Keywords: nerve regeneration, acetyl-11-keto-β-boswellic acid, peripheral nerve injury repair, sciatic nerve crush injury, Schwann cells, cell proliferation, ERK signaling pathway, PD98059, neural regeneration
Abstract
Frankincense can promote blood circulation. Acetyl-11-keto-β-boswellic acid (AKBA) is a small molecule with anti-inflammatory properties that is derived from Boswellia serrata. Here, we hypothesized that it may promote regeneration of injured sciatic nerve. To address this hypothesis, we established a rat model of sciatic nerve injury using a nerve clamping method. Rats were administered AKBA once every 2 days at doses of 1.5, 3, and 6 mg/kg by intraperitoneal injection for 30 days from the 1st day after injury. Sciatic nerve function was evaluated using the sciatic functional index. Degree of muscle atrophy was measured using the triceps surae muscle Cuadros index. Neuropathological changes were observed by hematoxylin-eosin staining. Western blot analysis was used to detect expression of phospho-extracellular signal-regulated kinase 1 and 2 (p-ERK1/2) in injured nerve. S100 immunoreactivity in injured nerve was detected by immunohistochemistry. In vivo experiments showed that 3 and 6 mg/kg AKBA significantly increased sciatic nerve index, Cuadros index of triceps muscle, p-ERK1/2 expression, and S100 immunoreactivity in injured sciatic nerve of sciatic nerve injury model rats. Furthermore, for in vitro experiments, Schwann cells were treated with AKBA at 0–20 μg/mL. Proliferation of Schwann cells was detected by Cell Counting Kit-8 colorimetry assay. The results showed that 2 μg/mL AKBA is the optimal therapeutic concentration. In addition, ERK phosphorylation levels increased following 2 μg/mL AKBA treatment. In the presence of the ERK1/2 inhibitor, PD98059 (2.5 μL/mL), the AKBA-induced increase in p-ERK1/2 protein expression was partially abrogated. In conclusion, our study shows that AKBA promotes peripheral nerve regeneration with ERK protein phosphorylation playing a key role in this process.
Introduction
Peripheral nerve injury (PNI) is common in clinical practice. Following PNI, the distal portion of the affected nerve de-generates (Stoll et al., 2002; Yu et al., 2016), followed by regrowth of the proximal nerve approximately 1 week after the initial injury (Raff et al., 2002; Coleman and Freeman, 2010). Schwann cells (SCs) promote nerve regeneration after axonal injury (Webber and Zochodne, 2010) by supplying neurotrophic factors. These promote axonal growth and also produce neurite-promoting factors that guide the regenerating axon (Dong et al., 1999). SCs at the site of PNI dedifferentiate to a progenitor-like state, then proliferate and clear axonal and myelin debris (Napoli et al., 2012; Wong et al., 2017). SCs can then differentiate, resulting in formation of Büngner bands (Namgung, 2014). These are unique columnar structures formed between SCs and the basement membrane, which guide the regrowing axon (Thomas and King, 1974; Wang et al., 2013), thus enabling repair of damaged peripheral nerves.
Mitogen-activated protein kinases 1 and 2 (also known as extracellular signal-regulated kinase (ERK)1/2) are signaling proteins that play a pivotal role in the cellular signal transduction network (Kitamura et al., 2011; Mårtensson et al., 2017). The ERK signaling pathway regulates a variety of cellular processes such as cellular proliferation, migration, differentiation, and apoptosis (Sun et al., 2015). Previous research has shown that nerve injury promotes activation of ERK signaling in SCs, both at the injury site and throughout the distal stump (Sheu et al., 2000). Moreover, activation of ERK signaling is sufficient to induce dedifferentiation of myelinated SCs in vitro (Harrisingh et al., 2004). Similar to many other processes, ERK signaling plays an important role in generation, development, and function of neural progenitor cells (Jaeger et al., 2011).
Frankincense has the effect of promoting blood circulation and tissue regeneration (Xu et al., 2009). We have previously shown that frankincense extract has potent neuroprotective properties, including promotion of crush-injured sci-atic nerve regeneration and improvement in sciatic nerve function (Jiang et al., 2016). Acetyl-11-keto-β-boswellic acid (AKBA) is a small molecule with anti-inflammatory properties that is derived from Boswellia serrata, a plant native to India and Pakistan. AKBA is a white powdery solid that has a molecular weight of 512.72, and belongs to the tricyclic triterpenoid compounds (Bairwa and Jachak, 2015).
We hypothesized that AKBA stimulates repair of PNI via activation of the ERK signaling pathway. Accordingly, the purpose of our present study was to investigate the functional effect of AKBA on PNI, which we assessed using a rat PNI model in vivo and SC proliferation assay in vitro.
Materials and Methods
In vivo experiments
Animals
A total of 120 healthy Sprague-Dawley rats (specific pathogen-free level; male and female), with body weights of 200–250 g and aged 2 months, were purchased from Harbin Medical University, Harbin, Heilongjiang Province, China (license No. SCXK (Hei) 2013-0001). Rats were maintained under SPF laboratory conditions on 12-hour light/dark cy-cles with free access to food and water. All procedures used in this study were approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University, China (No. 10224). Rats were randomly divided into four groups: PNI (n = 30, PNI only), low-dose AKBA (n = 30, PNI + 1.5 mg/kg AKBA), medium-dose AKBA (n = 30, PNI + 35 mg/kg AKBA), and high-dose AKBA (n = 30, PNI + 6 mg/kg AKBA).
