The G93A-SOD1 mice model and MRI diffusion as a preclinical tool to study amyotrophic lateral sclerosis (ALS): ALS is a progressive neurological disease characterized primarily by the development of limb paralysis, which eventually leads to lack of control on muscles under voluntary control and death within 3–5 years. Genetic heterogeneity and environmental factors play a critical role in the rate of disease progression and patients display faster declines once the symptoms have manifested. Since its original discovery, ALS has been associated with pathological alterations in motor neurons located in the spinal cord (SC), where neuronal loss by a mutation in the protein superoxide dismutase in parenthesis (mSOD1) and impairment in axonal connectivity, have been linked to early functional impairments. In addition, mechanisms of neuroinflammation, apoptosis, necroptosis and autophagy have been also implicated in the development of this disease. Among different animal models developed to study ALS, the transgenic G93A-SOD1 mouse has become recognized as a benchmark model for preclinical screening of ALS therapies. Furthermore, the progressive alterations in the locomotor phenotype expressed in this model closely resemble the progressive lower limb dysfunction of ALS patients. Among other imaging tools, MR diffusion tensor imaging (DTI) has emerged as a crucial, noninvasive and real time neuroimaging tool to gather information in ALS. One of the current concerns with the use of DTI is the lack of biological validation of the microstructural information given by this technique. Although clinical studies using DTI can provide a remarkable insight on the targets of neurodegeneration and disease course, they lack histological correlations. To address these shortcomings, preclinical models can be designed to validate the microstructural information unveiled by this particular MRI technique. Thus, the scope of this review is to describe how MRI diffusion and optical microscopy evaluate axonal structural changes at early stages of the disease in a preclinical model of ALS.
Use of DTI to study in vivo bioimaging markers in ALS: Considering the short interval from symptoms to patient death, there is a need for an imaging technique that is able to detect the loss of axonal connectivity at the earliest possible stage in order to preserve axonal function and improve patient outcomes. MRI is a useful tool to detect central nervous system (CNS) damage and monitor neurological treatments in the experimental models of neurological diseases (Gatto et al., 2015a). MRI techniques can not only look at gray matter (GM) and white matter (WM) degeneration damage. using macro-structural parameters (volume, cross-sectional areas) but also microstructural data by diffusion tensor imaging (DTI). In our current studies, anatomical imaging studies at early (presymptomatic) stages of the disease were not able to capture significant macrostructural changes in WM regions. Recently, DTI techniques have been well regarded by researchers to detect and monitor early changes in axonal organization (Kim et al., 2011; Underwood et al., 2011). Recent improvements in MRI diffusion protocols and increasing neurobiological research have increased our understanding of the underlying biological implication of these signals in ALS mice (Caron et al., 2015; Marcuzzo et al., 2017). However, research linking DTI and cellular changes in initial stages of ALS is scarce. To fill this gap in knowledge, our current investigations are focused on understanding the underlying microstructural changes captured by DTI using specific molecular and structural cellular markers. By the combined application of high MRI fields (9.4T) and optical fluorescence microscopy techniques, our group has studied the early MM changes in the G93A-SOD1 mice (Gatto et al., 2018). Results from our in vivo studies have shown that early alterations in the axonal organization can be revealed by DTI parameters such as fractional anisotropy (FA). However, our histological work with neurofilament stainings could not offer the necessary fine cellular details needed to proper evaluate individual axons (Figure 1a). In addition, little is known about the association between DTI scalars and changes in WM cellular structures and which of these parameters is the most relevant in the detection of the early microstructural alterations in ALS. In that regard, our results have demonstrated that the early changes in axial diffusion (AD) and radial diffusion (RD) are similarly affected. For instance, we have used changes in AD and RD to determine that mSOD1 simultaneously affects multiple cell populations across different WM compartments. Results from our studies showed that specific histological markers were related to changes of DTI-based parameters, some of them closely related to changes in axonal organization (FA) and neuronal loss (AD) and other to the presence of WM myelin anomalies (RD). Although the complex molecular mechanisms producing the structural differences between axonal networks at very early stages of ALS require further research (Morfini et al., 2013), neuropathological and imaging reports have demonstrated that alterations in the axonal connectivity is one of the key features in ALS.
Figure 1.
