Neural regeneration, or neuroregeneration, is a brain mechanism essential for rescuing cognitive functions pharmacologically against memory disorders and aging-related memory abnormality. In this short Perspective article, we intend to briefly present the essential roles of neuroregeneration and neural plasticity in learning and memory, memory disorders, and critical involvement in an effective treatment of memory disorders.
Learning and memory and neural plasticity: Our ability in learning and memory entirely depends on experience-induced neural plasticity to encode and store new memories. Neural plasticity refers to a set of synaptic and structural changes, including synaptogenesis, neurogenesis, synaptic plasticity, and synaptic/network remodeling in the brain, many of which involve changed activities of various signaling pathways underlying neuroregeneration. Neural plasticity is necessary in learning and memory, because signal transfer through postsynaptic potentials is very expensive in energy cost (using about 50% of the brain’s energy to drive signals along axons and across synapses even for the small number of functional synapses the brain keeps; Laughlin and Sejnowski, 2003). Rather than maintaining a vast, fixed network (costing more energy and space to operate), brains operate with remarkable efficiency by keeping the number of synapses at a low level (Hellwig, 2000), and with the capacity of initiating neural plasticity when cognition demands it. The ability of neural plasticity is thus central to a healthy cognitive function by directing the brain’s scarce resources to the required locations according to cognitive demands, while keeping a low number of functional synapses. Whenever the brains’ ability in neural plasticity and regeneration is jeopardized, learning and memory suffer. Indeed, we are facing an enormous medical challenge today in terms of how to maintaining neuroregeneration effectively (Ribas and Costa, 2017).
Neuroregeneration and memory impairment: An efficient brain dictates the very nature, due to the lack of abundance in functional connections of synapses the brain maintains, that learning and memory are much easier to be severely impaired than dramatically enhanced. Any brain injuries, disorders, or aging that jeopardizes neuroregeneration and remodeling are guaranteed to compromise the memory functions. Brains are susceptible to various types of injury ranging from trauma to neurodegenerative diseases: Alzheimer’s disease (AD), Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, the frontotemporal dementia, multiple sclerosis, and multiple system atrophy, and memory impairments due to traumatic brain injury, mental retardation, depression, alcohol-related dementia, and Creutzfeldt-Jakob disease. Neuroregeneration failure is largely responsible for these long-term deficits and poor functional recovery. Many of these memory disorders are linked to hippocampal dysfunction and synaptic deficiency (Sun et al., 2015). The adult hippocampus is very dynamic in synaptic plasticity and hosts a population of neural stem and progenitor cells (NSPCs) that proliferates throughout the mammalian life span. About 1400 newborn neurons are estimated to be added to the bilateral hippocampi of an adult human brain daily, accounting for about 1.8% of the total renewable neuronal population (Spalding et al., 2005), in addition to a very active and dynamic synaptic/structural plasticity. Impaired neural plasticity, especially synaptic deficiency, in the hippocampus strongly correlates with memory impairment in AD (Faure et al., 2011). Understanding the regulatory processes and the underlying mechanisms of adult neuroregeneration and remodeling in learning and memory formation is thus of paramount importance for the development of clinically effective memory therapeutics (Figure 1).
Figure 1.
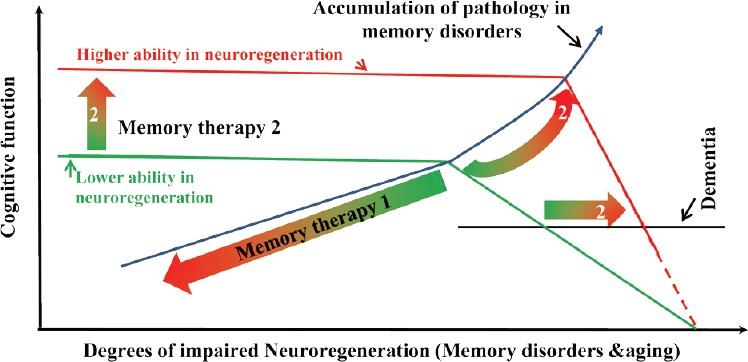
Roles of neuroregeneration in memory pharmacology.
