Abstract
Impairment of mental health is the most important risk factor for female sexual dysfunction. Women living with psychiatric illness, despite their frequent sexual difficulties, consider sexuality to be an important aspect of their quality of life. Antidepressant and antipsychotic medication, the neurobiology and symptoms of the illness, past trauma, difficulties in establishing relationships and stigmatization can all contribute to sexual dysfunction. Low sexual desire is strongly linked to depression. Lack of subjective arousal and pleasure are linked to trait anxiety: the sensations of physical sexual arousal may lead to fear rather than to pleasure. The most common type of sexual pain is 10 times more common in women with previous diagnoses of anxiety disorder. Clinicians often do not routinely inquire about their patients’ sexual concerns, particularly in the context of psychotic illness but careful assessment, diagnosis and explanation of their situation is necessary and in keeping with patients’ wishes. Evidence-based pharmacological and non-pharmacological interventions are available but poorly researched in the context of psychotic illness.
Keywords: antidepressant/ antipsychotic induced sexual dysfunction, female sexual dysfunction, psychiatric illness
Introduction
Psychiatric disease is the most important risk factor for women’s sexual dysfunction.1–4 This remains true in the context of chronic diseases known to interrupt the neurovascular basis of sexual physiology. Thus, depression, rather than the burden of physical disease or severity of complications, is the independent factor determining presence or absence of sexual dysfunction in women living with diabetes,5 multiple sclerosis,6 renal failure7 or rheumatic disease8 as well as those with a history of past childhood sexual abuse.9 A recent study of older women aged 50–99 years of age suggested that sexual health is linked more strongly to mental health than to physical function, stress or age itself.10 Nonetheless, poor mental health does not necessarily reduce the importance of sexual experience: a recent survey found that 43% of 1200 American women, including those with poor mental health, confirmed sexual health to be an important component of their quality of life, rating it as 4 or 5 on a 5-point Likert-type scale.11
The literature confirms that depression, anxiety and sexual dysfunction in women are related; however, the causal pathway is debated. Do depression and anxiety precipitate sexual dysfunction or is sexual dysfunction a frequent cause of mood disorder? The third possibility is that sexual dysfunctions, depression and anxiety disorders all result from an underlying vulnerability to both psychiatric disease and sexual dysfunction. Recent research studying this comorbidity over time showed results consistent with the last possibility, namely a shared underlying latent psychological vulnerability.12 This suggests that the presence of any one of the three risk factors will increase the odds of current or future symptoms of one or both of the other two, thus screening for all comorbidities needs to become routine.
The relationship between sexual dysfunction and psychotic disease in women is poorly understood. As with women experiencing depression and anxiety, women with schizophrenia and schizophrenia spectrum disorders have a very high burden of sexual dysfunction, with 60%–80% of women being affected.13–18 Antipsychotic medications, the symptoms of psychosis, institutionalization and societal stigma are all likely contributory factors. In comparison with women suffering from depression and anxiety, women with psychosis tend to have less social integration, more difficulty finding intimate partners and an overall lower level of functioning. This greater level of impairment has implications for both diagnosis and treatment of sexual dysfunction.
An additional challenge in addressing the sexual function of women with psychotic illness is that many clinicians may not feel comfortable speaking about sexuality with this patient population. Studies have shown that clinicians tend to underestimate the importance of the sexual aspects of their psychiatric patients’ lives and often do not inquire directly about sexual matters.18,19 A survey of British psychiatrists found that two-thirds do not inquire regularly about sexual function and only 17% of respondents felt competent assessing sexual concerns in their schizophrenic patients.19 Patients with psychotic illness feel this is an unmet treatment need and a cause for decreased quality of life.17,20–23
This review will identify the common sexual dysfunctions associated with depression, anxiety and psychosis as well as dysfunctions linked to their pharmacological treatment. We will outline evidence-based treatments of women’s Sexual Disorders including the management of medication-associated dysfunction. We will then discuss directions for future research.
Sexual response cycle
Research confirms women (and men) have many reasons to engage (or decline) partnered sex:24 the current model is often labeled an incentive-based sex response cycle.25 The two largest groups of reasons to be sexual are found to be those associated with a couple’s emotional intimacy and second, those to do with the expected pleasurable reward from the sexual event such that there need not necessarily be an initial sense of sexual urging/drive/innate desire.24 Instead, desire for sex itself can be experienced once arousal has occurred. This has been termed “responsive” or “triggered” desire. But to become aroused and desirous, there is need for appropriate sexual stimuli and context and the ability to pay attention to the stimuli and the sensations that follow.26 There is also a need to find the physical sensations and mental sexual excitement/arousal pleasurable. This will allow the intensity of sexual arousal to increase and ultimately lead to orgasm(s) on some or many occasions. Figure 1 shows the sexual response cycle in a diagrammatic form.