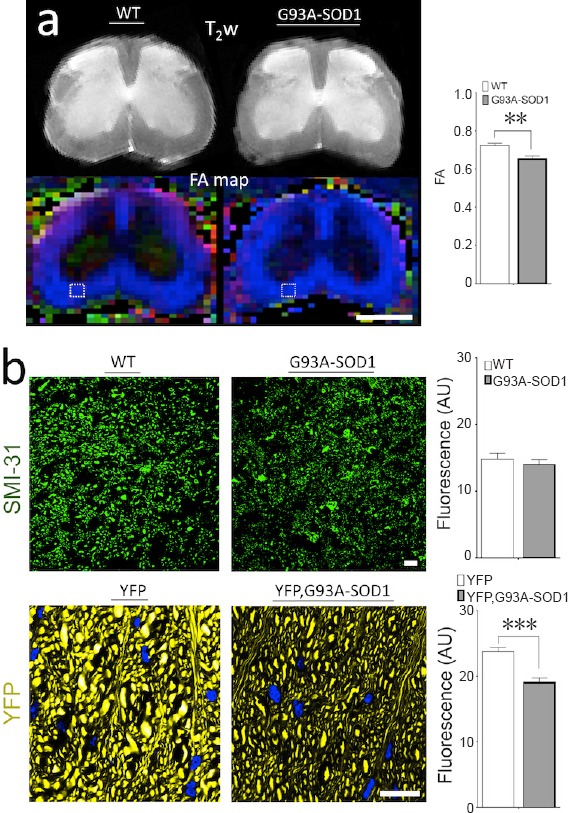
Diffusion tensor imaging (DTI) can detect early microstructural changes in white matter (WM) organization in spinal cord (SC) of the SOD1 mice.
(a) High-field MR diffusion T2-weighted (T2w) imaging and in vivo fractional anisotropy (FA) maps from axial lumbar sections in control mice (WT) and ALS mice (G93A-SOD1). Analysis of regions of interest (ROIs) were performed in the anterolateral funiculi (ALF) of the ventral SC WM based on previous ex vivo studies. Comparison between both groups showed a significant early decrease (P < 0.001) of FA (index of axonal organization) in the disease group. These results prove that presymptomatic anomalies in WM microstructure can be detected in vivo by DTI. (b) Microscopy analysis using immunohistochemistry (IHC) techniques from comparative WM ROIs using a phosphorylated neurofilament marker (SMI-31) at early stages of the disease demonstrated relative signs of WM structural anomalies in the ALS mice group. However, quantitative IHC methods, did not show significant differences of this marker in the ALS mice. To improve axonal visualization, we bred a line of mice expressing endogenous yellow fluorescence protein (YFP mouse) with ALS mice creating a fluorescent mice reporter (YFP, G93A-SOD1 mice). Confocal fluorescence microscopy at a higher magnification shows alteration in size and morphology of individual axons. Further ALF quantitative confocal fluorescence analysis demonstrated a significant reduction of the fluorescence levels in the YFP, G93A-SOD1 mice. Nuclear counterstaining with DAPI (blue). Statistical analysis performed with unpaired t-test analysis (n = 5 animals per group). **P < 0.01, ***P < 0.001. Scale bars: 1 cm in a, 10 μm in b. This review was partially based on previous work from Gatto et al. (2018).
DTI can evaluate presymptomatic alterations in ALS axonal connectivity: The CNS is a complex network of structurally interconnected regions thriving on the continuous integration of information across different regions of the brain and the SC. In our previous studies, tractography tractography techniques have provided an estimate of the axonal bundles trajectories and the complex biological rearrangement in WM architecture during early stages of ALS. However, the reconstructions of fiber tracks to evaluate qualitative changes in axonal organization have been restricted by biophysical factors, like the limited intrinsic resolution of the MRI signals (voxel size) as well as biological factors, such as variability and heterogeneity within CNS regions. Recent developments in MRI methods obtained from diffusion path probabilities provides estimates of quantitative connection strengths information across different WM regions (nodes) and enables the reconstruction of connectomes. Such elements display properties that are consistent with CNS networks mapped with other imaging modalities and have been validated by post-mortem brain studies. Additional quantitative metrics that reflect the diffusion properties connecting along edges of nearby voxels (Edge Weights) can be accounted as a method to reflect CNS connectivity strength (Colon-Perez et al., 2015). Such new approaches have been greatly contributed to our understanding of the impairment in SC connectivity centered in cervical and lumbar regions, consistent with the characteristic motor phenotype observed in mSOD1 mice as well as in the majority of ALS patients. Considering the alterations in multiple molecular, cellular and structural processes governing water diffusion across living tissues during the initial course of the disease, current mathematical models of MRI diffusion attenuation curves are far too simplistic to approximate the complex multi-cellular events occurring during any neurodegenerative process. Even though the development of new MRI methods to extract meaningful WM biomarkers to distinguish different forms of ALS are gaining momentum in the scientific community (Kolind et al., 2013), not many are focused towards revealing the significant heterogeneity in the biological tissues (Magin et al., 2013; Liang et al., 2016).
