Abstract
Background
HSP60-related immunological activities are found in normal-pressure glaucoma (NPG) patients, in whom an elevated intraocular pressure (IOP) found in primary open-angle glaucoma (POAG) is not observed. HSP60 was found in POAG and NPG patients, while anti-HSP60 level was mainly found to be higher in NPG patients. The purpose of this study was to compare the percentages of Th cells and levels of related cytokines, attempting to provide evidence to explain this discrepancy.
Material/Methods
Blood samples from POAG, NPG, and normal control (NC) groups were collected and peripheral blood monocytes were isolated and cultured with or without the stimulation of HSP60. Flow cytometry and enzyme-linked immunosorbent assay were used to assess the percentages of Th1, Th2, Th17, and Treg cells, as well as HSP60 antibody levels and related cytokine levels, before and after culture.
Results
Significantly higher titers of anti-HSP60 were observed only in NPG patients. Comparable Th1 and Th2 cell frequencies, IL-4 level, and IFN-γ level were found in POAG and NPG patients, while higher Treg cell frequency was only found in POAG patients. After culturing with HSP60, increased Th2 frequencies and decreased Th1 frequencies were observed in the POAG, NPG, and NC groups, while increased Treg frequency was only identified in the POAG and NC groups.
Conclusions
Different Th cell patterns were observed among POAG, NPG, and NC groups. Lack of induction of Treg cells and imbalance of the pro-inflammatory and anti-inflammatory response patterns of Th cells exist in some NPG patients.
MeSH Keywords: Chaperonin 60; Hydrocephalus, Normal Pressure; T-Lymphocytes, Regulatory
Background
Glaucoma is the leading irreversible eye disease in the world. The number of glaucoma patients will exceed 76 million by 2020, among which over 11 million will be bilaterally blind [1,2]. Elevation of intraocular pressure (IOP) is regarded the most important risk factor for the development of glaucoma, and IOP control is for now the only effective and well-accepted treatment. However, approximately 30% of glaucoma patients present without elevation of IOP [3–5]. Over 90% of Japanese open-angle glaucoma patients have normal IOP [6]. The Baltimore Eye Survey revealed that 20% of their POAG patients had an IOP of less than 21 mmHg on each of their first 3 visits, suggesting that normal-pressure eye disease may be more common than previously thought [3]. The efficacy of IOP control in normal-pressure glaucoma patients (NPG) has been reported; however, progression is still observed in many cases after lowering IOP. The existence of NPG patients has spurred further research to explore other possible risk factors for glaucoma to find effective treatments [7].
Accumulating evidence shows that heat-shock protein (HSP) 60-related autoimmunity is involved in many diseases, including inflammatory arthritis, type 1 diabetes, and multiple sclerosis [8–10], as well as in the development of NPG. Higher levels of HSP60 and its antibody have been found in the retina and serum/aqueous fluid of NPG patients, indicating involvement of HSP60-specific autoimmune activity [11–14]. Investigation of the association between involvement of HSP autoimmune activity and the signature event of glaucoma, which is retinal ganglion cell (RGC) loss, shows that HSP antibody can lead to changes in regulatory proteins and cytoskeleton of RGCs [15]. Furthermore, an experimental autoimmune glaucoma model has been successfully established and demonstrated that immunization with HSP60 can induce upregulated HSP60 antibody and significant RGC loss [16,17]. Although HSP60-specific autoimmune activity was observed in POAG and NPG, some findings suggest that the ways in which HSP autoimmunity is involved differ between POAG and NPG. Approximately 30% of patients with NPG have 1 or more immune-related diseases, which is not observed in POAG [18]. Unlike NPG, progression is limited in most cases of POAG if IOP is under control. More importantly, a higher titer of autoantibody to HSP60 was mainly found in patients with normal-pressure glaucoma, and less or no significant difference was found between POAG and control subjects in some studies [19].
Balanced responses of both pro- and anti-inflammatory Th cells are essential to prevent an immune response from turning into autoimmune disease. Recently, imbalances have been reported to be responsible for some autoimmune diseases, such as multiple sclerosis and type 1 diabetes, which are also associated with HSP60 [20,21]. Therefore, in this study, after evaluating the levels of HSP60 and its antibody in our POAG and NPG patients, we evaluated the percentages of the main Th cells involved in pro-inflammatory and anti-inflammatory responses, attempting to explain the discrepancy with regard to the presence of HSP60 and higher titers of HSP60 antibody in NPG patients compared to POAG patients.
Material and Methods
Study participants
This study was approved by the Institutional Review Board of our hospital, and carried out in accordance with the Declaration of Helsinki. After obtaining written informed consent, peripheral blood was collected from each participant, including glaucomatous patients and healthy volunteers.
Consecutive patients with POAG and NPG visiting Shanghai Ninth People’s Hospital between October 2013 and April 2015 were considered for inclusion in this study. Patients were followed clinically by one of the authors for a minimum of 2 years and received ophthalmologic examinations at least 4 times yearly.
The inclusion criteria for POAG consists of the presence of open iridocorneal angles, IOP higher than 21 mmHg, characteristically glaucomatous changes in visual fields, optic nerve cupping, retinal nerve fiber layer thinning, and the absence of other possible causes of optic neuropathy. Inclusion criteria for NPG are the same as for POAG except that no evidence showed IOP exceeded 21 mmHg, confirmed at least twice by 24-h IOP measurements. Normal control subjects had neither glaucoma nor any other ocular disease.
