Abstract
Background
Studies comparing the anastomotic leak rate in patients with an intrathoracic versus a cervical anastomosis after oesophagectomy are equivocal. The aim of this study was to compare clinical outcome after oesophagectomy in patients with an intrathoracic or cervical anastomosis, and to identify predictors of anastomotic leakage in a nationwide audit.
Methods
Between January 2011 and December 2015, all consecutive patients who underwent oesophagectomy for cancer were identified from the Dutch Upper Gastrointestinal Cancer Audit. For the comparison between an intrathoracic and cervical anastomosis, propensity score matching was used to adjust for potential confounders. Multivariable logistic regression modelling with backward stepwise selection was used to determine independent predictors of anastomotic leakage.
Results
Some 3348 patients were included. After propensity score matching, 654 patients were included in both the cervical and intrathoracic anastomosis groups. An intrathoracic anastomosis was associated with a lower leak rate than a cervical anastomosis (17·0 versus 21·9 per cent; P = 0·025). The percentage of patients with recurrent nerve paresis was also lower (0·6 versus 7·0 per cent; P < 0·001) and an intrathoracic anastomosis was associated with a shorter median hospital stay (12 versus 14 days; P = 0·001). Multivariable analysis revealed that ASA fitness grade III or higher, chronic obstructive pulmonary disease, cardiac arrhythmia, diabetes mellitus and proximal oesophageal tumours were independent predictors of anastomotic leakage.
Conclusion
An intrathoracic oesophagogastric anastomosis was associated with a lower anastomotic leak rate, lower rate of recurrent nerve paresis and a shorter hospital stay. Risk factors for anastomotic leak were co‐morbidities and proximal tumours.
Short abstract
Lower leak rates after intrathoracic anastomosis
Introduction
Oesophageal cancer is the sixth leading cause of cancer‐related mortality, and its incidence continues to increase every year1. According to international guidelines2, oesophagectomy is the cornerstone of curative treatment for non‐metastasized oesophageal cancer, often combined with neoadjuvant or perioperative chemo(radio)therapy. Improvement in surgical techniques, perioperative management and patient selection have resulted in a reduction in postoperative mortality after oesophagectomy3. However, anastomotic leakage remains relatively common, and is a major cause of morbidity and mortality. The percentage of patients with anastomotic leakage varies from 6 to 41 per cent4, 5, 6, 7, 8.
Several factors are associated with an increased risk of anastomotic leakage, including patient‐related characteristics5, 9, 10, intraoperative factors11, 12, 13, postoperative factors and surgical technique14, 15, 16. Controversy remains about the optimal anatomical location of the oesophagogastric anastomosis (intrathoracic versus cervical) after oesophagectomy. Several retrospective studies and one RCT reported increased leak rates in patients with a cervical anastomosis9, 17, 18. Other studies, including three RCTs19, 20, 21, did not show a statistically significant difference in leak rates. Some surgeons accept a possible higher leak rate associated with a cervical anastomosis, because a wider oncological resection margin can be achieved. Furthermore, in patients with an anastomotic leak, the sequela may be less severe for a cervical anastomosis than for an intrathoracic anastomosis22. Others advocate that an intrathoracic anastomosis is associated with a lower leak rate because the gastric tube is shorter and better vascularized9.
The scientific evidence for an association between the location of the anastomosis and risk of anastomotic leakage, postoperative morbidity and positive oncological resection margins after oesophagectomy is equivocal. Therefore, the primary aim of this study was to assess anastomotic leakage rates, postoperative morbidity and radical resection rates after oesophageal resection with either an intrathoracic or cervical oesophagogastric anastomosis. A secondary objective was to identify predictors of anastomotic leakage.
Methods
All patient data were obtained from the Dutch Upper Gastrointestinal Cancer Audit (DUCA), a registry of all patients undergoing surgery with curative intent for oesophageal or gastric cancer in the Netherlands. The DUCA is a subdivision of the Dutch Institute for Clinical Auditing, founded in 2011, with the objective to facilitate and organize the initiation of nationwide auditing in a uniform format. The DUCA collects data to monitor national guideline adherence and to provide surgical teams with reliable information on outcome measures. Participation is mandatory for all Dutch hospitals performing oesophageal resections, and data are registered for each patient during the hospital stay and until 30 days after discharge. Detailed descriptions of definitions used in the DUCA are provided in an online registry program to stimulate uniform data registration. An independent monitoring team audits the data to evaluate completeness and concordance. The organization of the DUCA has been described in more detail previously23.
Patients
All patients undergoing oesophagectomy for oesophageal cancer with gastric tube reconstruction between January 2011 and December 2015 were included. For the comparison between intrathoracic and cervical anastomoses, transhiatal resections were excluded because an intrathoracic anastomosis was never performed during this approach. All proximal tumours were also excluded because a cervical anastomosis is constructed in patients with a proximal tumour. To assess factors associated with anastomotic leakage, all patients were studied.
Treatment
Surgical treatment consisted of an open (both abdomen and chest), hybrid (abdomen minimally invasive and open chest) or totally minimally invasive transthoracic oesophagectomy followed by gastric tube construction with a cervical or intrathoracic anastomosis. Details of anastomotic techniques (stapled versus handsewn, end‐to‐side versus side‐to‐side) are not specified in the DUCA. Patients received neoadjuvant treatment according to national guidelines.
Outcome measures
Patient and treatment‐related characteristics were extracted from the DUCA. Histopathological, surgical and short‐term oncological outcomes were analysed. Surgical outcome parameters included: clinical or radiological anastomotic leakage, pulmonary complications, recurrent nerve paresis, surgical reintervention under general anaesthesia, duration of hospital stay, duration of ICU stay, mortality within 30 days after surgery and/or in‐hospital death, readmissions within 30 days after discharge, positive resection margin and number of retrieved lymph nodes.
