Abstract
Background/Aim:
Aberrant expression of CK20/CK7 is reported in a percentage of colorectal carcinomas (CRC); however, its relation to clinicopathological variables and survival data is still unclear. The objective of this study is to explore patterns of CK20/CK7 immunostaining in CRC and to analyse the diagnostic, prognostic, and predictive role of patterns of CK20/CK7 immunostaining.
Materials and Methods:
A total of 144 CRC cases were retrieved from the archives at the Department of Pathology, King Abdulaziz University, Jeddah, Saudi Arabia. Immunohistochemistry was performed using antibody to CK7 and CK20. Immunostaining was defined as low and high by using the extent of staining. The association of CK7 and CK20 with clinicopathological characteristics and survival.
Results:
CK20 was expressed in a higher percentage of CRC and nodal metastasis than CK7. No difference in CK7 and CK20 immunostaining in primary and metastasis carcinomas was found. Four patterns of CK20/CK7 were identified; CK20+/CK7− (60.4%), CK20+/CK7+ (2.1%), CK20−/CK7− (35.4%), and CK20−/CK7+ (2.1%). There was no statistically significant correlation between CK20/CK7 immunohistochemical profile and clinicopathological characteristics, prognosis, and survival was determined.
Conclusions:
Our results may support the heterogeneity of CRC. CRC showed four different subclasses following patterns of relative CK20/CK7 immunostaining. A considerable number of CRC expressed aberrant immune profile of CK20/CK7, which should be considered during diagnosing CRC in metastatic regions. Further studies on larger cohorts correlating different immunohistochemical cytokeratin profiles to molecular subtypes of CRC are recommended for better understanding of pathogenesis and behaviour of CRC.
Keywords: CRC, CK20, CK7, prognosis
INTRODUCTION
Worldwide, colorectal carcinoma (CRC) is associated with significant mortality and morbidity.[1] In Saudi Arabia, CRC accounted for 11.9% of all newly diagnosed patients in 2013. CRC ranked first among men and the third most common cancer among women.[2] Recently, molecular studies demonstrated that CRC is a heterogeneous group of diseases that develop through three main pathogenetic pathways; the chromosomal instability pathway (constituting 60–70% of sporadic CRC), the microsatellite instability (MSI) pathway (accounts for about 15% of sporadic CRC), and the CpG island methylation pathway. Tumours originating through these three pathways differ in precursor lesions, natural history, and pathological features. The immunohistochemical characteristics of molecular subsets of CRC are not well studied.[3,4,5,6]
The CK20+/CK7− profile is characteristic for colonic carcinoma and successfully used to distinguish it from tumours originating from breast, gynaecological tract, liver or lung. However, not all CRC expressed the usual cytokeratin profile, as some studies reported strong CK7 expression and conversely negative CK20 immunoexpression. CK20+/CK7− profile is expressed in about 75–95% of CRC, while the rest of cases show different profiles.[7,8,9,10,11] Previous studies correlated cytokeratin expression to clinicopathological characteristics of CRC. They found that loss of CK20 is associated with older age (above 56), right colonic tumour, higher grade, increased intratumoral lymphocytic infiltration (creating Crohn's disease-like infiltrate), mucinous histology, advanced tumour stage, presence of lymph node metastasis and worse overall and disease-free survival compared with patients with positive CK20 expression.[6,12,13]
The objective of this study was to explore patterns of CK20/CK7 immunostaining in primary CRC and nodal metastasis in a set of Saudi Arabian patients, and to analyse the diagnostic, prognostic and predictive role of variable patterns of CK20/CK7 immunostaining.
