endocytoscopy in differentiating neoplasia from non-neoplasia for colorectal diminutive polyps (≤ 5 mm). The diagnostic performance of endocytoscopy met the threshold of the Preservation and Incorporation of Valuable endoscopic Innovations statement for “resect-and-discard” and “diagnose-and-leave” strategies.
INTRODUCTION
Colorectal cancer (CRC) is a major cause of morbidity and mortality worldwide[1]. Resection of adenomas with colonoscopy has been shown to decrease the risk of subsequent CRC and CRC-related death[2,3].
Technological advances in colonoscopy have now made it possible to determine the histopathology of colorectal polyps in vivo, which may potentially reduce the cost of histopathological assessment of adenomas and avoid unnecessary polypectomy for hyperplastic polyps[4]. The American Society for Gastrointestinal Endoscopy (ASGE) has suggested key thresholds for assessing the histology of diminutive polyps (≤ 5 mm) using endoscopic technology in the Preservation and Incorporation of Valuable endoscopic Innovations (PIVI) document. This specified an agreement rate of ≥ 90% for assignment of post-polypectomy surveillance intervals and a negative predictive value of ≥ 90% for recto-sigmoid polyps with an adenomatous histology. However, it has been difficult for endoscopic real-time histologic assessment of diminutive polyps to meet the PIVI criteria, except for high-confidence assessment made by experts using narrow-band imaging (NBI)[5,6].
The single-Charge Coupled Device integrated-type endocytoscope, which will be newly launched in early 2018 by Olympus Medical Systems Corporation (Tokyo, Japan), is based on the technology of light contact microscopy (Figure 1). It has ultra-high magnification capability, allowing observation of both structural and cellular atypia in vivo and in real time[7-10]. The diagnostic accuracy of endocytoscopy for differentiating neoplastic from non-neoplastic colorectal polyps has already been reported to be 94.1%-100%[9-11]. A randomized study of colorectal lesions larger or equal to 5mm in diameter showed that the diagnostic accuracy of endocytoscopy was not inferior to that of biopsy[11]. Although it is expected that endocytoscopy will eventually be used as real-time histopathologic assessment, few studies have examined its diagnostic performance for diminutive polyps.
Figure 1.
Technology of light contact microscopy, endocytoscope.
Therefore, we conducted the present prospective study to clarify the diagnostic performance of endocytoscopy for differentiating neoplastic from non-neoplastic diminutive colorectal polyps.
MATERIALS AND METHODS
Patients
Consecutive adult patients 20 years of age or older who underwent total colonoscopy with an endocytoscope between October and December 2016 in Sano Hospital were enrolled in this study. The exclusion criteria were as follows: (1) Patients with a history of inflammatory bowel disease, hereditary polyposis syndrome, or Lynch syndrome; (2) patients with poor bowel preparation; and(3) patients without diminutive polyps. Written informed consent was obtained from all patients before the examination. The study protocol was approved by the institutional review board of Sano Hospital, Kobe, Japan. The clinical trial was registered in a clinical trial registry (UMIN 000023738).
Methods
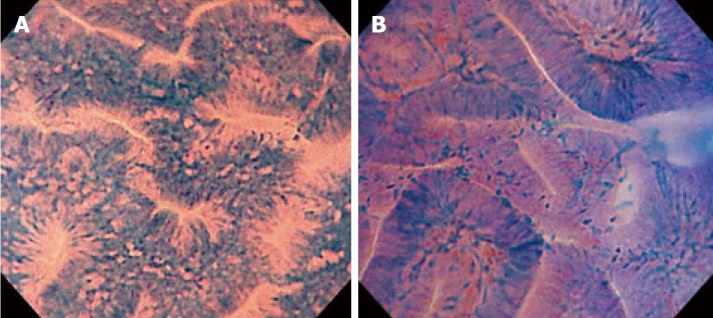
The procedures were performed by 6 endoscopists who had extensive experience in magnifying colonoscopy (> 1000 cases). All had only limited experience of endocytoscopy before the study. We employed a prototype endocytoscope (CF-Y0058-1) with capability of approximately 500-fold magnification, which had the same specifications as the one released by Olympus, with a LUCERA video processor. We used a distal attachment fitted to the tip of the endocytoscope, which facilitated stable attachment to the surface of the polyps in all cases. All diminutive colorectal polyps were detected by white light imaging and evaluated by endocytoscopy as follow. First, the lesions were stained with 0.05% crystal violet and 1% methylene blue for 30 seconds using a non-traumatic tube[12]. More dye was added if the staining of polyps was initially inadequate. Endoscopists were asked to assign an endocytoscopic (EC) classification (1a, 1b, 2, 3a, and 3b) for each polyp after staining[10]. The EC classification was based on the morphology of the lumina and the shape of nuclei in the lesions. EC1a was assigned to indicate normal mucosa, EC1b for hyperplastic polyp, EC2 for adenoma, and EC3a/3b for cancer. When many diminutive polyps were detected, only the first three were studied so as not to prolong the examination unnecessarily. Any inflammatory polyps were omitted from the study. When diminutive polyps were classified as EC1b or EC2, biopsy was performed (Figure 2). We then assessed the diagnostic performance of the endocytoscopy for EC2 and EC1b lesions by comparison with the corresponding histopathology of the biopsy specimen.