Figure 1.
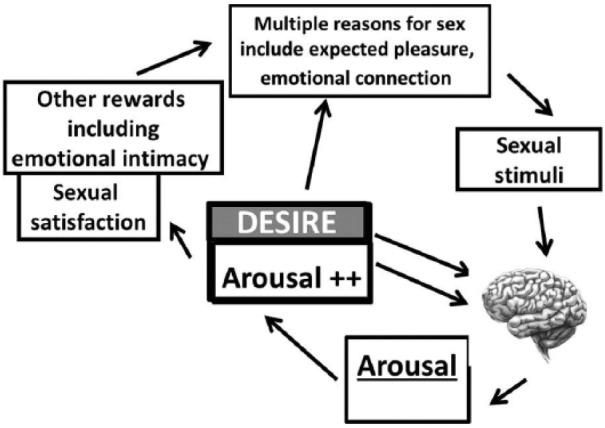
Incentive-based model of sexual response.
Human sexual response is depicted as a motivation-/incentive-based cycle of overlapping phases of variable order. A sense of desire may or may not be present initially: it can be triggered alongside the sexual arousal resulting from attending to sexual stimuli. Sexual arousal comprises subjective (pleasure/excitement/wanting more of the same), and physical (genital and non-genital responses) components. Psychological and biological factors influence the brain’s appraisal of the sexual stimuli. The sexual and non-sexual outcomes influence present and future sexual motivation.
Adapted from Basson.27
Female Sexual Disorders
Long standing concern and criticism of definitions of female Sexual Disorders as identified in the American Psychiatric Association’s Diagnostic and Statistical Manual Fourth Edition (DSM-IV) led to revised definitions published in 2013.28 Whereas previously sexual desire was deemed necessary prior to healthy sexual response in both men and women there is now acceptance of research confirming desire may follow and accompany a healthy sexual response of arousal from sexual stimuli.29 As well, arousal is now appreciated as a subjective excitement/pleasure—not only as a physical genital phenomenon. Thus, the definition of disordered female desire/interest and arousal has changed considerably: see Table 1. Due to lack of clear distinction between “vaginismus” and other causes of sexual pain on attempted or completed vaginal penetration, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) uses the umbrella term of genito-pelvic penetration pain disorder. These other causes include not only provoked vestibulodynia (PVD) which typically overlaps with “vaginismus”30 but other “more gynecological” entities, for example, lack of lubrication associated with estrogen lack, which often invoke the reflex pelvic muscle tightening response. There are now three criteria for diagnosing a Sexual Disorders: symptoms need to have persisted for a minimum of 6 months, be experienced in all or almost all (75%–100%) sexual encounters or have been persistent/ recurrent, and to have caused clinically significant distress. Unfortunately, validated questionnaires for diagnosing Sexual Disorders as per Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) are not yet available and so the prevalence of Sexual Disorders as currently understood is as yet unknown. A recent large British survey study, using proxy measures of DSM-5 Sexual Disorders found that while 22.8% of women reported one or more sexual difficulty including problematic orgasm, low sexual interest and arousal or painful sex, 3.6% of women met all three criteria for disorder.31
Table 1.
