Abstract
Background:
Colorectal cancer is one of the most common and significant malignancies in the world. YKL-40 (chitinase-3-like protein 1) is involved in cell proliferation, migration, inflammation, and tissue remodeling; and serum levels of YKL-40 are associated with patient outcome in various cancers. The aim of this study was to assess the potential clinical usage of YKL-40 pretreatment serum levels as a prognostic biomarker in rectal cancer.
Methods:
Concentrations of YKL-40 and standard tumor marker—Carcinoembryonic antigen (CEA)—were assessed in serum of 83 patients with rectal cancer without distant metastasis, and association with clinicopathological characteristics and disease-free and overall survival was evaluated.
Results:
Concentration of YKL-40 was significantly higher in serum of patients with rectal cancer compared to healthy controls (P = .0001), and YKL-40 levels were able to predict rectal cancer (area under the Receiver Operating Characteristic [ROC] curve = .769) with higher accuracy than CEA (area under the ROC curve = .728) in patients with early stage disease. Increased YKL-40 levels were significantly associated with age (P = .001); however, no association with other clinicopathological characteristics was observed. Finally, in patients with recurrence, the percentage of cases with increased concentration of YKL-40 was significantly higher than in patients without recurrence (P = .041), and Kaplan-Meier analysis demonstrated that elevated YKL-40 concentration is a predictor of poor overall survival in patients with rectal cancer.
Conclusion:
Pretreatment serum levels of YKL-40 may be a novel prognostic factor of overall and disease-free survival in patients with nonmetastatic colorectal cancer.
Keywords: rectal cancer, YKL-40, prognostic factor, biomarker
Introduction
Colorectal cancer is the third most commonly diagnosed cancer in men and the second most commonly diagnosed cancer in women worldwide.1 The overall risk of colorectal cancer is slowly declining, especially in elderly population aged 65 and over. That is in part due to changes in lifestyle-related risk factors (eg, smoking, diet) as well as due to introduction of new screening methods for early detection and improved treatment options.2 Currently, the main predictor of overall and recurrence-free survival is the tumor stage at the time of the diagnosis. Depending on the clinical stage determined in accordance with the TNM (tumour, lymph nodes, metastasis) classification, patients are stratified into prognostic groups and offered most appropriate course of treatment. However, often patients within the same prognostic group and in the same stage of tumor progression can undergo a different course of the disease. Detection of early stage tumors and identification of biomarkers to predict patients’ response to treatment at the diagnosis are crucial to reduce the mortality of colorectal cancer.
In recent years, researchers have been trying to identify new prognostic biomarkers, which will help to better stratify patients and provide more personalized treatment strategies. Development of prognostic and predictive biomarkers that can be also used as potential drug targets is essential to improve current clinical practice and patient outcomes. Cytokines, metalloproteinases, and other molecules are of special interest, as their increased activity has been implicated in promoting tumor growth and proliferation and their expression levels can be easily assessed in patients’ blood.3–5 A number of recent studies have shown that Chitinase-3-like protein 1 (CHI3L1) also known as YKL-40 could be a potential candidate biomarker and therapeutic target in cancer. YKL-40 is a highly conserved glycoprotein binding heparin that is produced by different immune cells, such as active neutrophils, macrophages, as well as by tumor-associated macrophages and tumor cells.6 YKL-40 encoding gene is located on chromosome 1q32.1 and its increased expression has been observed in normal cells exhibiting high proliferation and differentiation7 and in cells with high cellular activity.8 In cancer, the YKL-40 gene overexpression has been shown in glioblastoma, in papillary thyroid carcinoma, and in chondrosarcoma.9 Interestingly, immunohistochemistry has shown that high tissue expression of YKL-40 protein is associated with higher tumor grade and poor differentiation in breast cancer.10 In glioblastoma, increased YKL-40 protein expression was associated with poorer radiation response, shorter survival, and loss of chromosome 10, which is a common genetic aberration in this type of cancer.11
Several studies have shown that serum levels of YKL-40 are elevated in a number of solid tumors, such as breast, colon, prostate, lung, kidney, ovary, melanoma, and in leukemia.12–19 Elevated patient serum concentrations of YKL-40 are often predictive of advanced tumor stage, limited response to therapy, and poor prognosis.20–24 This association between increased levels of YKL-40 and poor patient outcomes, as well as its previously reported function in inflammation and tissue remodeling, suggests that YKL-40 plays a potential role in promoting tumor growth.4 However, the exact role of YKL-40 in cancer development and progression is not yet fully known. It has been shown to promote cancer angiogenesis in breast, colon, and glioblastoma cancer cells in vivo.25–27 Moreover, overexpression of YKL-40 induces cell proliferation and migration, inhibits cell differentiation, and is involved in extracellular matrix reconstruction in various types of cancer.28,29