Figure 2.
Typical images of endocytoscopic (EC) 1b and EC2. A: EC1b shows narrow serrated lumina and small roundish nuclei; B: EC2 shows slit-like smooth lumina and uniform fusiform or roundish nuclei.
The location, size and shape (Paris classification) of all polyps and the examination time including dye staining were recorded[13]. The biopsy specimens were diagnosed histopathologically by an experienced gastrointestinal histopathologist (T.F) according to the criteria of the World Health Organization without endoscopic diagnosis[14]. We prospectively evaluated the diagnostic performance of endocytoscopy for differentiating between neoplastic and non-neoplastic polyps.
Statistical analysis
Continuous variables are expressed as mean ± SD. In this study, the 95% confidence interval (95%CI) for diagnostic performance was calculated.
RESULTS
During the study period, 71 patients underwent total colonoscopy using an endocytoscope. Among these patients, 4 with a history of ulcerative colitis, 3 with poor bowel preparation, and 25 without diminutive polyps were excluded, leaving a total of 39 patients with 63 diminutive polyps for analysis. Table 1 shows the characteristics of the eligible patients and resected polyps. All eligible polyps were evaluated by endocytoscopy. The mean polyp size was 3.3 ± 0.9 mm. Among the 63 diminutive polyps, 60 were flat and 3 were pedunculated. The mean duration of EC observation, including the time taken for staining with crystal violet and methylene blue, was 3.0 ± 1.9 min. The relationship between endoscopic diagnosis using the EC classification and pathological diagnosis of colorectal diminutive polyps in terms of the ability to differentiate between adenoma and hyperplastic polyp is shown in Table 2. The sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of EC2 for adenoma compared with EC1b for hyperplastic polyp were 98.0%, 92.3%, 96.8%, 98.0% and 92.3%, respectively. There were two cases of disagreement between the endoscopic and histopathological diagnoses.
Table 1.
Characteristics of the studied patients and resected polyps
| Total patients, n | 39 |
| Male/female | 23/16 |
| Age (mean ± SD, yr) | 65.8 ± 13.1 |
| Polyps, n | 63 |
| Size (mean ± SD, mm) | 3.3 ± 0.9 |
| Location, right/left | 33/30 |
| Shape, protruding/flat/depressed | 3/60/0 |
| Histopathology | |
| Hyperplastic polyp | 13 |
| Adenoma | 50 |
Table 2.
Diagnostic performance of endocytoscopy for differentiating adenoma from hyperplastic polyp among diminutive colorectal polyps
| EC classification | Hyperplastic polyp | Adenoma |
| n | n | |
| EC 1b | 12 | 1 |
| EC 2 | 1 | 49 |
Sensitivity 98.0% (95%CI: 89.3%-99.9%); Specificity 92.3% (95%CI: 64.0%-99.8%); Accuracy 96.8% (95%CI: 89.0%-99.6%); Positive predictive value 98.0% (95%CI: 89.3%-99.9%); Negative predictive value 92.3% (95%CI: 64.0%-99.8%). EC: Endocytoscopic.
DISCUSSION
In this study, endocytoscopy achieved a high diagnostic performance for differentiating neoplastic from non-neoplastic diminutive colorectal polyps. To our knowledge, this is the first prospective study to have evaluated the performance of endocytoscopy for diminutive polyps in real time.
