Abstract
To improve health services' quantity and quality, African countries are increasingly engaging in performance‐based financing (PBF) interventions. Studies to understand their implementation in francophone West Africa are rare. This study analysed PBF implementation in Burkina Faso 12 months post‐launch in late 2014.
The design was a multiple and contrasted case study involving 18 cases (health centres). Empirical data were collected from observations, informal (n = 224) and formal (n = 459) interviews, and documents.
Outside the circle of persons trained in PBF, few in the community had knowledge of it. In some health centres, the fact that staff were receiving bonuses was intentionally not announced to populations and community leaders. Most local actors thought PBF was just another project, but the majority appreciated it. There were significant delays in setting up agencies for performance monitoring, auditing, and contracting, as well as in the payment. The first audits led rapidly to coping strategies among health workers and occasionally to some staging beforehand. No community‐based audits had yet been done. Distribution of bonuses varied from one centre to another.
This study shows the importance of understanding the implementation of public health interventions in Africa and of uncovering coping strategies.
Keywords: Burkina Faso, implementation, multiple‐case study, performance‐based financing, process evaluation
1. INTRODUCTION
Over the past several years in Africa, many countries have embarked on performance‐based financing (PBF) interventions. While the theoretical foundations of the approach are still under debate,1 it involves linking the funding of health centres and the payment of bonuses to health personnel with their achievement of indicators of quantity and often quality of care that have been defined beforehand.2 These indicators are monitored by independent actors. Scientific evidence for the effectiveness or efficiency of PBF remains limited in both the North3 and the South.4, 5 Studies on its implementation are still very rare in francophone West Africa,6 although some are beginning to emerge elsewhere in Africa.7, 8, 9, 10, 11 These studies have variable methods and results, but all confirm the existence of a knowledge gap regarding PBF implementation in Africa. Some even point to a trend in the PBF literature of a “well‐known publication bias in favour of success stories.”9 Nevertheless, it is essential that we enter into the “black box” of PBF and gain a deeper understanding of the functioning of this complex intervention.9, 12, 13 Studies that are not funded by the agencies organizing PBF are still very rare in Africa,4 as are studies on the implementation of such interventions.14, 15
In Burkina Faso, following a prepilot project in 3 districts,16 the Ministry of Health and the World Bank deployed a PBF intervention in 12 additional districts in January 2014. Burkina Faso decided to innovate by including strategies aimed at improving equity and supporting demand for services. Accordingly, 10 districts organized, in certain randomly selected health centres, a community‐based process for selecting indigents to be exempted from service fees, while 2 other districts organized a community‐based health insurance plan associated with this exemption. Thus, all patients continue to pay point‐of‐service user fees, except for indigents who have been exempted and pregnant women, whose deliveries were subsidized by the State beginning in 2006 and have been free since 2016. With the support of World Bank consultants, an implementation guide for the intervention, with over 300 pages, was drafted and is available.17
A central unit, called the PBF Technical Service (ST‐FBR—Service technique—financement basé sur les résultats), was created to support the implementation of PBF. Its role is to develop and monitor indicators, supervise audits, organize training workshops, and analyse performance data. Contracting and auditing agencies (ACVs—Agence de contractualisation et de vérification) were set up to audit health centres' quantitative results, support health workers in using PBF tools, enter data, and submit the health centres' invoices for verification. The ACVs conduct monthly quantitative audits to count the numbers of targeted services provided (approximately 23 indicators). Quality audits are conducted quarterly on several quality dimensions, such as the conditions for patient intake, availability of supplies, document maintenance, drug management, and financial management (approximately 140 indicators). The latter requires collaboration with members of health centre management committees (COGESs—Comité de gestion) because of their involvement in managing the centres' accounts. Various tools (eg, performance improvement plan and rating tool) are in place to support planning in relation to activities and finances. Quantity indicators are purchased for a fixed price, while payments for quality indicators depends on achieving a minimal target.
To conduct an impact evaluation funded by the World Bank, the PBF project was based on a randomized controlled trial of primary health centres (CSPSs—Centres de santé et de promotion sociale). Four randomization categories were defined: (1) PBF1: The health centre is paid fixed prices for activity indicators achieved, but these prices may fluctuate over the course of the project; (2) PBF2: PBF1 coupled with a community‐based selection of indigents to be exempted from fees for services, which are purchased at a moderate price; (3) PBF3: PBF2, with a higher purchase price for indigents, aimed at encouraging innovative initiatives to increase their use of services; and (4) PBF4: PBF1 linked with a community‐based health insurance programme coupled with a community‐based indigent selection process. This last PBF component has been implemented only in one district.
For all 4 modalities, an “equity bonus” was put in place between the districts and health centres. Thus, the purchase prices vary considerably among the health centres, to take into account particularly their distance from the district's main city and the working conditions (resource needs). For example, the price for a curative consultation for someone over the age of 5 years will range from 100 to 180 CFA francs (approximately 0.15 to 0.27 euros) within the CSPSs of a same district. Details are available in the implementation guide.17
The implementation fidelity analysis for the PBF project, conducted 12 months after its launch, showed that, in general, the important and essential elements of the intervention theory were implemented.18 However, we noted some implementation gaps and delays in terms of performance audits and payment of PBF subsidies, which to some extent compromised the theoretical effectiveness of the incentive rationale. While this fidelity analysis is essential, it is not sufficient on its own for understanding the intervention's functioning in all its subtleties.15 We therefore conducted a qualitative analysis of PBF implementation in Burkina Faso.