Statistical analysis
Patient and treatment‐related characteristics are described as count with percentages, mean(s.d.) or median (range), as appropriate. Missing values were encountered in 217 patients for eight variables; the percentage of missing values per variable was limited (range 0·0 to 4·9 per variable). It was not possible to recover missing data because patient and hospital identity are concealed in the DUCA. Missing data were considered at random and handled using imputation with the iterative Markov chain Monte Carlo method (5 iterations)24.
To account for the effect of possible confounders on outcomes, propensity score matching was performed for the analysis of intrathoracic versus cervical anastomosis. First, propensity scores (the probability, ranging from 0 to 1, that a patient was assigned to an intrathoracic or cervical anastomosis) were derived using a logistic regression model, which included all patient and treatment‐related characteristics presented in Table 1. One‐to‐one propensity score matching was performed with nearest‐neighbour matching without replacement, using a calliper width of 0·25 multiplied by the standard deviation of the estimated propensity score25. Balance in measured patient and treatment‐related characteristics of the matched cohort was assessed using standardized mean differences, with differences of less than 10 per cent and close to 0 per cent taken to indicate good balance26. To evaluate the significance of differences between the two treatment groups, the χ2 test was used for categorical variables, and the Student's t test and Mann–Whitney U test for continuous variables with a normal and skewed distribution respectively. Logistic regression analysis was used to stratify by type of surgery (open versus total minimally invasive approach) in the propensity‐matched cohort by adding an interaction term between surgical approach and anastomotic location for each outcome. For this analysis, hybrid procedures were added to the minimally invasive group.
Table 1.
Patient and treatment‐related characteristics according to location of the anastomosis, before and after propensity score matching
| Before matching (n = 2086) | SMD (%) | After matching (n = 1308) | SMD (%) | ||||
|---|---|---|---|---|---|---|---|
| Intrathoracic anastomosis (n = 928) | Cervical anastomosis (n = 1158) | Intrathoracic anastomosis (n = 654) | Cervical anastomosis (n = 654) | ||||
| Age (years)* | 64·1(9·0) | 63·9(8·6) | 2·7 | 64·1(8·9) | 64·0(8·3) | 2·7 | |
| Sex | M | 779 (83·9) | 835 (72·1) | 32·2 | 526 (80·4) | 533 (81·5) | 2·9 |
| F | 149 (16·1) | 323 (27·9) | 128 (19·6) | 121 (18·5) | |||
| BMI (kg/m2)* | 26·2(4·2) | 25·4(4·2) | 21·0 | 26·0(4·2) | 26·2(4·2) | 1·2 | |
| ASA fitness grade | I | 150 (16·2) | 215 (18·6) | 9·0 | 110 (16·8) | 109 (16·7) | 5·0 |
| II | 571 (61·5) | 726 (62·7) | 423 (64·7) | 408 (62·4) | |||
| III | 207 (22·3) | 212 (18·3) | 121 (18·5) | 134 (20·5) | |||
| IV | 0 (0) | 5 (0·4) | 0 (0) | 3 (0·5) | |||
| COPD | No | 807 (87·0) | 1021 (88·2) | 3·6 | 574 (87·8) | 568 (86·9) | 2·7 |
| Yes | 121 (13·0) | 137 (11·8) | 80 (12·2) | 86 (13·1) | |||
| Coronary artery disease | No | 830 (89·4) | 1063 (91·8) | 7·7 | 593 (90·7) | 597 (91·3) | 2·0 |
| Yes | 98 (10·6) | 95 (8·2) | 61 (9·3) | 57 (8·7) | |||
| History of myocardial infarction | No | 861 (92·8) | 1102 (95·2) | 9·2 | 616 (94·2) | 614 (93·9) | 1·2 |
| Yes | 67 (7·2) | 56 (4·8) | 38 (5·8) | 40 (6·1) | |||
| History of arrhythmia | No | 844 (90·9) | 1072 (92·6) | 5·7 | 598 (91·4) | 598 (91·4) | 0·0 |
| Yes | 84 (9·1) | 86 (7·4) | 56 (8·6) | 56 (8·6) | |||
| Hypertension | No | 643 (69·3) | 793 (68·5) | 1·8 | 449 (68·7) | 449 (68·7) | 0·0 |
| Yes | 285 (30·7) | 365 (31·5) | 205 (31·3) | 205 (31·3) | |||
| Peripheral vascular disease | No | 891 (96·0) | 1126 (97·2) | 6·3 | 634 (96·9) | 634 (96·9) | 0·0 |
| Yes | 37 (4·0) | 32 (2·8) | 20 (3·1) | 20 (3·1) | |||
| Diabetes mellitus | No | 780 (84·1) | 1010 (87·2) | 8·6 | 553 (84·6) | 561 (85·8) | 3·3 |
| Yes | 148 (15·9) | 148 (12·8) | 101 (15·4) | 93 (14·2) | |||
| History of stroke | No | 872 (94·0) | 1102 (95·2) | 5·0 | 618 (94·5) | 615 (94·0) | 1·9 |
| Yes | 56 (6·0) | 56 (4·8) | 36 (5·5) | 39 (6·0) | |||
