Abstract
Background/Aim:
Vitamin D deficiency is common in irritable bowel syndrome (IBS). There is growing interest in the role of vitamin D in pediatric IBS. We aimed to evaluate the effect of vitamin D supplementation in adolescents with IBS and vitamin D deficiency.
Patients and Methods:
One hundred and twelve adolescents with IBS and vitamin D deficiency were randomly divided into two groups of matched age and sex. The first group received oral vitamin D3 2000IU/day for 6 months and the second group received placebo for 6 months. Vitamin D status as well as different IBS score systems (IBS-SSS, IBS-QoL, and total score) were evaluated before and 6 months after treatment.
Results:
IBS patients who received vitamin D supplementation for 6 months showed significant improvement in IBS-SSS (P < 0.001), IBS-QoL (P < 0.001), and total score (P = 0.02) compared to IBS placebo group. IBS patients treated with vitamin D showed two folds increase in their serum vitamin D levels (from 17.2 ± 1.3 to 39 ± 3.3) ng/ml with P < 0.001. While in the placebo group, their serum vitamin D levels were not significantly changed (P = 0.66). Vitamin D was tolerated well without any recorded adverse effects during the study period.
Conclusion:
Vitamin D supplementation can be effective in treating adolescents with IBS and vitamin D deficiency.
Keywords: Adolescents, irritable bowel syndrome, vitamin D
INTRODUCTION
Irritable bowel syndrome (IBS) is the most prevalent gastrointestinal problem which affects quality of life.[1] It is a functional gastrointestinal disorder characterized by varying degrees of abdominal pain or discomfort, abdominal distension, altered bowel habits, and flatulence and can be divided into three subtypes according to bowel habits –IBS with diarrhea (IBS-D), IBS with constipation (IBS-C), and IBS with mixed bowel habits (IBS-M). Incidence of IBS has been increasing over last two decades.[2]
IBS pathogenesis is poorly recognized and many theories have been proposed to explain its pathogenesis.[3] Low-grade mucosal inflammation, bacterial overgrowth, immune activation, visceral hypersensitivity, and disturbed gastrointestinal motility are possible mechanisms of pathogenesis.[4,5] Dysregulation of brain-gut axis function is becoming an acceptable theory to explain IBS through different mechanisms including neurotransmitters found in the brain and gut e.g., cholecystokinin, vasoactive intestinal peptide, and serotonin.[6] Moreover, food allergy or vitamin deficiency may play a role.[7,8,9] Higher incidence of IBS was recorded in patients with anxiety, major depression, personality disorders, and hysteria; moreover, IBS itself can cause anxiety and depression.[10,11]
Recent studies have suggested a relationship between vitamin D and IBS. Vitamin D has a potential role as immune modulator, anti-inflammatory, and anti-microbial agent that can explain its role in IBS.[12] Furthermore, vitamin D receptors (VDR) are expressed in the gut affecting gut function, motility, and IBS symptoms.[13] Moreover, depression, which initiates or aggravates IBS symptoms, is more common in vitamin D deficiency.[14] Low vitamin D level was recorded in IBS patients in many studies.[15,16] This deficiency can be attributed to many factors as altered digestive pattern or intolerance to dairy and fatty food. Supplementation of vitamin D in adult IBS patients significantly improves their symptoms and quality of life.[2,17] However, similar studies are lacking in pediatric patients. To our knowledge, this is the first study to evaluate the effect of vitamin D supplementation in adolescents with IBS.
MATERIALS AND METHODS
This is a prospective randomized controlled trial that was carried out from April 2015 to April 2017 on 112 adolescent IBS patients with vitamin D deficiency aged 14–18 years who were selected from the outpatient clinics of Pediatrics Departments, Tanta University Hospital. The study was approved by the local ethical committee of Faculty of Medicine, Tanta University. The study was performed in accordance with the Helsinki Declaration of 1975. Written informed consent was signed by parents of all participants involved in the study. The study was registered at www.pactr.org (PACTR201706002337124).
Inclusion criteria: IBS adolescents aged 14–18 years diagnosed with ROME III criteria for diagnosis of childhood irritable bowel syndrome [9] and with vitamin D serum level less than 20 ng/ml.