2. METHODS
This study fits within the streams of interventional research19 and evaluation based on intervention theory20 recommended for PBF analysis. We also drew from the conceptual approaches of the study of public policy implementation.15, 21, 22, 23 The PBF intervention theory was described in detail in the published protocol24 and guided our data collection and analysis, as recommended for policy analysis in low‐ and middle‐income countries.22 With respect to design, we are part of the third generation of studies on the implementation of interventions, in using multiple case studies in particular25 and in taking into account recommendations for improving the quality of qualitative studies, specifically regarding PBF.26
We conducted a contrasted multiple case study with several nested levels of analysis corresponding to health centres and the groups of actors involved.27 The selection of cases was explained in the protocol.24 The study was conducted in 3 different health districts (Appendix A) to represent the diversity of PBF implementation processes. Data were collected in a regional hospital, 2 district hospitals, and 18 primary care health centres (CSPSs). The analysis for the hospitals is reported in a separate article because the context is different. A discussion group with members of the 3 district management teams (ECDs—Équipes cadre du district), supported by a statistical analysis of routine data, was used to classify the CSPSs according to their level of performance before the PBF launch. Performance here was understood in terms of indicators of services use that were available from these routine data, whose quality was verified. In the 2 districts that organized PBF1, PBF2, and PBF3, one performing and one non‐performing CSPS were selected for each type (n = 3 × 2 × 2). In the district organizing PBF1 and PBF4, one high‐performing, one average‐performing, and one non‐performing CSPS were selected (n = 3 × 2) (see Figure 1). In each district, the research was under the responsibility of an experienced researcher from the country, a social anthropologist, who coordinated the data collection conducted by 4 research assistants. Each of these assistants was responsible for collecting data in 2 or 3 CSPSs of the same PBF type, except in the case of the hospitals, for which one assistant per district did all the data collection. All of them spent 10 to 15 days in each village or city where the health centres were located. Data collection was done between November 2014 and February 2015. At the start of the research project, all the researchers and assistants attended a 3‐day workshop to coordinate the process.
Figure 1.
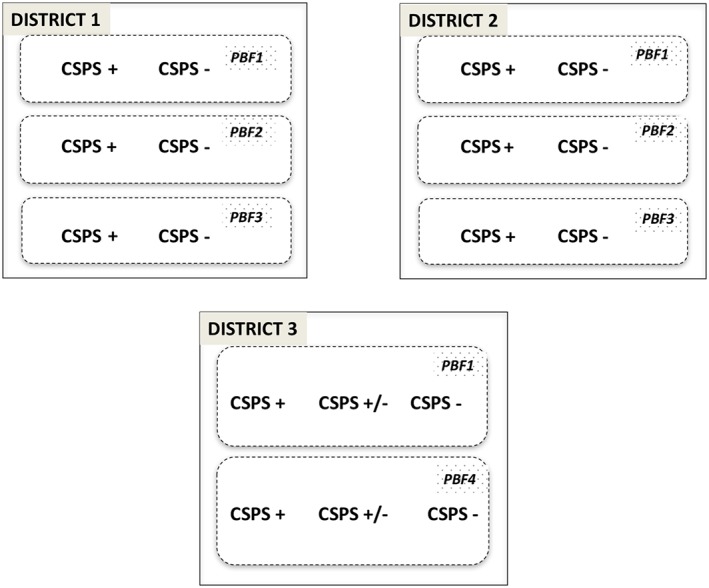
18 case studies in 3 health districts, adapted from Ridde et al24
Four qualitative data collection instruments were used.
Observations (participant and non‐participant) were conducted in the villages (markets, community meetings, etc) to write a descriptive monograph of the local context (villages covered by the CSPSs and the district for the CMA‐Centre médical avec antenne chirurgical, hôpital de district), as well as in the health centres to study interactions between patients and health workers during consultations, follow‐up visits, and supervision, etc. Each assistant recorded the results of these observations in a research diary.
Individual formal interviews were conducted using a guide that had been tested in advance, which was tailored to the different intervention modalities (mutual insurance and indigents) and addressed the dimensions of the intervention theory. The interviews were recorded with the participants' informed consent. The interviewees were selected, after several days of research assistant presence at the site, based on their availability and capacity to shed useful light on the PBF intervention. The selection was based on the sampling principle of seeking variation and intensity within groups of strategic actors28: health workers, community health workers, members of management committees (COGESs), elected officials and representatives of local associations, users, and populations. Table 1 shows the distribution of the 459 individual formal interviews by district. On average, we conducted 22 interviews per catchment area for each health centre.
Individual informal interviews were also conducted. In contrast to the interviews described above, these were unplanned and not recorded, but essential to the understanding of PBF in a development assistance context.29 Interviewees were selected as opportunities arose28 and based on what they might bring to the understanding of the situation under study. Notes were written after the interviews to capture the most important elements of the discussion. The last row of Table 1 presents the distribution of the 224 individual informal interviews by district.
Numerous documents were analysed: reports of activities and training sessions, training materials, implementation guide, consultation registers, etc. Summaries of key elements were noted in the logbooks of the assistants and researchers.
Table 1.
Total numbers of interviews per district
| District 1 | District 2 | District 3 | |
|---|---|---|---|
| District management team members | 8 | 2 | 5 |
| Health professionals | 36 | 46 | 52 |
| Community health workers | 8 | 12 | 22 |
| COGES members | 14 | 27 | 25 |
| Elected officials, NGOs, local leaders | 28 | 20 | 52 |
| Service users | 17 | 23 | 59 |
| Total formal interviews | 114 | 130 | 215 |
| Total informal interviews | 168 | 30 | 26 |
All the interviews were fully transcribed (mostly by the assistants) then coded using the QDA Miner Lite 4 software. The assistants were trained on the software just before the analysis during a workshop at which the preliminary results were discussed and compared. The training also provided an opportunity to test and adapt the coding tree and to develop a shared understanding to facilitate the data analysis, which was done using a deductive‐inductive type of framework analysis30 regarding the intervention theory. The assistants wrote a monograph and a summary report of their case studies (n = 4 assistants in the 3 districts), which was subjected to cross‐sectional analysis by the 3 researchers in charge (n = 3 reports). Finally, a cross‐sectional analysis of the 3 districts was performed by the principal investigator and validated by all the co‐researchers, and is the subject of this article.
The research protocol was approved by health research ethics committees of Burkina Faso (2014‐02‐14) and of the University of Montreal Hospital Research Centre (13.158). Participants participated in interviews on a voluntary basis and provided informed consent.
3. RESULTS
Given the wide scope of our research, the presentation of results here is focused on the essential general elements of the PBF implementation analysis. The following sections present the results by themes, as there were few differences between the cases or contexts, but any differences that were observed are described.
3.1. Launch and CSPS randomization
The introduction of PBF at the local level followed the standard process of most public health interventions in Burkina Faso, from the top down: information sessions, cascade‐type training sessions, distribution of materials, etc. However, a major recollection of the health workers concerned the activity of randomly assigning CSPSs to the different types of PBF modalities, which was done in late 2013 and early 2014. As stipulated by the World Bank for its impact evaluation, a quasi‐experimental design was used to put the intervention in place. The managers of each CSPS in a same district were assembled in one room to draw from a hat the type of PBF they would implement. They were given beforehand a detailed explanation of the concept, rationale, and functioning of the randomization process.