These comprehensive studies suggest that YKL-40 may serve as a potential novel therapeutic target. Interestingly, treatment with anti-YKL-40 antibody in combination with other treatment strategies resulted in inhibition of tumor growth, angiogenesis, and progression in various types of cancer.30 In glioblastoma, YKL-40 neutralizing antibody treatment led to inhibition of tumor angiogenesis and progression, while treatment with anti-YKL-40 in combination with radiation resulted in inhibition of tumor growth and increased survival in mouse xenograft models.26,31
In colorectal cancer, multiple studies have demonstrated the emerging role of YKL-40 levels in risk prediction and as an independent prognostic biomarker.19,32,33 Preoperative serum YKL-40 levels have been shown to be associated with higher tumor stage in patients with colorectal cancer and were significantly decreased after the tumor was removed.34,35 High serum YKL-40 concentration after surgery was related to poor outcome in patients with colorectal cancer, suggesting its potential value for disease monitoring.35 Protein and gene tissue expression of YKL-40 has been previously shown to be upregulated in tumor samples from patients with colorectal cancer compared to normal controls. Additionally, high expression of YKL-40 has been shown to be associated with increased number of infiltrating macrophages, increased density of microvessels, and increased tumor growth in colorectal cancer, suggesting that its inhibition may be a novel therapeutic strategy in colorectal cancer.36 Therefore, further validation of YKL-40 role as a predictive and prognostic biomarker in colorectal cancer is needed. Above-mentioned studies included patients with colorectal cancer with different stages of the disease, with only a limited number of studies employing patient stratification based on tumor location into their study design. To address this important gap in YKL-40 research in colorectal cancer, we evaluated the prognostic and predictive value of YKL-40 serum concentrations in a homogenous group of patients with rectal cancer without distant metastasis.
Patients and Methods
Serum samples were obtained from 83 patients with colorectal cancer, including 24 women and 59 men, all between 25 and 82 years of age (median 65 years). Samples were collected from patients with rectal cancer who underwent a radical surgery between 2008 and 2011 at the Maria Sklodowska-Curie Memorial Cancer Centre. The study was approved by the Regional Committee for the Ethics of Scientific Studies of Cancer Centre and Institute of Oncology in Warsaw, Poland, reference number: 1/2008. The clinical follow-up of the patients was carried out until the year 2016, median was about 7 years. During the follow-up period, 25 patients had relapse and 18 patients died. Seven patients were excluded from the overall survival (OS) and disease-free survival (DFS) analysis due to the lack of follow-up. Tumors were staged in accordance with TNM staging system. Patients with distant metastases were excluded from this study. The selection of patients and the method of treatment were performed as described previously.37 Clinical and histopathological characteristics of all patients are presented in Table 1.
Table 1.
Clinicopathological Characteristics of Patients With Rectal Cancer.
| Characteristics | Number of Patients | Percentage of Patients |
|---|---|---|
| Gender | ||
| Women | 24/83 | 29 |
| Men | 59/83 | 71 |
| Tumor size (T) | ||
| pT1 | 2/83 | 2 |
| pT2 | 24/83 | 29 |
| pT3 | 57/83 | 69 |
| pT4 | 0/83 | 0 |
| Lymph node status (N) | ||
| pN0 | 49/83 | 59 |
| pN1 | 23/83 | 28 |
| pN2 | 11/83 | 13 |
| Distance metastasis status (M) | ||
| M0 | 83/83 | 100 |
| Tumor grade (G) | ||
| G1 | 2/83 | 2 |
| G2 | 19/83 | 23 |
| G3 | 7/83 | 9 |
| Gx | 55/83 | 66 |
| Recurrence | ||
| Yes | 25/76 | 33 |
| No | 51/76 | 67 |
| Survival status | ||
| Alive | 58/76 | 76 |
| Dead | 18/76 | 24 |
The control group consisted of 38 healthy individuals, 25 female and 13 male with a median age of 61 years (range: 25-78 years). All individuals included in the control group were healthy (not taking any medicine, without any clinical signs or symptoms of cancer, liver, joints, metabolic or hormonal disease). All healthy individuals included in the study were recruited at the Maria Skolodowska-Curie Cancer Center. Venous blood collected from healthy controls was processed in the same way as for patients in order to standardize clotting conditions. All sera were separated within 1 hour after blood collection and stored in −70°C until assayed. A cutoff point of YKL-40 was established based on its concentration in the control group, taking the 95th percentile (Table 2).
Table 2.