Fluorescent transgenic mice as a tool to evaluate axonal morphology: Although current immuno-histochemical (IHC) techniques can be used to evaluate WM axons, this biochemical approach is limited in morphological details. Since their development, the use of YFP mice reporters have gained popularity due to its simplicity and practicality of imaging acquisition and analysis. To enhance the visualization of sole axonal structures, we applied novel in vivo genetic fluorescent imaging tools. Based on previous investigations, the use of endogenously expressed fluorescent genes in transgenic mouse models, such as yellow fluorescent proteins (YFP) has been our preferred approach to observe individual axonal changes in other neurodegenerative diseases (Gatto et al., 2015b). Using this live mosaic fluorescent expression driven by a neuron-specific gene promoter, axonal trajectories in the SC can be visualized from their origins in specific layers of the cerebral cortex to axonal populations within the WM (YFP, G93ASOD1 mice), retrieving detailed information of anomalies in the axonal organization and validate the microstructural changes (Figure 1b).
Biological and technical limitations in preclinical ALS studies: It is now recognized that the SOD1 model has many problems with regards to developing new therapies and so far almost all drugs that were successful in ALS mice models later failed in humans. Moreover, the transgenic G93A-SOD1 mouse has been used frequently for pre-clinical screening of ALS-therapies but needs to be analyzed with care in regards to later translation of therapies. From a technical standpoint, the application of MRI methods to examine the SC in vivo presents multiple challenges. Due to the relative small size of the SC, a higher spatial resolution is usually required to obtain proper signal and contrast-to-noise ratios. Other factors are known to affect the quality of the information gathered by DTI: (1) physiological motions (respiration, heart beats) create ghosting artifacts and partial volume effects due to the surrounding cerebrospinal fluid, (2) chemical shift artifacts arising from the epidural fat and other nearby structures, and (3) geometric distortions arising from magnetic field inhomogeneity in nearby intervertebral disks and lungs, among many others. In addition, in vivo studies require a short scanning time, which also limits the achievable resolution. In terms of histological preparation, it is also possible that the fixation and processing of the samples may alter the cellular structure and membrane of the nervous tissue leading to histological damage. However, one of the advantages of the preclinical models is the possibility to obtain littermates-control animals. Processing samples simultaneously from both animal groups, we can investigate the specific microstructural changes associated with the disease and exclude the influence of other unknown variables and histological artifacts. Above all, our approach has been dedicated to limit such shortcomings, including the examination of comparable histological regions by optical microscopy techniques with higher imaging and spatial resolutions.
Conclusions: Our current studies have demonstrated that MRI diffusion is an ideal imaging tool to explore the early microstructural WM anomalies in ALS. In combination with optical microscopy techniques, DTI enhances our knowledge of the microstructural anomalies during early stages of the disease. Interruption of axonal communications plays a key role in the functional decay seen in ALS patients. Thus, understanding of the microstructural changes is paramount to effectively detect and monitor disease progression. MRI diffusion information obtained by simple diffusion models in preclinical and clinical scenarios are still falling short in their ability to describe complex multicellular changes in ALS. Our future prospects include the design of new approaches to improve the detection of early microstructural WM changes in other preclinical mice models. Ultimately, it is our hope that the understanding and validation of this imaging technique will contribute towards the development of novel therapeutic strategies in neural regeneration research.
This work was provided by the Chicago Biomedical Consortium's Postdoctoral Research Award, No. 085740.
Footnotes
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer review reports:
Reviewer 1: Jan C. Koch, University Medicine Gottingen, Germany.
Comments to author: In the current manuscript, the authors summarize their recent findings on diffusion tensor imaging of axonal tracts in the ALS model mouse SOD1-G93A published in Brain Res, 2017.
Reviewer 2: Adonis Sfera, Patton State Hospital, USA.
Comments to author: This article reports original research in ALS on transgenic mice. The article describes MRI diffusion (DTI) in combination with optical microscopy as a tool for detecting early markers of ALS. As a clinician (psychiatrist) I congratulate the authors for trying to identify biological markers for this terrible condition.
Reviewer 3: Marcondes C. França Jr, Universidade Estadual de Campinas, Brazil.