The health workers were divided in their views on this process. Some said this random selection had been decided, there was no point in fighting it, or that the distribution had been left to chance rather than being deliberate and biased by personal connections. Others found the process unfair, either because the luck of the draw gave them PBF1, for which performance‐related payments were lower and there was no indigent care, or, alternatively, they would have preferred that the intervention be implemented for everyone, everywhere, without selection. In fact, indigent care is useful everywhere if properly implemented: “What PBF is doing there, it's not good! It should do the same thing everywhere.” At the randomization workshop, the explanation of the different types of PBF was complicated, even though most of the health workers had previously undergone training. They had already worked out, however, that PBF3 could be more advantageous for them. In one region, a nurse exclaimed, in the presence of colleagues, about the consequences of the random draw: “We should have been warned before coming; we would have prepared ourselves for the trauma,” which made everyone laugh. As each health worker then drew their lots, there were cries and exclamations depending on the type of PBF drawn. One manager tried to joke about a CSPS: “Too bad, eh? It's in PBF1,” while a nurse said he “suffered from being in PBF1.”
3.2. Knowledge about PBF
In keeping with a “cascade training” approach organized on a very wide scale (including sending certain managers outside of the country for training), all those involved were the best informed on the general principles and functioning of PBF. Every health centre designated someone as the PBF focal point who was trained and was expected to share the information. Outside of that circle, however, the level of knowledge declined, to the point of being non‐existent for the population or community leaders. Health workers who were absent during the training or those working in health centres where the information had not been shared (particularly because of internal conflicts) were not really aware of the details. Worker mobility also reduced the transmission of information, as new workers were not trained and others left to work in CSPSs in other regions where there was no PBF.
In some CSPSs, health workers and COGES leaders decided to conceal, or simply not mention, the PBF functioning to the population and/or to community health workers, especially regarding staff bonuses. In general, however, the complexity of the PBF functioning was not completely understood. Health workers retained mainly the fact that there were performance indicators to be achieved, activities were being monitored, and their remuneration had been changed to provide added “incentives.”
“Now we're told, you give results and we give you the funds; it's based on the work. Now it's, produce this and we give you that.”
Overall, those we encountered who knew about PBF viewed it as just another “project,” like so many others that had come before and would come after it. As such, they knew it was temporary, funded and directed by international aid, and that it could provoke any number of monopolizing strategies.
3.3. Assessments
The great majority of those who knew about the existence of PBF in Burkina Faso and whom we met 12 months after its launch in the 3 districts viewed this new intervention positively. The health workers saw several advantages: subsidies that could help reinforce the health centre and its financial capacities, but also increases in health workers' motivation and income, stronger team work, and a return to higher quality work standards, leading to better relationships with patients and improved service use. Sometimes, however, we noted among certain health workers a focus on the bonus as a way of raising their income, to the detriment of their objective of improving the quality of care. The care of indigents that was announced and undertaken (community‐based selection) was particularly well appreciated by the actors. The few users who knew about the existence of PBF in Burkina Faso said they were now being better treated and that more time was spent on them in consultations.
“Since PBF was introduced, we've gotten better treatment. Before, you went, and the consultation took no time. Now … they take the time to ask you questions to find out what's really going on with you.”
3.4. Performance contracts, monitoring, and auditing
With few exceptions, most of the health centres had not yet signed the performance contracts between health centres and the contracting and auditing agencies (ACVs). They were unable to do it all at the same time, but instead did it over the first year of implementation. In one CSPS, the contract was signed 1 year after PBF was launched. These delays due to cumbersome administrative procedures built up over time (recruitment of ACVs, signing of contracts, etc), such that local actors did not really know how the contracts, indicators, and performance improvement plans were supposed to work; these were essential for planning how the centres would invest PBF subsidies and other income to improve services. For example, in one CSPS, the care providers did not know that the legibility of the registers would be evaluated, which caused them to lose points in the first audits. Some of them improved after receiving advice. Thus, the CSPSs' activities were purchased during that first year of PBF implementation, but they received their subsidies only much later, and without reference to the performance contract, which had not been signed.
The ACVs were recruited in January 2014 but only began operations in August 2014. Until then, district civil servants were trained to serve as temporary quantity auditors. Then, when most of the ACVs recruited them, they did not give up their positions as civil servants in the district teams, but instead combined their functions. Because of this, the health centre personnel were sometimes unable to distinguish between the actors in charge of audits, as both types of audits were done by the members of the district management teams (ECD) recruited by the auditing agencies.
Audits were done fairly regularly. The CSPSs received monthly visits for the quantitative audits and quarterly visits for qualitative audits. Quantitative audits involved counting the service utilization data recorded in the registers and comparing them against the numbers reported by the service providers.
“When they come to do the quantitative evaluation, it's the consultations for the month that are counted, from the first to the last consultation, down to the last sick person, in fact. They check everything to make sure they don't count the same person twice ….”
The health workers did a manual count of the different types of services provided. Then they reported the quantity on the ACV's quantity form. The auditor also manually counted the number of different types of consultations to validate the numbers (see example in Appendix B). The form compared them and showed the difference. If the difference between the quantity reported by health workers and the quantity validated by the auditor was 10% or more in either direction, that indicator was annulled and no subsidy was provided for it. Then the medical auditor sent the form to the ACV office to be entered into the PBF portal. After this data entry, the ST‐FBR validated the results on the portal.
“In the beginning we were seen as police, which was probably why they didn't welcome us very warmly. But we managed to convey the message that our role is to help CSPSs get as much money as possible to function better.”
The first audits were followed by team discussions everywhere and rapid readjustments by health workers to ensure subsequent ones would be better, to receive the maximum subsidies possible, because, “when PBF comes to audit, they're too rigorous; one small mistake, there's no forgiveness in PBF!” However, the quantitative audit was often a source of contention (between auditors and health workers, between care providers and support staff, and among care providers).
Monitoring of care quality was performed by the health system actors. Members of ECDs (eg, physician, midwife, and pharmacist) monitored the quality of services provided by the CSPSs, while regional hospital (CHR—Centre hospitalier régional) teams monitored those provided by district hospitals. CHR audits were conducted by peers. To receive a quality bonus, CSPSs had to have a minimal score of 50%. For health centres designated to receive the quality bonus, that bonus was calculated using a multiplicative formula that took into account the overall quality score and the total amount of quantity subsidies. Some CSPSs in the districts involved had not managed to obtain that score in the first 3 audits.