MATERIALS AND METHODS
Patients
One hundred and forty-four retrospective CRCs and 49 corresponding regional nodal metastasis constituted the material of the present study. Pathological materials were collected from archives of Pathology Department, King Abdulaziz University Hospital, Jeddah, Saudi Arabia in the period 1995–2012. The diagnosis of CRC was confirmed after excluding the possibility of carcinomas of non-colonic origin by re-evaluation of clinical presentation, endoscopic and radiological findings as well as serum tumour markers (CEA, CA19-9, CA125 and AFP) and immunohistochemistry for Cdx 2, vimentin, Ca-125 whenever needed. Clinical data was retrieved from the patients' records. Clinicopathological parameters of patients are summarised in Table 1. Postoperative pathological staging was performed according to the AJCC staging system.[14] The study was approved by the Research Committee of the Biomedical Ethics Unit, Faculty of Medicine, King AbdulazizUniversity, Jeddah, Saudi Arabia. All patients included in this study gave an informed written consent for utilisation of their material in research.
Table 1.
Clinicopathological parameters of patients (n=144)
Tissue microarray construction
Tissue microarray (TMA) was constructed from CRC and nodal metastasis paraffin blocks using an automated tissue arrayer (MASTER 3D HISTECH). Two tissue cores (each 1.5 mm) were punched from two different target areas of each donor block. TMA blocks then sliced into 4-μm thick sections and mounted on sialinated slides for further immunohistochemistry.
Immunohistochemistry of tissue microarray
Immunoperoxidase technique was performed using monoclonal mouse Anti-Human CK-20 and monoclonal mouse Anti-Human CK-7 (Dako Cytomation Norden A/S, Glostrup, Denmark, dilution 1:100 each). An automatic immunostainer (Ventana Bench Mark XT, Ventana Inc., Tucson, AZ) was used following the manufacturer's instructions. In each analysis, positive controls consisted of CRC samples previously shown to stain with CK20 and thyroid tissue known to be positive to CK7. Tris-buffered saline in place of the primary antibody was used as a negative control.
Interpretation of immunohistochemical staining
Cells were considered positive for CK20 and CK7 when distinct cytoplasm and/or cell membrane yellow to brown staining was identified. Percentage of positive cells were recorded in a semiquantitative method according to a scale from 1 to 4; score 4 (staining in >50% of tumour cells), score 3 (staining in 20–50% of cells), score 2 (staining of 5–20% of cells), while score 1 (<5% staining). When CK20 immunostaining was dichotomized for statistical risk assessment, scores 1 and 2 were defined as low immunostaining, while scores 3 and 4 were included in high immunostaining category. In case of CK7, score 1 was considered as low immunostaining, while scores 2, 3 and 4 were considered as high immunostaining. In addition, the combination of immunostaining of CK20/CK7 was considered into four classes as follows: CK20+/CK7−, CK20−/CK7−, CK20+/CK7+, and CK20−/CK7−. Negative was defined as low immunostaining and positive was defined as high immunostaining.
Statistical analysis
Mann–Whitney test was used to test the difference between two variables. The Kruskal–Wallis test was used to demonstrate the association between three or more groups of patients. Non-parametric Chi-square was used to test variance along one variable. Logistic regression analysis was used to predict nodal metastasis, distant metastasis, surgical resection margins, and lymphovascular invasion. Disease-free survival probabilities were tested by Kaplan–Meier procedure. Disease-free survival time was calculated from the date of pathological diagnosis to the occurrence of relapse. Statistical procedures were performed using SPSS® version 16.0 (SPSS, Chicago, IL, USA). Statistical significance was determined at P value of ≤0.05 and was two-sided.
RESULTS
CK20 and CK7 immunostaining profile
Positive cytoplasmic and membranous immunostaining of CK20 was seen in 62.5% of primary CRC and 63.5% of regional nodal metastasis [Figure 1 and Table 2]. CK7 immunostaining was seen in 5.6% of primary CRC and in 4% of regional nodal metastasis [Figure 1 and Table 2]. In primary CRC, CK20 immunostaining was statistically significantly higher than CK7 immunostaining (P < 0.001). On the other hand, there was no statistically significant difference in CK20/CK7 immunostaining noted between CRC and lymph node metastasis. The combination of CK20/CK7 immunoprofile showed that the CK20+/CK7− profile was the highest followed by CK20−/CK7− then CK20+/CK7+ and CK20−/CK7+ (2.1%). Details of results are shown in Table 3.
