Recently there have been exciting research advances in neuroprotective therapies for ischemic stroke. In the past, the search for neuroprotective agents has been fraught with failure at the clinical trials stage due to numerous factors, including subject heterogeneity and improper therapeutic windows (Tymianski, 2017). Moreover, it is becoming clearer that the complex and evolving pathobiology of stroke requires multimodal therapeutic approaches capable of modulating the numerous axes that contribute to ischemia/reperfusion damage, rather than targeting a single axis (Bernstock et al., 2018a). With the success of recent endovascular thrombectomy (EVT) trials, it has been suggested that clinical trials of EVT with adjunct neuroprotection can overcome past difficulties and maximize the effect size by using imaging to reduce patient heterogeneity (i.e., selecting those with large vessel occlusions, small ischemic cores, and good collateral circulation), restoring perfusion using better EVT devices, and enrolling patients in the correct therapeutic window (i.e., when they still have salvageable brain tissue) (Tymianski, 2017). Considering the opportunity that this represents for new, better clinical trials of neuroprotective agents, the search is on for high-potential compounds that may be investigated in these future studies.
Of particular interest are potential therapies centered on the modulation of protein SUMOylation, a post-translational modification that regulates a myriad of diverse pathways in the cell (Bernstock et al., 2018a). A little under a decade ago, it was discovered that 13-lined ground squirrels (Ictidomys tridecemlineatus) demonstrated extreme global levels of SUMOylated proteins in their brains during hibernation torpor, a state that is in and of itself effectively a model of “natural tolerance” to ischemia-like conditions (Bernstock et al., 2018a). Following this landmark observation, numerous in vitro and in vivo models have demonstrated that increasing global protein SUMOylation leads to an induction of ischemic tolerance (Bernstock et al., 2018a). Naturally, it was of great clinical relevance to search for small molecules that would be capable of pharmacologically modulating SUMOylation, in the hopes of developing novel therapies for a pathology with a marked worldwide disease burden.
SUMO is primarily found in three isoforms, with SUMO2 and SUMO3 sharing 96% homology. In brief, the SUMOylation pathway is as follows: first, the SUMO-specific proteases (SENPs) cleave the immature SUMO precursor to produce the functional SUMO form (Flotho and Melchior, 2013). As the initial (ATP-dependent) step in SUMO-conjugation, the SUMO E1 enzyme (a heterodimer of SUMO activating enzyme (SAE)1/2) forms a covalent thioester with SUMO (Flotho and Melchior, 2013). Following that, SUMO is transferred to the catalytic domain of the SUMO E2 enzyme, Ubc9, which then forges an isopeptide bond between SUMO and the target SUMO-substrate protein (in some cases, a target-specific E3 ligase may aid the association of the SUMO-Ubc9 intermediate to the target) (Flotho and Melchior, 2013). The immediate effects of SUMOylation include promotion or inhibition of protein-protein interactions, alteration of the target’s conformational state, and regulation of the target’s stability by inhibiting or promoting ubiquitination (Flotho and Melchior, 2013; Bernstock et al., 2018a). Finally, removal of SUMO from the target protein (i.e., deconjugation) is effected by the isopeptidase activity of the SENP family; a few other SUMO-deconjugating proteins have been identified, but their activity is highly substrate-specific. Overall, the cycling of the SUMO pathway from conjugation through deconjugation is dynamic and rapid (Flotho and Melchior, 2013).
When considering druggable targets of the SUMO pathway, there are certain features of this post-translational modification that lend themselves easily towards modulating global SUMOylation. Unlike ubiquitination, SUMOylation limits itself to one E1 activating enzyme (the heterodimer SAE1/2) and one E2 conjugase (Ubc9) — thus, targeting each of these components of the SUMO-conjugation machinery is likely to effect significant changes in levels of SUMOylated proteins. Past in vitro and in vivo studies have leveraged this principle in order to effectively investigate the upregulation of SUMO-conjugation and protection against oxygen-glucose deprivation (OGD) (an in vitro model of ischemic stroke) or middle cerebral artery occlusion (MCAO), such as by constitutively overexpressing Ubc9 in transgenic mice which later demonstrated improved outcomes after MCAO compared to wild-type mice. Certain microRNAs (i.e., miRNA-182 and 183) have also been identified as inhibitors of SUMOylation and pharmacological inhibition of these miRNAs represents another druggable axis. Lastly, the SENP protein family — as SENPs are capable of cleaving SUMO from SUMOylated proteins regardless of the protein’s identity (only having preference for specific SUMO isoforms), these enzymes, particularly SENP1–3, may also be targeted to modulate global protein SUMOylation (Bernstock et al., 2018a).