“So far, only one health centre in five has gotten a score of 50% or higher… some have had 40% or 49%.”
Most of the health workers, at least in their statements, appreciated the audits, which they often saw as formative supervisions and occasions for constructive discussions. However, they sometimes complained of certain auditors' haughty and condescending attitudes, which were not very conducive to learning:
“They drill down into the details to judge, and yet they're not supposed to do that, and generally auditors don't like being contested.” Some elements monitored were perceived as exaggerated: “… if they ask me to keep the courtyard clean, I can understand, but this thing about looking for dust, well … [group laughing] ah! Often they even go looking with ball‐point pens in our premises; really ….”
In addition, in a few CSPSs in several districts, we observed the scene being prepared in anticipation of the audit, as audits were always announced or simply known, since this information circulated rapidly in these settings, where the level of social connection is high. For example, the evening before the audit, health workers cleaned the health centre, disposed of garbage, installed mosquito nets over beds, or replaced defective mattresses of patients with those of the health workers. Some patients who were under observation were discharged so that the care providers would not “lose points because of their lack of cleanliness.” Likewise, some data were added to the registers, which were part of the audit, but our observations showed that these data were not real. Examples of this included recording patients' vital signs (temperature, blood pressure, and weight) or filling out partograms, which some health workers privately called “postograms” (ie, completed after the fact), as was reported to us in the informal interviews.
After 1 year of implementation, the community audits to gauge users' satisfaction and assess the veracity of the patients' names and services recorded in the registers had not yet been done in any of the 3 districts, contrary to what had been planned. Nor had any cross‐checking been done in the health centres of those districts to prove the reliability of the results of the quality and quantity audits conducted by the different auditing structures (ACVs and quality teams).
3.5. Payment of individual bonuses to health personnel
For the health workers, the payment of individual bonuses was essential. Some workers thus referred to the subsidy transmitted to purchase services as “PBF incentives.” However, only a portion of these subsidies went directly to health workers, according to a complex price schedule based on several criteria (seniority, responsibility, absences, and individual activity). As stipulated in the implementation guide, this bonus could not exceed 30% of the overall income of the health centre.17 An Excel file was given to all health centre managers, but the content of that instrument changed several times, which did not facilitate the health workers' comprehension. Everywhere, the arrival and distribution of the different types of financial incentives, including bonuses, were significantly delayed (more than 5 months), whereas health workers had been told some would be monthly. The consequences could be the opposite of what was intended:
“… they didn't respect what they said. So, some of the others were even discouraged because of that.”
The distribution of individual bonuses varied considerably from one health centre to another and was very dependent on local leadership and the usual power issues related to money. In some centres, there was a total lack of transparency. Health workers signed a sheet showing their bonus level, but the manager hid the amounts that the others were receiving, which should not have been done. When, inadvertently, the workers then found out the bonus amounts, which differed among them, they sometimes expressed a sense of injustice. Elsewhere, in one high‐performing CSPS, the manager decided to involve all members of the team in calculating the bonuses in a fully transparent process. In another CSPS, however, in an attempt to avoid internal conflicts, the manager decided to disregard the recommendations of the procedure manual and attributed the same performance score to all workers, as well as the same number of days of absence, regardless of the reality. This did not quite, however, achieve the desired result:
“As for this score, here, these are the scores they give you, but often you see that it's not fair, because not everyone deserves the same thing. But, hey, the manager has to give us all the same score so that no one can say, why did this one or that one get such‐and‐such a score?”
In its design, PBF made no provisions for bonuses to be given to COGES members, yet they managed the budget, the CSPSs' bank account, and community mobilization. Most health centres respected this rule, but others contested it because they felt it was unfair, given the important work performed by these members of the local community. They were also the ones who took delivery of the major subsidies coming in for services purchased under the PBF intervention. In this respect, one COGES president observed:
“The PBF needs to think about motivating COGES members, too. How is someone supposed to go withdraw more than 400,000 francs [600 Euros] and then give it to the workers, so they can share it among themselves!”
However, they were quickly brought back to order by the central authorities and had to officially desist.
4. DISCUSSION
This study is the first in Burkina Faso to analyse PBF implementation and one of the very few published on the subject in francophone West Africa. It is a response to needs expressed by many researchers to understand the “why” and “how” of PBF.6, 11, 13, 31
The results showed that adaptation and change are the norm rather than the exception in the implementation of public health interventions, which clearly presents challenges for evaluation.32, 33 While this PBF intervention is very centralized and its contours perfectly planned and defined in a very detailed implementation guide,17 when confronted with real‐life contexts, actors, and events, some things did not unfold as planned. Echoing the conclusions of Hawe,34 and that of Macq and Chiem specifically concerning the PBF intervention,31 it thus represents just one event among many others in the complex system that is Burkina Faso's health system. Front‐line workers do not know all the details of the intervention, and the central actors and funding agency are unable to control its functioning as a whole, which raises questions about the choice of an randomized controlled trial (RCT) evaluation design rather than an approach that would better take into account the real‐life context.33
The delays in paying bonuses (similar to those experienced in Benin11), when bonuses are at the heart of the theoretical and practical argument underpinning the intervention, are a prime example of the difficulties of organizing everything beforehand. Moreover, we saw that, as seen in Tanzania,35 Benin,6 and Sierra Leone,36 issues around the distribution of individual bonuses in health centres are crucial, raising notions of social justice and equity. Furthermore, PBF in Burkina Faso operates on a “carrot and carrot” approach, to paraphrase the experts, where no sanction appears possible or likely (and is perhaps not at all realistic in the West African context, in contrast to Rwanda,37 at least in the experts' opinions2). We might therefore wonder whether the underlying theoretical rationale is not simply a pretext, which some might consider valid, to provide additional resources to the operational level of the health system, which in fact needs them. But is this incentive‐based logic worth the price, when other options to solve the health system's problems have not all been tested1 and PBF efficiency remains to be proven?4, 11 The current state of knowledge is not yet adequate to answer this question in Africa.