Exclusion criteria for the POAG and NPG candidates included: a history of any other ocular disease; use of contact lenses; ophthalmic surgical procedures or intraocular laser surgery in the past 1 year before this study; and a history of any kind of immunologic disease and immune-mediated disorder, including inflammation within the last 6 months, and cancer. Exclusion criteria for the NC group were the same as for the other 2 groups.
We screened 174 patients with POAG or NPG, as well as 40 healthy volunteers, during the study period. All candidates underwent thorough ocular examinations, including visual acuity, slit-lamp biomicroscopy, ophthalmoscopy, Goldmann applanation tonometry, visual field testing, and optical coherence topography. Results of examinations of the more severe eye were included in the comparison if both eyes were diagnosed as having glaucoma.
Blood sampling
We collected 6 mL of fresh peripheral blood into Vacutainer tubes (Becton Dickinson Systems, San Jose, CA) containing EDTA from each participant. After serum was isolated and stored at −80°, peripheral blood mononuclear cells (PBMCs) were isolated with the Ficoll-Hypaque density gradient (density 1.077; Biochrom, Berlin, Germany) centrifugation method. Isolated PBMCs were then resuspended in RPMI 1640 medium (Gibco, Grand Island, NY) that contained 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, 100 μg/mL streptomycin, 100 units/mL penicillin, and 10% heat-inactivated fetal bovine serum.
Cell culture and stimulation
Isolated PBMCs were plated at a density of 1.0×106/well and cultured in the absence or presence of HSP60 (2.5 ng/ml, Enzo Life Sciences Inc., Farmingdale, NY) for 2 days. Cells were cultured and harvested for flow cytometry for a direct culture assay as described by Kepitein and de Kleer [22,23], in which the peripheral blood monocytes were isolated and cultured with or without the stimulation of HSP60, and at different time points of culture, monocytes were collected for flow cytometry analysis.
Flow cytometry
Prior to Th1, Th2, and Th17 analysis, cells were first stimulated for 5 h with a cell stimulation cocktail, which included protein transport inhibitors Phorbol 12-myristate 13-acetate (PMA), ionomycin, Brefeldin A, and monensin (eBioscience, San Diego, CA), according to the manufacturer’s protocol. Then, cells were extracellularly stained with anti-human CD4 antibody, consecutively fixed and permeabilized (BD Biosciences, San Jose, CA, USA), and intracellularly stained with anti-human interferon-γ, interleukin (IL)-4, and IL-17 antibody (eBioscience, San Diego, CA). For Tregs analysis, no stimulation with cell stimulation cocktail was needed. Monoclonal antibodies (mAbs) specific for anti-human CD4 and CD25 were used for surface staining, and anti-human FoxP3 antibody (eBioscience, San Diego, CA) was used for intracellular staining after fixation and permeabilization (eBioscience, San Diego, CA). Data were acquired using a FACS Calibur system (BD Biosciences) and analyzed by FlowJo software (Tree Star, Inc.).
Enzyme-linked immunosorbent assay
Serum HSP60 antibody titers were measured using an in-house enzyme-linked immunosorbent assay (ELISA) described by Ghayour-Mobarhan [23]. Briefly, 96-well micro-titer plates (Costar, Corning, NY) were coated with 100 ng per well of low-endotoxin recombinant human HSP60 (Enzo Life Sciences Inc., Farmingdale, NY) dissolved in 100 μl carbonate buffer, and incubated overnight at 4°C under humidified conditions. The wells were then washed 3 times with phosphate-buffered saline (PBS) containing 0.05% Tween-20 (PBST). Non-specific binding was reduced by blocking each well with 10% normal goat serum in PBS, and 200 μl was added to each well, and incubated overnight at 4°C under humidified conditions. Wells were washed 3 times with PBS. Serum was diluted 1: 30 with 1% goat serum in PBS, and 100 μl was added to the each well in duplicate and then incubated for 2 h at room temperature. After washing 3 times in PBST, 100 μl peroxide conjugated-goat anti-human IgG (BD Biosciences, San Jose, CA) diluted 1: 5000 with 1% goat serum in PBS was added to each well and incubated for 45 min at room temperature. After washing 3 times in PBST and 2 times in PBS, 100 μl of tetramethylbenzidine (TMB) substrate (100 μl of 6 mg/ml TMB in DMSO was added to 10 ml of 50 mM acetate buffer, pH 4.5) was added per well, and the plates were incubated for 10 min in the dark at room temperature. Then, 100 ml 2M H2SO4 was added to each well to stop the reaction. Optical density values were read at 450 nm as a reference length.
Serum levels of cytokines, including IFN-γ, IL-4, IL-10, IL-17, and TGF-β1, were measured with ELISA kits (eBioscience, San Diego, CA) according to the manufacturer’s protocols.
Statistical analysis
Analyses were performed using SPSS software (version 17.0, SPSS, Chicago, IL). The differences in the results of ophthalmic examinations between POAG and NPG groups were calculated using the t test, signed-rank test, or chi-square test. One-way analysis of variance with Bonferroni post hoc analysis was used when comparing results of ELISA and flow cytometry. P values <0.05 were considered statistically significant.
Results
Subjects
Finally, 35 patients were included in the POAG group, 19 patients in the NPG group, and 27 normal participants in the NC group. Demographic and clinical information of patients is shown in Table 1. All 3 groups are age- and sex-matched.