| Thromboembolic events | No | 887 (95·6) | 1120 (96·7) | 5·5 | 632 (96·6) | 631 (96·5) | 0·7 |
| Yes | 41 (4·4) | 38 (3·3) | 22 (3·4) | 23 (3·5) | |||
| Endocrine disorder | No | 895 (96·4) | 1113 (96·1) | 1·8 | 630 (96·3) | 628 (96·0) | 1·7 |
| Yes | 33 (3·6) | 45 (3·9) | 24 (3·7) | 26 (4·0) | |||
| Previous abdominal or thoracic surgery | No | 657 (70·8) | 822 (71·0) | 0·4 | 470 (71·9) | 475 (72·6) | 1·7 |
| Yes | 271 (29·2) | 336 (29·0) | 184 (28·1) | 179 (27·4) | |||
| Histology | ADC | 814 (87·7) | 723 (62·4) | 77·0 | 545 (83·3) | 533 (81·5) | 5·6 |
| SCC | 96 (10·3) | 392 (33·9) | 92 (14·1) | 104 (15·9) | |||
| Other | 18 (1·9) | 43 (3·7) | 17 (2·6) | 17 (2·6) | |||
| Tumour location† | Middle | 42 (4·5) | 319 (27·5) | 110·7 | 42 (6·4) | 47 (7·2) | 3·7 |
| Distal | 886 (95·5) | 839 (72·5) | 612 (93·6) | 607 (92·8) | |||
| cT category | T1 | 49 (5·3) | 64 (5·5) | 6·4 | 35 (5·4) | 42 (6·4) | 0·3 |
| T2 | 190 (20·5) | 216 (18·7) | 125 (19·1) | 117 (17·9) | |||
| T3 | 665 (71·7) | 819 (70·7) | 474 (72·5) | 470 (71·9) | |||
| T4 | 24 (2·6) | 59 (5·1) | 20 (3·1) | 25 (3·8) | |||
| cN category | N0 | 364 (39·2) | 370 (32·0) | 18·9 | 230 (35·2) | 242 (37·0) | 0·4 |
| N1 | 388 (41·8) | 503 (43·4) | 283 (43·3) | 268 (41·0) | |||
| N2 | 156 (16·8) | 238 (20·6) | 125 (19·1) | 117 (17·9) | |||
| N3 | 20 (2·2) | 47 (4·1) | 16 (2·4) | 27 (4·1) | |||
| Neoadjuvant therapy | No | 79 (8·5) | 95 (8·2) | 0·4 | 50 (7·6) | 54 (8·3) | 1·3 |
| nCT | 52 (5·6) | 75 (6·5) | 49 (7·5) | 46 (7·0) | |||
| nCRT | 797 (85·9) | 988 (85·3) | 555 (84·9) | 554 (84·7) | |||
| Type of surgery | Open | 199 (21·4) | 355 (30·7) | 22·4 | 161 (24·6) | 170 (26·0) | 3·4 |
| MI | 716 (77·2) | 791 (68·3) | 487 (74·5) | 479 (73·2) | |||
| Hybrid | 13 (1·4) | 12 (1·0) | 6 (0·9) | 5 (0·8) | |||
| Year of surgery | 2015 | 328 (35·3) | 225 (19·4) | 61·7 | 174 (26·6) | 164 (25·1) | 1·9 |
| 2014 | 248 (26·7) | 234 (20·2) | 157 (24·0) | 178 (27·2) | |||
| 2013 | 190 (20·5) | 212 (18·3) | 164 (25·1) | 135 (20·6) | |||
| 2012 | 108 (11·6) | 279 (24·1) | 105 (16·1) | 125 (19·1) | |||
| 2011 | 54 (5·8) | 208 (18·0) | 54 (8·3) | 52 (8·0) | |||
Values in parentheses are percentages unless indicated otherwise;
values are mean(s.d.). Patients with proximal tumours and those who underwent a transhiatal resection were excluded from the analysis. All variables presented in Table 1 were used for propensity matching.
Middle indicates 24–32 cm from teeth, and distal more than 32 cm from teeth. SMD, standardized mean difference; COPD, chronic obstructive pulmonary disease; ADC, adenocarcinoma; SCC, squamous cell carcinoma; nCT, neoadjuvant chemotherapy; nCRT, neoadjuvant chemoradiotherapy; MI, minimally invasive.
The potential association between preoperative patient characteristics and anastomotic leakage was evaluated using univariable analyses in all patients (also including proximal tumours and patients who underwent a transhiatal resection). Variables with P < 0·250 in univariable analysis were entered into a multivariable logistic regression model with backward stepwise selection to determine independent predictors of anastomotic leakage. Multivariable Poisson regression with log link and robust error variance of the final model was used to determine relative risk (RR) estimates with 95 per cent confidence intervals.
Statistical analyses were undertaken using SPSS® version 23.0 (IBM, Armonk, New York, USA), and R 3.1.2 open‐source software with MatchIt and optmatch, sandwich, lmtest and Mice packages (http://www.r-project.org). P < 0·050 was considered statistically significant.
Results
Of 3348 patients selected for the study, 2086 were included in the comparison between an intrathoracic anastomosis (928) and a cervical anastomosis (1158) (Fig. 1). Patients were predominantly men (77·4 per cent), and the mean(s.d.) age was 64·6(9·0) years. The percentage of patients with an intrathoracic anastomosis increased during the study interval from 20·6 per cent in 2011 to 59·3 per cent in 2015. Patient and treatment‐related characteristics according to location of the anastomosis are shown in Table 1. After propensity matching, 654 patients were included in both groups and all baseline variables including year of surgery were equally distributed (SMD less than 10 per cent).
Figure 1.