In addition, several elements of the content (payment methods, rates, etc) evolved as the months went by, which is a positive factor in terms of its adaptation to context,38, 39 but which is not without consequences with respect to its understanding and uptake by the actors, as has been evident here in Burkina Faso and elsewhere in Africa.6, 13, 38 On the other hand, several elements of the local context and of the health system appear not to have been sufficiently considered, such as the presence of medical support personnel in health centres, whose activities are not all taken into consideration in calculating the subsidies, and the essential role of COGES members, who receive no bonuses, etc. These latter elements certainly contribute to the actors' perception that PBF is just one more development project, like so many others, rather than as a systemic reform. The fact that the technical unit charged with overseeing the project is only an administrative service in a department of the Ministry of Health, with no real autonomy in terms of management or decision‐making, encourages this perception among health workers. Despite a study on this subject, the reasons behind this strategic choice remain unknown.40 Whereas the World Bank and the government had no qualms about funding this new and ambitious intervention, the State ran up against its own bureaucracy, which was not well suited to managing it. The willingness to integrate it into the State's usual operations is laudable, in contrast to PBF projects elsewhere that are often organized in parallel,40, 41 but in the present case, it had to grapple with an entrenched organization in Africa that could not easily be changed within a few months.42 The institutional arrangements needed for PBF are complex,43 and it is questionable whether African states are able to adapt themselves. The subsidies for assisted deliveries had already encountered these challenges in Burkina Faso.44 As with the user fee exemption policies of the 2000s in Africa,45 and despite the PBF theory's declared objective of introducing greater management autonomy, we may wonder whether implementing PBF in this specific context of Burkina Faso might not, in fact, be a new attempt by the State at centralization, which runs counter to the decentralization reforms of the 1990s. Perhaps Burkina Faso's context and the organization of its health system simply do not lend themselves to this type of intervention? The question here is one of coherence between health policies and political will, a positive example of which has been described particularly in Rwanda, and a more difficult one in Niger.37 Taking the example of Rwanda, in fact, Booth and Cammack showed that “… performance‐based financing […] are favoured remedies at the World Bank and pure products of traditional top‐down principal‐agent strategies … they work in Rwanda because of specific contextual and design features.” 37(p69) However, PBF is not exclusively tied to the World Bank and the top‐down approach; other more decentralized and participative models also exist, as in Mali, for example.41
In addition, the actors' perceptions, as well as the implementation methods, are similar to those of a (new) development project, not very integrated and organized according to the traditional top‐down model, as is often the case.6, 21 In the context of this World Bank funded project, this takes the form of a very standardized intervention led by a technical cell of experts40 who are directly linked to the funding agency and organized in a top‐down approach. However, we also saw that front‐line actors used whatever room they had to manoeuvre to adapt the policy to their local contexts, as Lipsy23 has been saying for a long time. Aside from the unanticipated effects that we will describe in subsequent articles, the data showed that if they stray too far from the original content (design) of PBF—such as with bonuses for non‐official workers—they are quickly called to order, thus confirming the pyramidal nature of the implementation in our study context. Framed within Matland's proposed matrix of implementation types, the PBF in Burkina Faso would appear to be a “political implementation,” in which “implementation outcomes are decided by power.” 46(p163) In effect, while there is not much ambiguity in the objectives being targeted by PBF, the choice of policy instrument remains controversial. Matland suggests that, in this type of implementation, “remunerative mechanisms will predominate,” 46(p163) which appears to be the case here. Performance‐based financing has been implemented primarily because the World Bank wants it and has very sizeable funds that are difficult to refuse in the national context—which was nevertheless done, for example, in Chad47—and a coalition of international and national actors was created to help disseminate it.
The article presents the results of a cross‐sectional data collection, showing the situation at a given point in time and not its evolution (even though adaptations over the course of the 12 months were observed). We wish to share the results without waiting for the other phases because studies on this subject are so rare in francophone West Africa. The next phases of our research programme will shed more light on changes in the intervention, its adaptation, and how the context and actors behave over time. Longitudinal case studies are, in fact, essential for understanding policies but are difficult to organize.21, 24, 25 The strength of our analysis is that it is based on an empirical triangulation of a large sample of data and contrasted cases, as well as on an interdisciplinary collaboration that ensures the quality of the data. All the assistants were trained and supervised to ensure the rigour of their work, and the researchers' interpretations were solid, thanks to their deep knowledge of Burkina Faso's context and health policies. In addition, the results were widely shared and validated by numerous PBF stakeholders in the country. We therefore consider that they are transferable to all districts of the country that are involved in implementing PBF and will certainly be helpful in gaining a better understanding of the impact evaluation results.
5. CONCLUSION
Performance‐based financing is an option that is being increasingly promoted in Africa, even though the evidence of its effectiveness remains limited and knowledge about the challenges involved in its implementation is still scarce. After a pilot project in 3 districts, Burkina Faso decided to deploy this complex intervention on a large scale. This first study on its implementation showed that Burkina Faso faces the same challenges as other African countries.13 After nearly 12 months, the actors and those in charge of the intervention had adapted to the local contexts, despite a relatively rigid and standardized process and differences in material and human conditions among the health centres involved. However, for some dimensions of the intervention, less attention was paid to context, such as the role of community leaders, the distribution of bonuses, or significant delays in payment. The study also showed the importance of opening the “black box” to understand the intervention and expose its adaptations in local contexts.48 Future analyses will shed light on how the intervention evolved over time (“what Wildavsky and Majone call ‘implementation as evolution’”21), to take into account all these challenges, to be as effective as possible.
CONFLICTS OF INTEREST
VR is a co‐researcher on the baseline and end line study of the impact evaluation of PBF in Burkina Faso but has received no salary from the funder (World Bank) for this activity. The authors have no conflicts of interests regarding the publication of this paper.
ACKNOWLEDGMENTS
We would like to thank Élisabeth Paul, Aloys Zongo, Lara Gautier, and Jean‐Pierre Olivier de Sardan for their attentive and constructive review of a previous version of this article. This article was produced based on data collected by numerous surveyors under our supervision, whom we would like to thank warmly here: Idriss Ali Gali Gali, Assita Keita, Vincent Koudougou, Vincent‐Paul Sanon, Guillaume Kambire, Soulehimane Ouedraogo, Saliou Sanogo, Mariatou Zongo, Elodie Ilboudo, Maïmouna Sanou, Abdram Sow, Nestor Zante, and Oriane Bodson. The authors express their gratitude to the members of the PBF technical service, the district‐level managers, and participants for their contribution to this study. The authors would also like to thank Donna Riley for translation and editing support.
This work was supported by the Canadian Institutes of Health Research (CIHR), who funded the program (ROH‐115213). The research project is part of the “Community research studies and interventions for health equity in Burkina Faso.” Sponsors did not have a role in the study design; the collection, analysis, and interpretation of data; the writing of the report; and the decision to submit the article for publication. AMTT received a training bursary from the Canadian Institutes of Health Research (CIHR). VR holds a CIHR‐funded Research Chair in Applied Public Health (CPP‐137901).