The diagnostic performance of endocytoscopy for diminutive polyps met the PIVI criteria for assessment of histology. A meta-analysis of studies that had evaluated image-enhanced endoscopy techniques such as NBI, i-SCAN, and Fuji Intelligent Chromo Endoscopy concluded that the diagnostic performance of NBI met the threshold only when assessments could be made with high confidence[6]. The rate of high confidence for NBI-based diagnosis without magnification is reported to range from 75% to 80%[4,15,16]. Endocytoscopy was able to achieve a high diagnostic performance for all diminutive colorectal polyps regardless of confidence level.
However, the diagnostic performance in our present study tended to be slightly lower than that in previous studies of endocytoscopy[9,10]. There are several possible reasons for this difference. First, although some previous studies using endocytoscopy demonstrated a diagnostic accuracy of almost 100% in differentiating neoplasia from non-neoplasia, most of the polyps analyzed were small (6-9 mm) or large (≥ 10 mm). It is generally more difficult to diagnose diminutive polyps accurately than in the case for small or large polyps because any neoplastic changes in the former are expected to be minimal. Second, some earlier studies excluded lesions that were not imaged well by endocytoscopy. In order to obtain a fully magnified image with an endocytoscope, the scope should physically be in contact to the lesion. Factors including colonic peristalsis, movement due to breathing and heartbeat often make it difficult to obtain evaluable images using endocytoscopy. Kudo et al[10] reported that endocytoscopy yielded sufficiently clear images in 95.5% of the cases they studied. These factors also need to be considered when discussing the diagnostic performance of endoscopic instruments.
In the present study, there were two cases of disagreement between the EC diagnosis and the histopathological diagnosis made by biopsy. We investigated the causes of disagreement in these two cases. In one of them, although the endoscopist diagnosed the polyp as EC1b, the pictures taken suggested that it should have been diagnosed as EC2 (Figure 3). This suggests the need for more experience with real-time evaluation of 500-fold magnification images for assessment of cellular and nuclear morphology. In the other case, the polyp had a shallow depressed area on its surface, and the EC diagnosis of EC2 was based on the features in this depressed area. Histopathologic evaluation demonstrated a hyperplastic polyp, but a section of the pathology specimen revealed no depressed area (Figure 4). We speculate that this discrepancy resulted from inadequate resection of the biopsy specimen, which did not provide the part showing adenomatous change.
Figure 3.
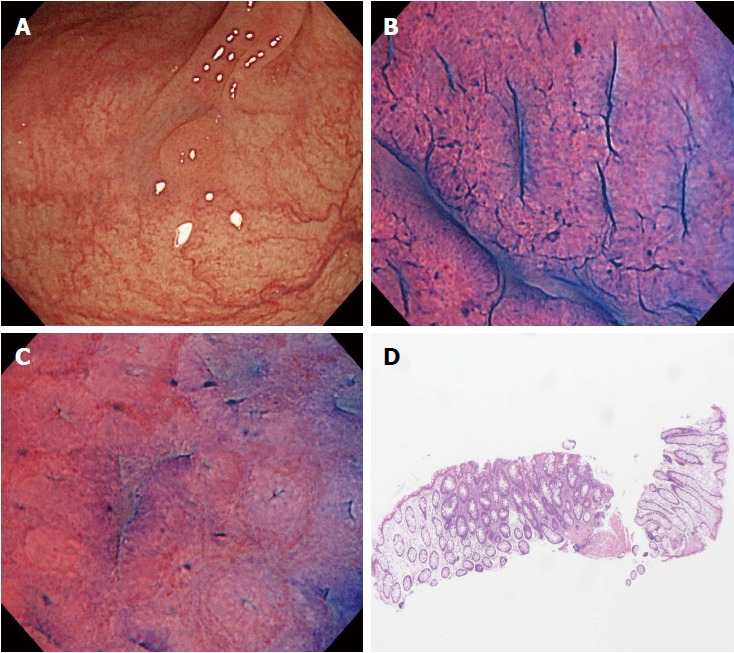
A case of disagreement between endocytoscopic (EC) 1b diagnosis and histopathologic diagnosis (adenoma). A: Flat-type lesion (0-IIa) 3 mm in size; B: The polyp was located in the sigmoid colon. The EC images showed slit-like smooth lumina; C: The EC images showed roundish lumina; D: The nuclei were not clear due to inadequate staining. An endoscopist diagnosed the polyp as EC1b. Histologic examination revealed an adenomatous polyp.