Table 1.
Comparison of demographic and clinical data of POAG and NPG patients.
| POAG (n=35) | NPG (n=19) | p-Value | |
|---|---|---|---|
| Gender (Male: Female) | 26: 9 | 14: 5 | 0.962* |
| Age (years) | 41.23±11.42 | 45.89±9.28 | 0.133** |
| Use of prostaglandin analogs | 33 (94.29%) | 16 (84.21%) | 0.227* |
| IOP (mmHg) | 18 (16–22) | 15 (14–17) | 0.002*** |
| C/D ratio | 0.9 (0.8–0.9) | 0.8 (0.8–0.9) | 0.970*** |
Chi-square test;
Student’s t-test, data are expressed as means and standard deviations;
Wilcoxon rank sum test, data are expressed as medians and interquartile ranges;
POAG – primary open angle glaucoma; NPG – normal pressure glaucoma; IOP – intraocular pressure; C/D ratio – cup-to-disc ratio.
Higher antibody titers were found in NPG group
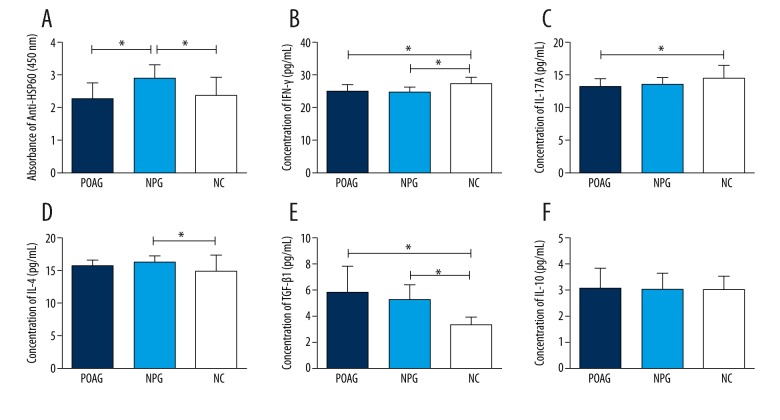
Controversial results have been obtained in different studies with regard to the level of HSP60 antibody in POAG, suggesting the HSP60 antibody level might vary in different groups of POAG patients. Therefore, we tested the HSP60 antibody level in our patients and based the subsequent analysis on the results. The titer of anti-HSP60 antibody was measured using an in-house ELISA within 1 week after the serum was collected and stored at −80°. Serum titer of anti-HSP60 antibody was significantly higher in NPG patients than in POAG patients or normal participants (Figure 1), indicating that in these 3 groups of subjects, higher level of anti-HSP60 was mainly found in NPG, and anti-HSP60 level was not higher in POAG, compared to normal controls.
Figure 1.
Comparison of serum levels of anti-HSP60, IFN-γ, IL-4, IL-17A, TGF-β1, and IL-10 between the primary open-angle glaucoma (POAG) group, the normal-pressure glaucoma (NPG) group, and the normal control (NC) group. (A) The absorbance of anti-HSP60 was higher in the NPG group than in the NC group (P<0.05). No significant difference was found between the POAG and NC groups. (B) Serum levels of IFN-γ were lower in the POAG and NPG groups (P<0.05). (C) The levels of IL-17A were lower in the POAG and NPG groups, and the difference was significant between POAG and NC groups (P<0.05). (D) Serum level of IL-4 was significant higher in the NPG group than in the NC group (P<0.05). The elevation of IL-4 was not significant in the POAG group compared to the NC group (P>0.05). (E) The levels of TGF-β1 were significantly higher in the POAG and NPG groups (P<0.05). (F) No significant difference in IL-10 level was observed between groups. Dark blue column: POAG group; light blue column: NPG group; white column: NC group. Statistical significance was analyzed using the t test. * P <0.05.
Imbalance between pro- and anti-inflammatory responses of Th cells in POAG and NPG
It has been confirmed by previous reports that elevated expression of HSP60 was found in retinas of both POAG and NPG patients, which means both group of patients were exposed to the antigen HSP60. Since we also observed in our patients that the anti-HSP60 level was only higher in the NPG group, we hypothesized that after the exposure to HSP60, different immune events in POAG and NPG may occur and lead to differences in antibody reactivity. Imbalance between pro- and anti-inflammatory responses of Th cells is associated with HSP60-related autoimmune diseases, so we evaluated the frequencies of Th cells and levels of related cytokines.
Circulating pro-inflammatory Th cell frequencies
Th2 frequency was increased in both POAG and NPG patients.