PNI animal model establishment
Rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (3.5 mL/kg of body weight), followed by shearing and disinfection of the skin prior to surgery. The sciatic nerve of the right leg was exposed and clamped three times for 10 seconds each using 2-mm-wide pincers (Shanghai Medical Devices (Group) Co., Ltd., Shanghai, China). Location of the distal portion of the injured nerve was marked by a 10-0 nylon microscopic suture. The incision was then closed in layers (muscle and skin) using absorbable sutures. All operations were performed on the right hind limb, while the left hind limb served as the non-operated control. All surgeries were completed by one person under aseptic conditions. The experimenter was blinded to treatment (Luís et al., 2008).
Drug intervention
All rats received intraperitoneal injection of AKBA (Shanghai Yuan-Ye BioTech Co., Ltd., Shanghai, China) dissolved in physiological saline solution to the appropriate concentration, with equivalent volumes of solution on the first day after surgery and every two days after that for a duration of 30 days. AKBA doses in the low-, medium-, and high-dose AKBA groups were 1.5, 3, and 6 mg/kg body weight, respectively. Rats in the PNI group were given an equivalent volume of physiological saline (4 mL/kg).
Sciatic functional index (SFI) measurement
Evaluation of SFI was performed at days 10, 20, and 30 after injury to determine the level of motor function following nerve injury (de Medinaceli et al., 1982). Rat hind limbs were dipped in water-soluble blue ink, and the rat allowed to walk down a track, leaving its hind footprints on the paper. A minimum of five complete clear footprints was required to calculate SFI, which was calculated as follows:
SFI = 109.5 (ETS – NTS) / NTS–38.3 (EPL – NPL) / NPL + 13.3 (EIT – NIT) / NIT – 8.8
Where PL is distance between the heel and third fingertip, TS is distance between the first toe and fifth finger, IT is distance between the second toe and fourth toe (Figure 1), E is sciatic nerve crush side, and N is normal side.
Figure 1.
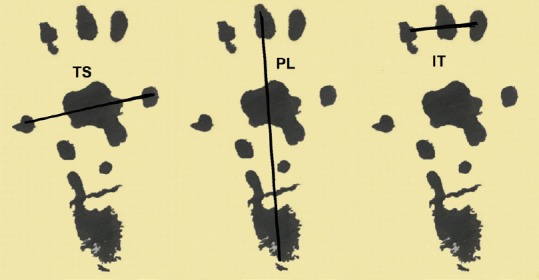
Rat toe measurement method.
TS: Distance between the first toe and fifth finger; PL: distance between the heel and third fingertip; IT: distance between the second toe and fourth toe.
The extent of functional recovery was assessed by calculating the ratio. SFI values of 0 are considered to indicate a healthy level of function, while −100 indicates the most severe level of injury.
Triceps surae muscle Cuadros index (TSCI) measurement
TSCI is a reliable method of evaluating the extent of neurological recovery, and reflects the degree of muscle atrophy (Cuadros and Granatir, 1987). The entire length of the triceps surae muscle of the right hind limb was excised at the point of the tendon on days 10, 20, and 30 following surgery, and weighed using an electronic balance (Sartorius Mechatronics T&H GmbH, Beijing, China). To quantify the extent of nerve injury, the ratio of triceps surae muscle weight to body weight was then calculated by the Cuadros method: calf triceps surae muscle mass / body mass × 100 (Sang et al., 2008), (Koryak, 1995).
Hematoxylin and eosin (H&E) staining
Crushed nerve samples (about 1 cm in length) were excised on day 30 after injury. Samples were fixed in 10% formaldehyde solution for > 72 hours, followed by step-by-step dehydration in ethyl alcohol solution before mounting on glass slides. Next, H&E staining was performed and samples observed using a light microscope (Beijing Ou Bo Tong Optical Technology Co., Ltd., Beijing, China).
Western blot assay
Protein was extracted from excised, injured nerves on days 10, 20, and 30 after injury. Levels of ERK1/2 and phospho-ERK1/2 (P-ERK1/2) were then quantified by western blot assay. Protein samples were separated using 10% sodium dodecyl sulfatepolyacrylamide gel electrophoresis, followed by electroblotting onto polyvinylidene fluoride (PVDF) membranes. Membranes were blocked by incubation in 5% nonfat milk solution for 2 hours. Membranes with attached transferred proteins were incubated with rabbit anti-ERK1/2 antibody (1:1,000; Cell Signaling Technology, Danvers, MA, USA) and rabbit anti-P-ERK1/2 antibody (1:1,000; Cell Signaling Technology) overnight at 4°C, followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:2,000; Cell Signaling Technology) for 2 hours at 37°C. Reaction products were visualized by chemiluminescence detection using an ECL Western Blotting Detection System (GE Healthcare, Piscataway, NJ, USA). Quantification of relative band densities was performed by scanning densitometry using ImageJ software (National Institute of Health, Bethesda, MD, USA). Relative expression of P-ERK 1/2 was expressed as an optical density value ratio of P-ERK 1/2 to ERK 1/2. A similar procedure was performed using mouse anti-lyceraldehyde-3-phosphate dehydrogenase (GAPDH) primary antibody (1:1,000, Cell Signaling Technology), with GAPDH protein used as an internal control.