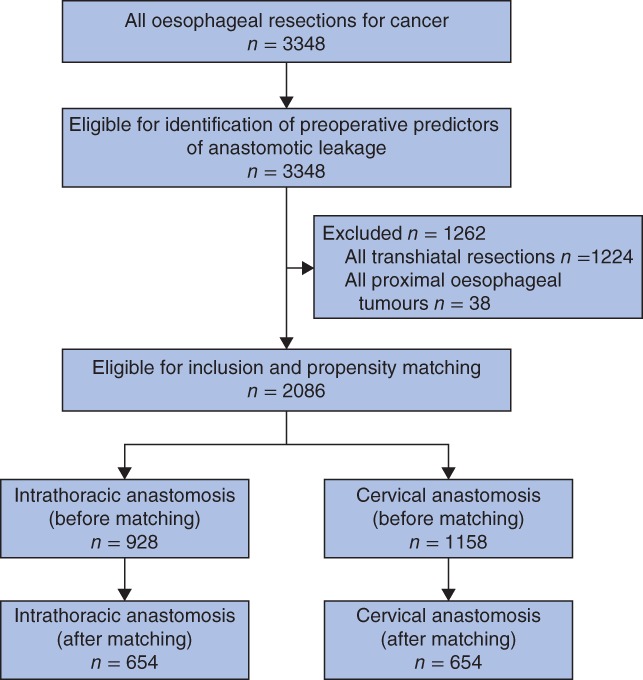
Study flow chart
Intrathoracic versus cervical anastomoses
Postoperative complications and pathological data are shown in Table 2. Anastomotic leakage was less frequent in patients who underwent an intrathoracic anastomosis than in those with a cervical anastomosis: 111 of 654 (17·0 per cent) versus 143 of 654 (21·9 per cent) respectively (P = 0·025). Recurrent nerve paresis occurred less often in patients with an intrathoracic anastomosis: 4 of 654 (0·6 per cent) versus 46 of 654 (7·0 per cent) respectively (P < 0·001). The median duration of hospital stay was shorter in patients with an intrathoracic anastomosis: 12 (range 3–145) versus 14 (4–386) days (P < 0·001). Surgical reinterventions, duration of ICU stay, in‐hospital mortality and number of readmissions were comparable between the two groups. The associations between location of the anastomosis and outcome parameters were not statistically significant when stratified by type of surgical approach (P for interaction > 0·050) (Table 2).
Table 2.
Outcome after oesophagectomy according to location of the anastomosis, before and after propensity score matching
| Before matching (n = 2086) | P ‡‡ | After matching (n = 1308) | P ‡‡ | P for interactionby type of surgery¶¶ | |||
|---|---|---|---|---|---|---|---|
| Intrathoracic anastomosis (n = 928) | Cervical anastomosis (n = 1158) | Intrathoracic anastomosis (n = 654) | Cervical anastomosis (n = 654) | ||||
| Anastomotic leakage† | 0·230 | 0·025 | 0·523 | ||||
| No | 756 (81·5) | 919 (79·4) | 543 (83·0) | 511 (78·1) | |||
| Yes | 172 (18·5) | 239 (20·6) | 111 (17·0) | 143 (21·9) | |||
| Pulmonary complications‡ | 0·688 | 0·408 | 0·267 | ||||
| No | 598 (64·4) | 756 (65·3) | 421 (64·4) | 432 (66·1) | |||
| Yes | 330 (35·6) | 402 (34·7) | 233 (35·6) | 222 (33·9) | |||
| Recurrent nerve paresis§ | < 0·001 | < 0·001 | 0·086 | ||||
| No | 924 (99·6) | 1063 (91·8) | 650 (99·4) | 608 (93·0) | |||
| Yes | 4 (0·4) | 95 (8·2) | 4 (0·6) | 46 (7·0) | |||
| Surgical reintervention¶ | 0·340 | 0·444 | 0·055 | ||||
| No | 783 (84·4) | 959 (82·8) | 558 (85·3) | 548 (83·8) | |||
| Yes | 145 (15·6) | 199 (17·2) | 96 (14·7) | 106 (16·2) | |||
| Duration of hospital stay (days)* | 12 (3–172) | 14 (3–386) | < 0·001§§ | 12 (3–145) | 14 (4–386) | < 0·001§§ | 0·427 |
| Duration of ICU stay (days)* | 2 (0–125) | 2 (0–155) | 0·024§§ | 2 (0–125) | 2 (0–155) | 0·123§§ | 0·493 |
| Postoperative death# | 0·749 | 0·458 | 0·061 | ||||
| No | 889 (95·8) | 1106 (95·5) | 633 (96·8) | 628 (96·0) | |||
| Yes | 39 (4·2) | 52 (4·5) | 21 (3·2) | 26 (4·0) | |||
| Readmission** | 0·991 | ||||||
| No | 794 (85·6) | 991 (85·6) | 556 (85·0) | 547 (83·6) | 0·494 | 0·674 | |
| Yes | 134 (14·4) | 167 (14·4) | 98 (15·0) | 107 (16·4) | |||
| Positive resection margin†† | 0·497 | 0·618 | 0·780 | ||||
| No | 877 (94·5) | 1102 (95·2) | 618 (94·5) | 622 (95·1) | |||
| Yes | 51 (5·5) | 56 (4·8) | 36 (5·5) | 32 (4·9) | |||
| No. of lymph nodes harvested | 0·752 | 0·223 | 0·070 | ||||
| < 20 | 444 (47·8) | 546 (47·2) | 356 (54·4) | 334 (51·1) | |||
| ≥ 20 | 484 (52·2) | 612 (52·8) | 298 (45·6) | 320 (48·9) | |||
Values in parentheses are percentages unless indicated otherwise;
values are median (range).
Any clinically or radiologically proven anastomotic leakage.
Clinically proven pneumonia, pleural effusion leading to drainage, pleural empyema, acute respiratory distress syndrome or reintubation.
Any vocal cord dysfunction after resection.
Any postoperative surgical reintervention under general anesthesia.
Death during initial hospital admission or within 30 days after surgery.
Readmission to hospital within 30 days after initial discharge.
The resection margin was evaluated using the College of American Pathologists criteria27.
χ2 test, except
Mann–Whitney U test.
Stratified analysis for type of surgery (open approach versus minimally invasive approach).
Among patients with an anastomotic leak, there was no significant difference between the anastomosis groups in the percentage of patients who had a surgical reintervention (53·2 per cent of patients with an intrathoracic anastomosis versus 44·8 per cent with a cervical anastomosis; P = 0·184) or in‐hospital mortality (8·1 versus 10·5 per cent respectively; P = 0·520). Duration of hospital stay (median 40 (range 9–132) versus 28 (4–132) days; P < 0·001) and length of ICU stay (median 8 (1–111) versus 4 (1–155) days; P = 0·021) were longer after an intrathoracic compared with a cervical anastomotic leak.