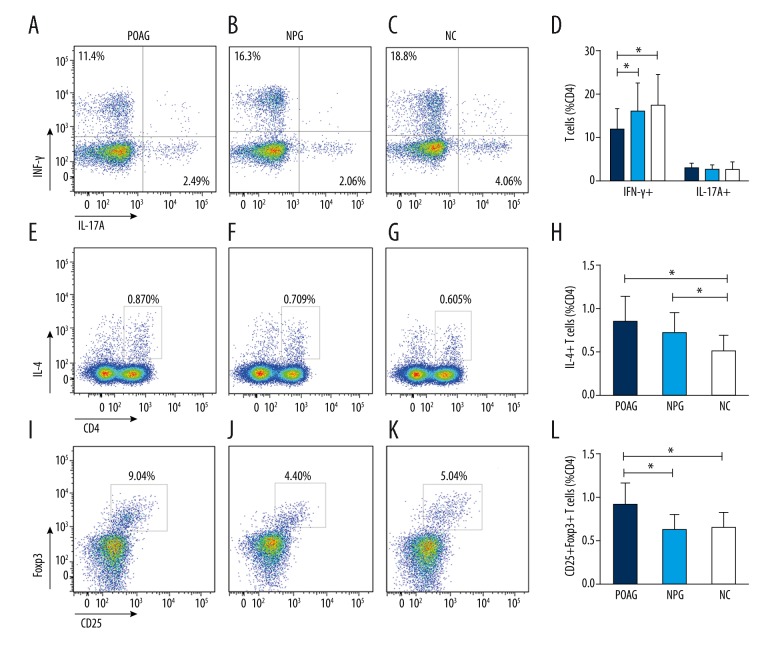
The proportion of Th2 cells is expressed as the percentage of all CD4+ T cells. The frequency of Th2 cells was evidently increased in the peripheral blood of patients in the POAG and NPG groups compared to the NC group (POAG group: 0.849±0.299; NPG group: 0.726±0.227; NC group: 0.507±0.18; p=0.000; Figure 2). No significant difference was found between the POAG and NPG group (p>0.05).
Figure 2.
Comparison of peripheral frequencies of Th1 (CD4+IFN-γ+ T cells), Th2 (CD4+IL-4+ T cells), Th17 (CD4+IL-17A+ T cells), and Treg (CD4+CD25+Foxp3+ T cells) between the primary open-angle glaucoma (POAG) group, the normal-pressure glaucoma (NPG) group, and the normal control (NC) group. (A–D) Representative experiments of the frequencies of Th1 and Th17 cells and comparison between groups. Frequencies of Th1 cells were lower in the POAG and NPG groups compared to the NC group. The difference was significant between the POAG and NC groups (P<0.05). No significant difference in the frequencies of Th17 cells was observed between groups. (E–H) Representative experiments on the frequencies of Th2 cells and comparison between groups. Frequencies of Th2 cells were significantly higher in the POAG and NPG groups compared to the NC group (P<0.05). (I–L) Representative experiments on the frequencies of Treg cells and comparison between groups. The frequency of Treg cells was higher in the POAG group (P<0.05) but not in the NPG group, compared to the NC group. Dark blue column: POAG group; Light blue column: NPG group; whitr column: NC group. Statistical significance was analyzed using the t test. * P<0.05.
Th1 frequency was decreased in POAG patients
Unlike Th2 cells, Th1 cells are pertinent to cell immunity. Cytokines secreted by Th2 cells can suppress the proliferation of Th1 cells. Compared to the other 2 groups, the frequency of Th1 cells was obviously decreased in the POAG group (POAG group: 11.95±4.5; NPG group: 16.13±6.41; NC group: 17.53±6.89; p=0.001; Figure 2). Although the frequency of Th1 cells was lower in the NPG group than in the NC group, no significant difference was observed (p>0.05).
No obvious difference in frequency of Th17 was observed between groups
Th17 cells are an important pro-inflammatory component and have been shown to promote inflammation in some diseases. The proportion of Th17 cells is expressed as the percentage of CD4+ T cells. The frequency of Th17 cells was higher in the POAG group compared to that in the other groups, but the difference was not statistically significant (POAG group: 3.04±1.01; NPG group: 2.71±1.07; NC group: 2.82±1.65; p=0.624; Figure 2).
Circulating anti-inflammatory Th cells frequencies
Frequency of Treg cells is expressed as CD25+Foxp3+ T cells of all CD4+ T cells. As shown in Figure 2, distinctly increased frequency of Treg was only observed in the POAG group, compared to that in the NC group (POAG group: 9.1±2.48; NPG group: 6.29±1.74; NC group: 6.53±1.7; p=0.000; Figure 2). No significant difference was found between the NPG group and NC group (p>0.05)
Serum levels of pro-inflammatory and anti-inflammatory cytokines
Th1 cells are characterized by the production and release of interferon gamma (IFN-γ). The levels of IFN-γ in the POAG group (24.96±1.94 pg/ml, p<0.05, Figure 1) and the NPG group (24.65±1.50 pg/ml, p<0.05) were lower than in the NC group (27.33±1.98 pg/ml). Interleukin 4 is the signature cytokine of Th2 cells. The levels of IL-4 were higher in the POAG group (15.59±0.87 pg/ml, p>0.05) and especially in the NPG group (16.23±0.91 pg/ml, p<0.05), compared to that in the NC group (14.87±2.30 pg/ml). The level of IL-17A in the POAG group was lower than in the NC group (13.17±1.10 pg/ml vs. 14.55±1.98 pg/ml, p<0.05), and no significant decrease was observed in the NPG group (13.52±1.08 pg/ml). The level of Treg-related cytokines TGF-β1 was higher in the POAG group (5.83±1.93 ng/ml) and NPG group (5.21±1.13ng/ml) compared to the NC group (3.33±0.59 pg/ml). No significant difference was found in the level of IL-10 among groups (3.10±0.75 pg/ml, 3.05±0.62 pg/ml, 3.03±0.55 pg/ml, p>0.05).
