The initial trauma to the spinal cord is just the starting point for a cascade of endogenous events that will collectively determine the injury extension. These secondary events include, but are not limited to: glutamate excitoxicity, induction of apoptotic pathways, ionic imbalances and the development of a strong and dysfunctional inflammatory response. The secondary injury is associated to an aggravation of neuronal damage increasing the extent of neurological deficits (Ek et al., 2010).
The delayed secondary injury, lasting hours, days or even months, represents an attractive time-window of opportunity for neuroprotective therapeutic interventions.
The main purpose of such therapies is to prevent or antagonize secondary events therefore protecting spared/surviving neural tissue and avoiding the extension of neurological impairments (Vasconcelos et al., 2016).
The strong inflammatory response developed after spinal cord injury (SCI) is overall regarded as a detrimental phenomenon to recovery and has been a target for the development of therapeutics for SCI.
The infiltration of immune cells within the nervous system parenchyma held a long-lasting bad reputation based on other neurological diseases whose etiology is characterized by an abnormal immune infiltration into the central nervous system (CNS) parenchyma. Moreover, particular anatomical features of the CNS that reduce neuroimmune interactions, such as the presence of barriers (blood-brain barrier, blood-spinal cord barrier and the choroid plexus-barrier) sustain the notion that CNS tissue should be protected from immune cells to maintain its homeostasis and integrity.
Most of the therapies targeting inflammation developed in the last decades were anti-inflammatory strategies suppressing overall the immune response.
In fact the only approved therapy for treating spinal cord injury is methylprednisolone, a potent anti-inflammatory drug. However, effectiveness of methylprednisolone in spinal cord injury management is being largely questioned by studies showing reduced benefits of the routine administration of this drug and the higher prevalence of complications such as gastrointestinal bleedings or the higher susceptibility to infections (Evaniew et al., 2016). These findings have led to guidelines recommendation of discontinuing methylprednisolone administration in SCI patients (Evaniew et al., 2016). Curiously, in some clinical contexts, acute infusion of methylprednisolone is still an option even though the ratio between the benefits versus side effects being very small and the reason is simple - there is no better alternative, which illustrates the urgency of developing better therapies.
One possibility for the failure of the anti-inflammatory strategies is the fact that acute inflammation is integrated and needed for a successful tissue repair response. In line with this idea, studies on zebrafish, an animal model capable of fully recover from a SCI, demonstrated that inflammation plays a vital role in the regeneration process (Kyritsis et al., 2012). In this study, zebrafish were no longer able to recover from a SCI, when acute inflammation was experimentally blocked (Kyritsis et al., 2012).
A successful tissue repair response depends also on a rapid triggering of inflammation followed by a complex orchestration of different cells and molecular events that unfold in a temporal controlled manner. Acute inflammation consists in the infiltration of inflammatory cells specialized in tissue defense, cleaning and repair. One key cell in this process is the macrophage. Macrophages derive from monocytes and can acquire a diverse spectrum of activation states with different functionalities. The type of macrophage activation can range from the most proinflammatory, or classically activated phenotype (M1) to the anti-inflammatory or alternatively activated phenotype (M2).
The immune response to SCI at the initial stages has some resemblances of that in non-CNS injured tissues, with a rapid development of inflammation activating both endogenous (microglia) and infiltrating macrophages (Shechter and Schwartz, 2013). At the lesion epicenter a mix of immune cells exhibiting different activation profiles (M1 and M2) can be found shortly after primary injury (Kigerl et al., 2009), which may be important for cleaning debris and fighting possible pathogens. In an efficient immune response to injury, macrophages should progressively adopt or be replaced by cells exhibiting activation states associated with tissue repair and regeneration (more M2 polarized) (Figure 1). However in SCI, M1 macrophages quickly become the predominant cell type (Kigerl et al., 2009) with concomitant disappearing of M2 macrophages (Figure 1). This response is associated with fibrosis, oxidative damage and further neurodegeneration contributing for the regeneration failure.
Figure 1.