Definitions of Sexual Disorders in women, American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5).28
| Female sexual interest/arousal disorder |
|---|
| Lack of sexual interest/arousal for a minimum duration of 6 months as manifested by at least three of the following indicators: |
| 1. Absent/reduced frequency or intensity of interest in sexual activity |
| 2. Absent/reduced frequency or intensity of sexual/erotic thoughts or fantasies |
| 3. Absence or reduced frequency of initiation of sexual activity and is typically unreceptive to a partner’s attempts to initiate |
| 4. Absent/reduced frequency or intensity of sexual excitement/pleasure during sexual activity on all or almost all (approximately 75%) sexual encounters |
| 5. Sexual interest/arousal is absent or infrequently elicited by any internal or external sexual/erotic cues (e.g. written, verbal, visual, etc.) |
| 6. Absent/reduced frequency or intensity of genital and/or nongenital sensations during sexual activity on all or almost all (approximately 75%) sexual encounters |
| Female orgasmic disorder |
| At least one of the two following symptoms where the symptom(s) must have been present for a minimum duration of approximately 6 months and be experienced on all or almost all (approximately 75%) occasions of sexual activity: |
| 1. Marked delay in, marked infrequency, or absence of, orgasm |
| 2. Markedly reduced intensity of orgasmic sensation |
| Genitopelvic pain/penetration disorder |
| Persistent or recurrent difficulties for a minimum duration of approximately 6 months with one or more of the following: |
| 1. Marked difficulty having vaginal intercourse/penetration |
| 2. Marked vulvovaginal or pelvic pain during vaginal intercourse/penetration attempts |
| 3. Marked fear or anxiety either about vulvovaginal or pelvic pain on vaginal penetration |
| 4. Marked tensing or tightening of the pelvic floor muscles during attempted vaginal penetration |
Notwithstanding the limitations of previous research into the epidemiology of female sexual dysfunction in light of the new definitions, its brief summary may still be helpful. The larger surveys conducted during the last 10 years found approximately 10% of women reported ongoing sexual dysfunction that caused them distress, while a further 20% reported less disturbing sexual problems.1,4,32 A general muting of response—low desire, low subjective arousal along with infrequent or absent orgasm was the most common presentation in many surveys.33 Seven year longitudinal study of Australian women showed desire to decrease with age and with menopause.34 Studies of sexual function after natural and surgical menopause suggest desire is similar32,35 but distress about lowered desire is greater after surgical menopause.32 Although desire was seen to lessen with older age, women’s associated distress decreased such that the prevalence of DSM-IV “hypoactive sexual desire disorder” was thought to vary little with age.36
Postmenopausal vaginal dryness and associated dyspareunia was found to affect some 15% to 30% of women with marked cultural differences to the extent that this lead to bothersome sexual difficulties.37 Lack of lubrication and associated dyspareunia was reported by 5%–25% of younger women again with marked cultural differences leading to resulting sexual distress Leiblum et al.37 Dyspareunia from PVD, the most common cause of dyspareunia in premenopausal women, is thought to affect some 15%–18% of women.30 Isolated lack of orgasm despite high arousal is of uncertain prevalence because studies generally include women with low arousal alongside their lack of orgasm.
Recent reviews conclude that the lessening of sexual activities during pregnancy,38 especially during the third trimester, and postpartum39 are due to many psychological and physiological factors, are logical and not indicative of ongoing Sexual Disorders.
Critique of the various survey instruments used in clinical sexual research is beyond the scope of this review. However, the most widely used survey instrument, the Female Sexual Function Index (FSFI)40 which asks about sexual experience in the previous 4 weeks has some limitations. Although the FSFI’s internal consistency is good with a Cronbach’s alpha coefficient 0.82, and a test–retest reliability of 0.79–0.86, the questionnaire does focus on initial sexual desire and thus penalizes women with mostly responsive desire triggered along with arousal.29 Moreover the appropriateness of relying only on the past 4 weeks which for many reasons, including partner absence, may not represent a woman’s usual experiences, has been highly criticized.41
Depression
In addition to consistently identifying the strong link between depression and women’s reduced interest or desire for partnered sex, epidemiological studies confirm depression’s negative effects upon orgasmic experience,42 and its strong association with increased sexual risk behaviors.43
A recent British survey of 6669 women using proxy measures of DSM-5 items found current depression to increase the risk of sexual dysfunction with an odds ratio of 3.12.4 The anhedonia of depression has been shown to be particularly linked to muting of desire and response as well as to the risk of sexual pain.44 The most common form of chronic dyspareunia, (chronic dyspareunia now being subsumed under the more broad DSM-5 nomenclature of “Genital Penetration Pain Disorder”), namely PVD, is three times more common in women with a premorbid diagnosis of depression.45 Even in the absence of a clinical depression, negative mood has been found to impair sexual function,33 while positive or negative sexual experiences were found to modulate mood the day after the sexual engagement.46,47 Studies which control for current mood (as well as for medications, marital state and substance abuse), such as the Study of Women’s Health Across the Nation (SWAN), confirm a history of recurrent depression to be associated with reduced sexual arousal and reduced sexual pleasure.48
Review of the currently accepted model of human sexual response cycle clarifies its potential major disruption in the presence of depression, see Figure 2.
Figure 2.