Exclusion criteria: adolescents with recent use of antibiotics (4 weeks), recent change in IBS therapy, gastrointestinal infection, history of gut surgery or radiation, celiac disease, chronic drug therapy that interfere with vitamin D metabolism such as antiepileptics and glucocorticoids, overweight, or underweight adolescents according to the centile curve,[18] chronic disease such as renal failure or diabetes mellitus, and patients taking vitamin D supplementation. Our participants were randomly divided into two groups:
Group 1: included 56 IBS adolescents who received oral vitamin D3 (2000 IU/day) in the form of two drops daily for 6 months where one drop contains 1000 IU of vitamin D3.
Group II: included 56 IBS adolescent patients who received placebo in the form of two drops daily of glucose 5% placed in the same container as vitamin D3.
Participants who met the inclusion criteria were randomized to one of the two study groups according to computer-generated random numbers in a 1:1 ratio using a random block size of 6. The random number list was generated by QuickCalc GraphPad Software Inc., La Jolla, CA, USA. The randomization was performed by an independent statistician. Allocation concealment was done by sequentially numbered sealed opaque envelopes. After the written consent was signed, the sealed opaque envelope was opened and the patient was enrolled into the respective group. All treating staff and outcome assessors were blinded to the treatment group.
All participants were subjected to the following:
Complete history taking, especially dietetic history of last 4 days (3 working days and one vacation day) was recorded using food frequency questionnaire (FFQ) and filled by direct interview, and then calculated using dietary calculator software
Complete clinical examination including anthropometric measurements such as weight, height, and body mass index (BMI)
IBS symptoms severity score (IBS-SSS) questionnaire [19] was filled at the start of the study, 3 months, and 6 months after treatment by all IBS participants. IBS-SSS consists of 5 items (severity and frequency of abdominal pain, bloating, satisfaction with bowel habits, and quality of life) collected by direct interview using visual analog scale (VAS). Each item was scored on a scale from 0–100. Score below 75 means that the patient is in remission. The mild, moderate, and severe boundary scores are 75–175,175–300, and above 300, respectively. A decrease in the score of 50 or more was considered significant improvement
IBS quality of life (IBS-QoL) questionnaire [20] was also filled at the start of the study, 3 months, and 6 months after treatment by all IBS participants. Effectiveness, reliability, and sensitivity of IBS-SSS to treatment are verified by IBS-QoL which has 34 items, based on 5-choice scale (0–4) and the summed total score was transformed to a 100 scale ranging from 0 (lowest) to 100 (highest)
A total score of IBS measured by a VAS of 100 scale to assess the total impact of IBS symptoms on the quality of life was evaluated at the same frequency as IBS-SSS and IBS-QoL scores
Routine laboratory investigations in the form of complete blood count (CBC), erythrocyte sedimentation rate (ESR), serum calcium, random blood sugar, renal and hepatic functions, serum proteins, urine, and stool analysis. Fecal calprotectin in stool was measured in all included patients to exclude patients with inflammatory bowel disease
Serum level of 25 (OH)D was measured for all participants at the start of the study and 6 months after treatment. For measuring serum level of vitamin D, whole blood was collected by standard venipuncture in VACUETTE® Blood Collection Tubes (Greiner Bio-One, Kremsmuenster, Austria) containing K2EDTA and containing clot activator/Sep. Serum samples were left to clot 5 to 10 minutes at room temperature and were centrifuged at 1000× g for 10 minutes. A part of the serum samples was used for routine laboratory investigations and the other part was stored at −20°C till the time of 25 (OH)D assay. 25 (OH)D was measured using vitamin D total assay kit (25-hydroxyvitamin D, Catalog No: 05894913190). Vitamin D assay was performed with cobase 602 analyzer (Roche Diagnostics, USA). Subjects with serum levels of vitamin D (less than20 ng/ml) were considered deficient in vitamin D according to endocrine society clinical practice guideline [21] and included in the study.
All participants were instructed to record a diary (recording adherence to therapy, bowel habits, any side effects of the drug, abdominal pain, and other abdominal symptoms). Moreover, we followed the participants by phone weekly for compliance and any complications and to remind them of visit dates, besides face to face meeting every 3 months to provide study-materials and to get feedback. Furthermore, all patients were asked to return the used packages of drugs during the follow-up visit to ensure compliance to treatment.
All symptoms, serum calcium, serum vitamin D levels, and the three IBS scores were evaluated before and 6 months after treatment and any side effects of the drug were recorded during face-to-face meetings. If any adverse effects were reported during the study, both serum vitamin D level, and serum calcium levels were checked. If vitamin D exceeded 100 ng/ml and serum calcium exceeded 11 mg/dl, the study was stopped for that patient and further evaluation of kidney function was done and treatment was given, as required.