Serum Concentration of CEA and YKL-40 in Control Group and Patients With Rectal Cancer.
| Variable | Control Group (n = 38) | Patients (n = 83) | |||||
|---|---|---|---|---|---|---|---|
| Median | Range | Cutoff | Median | Range | % | P Value | |
| CEA | 1.1 | 0.5-3.1 | 5 | 2.7 | 0.5-128.2 | 35 | .001a |
| YKL-40 | 25.9 | 11.5-49.4 | 44.6 | 47.9 | 12.12-210.4 | 54 | .001a |
a P < .05 was considered significant.
Venous blood was taken from patients before treatment. In order to standardize clotting conditions, all sera were separated within 1 hour after blood collection and stored in −70°C until assayed. The serum concentration of YKL-40 was determined using an enzyme-linked immunosorbent assay with R&D System. Concentration of CEA was used as a standard tumor marker and determined using chemiluminescence method with Architect i2000 sr, (Abbot Diagnostics, IL, USA), according to manufacturer’s recommendations.
The Mann-Whitney U test, χ2 test, and Spearman’s rank correlation coefficient were used. Pairwise nonparametric comparisons of the neighboring area under the ROC curves (AUCs) were performed by the Wilcoxon signed rank test. The DFS and OS analyses were performed using Kaplan-Meier method, applying one-way analysis with the use of log-rank test to compare survival curves. Univariate and multivariate analyses were performed using Cox’s regression model.
Results
Control cutoff value for YKL-40 serum concentration (44.6 pg/mL) was established based on its levels in the control group of healthy individuals, taking the 95th percentile.
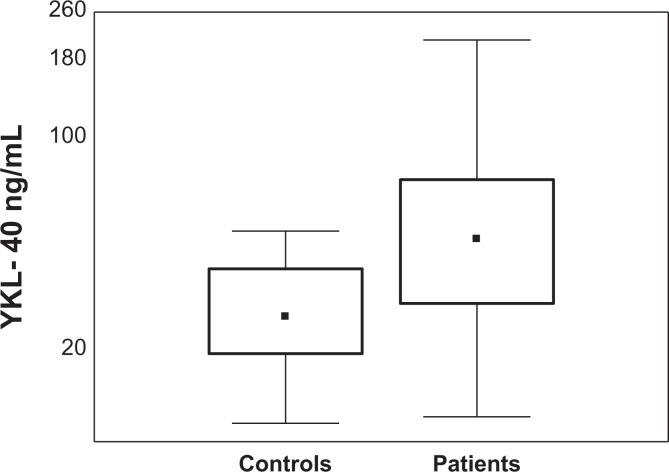
In nonmetastatic patients with rectal cancer, pretreatment serum YKL-40 concentrations were increased in 54% of cases, with median concentration (47.9 pg/mL) above the cutoff point. In comparison, only 35% of patients shown increased concentrations of the standard CEA marker, with median concentration below the cutoff point according to manufacturer’s recommendations (median value: 2.7 ng/mL, cutoff value: 5 ng/mL; Table 2). Serum levels of YKL-40 were significantly increased in patients with rectal cancer compared to the control group of healthy individuals (P = .0001; Figure 1).
Figure 1.
Serum YKL-40 pretreatment levels in control group and in patients with early stage rectal cancer.
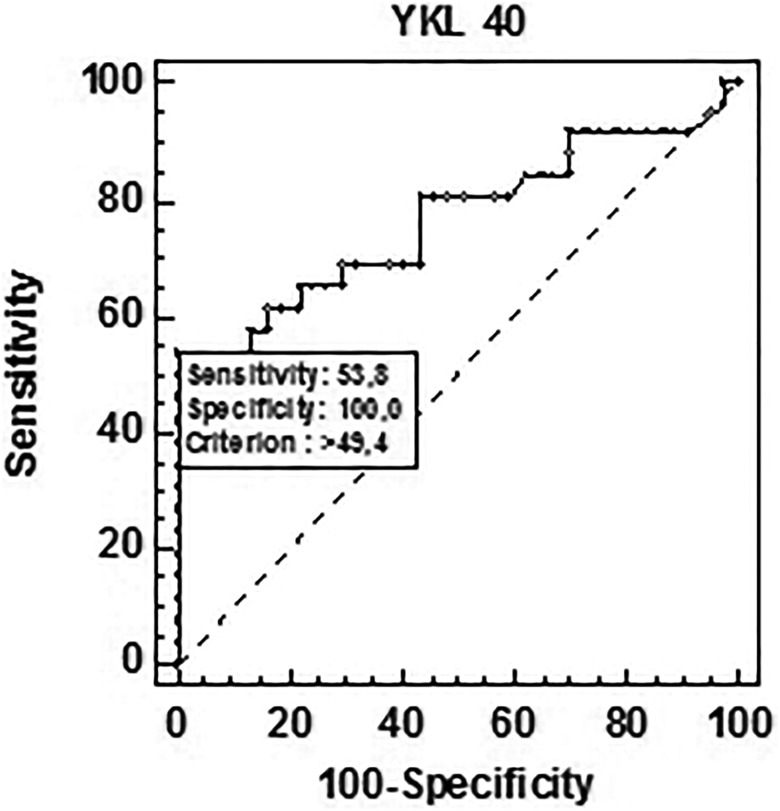
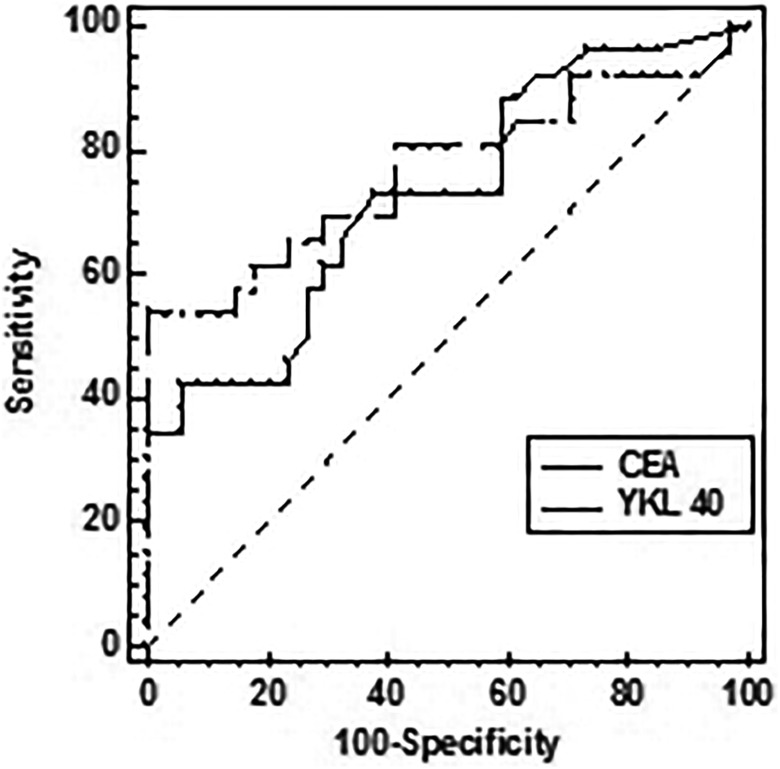