HSP60-induced changes of Th cell frequencies in NPG were different from those in POAG and NC
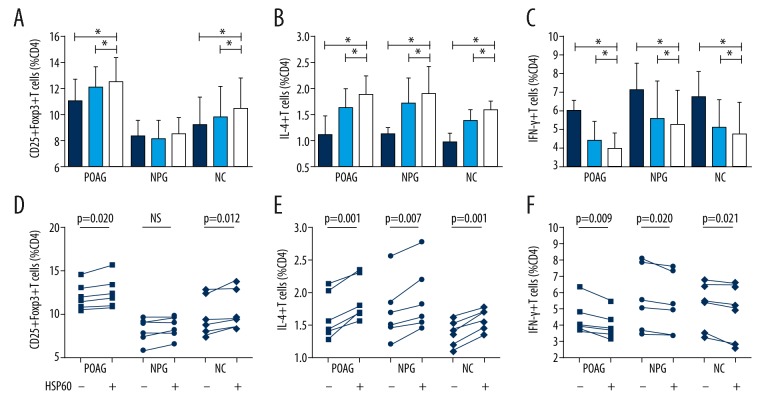
Higher titer of anti-HSP60 was observed only in NPG patients, which supports the presence of HSP60 as an antigen in NPG. We also observed the different pro- and anti-inflammatory Th cell response patterns in POAG and NPG patients. However, it was still unknown whether the presence of HSP60 was associated with the different Th cell patterns or if they were merely coexisting in glaucoma patients. Therefore, we stimulated PBMCs isolated from patients and normal participants with HSP60 for 2 days to observe whether HSP60 would result in Th cell response patterns similar to those we had found previously. Frequencies of Th1, Th2, and Treg cells with or without the stimulation of HSP60 are shown in Figure 3. The frequencies of Th2 cells increased more significantly with the stimulation of HSP60 in POAG, NPG, and NC groups than without HSP60 stimulation (1.89±0.34 vs. 1.63±0.36, p=0.001; 1.90±0.50 vs. 1.71±0.47, p=0.007; 1.59±0.17 vs. 1.38±0.20, p=0.001). The frequencies of Th1 cells were lower with the stimulation of HSP60 in POAG, NPG, and NC groups (3.98±0.85 vs. 4.44±1.02, p=0.028; 5.29±1.89 vs. 5.61±2.00, p=0.02; 4.79±1.69 vs. 5.14±1.46, p=0.021). However, more significantly increased frequencies of Treg cells after stimulation of HSP60 were only observed in the POAG and NC groups (12.51±1.78 vs. 12.05±1.49, p=0.02; 10.47±2.28 vs. 9.81±2.24, p=0.012). In the NPG group, no significant difference in the frequencies of Treg cells was observed with or without the stimulation of HSP60 (8.52±1.19 vs. 8.17±1.38, p=0.055), and no significant elevation was observed between basic Treg cell frequency and the frequency after 2-day culture with HSP60 stimulation (8.37±1.18 vs. 8.52±1.19, p=0.485).
Figure 3.
Frequencies of Th1(CD4+IFN-γ+ T cells), Th2 (CD4+IL-4+ T cells), and Treg (CD4+CD25+Foxp3+ T cells) cultured for 2 days with or without the stimulation of HSP60. (A, D) Frequencies of Treg cells increased more with the stimulation of HSP60 in primary open-angle glaucoma patients and normal subjects. In normal-pressure glaucoma patients, no significant increase of Treg cells was found after 2-day culture with or without HSP60 stimulation. (B, E) Frequencies of Th2 cells increased more significantly with the stimulation of HSP60 in all groups. (C, F) Frequencies of Th1 cells decreased more significantly with the stimulation of HSP60 in all groups. POAG – primary open-angle glaucoma; NPG – normal-pressure glaucoma; NC – normal control. Dark blue column: basic Th cell frequency; light blue column: Th cell frequency on day 2, without HSP60 stimulation; white column: Th cell frequency on day 2, with HSP60 stimulation. Statistical significance was analyzed using a paired t test or signed-rank test (n=6). * P<0.05.
Discussion
Accumulating evidence on immune response to HSP has been found in glaucoma patients and animal models. Investigations into the immune pathogenesis may increase the probability of finding additional treatments for NPG patients. In 1998, Tezel et al. found increased titers of serum antibodies to HSP60 mainly in NPG patients [19]. In the present study, similar results were obtained, showing that titers of anti-HSP60 were also higher in the NPG group compared to the NC group, and no significant difference was observed between the POAG and NC groups. It has been reported that greater intensity of immunostaining and more labeled cells for HSP60 were found in postmortem eyes of POAG and NPG patients [11,24]. If they were exposed to the same antigen, why were elevated titers of anti-HSP60 found only in the NPG patients?
When confronted with antigens like HSP60, Th cells can differentiate into various subpopulations of T cells with specific functions and properties, including pro- and anti-inflammatory Th cell subsets. Th1 and Th2 cells are 2 kinds of traditional pro-inflammatory Th cells. Th1 cells are characterized by the production and release of IFN-γ and are involved in cellular immune response. Th2 cells are mostly involved in humoral immune response and help B cells to produce antibodies. Recently, Th17 cells were recognized as a novel subset of pro-inflammatory Th cells, providing protection against bacterial infection. In contrast, Treg cells play an important anti-inflammatory role in maintaining the peripheral tolerance, controlled by transcription factor Foxp3 and differentiated in response to TGF-β [25]. Balanced function of pro- and anti-inflammatory components is essential to ensure that the immune response is kept under tight control and the chance of developing immunopathy is diminished. The imbalance has been found to be a pathogenic mechanism leading to the onset of autoimmune phenomena, and the abnormal activation of pro-inflammatory cells may be associated with deficiencies in the anti-inflammatory components [26,27].