Immunohistochemical analysis
S100 can be used as a biomarker of SC activation (Mata et al., 1990). Distal sciatic nerve segments were removed on day 30 after injury. Samples were embedded in paraffin sections followed by immunohistochemical staining with rabbit anti-S100 antibody (1:400, Wuhan Boster Biological Engineering Co., Ltd., Wuhan, China). The ratio of integrated optical density to area on each section was calculated using Image-Pro Plus 6.0 software (Media Cybernetics, Bethesda, MD, USA).
In vitro experiments
Proliferation assay
Cultured SCs at the fifth passage (purchased from the Chinese Academy of Sciences, Shanghai, China) were used for cytotoxicity assays. Cells were randomly divided into experimental and control groups. 1.0 × 105 quiescent SCs (100,000 per well) were grown in 96-well culture plates. Cells were incubated in Dulbecco’s Minimum Essential Medium (DMEM) supplemented with 10% fetal bovine serum. AKBA concentration in the culture medium of experimental groups was 1.25, 2.5, 5, 10, or 20 µg/mL. Cells in the control group were incubated with an equivalent volume of physiological saline solution containing no AKBA. SCs were incubated for 24 hours at 37°C in an atmosphere containing 5% CO2. Ten µL of Cell Counting Kit-8 (CCK-8) solution (5 mg/mL) was added to each well 4 hours prior to the cell viability assay. Next, the liquid was removed and 200 µL DMEM added. The absorbance level in each well was measured at a wave length of 490 nm using an enzyme-linked immunosorbent assay reader (Bio-Rad, Tokyo, Japan). For the proliferation assay, AKBA concentration in the culture medium of experimental groups was 1, 2, or 4 µg/mL, and was incubated for 12, 24, 36, or 48 hours. All other procedures were the same as those described for the cytotoxicity assay.
Expression of ERK1/2 and p-ERK1/2 in cultured SCs
SCs were cultured in AKBA (2 µg/mL) in 6-well plates for 0, 6, 12, or 24 hours. At each timepoint, cells were harvested on ice using a cell scraper, lysed in 1 mL radioimmunoprecipitation assay (RIPA) buffer containing 10 µL phenyl-methylsulfonyl fluoride and 10 µL Na3 VO4, incubated at 4°C for 30 minutes, and then centrifuged at 15,000 × g for 15 minutes. Supernatants were boiled and then mixed with sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer. Levels of p-ERK/ERK was determined by western blot assay.
PD98059 (Cell Signaling Technology) is regarded as an ERK signaling inhibitor that reduces ERK phosphorylation levels. The four experimental groups included a control group (without AKBA and PD98059), inhibitor group (2.5 μL/mL PD98059 only), drug-treated group (2 µg/mL AKBA only), and drug-inhibitor group (2 µg/mL AKBA + 2.5 μL/mL PD98059). Inhibitor was diluted in complete medium before being exposed to cells for 24 hours. Levels of p-ERK/ERK were determined by western blot assay, with cell viability assessed by CCK-8 colorimetry assay in all groups.
Statistical analysis
Mean values from different treatment groups were compared by t-test, which was calculated using GraphPad Prism 5.0 statistical analysis software (GraphPad Software, Inc., La Jolla, CA, USA). P values < 0.05 were considered to indicate statistically significant differences.
Results
In vivo experiments
Effect of AKBA on sciatic nerve function in the rat sciatic nerve injury model
Motor function was evaluated on days 10, 20, and 30 after sciatic nerve crush injury. Gradual recovery of sciatic nerve function was observed in rats from all groups. However, sciatic nerve function remained poor in the PNI group (Figure 2). Sciatic nerve function was significantly improved in rats in the high-dose AKBA group at days 20 and 30 compared with the PNI group (P < 0.01). Similarly, sciatic nerve function was significantly improved in rats in the medium-dose AKBA group compared with the PNI group (P < 0.05). However, no significant differences in sciatic nerve function were observed between rats in the low-dose AKBA group compared with PNI at any time point.
Figure 2.
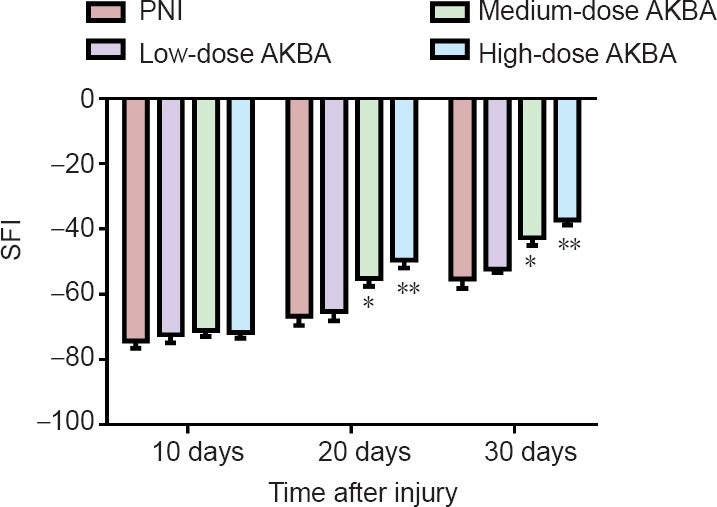
Effect of acetyl-11-keto-β-boswellic acid (AKBA) on sciatic functional index (SFI) in the rat sciatic nerve injury model.