Ability in neuroregeneration represents one of the core features of the cognitive function, which is impaired by damaging neuropathology in memory disorders or reduced in aging. Memory therapy 1 is the ‘conventional’ therapeutic strategy, which antagonizes the neuropathology of memory disorders and of memory decline in aging. Memory pharmacology 2 enhances the ability in neuroregeneration, so that cognitive function is maintained to a much greater amount of pathology before the function starts to drop (Memory therapy 2) at a much later time (2). The clinical diagnosis of dementia would also occur later (2), at greater neuropathology, but probably at greater rate of progression.
Neuroregeneration and memory pharmacology: Although adult brains have some abilities in neuroregeneration upon injuries, the neural repair, or neuroregeneration, is, unfortunately, generally too slow and insufficient. Therefore, memory impairments due to brain damage are usually permanent and incapacitating. Currently, donepezil, galantamine, rivastigmine, and memantine are the four food and drug administration (FDA)-approved drugs available for AD treatment. They can only provide symptomatic, short-term benefit (without changing the rate of disorder progression) and no specific treatments are available for other memory disorders. Fortunately, however, it has been well established that adult brain regions have an endogenous capacity in reserve, which could be unmasked and enhanced through activation of the signal pathway(s) (Darian-Smith and Gilbert, 1994). The spine turnover, for example, could potentially reach a level three times as high as that of the control (Keck et al., 2008). The dynamic structures include the hippocampal CA1 (Attardo et al., 2015), the core structure involved in many types of memory formation, such as episodic memory. Several approaches are likely to facilitate neuroregeneration for a functional recovery from memory disorders.
First, activities of neural and endothelial growth factors can be enhanced to facilitate neuroregeneration and remodeling. Brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF), for examples, are essential molecules for neural plasticity. The signal pathway can be activated to certain degrees by physical exercise and/or by environmental enrichment. But more effectively, some selective activators of protein kinase C (PKC) isoforms have been shown to enhance BDNF expression and activity in the hippocampus, activity of the growth-associated protein-43 (GAP-43), and to facilitate neuroregeneration and remodeling. Their therapeutic impacts have been shown in several models of memory disorders, such as AD model mice, fragile X mental retardation mice, vascular cognitive impairments in rats, and depression-induced spatial memory impairment in rats (Sun et al., 2015). GAP-43 is one of several proteins that sub-serve neuroregeneration and synaptic plasticity. The PKC-GAP-43 pathway contributes to an F-actin based stabilization of new synapses. In AD and aging, PKC-mediated phosphorylation of GAP-43 is decreased, but can beactivated through PKC-regulated phosphorylation (at Ser41). Activation of some PKC isoforms can also stabilize GAP-43 mRNA or other mRNAs involved in axonal outgrowth and neuroregeneration through the ELAV-like protein 4 (HuD). Recent studies show that adult hippocampal NSPCs shape their environment through secreting large quantities of the essential growth factor VEGF. This self-derived VEGF is functionally relevant for maintaining the neurogenic niche since inducible, NSPC-specific loss of VEGF leads to impaired stem cell maintenance (Kirby et al., 2015).
Second, histone deacetylase (HADC) inhibitors have been found to have promising neuroprotective potential against memory disorders, including AD, although many of the inhibitors have shown undesirable side effects. Histones are building blocks of the nucleosome and their acetylation/deacetylation plays a critical role in learning and memory functions. The level of histone acetylation is regulated by the dynamic equilibrium histone-acetyltransferase and HDACs. This acetylation homeostasis is disturbed in various neurodegenerative diseases. Generally speaking, histone deacetylation represses gene transcription while histone acetylation makes chromatin more loosely packed to activate transcription. It has been shown that some potent HDAC inhibitors can reverse AD-like changes in an animal model (Fischer et al., 2010).
Third, enhancing synaptic plasticity alone might not be enough to overcome the functional impacts after a significant loss of neurons, especially in the late stage of memory disorders. Replacement of lost neurons can be achieved through neurogenesis and/or neural stem cells. NSPCs are self-renewing, multipotent cells that generate the neurons and glia of the nervous system. Grafting neural stem cells into the hippocampus of aged animals has been found to form synaptic connections with hosting neurons and improves memory functions in AD models. The underlying mechanism may actually involve induced neuroregeneration, resulting fromneurotrophic factors secreted by the stem cells to increase synaptic density and modulate neural plasticity and neurogenesis. The potential usage of neural stem cells in clinics depends on how well their undesired impacts can be minimized (such as abnormal growth, misdirected growth, immune reaction, and long-term behavioral changes).