The primary outcome was to assess IBS symptoms and severity score after treatment with vitamin D for 6 months. Secondary outcome was to assess serum vitamin D levels and QoL in IBS patients before and after vitamin D supplementation.
Statistical analysis
Sample size of 45 IBS patients in each group was required to achieve power of 80% with alpha = 0.05 to detect a difference of 60 in the mean of IBS-SSS (the primary outcome) based on a previous study.[22] We recruited more than the estimated sample size as we assumed that not all our patients would be compliant with the treatment and hence we increased the sample size in consideration of withdrawal of any patient, which would undermine our results. Moreover, all our patients in group 1 had vitamin D deficiency and were instructed to take therapeutic vitamin D treatment with or without involvement in our study. Collected data were analyzed using SPSS version 17 (SPSS Inc., Chicago, IL). Continuous data were expressed as mean ± standard deviation. A paired t-test was used for comparison in the same group before and after treatment. Independent t-test was used for comparison between group 1 and group 2. Categorical variables were expressed as number and percentages and analyzed using Chi-square test. Pearson correlation was done to evaluate the correlation between serum level of 25 (OH)D before and after treatment with IBS scores. Statistical significance was defined as P < 0.05.
RESULTS
Of 130 IBS patients assessed for eligibility, only 112 patients were enrolled in the study and 56 were randomly allocated to either active treatment group or placebo group between April 2015 and April 2017. Among 112 patients enrolled in the study, and none discontinued the intervention or were lost in follow-up [Figure 1].
Figure 1.
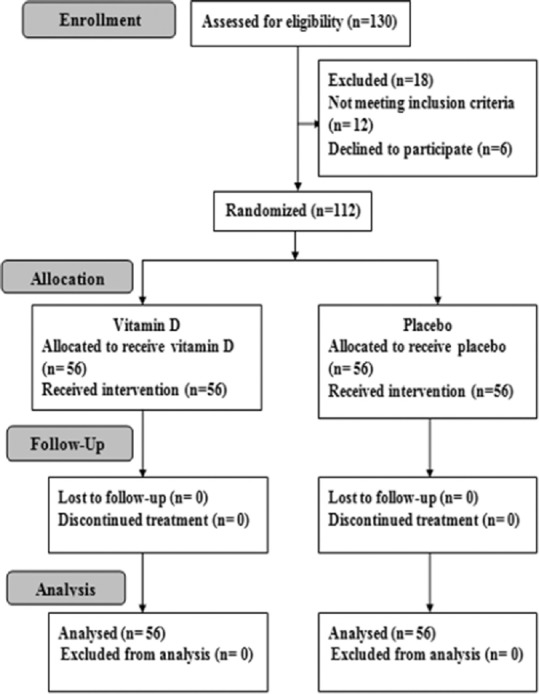
Flow chart of the study
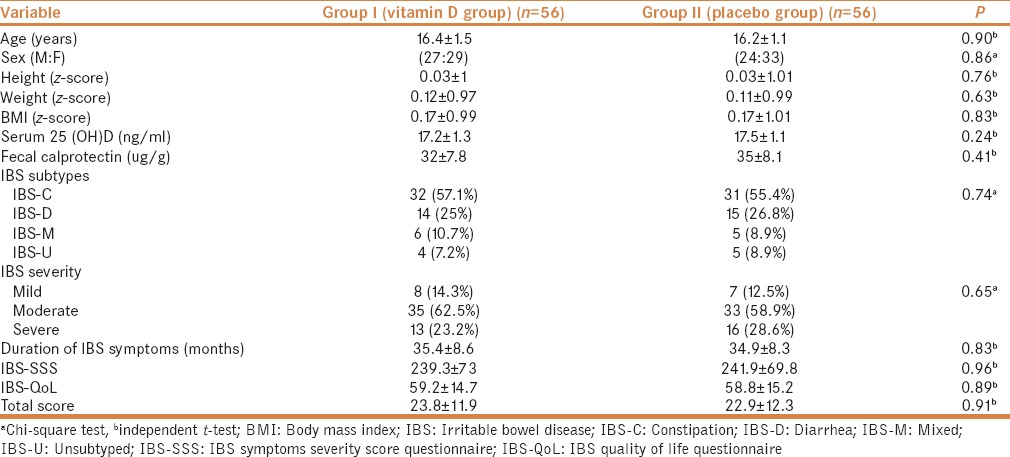
There was no significant difference between the treatment group and placebo group before treatment regarding demographic data, anthropometric measurements, clinical characteristics, serum 25 (OH)D level, IBS Subtypes, IBS severity, IBS-SSS, IBS-QoL, and total score [Table 1].