In our study, significantly activated pro-inflammatory responses were observed in the POAG and NPG groups, compared to the NC group. Frequencies of Th2 cells were elevated in the POAG and NPG groups, indicating the existence of Th2-driven immune response. However, frequencies of Th1 cells were lower in both groups than in normal controls. The transcription factors of Th2 and Th1 cells – GATA-3 and T-bet – are characterized by mutual antagonism, and the production of IL-4 by Th2 antagonizes Th1 polarization. Therefore, decreased Th1 frequency may also support the activation of Th2-driven immune response. Consistently, higher levels of IL-4 and lower levels of IFN-γ were found in both glaucoma groups. However, significantly increased frequency of the anti-inflammatory component – Treg cells – was only found in the POAG group, and no significant difference was observed between the NPG and NC groups. The importance of Treg cells in the suppression of potentially harmful and excessive immune responses has been established by many investigations [28], and Treg alterations have been identified in several autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus [29–31]. It has been reported that Th2 cell-driven immune responses are suppressed by Treg cells via a CK2-controlled mechanisms or IRF4-controlled mechanisms [32,33]. The lack of increased Treg frequency in the NPG group were observed together with increased Th2 frequency and higher titers of anti-HSP antibodies, implying that the lack of suppression by Treg cells may be ascribed to the increased Th2 cell-driven immune response and consequent RGC apoptosis.
The increased Th2 frequency with decreased Th1 frequency in our study is evidence of weakly immunogenic conditions, because weak antigenic stimulation upregulates expression of a transcription factor GATA-3, which in turn drives differentiation of naïve T cells into Th2 effectors. Although Treg cells usually exert their influence by suppressing the proliferation and cytokine production of effector T cells, under steady-state or weakly immunogenic conditions, Treg cells act principally to inhibit effector function, whereas priming and effector differentiation appears to be unaltered [34,35]. This may explain why an increased Th2 frequency without elevated IL-4 was found in the peripheral blood of patients in the POAG group. When we evaluated Treg-related cytokines – TGF-β1 and IL-10 – the levels of these 2 cytokines were not consistent with the alteration in frequencies of Treg cells. TGF-β1 is a very important suppressive cytokine secreted by Treg cells and can induce Foxp3 expression and a Treg phenotype; however, the serum level of TGF-β1 can be influenced by many other factors. Konstas reported that latanoprost monotherapy has a marked effect on the aqueous concentration of TGF-β1 [36]. Kuchtey et al. recently reported the significant elevation of systemic TGF-β1 in POAG patients as compared to controls [37], which was also confirmed in our study. Microfibril deficiencies are associated with increased TGF-β signaling and higher systemic TGF-β1 concentrations [38,39], and microfibril genes are reported to be associated with glaucoma [40,41], so we hypothesized that the microfibril defects lead to elevation of IOP TGF-β1 in glaucoma patients. Microfibrils are found in the trabecular meshwork, the inner wall of Schlemm’s canal, the iris, the ciliary body, and in the optic nerve head [42,43]. Thus, the microfibril defects may cause elevation of TGF-β1 in both POAG and NPG. The fact that no difference in IL-10 level was observed between groups may show that IL-10-secreting CD4+ Treg cells are not the primary functioning Treg cells in glaucoma patients.
To further investigate if the pattern of pro- and anti-inflammation Th cells was associated with HSP60, we cultured PBMC isolated from POAG and NPG patients and normal participants, with or without the stimulation of HSP60 for 2 days. More significantly elevated Th2 frequencies were observed with the stimulation of HSP60 in all glaucoma patients and normal participants. However, in NPG patients, the frequency of Treg cells was not obviously elevated after 2 days, with or without the HSP60 stimulation, and the Treg cell frequency even decreased in 2 patients. Zhou and Dominguez reported that the frequency of Treg cells increased because HSP60 can induce Treg cells [44,45]. Therefore, our results indicate that HSP60 can induce Th cell patterns similar to those we had earlier observed in glaucoma patients and normal participants. More importantly, in our study, the absence of increased frequency of Treg cells shows that Treg deficiency might exist in NPG patients. This deficiency might lead to an imbalance between pro- and anti-inflammatory components, which could consequently cause the activation of Th2-driven immune response and elevated titers of anti-HSP60 in NPG patients. Efforts have been made to investigate Treg-inducing adjuvant, which may be a potential treatment for some NPG patients.
There are 2 main limitations to our study. First, more samples are needed to determine whether Treg deficiency exists only in the minority of NPG patients or in most of them. Second, in vivo experiments with animal models are needed to determine whether the absence of activated and functional Treg will cause NPG-like immune response and RGC loss. Further elucidation of the role of immune responses in glaucoma will inspire the discovery of novel pathogenic mechanism and even diagnostic and therapeutic strategies in glaucoma.
Conclusions
Different Th cell patterns were observed among POAG, NPG, and NC groups. Lack of induction of Treg cells and imbalance of the pro-inflammatory and anti-inflammatory response patterns of Th cells exist at least in some NPG patients.
Footnotes
Source of support: This research was supported by the National Natural Science Foundation of China (No. 81670845), and the Science and Technology Commission of Shanghai Municipality, Shanghai, China (No. 11419960600)
Conflicts of interests
None.