Figure 1.
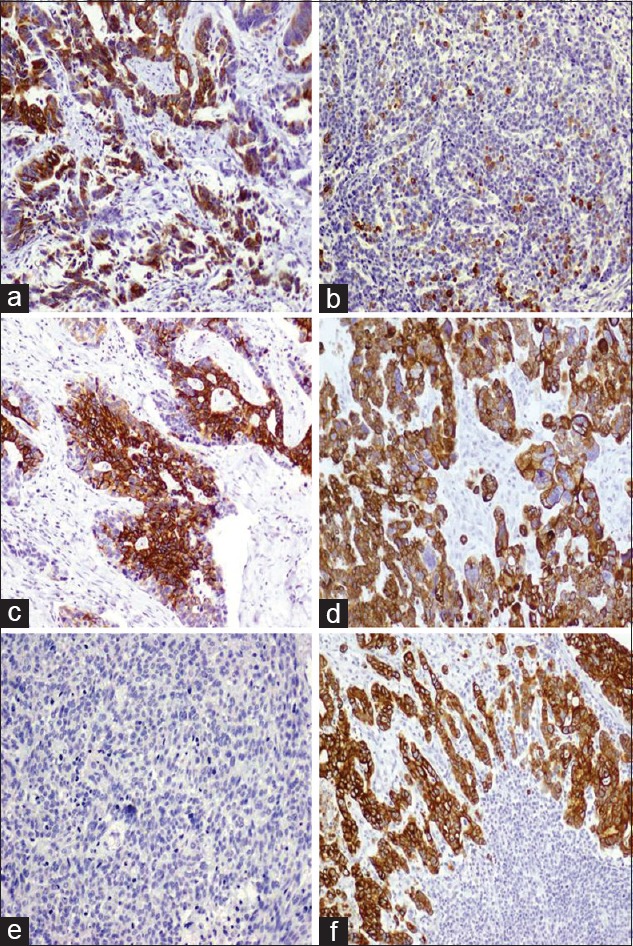
CK20 CK7 immunostaining in CRC (a) Strong CK20 immunostaining in a moderately differentiated CRC. (b) Negative CK20 reactivity in poorly differentiated CRC. (c) Strong and diffuse staining in metastatic CRC to lymph nodes. (d) Strong cytoplasmic staining in a poorly differentiated CRC. (e) Negative CK7 reactivity in a poorly differentiated CRC. (f) Strong cytoplasmic staining to CK7 in a moderately differentiated CRC carcinoma metastatic to lymph node. Original magnification ×100. Immunostaining labelling was done with anti-CK20 and anti-CK7 with diaminobenzidine used as the chromogen and haematoxylin as counterstain
Table 2.
Categories of CK20 and CK7 immunostaining

Table 3.
Differential CK20/CK7 immunostaining in primary CRC patients

Relation between CK20/CK7 immunostaining and prognosis
There was no association between CK20/or CK7 immunostaining and clinicopathological features. Regression analysis revealed no predictive or prognostic value of CK20 and/or CK7 immunostaining in CRC [Table 4]. CK20 immunostaining was not related to disease free survival, Log rank (Mantel-cox) = 1.435, P value = 0.231 as was CK7 immunostaining; Log rank (Mantel-cox) = 0.000, P value = 0.996 [Figure 2].
Table 4.
Regression analysis for CK7 and CK20 immunostaining
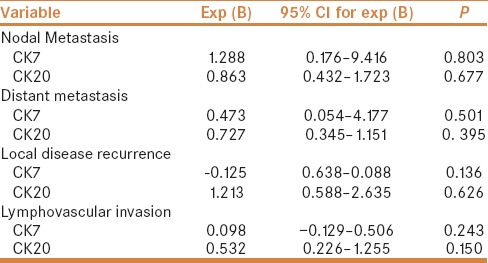
Figure 2.