Conclusions: The brain is continuously rebuilding itself cognitively, involving activity-dependent changes in synaptic strengths and synaptic remodeling, or structural plasticity. Neural plasticity includes a mechanism of neurogenesis and remodeling connectivity between neurons. The continuous addition and turnover of neurons and synapses occur throughout life in some brain regions (Sailor et al., 2017), including the hippocampus and representing an endogenous system on which pharmacological agents can potentially target to achieve therapeutic impacts. The promotion of robust neuroregeneration in adult mammalian brains holds great therapeutic potential for memory disorders. Specific manipulations have unmasked a hidden capacity for structural plasticity. Genetic manipulation approaches (Ribas and Costa, 2017) may be used to regulate the intrinsic neuroregenerative capacity. Rescuing memory functions from memory disorders or aging-related impairment and dementia may be achieved through facilitating signal pathways that promote neuroregeneration, through targeting the endogenous inhibitory pathways of PKC, GAP-43, VEGF, or other molecules that promote neuroregeneration, or both. More isoform-selective HDAC inhibitors may be developed for treating memory disorders in the future. There are also many other signal molecules/pathways, although not-mentioned in this short article, that might be targeted for developing memory therapeutics. Lastly, it is equally important to emphasize here that cognitive process is extremely delicate, changes of neural plasticity and activities of the underlying signal pathways, either excessive or inadequate, may be deleterious to learning and memory functions.
Footnotes
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Hernández-Echeagaray Elizabeth, Universidad Nacional Autonoma de Mexico, Mexico.
References
- Attardo A, Fitzgerald JE, Schnitzer MJ. Impermanence of dendritic spines in live adult CA1 hippocampus. Nature. 2015;523:592–596. doi: 10.1038/nature14467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darian-Smith C, Gilbert CD. Axonal sprouting accompanies functional reorganization in adult cat striate cortex. Nature. 1994;368:737–740. doi: 10.1038/368737a0. [DOI] [PubMed] [Google Scholar]
- Faure A, Verret L, Bozon B, El Tannir El Tayara N, Ly M, Kober F, Dhenain M, Rampon C, Delatour B. Impaired neurogenesis, neuronal loss, and brain functional deficits in the APPxPS1-Ki mouse model of Alzheimer’s disease. Neurobiol Aging. 2011;32:407–418. doi: 10.1016/j.neurobiolaging.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Mungenast A, Tsai LH. Targeting the correct HDAC(s) to treat cognitive disorders. Trends Pharmacol Sci. 2010;31:605–617. doi: 10.1016/j.tips.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Hellwig B. A quantitative analysis of the local connectivity between pyramidal neurons in layers 2/3 of the rat visual cortex. Biol Cybern. 2000;82:111–121. doi: 10.1007/PL00007964. [DOI] [PubMed] [Google Scholar]
- Keck T, Mrsic-Flogel TD, Vaz Afonso M, Eysel UT, Bonhoeffer T, Hubener M. Massive restructuring of neuronal circuits during functional reorganization of adult visual cortex. Nat Neurosci. 2008;11:1162–1167. doi: 10.1038/nn.2181. [DOI] [PubMed] [Google Scholar]
- Kirby ED, Kuwahara AA, Messer RL, Wyss-Coray T. Adult hippocampal neural stem and progenitor cells regulate the neurogenic niche by secreting VEGF. Proc Natl Acad Sci U S A. 2015;112:4128–4133. doi: 10.1073/pnas.1422448112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin SB, Sejnowski TJ. Communication in neuronal networks. Science. 2003;301:1870–1874. doi: 10.1126/science.1089662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas VT, Costa MR. Gene Manipulation Strategies to Identify Molecular Regulators of Axon Regeneration in the Central Nervous System. Front Cell Neurosci. 2017;11:231. doi: 10.3389/fncel.2017.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailor KA, Schinder AF, Lledo PM. Adult neurogenesis beyond the niche: its potential for driving brain plasticity. Curr Opin Neurobiol. 2017;42:111–117. doi: 10.1016/j.conb.2016.12.001. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Bhardwaj RD, Buchholz BA, Druid H, Frisen J. Retrospective birth dating of cells in humans. Cell. 2005;122:133–143. doi: 10.1016/j.cell.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Sun MK, Nelson TJ, Alkon DL. Towards universal therapeutics for memory disorders. Trends Pharmacol Sci. 2015;36:384–394. doi: 10.1016/j.tips.2015.04.004. [DOI] [PubMed] [Google Scholar]