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–67. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Sommer A, Tielsch JM, Katz J, et al. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch Ophthalmol. 1991;109:1090–95. doi: 10.1001/archopht.1991.01080080050026. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell P, Smith W, Attebo K, Healey PR. Prevalence of open-angle glaucoma in Australia. The Blue Mountains Eye Study. Ophthalmology. 1996;103:1661–69. doi: 10.1016/s0161-6420(96)30449-1. [DOI] [PubMed] [Google Scholar]
- 5.Dielemans I, Vingerling JR, Wolfs RC, et al. The prevalence of primary open-angle glaucoma in a population based study in the Netherlands. The Rotterdam Study. Ophthalmology. 1994;101:1851–55. doi: 10.1016/s0161-6420(94)31090-6. [DOI] [PubMed] [Google Scholar]
- 6.Iwase A, Araie M, Tomidokoro A, et al. Prevalence and causes of low vision and blindness in a Japanese adult population: The Tajimi Study. Ophthalmology. 2006;113:1354–62. doi: 10.1016/j.ophtha.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Bell K, Gramlich OW, Von Thun Und Hohenstein-Blaul N, et al. Does autoimmunity play a part in the pathogenesis of glaucoma? Prog Retin Eye Res. 2013;36:199–216. doi: 10.1016/j.preteyeres.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Ulmansky R, Landstein D, Moallem E, et al. A humanized monoclonal antibody against heat shock protein 60 suppresses murine arthritis and colitis and skews the cytokine balance toward an anti-inflammatory response. J Immuno. 2015;194:5103–9. doi: 10.4049/jimmunol.1500023. [DOI] [PubMed] [Google Scholar]
- 9.Sarikonda G, Sachithanantham S, Miller JF, et al. The Hsp60 peptide p277 enhances anti-CD3 mediated diabetes remission in non-obese diabetic mice. J Autoimmun. 2015;59:61–66. doi: 10.1016/j.jaut.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Elfaitouri A, Herrmann B, Bolin-Wiener A, et al. Epitopes of microbial and human heat shock protein 60 and their recognition in myalgic encephalomyelitis. PLoS One. 2013;8:e81155. doi: 10.1371/journal.pone.0081155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tezel G, Hernandez R, Wax MB. Immunostaining of heat shock proteins in the retina and optic nerve head of normal and glaucomatous eyes. Arch Ophthalmol. 2000;118:511–18. doi: 10.1001/archopht.118.4.511. [DOI] [PubMed] [Google Scholar]
- 12.Wax MB, Tezel G, Kawase K, Kitazawa Y. Serum autoantibodies to heat shock proteins in glaucoma patients from Japan and the United States. Ophthalmology. 2001;108:296–302. doi: 10.1016/s0161-6420(00)00525-x. [DOI] [PubMed] [Google Scholar]
- 13.Bell K, Funke S, Pfeiffer N, Grus FH. Serum and antibodies of glaucoma patients lead to changes in the proteome, especially cell regulatory proteins, in retinal cells. PloS One. 2012;7:e46910. doi: 10.1371/journal.pone.0046910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joachim SC, Wuenschig D, Pfeiffer N, Grus FH. IgG antibody patterns in aqueous humor of patients with primary open angle glaucoma and pseudoexfoliation glaucoma. Mol Vis. 2007;13:1573–79. [PubMed] [Google Scholar]
- 15.Tezel G, Wax MB. The mechanisms of hsp27 antibody-mediated apoptosis in retinal neuronal cells. J Neurosci. 2000;20:3552–62. doi: 10.1523/JNEUROSCI.20-10-03552.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joachim SC, Wax MB, Seidel P, et al. Enhanced characterization of serum autoantibody reactivity following HSP 60 immunization in a rat model of experimental autoimmune glaucoma. Curr Eye Res. 2010;35:900–8. doi: 10.3109/02713683.2010.495829. [DOI] [PubMed] [Google Scholar]
- 17.Wax MB, Tezel G, Yang J, et al. Induced autoimmunity to heat shock proteins elicits glaucomatous loss of retinal ganglion cell neurons via activated T-cell-derived fas-ligand. J Neurosci. 2008;28:12085–96. doi: 10.1523/JNEUROSCI.3200-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cartwright MJ, Grajewski AL, Friedberg ML, et al. Immune-related disease and normal-tension glaucoma. A case-control study. Arch Ophthalmol. 1992;110:500–2. doi: 10.1001/archopht.1992.01080160078035. [DOI] [PubMed] [Google Scholar]
- 19.Tezel G, Seigel GM, Wax MB. Autoantibodies to small heat shock proteins in glaucoma. Invest Ophthalmol Vis Sci. 1998;39:2277–87. [PubMed] [Google Scholar]
- 20.Ivanova EA, Orekhov AN. T helper lymphocyte subsets and plasticity in autoimmunity and cancer: An overview. Biomed Res Int. 2015;2015:327470. doi: 10.1155/2015/327470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, You C, Zhang Z, et al. Roles of Treg/Th17 cell imbalance and neuronal damage in the visual dysfunction observed in experimental autoimmune optic neuritis chronologically. Neuromolecular Med. 2015;17:391–403. doi: 10.1007/s12017-015-8368-4. [DOI] [PubMed] [Google Scholar]
- 22.Kapitein B, Aalberse JA, Klein MR, et al. Recognition of self-heat shock protein 60 by T cells from patients with atopic dermatitis. Cell Stress Chaperones. 2013;18:87–95. doi: 10.1007/s12192-012-0361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Kleer IM, Kamphuis SM, Rijkers GT, et al. The spontaneous remission of juvenile idiopathic arthritis is characterized by CD30+ T cells directed to human heat-shock protein 60 capable of producing the regulatory cytokine interleukin-10. Arthritis Rheum. 2003;48:2001–10. doi: 10.1002/art.11174. [DOI] [PubMed] [Google Scholar]