The potential diagnostic utility of YKL-40 concentrations was evaluated using ROC curve analysis in patients with rectal cancer and healthy individuals. The AUC of .757 (95% CI: 0.673-0.842) confirmed its suitability to discriminate between patients and controls (Figure 2). At the cutoff point of 44.8 pg/mL, the diagnostic sensitivity and specificity for this group amounted to 52.0% and 97.3%, respectively. By comparing the diagnostic potential of YKL-40 to the standard tumor marker CEA in patients with low-grade tumors (T1, T2; N0) versus normal controls, it was shown that the AUC for YKL-40 was greater (AUC = 0.769; 95% CI: 0.638-0.900) than that for CEA (AUC = 0.728; 95% CI: 0.597-0.859). The diagnostic sensitivity of YKL-40 marker in this group constituted 53.8% at a cutoff point of 49.4 ng/mL, whereas for CEA was lower and amounted to 42.3% at a cutoff point of 2.6 ng/mL (Figure 3). However, the differences between the two markers were not statistically significant.
Figure 2.
The area under the ROC curve of YKL-40 assays in patients vs control group.
Figure 3.
Potential of YKL-40 and CEA serum concentrations to discriminate between patients with rectal cancer with low-grade tumors and healthy individuals based on the area under the curve (AUC). Pairwise nonparametric comparisons of the neighboring AUCs were performed by the Wilcoxon test.
Next, we analyzed the relationship between the concentration of YKL-40 biomarker and clinicopathological parameters (T, N), gender and patient age at diagnosis. By evaluating the association between YKL-40 concentration and tumor size and lymph node status, we have shown that a higher percentage of patients with T3 tumors had increased YKL-40 levels (58%), compared to patients with T1 + T2 tumors (50%; Table 3). Similar association was observed in relation to lymph node status, with 52% of N1-N2 patients having elevated levels of YKL-40 in comparison to 44.7% of N0 patients (Table 3). These differences were not statistically significant. Due to small number of patients with determined histologic grade (G), the association between YKL-40 concentration and G parameter was not analyzed. When analyzing the relationship between YKL-40 concentration and patient gender, we observed that similar percentage of patients in both genders had increased concentrations; however, in women, the median concentration was higher (average value: 67.4 pg/mL) than in men (average value: 46.2 pg/mL).
Table 3.