Sexual response cycle is potentially weakened by depression at all points in an incentive-based model of sexual response.
Depression diminishes sexual incentives: anhedonia lessens the wanting of physical pleasure; depression reduces emotional intimacy—a major sexual incentive for women. There is little effort to secure needed sexual stimuli and sexual context. Sexual information processing in the brain is severely compromised by poor concentration and non-erotic thoughts and emotions leading to minimal arousal and no triggered desire. Neurotransmitters modulating sexual arousal are altered in depression. Outcome is unsatisfactory physically and emotionally and does not motivate further sexual interaction.
Many of the factors involved in partnered sex, including the relationship itself, the need to communicate sexual needs, the need to take care of the partner’s sexual satisfaction, concern about outcome and dealing with feelings of inadequacy, do not apply to sex alone. Self-stimulation/masturbation may continue in the presence of depression.48
Patients with bipolar disorder may have a different profile of sexual dysfunction than patients with unipolar depression. Research has found that patients with bipolar disorder experience difficulties with desire, arousal and achieving orgasm, but they also have increased risky sexual behaviors and more frequently changing partners as compared to patients with unipolar depression.49 This same study also found that all types of sexual dysfunction in bipolar men and women were associated with suicide plans, attempts and thoughts of death.49 The link between sexual dysfunction and suicidality was more subtle in patients with unipolar disease: impaired desire was related to thoughts of death.
Anxiety disorders
Epidemiological studies confirm anxiety disorders to be risk factors for low sexual desire and arousal50–54 with more recent research strongly linking aspects of anxiety with orgasmic difficulties42 and with sexual pain.45 The increased sympathetic nervous system activity of sexual arousal, while increasing women’s genital congestion, involves non-genital sensations which could be misinterpreted as threatening by an anxious woman, thereby negating any potential sexual pleasure.50 Laboratory studies confirm that women’s subjective arousal from an erotic film decreases if there is a preceding anxiety-evoking film, while genital congestion may increase.55 This is in keeping with the generally poor correlation between women’s genital sexual arousal/congestion and subjective arousal.56 These physical sensations from increased sympathetic drive, including shortness of breath, increased temperature, muscle tension and palpitations, have been termed “anxious arousal.” Trait anxiety is linked to anxiety sensitivity, that is, fear of the anxiety response itself and misinterpretation of these sensations: thus, it is postulated that a highly anxious woman is unlikely to experience pleasure from the physical sensations of sexual arousal.50
Keeping in mind the sexual response cycle, it is understandable that both sexual and nonsexual worries can be potent distractors when women with anxiety disorders are attempting to be sexual, limiting their arousal57 (and therefore frequency of orgasm) and likelihood of triggering desire. Brain imaging, albeit mainly focused on men, confirms marked deactivation to remove tonic sexual disinhibition, that is, necessary for orgasm to take place.58 Thoughts of potential harm from letting go of control and becoming vulnerable would logically preclude orgasm as would compulsive thoughts of orderliness and tidiness. Research confirms the inhibitory effect of trait anxiety on women’s subjective arousal in a laboratory setting but more strongly with partnered sexual experiences.50 Longitudinal study of women from age 21 to 50 years showed orgasmic difficulties to be associated with obsessive-compulsive features.42 Also noted was a detrimental effect on desire and orgasm from phobic anxiety, somatization and panic disorder.42 Obsessive compulsive disorder has been shown to be likely more detrimental than social anxiety or generalized anxiety disorder.52,59
Importantly, the experience of “anxious arousal” has been shown to be linked to women’s sexual pain, reduced subjective arousal and impaired lubrication.46 PVD is some 10 times more common in women with a premorbid history of anxiety disorder.45 A recent twin study confirmed a strong association between women’s sexual pain and anxiety sensitivity.60 PVD is a chronic pain syndrome associated with both peripheral and central sensitization of the nervous system and typically co-morbid with other chronic pain conditions including temporomandibular joint pain, irritable bowel syndrome, fibromyalgia and interstitial cystitis. As well as clinically diagnosed anxiety disorders, certain personality traits also appear to be risk factors. These include overly conscientiousness, hypervigilance to pain, fear of negative evaluation by others, and pain catastrophizing, the latter being significantly correlated with pain intensity during intercourse.61 It is thought that these internal stressors may modulate pain circuitry and be involved in central sensitization of the nervous system.62 The resulting damage to self-image and sexual self-confidence and the increased burden of guilt and responsibility for deterioration of relationships63 from the inability to have penetrative sex only add to the woman’s stress, and maintain the vicious cycle.60,64