Figure 4.
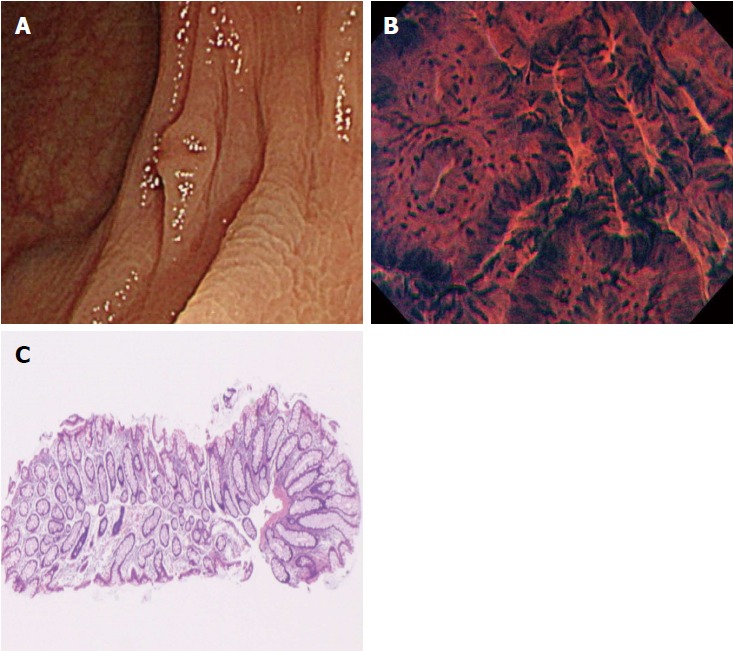
A case of disagreement between endocytoscopic (EC) 2 diagnosis and histopathologic diagnosis (hyperplastic polyp). A: Flat-type lesion (0-IIa) with a shallow depressed area, 3 mm in size; B: The polyp was located in the transverse colon. Endoscopic imaging in the shallow depressed area showed typical EC2 features with slit-like smooth lumina and uniform fusiform and roundish nuclei; C: The polyp was diagnosed as EC2. Histologic examination revealed a hyperplastic polyp without a depressed area.
Because endocytoscopy has a high diagnostic performance for diminutive polyps, it is applicable as a means of assessing real-time histopathology. However, to justify the clinical application of endocytoscopy, clarification of its disadvantages is essential. In the present study, it took approximately 3 min to observe each lesion, including the time required for staining. For this reason, it is not realistic to carry out endocytoscopy for all lesions when many polyps were detected. Solving this problem represents a significant challenge.
This study had some limitations. First, because we were using a prototype endocytoscope that was available to our institute for only a limited period, the number of samples was too small. Second, this study was performed at a single community-based hospital. Additional multicenter studies will be needed to confirm the present results. Third, all colonoscopists who performed endocytoscopy had enough experience of magnifying colonoscopy. The assessment during colonoscopy is a different experience from evaluations of only endoscopic still images. Endoscopists are required to obtain clear evaluable images in a colonic environment that is moving or contains remnant stool material[17,18]. It remains to be examined whether non-experts in magnifying endoscopy can deliver highly reliable diagnoses using endocytoscopy.
We conclude from this study that endocytoscopy can achieve high diagnostic performance in terms of differentiating neoplastic from non-neoplastic diminutive colorectal polyps. Real-time histopathologic assessment by endocytoscopy in vivo has the potential to replace the conventional diagnosis by histopathologists in vitro.
ARTICLE HIGHLIGHTS
Research background
Endocytoscopy, which will be newly launched in 2018, allows us to observe both structural and cellular atypia in vivo. The Preservation and Incorporation of Valuable endoscopic Innovations (PIVI) document described key thresholds for assessing the histology of diminutive polyps (≤ 5 mm) using endoscopic technology. However, few studies about endocytoscopy have examined its diagnostic performance for diminutive colorectal polyps.
Research motivation
This study before the launch of endocytoscopy can help to understand the usefulness of endocytoscopy.