Association Between YKL-40 Serum Levels and Clinicopathologic Characteristics in Patients With Rectal Cancer.
| Characteristics | YKL-40 [pg/mL] | ||
|---|---|---|---|
| Median | Number and % Above Cutoff | P Value | |
| All | 47.9 | (45/83) 54 | |
| Gender | |||
| Women | 67.4 | (13/24) 54 | NS |
| Men | 46.2 | (32/59) 54 | |
| Age, years | |||
| Age < 65 | 35.4 | (17/41) 41 | .001a |
| Age > 65 | 60.8 | (29/42) 69 | |
| T Stage | |||
| T1 + T2 | 41.8 | (13/26) 50 | NS |
| T3 | 46.6 | (33/57) 58 | |
| N stage | |||
| N0 | 44.7 | (25/49) 51 | .066 |
| N1 + N2 | 52.1 | (21/34) 62 | |
a P < .05 was considered significant. Abbreviation: NS, not significant.
Significant differences in YKL-40 concentrations were observed in association with patient age at diagnosis. Patients aged 65 years and over had higher serum levels of YKL-40 compared to younger patients (P = .001; Table 3). Spearman correlation coefficient analysis confirmed the significant correlation between YKL-40 levels and the age at diagnosis (P = .001; R = .35).
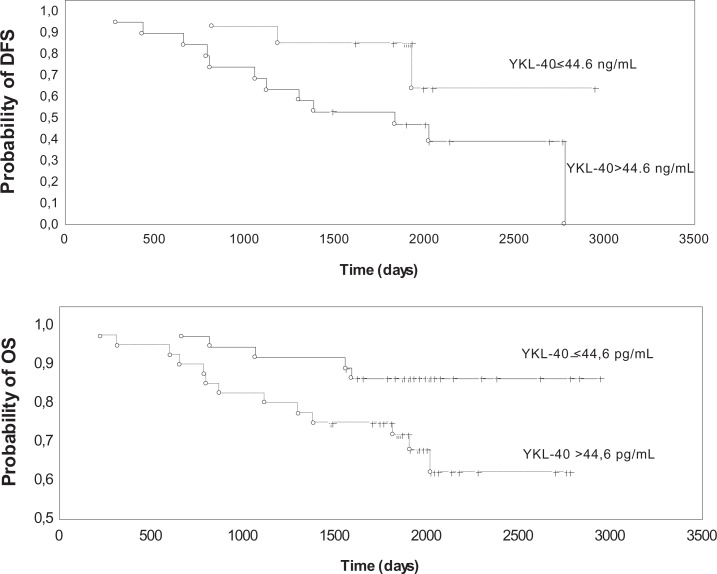
Finally, we assessed the potential prognostic value of YKL-40 concentration (cutoff value: 44.6 pg/mL) in relation to DFS and OS (Figure 4). Follow-up data of at least 8 years were available for 76 patients without distant metastases, treated with preoperative radiotherapy and then subjected to radical surgery. The median follow-up time was 2431 days. Tumor recurrence was observed in 25 cases (33% of all patients), and the median time to recurrence was about 5 years (2013 days). Log-rank univariate analysis did not show any association between clinicopathological parameters, YKL-40 and DFS. Majority (60%) of recurrences occurred in patients with advanced, third stage disease. In this group of patients, DFS was associated with YKL-40 levels (P = .044) as shown by the log-rank univariate analysis (Figure 3). In patients with recurrence, the percentage of cases with increased concentration of YKL-40 was significantly higher (68%) than in patients without recurrence (43%; P = .041; χ2 = 4.15).
Figure 4.
Disease-free survival (DFS) and overall survival (OS) of patients with rectal cancer stratified by serum levels of YKL-40.
The median OS was 5.15 years (1881 days), with 18 patients who have died during follow-up (24% of all patients). YKL-40 concentration had a significant impact on OS as confirmed by χ2 test. Increased concentration of YKL-40 was observed in over 72% of patients who died, compared to 45% of living patients (P = .042; χ2 = 4.13). In log-rank analysis, tumor size (P = .015), lymph node status (P = .021), and YKL-40 concentrations (P = .040) showed significant prognostic value. However, in multivariate analysis, the most notable association among the evaluated parameters (tumor size—P = .13), lymph node status—P = .343) was observed for YKL-40 (P = .09), although it did not achieve statistical significance (Figure 3). Patient’s gender and age did not provide any prognostic value in relation to OS or DFS.
Discussion
Identification of prognostic biomarkers remains a major challenge in choosing an appropriate treatment strategy for patients with colorectal cancer. Standard prognostic factors have limited value for identification of patients with an increased risk of local or distant recurrence. Accurate assessment of currently used prognostic factors such as tumor size, the infiltration of intestine wall, the stage of tumor differentiation, vascularization, and lymph node status is possible, in most cases, using postoperative material. In recent years, many studies have sought to determine biomarkers that can be easily assessed in patient’s serum to allow for prognostic assessment before treatment and to identify patients requiring more aggressive treatment, improving the OS rate.