Table 1.
Demographic data and clinical characteristics in IBS patients’ groups before start of the treatment
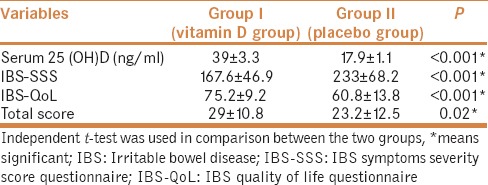
At the end of our study, IBS patients who received vitamin D had a significantly higher serum vitamin D compared to placebo patients (P < 0.001) with no reported side effects. IBS-SSS, IBS-QoL, and total scores were significantly improved in vitamin D treatment group than placebo group (P < 0.001, P < 0.001, P = 0.02), respectively [Table 2.]
Table 2.
Vitamin D levels, IBS-SSS, IBS-QOL, and total score in IBS patients’ groups at the end of the study
After 6 months of treatment, serum 25 (OH)D level was significantly increased in vitamin D group from (17.2 ± 1.3) to (39 ± 3.3) with P = 0.001. While in the placebo group, serum level of 25 (OH)D was not significantly changed (P = 0.66) [Table 3]. Vitamin D was well tolerated with no recorded side effects.
Table 3.
Clinical characteristics and vitamin D levels in IBS patients’ groups before and after treatment
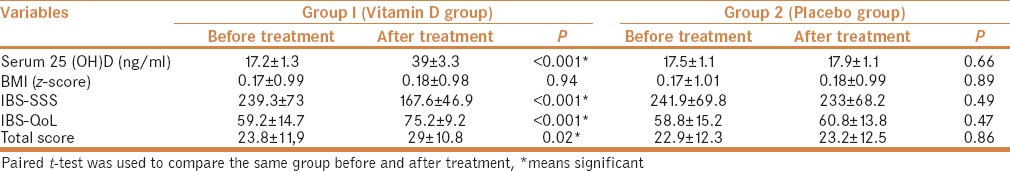
IBS-SSS was significantly improved in vitamin D group after treatment than before treatment (167.6 ± 46.9 vs 239.3 ± 73) with P value <0.001. However, there was no significant change in IBS-SSS in placebo group. IBS-QoL was significantly improved in vitamin D group after treatment (P = 0.001). While some improvement occurred in placebo group post-intervention regarding IBS-QoL, that improvement was of no statistical significance (P = 0.47). Consequently, total score was significantly improved in vitamin D group (P = 0.02). However, no significant improvement occurred in placebo group (P = 0.86) [Table 3].
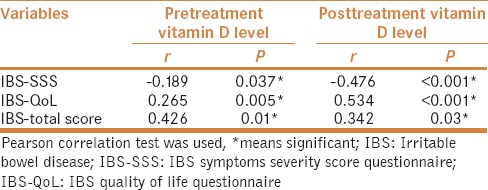
Pearson correlation revealed there was significant negative correlation between pretreatment serum level of 25 (OH)D and IBS-SSS (P =< 0.05), while there was significant positive correlation between pretreatment serum level of 25 (OH)D and both IBS-QoL and total score. Similarly, there was significant negative correlation between posttreatment serum level of 25 (OH)D and IBS-SSS (P < 0.001), while there was significant positive correlation between posttreatment serum level of 25 (OH)D and both IBS-QoL and total score [Table 4].
Table 4.
Correlations between pre and posttreatment vitamin D level in IBS treated patients and different IBS scores
DISCUSSION
Irritable bowel disease is a common health problem affecting nearly all age groups all over the world. Pathogenesis of IBS is still not well understood. Most studies regarding pathogenesis and management of IBS were done in adult patients, and to our knowledge, this is the first study evaluating the effect of vitamin D supplementation on adolescent IBS patients.
In our study, IBS adolescent patients who received vitamin D had a significantly higher serum vitamin D compared to placebo group. In addition, there was significant improvement in the clinical status in adolescents with IBS who received vitamin D evaluated by the three scores (IBS-SSS, IBS-QoL, and total score) compared to placebo group. Such improvement has been recorded before but only in adults.[2,17,22]
Even though the exact mechanism of how vitamin D supplementation improves symptoms and scores in IBS patients is still unknown, many explanations have been suggested. One explanation focuses on anxiety and depression as major psychological elements in the onset and progress of IBS symptoms that also affects their QoL; numerous studies also found a link between vitamin D deficiency and depression, with supplementation of vitamin D in these patients leading to improvement in their anxiety and depression.[23,24] Thus, vitamin D supplementation in IBS patients can act through ameliorating their anxiety, stress, and depression, and consequently, improving symptoms and quality of life.