Higher SFI represents greater sciatic function. Data were expressed as the mean ± SD and analyzed by t-test. *P < 0.05, ** P < 0.01, vs. PNI group. PNI group: Peripheral nerve injury (PNI) model only; low-dose AKBA group: PNI model + 1.5 mg/kg AKBA for 30 days; medium-dose AKBA group: PNI model + 3 mg/kg AKBA for 30 days; and high-dose AKBA group: PNI model + 6 mg/kg AKBA for 30 days. Sciatic nerve function was measured at 10, 20, and 30 days after surgery by SFI.
Effect of AKBA on TSCI in the rat sciatic nerve injury model
The effect of AKBA on sciatic nerve function was measured by TSCI on days 10, 20, and 30 after nerve injury (Figure 3). Significantly higher TSCI (indicating superior sciatic nerve function) was observed in the high-dose AKBA group at day 10 compared with the PNI group (P < 0.05). Furthermore, TSCI was significantly higher in the medium-dose and high-dose AKBA groups at days 20 and 30 after surgery compared with the PNI group (P < 0.05).
Figure 3.
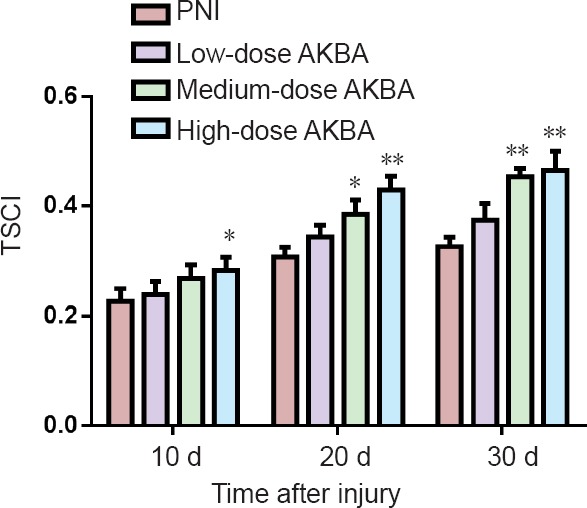
Effect of acetyl-11-keto-β-boswellic acid (AKBA) on triceps surae muscle Cuadros index (TSCI) in the rat sciatic nerve injury model.
Higher TSCI indicates superior sciatic nerve function. Data were expressed as the mean ± SD and analyzed by t-test. *P < 0.05, **P < 0.01, vs. PNI group. PNI group: Peripheral nerve injury (PNI) model only; low-dose AKBA group: PNI model + 1.5 mg/kg AKBA for 30 days; medium-dose AKBA group: PNI model + 3 mg/kg AKBA for 30 days; and high-dose AKBA group: PNI model + 6 mg/kg AKBA for 30 days. Sciatic nerve function was measured on days (d) 10, 20, and 30 after injury using TSCI.
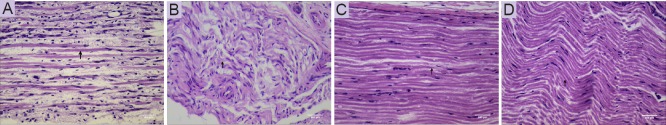
Effect of AKBA on pathological changes of injured sciatic nerve in the rat sciatic nerve injury model
Injured sciatic nerves were examined histologically by H&E staining of samples obtained at 30 days after surgery. Light microscopy showed that injured sciatic nerves were swollen with a deliquescent appearance and low density of nerve fibers in the PNI group (Figure 4A). Further, injured sciatic nerves from the low-dose AKBA group showed signs of segmental nerve fiber bending (Figure 4B). Samples from the medium-dose AKBA group revealed swollen and deli-quescent nerve fibers, with more nerve fibers compared with the PNI group (Figure 4C). Minor swelling of nerve fibers was observed in sciatic nerve samples from rats in the high-dose AKBA group (Figure 4D).
Figure 4.
Effect of acetyl-11-keto-β-boswellic acid (AKBA) on pathological changes of injured nerve in the rat sciatic nerve injury model at 30 days after surgery (hematoxylin & eosin staining).
(A) Peripheral nerve injury (PNI) group: PNI model only, injured sciatic nerves were swollen with a deliquescent appearance. (B) Low-dose AKBA group: PNI model + 1.5 mg/kg AKBA for 30 days, injured sciatic nerves revealed signs of segmental nerve fiber bending. (C) Medium-dose AKBA group: PNI model + 3 mg/kg AKBA for 30 days, injured sciatic nerves were swollen with deliquescent nerve fibers. (D) High-dose AKBA group: PNI model + 6 mg/kg AKBA for 30 days, minor swelling of nerve fibres was observed. Arrows indicate nerve fibres. Scale bars: 240 μm.