Here, we assessed the clinical utility of CHI3L1, also known as YKL-40 in serum of patients with rectal cancer without distant metastases. We observed significantly higher levels of this biomarker in serum from cancer patients compared healthy controls. Moreover, we demonstrated that the percentage of patients with increased YKL-40 concentrations compared to healthy controls was considerably higher than that of standard CEA tumor marker. ROC curves analysis validated the diagnostic potential of YKL-40 serum levels and showed that its sensitivity and specificity in patients with low tumor stage was higher than that of the standard tumor marker CEA. This is in agreement with Johansen et al, who compared diagnostic sensitivity of both YKL-40 and CEA in patients with colorectal cancer, including those with rectal cancer and in healthy individuals demonstrating similar AUC values of both markers and higher AUC values for YKL-40. Additionally, increased concentrations of YKL-40 are associated with a much greater risk of colorectal cancer, which is an indication for colonoscopy.32
No significant association between YKL-40 biomarker concentrations and clinicopathological parameters, including tumor size, lymph node status, and gender, was found. However, we observed higher median concentrations and more frequently increased values of YKL-40 in patients with T3 and positive lymph nodes (N1-N2) than in patients with less advanced disease. Moreover, median YKL-40 concentration was significantly increased in female compared to male patients. This is supported by previous studies that reported significantly higher levels of YKL-40 in patients with colorectal cancer in comparison to control group and no association between YKL-40 concentration and evaluated clinicopathological parameters.33 Our work demonstrated a positive correlation of YKL-40 levels with patient’s age; in patients aged 65 years and over, the serum YKL-40 concentrations were higher than in younger individuals. Other studies describe similar effect of age on the concentration of YKL-40 not only in patients with colorectal cancer but also in other tumors.19,32,33
Currently, the role of YKL-40 as a prognostic factor in patients with colorectal cancer is not fully known. In our study, after 8 years of follow-up, we did not observe any association between YKL-40 levels and DFS. However, we discovered that concentrations of YKL-40 were more often significantly increased in patients with recurrence compared to patients without recurrence. Since most patients with recurrence had very advanced tumors (III stage), the prognostic value of YKL-40 was further analyzed in these patients. In this patient group, we observed a significant relationship between YKL-40 levels and DFS, with increased concentrations of YKL-40 being associated with poor DFS. In all patients included in this study, the univariate analysis demonstrated that YKL-40 concentration is associated with OS, suggesting its potential prognostic value. Similarly, tumor size and lymph node status were associated with OS; however, in multivariate analysis, only YKL-40 showed a weak association with OS. These results suggest that elevated pretreatment serum levels of YKL-40 may be a novel prognostic factor of OS in patients with colorectal cancer and disease-free survival in patients with nonadvanced tumors.
In this study, we only included patients who had local tumors at the time of the diagnosis, excluding patients who presented with metastatic tumors. However, previous studies have confirmed the prognostic value of YKL-40 concentrations in patients with colorectal cancer regardless of tumor location (local or distant) and in all stages of clinical advancement.34,35 Evaluation of prognostic factors in predefined patient’s groups is now of great clinical interest, and several studies have assessed the prognostic value of YKL-40 in different cohorts of patients with colorectal cancer. One of such studies, performed by Tarpgaard et al, confirmed the prognostic value of YKL-40 in patients with colorectal cancer with distant metastases.38 In another study, authors demonstrated the association between the expression of YKL-40 and c-Met and the response to chemoradiotherapy in patients with locally advanced rectal cancer. Higher levels of both prognostic factors were associated with partial or total lack of response to treatment.39
YKL-40 is an attractive candidate for development of anticancer therapies as it is involved in tumor angiogenesis. Several studies have assessed the possibility of using YKL-40 blocking antibodies in cancer treatment.26 Inhibition of YKL-40 activity resulted in blocking of key pathways related to proliferation and migration of tumor cells.20,29 Together with our results showing the prognostic potential of YKL-40, these findings suggest that YKL-40 might be used both as a therapeutic target and as a biomarker for monitoring the treatment course of colorectal cancer.39 Further studies into the functional role of YKL-40 in colorectal cancer could improve the current understanding of the mechanism of the cancer development and progression.
In conclusion, we have shown that increased pretreatment serum concentrations of YKL-40 in patients with colorectal cancer with local, nonmetastatic tumors are associated with poor DFS and OS. Therefore, serum levels of YKL-40 might be used as a prognostic biomarker, in addition to currently used standard markers, to enhance the current stratification of patients with colorectal cancer and to facilitate better clinical decisions and improve patient outcomes.
Abbreviations
- AUC
area under the ROC curve
- CHI3L1
chitinase-3-like protein 1
- DFS
disease-free survival
- OS
overall survival.