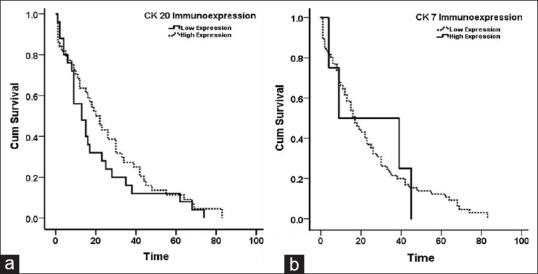
Disease-free survival (a) According to CK20 immunostaining; Log rank (Mantel-cox) = 1.435, P value = 0.231. (b) According to CK7 staining; Log rank (Mantel-cox) = 0.000, P value = 0.996. Time is calculated in months
DISCUSSION
Relative expression of CK20/CK7 is an approved diagnostic tool to help determine site of origin in metastatic carcinomas. CK20 is specific for colonic, urothelial and Merckel cell carcinoma. On the other hand, CK7 is characteristic of glandular malignancies originating from breast, respiratory tract, biliary tract and Mullarian epithelium. CK7 expression in CRC is rare and positivity considered as exclusion criteria to tumours of CRC origin.[7,8,11] Occasionally, loss of expression of CK20 and conversely positive expression of CK 7 was noted in some CRC. The value of this aberrant expression is still unclear.[15,16,17] We studied the immunostaining patterns of CK20/CK7 in 144 CRC and in 49 lymph node metastasis. In primary CRC, CK20 was positive in 62.5%, while CK7 was positive in 5.6%. In nodal metastasis, CK 20 and CK 7 showed positivity in 63.5% and 4.1%, respectively.
In the current study, we classified CRC tumours into four groups according to different CK20/CK7 immunostaining profiles. The most common profile was the usual CK20+/CK7− (60.4%), followed by negativity to both markers (35.4%), followed by positivity to both markers (2.1%) and the CK20−/CK7+ profile (2.1%). Our results are comparable to previous studies [7,15,16] with minor discrepancy. In the present CRC tumours showed increased percentage of CK 20 negativity and reduced percentage CK7 positivity. The reason for this discrepancy could be explained by difference in the studied population, technical variations in immunohistochemical procedure or interpretation criteria. The reason behind unusual immunostaining of CK20 and CK7 is still unclear. Recent molecular studies categorised CRC into microsatellite stable and microsatellite instable tumours. Many studies have attempted to find the relationship between immunophenotypic and molecular backgrounds of CRC.[18,19,20,21] Others studied the CK20/CK7 expression in 44 CRC cases in relation to molecular subtypes.[19,20] They concluded that reduction or total absence of CK20 is a phenotypic characteristic of CRC originating through MSI. On the other hand, Gurzu and Jung [12] a conducted similar study and reported that microsatellite instability-CRCs are associated with diffuse expression of CK7 and absence of CK20. The above results can explain the reason behind aberrant expression of CK20 and CK7 in percentage of CRC. We compared percentage of four CK20/CK7 profiles in CRC obtained in the present research to some previous researches in Table 5.
Table 5.
Comparison between percentage of differential immunostaining of CK20 and CK7 in some previous reports

The relative expression of CK20/CK7 in malignant tissue was compared to clinicopathological characteristics. We found no difference in CK20 and CK7 immunostaining by age, sex, tumour location, size, histological grade, presence of lymphovascular invasion, positive margins, lymph node status or tumour stage or tumour progression indicators. Our results are concordant with those of Hernandez et al, except that their findings showed an association between CK20+/CK7+ profile and advanced CRC in comparison to early stage CRC, which commonly show CK20+/CK7− profile.[16] On the other hand, Park et al claimed that CK20−/CK7+ profile is associated more with right side CRC than left side tumours, and most cases consisted of high-grade carcinoma.[22] Bressenot et al suggested that aberrant expression of CK20 and CK7 is related to tumour progression, based on studying one of his cases.[23] The case was of a 70-year-old lady presenting with high-grade CRC, stage T3, N2, MX. The primary tumour was CK7+/CK20−, whereas, tumour metastasizing to lymph nodes showed variable profiles of CK20+/CK7− and CK20−/CK7−. Moreover, the authors suggested that aberrant CK20/CK7 profile is usually found in CRC metastasizing to lung, ovary and endometrium.