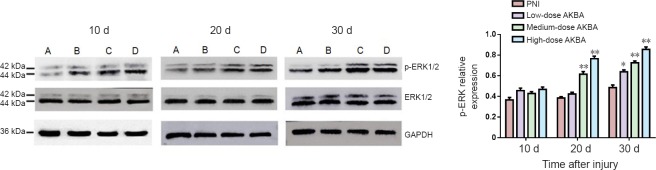
Effect of AKBA on p-ERK1/2 expression in injured sciatic nerve in the rat sciatic nerve damage model
Expression of ERK1/2 and p-ERK1/2 was investigated at days 10, 20, and 30 after injury. Expression of ERK1/2 was virtually unchanged at all time points in the injured nerve of rats from different groups (data not shown). Significant increases in p-ERK1/2 expression were observed in the medium-dose and high-dose AKBA groups compared with the PNI group at day 20 after surgery (P < 0.05). p-ERK1/2 expression was significantly higher in all AKBA treatment groups compared with the PNI group at 30 days after surgery (P < 0.05; Figure 5).
Figure 5.
Effect of acetyl-11-keto-β-boswellic acid (AKBA) on phospho-extracellular signal-regulated kinase 1 and 2 (p-ERK1/2) relative expression levels detected by western blot assay of injured nerve from the rat sciatic nerve injury model.
p-ERK1/2 expression was investigated in the rat sciatic nerve at 10, 20, and 30 days after surgery. Relative expression of p-ERK 1/2 was expressed by a ratio of the optical density values of P-ERK 1/2 to ERK 1/2. (A) PNI group: peripheral nerve injury (PNI) model only; (B) low-dose AKBA group: PNI model + 1.5 mg/kg AKBA for 30 days; (C) medium-dose AKBA group: PNI model + 3 mg/kg AKBA for 30 days; and (D) high-dose AKBA group: PNI model + 6 mg/kg AKBA for 30 days. Data were expressed as the mean ± SD and analyzed by t-test. *P < 0.05, **P < 0.01, vs. PNI group. d: Days.
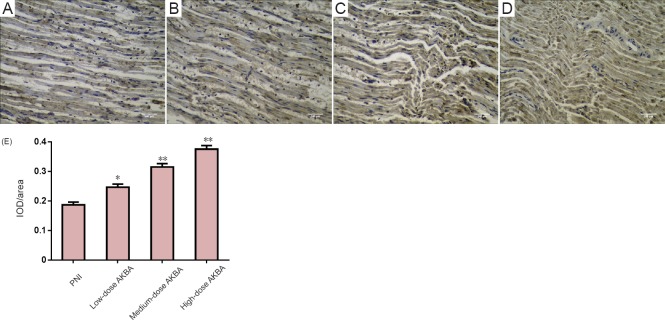
Effect of AKBA on S100 immunoreactivity in injured sciatic nerve in the rat sciatic nerve injury model
S100 immunoreactivity was detected in the injured sciatic nerve at day 30 after surgery (Figure 6). A significant increase was observed in S100 immunoreactivity in both the medium- and high-dose AKBA groups compared with the PNI group at 30 days after surgery (P < 0.05).
Figure 6.
Effect of acetyl-11-keto-β-boswellic acid (AKBA) on S100 immunohistochemistry in injured nerve from the rat sciatic nerve injury model at 30 days after surgery.
(A–D) S100 immunohistochemistry in rat sciatic nerve (original magnification, 400×). S100 immunoreactivity is brown. (A) PNI group: peripheral nerve injury (PNI) model only; (B) low-dose AKBA group: PNI model + 1.5 mg/kg AKBA for 30 days; (C) medium-dose AKBA group: PNI model + 3 mg/kg AKBA for 30 days; and (D) high-dose AKBA group: PNI model + 6 mg/kg AKBA for 30 days. Scale bars: 240 μm. (E) S100-positive cells in the PNI and AKBA groups. Data were expressed as the mean ± SD and analyzed by t-test. *P < 0.05, **P < 0.01, vs. PNI group.
In vitro experiments
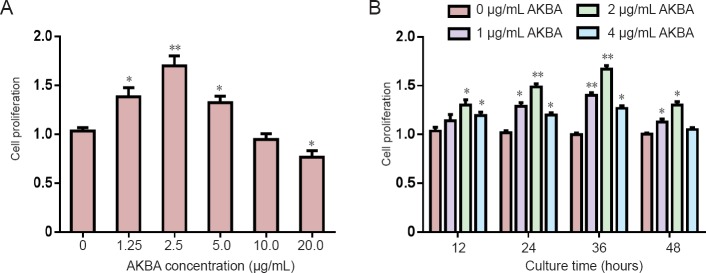
Effect of AKBA on cytotoxicity and proliferation of SCs
AKBA promoted SC proliferation in a concentration range of 1.25–5 µg/mL. However, SC proliferation was inhibited by AKBA at a concentration of > 5 µg/mL (Figure 7A). Overall, 2 µg/mL AKBA was considered the best treatment concentration (Figure 7B).
Figure 7.
Effect of acetyl-11-keto-β-boswellic acid (AKBA) on cytotoxicity and proliferation of cultured Schwann cells (Cell Counting Kit-8 colorimetry assay).
(A) Proliferation of Schwann cells during 24-hour incubation with AKBA. (B) Proliferation of Schwann cells at different time points. Cell proliferation was expressed as a ratio of optical density values of target group to control group (0 µg/mL). Data were expressed as the mean ± SD and analyzed by t-test. *P < 0.05, **P < 0.01, vs. control group (0 µg/mL).