Predictors of anastomotic leakage
Some 656 of 3348 patients (19·6 per cent) had an anastomotic leak (Table 3, Fig. 1). The median duration of hospital stay was 26 (range 3–200) days in patients with anastomotic leakage compared with 11 (1–386) days in patients without an anastomotic leak (P < 0·001). Mortality rates were 9·1 and 2·7 per cent respectively (P = 0·001).
Table 3.
Characteristics of 3348 patients with oesophageal cancer according to anastomotic leakage
| No anastomotic leakage (n = 2692)† | Anastomotic leakage (n = 656)† | P ¶ | Missing data‡ | ||
|---|---|---|---|---|---|
| Age (years)* | 64·4(9·1) | 65·4(8·5) | 0·014# | 11 (0·3) | |
| Sex | M | 2087 (77·5) | 519 (79·1) | 0·379 | 1 (0·0) |
| F | 605 (22·5) | 137 (20·9) | |||
| BMI (kg/m2)* | 25·9(4·4) | 26·1(4·3) | 30 (0·9) | ||
| ASA fitness grade | I | 487 (18·1) | 90 (13·7) | < 0·001 | 23 (0·7) |
| II | 1656 (61·5) | 369 (56·3) | |||
| III | 541 (20·1) | 191 (29·1) | |||
| IV | 8 (0·3) | 6 (0·9) | |||
| COPD | No | 2348 (87·2) | 540 (82·3) | 0·001 | 0 (0) |
| Yes | 344 (12·8) | 116 (17·7) | |||
| Coronary artery disease | No | 2440 (90·6) | 576 (87·8) | 0·029 | 0 (0) |
| Yes | 252 (9·4) | 80 (12·2) | |||
| History of myocardial infarction | No | 2522 (93·7) | 597 (91·0) | 0·015 | 0 (0) |
| Yes | 170 (6·3) | 59 (9·0) | |||
| History of arrhythmia | No | 2490 (92·5) | 582 (88·7) | 0·002 | 0 (0) |
| Yes | 202 (7·5) | 74 (11·3) | |||
| Hypertension | No | 1840 (68·4) | 417 (63·6) | 0·019 | 0 (0) |
| Yes | 852 (31·6) | 239 (36·4) | |||
| Peripheral vascular disease | No | 2593 (96·3) | 626 (95·4) | 0·285 | 0 (0) |
| Yes | 99 (3·7) | 30 (4·6) | |||
| Diabetes mellitus | No | 2302 (85·5) | 524 (79·9) | < 0·001 | 0 (0) |
| Yes | 390 (14·5) | 132 (20·1) | |||
| History of stroke | No | 2535 (94·2) | 609 (92·8) | 0·201 | 0 (0) |
| Yes | 157 (5·8) | 47 (7·2) | |||
| Thromboembolic events | No | 2578 (95·8) | 630 (96·0) | 0·756 | 0 (0) |
| Yes | 114 (4·2) | 26 (4·0) | |||
| Endocrine disorder | No | 2604 (96·7) | 626 (95·4) | 0·104 | 0 (0) |
| Yes | 88 (3·3) | 30 (4·6) | |||
| Previous abdominal or thoracic surgery | No | 1881 (69·9) | 457 (69·7) | 0·917 | 0 (0) |
| Yes | 811 (30·1) | 199 (30·3) | |||
| Histology | ADC | 2095 (77·8) | 505 (77·0) | 0·849 | 20 (0·6) |
| SCC | 528 (19·6) | 135 (20·6) | |||
| Other | 69 (2·6) | 16 (2·4) | |||
| Tumour location§ | Proximal | 28 (1·0) | 16 (2·4) | 0·015 | 23 (0·7) |
| Middle | 312 (11·6) | 81 (12·3) | |||
| Distal | 2352 (87·4) | 559 (85·2) | |||
| cT category | T1 | 154 (5·7) | 41 (6·3) | 0·568 | 163 (4·9) |
| T2 | 543 (20·2) | 126 (19·2) | |||
| T3 | 1896 (70·4) | 458 (69·8) | |||
| T4 | 99 (3·7) | 31 (4·7) | |||
| cN category | N0 | 986 (36·6) | 234 (35·7) | 0·811 | 116 (3·5) |
| N1 | 1131 (42·0) | 287 (43·8) | |||
| N2 | 492 (18·3) | 118 (18·0) | |||
| N3 | 83 (3·1) | 17 (2·6) | |||
| Neoadjuvant therapy | No | 260 (9·7) | 73 (11·1) | 0·062 | 0 (0) |
| nCT | 205 (7·6) | 34 (5·2) | |||
| nCRT | 2227 (82·7) | 549 (83·7) | |||
Values in parentheses are percentages unless indicated otherwise;
values are mean(s.d.).
Data set after imputation.
Data missing for each variable before imputation.
Proximal indicates less than 24 cm from teeth, middle indicates 24–32 cm from teeth, and distal more than 32 cm from teeth. COPD, chronic obstructive pulmonary disease; ADC, adenocarcinoma; SCC, squamous cell carcinoma; nCT, neoadjuvant chemotherapy; nCRT, neoadjuvant chemoradiotherapy.
χ2 test, except #Student's t test.
Univariable analysis revealed that anastomotic leakage was associated with several patient‐related factors including age, ASA fitness grade, tumour location and co‐morbidities (Table 3). Tumour histology, TNM stage and neoadjuvant therapy did not differ between the groups with or without an anastomotic leak.