It is reported that there is a state of chronic low mucosal inflammation in IBS caused by activated mast cells, T lymphocytes, and proinflammatory cytokines that altered colonic distension causing most IBS symptoms. Moreover, inflammation causes an increase in the sensitivity of nervous system and consequently increases gut hypersensitivity and perception of abdominal pain. Nevertheless, vitamin D has a potential anti-inflammatory, antimicrobial, and immunomodulator effect that helps the state of low-grade mucosal inflammation and altered immunity presented in IBS patients.[12]
Vitamin D receptor (VDR) is expressed through the nervous system and in the gut where its activation is linked to neurotransmitter levels, serotonin synthesis, intestinal epithelial barrier function, and bowel inflammation.[13,25,26,27] Moreover, binding of 25 (OH)D-VDR complex results in the expression of 1-alpha-hydroxylase which converts 25 (OH)D to 1, 25-dihydroxyvitamin D.[28] This 25 (OH)D metabolite has been shown to upregulate neutrophins which in turn promote survival and differentiation of nerve cells.[29] Hence, vitamin D may directly affect neurological development, gut function, and consequently improveIBS symptoms and QoL, as shown in the results of our study.
In a study on IBS adult patients, vitamin D supplementation was provided for 6 months at a dose of 50,000 IU weekly that effectively raised serum 25 (OH)D3 levels more than 2.5 times than the baseline and significantly reduced the mean IBS-SSS score in vitamin D treatment group.[14] This was similar to our results, however, the mean rise of serum levels of vitamin D in their study was more than ours that could be due to higher dose of vitamin D used in their study. Contrary to our results, Tazzyman et al.[30] reported no significant improvement in IBS symptoms after 12 weeks of vitamin D supplementation in adults with IBS. That study was a pilot study (included only 50 patients in three arms) and was therefore underpowered to provide significant findings. Moreover, participants were recruited following a poster campaign at a university and this would only assess a selected group of people. In addition, short duration of supplementation (3 months) could be another cause of negative results besides age variation in their study unlike our study.
Limitation of the study
This is a one-centre study; however, our hospital is a tertiary centre that received referral from a wide range of area. Variation in the time of the year could affect vitamin D status; however, our country is sunny most of the year. The findings of our study are limited to our population and further studies are needed in different pediatric age groups. We used normal vitamin D dose to treat deficiency in our study, and further studies comparing the effects of different doses of vitamin D on children with IBS are needed to develop a protocol for supplementation.
CONCLUSION
Vitamin D supplementation can be effective in treating adolescents with IBS. Larger studies in other ethnicities are needed to examine the effectiveness of Vitamin D supplementation in treating IBS and to determine the optimal protocol of vitamin D supplementation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ahlawat R, Weinstein T, Pettei MJ. Vitamin D in pediatric gastrointestinal disease. Curr Opin Pediatr. 2017;29:122–7. doi: 10.1097/MOP.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 2.Sprake EF, Grant VA, Corfe BM. Vitamin D3 as a novel treatment for irritable bowel syndrome: Single case leads to critical analysis of patient-centred data. BMJ Case Rep. 2012;007223 doi: 10.1136/bcr-2012-007223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oświęcimska J, Szymlak A, Roczniak W, Girczys-Połedniok K, Kwiecień J. New insights into the pathogenesis and treatment of irritable bowel syndrome. Adv Med Sci. 2017;62:17–30. doi: 10.1016/j.advms.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Parkes GC, Brostoff J, Whelan K, Sanderson JD. Gastrointestinal microbiota in irritable bowel syndrome: Their role in its pathogenesis and treatment. Am J Gastroenterol. 2008;103:1557–67. doi: 10.1111/j.1572-0241.2008.01869.x. [DOI] [PubMed] [Google Scholar]
- 5.Carding S, Verbeke K, Vipond DT, Corfe BM, Owen L. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:26191. doi: 10.3402/mehd.v26.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 7.O'Malley T, Heuberger R. Vitamin D status and supplementation in pediatric gastrointestinal disease. J Spec Pediatr Nurs. 2011;16:140–50. doi: 10.1111/j.1744-6155.2011.00280.x. [DOI] [PubMed] [Google Scholar]