AUTHORS' CONTRIBUTIONS
VR conceived the study protocol with the support of MY, PAS, SZ, and AMTT. VR analyzed the data based on the case reports and wrote the first draft of the manuscript with the support of MY, PAS, SZ, and AMTT. MY, PAS, and SZ supervised the data collection in one district, analyzed the data, wrote the district case study report, and critically reviewed the manuscript. AMTT helped develop the study protocol, trained the research assistants to use QDA Miner, interpreted the results, and critically reviewed the manuscript. All authors read and approved the final manuscript.
APPENDIX A.
A.1.
Characteristics of selected cases by district in 2015
District 1
| PBF1 | PBF2 | PBF3 | |||||
|---|---|---|---|---|---|---|---|
| CSPS1 | CSPS2 | CSPS3 | CSPS4 | CMA | CSPS5 | CSPS6 | |
| Starting performance | Quintile 4 | Quintile 2 | Quintile 5 | Quintile 1 | NA | Quintile 3 | Quintile 1 |
| Year opened | 2009 | 2003 | 1964 | 1958 | 1964 | 2009 | 2007 |
| Villages/sectors | 22 | 6 | 9 | 5 | 46 | 7 | 8 |
| Population | 10 942 | 3701 | 13 656 | 4066 | 58 091 | 7800 | 3600 |
| Health workers | 6 | 5 | 6 | 4 | 151 | 3 | 3 |
| Support personnel | 3 | 3 | 3 | 3 | 3 | 3 | |
| COGES members | 8 | 2 active | 2 active | 4 active | 4 | ||
| Distance from referral centre | 5 km | 25 km | 30 km | 8 km | 40 km | 22 km | |
| Contacts/inhabitant/year | 0.42 | 1.06 | 0.76 | 0.81 | 0.53 | 0.82 | |
District 2
| PBF1 | PBF2 | PBF3 | |||||
|---|---|---|---|---|---|---|---|
| CSPS 1 | CSPS 2 | CSPS 3 | CSPS 4 | CHR | CSPS5 | CSPS6 | |
| Starting performance | Quintile 3 | Quintile 1 | Quintile 2 | Quintile 1 | NA | Quintile 5 | Quintile 1 |
| Year opened | 1988 | 1993 | 1990 | 1988 | 1954 | 2000 | 1997 |
| Villages/sectors | 05 | 07 | 10 | 08 | 821 | 06 | 13 |
| Population | 10 484 | 14 169 | 11 033 | 8936 | 1 421 253 | 6299 | 10 714 |
| Health workers | 4 | 5 | 4 | 3 | 210 | 4 | 7 |
| Support personnel | 3 | 4 | 4 | 4 | 46 | 4 | 3 |
| Distance from referral centre | 45 km | 35 km | 12 km | 25 km | 0 km | 30 km | 20 km |
| Contacts/inhabitant/year | 0.37 | 0.61 | |||||
District 3
| PBF1 | PBF4 | |||||
|---|---|---|---|---|---|---|
| CSPS 1 | CSPS 2 | CSPS 3 | CSPS 4 | CSPS 5 | CSPS6 | |
| Starting performance | Average | Low | High | Average | Low | High |
| Year opened | 1960 | 2011 | 2004 | 1986 | 1983 | 1994 |
| Villages/sectors | 10 | 4 | 3 | 5 | 6 | 1 |
| Population | 15 132 | 7732 | 10 383 | 10 738 | 4977 | 10 884 |
| Health workers | 6 | 3 | 6 | 6 | 4 | 5 |
| Support personnel | 3 | 2 | 5 | 3 | 3 | 3 |
| Distance from referral centre | 57 km | 90 km | 30 km | 45 km | 90 km | 20 km |
| Contacts/inhabitant/year | 0.56 | 0.31 | 0.82 | 0.84 | 0.083 | 1.35 |
APPENDIX B.
B.1.
Examples of indicators and unit prices for a health centre quantity audit in the first arm of the intervention (PBF1) in January 2014
| Indicators | Unit price (CFA francs) | Quantity reported by health workers | Quantity validated by auditor | |
|---|---|---|---|---|
| 1 | Number of new consultations for patients aged 5 years + seen in curative nursing visits | 130 | 156 | 226 |
| 2 | Number of new consultations for patients under 5 year seen in curative nursing visits | 195 | 160 | 91 |
| 3 | Number of patient days under observation | 325 | 13 | 0 |
| 4 | Number of counter‐referrals received | 1300 | 2 | 2 |
| 5 | Number of children completely vaccinated | 390 | 9 | 8 |
| 6 | Number of pregnant women having received VAT2 tetanus vaccine or higher during the month | 325 | 26 | 17 |
| 7 | Number of pregnant women (new and previously enrolled) seen in prenatal consultation | 520 | 39 | 39 |
| 8 | Number of pregnant women (new and previously enrolled) seen in postnatal consultation (D6‐D8 and W6‐W8) | 650 | 9 | 9 |
| 9 | Number of deliveries carried out during the month | 1950 | 7 | 5 |
| 10 | Number of women (new and previously enrolled) seen during the month for family planning visits and using oral or injectable contraceptives | 650 | 22 | 21 |
| 11 | Number of women (new and previously enrolled) seen during the month for family planning visits and using long‐term contraceptives (IUD) | 1300 | 4 | 4 |
| 12 | Number of newly registered 0‐11 month‐olds seen in well‐baby visits | 130 | 10 | 10 |
| 13 | Number of children aged 12‐23 months seen in well‐baby visits | 325 | 11 | 14 |
| 14 | Number of children aged 6‐59 months treated for moderate acute malnutrition (MAM) | 390 | 2 | 2 |
| 15 | Number of children aged 6‐59 months treated for severe acute malnutrition (SAM) without complications | 975 | 6 | 6 |
| 16 | Number of home visits performed | 3900 | 0 | 0 |
| 17 | Number of people having undergone voluntary HIV screening (not including women screened as part of PMTCT) | 650 | 26 | 26 |
| 18 | Number of pregnant women having undergone HIV screening as part of PMTCT | 650 | 11 | 11 |
| 19 | Number of HIV+ mothers receiving complete prophylactic treatment with ART | 3250 | 0 | 0 |
| 20 | Number of newborns with HIV+ mothers taken into treatment | 3900 | 1 | 1 |
| 21 | Number of PLWHA on ART being followed | 1300 | 0 | 0 |
| 22 | Number of cases of PTB+ (new and relapsed) identified | 7800 | 0 | 0 |
| 23 | Number of cases of tuberculosis (all forms) treated and declared cured or treatment terminated | 11050 | 0 | 0 |
Description: Examples of indicators and their unit prices for a health centre quantity audit in the first arm of the intervention (PBF1) in January 2014. The quantity reported by the health workers is compared to the quantity validated by the auditor.