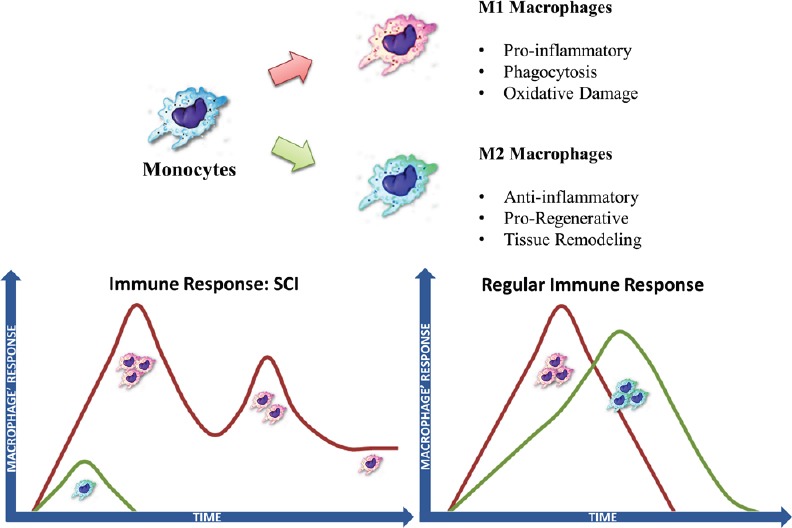
Comparison of the type of monocyte/macrophage response in spinal cord injury (SCI) with non-central nervous system (CNS) tissue.
Monocytes can differentiate into macrophages with different phenotypes. Macrophages can display different activation states. Macrophage activation can range from classical M1 activation at one pole - the most pro-inflammatory macrophages, to M2 alternative activation with anti-inflammatory effects and associated with tissue repair. The temporal dynamics of macrophage infiltration and activation phenotyope after injury is different in the spinal cord compared to non-CNS tissue.
As more as we understand the functional role of the inflammatory response, the more evident becomes that this response can support both beneficial and detrimental effects to recovery.
The increased understanding of SCI pathophysiology and associated inflammatory response is shifting the research focus from developing anti-inflammatory to immunomodulatory therapies. The main aim of immunomodulatory strategies is at boosting immune cells displaying repairing/regenerative while abrogating pro-inflammatory cells with strong oxidative response.
Currently, different strategies such as the use of biomaterials, cell-based therapies or molecular therapies have demonstrated promising immunomodulatory potential in pre-clinical models of SCI.
One such example is the use of scaffolds to form a biomaterial bridge serving as a pathway for regenerating neurons to bypass the lesion. While the primary aim of this sort of strategy is to optimize and direct axonal growth, the potential immunomodulatory ability has raise attention when designing these biomaterials. It has been shown that the mechanical properties of scaffolds can affect the number of immune cells infiltration but also the type of both innate and adaptive cells activation (Dumont et al., 2016). Scaffolds specific features such as pore size or geometry can facilitate wound healing properties of macrophages.
Cell-based therapies also hold promise as a neuroprotective strategy for SCI recovery. This type of therapy mainly aims at re-populating the injury site, but has also demonstrated an interesting function at modulating the inflammatory environment. Both neural stem cells and mesenchymal stem cells have demonstrated a potential immunomodulatory role either through cell-to-cell contact but specially through the release of soluble factors (DePaul et al., 2015; Dumont et al., 2016). Some of the immunomodulatory effects are the ability to decrease the amount of macrophage infiltration, increase the release of anti-inflammatory cytokines while decreasing pro-inflammatory cytokines, to promote alternatively activation of macrophages or to promote T regulatory cells (DePaul et al., 2015; Dumont et al., 2016).
Other strategies rely on the manipulation of specific molecules to modulate inflammation to favour recovery (Gensel et al., 2015; Kwon et al., 2015; Francos-Quijorna et al., 2016; Lima et al., 2017). For example, it has been shown that depending on the type of receptor activated in macrophages they can display neuroprotective or neurotoxic effects in SCI. The local administration of TLR2 agonist boosts macrophage reaction while protecting the tissue after SCI (Gensel et al., 2015) demonstrating the importance of local molecular signals for macrophages engaging activation phenotypes associated with tissue repair.