CONCLUSIONS
Our results may help in demonstrating the heterogeneity of CRC. According to CK20/CK7 immunostaining, CRC were categorised into four different subclasses. Unfortunately, we could not correlate between these subclasses and clinicopathological variables, survival outcome or prognostic data. A considerable number of CRC expressed aberrant immunoprofiles of CK20/CK7, which should be considered during diagnosing carcinomas in metastatic regions. Further studies on larger cohorts correlating different immunohistochemical cytokeratin profiles to molecular subtypes of CRC are recommended for better understanding of pathogenesis, and different behaviour of CRC, which dictates the likelihood response to traditional and targeted therapeutic agents.
Clinical practice points
CRC shows different combinations of CK20/CK7 immunostaining.
The reason behind unusual immunostaining of CK20 and CK7 is still unclear.
Aberrant CK20/CK7 immunostaining should be considered when used in diagnosis of metastatic CRC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH)-King Abdulaziz City for Science and Technology - the Kingdom of Saudi Arabia - award number (11-BIO1524-03). The authors also, acknowledge with thanks Science and Technology Unit, King Abdulaziz University for technical support.
REFERENCES
- 1.Ross WA. Colorectal cancer screening in evolution: Japan and the USA. J Gastroenterol Hepatol. 2010;25(Suppl 1):S49–56. doi: 10.1111/j.1440-1746.2010.06221.x. [DOI] [PubMed] [Google Scholar]
- 2.Al-Madouj A, Alshahrani Z, Alrawaji A, Hayder M, Al-Shridah M, Al-Shamrani, T, et al. Cancer Incidence Report. Cancer Registry. 2016. [Last accessed on Dec 20 2017]. pp. 1–88. Available from: http://www.chs.gov.sa/Ar/HealthCenters/NCC/CancerRegistry/CancerRegistryReports/2013.pdf.
- 3.Al-Sohaily S, Biankin A, Leong R, Kohonen-Corish M, Warusavitarne J. Molecular pathways in colorectal cancer. J Gastroenterol Hepatol. 2012;27:1423–31. doi: 10.1111/j.1440-1746.2012.07200.x. [DOI] [PubMed] [Google Scholar]
- 4.Mojarad EN, Kuppen PJ, Aghdaei HA, Zali MR. The CpG island methylator phenotype (CIMP) in colorectal cancer. Gastroenterol Hepatol Bed Bench. 2013;6:120–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Worthley DL, Leggett BA. Colorectal cancer: Molecular features and clinical opportunities. Clin Biochem Rev. 2010;31:31–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113–30. doi: 10.1111/j.1365-2559.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- 7.Bayrak R, Yenidunya S, Haltas H. Cytokeratin 7 and cytokeratin 20 expression in colorectal adenocarcinomas. Pathol Res Pract. 2011;207:156–60. doi: 10.1016/j.prp.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Karantza V. Keratins in health and cancer: More than mere epithelial cell markers. Oncogene. 2011;30:127–38. doi: 10.1038/onc.2010.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park SY, Kim BH, Kim JH, Lee S, Kang GH. Panels of immunohistochemical markers help determine primary sites of metastatic adenocarcinoma. Arch Pathol Lab Med. 2007;131:1561–7. doi: 10.5858/2007-131-1561-POIMHD. [DOI] [PubMed] [Google Scholar]
- 10.Shin JH, Bae JH, Lee A, Jung CK, Yim HW, Park JS, et al. CK7, CK20, CDX2 and MUC2 Immunohistochemical staining used to distinguish metastatic colorectal carcinoma involving ovary from primary ovarian mucinous adenocarcinoma. Jpn J Clin Oncol. 2010;40:208–13. doi: 10.1093/jjco/hyp150. [DOI] [PubMed] [Google Scholar]