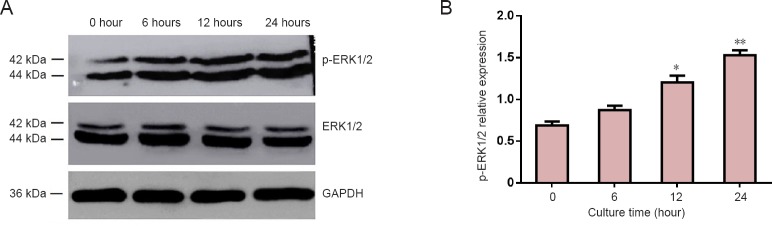
Effect of AKBA on p-ERK1/2 expression in SCs
At all time points, ERK1/2 expression was virtually unchanged in cultured SCs from different groups (Figure 8A). Significant increases in p-ERK1/2 expression were observed following 6, 12, or 24 hour-incubation with AKBA (2 µg/mL) compared with p-ERK1/2 expression at 0 hour (P < 0.05; Figure 8B). Expression of p-ERK1/2 was significantly en-hanced after AKBA treatment in a time-dependent manner.
Figure 8.
Effect of acetyl-11-keto-β-boswellic acid (AKBA) on relative phospho-extracellular signal-regulated kinase 1 and 2 (p-ERK1/2) expression in Schwann cells.
(A) Schwann cells were incubated with 2 µg/mL AKBA at 0, 6, 12, and 24 hours followed by western blot assay. (B) p-ERK1/2 expression was quantified at each time point. Relative expression of p-ERK1/2 was expressed by a ratio of optical density values of p-ERK1/2 to ERK1/2. Data were expressed as the mean ± SD and analyzed by t-test. *P < 0.05, **P < 0.01, vs. control group (0 hours).
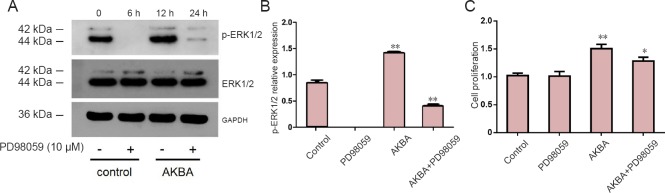
In the presence of the ERK1/2 inhibitor, PD98059, the AKBA-induced increase in p-ERK1/2 protein expression was partially abrogated (Figure 9A, B). Similarly, the AKBA-induced increase in cell proliferation was partially abrogated, but still significantly higher compared with the control group (Figure 9C).
Figure 9.
Effect of on acetyl-11-keto-β-boswellic acid (AKBA) or PD98059 on phospho-extracellular signal-regulated kinase 1 and 2 (p-ERK1/2) expression and cell proliferation of Schwann cells.
(A) p-ERK1/2 expression in Schwann cells treated without AKBA or PD98059 (control group), with ERK1/2 inhibitor PD98059 only, 2 µg/mL AKBA only, or 2 µg/mL AKBA + 2.5 μL/mL PD98059. (B) p-ERK1/2 expression was quantified from different groups by western blot assay. Relative p-ERK 1/2 expression was expressed by a ratio of optical density values of p-ERK1/2 to ERK1/2. (C) Quantitative results of cell proliferation detected by CCK-8 colorimetry assay. Cell proliferation was expressed as a ratio of optical density values of target group to control group (0 µg/mL). Data were expressed as the mean ± SD and analyzed by t-test. *P < 0.05, **P < 0.01, vs. control group.
Discussion
Our in vivo experiments show that the regenerative ability of injured peripheral nerve increases with increasing AKBA concentration. Thus, this effect of AKBA on injured peripheral nerve is dose-dependent, although needs further investigation. We also determined the effect of AKBA at a range of concentrations on SC growth in vitro. Our results show that AKBA at a concentration range of 1.25–5 μg/mL significantly promotes fibroblast proliferation, peaking at 2 μg/mL. However, AKBA inhibited cell proliferation when the concentration exceeded 20 µg/mL. Many research studies have demonstrated that natural plant extracts can accelerate cell proliferation, playing a positive role within an optimal concentration range (Li et al., 2001). However, negative effects using the same extracts are often observed when concentrations are too high. Studying the inhibitory effect of AKBA has shown that at a high concentration, AKBA is able to inhibit the inflammatory response via inhibition of the nuclear factor kappa B (NF-κB) signaling pathway (Takada et al., 2006), and is also protective against ischemia/reperfusion injury (Ding et al., 2014). Interestingly, in this study, we show that AKBA at an optimized concentration can activate the Ras/ERK signaling pathway, which increases SC proliferation. It is well known that SCs play a vital role in repair of PNI.
ERK1/2 is one of the most widely studied proteins, and its relationship with proliferation has been shown in many cell types (He et al., 2007; Zhao et al., 2010). Here, we show that AKBA treatment increases p-ERK1/2 expression in SCs. PD98059 inhibits either basal mitogen-activated protein kinase kinase 1 (MEK1) or partially activated MEK produced by mutation of serine at residues 218 and 222 to glutamate (MEK-2E). PD98059 does not inhibit the mitogen-activated protein kinase (MAPK) homologues, c-Jun N-terminal kinase (JNK) and P38. PD98059 is highly selective against MEK and does not inhibit the activity of many other kinase including Raf kinase, cyclic adenosine monophosphate-dependent kinase, protein kinase C, v-Src, epidermal growth factor (EGF) receptor kinase, insulin receptor kinase, platelet derived growth factor (PDGF) receptor kinase, and phosphatidylinositol 3-kinase. PD98059 inhibits PDGF-stimulated activation of MAPK (Dudley et al., 1995). SC proliferation is associated with ERK signaling through inhibition of ERK1/2 phosphorylation (Virdee and Tolkovsky, 1996). In fact, ERK1/2 expression is clearly observed in sciatic nerves undergoing repair (Mårtensson et al., 2007; Tsuda et al., 2011). Activation of p-ERK signaling has also been shown to promote proliferation of SCs (Tsuda et al., 2011). These findings are consistent with our results.