Comments to author: This invited article addresses the potential role of DTI in the assessment of white matter damage in animal models of ALS. This is certainly a hot topic in the investigation of ALS with several (human and animal) studies exploring this kind of MRI technique for diagnostic or prognostic purposes. Nevertheless, very few reports tried to correlate DTI-based parameters with histological findings, especially at the spinal level (mostly due to technical limitations for image acquisition). Then, I believe that the current paper adds to this specific aspect of ALS-related literature.
References
- Caron I, Micotti E, Paladini A, Merlino G, Plebani L, Forloni G, Modo M, Bendotti C. Comparative magnetic resonance imaging and histopathological correlates in two SOD1 transgenic mouse models of amyotrophic lateral sclerosis. PLoS One. 2015;10:e0132159. doi: 10.1371/journal.pone.0132159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon-Perez LM, Spindler C, Goicochea S, Triplett W, Parekh M, Montie E, Carney PR, Price C, Mareci TH. Dimensionless, scale invariant, edge weight metric for the study of complex structural networks. PLoS One. 2015;10:e0131493. doi: 10.1371/journal.pone.0131493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto R, Chauhan M, Chauhan N. Anti-edema effects of rhEpo in experimental traumatic brain injury. Restor Neurol Neurosci. 2015a;33:927–941. doi: 10.3233/RNN-150577. [DOI] [PubMed] [Google Scholar]
- Gatto RG, Li W, Magin RL. Diffusion tensor imaging identifies presymptomatic axonal degeneration in the spinal cord of ALS mice. Brain Res. 2018;1679:45–52. doi: 10.1016/j.brainres.2017.11.017. [DOI] [PubMed] [Google Scholar]
- Gatto RG, Chu Y, Ye AQ, Price SD, Tavassoli E, Buenaventura A, Brady ST, Magin RL, Kordower JH, Morfini GA. Analysis of YFP(J16)-R6/2 reporter mice and postmortem brains reveals early pathology and increased vulnerability of callosal axons in Huntington’s disease. Hum Mol Genet. 2015b;24:5285–5298. doi: 10.1093/hmg/ddv248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Wu TH, Budde MD, Lee JM, Song SK. Noninvasive detection of brainstem and spinal cord axonal degeneration in an amyotrophic lateral sclerosis mouse model. NMR Biomed. 2011;24:163–169. doi: 10.1002/nbm.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolind S, Sharma R, Knight S, Johansen-Berg H, Talbot K, Turner MR. Myelin imaging in amyotrophic and primary lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:562–573. doi: 10.3109/21678421.2013.794843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Ye AQ, Chen W, Gatto RG, Colon-Perez L, Mareci TH, Magin RL. A fractal derivative model for the characterization of anomalous diffusion in magnetic resonance imaging. Commun Nonlinear Sci Numer Simul. 2016;39:529–537. [Google Scholar]
- Magin RL, Ingo C, Colon-Perez L, Triplett W, Mareci TH. Characterization of anomalous diffusion in porous biological tissues using fractional order derivatives and entropy. Microporous Mesoporous Mater. 2013;178:39–43. doi: 10.1016/j.micromeso.2013.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcuzzo S, Bonanno S, Figini M, Scotti A, Zucca I, Minati L, Riva N, Domi T, Fossaghi A, Quattrini A, Galbardi B, D’Alessandro S, Bruzzone MG, Garcia-Verdugo JM, Moreno-Manzano V, Mantegazza R, Bernasconi P. A longitudinal DTI and histological study of the spinal cord reveals early pathological alterations in G93A-SOD1 mouse model of amyotrophic lateral sclerosis. Exp Neurol. 2017;293:43–52. doi: 10.1016/j.expneurol.2017.03.018. [DOI] [PubMed] [Google Scholar]
- Morfini GA, Bosco DA, Brown H, Gatto R, Kaminska A, Song Y, Molla L, Baker L, Marangoni MN, Berth S, Tavassoli E, Bagnato C, Tiwari A, Hayward LJ, Pigino GF, Watterson DM, Huang CF, Banker G, Brown RH, Jr, Brady ST. Inhibition of fast axonal transport by pathogenic SOD1 involves activation of p38 MAP kinase. PLoS One. 2013;8:e65235. doi: 10.1371/journal.pone.0065235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood CK, Kurniawan ND, Butler TJ, Cowin GJ, Wallace RH. Non-invasive diffusion tensor imaging detects white matter degeneration in the spinal cord of a mouse model of amyotrophic lateral sclerosis. Neuroimage. 2011;55:455–461. doi: 10.1016/j.neuroimage.2010.12.044. [DOI] [PubMed] [Google Scholar]