Research objectives
To clarify the diagnostic performance of endocytoscopy for differentiating neoplastic from non-neoplastic diminutive colorectal polyps.
Research methods
We prospectively recruited patients who underwent endocytoscopy between October and December 2016 at Sano Hospital. Diminutive polyps were evaluated by endocytoscopy after being stained with 0.05% crystal violet and 1% methylene blue. The diminutive polyps were classified according to the endocytoscopic (EC) classification. Endoscopists have assessed the diagnostic performance of endocytoscopy for EC2 (indicator of adenoma) and EC1b (indicator of hyperplastic polyp) lesions by comparison with the histopathology of the biopsy specimen.
Research results
A total of 39 patients with 63 diminutive polyps were analyzed. The accuracy and negative predictive value of EC2 for adenoma compared with EC1b for hyperplastic polyp were 98.0% and 92.3%. Endocytoscopy showed a high diagnostic performance for differentiating between neoplastic and non-neoplastic colorectal diminutive polyps. However, because endocytoscope was available to our institute for only a limited period, the number of samples was small.
Research conclusions
The diagnostic performance of endocytoscopy for colorectal diminutive polyps met the PIVI criteria for assessment of histology.
Research perspectives
Real-time histopathologic assessment by endocytoscopy has the potential to save histopathological diagnosis. Additional multicenter studies are needed to confirm our result.
Footnotes
Institutional review board statement: This study was approved by the Institutional Review Board at Sano Hospital.
Informed consent statement: Written informed consent for the procedures was obtained from all patients.
Conflict-of-interest statement: None.
Data sharing statement: No additional data are available.
Manuscript source: Invited manuscript
Peer-review started: December 17, 2017
First decision: January 6, 2018
Article in press: March 6, 2018
Specialty type: Oncology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Karakoyun K, Sun LM S- Editor: Cui LJ L- Editor: A E- Editor: Wang CH
Contributor Information
Takahiro Utsumi, Gastrointestinal Center and Institute of Minimally Invasive Endoscopic Care (iMEC), Sano Hospital, Hyogo 655-0031, Japan.
Yasushi Sano, Gastrointestinal Center and Institute of Minimally Invasive Endoscopic Care (iMEC), Sano Hospital, Hyogo 655-0031, Japan. ys_endoscopy@hotmail.com.
Mineo Iwatate, Gastrointestinal Center and Institute of Minimally Invasive Endoscopic Care (iMEC), Sano Hospital, Hyogo 655-0031, Japan.
Hironori Sunakawa, Gastrointestinal Center and Institute of Minimally Invasive Endoscopic Care (iMEC), Sano Hospital, Hyogo 655-0031, Japan.
Akira Teramoto, Gastrointestinal Center and Institute of Minimally Invasive Endoscopic Care (iMEC), Sano Hospital, Hyogo 655-0031, Japan.
Daizen Hirata, Gastrointestinal Center and Institute of Minimally Invasive Endoscopic Care (iMEC), Sano Hospital, Hyogo 655-0031, Japan.
Santa Hattori, Gastrointestinal Center and Institute of Minimally Invasive Endoscopic Care (iMEC), Sano Hospital, Hyogo 655-0031, Japan.
Wataru Sano, Gastrointestinal Center and Institute of Minimally Invasive Endoscopic Care (iMEC), Sano Hospital, Hyogo 655-0031, Japan.
Noriaki Hasuike, Gastrointestinal Center and Institute of Minimally Invasive Endoscopic Care (iMEC), Sano Hospital, Hyogo 655-0031, Japan.
Kazuhito Ichikawa, Department of Pathology, Shinko Hospital, Hyogo 651-0072, Japan.