- 8.Rao SS, Yu S, Fedewa A. Systematic review: Dietary fibre and FODMAP-restricted diet in the management of constipation and irritable bowel syndrome. Aliment Pharmacol Ther. 2015;41:1256–70. doi: 10.1111/apt.13167. [DOI] [PubMed] [Google Scholar]
- 9.Drossman DA. The functional gastrointestinal disorders and the ROME III process. Gastroenterology. 2006;130:1377–90. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka Y, Kanazawa M, Fukudo S, Drossman Da. Biopsychosocial model of irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:131–9. doi: 10.5056/jnm.2011.17.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fond G, Loundou A, Hamdani N. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): A systemic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci. 2014;264:651–60. doi: 10.1007/s00406-014-0502-z. [DOI] [PubMed] [Google Scholar]
- 12.Coussens AK, Martineau AR, Wilkinson RJ. Anti-inflammatory and antimicrobial action in combating TB/HIV. Scientifica. 2014;2014:903680. doi: 10.1155/2014/903680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;249:208–16. doi: 10.1152/ajpgi.00398.2007. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong D, Meenagh G, Bickle I, Lee AS, Curran ES, Finch MB. Vitamin D deficiency is associated with anxiety and depression in fibromyalgia. Clin Rheumatol. 2007;26:551–4. doi: 10.1007/s10067-006-0348-5. [DOI] [PubMed] [Google Scholar]
- 15.Khayyat Y, Attar S. Vitamin D deficiency in patients with Irritable Bowel Syndrome: Does it exist. Oman Med J. 2015;30:115–8. doi: 10.5001/omj.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nwosu BU, Maranda L, Candela N. Vitamin D status in pediatric irritable bowel syndrome. PLoS One. 2017;12:e0172183. doi: 10.1371/journal.pone.0172183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abbasnezhad A, Amani R, Hajiani E, Alavinejad P, Cheraghian B, Ghadiri A. Effect of vitamin D on gastrointestinal symptoms and health-related quality of life in irritable bowel syndrome patients: A randomized double-blind clinical trial. Neurogastroenterol Motil. 2016;28:1533–44. doi: 10.1111/nmo.12851. [DOI] [PubMed] [Google Scholar]
- 18.Cole TG, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ. 2000;320:1240–3. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francis CY, Moris JF, Whorwell PJ. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 20.Bao C, Zhang J, Liu J, Liu H, Wu L, Shi Y, et al. Moxibustion treatment for diarrhea-predominant irritable bowel syndrome: Study protocol for a randomized controlled trial. BMC Complement Altern Med. 2016;16:408. doi: 10.1186/s12906-016-1386-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 22.Jalili M, Hekmatdoost A, Vahedi H, Poustchi H, Khademi B, Saadi M, et al. Co-Administration of Soy Isoflavones and Vitamin D in Management of Irritable Bowel Disease. PloS One. 2016;11:e 0158545. doi: 10.1371/journal.pone.0158545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerr DC, Zava DT, Piper WT, Saturn SR, Frei B, Gombart AF. Association between vitamin D levels and depressive symptoms in healthy young adult women. Psychiatry Res. 2015;227:46–51. doi: 10.1016/j.psychres.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han B, Lyu Y, Sun H, Wei Y, He J. Low serum levels of vitamin D are associated with post-stroke depression. Eur J Neurol. 2015;28:1269–74. doi: 10.1111/ene.12607. [DOI] [PubMed] [Google Scholar]
- 25.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377–90. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Patric RP, Ames BN. Vitamin D hormone regulates serotonin synthesis. Part 1: Relevance for autism. FASEB J. 2014;28:2398–413. doi: 10.1096/fj.13-246546. [DOI] [PubMed] [Google Scholar]
- 27.Li YC, Chen Y, Du J. Critical roles of intestinal epithelial vitamin D receptor signaling in controlling gut mucosal inflammation. J Steroid Biochem Mol Biol. 2015;148:179–83. doi: 10.1016/j.jsbmb.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, et al. Extra renal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–94. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 29.McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction. FASEB J. 2008;22:982–1001. doi: 10.1096/fj.07-9326rev. [DOI] [PubMed] [Google Scholar]
- 30.Tazzyman S, Richards N, Trueman AR, Evans AL, Grant VA, Garaiova I, et al. Vitamin D associates with improved quality of life in participants with irritable bowel syndrome: Outcomes from a pilot trial. BMJ Open Gastroenterol. 2015;2:e000052. doi: 10.1136/bmjgast-2015-000052. [DOI] [PMC free article] [PubMed] [Google Scholar]