Independent predictors of the development of anastomotic leakage included: an ASA grade of III (RR 1·31, 95 per cent c.i. 1·09 to 1·78; P = 0·009) or IV (RR 1·98, 1·27 to 3·64; P = 0·026), history of chronic obstructive pulmonary disease (COPD) (RR 1·21, 1·02 to 1·45; P = 0·031), history of cardiac arrhythmia (RR 1·25, 1·01 to 1·55, P = 0·044), diabetes mellitus (RR 1·26, 1·06 to 1·49; P = 0·009) and tumour of the proximal oesophagus (RR 1·86, 1·25 to 2·77; P = 0·022) (Table 4).
Table 4.
Results of multivariable logistic regression analysis assessing preoperative risk of developing anastomotic leakage after oesophagectomy in 3348 patients with oesophageal cancer
| Relative risk | P | |
|---|---|---|
| ASA fitness grade | ||
| I | 1·00 (reference) | |
| II | 0·99 (0·77, 1·30) | 0·990 |
| III | 1·31 (1·09, 1·78) | 0·009 |
| IV | 1·98 (1·27, 3·64) | 0·026 |
| COPD | 1·21 (1·02, 1·45) | 0·031 |
| History of arrhythmia | 1·25 (1·01, 1·55) | 0·044 |
| Diabetes mellitus | 1·26 (1·06, 1·49) | 0·009 |
| Tumour location | ||
| Proximal | 1·86 (1·25, 2·77) | 0·022 |
| Middle | 1·07 (0·87, 1·32) | 0·514 |
| Distal | 1·00 (reference) |
Values in parentheses are 95 per cent confidence intervals. COPD, chronic obstructive pulmonary disease.
Discussion
This nationwide multicentre cohort study compared clinical outcome in patients with an intrathoracic versus cervical anastomosis following oesophagectomy. An intrathoracic anastomosis was associated with lower rates of anastomotic leak and recurrent nerve paresis. This may explain the observed shorter duration of hospital stay among patients with an intrathoracic anastomosis than for those with a cervical anastomosis. However, among patients with an anastomotic leak, ICU and hospital stay was longer in the group with an intrathoracic anastomosis. Independent risk factors for anastomotic leakage include an ASA fitness grade of III or IV, history of cardiac arrhythmia, COPD, diabetes mellitus and proximally located tumours.
Four RCTs18, 19, 20, 21 have investigated clinical outcome in patients with an intrathoracic or cervical anastomosis. The results of these studies are equivocal regarding which anastomotic technique is preferred in reducing the risk of anastomotic leakage. The conflicting results can be explained by these studies being underpowered, with few events (range 2–13 per study), resulting in uncertain estimates. Other methodological shortcomings are the large degree of variation in surgical approaches, definitions of anastomotic leakage, and variation in stapled and hand‐sutured anastomosis. Two meta‐analyses15, 28, including 298 patients, found that anastomotic leakage occurred less often after an intrathoracic anastomosis than a cervical anastomosis. The present results are in line with these analyses.
Although the anastomotic leak rate was lower in patients with an intrathoracic anastomosis, and this may appear the preferred location of the oesophagogastric anastomosis, leak rates in the present study are high compared with those in other studies5, 9. Leak rates after an intrathoracic and cervical anastomosis range from 9 to 21 per cent9, 17, 29, 30 and 8 to 35 per cent9, 17, 29, 31 respectively. Some studies included only clinically relevant or radiologically proven anastomotic leaks, whereas others included both32. This discrepancy in definitions makes it difficult to compare leak rates between studies. In the present study, the definition of anastomotic leakage remained the same throughout the study, and included (subtle) clinical and radiological signs of leakage.
Between 2011 and 2016, some centres moved from a cervical to an intrathoracic anastomosis; 20·6 per cent of anastomoses were intrathoracic in 2011 and 59·3 per cent in 2015. The introduction of an intrathoracic anastomosis is associated with a learning curve33, 34. Furthermore, the proportion of minimally invasive procedures increased from 53·1 to 85·4 per cent during the study period. The introduction of minimally invasive oesophagectomy is also associated with a learning curve when looking at reinterventions, morbidity and mortality35, 36. Although there were no differences in year of surgery and type of surgery between the groups after propensity score matching, a potential learning curve may explain the high leak rate in the present study.
Despite a reduced risk of leakage and shorter hospital stay after an intrathoracic anastomosis, some surgeons prefer a cervical anastomosis. The possibility of a wider resection margin and less severe complications in patients with an anastomotic leak are claimed benefits of a cervical anastomosis37. The present study demonstrated that an anastomotic leak in a patient with an intrathoracic anastomosis led to a longer intensive care and hospital stay. This suggests that the clinical course in patients with an intrathoracic anastomotic leak is indeed more severe38. There were no differences in R0 resection rates, surgical reinterventions and postoperative mortality between intrathoracic and cervical anastomoses. The safety of the intrathoracic technique is supported by a recent meta‐analysis28 that found no difference in in‐hospital mortality between intrathoracic and cervical anastomoses.
Previous studies9, 15, 28, 39 have defined factors associated with anastomotic leakage after an intrathoracic anastomosis. Factors resulting in poor tissue perfusion and vascular impairment are considered important5, 9, 10, 40. This is in accordance with the present findings, as COPD, diabetes mellitus, ASA grades of III and IV, and proximal tumours were identified as independent risk factors for the development of anastomotic leakage. Although the present study did not identify risk factors other than those already described in the literature, these findings suggest that it may be important to improve the preoperative physical status of high‐risk patients before oesophagectomy.
Strengths of this study include the population‐based design, the adjustment for important confounders, and the relatively large sample size. Furthermore, data from the DUCA are collected prospectively, and controlled for completeness and validity by an independent monitoring team. There are also limitations to this study, including its retrospective design and lack of randomization. Although propensity score matching was performed, the inability of propensity score matching to adjust for unknown confounders (such as surgical decision‐making) is a limitation. Details of anastomotic techniques that may influence the healing of the anastomosis are not recorded in the DUCA. In addition, centre‐ and surgeon‐specific data on leak rates were not available for the purpose of this study, although these data are available for the individual centres. At present, a randomized trial41 comparing the intrathoracic and cervical approach is under way that should resolve these limitations and make an important contribution to the current literature.