With recent advancements in available technologies, as well as the investment of millions of dollars into facilities and collaborative consortiums for drug discovery, repurposing, and repositioning, the future looks bright and promising for developing effective therapies. Powerful tools that can be applied to myriad pathologies, including rare and neglected diseases, are being improved with each day; searching for neuroprotectants that act through modulating SUMOylation is but one approach. The number of screens that have been reported continues to expand and new strategies such as drug combination screens and rapid computerized approaches increase successful drug repositioning (Sun et al., 2016). Using these new technologies, and components/interactors of the SUMO-conjugation pathway as screening targets, recent drug repurposing/discovery efforts have resulted in promising leads. An AlphaScreen-based assay using SUMO1 and Ran GTPase-activating protein as the substrates identified a lead compound, N106, as an activator of SUMOylation through interaction with SAE1. While currently being investigated as a treatment for heart failure, future studies may explore its ability to cross the blood-brain barrier and, thusly, its potential to be translated into a neuroprotective drug (Kho et al., 2015). Numerous compounds screened against miRNAs 182 and 183, including histone de-acetylase inhibitors and synthetic retinoids, have been shown to increase global SUMOylation and induce protection against OGD (Bernstock et al., 2016). Whereas earlier screening strategies targeting the SENPs have produced lackluster results (Bernstock et al., 2018a), a newly-developed quantitative high-throughput screening paradigm using a physiologically-relevant SENP substrate has identified compounds that are SENP inhibitors capable of increasing global SUMOylation in vitro and inducing protection against OGD; the utility of such an approach having originally been demonstrated by our group (i.e., neuroprotection induced via the inhibition of SENPs) (Lee et al., 2016; Bernstock et al., 2018b).
This screening paradigm has been further iteratively developed and refined with the addition of several orthogonal assays in order to maximize its utility. Following the initial AlphaScreen-based assay, a cell-free assay comprising recombinant human SENP2 catalytic domain and a recombinant SUMO2-SUMO3 substrate was employed to confirm the inhibitory effects of identified compounds (Bernstock et al., 2018b). As the ultimate goal of the screen was to identify compounds that could be developed into clinically-useful therapies, an ATP-content-based toxicity screen filtered out dangerous, cytotoxic compounds (Bernstock et al., 2018b). The cellular thermal shift assay (CETSA) was then used to assess engagement of the target enzyme in cells by the small molecules of interest, based on the simple but useful principle of a protein being thermostabilized by a ligand (i.e., shifting the melting point upwards) (Bernstock et al., 2018b). Software-based in silico models of SUMO/SUMO-target interactions for compounds confirmed by CETSA, while ultimately not included as a triaging step, beautifully illustrated low-energy binding poses for all confirmed compounds (Bernstock et al., 2018b). A small handful of compounds, the highest-potential remainder out of the thousands in the compound libraries, finally entered functional assays: determination of their effects on global protein SUMOylation in cell culture, and, of those compounds that successfully increased SUMOylation, evaluation of their protective efficacy against OGD. Two compounds, ebselen and 6-thioguanine, were identified; ebselen was then injected into mice, and was shown to increase levels of SUMOylated proteins in the brain (Bernstock et al., 2018b). Ultimately, the end product is a powerful screening platform that is capable of effectively identifying SENP2 inhibitors that can increase global SUMOylation in vitro and in vivo and effect protection against OGD; notably, it might also be effectively adapted for SENP1 (Bernstock et al., 2018b). Future efforts should employ larger compound libraries in the initial screen, as well as leverage medicinal chemistry to optimize any compounds identified as potential neuroprotectants, eventually leading into the aforementioned EVT-adjuvant neuroprotection clinical trials.
Beyond ischemic stroke, pharmacologic modulation of global SUMOylation has a potential role in the treatment of other diseases as well. Whereas increased protein SUMOylation effects neuroprotection against stroke, inhibition of protein SUMOylation is increasingly becoming a viable strategy for the treatment of diseases such as cancer (Bernstock et al., 2018a). Of note, numerous cell-cycle regulators that are oncogenes or tumor suppressors are regulated through SUMOylation, and dysregulation of the SUMO-conjugating and SUMO-deconjugating activities has severe consequences for proliferation and genomic stability; consequently, a number of cancers, including glioblastoma (GBM), are dependent on SUMOylation machinery (Eifler and Vertegaal, 2015). Thus, drug screening strategies may also be employed to discover/repurpose small molecules that are capable of downregulating protein SUMOylation. For instance, recently, topotecan was identified as a potent inhibitor of global SUMOylation in GBM, neuroblastomas, and rat cortical neurons, with downstream effects on mitotic progression and metabolism in GBM havening been demonstrated (Bernstock et al., 2017). Another compound, ML-792, has been identified as a potent and selective inhibitor of SAE2, and is highly toxic to cell lines exhibiting amplified Myc. As loss of SAE1/2 function drives synthetic lethality with Myc-hyperactivation, the therapeutic potential of inhibiting SUMOylation in Myc-driven cancers is an exciting area of research (Schneekloth, 2017). Another compound, spectomycin B1, has been identified as a Ubc9 inhibitor, a position where it can markedly inhibit SUMOylation (Hirohama et al., 2013) and may therefore ultimately be employed as an adjuvant chemotherapeutic.
However, an important caveat is that the SUMO pathway also regulates myriad homeostatic pathways/responses (Bernstock et al., 2018a). For example, SUMOylation has also been implicated in emotionality and cognition, particularly with regard to anxiety, episodic memory, and emotional memory (Bernstock et al., 2018a). Therefore, interventions upregulating or downregulating the SUMO machinery must strike a careful balance. In summary, drugging SUMOylation clearly warrants continued attention in an effort to develop novel neuroprotective and oncologic therapeutics approaches for patients and families in need (Figure 1).