Ridde V, Yaogo M, Zongo S, Somé P‐A, Turcotte‐Tremblay A‐M. Twelve months of implementation of health care performance‐based financing in Burkina Faso: A qualitative multiple case study. Int J Health Plann Mgmt. 2018;33:e153–e167. https://doi.org/10.1002/hpm.2439
REFERENCES
- 1. Paul E, Renmans D. Performance‐based financing in the heath sector in low‐ and middle‐income countries: is there anything whereof it may be said, see, this is new? Int J Health Plann Manage. 2017. https://doi.org/10.1002/hpm.2409 [DOI] [PubMed] [Google Scholar]
- 2. Fritsche GB, World Bank . Performance‐Based Financing Toolkit. Washington, DC: The World Bank; 2014. [Google Scholar]
- 3. Milstein R, Schreyoegg J. Pay for performance in the inpatient sector: a review of 34 P4P programs in 14 OECD countries. Health Policy. 2016;120(10):1125‐1140. https://doi.org/10.1016/j.healthpol.2016.08.009 [DOI] [PubMed] [Google Scholar]
- 4. Turcotte‐Tremblay A‐M, Spagnolo J, De Allegri M, Ridde V. Does performance‐based financing increase value for money in low‐ and middle‐income countries? A systematic review. Health Econ Rev. 2016;6(1). https://doi.org/10.1186/s13561-016-0103-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Witter S, Fretheim A, Kessy F, Lindahl A. Paying for performance to improve the delivery of health interventions in low‐ and middle‐income countries. Cochrane Database Syst Rev. 2012; CD007899. doi: https://doi.org/10.1002/14651858.CD007899.pub2 [DOI] [PubMed] [Google Scholar]
- 6. Paul E, Sossouhounto N, Eclou DS. Local stakeholders' perceptions about the introduction of performance‐based financing in Benin: a case study in two health districts. Int J Health Policy Manag. 2014;3(4):207‐214. https://doi.org/10.15171/ijhpm.2014.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anselmi L, Binyaruka P, Borghi J. Understanding causal pathways within health systems policy evaluation through mediation analysis: an application to payment for performance (P4P) in Tanzania. Implement Sci. 2017;12(1). https://doi.org/10.1186/s13012-016-0540-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wilhelm DJ, Brenner S, Muula AS, De Allegri M. A qualitative study assessing the acceptability and adoption of implementing a results based financing intervention to improve maternal and neonatal health in Malawi. BMC Health Serv Res. 2016;16(1). https://doi.org/10.1186/s12913-016-1652-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ssengooba F, McPake B, Palmer N. Why performance‐based contracting failed in Uganda—an “open‐box” evaluation of a complex health system intervention. Soc Sci Med. 2012;75(2):377‐383. https://doi.org/10.1016/j.socscimed.2012.02.050 [DOI] [PubMed] [Google Scholar]
- 10. Renmans D, Holvoet N, Orach CG, Criel B. Opening the “black box” of performance‐based financing in low‐ and lower middle‐income countries: a review of the literature. Health Policy Plan. 2016;31(9):1297‐1309. https://doi.org/10.1093/heapol/czw045 [DOI] [PubMed] [Google Scholar]
- 11. Antony M, Bertone MP, Barthes O. Exploring implementation practices in results‐based financing: the case of the verification in Benin. BMC Health Serv Res. 2017;17(1). https://doi.org/10.1186/s12913-017-2148-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Macq J, Chiem J‐C. Looking at the effects of performance‐based financing through a complex adaptive systems lens. Bull World Health Organ. 2011;89(9):699‐700. https://doi.org/10.2471/BLT.11.089920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Renmans D, Holvoet N, Orach CG, Criel B. Opening the “black box” of performance‐based financing in low‐ and lower middle‐income countries: a review of the literature. Health Policy Plan. 2016;31(9):1297‐1309. https://doi.org/10.1093/heapol/czw045 [DOI] [PubMed] [Google Scholar]
- 14. Saetren H. Facts and myths about research on public policy implementation: out‐of‐fashion, allegedly dead, but still very much alive and relevant. Policy Stud J. 2005;33(4):559‐582. [Google Scholar]
- 15. Ridde V. Need for more and better implementation science in global health. BMJ Glob Health. 2016;1(2): e000115. doi:https://doi.org/10.1136/bmjgh-2016-000115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Steenland M, Robyn PJ, Compaore P, et al. Performance‐based financing to increase utilization of maternal health services: evidence from Burkina Faso. SSM ‐ Popul Health. 2017;3:179‐184. https://doi.org/10.1016/j.ssmph.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. ministère de la Santé M. Guide de mise en oeuvre du financement basé sur les résultats dans le secteur de la Santé. Ouagadougou: Burkina Faso; 2013. [Google Scholar]
- 18. Bodson O, Barro A, Turcotte‐Tremblay A‐M, Zante N, Ridde V. Assessing Implementation Fidelity of a Results‐Based Financing Intervention in Burkina Faso. Young Researchers Posters, World Health Summit, Geneva; June 2016. http://www.equitesante.org/wp-content/uploads/2016/06/test-poster-Londres-Juin9-V7.pdf. Accessed May 15, 2017. [Google Scholar]
- 19. Hawe P, Di Ruggiero E, Cohen E. Frequently asked questions about population health intervention research. Can J Public Health. 2012;103(6):e468‐e471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weiss CH. Evaluation: Methods for Studying Programs and Policies. 2nd ed. Prentice Hall: Upper Saddle River, NJ; 1998. [Google Scholar]
- 21. Hill MJ, Hupe PL. Implementing Public Policy: An Introduction to the Study of Operational Governance. 3rd ed. Thousand Oaks, CA: SAGE Publications; 2014. [Google Scholar]
- 22. Erasmus E, Orgill M, Schneider H, Gilson L. Mapping the existing body of health policy implementation research in lower income settings: what is covered and what are the gaps? Health Policy Plan. 2014;29(Suppl 3):iii35‐iii50. https://doi.org/10.1093/heapol/czu063 [DOI] [PubMed] [Google Scholar]
- 23. Lipsky M. Street‐Level Bureaucracy. Dilemmas of the Individual in Public Services. New York: Russell Sage Foundation; 2010. [Google Scholar]