Another demonstration on the importance of the molecular environment for the type of immune response to SCI is the study by Kwon et al. showing that the presence of the chemokine, chemokine (C-C motif) ligand 2 (CCL2), at the injury drives a proregenerative activation phenotype by macrophages (Kwon et al., 2015).
Molecules involved in the polarization of M2 macrophages have also shown to exert neuroprotective effects after SCI. For instance, the local administration of interleukin 4 (IL-4), an interleukin known to be central for M2 activation of macrophages have shown to benefit recovery (Francos-Quijorna et al., 2016).
For most of the current immunomodulatory strategies the achievement of both histological and motor recovery after SCI are still far from ideal. One possibility is that most of these strategies only target the early inflammatory events not taking into account the temporal dynamics of the inflammatory response (Figure 1). One example illustrating the importance of the temporal dynamics of the inflammatory response for the success of immunomodulatory strategies is related to IL-4 therapy. A delayed administration of IL-4 48 hours after injury is able to skew macrophages/microglia to a M2 phenotype contrasting with the acute administration that has no immunomodulatory effect (Francos-Quijorna et al., 2016).
One major drawback of delaying the administration of the immunomodulatory drug is the fact of having to repeat a surgical procedure after the lesion for local delivery. Systemic administration while overcoming the need of repeated surgeries can lead to other complications such as off-target effects or reduced amount of drug reaching the lesion site.
Our lab have now demonstrated, using a rat model of contusion thoracic SCI, that a repeated scheme of systemic administration of IL-4 for a week also exert neuroprotective effects (Lima et al., 2017) with no apparent complications. Systemic delivery is interesting since they are less invasive avoiding the unnecessary extra manipulation of delicate nervous tissue. The major drawback of cytokine therapies however, is that these proteins are known for their short half-life, potentially hampering the therapeutic efficacy of systemic delivery strategies. Our results showed that systemic administration of IL-4 promoted a significant elevation on the serum concentration of interleukin 10 (IL-10), an anti-inflammatory cytokine. Perhaps, more interesting, were the local effects of the treatment. Systemic administration of IL-4 was able to reduce the number of macrophages/microglia at the spinal cord. Still not understood is if this effect of IL-4 on macrophage reduction is due to less infiltration, less survival or increased drainage.
Importantly, in treated animals, macrophages displayed more ramifications while the macrophages from non-treated animals displayed a more rounded shape. Macrophages displaying a round shape are generally associated with pro-inflammatory phenotypes.
Consistent with a neuroprotective effect of our immunomodulatory strategy we found the presence of more neurons at the epicenter of the lesion after IL-4 treatment. The finding that IL-4 treatment was able to rescue surviving neurons at the lesion epicenter is particularly interesting since our model represents a severe lesion, and after two months of the injury almost no neurons can be found at the epicenter of the lesion from non-treated animals.
Although systemic IL-4 treatment led to interesting alterations at the histological level, the modest improvement seen at the functional level illustrates the need to improve current available immunomodulatory strategies.
Research efforts in the field are being placed in the optimization of current immunomodulatory strategies as their therapeutic value as an individual therapy is still unsatisfactory for full SCI recovery.
Moreover, advancements in the field also demonstrated the highly dynamic nature of this response with specific immune cell infiltration peaking at different time-points after the initial injury. Future immunomodulatory therapies should reflect the temporal dynamics of the inflammatory response and be administered accordingly to the inflammatory event targeted. For example, therapies targeting infiltrating macrophages should be administered 2–3 days after the injury since it is when macrophage infiltration peaks, or therapies targeted to adaptive responses should be administered at later time points.
For the specific case of systemic therapies the use of bioengineered solutions to target delivery or to avoid the rapid degradation of molecules in circulation can be an attractive option to optimize current therapies using bioactive molecules.
Spinal cord injury is complex triggering many pathological processes beyond inflammation. Most likely, therapeutic strategies in the future should reflect this pathological complexity, combining different approaches targeting different secondary events or regenerative strategies to maximize recovery potential.