Figure 1.
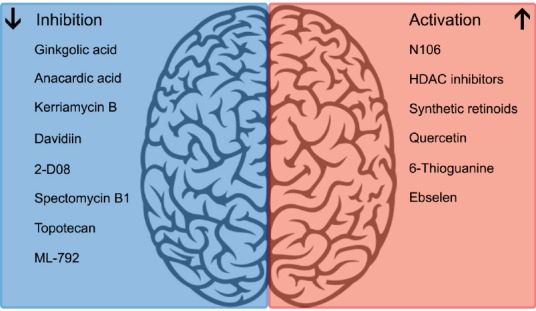
Pharmacological modulators of SUMOylation.
Footnotes
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer review report:
Reviewer: Hong Chen, Huazhong University of Science and Technology, China.
Comments to authors: In the present study authors describe that targeting SUMOs may represent the potential therapies for ischemic stroke or cancer. SUMOylation regulates almost all major cellular pathways through activation and repression. In general, this study was well written and nicely summarized.
References
- Bernstock JD, Yang W, Ye DG, Shen Y, Pluchino S, Lee YJ, Hallenbeck JM, Paschen W. SUMOylation in brain ischemia: Patterns, targets, and translational implications. J Cereb Blood Flow Metab. 2018a;38:5–16. doi: 10.1177/0271678X17742260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstock JD, Lee YJ, Peruzzotti-Jametti L, Southall N, Johnson KR, Maric D, Volpe G, Kouznetsova J, Zheng W, Pluchino S, Hallenbeck JM. A novel quantitative high-throughput screen identifies drugs that both activate SUMO conjugation via the inhibition of microRNAs 182 and 183 and facilitate neuroprotection in a model of oxygen and glucose deprivation. J Cereb Blood Flow Metab. 2016;36:426–441. doi: 10.1177/0271678X15609939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstock JD, Ye D, Gessler FA, Lee YJ, Peruzzotti-Jametti L, Baumgarten P, Johnson KR, Maric D, Yang W, Kögel D, Pluchino S, Hallenbeck JM. Topotecan is a potent inhibitor of SUMOylation in glioblastoma multiforme and alters both cellular replication and metabolic programming. Sci Rep. 2017;7:7425. doi: 10.1038/s41598-017-07631-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstock JD, Ye D, Smith JA, Lee YJ, Gessler FA, Yasgar A, Kouznetsova J, Jadhav A, Wang Z, Pluchino S, Zheng W, Simeonov A, Hallenbeck JM, Yang W. Quantitative high-throughput screening identifies cytoprotective molecules that enhance SUMO conjugation via the inhibition of SUMO-specific protease (SENP)2. FASEB J. 2018b doi: 10.1096/fj.201700711R. doi: 10.1096/fj.201700711R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eifler K, Vertegaal AC. SUMOylation-mediated regulation of cell cycle progression and cancer. Trends Biochem Sci. 2015;40:779–793. doi: 10.1016/j.tibs.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotho A, Melchior F. Sumoylation: a regulatory protein modification in health and disease. Annu Rev Biochem. 2013;82:357–385. doi: 10.1146/annurev-biochem-061909-093311. [DOI] [PubMed] [Google Scholar]
- Hirohama M, Kumar A, Fukuda I, Matsuoka S, Igarashi Y, Saitoh H, Takagi M, Shin-ya K, Honda K, Kondoh Y, Saito T, Nakao Y, Osada H, Zhang KY, Yoshida M, Ito A. Spectomycin B1 as a novel SUMOylation inhibitor that directly binds to SUMO E2. ACS Chem Biol. 2013;8:2635–2642. doi: 10.1021/cb400630z. [DOI] [PubMed] [Google Scholar]
- Kho C, Lee A, Jeong D, Oh JG, Gorski PA, Fish K, Sanchez R, DeVita RJ, Christensen G, Dahl R, Hajjar RJ. Small-molecule activation of SERCA2a SUMOylation for the treatment of heart failure. Nat Commun. 2015;6:7229. doi: 10.1038/ncomms8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Bernstock JD, Nagaraja N, Ko B, Hallenbeck JM. Global SUMOylation facilitates the multimodal neuroprotection afforded by quercetin against the deleterious effects of oxygen/glucose deprivation and the restoration of oxygen/glucose. J Neurochem. 2016;138:101–116. doi: 10.1111/jnc.13643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneekloth JS Jr. Drug discovery: Controlling protein SUMOylation. Nat Chem Biol. 2017;13:1141–1142. doi: 10.1038/nchembio.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Sanderson PE, Zheng W. Drug combination therapy increases successful drug repositioning. Drug Discov Today. 2016;21:1189–1195. doi: 10.1016/j.drudis.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymianski M. Combining neuroprotection with endovascular treatment of acute stroke: is there hope? Stroke. 2017;48:1700–1705. doi: 10.1161/STROKEAHA.117.017040. [DOI] [PubMed] [Google Scholar]