Our in vivo experiments show that AKBA significantly promotes peripheral nerve repair and SC proliferation after rat sciatic nerve injury. This is consistent with our in vitro experiments. Thus, we propose a hypothesis that increased p-ERK1/2 expression promotes cell proliferation while stimulating cell secreted active factors to promote repair of PNI. Although this study shows that AKBA promotes repair of PNI, the mechanisms involved remain unclear. In future studies, we will improve the rat sciatic nerve injury model (e.g., cutting the sciatic nerve) or use a different mode of administra-tion to further determine its mechanism.
Footnotes
Conflicts of interest: None declared.
Financial support: None.
Research ethics: All of the procedures used in this study were approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
(Copyedited by Yu J, Li CH, Song LP, Zhao M.)
References
- Bairwa K, Jachak SM. Development and optimisation of 3-Acetyl-11-keto-beta-boswellic acid loaded poly-lactic-co-glycolic acid-nanoparticles with enhanced oral bioavailability and in-vivo anti-inflammatory activity in rats. J Pharm Pharmacol. 2015;67:1188–1197. doi: 10.1111/jphp.12420. [DOI] [PubMed] [Google Scholar]
- Coleman MP, Freeman MR. Wallerian degeneration, wld(s), and nmnat. Annu Rev Neurosci. 2010;33:245–267. doi: 10.1146/annurev-neuro-060909-153248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadros CL, Granatir CE. Nerve regeneration through a synthetic microporous tube (expanded polytetrafluoroethylene): experimental study in the sciatic nerve of the rat. Microsurgery. 1987;8:41–47. doi: 10.1002/micr.1920080202. [DOI] [PubMed] [Google Scholar]
- de Medinaceli L, Freed WJ, Wyatt RJ. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol. 1982;77:634–643. doi: 10.1016/0014-4886(82)90234-5. [DOI] [PubMed] [Google Scholar]
- Ding Y, Chen M, Wang M, Wang M, Zhang T, Park J, Zhu Y, Guo C, Jia Y, Li Y, Wen A. Neuroprotection by acetyl-11-keto-beta-Boswellic acid, in ischemic brain injury involves the Nrf2/HO-1 defense pathway. Sci Rep. 2014;4:7002. doi: 10.1038/srep07002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Sinanan A, Parkinson D, Parmantier E, Mirsky R, Jessen KR. Schwann cell development in embryonic mouse nerves. J Neurosci Res. 1999;56:334–348. doi: 10.1002/(SICI)1097-4547(19990515)56:4<334::AID-JNR2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci U S A. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrisingh MC, Perez-Nadales E, Parkinson DB, Malcolm DS, Mudge AW, Lloyd AC. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. EMBO J. 2004;23:3061–3071. doi: 10.1038/sj.emboj.7600309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XQ, Chen R, Yang P, Li AP, Zhou JW, Liu QZ. Biphasic effect of arsenite on cell proliferation and apoptosis is associated with the activation of JNK and ERK1/2 in human embryo lung fibroblast cells. Toxicol Appl Pharmacol. 2007;220:18–24. doi: 10.1016/j.taap.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Jaeger I, Arber C, Risner-Janiczek JR, Kuechler J, Pritzsche D, Chen IC, Naveenan T, Ungless MA, Li M. Temporally controlled modulation of FGF/ERK signaling directs midbrain dopaminergic neural progenitor fate in mouse and human pluripotent stem cells. Development. 2011;138:4363–4374. doi: 10.1242/dev.066746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Ma J, Wei Q, Feng X, Qiao L, Liu L, Zhang B, Yu W. Effect of frankincense extract on nerve recovery in the rat sciatic nerve damage model. Evid Based Complement Alternat Med. 2016;2016:3617216. doi: 10.1155/2016/3617216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K, Fujiyoshi K, Yamane J, Toyota F, Hikishima K, Nomura T, Funakoshi H, Nakamura T, Aoki M, Toyama Y, Okano H, Nakamura M. Human hepatocyte growth factor promotes functional recovery in primates after spinal cord injury. PLoS One. 2011;6:e27706. doi: 10.1371/journal.pone.0027706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koryak Y. Contractile properties of the human triceps surae muscle during simulated weightlessness. Eur J Appl Physiol Occup Physiol. 1995;70:344–350. doi: 10.1007/BF00865032. [DOI] [PubMed] [Google Scholar]
- Li F, Wang D, Jiang Z, Gao X, Zhao H. Activity stimulating osteoblast-like cells proliferation of some traditional Chinese medicinal herbs and other plants. Pharm Biol. 2001;39:351–356. [Google Scholar]
- Luís AL, Rodrigues JM, Geuna S, Amado S, Simões MJ, Fregnan F, Ferreira AJ, Veloso AP, Armada-da-Silva PA, Varejão AS, Maurício AC. Neural cell transplantation effects on sciatic nerve regeneration after a standardized crush injury in the rat. Microsurgery. 2008;28:458–470. doi: 10.1002/micr.20524. [DOI] [PubMed] [Google Scholar]