- 24. Ridde V, Turcotte‐Tremblay A‐M, Souares A, et al. Protocol for the process evaluation of interventions combining performance‐based financing with health equity in Burkina Faso. Implement Sci. 2014;9(1):149 https://doi.org/10.1186/s13012-014-0149-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saetren H. Implementing the third generation research paradigm in policy implementation research: an empirical assessment. Public Policy Adm. 2014;29(2):84‐105. https://doi.org/10.1177/0952076713513487 [Google Scholar]
- 26. Cataldo F, Kielmann K. Qualitative Research to Enhance the Evaluation of Results‐Based Financing Programmes: The Promise and the Reality. Washington, DC: World Bank Group; 2016. [Google Scholar]
- 27. Yin RK. Case Study Research: Design and Methods. 4th ed. Los Angeles, CA: Sage Publications; 2009. [Google Scholar]
- 28. Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health. 2015;42(5):533‐544. https://doi.org/10.1007/s10488-013-0528-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Olivier de Sardan J‐P. Epistemology, Fieldwork, and Anthropology. London: Palgrave; 2015. [Google Scholar]
- 30. Ritchie J, Spenzer L. Qualitative data analysis for applied policy research In: Bryman A, Burgess RG, eds. Analyzing Qualitative Data. London and New York: Routledge; 1994:173‐194. [Google Scholar]
- 31. Macq J, Chiem J‐C. Looking at the effects of performance‐based financing through a complex adaptive systems lens. Bull World Health Organ. 2011;89(9):699‐700. https://doi.org/10.2471/BLT.11.089920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Perez D, Lefevre P, Castro M, et al. Diffusion of community empowerment strategies for Aedes aegypti control in Cuba: a muddling through experience. Soc Sci Med. 2013;84:44‐52. https://doi.org/10.1016/j.socscimed.2013.02.003 [DOI] [PubMed] [Google Scholar]
- 33. Ridde V, Robert E. Real World Evaluation Strategies; 2014. http://www.oxfordbibliographies.com/display/id/obo-9780199756797-0140. Accessed September 8, 2016. [Google Scholar]
- 34. Hawe P, Shiell A, Riley T. Theorising interventions as events in systems. Am J Community Psychol. 2009;43(3–4):267‐276. https://doi.org/10.1007/s10464-009-9229-9 [DOI] [PubMed] [Google Scholar]
- 35. Chimhutu V, Songstad NG, Tjomsland M, Mrisho M, Moland KM. The inescapable question of fairness in pay‐for‐performance bonus distribution: a qualitative study of health workers' experiences in Tanzania. Glob Health. 2016;12(1):77 https://doi.org/10.1186/s12992-016-0213-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bertone MP, Lagarde M, Witter S. Performance‐based financing in the context of the complex remuneration of health workers: findings from a mixed‐method study in rural Sierra Leone. BMC Health Serv Res. 2016;16(1). https://doi.org/10.1186/s12913-016-1546-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Booth D, Cammack D. Governance for Development in Africa. Solving Collective Action Problems. New York: Zed Books; 2013. [Google Scholar]
- 38. Ogundeji YK, Jackson C, Sheldon T, Olubajo O, Ihebuzor N. Pay for performance in Nigeria: the influence of context and implementation on results. Health Policy Plan. 2016;31(8):955‐963. https://doi.org/10.1093/heapol/czw016 [DOI] [PubMed] [Google Scholar]
- 39. Shoveller J, Viehbeck S, Di Ruggiero E, Greyson D, Thomson K, Knight R. A critical examination of representations of context within research on population health interventions. Crit Public Health. December 2015;1‐14. https://doi.org/10.1080/09581596.2015.1117577 [Google Scholar]
- 40. Zida A, Lavis JN, Sewankambo NK, Kouyate B, Moat K, Shearer J. Analysis of the policymaking process in Burkina Faso's health sector: case studies of the creation of two health system support units. Health Res Policy Syst. 2017;15(1). https://doi.org/10.1186/s12961-017-0173-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seppey M, Ridde V, Toure L, Coulibaly A. Sustainability of a results‐based financing pilot project: case study in two health districts in Mali. November 2016. http://www.equitesante.org/wp-content/uploads/2016/11/Sustainability-of-a-performance-based-financing-pilot-project.pdf. Accessed May 15, 2017. [DOI] [PMC free article] [PubMed]
- 42. Bierschenk T, Olivier de Sardan J‐P. (Eds). States at Work: Dynamics of African Bureaucracies. Leiden; Boston: Brill; 2014. [Google Scholar]
- 43. Bertone MP, Meessen B. Studying the link between institutions and health system performance: a framework and an illustration with the analysis of two performance‐based financing schemes in Burundi. Health Policy Plan. 2013;28(8):847‐857. https://doi.org/10.1093/heapol/czs124 [DOI] [PubMed] [Google Scholar]
- 44. Ridde V, Richard F, Bicaba A, Queuille L, Conombo G. The national subsidy for deliveries and emergency obstetric care in Burkina Faso. Health Policy Plan. 2011;26(Suppl 2):ii30‐ii40. [DOI] [PubMed] [Google Scholar]
- 45. Olivier de Sardan J‐P, Ridde V. Public policies and health systems in Sahelian Africa: theoretical context and empirical specificity. BMC Health Serv Res. 2015;15(Suppl 3):S3 https://doi.org/10.1186/1472-6963-15-S3-S3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Matland RE. Synthesizing the implementation literature: the ambiguity‐conflict model of policy implementation. J Public Adm Res Theory. 1995;5(2):145‐174. [Google Scholar]
- 47. Kiendrébéogo JA, Berthé A, Yonli L, Béchir M, Shroff ZC, Meessen B. Why performance‐based financing in Chad failed to emerge on the national policy agenda. Health Syst Reform. 2017;3(2):80‐90. https://doi.org/10.1080/23288604.2017.1280115 [DOI] [PubMed] [Google Scholar]
- 48. Ridde V, Olivier de Sardan J‐P. La mise en oeuvre des interventions de santé publique en Afrique: un thème stratégique négligé. Médecine Santé Trop. 2017;27:6‐9. [DOI] [PubMed] [Google Scholar]