Footnotes
Authors’ Note: All procedures performed in studies involving human participants were in accordance with ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by SN/GW08/2015.
ORCID iD: Malgorzata Fuksiewicz, PhD  http://orcid.org/0000-0001-9625-423X
http://orcid.org/0000-0001-9625-423X
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. [DOI] [PubMed] [Google Scholar]
- 2. Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–117. [DOI] [PubMed] [Google Scholar]
- 3. Fuksiewicz M, Kotowicz B, Rutkowski A, Kowalska M. The matrix metalloproteinase-7 and pro-enzyme of metalloproteinase-1 as a potential marker for patients with rectal cancer without distant metastasis. Tumour Biol. 2015;36(5):3629–3635. [DOI] [PubMed] [Google Scholar]
- 4. Szczepanik AM, Siedlar M, Szura M, et al. Preoperative serum chemokine (C-C motif) ligand 2 levels and prognosis in colorectal cancer. Pol Arch Med Wewn. 2015;125(6):443–451. [DOI] [PubMed] [Google Scholar]
- 5. Mehta RS, Chong DQ, Song M, et al. Association between plasma levels of macrophage inhibitory cytokine-1 before diagnosis of colorectal cancer and mortality. Gastroenterology. 2015;149(3):614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johansen JS, Schultz NA, Jensen BV. Plasma YKL-40: a potential new cancer biomarker? Future Oncol. 2009;5(7):1065–1082. [DOI] [PubMed] [Google Scholar]
- 7. Johansen JS, Hoyer PE, Larsen LA, Price PA, Mollgard K. YKL-40 protein expression in the early developing human musculoskeletal system. J Histochem Cytochem. 2007;55(12):1213–1228. [DOI] [PubMed] [Google Scholar]
- 8. Ringsholt M, Hogdall EV, Johansen JS, Price PA, Christensen LH. YKL-40 protein expression in normal adult human tissues—an immunohistochemical study. J Mol Histol. 2007;38(1):33–43. [DOI] [PubMed] [Google Scholar]
- 9. Johansen JS, Jensen BV, Roslind A, Nielsen D, Price PA. Serum YKL-40, a new prognostic biomarker in cancer patients? Cancer Epidemiol Biomarkers Prev. 2006;15(2):194–202. [DOI] [PubMed] [Google Scholar]
- 10. Shao R, Cao QJ, Arenas RB, Bigelow C, Bentley B, Yan W. Breast cancer expression of YKL-40 correlates with tumour grade, poor differentiation, and other cancer markers. Br J Cancer. 2011;105(8):1203–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pelloski CE, Mahajan A, Maor M, et al. YKL-40 expression is associated with poorer response to radiation and shorter overall survival in glioblastoma. Clin Cancer Res. 2005;11(9):3326–3334. [DOI] [PubMed] [Google Scholar]
- 12. Jensen BV, Johansen JS, Price PA. High levels of serum HER-2/neu and YKL-40 independently reflect aggressiveness of metastatic breast cancer. Clin Cancer Res. 2003;9(12):4423–4434. [PubMed] [Google Scholar]
- 13. Johansen JS, Christensen IJ, Riisbro R, et al. High serum YKL-40 levels in patients with primary breast cancer is related to short recurrence free survival. Breast Cancer Res Treat. 2003;80(1):15–21. [DOI] [PubMed] [Google Scholar]
- 14. Bergmann OJ, Johansen JS, Klausen TW, et al. High serum concentration of YKL-40 is associated with short survival in patients with acute myeloid leukemia. Clin Cancer Res. 2005;11(24 pt 1):8644–8652. [DOI] [PubMed] [Google Scholar]
- 15. Krogh M, Christensen I, Bouwhuis M, et al. Prognostic and predictive value of YKL-40 in stage IIB-III melanoma. Melanoma Res. 2016;26(4):367–376. [DOI] [PubMed] [Google Scholar]
- 16. Hogdall EV, Ringsholt M, Hogdall CK, et al. YKL-40 tissue expression and plasma levels in patients with ovarian cancer. BMC Cancer. 2009;9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boisen MK, Madsen CV, Dehlendorff C, Jakobsen A, Johansen JS, Steffensen KD. The prognostic value of plasma ykl-40 in patients with chemotherapy-resistant ovarian cancer treated with bevacizumab. Int J Gynecol Cancer. 2016;26(8):1390–1398. [DOI] [PubMed] [Google Scholar]
- 18. Chen J, Liu X, Pan YQ, et al. Analysis of diagnostic value of YKL-40 in ovarian cancer [published online ahead of print April 21, 2016]. Int J Gynecol Cancer. 2016. [DOI] [PubMed] [Google Scholar]
- 19. Vom Dorp F, Tschirdewahn S, Niedworok C, et al. Circulating and tissue expression levels of YKL-40 in renal cell cancer. J Urol. 2016;195(4 pt 1):1120–1125. [DOI] [PubMed] [Google Scholar]