Takahiro Fujimori, Department of Pathology, Shinko Hospital, Hyogo 651-0072, Japan.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 3.Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF, et al. Colonoscopic polypectomy and long-term prevention of colorectal cancer deaths. N Engl J Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ignjatovic A, East JE, Suzuki N, Vance M, Guenther T, Saunders BP. Optical diagnosis of small colorectal polyps at routine colonoscopy (Detect InSpect ChAracterise Resect and Discard; DISCARD trial): a prospective cohort study. Lancet Oncol. 2009;10:1171–1178. doi: 10.1016/S1470-2045(09)70329-8. [DOI] [PubMed] [Google Scholar]
- 5.Rex DK, Kahi C, O’Brien M, Levin TR, Pohl H, Rastogi A, Burgart L, Imperiale T, Ladabaum U, Cohen J, et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2011;73:419–422. doi: 10.1016/j.gie.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 6.ASGE Technology Committee, Abu Dayyeh BK, Thosani N, Konda V, Wallace MB, Rex DK, Chauhan SS, Hwang JH, Komanduri S, Manfredi M, Maple JT, Murad FM, Siddiqui UD, Banerjee S. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2015;81:502.e1–502.e16. doi: 10.1016/j.gie.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Cipolletta L, Bianco MA, Rotondano G, Piscopo R, Meucci C, Prisco A, Cipolletta F, de Gregorio A, Salvati A. Endocytoscopy can identify dysplasia in aberrant crypt foci of the colorectum: a prospective in vivo study. Endoscopy. 2009;41:129–132. doi: 10.1055/s-0028-1103452. [DOI] [PubMed] [Google Scholar]
- 8.Inoue H, Kazawa T, Sato Y, Satodate H, Sasajima K, Kudo SE, Shiokawa A. In vivo observation of living cancer cells in the esophagus, stomach, and colon using catheter-type contact endoscope, “Endo-Cytoscopy system”. Gastrointest Endosc Clin N Am. 2004;14:589–594, x-xi. doi: 10.1016/j.giec.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Sasajima K, Kudo SE, Inoue H, Takeuchi T, Kashida H, Hidaka E, Kawachi H, Sakashita M, Tanaka J, Shiokawa A. Real-time in vivo virtual histology of colorectal lesions when using the endocytoscopy system. Gastrointest Endosc. 2006;63:1010–1017. doi: 10.1016/j.gie.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Kudo SE, Wakamura K, Ikehara N, Mori Y, Inoue H, Hamatani S. Diagnosis of colorectal lesions with a novel endocytoscopic classification - a pilot study. Endoscopy. 2011;43:869–875. doi: 10.1055/s-0030-1256663. [DOI] [PubMed] [Google Scholar]
- 11.Mori Y, Kudo S, Ikehara N, Wakamura K, Wada Y, Kutsukawa M, Misawa M, Kudo T, Kobayashi Y, Miyachi H, et al. Comprehensive diagnostic ability of endocytoscopy compared with biopsy for colorectal neoplasms: a prospective randomized noninferiority trial. Endoscopy. 2013;45:98–105. doi: 10.1055/s-0032-1325932. [DOI] [PubMed] [Google Scholar]
- 12.Ichimasa K, Kudo SE, Mori Y, Wakamura K, Ikehara N, Kutsukawa M, Takeda K, Misawa M, Kudo T, Miyachi H, et al. Double staining with crystal violet and methylene blue is appropriate for colonic endocytoscopy: an in vivo prospective pilot study. Dig Endosc. 2014;26:403–408. doi: 10.1111/den.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3–43. doi: 10.1016/s0016-5107(03)02159-x. [DOI] [PubMed] [Google Scholar]
- 14.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon: IARC press;; 2010. [Google Scholar]
- 15.Rex DK. Narrow-band imaging without optical magnification for histologic analysis of colorectal polyps. Gastroenterology. 2009;136:1174–1181. doi: 10.1053/j.gastro.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Hewett DG, Kaltenbach T, Sano Y, Tanaka S, Saunders BP, Ponchon T, Soetikno R, Rex DK. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology. 2012;143:599–607.e1. doi: 10.1053/j.gastro.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Ladabaum U, Fioritto A, Mitani A, Desai M, Kim JP, Rex DK, Imperiale T, Gunaratnam N. Real-time optical biopsy of colon polyps with narrow band imaging in community practice does not yet meet key thresholds for clinical decisions. Gastroenterology. 2013;144:81–91. doi: 10.1053/j.gastro.2012.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuiper T, Marsman WA, Jansen JM, van Soest EJ, Haan YC, Bakker GJ, Fockens P, Dekker E. Accuracy for optical diagnosis of small colorectal polyps in nonacademic settings. Clin Gastroenterol Hepatol. 2012;10:1016–1020; quiz e79. doi: 10.1016/j.cgh.2012.05.004. [DOI] [PubMed] [Google Scholar]