- Mårtensson L, Gustavsson P, Dahlin LB, Kanje M. Activation of extracellular-signal-regulated kinase-1/2 precedes and is required for injury-induced Schwann cell proliferation. Neuroreport. 2007;18:957–961. doi: 10.1097/WNR.0b013e32819f8f27. [DOI] [PubMed] [Google Scholar]
- Mårtensson LB, Blom CL, Dahlin LB. Ca2+ involvement in activation of extracellular-signal-regulated-kinase 1/2 and m-calpain after axotomy of the sciatic nerve. Neural Regen Res. 2017;12:623–628. doi: 10.4103/1673-5374.205103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata M, Alessi D, Fink DJ. S100 is preferentially distributed in myelin-forming Schwann cells. J Neurocytol. 1990;19:432–442. doi: 10.1007/BF01188409. [DOI] [PubMed] [Google Scholar]
- Namgung U. The role of Schwann cell-axon interaction in peripheral nerve regeneration. Cells Tissues Organs. 2014;200:6–12. doi: 10.1159/000370324. [DOI] [PubMed] [Google Scholar]
- Napoli I, Noon LA, Ribeiro S, Kerai AP, Parrinello S, Rosenberg LH, Collins MJ, Harrisingh MC, White IJ, Woodhoo A, Lloyd AC. A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron. 2012;73:729–742. doi: 10.1016/j.neuron.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- Sang QL, Wei Z, Xu ZM, Liu B, Li M, Yin WT. Effect of Astragalus polysaccharides on sciatic nerve regeneration in rats. Shizhen Guoyi Guoyao. 2008;19:851–853. [Google Scholar]
- Sheu JY, Kulhanek DJ, Eckenstein FP. Differential patterns of ERK and STAT3 phosphorylation after sciatic nerve transection in the rat. Exp Neurol. 2000;166:392–402. doi: 10.1006/exnr.2000.7508. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S, Myers RR. Degeneration and regeneration of the peripheral nervous system: from Augustus Waller’s observations to neuroinflammation. J Peripher Nerv Syst. 2002;7:13–27. doi: 10.1046/j.1529-8027.2002.02002.x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35:600–604. doi: 10.3109/10799893.2015.1030412. [DOI] [PubMed] [Google Scholar]
- Takada Y, Ichikawa H, Badmaev V, Aggarwal BB. Acetyl-11-keto-beta-boswellic acid potentiates apoptosis, inhibits invasion, and abolishes osteoclastogenesis by suppressing NF-kappa B and NF-kappa B-regulated gene expression. J Immunol. 2006;176:3127–3140. doi: 10.4049/jimmunol.176.5.3127. [DOI] [PubMed] [Google Scholar]
- Thomas PK, King RH. The degeneration of unmyelinated axons following nerve section: an ultrastructural study. J Neurocytol. 1974;3:497–512. doi: 10.1007/BF01098736. [DOI] [PubMed] [Google Scholar]
- Tsuda Y, Kanje M, Dahlin LB. Axonal outgrowth is associated with increased ERK 1/2 activation but decreased caspase 3 linked cell death in Schwann cells after immediate nerve repair in rats. BMC Neurosci. 2011;12:12. doi: 10.1186/1471-2202-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virdee K, Tolkovsky AM. Inhibition of p42 and p44 mitogen-activated protein kinase activity by PD98059 does not suppress nerve growth factor-induced survival of sympathetic neurones. J Neurochem. 1996;67:1801–1805. doi: 10.1046/j.1471-4159.1996.67051801.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhang P, Kou Y, Yin X, Han N, Jiang B. Hedysari extract improves regeneration after peripheral nerve injury by enhancing the amplification effect. PLoS One. 2013;8:e67921. doi: 10.1371/journal.pone.0067921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber C, Zochodne D. The nerve regenerative microenvironment: early behavior and partnership of axons and Schwann cells. Exp Neurol. 2010;223:51–59. doi: 10.1016/j.expneurol.2009.05.037. [DOI] [PubMed] [Google Scholar]
- Wong KM, Babetto E, Beirowski B. Axon degeneration: make the Schwann cell great again. Neural Regen Res. 2017;12:518–524. doi: 10.4103/1673-5374.205000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu RS, Zong XH, Li XG. Controlled clinical trials of therapeutic effects of Chinese herbs promoting blood circulation and removing blood stasis on the treatment of reflex sympathetic dystrophy with type of stagnation of vital energy and blood stasis. Zhongguo Gu Shang. 2009;22:920–922. [PubMed] [Google Scholar]
- Yu J, Gu X, Yi S. Ingenuity pathway analysis of gene expression profiles in distal nerve stump following nerve injury: insights into Wallerian degeneration. Front Cell Neurosci. 2016;10:274. doi: 10.3389/fncel.2016.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Yang XH. Effects of JAK2-dependent ERK signal pathway on erythropoietin-induced functional activation of bone marrow-derived endothelial progenitor cells. Zhongguo Zuzhi Gongcheng Yanjiu. 2010;14:9203–9207. [Google Scholar]