Acknowledgements
J.A.H.G. and L.G. contributed equally to this work. The authors thank all surgeons, registrars, physician assistants and administrative nurses for data registration in the DUCA database, as well as the DUCA group for scientific input. This paper reports the results of a preregistered study with complete analysis plans (https://www.dica.nl/duca/onderzoek). The authors certify that the results of all preregistered analyses are reported. Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the DUCA at onderzoek@dica.nl.
Disclosure: The authors declare no conflict of interest.
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) – Esophageal and Esophagogastric Junction Cancers Version 3; 2017. http://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf [accessed 21 September 2017]. [Google Scholar]
- 3. Lagarde SM, de Boer JD, ten Kate FJ, Busch OR, Obertop H, van Lanschot JJ. Postoperative complications after esophagectomy for adenocarcinoma of the esophagus are related to timing of death due to recurrence. Ann Surg 2008; 247: 71–76. [DOI] [PubMed] [Google Scholar]
- 4. Parekh K, Iannettoni MD. Complications of esophageal resection and reconstruction. Semin Thorac Cardiovasc Surg 2007; 19: 79–88. [DOI] [PubMed] [Google Scholar]
- 5. Gronnier C, Tréchot B, Duhamel A, Mabrut J‐Y, Bail J‐P, Carrere N et al Impact of neoadjuvant chemoradiotherapy on postoperative outcomes after esophageal cancer resection: results of a European multicenter study. Ann Surg 2014; 260: 761–764. [DOI] [PubMed] [Google Scholar]
- 6. Hulscher JB, van Sandick JW, de Boer AG, Wijnhoven BP, Tijssen JG, Fockens P et al Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002; 347: 1662–1669. [DOI] [PubMed] [Google Scholar]
- 7. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP et al Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366: 2074–2084. [DOI] [PubMed] [Google Scholar]
- 8. Kumagai K, Rouvelas I, Tsai JA, Mariosa D, Klevebro F, Lindblad M et al Meta‐analysis of postoperative morbidity and perioperative mortality in patients receiving neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal and gastro‐oesophageal junctional cancers. Br J Surg 2014; 101: 321–338. [DOI] [PubMed] [Google Scholar]
- 9. Kassis ES, Kosinski AS, Ross PJ, Koppes KE, Donahue JM, Daniel VC. Predictors of anastomotic leak after esophagectomy: an analysis of the Society of Thoracic Surgeons General Thoracic Database. Ann Thorac Surg 2013; 96: 1919–1926. [DOI] [PubMed] [Google Scholar]
- 10. Goense L, van Rossum PS, Weijs TJ, van Det MJ, Nieuwenhuijzen GA, Luyer MD et al Aortic calcification increases the risk of anastomotic leakage after lvor‐Lewis esophagectomy. Ann Thorac Surg 2016; 102: 247–252. [DOI] [PubMed] [Google Scholar]
- 11. Goense L, van Rossum PSN, Tromp M, Joore HC, van Dijk D, Kroese AC et al Intraoperative and postoperative risk factors for anastomotic leakage and pneumonia after esophagectomy for cancer. Dis Esophagus 2016; 30: 1–10. [DOI] [PubMed] [Google Scholar]
- 12. Michelet P, D'Journo X‐B, Roch A, Papazian L, Ragni J, Thomas P et al Perioperative risk factors for anastomotic leakage after esophagectomy: influence of thoracic epidural analgesia. Chest 2005; 128: 3461–3466. [DOI] [PubMed] [Google Scholar]
- 13. Kusano C, Baba M, Takao S, Sane S, Shimada M, Shirao K et al Oxygen delivery as a factor in the development of fatal postoperative complications after oesophagectomy. Br J Surg 1997; 84: 252–257. [PubMed] [Google Scholar]
- 14. Haverkamp L, van der Sluis PC, Verhage RJJ, Siersema PD, Ruurda JP, van Hillegersberg R. End‐to‐end cervical esophagogastric anastomoses are associated with a higher number of strictures compared with end‐to‐side anastomoses. J Gastrointest Surg 2013; 17: 872–876. [DOI] [PubMed] [Google Scholar]
- 15. Markar SR, Arya S, Karthikesalingam A, Hanna GB. Technical factors that affect anastomotic integrity following esophagectomy: systematic review and meta‐analysis. Ann Surg Oncol 2013; 20: 4274–4281. [DOI] [PubMed] [Google Scholar]
- 16. Markar SR, Karthikesalingam A, Vyas S, Hashemi M, Winslet M. Hand‐sewn versus stapled oesophago‐gastric anastomosis: systematic review and meta‐analysis. J Gastrointest Surg 2011; 15: 876–884. [DOI] [PubMed] [Google Scholar]
- 17. Klink CD, Binnebösel M, Otto J, Boehm G, von Trotha KT, Hilgers R‐D et al Intrathoracic versus cervical anastomosis after resection of esophageal cancer: a matched pair analysis of 72 patients in a single center study. World J Surg Oncol 2012; 10: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chasseray VM, Kiroff GK, Buard JL, Launois B. Cervical or thoracic anastomosis for esophagectomy for carcinoma. Surg Gynecol Obstet 1989; 169: 55–62. [PubMed] [Google Scholar]
- 19. Walther B, Johansson J, Johnsson F, Von Holstein CS, Zilling T. Cervical or thoracic anastomosis after esophageal resection and gastric tube reconstruction: a prospective randomized trial comparing sutured neck anastomosis with stapled intrathoracic anastomosis. Ann Surg 2003; 238: 803–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Okuyama M, Motoyama S, Suzuki H, Saito R, Maruyama K, Ogawa J‐I. Hand‐sewn cervical anastomosis versus stapled intrathoracic anastomosis after esophagectomy for middle or lower thoracic esophageal cancer: a prospective randomized controlled study. Surg Today 2007; 37: 947–952. [DOI] [PubMed] [Google Scholar]