- 24.Gramlich OW, Beck S, von Thun Und Hohenstein-Blaul N, et al. Enhanced insight into the autoimmune component of glaucoma: IgG autoantibody accumulation and pro-inflammatory conditions in human glaucomatous retina. PLoS One. 2013;8:e57557. doi: 10.1371/journal.pone.0057557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mian MO, Barhoumi T, Briet M, et al. Deficiency of T-regulatory cells exaggerates angiotensin II-induced microvascular injury by enhancing immune responses. J Hypertens. 2016;34:97–108. doi: 10.1097/HJH.0000000000000761. [DOI] [PubMed] [Google Scholar]
- 27.Feger U, Luther C, Poeschel S, et al. Increased frequency of CD4+ CD25+ regulatory T cells in the cerebrospinal fluid but not in the blood of multiple sclerosis patients. Clin Exp Immunol. 2007;147:412–18. doi: 10.1111/j.1365-2249.2006.03271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: Role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–90. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 30.van Amelsfort JM, Jacobs KM, Bijlsma JW, et al. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: Differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–85. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- 31.Lyss uk EY, Torgashina AV, Soloviev SK, et al. Reduced number and function of CD4+CD25highFoxP3+ regulatory T cells in patients with systemic lupus erythematosus. Adv Exp Med Biol. 2007;601:113–19. [PubMed] [Google Scholar]
- 32.Zheng Y, Chaudhry A, Kas A, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control Th2 responses. Nature. 2009;458:351–56. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ulges A, Klein M, Reuter S, et al. Protein kinase CK2 enables regulatory T cells to suppress excessive Th2 responses in vivo. Nat Immunol. 2015;16:267–75. doi: 10.1038/ni.3083. [DOI] [PubMed] [Google Scholar]
- 34.Chen ML, Pittet MJ, Gorelik L, et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci USA. 2005;102:419–24. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mempel TR, Pittet MJ, Khazaie K, et al. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity. 2006;25:129–41. doi: 10.1016/j.immuni.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 36.Konstas AG, Koliakos GG, Karabatsas CH, et al. Latanoprost therapy reduces the levels of TGF beta 1 and gelatinases in the aqueous humour of patients with exfoliative glaucoma. Exp Eye Res. 2006;82:319–22. doi: 10.1016/j.exer.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Kuchtey J, Kunkel J, Burgess LG, et al. Elevated transforming growth factor beta1 in plasma of primary open-angle glaucoma patients. Invest Ophthalmol Vis Sci. 2014;55:5291–97. doi: 10.1167/iovs.14-14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matt P, Schoenhoff F, Habashi J, et al. Circulating transforming growth factor-beta in Marfan syndrome. Circulation. 2009;120:526–32. doi: 10.1161/CIRCULATIONAHA.108.841981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loeys BL, Gerber EE, Riegert-Johnson D, et al. Mutations in fibrillin-1 cause congenital scleroderma: Stiff skin syndrome. Sci Transl Med. 2010;2:23ra20. doi: 10.1126/scitranslmed.3000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ali M, McKibbin M, Booth A, et al. Null mutations in LTBP2 cause primary congenital glaucoma. Am J Hum Genet. 2009;84:664–71. doi: 10.1016/j.ajhg.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jelodari-Mamaghani S, Haji-Seyed-Javadi R, Suri F, et al. Contribution of the latent transforming growth factor-beta binding protein 2 gene to etiology of primary open angle glaucoma and pseudoexfoliation syndrome. Mol Vis. 2013;19:333–47. [PMC free article] [PubMed] [Google Scholar]
- 42.Wheatley HM, Traboulsi EI, Flowers BE, et al. Immunohistochemical localization of fibrillin in human ocular tissues. Relevance to the Marfan syndrome. Arch Ophthalmol. 1995;113:103–9. doi: 10.1001/archopht.1995.01100010105028. [DOI] [PubMed] [Google Scholar]
- 43.Hann CR, Fautsch MP. The elastin fiber system between and adjacent to collector channels in the human juxtacanalicular tissue. Invest Ophthalmol Vis Sci. 2011;52:45–50. doi: 10.1167/iovs.10-5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou S, Jin X, Chen X, et al. Heat shock protein 60 in eggs specifically induces tregs and reduces liver immunopathology in mice with schistosomiasis Japonica. PLoS One. 2015;10:e0139133. doi: 10.1371/journal.pone.0139133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dominguez Mdel C, Lorenzo N, Barbera A, et al. An altered peptide ligand corresponding to a novel epitope from heat-shock protein 60 induces regulatory T cells and suppresses pathogenic response in an animal model of adjuvant-induced arthritis. Autoimmunity. 2011;44:471–82. doi: 10.3109/08916934.2010.550590. [DOI] [PubMed] [Google Scholar]