- 11.Wong HH, Chu P. Immunohistochemical features of the gastrointestinal tract tumors. J Gastrointest Oncol. 2012;3:262–84. doi: 10.3978/j.issn.2078-6891.2012.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gurzu S, Jung I. Aberrant pattern of the cytokeratin 7/cytokeratin 20 immunophenotype in colorectal adenocarcinomas with BRAF mutations. Pathol Res Pract. 2012;208:163–6. doi: 10.1016/j.prp.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg R, Hoos A, Mueller J, Baier P, Stricker D, Werner M, et al. Prognostic significance of cytokeratin-20 reverse transcriptase polymerase chain reaction in lymph nodes of node-negative colorectal cancer patients. J Clin Oncol. 2002;20:1049–55. doi: 10.1200/JCO.2002.20.4.1049. [DOI] [PubMed] [Google Scholar]
- 14.Edge S, Byrd D, Compton C. AJCC Cancer Staging Handbook. 7th edition edn. New York: Springer; 2010. [Google Scholar]
- 15.Bayrak R, Haltas H, Yenidunya S. The value of CDX2 and cytokeratins 7 and 20 expression in differentiating colorectal adenocarcinomas from extraintestinal gastrointestinal adenocarcinomas: Cytokeratin 7-/20+phenotype is more specific than CDX2 antibody. Diagn Pathol. 2012;7:9. doi: 10.1186/1746-1596-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez BY, Frierson HF, Moskaluk CA, Li YJ, Clegg L, Cote TR, et al. CK20 and CK7 protein expression in colorectal cancer: Demonstration of the utility of a population-based tissue microarray. Hum Pathol. 2005;36:275–81. doi: 10.1016/j.humpath.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Saad RS, Silverman JF, Khalifa MA, Rowsell C. CDX2, cytokeratins 7 and 20 immunoreactivity in rectal adenocarcinoma. Appl Immunohistochem Mol Morphol. 2009;17:196–201. doi: 10.1097/PAI.0b013e31819268f2. [DOI] [PubMed] [Google Scholar]
- 18.Kim JH, Rhee YY, Bae JM, Cho NY, Kang GH. Loss of CDX2/CK20 expression is associated with poorly differentiated carcinoma, the CpG island methylator phenotype, and adverse prognosis in microsatellite-unstable colorectal cancer. Am J Surg Pathol. 2013;37:1532–41. doi: 10.1097/PAS.0b013e31829ab1c1. [DOI] [PubMed] [Google Scholar]
- 19.Lugli A, Tzankov A, Zlobec I, Terracciano LM. Differential diagnostic and functional role of the multi-marker phenotype CDX2/CK20/CK7 in colorectal cancer stratified by mismatch repair status. Mod Pathol. 2008;21:1403–12. doi: 10.1038/modpathol.2008.117. [DOI] [PubMed] [Google Scholar]
- 20.McGregor DK, Wu TT, Rashid A, Luthra R, Hamilton SR. Reduced expression of cytokeratin 20 in colorectal carcinomas with high levels of microsatellite instability. Am J Surg Pathol. 2004;28:712–8. doi: 10.1097/01.pas.0000126757.58474.12. [DOI] [PubMed] [Google Scholar]
- 21.Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-the stable evidence. Nat Rev Clin Oncol. 2010;7:153–62. doi: 10.1038/nrclinonc.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park SY, Kim HS, Hong EK, Kim WH. Expression of cytokeratins 7 and 20 in primary carcinomas of the stomach and colorectum and their value in the differential diagnosis of metastatic carcinomas to the ovary. Hum Pathol. 2002;33:1078–85. doi: 10.1053/hupa.2002.129422. [DOI] [PubMed] [Google Scholar]
- 23.Bressenot A, Zimmer O. CK20/CK7 protein expression in colorectal cancer: A marker for progression of colorectal cancer. Hum Pathol. 2008;39:1714. doi: 10.1016/j.humpath.2008.07.010. author reply 1715. [DOI] [PubMed] [Google Scholar]