- 21. Ribet M, Debrueres B, Lecomte‐Houcke M. Resection for advanced cancer of the thoracic esophagus: cervical or thoracic anastomosis? Late results of a prospective randomized study. J Thorac Cardiovasc Surg 1992; 103: 784–789. [PubMed] [Google Scholar]
- 22. Urschel JD. Esophagogastrostomy anastomotic leaks complicating esophagectomy: a review. Am J Surg 1995; 169: 634–640. [DOI] [PubMed] [Google Scholar]
- 23. Busweiler LAD, Wijnhoven BPL, van Berge Henegouwen MI, Henneman D, van Grieken NCT, Wouters MWJM et al Early outcomes from the Dutch Upper Gastrointestinal Cancer Audit. Br J Surg 2016; 103: 1855–1863. [DOI] [PubMed] [Google Scholar]
- 24. Sterne JAC, White IR, Carlin JB, Spratt M, Royston P, Kenward MG et al Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009; 338: 2393–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Austin PC. Optimal caliper widths for propensity‐score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011; 10: 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Austin PC. Assessing balance in measured baseline covariates when using many‐to‐one matching on the propensity‐score. Pharmacoepidemiol Drug Saf 2008; 17: 1218–1225. [DOI] [PubMed] [Google Scholar]
- 27. College of American Pathologists . Protocol for the Examination of Specimens From Patients With Carcinoma of the Esophagus, Version 3.2.0.0. http://www.cap.org/cancerprotocols [accessed 8 October 2017]. [Google Scholar]
- 28. Biere SSAY, Maas KW, Cuesta MA, van der Peet DL. Cervical or thoracic anastomosis after esophagectomy for cancer: a systematic review and meta‐analysis. Dig Surg 2011; 28: 29–35. [DOI] [PubMed] [Google Scholar]
- 29. Zhai C, Liu Y, Li W, Xu T, Yang G, Lu H et al A comparison of short‐term outcomes between Ivor‐Lewis and McKeown minimally invasive esophagectomy. J Thorac Dis 2015; 7: 2352–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Workum F, van der Maas J, van den Wildenberg FJH, Polat F, Kouwenhoven EA, van Det MJ et al Improved functional results after minimally invasive esophagectomy: intrathoracic versus cervical anastomosis. Ann Thorac Surg 2017; 103: 267–273. [DOI] [PubMed] [Google Scholar]
- 31. Saluja SS, Ray S, Pal S, Sanyal S, Agrawal N, Dash NR et al Randomized trial comparing side‐to‐side stapled and hand‐sewn esophagogastric anastomosis in neck. J Gastrointest Surg 2012; 16: 1287–1295. [DOI] [PubMed] [Google Scholar]
- 32. Bruce J, Krukowski ZH, Al‐Khairy G, Russell EM, Park KGM. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Br J Surg 2001; 88: 1157–1168. [DOI] [PubMed] [Google Scholar]
- 33. Mungo B, Lidor AO, Stem M, Molena D. Early experience and lessons learned in a new minimally invasive esophagectomy program. Surg Endosc 2016; 30: 1692–1698. [DOI] [PubMed] [Google Scholar]
- 34. van Workum F, van den Wildenberg FJH, Polat F, de Wilt JHW, Rosman C. Minimally invasive oesophagectomy: preliminary results after introduction of an intrathoracic anastomosis. Dig Surg 2014; 31: 95–103. [DOI] [PubMed] [Google Scholar]
- 35. Mackenzie H, Markar SR, Askari A, Ni M, Faiz O, Hanna GB. National proficiency‐gain curves for minimally invasive gastrointestinal cancer surgery. Br J Surg 2016; 103: 88–96. [DOI] [PubMed] [Google Scholar]
- 36. Sihag S, Kosinski AS, Gaissert HA, Wright CD, Schipper PH. Minimally invasive versus open esophagectomy for esophageal cancer: a comparison of early surgical outcomes from the Society of Thoracic Surgeons National Database. Ann Thorac Surg 2016; 101: 1281–1289. [DOI] [PubMed] [Google Scholar]
- 37. Blewett CJ, Miller JD, Young JE, Bennett WF, Urschel JD. Anastomotic leaks after esophagectomy for esophageal cancer: a comparison of thoracic and cervical anastomoses. Ann Thorac Cardiovasc Surg 2001; 7: 75–78. [PubMed] [Google Scholar]
- 38. van Rossum PSN, Haverkamp L, Carvello M, Ruurda JP, van Hillegersberg R. Management and outcome of cervical versus intrathoracic manifestation of cervical anastomotic leakage after transthoracic esophagectomy for cancer. Dis Esophagus 2017; 30: 1–8. [DOI] [PubMed] [Google Scholar]
- 39. Wiggins T, Markar SR, Arya S, Hanna GB. Anastomotic reinforcement with omentoplasty following gastrointestinal anastomosis: a systematic review and meta‐analysis. Surg Oncol 2015; 24: 181–186. [DOI] [PubMed] [Google Scholar]
- 40. van Rossum PSN, Haverkamp L, Verkooijen HM, van Leeuwen MS, van Hillegersberg R, Ruurda JP. Calcification of arteries supplying the gastric tube: a new risk factor for anastomotic leakage after esophageal surgery. Radiology 2015; 274: 124–132. [DOI] [PubMed] [Google Scholar]
- 41. van Workum F, Bouwense SAW, Luyer MDP, Nieuwenhuijzen GAP, van der Peet DL, Daams F et al Intrathoracic versus Cervical ANastomosis after minimally invasive esophagectomy for esophageal cancer: study protocol of the ICAN randomized controlled trial. Trials 2016; 17: 505. [DOI] [PMC free article] [PubMed] [Google Scholar]