- 20. Schultz NA, Johansen JS. YKL-40-A Protein in the field of translational medicine: a role as a biomarker in cancer patients? Cancers (Basel). 2010;2(3):1453–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dupont J, Tanwar MK, Thaler HT, et al. Early detection and prognosis of ovarian cancer using serum YKL-40. J Clin Oncol. 2004;22(16):3330–3339. [DOI] [PubMed] [Google Scholar]
- 22. Lugowska I, Kowalska M, Fuksiewicz M, et al. Serum markers in early-stage and locally advanced melanoma. Tumour Biol. 2015;36(11):8277–8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmidt H, Johansen JS, Sjoegren P, et al. Serum YKL-40 predicts relapse-free and overall survival in patients with American Joint Committee on Cancer stage I and II melanoma. J Clin Oncol. 2006;24(5):798–804. [DOI] [PubMed] [Google Scholar]
- 24. Mygind ND, Iversen K, Kober L, et al. The inflammatory biomarker YKL-40 at admission is a strong predictor of overall mortality. J Intern Med. 2013;273(2):205–216. [DOI] [PubMed] [Google Scholar]
- 25. Shao R, Hamel K, Petersen L, et al. YKL-40, a secreted glycoprotein, promotes tumor angiogenesis. Oncogene. 2009;28(50):4456–4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Faibish M, Francescone R, Bentley B, Yan W, Shao R. A YKL-40-neutralizing antibody blocks tumor angiogenesis and progression: a potential therapeutic agent in cancers. Mol Cancer Ther. 2011;10(5):742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Francescone RA, Scully S, Faibish M, et al. Role of YKL-40 in the angiogenesis, radioresistance, and progression of glioblastoma. J Biol Chem. 2011;286(17):15332–15343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jeet V, Tevz G, Lehman M, Hollier B, Nelson C. Elevated YKL40 is associated with advanced prostate cancer (PCa) and positively regulates invasion and migration of PCa cells. Endocr Relat Cancer. 2014;21(5):723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shao R, Taylor SL, Oh DS, Schwartz LM. Vascular heterogeneity and targeting: the role of YKL-40 in glioblastoma vascularization. Oncotarget. 2015;6(38):40507–40518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shao R. YKL-40 acts as an angiogenic factor to promote tumor angiogenesis. Front Physiol. 2013;4:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shao R, Francescone R, Ngernyuang N, et al. Anti-YKL-40 antibody and ionizing irradiation synergistically inhibit tumor vascularization and malignancy in glioblastoma. Carcinogenesis. 2014;35(2):373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Johansen JS, Christensen IJ, Jorgensen LN, et al. Serum YKL-40 in risk assessment for colorectal cancer: a prospective study of 4,496 subjects at risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2015;24(3):621–626. [DOI] [PubMed] [Google Scholar]
- 33. Liu X, Zhang Y, Zhu Z, Ha M, Wang Y. Elevated pretreatment serum concentration of YKL-40: an independent prognostic biomarker for poor survival in patients with colorectal cancer. Med Oncol. 2014;31(8):85. [DOI] [PubMed] [Google Scholar]
- 34. Cintin C, Johansen JS, Christensen IJ, Price PA, Sorensen S, Nielsen HJ. Serum YKL-40 and colorectal cancer. Br J Cancer. 1999;79(9-10):1494–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cintin C, Johansen JS, Christensen IJ, Price PA, Sorensen S, Nielsen HJ. High serum YKL-40 level after surgery for colorectal carcinoma is related to short survival. Cancer. 2002;95(2):267–274. [DOI] [PubMed] [Google Scholar]
- 36. Kawada M, Seno H, Kanda K, et al. Chitinase 3-like 1 promotes macrophage recruitment and angiogenesis in colorectal cancer. Oncogene. 2012;31(26):3111–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rutkowski A, Zajac L, Pietrzak L, et al. Surgical site infections following short-term radiotherapy and total mesorectal excision: results of a randomized study examining the role of gentamicin collagen implant in rectal cancer surgery. Tech Coloproctol. 2014;18(10):921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tarpgaard LS, Guren TK, Glimelius B, et al. Plasma YKL-40 in patients with metastatic colorectal cancer treated with first line oxaliplatin-based regimen with or without cetuximab: RESULTS from the NORDIC VII Study. PLoS One. 2014;9(2):e87746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Senetta R, Duregon E, Sonetto C, et al. YKL-40/c-Met expression in rectal cancer biopsies predicts tumor regression following neoadjuvant chemoradiotherapy: a multi-institutional study. PLoS One. 2015;10(4):e0123759. [DOI] [PMC free article] [PubMed] [Google Scholar]