The study was supported by Prémios Santa Casa Neurociências—Prize Meloe Castro for Spinal Cord Injury Research; Portuguese Foundation for Science and Technology (Financiado no âmbito do Projecto 3599—Promover a Produção Científica e Desenvolvimento Tecnológico e a Constituição de Redes Temáticas (3599-PPCDT), project: PTDC/DTP-FTO/5109/2014; Post-Doctoral fellowship—SFRH/BPD/97701/2013—to N.A. Silva; IF Development Grant to A. J. Salgado).
We acknowledge José Graça for his contribution on making Figure 1.
Footnotes
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer review report:
Reviewer: Melissa Renee Andrews, University of St Andrews, UK.
Comments to authors: The article is a good synopsis of inflammation and immunomodulation after spinal cord injury.
References
- DePaul MA, Palmer M, Lang BT, Cutrone R, Tran AP, Madalena KM, Bogaerts A, Hamilton JA, Deans RJ, Mays RW, Busch SA, Silver J. Intravenous multipotent adult progenitor cell treatment decreases inflammation leading to functional recovery following spinal cord injury. Sci Rep. 2015;5:16795. doi: 10.1038/srep16795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont CM, Margul DJ, Shea LD. Tissue Engineering approaches to modulate the inflammatory milieu following spinal cord injury. Cells Tissues Organs. 2016;202:52–66. doi: 10.1159/000446646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek CJ, Habgood MD, Callaway JK, Dennis R, Dziegielewska KM, Johansson PA, Potter A, Wheaton B, Saunders NR. Spatio-temporal progression of grey and white matter damage following contusion injury in rat spinal cord. PLoS One. 2010;5:e12021. doi: 10.1371/journal.pone.0012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evaniew N, Belley-Côté EP, Fallah N, Noonan VK, Rivers CS, Dvorak MF. Methylprednisolone for the treatment of patients with acute spinal cord injuries: a systematic review and meta-analysis. J Neurotrauma. 2016;33:468–481. doi: 10.1089/neu.2015.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francos-Quijorna I, Amo-Aparicio J, Martinez-Muriana A, Lopez-Vales R. IL-4 drives microglia and macrophages toward a phenotype conducive for tissue repair and functional recovery after spinal cord injury. Glia. 2016;64:2079–2092. doi: 10.1002/glia.23041. [DOI] [PubMed] [Google Scholar]
- Gensel JC, Wang Y, Guan Z, Beckwith KA, Braun KJ, Wei P, McTigue DM, Popovich PG. Toll-like receptors and dectin-1, a C-type lectin receptor, trigger divergent functions in CNS macrophages. J Neurosci. 2015;35:9966–9976. doi: 10.1523/JNEUROSCI.0337-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon MJ, Shin HY, Cui Y, Kim H, Thi AH, Choi JY, Kim EY, Hwang DH, Kim BG. CCL2 mediates neuron-macrophage interactions to drive proregenerative macrophage activation following preconditioning injury. J Neurosci. 2015;35:15934–15947. doi: 10.1523/JNEUROSCI.1924-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyritsis N, Kizil C, Zocher S, Kroehne V, Kaslin J, Freudenreich D, Iltzsche A, Brand M. Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science. 2012;338:1353–1356. doi: 10.1126/science.1228773. [DOI] [PubMed] [Google Scholar]
- Lima R, Monteiro S, Lopes JP, Barradas P, Vasconcelos NL, Gomes ED, Assunção-Silva RC, Teixeira FG, Morais M, Sousa N, Salgado AJ, Silva NA. Systemic interleukin-4 administration after spinal cord injury modulates inflammation and promotes neuroprotection. Pharmaceuticals (Basel) 2017:10. doi: 10.3390/ph10040083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R, Schwartz M. CNS sterile injury: just another wound healing? Trends Mol Med. 2013;19:135–143. doi: 10.1016/j.molmed.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Vasconcelos NL, Gomes ED, Oliveira EP, Silva CJ, Lima R, Sousa N, Salgado AJ, Silva NA. Combining neuroprotective agents: effect of riluzole and magnesium in a rat model of thoracic spinal cord injury. Spine J. 2016;16:1015–1024. doi: 10.1016/j.spinee.2016.04.013. [DOI] [PubMed] [Google Scholar]