Antidepressant induced sexual dysfunction
The frequency of sexual dysfunction due to antidepressant medications is difficult to assess, given that the adverse sexual effects of anxiety and depression are comparable to the side effects of antidepressant medications. Patient surveys may not be able to differentiate between these two etiologies.65 Many studies also rely on spontaneous patient report, and research has shown that incidence of sexual side effects is higher when patients are directly asked and a validated scale is used.66 Another methodological problem is that many studies record the responses of patients who have no sexual life as indicating that they have no sexual disturbances from their medications.67 With these limitations in mind, it is worth noting that self-reported sexual dysfunction among patients using antidepressants is extremely common. One recent study of long-term users of antidepressants found that 71.8% of respondents reported adverse sexual effects.68 A meta-analysis from 2013 reported prevalence rates ranging from 50% to 70%.69
Medication-associated sexual dysfunction is a serious concern in both the initial treatment of depression and anxiety and in longer term maintenance therapy.69 Unfortunately, the sexual side effects tend to occur within the first 3 weeks of treatment before benefit on mood is obtained, thereby risking early discontinuance. In the longer term, women whose mood disorder is in remission may find they are balancing continuing the medication to sustain their mood with discontinuing medication so as to improve their sexual lives. Fortunately, there are now choices and therapeutic interventions to limit the muting of women’s sexual response from antidepressants.
Studies suggest that sexual dysfunction is most likely when the mechanism of drug action is focused on the blockade of the reuptake of serotonin at 5-HT receptors—especially 5HT2 subtypes, whereas 5-HT1A receptor activity appears to be is pro-sexual.69,70 Medications that focus on increasing the uptake of norepinephrine or dopamine or blocking 5-HT2 receptors tend to have minimal negative sexual side effects.71 Suppression of sexual desire and arousal is commonly reported as is delay or absence of orgasm.69 Impaired lubrication and subsequent pain and discomfort are less frequent complaints.
Mechanisms of action and risk of antidepressant induced sexual dysfunction
Table 2 indicates presumed mechanism of interference with sexual response with different classes of antidepressants as well as approximate risk of medication-associated dysfunction.72–83
Table 2.
Mechanisms of action and risk of antidepressant induced sexual dysfunction.
| Drug | Main relevant mechanisms | AISD risk | Comments |
|---|---|---|---|
| SSRIsa,b | block 5HT reuptake | High | meta-analyses: risk similar |
| SNRIsc | block 5HT reuptake, noradrenergic | Medium | desvenlafaxine & duloxetine ? lower risk |
| MAOIsd | dopaminergic, noradrenergic but serotoninergic |
Medium | Td selegiline ? low risk |
| Quetiapinee | antagonizes D1, D2, 5HT2, 5HT1A | Medium | ? < than schizophrenia dosage |
| Mirtazapinef | noradrenergic, serotoninergic but blocks 5HT2, dopaminergic | Low | Weight gain issue |
| Bupropiong | dopaminergic, noradrenergic | Very low | |
| Trazadoneh | 5HT2A/5HT2 C antagonism, weakly blocks 5HT reuptake |
Very low | |
| Meclobamidei | reversible MAOI | Very low | |
| Vilazadonej | SSRI plus HT1A partial agonist | Negligible | More study needed |
| Vortioxetinek | “multimodal”: inhibits serotonin transporter, agonist 5HT1A | Negligible | More study needed |
| Aripiprazolel | partial agonist D2, 5HT1A, antagonist 5HT2A, spares prolactin | Negligible | More study needed |
| Lithiumm | unclear | Medium | More study needed |
AISD: antidepressant induced sexual dysfunction; MAOI: monoamine oxidase inhibitor; SNRI: serotonin-norepinephrine reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor.
Comparative studies of SSRIs have not consistently shown any statistical difference in their potential to cause sexual side effects.69
Case reports of increased desire, spontaneous orgasms and orgasms provoked by exercise from fluoxetine.72
Reports are conflicting, some consensus that there are fewer sexually negative effects from SNRIs than from SSRIs, particularly in the case of duloxetine.73
A recent formulation of transdermal selegiline is reported to be comparable to placebo in terms of sexual side effects.74
Clayton et al.75
Montejo et al.76
Pereira et al.77
Boyarsky and Hirshfeld.78
Baldwin.79
Clayton et al.80
Jacobsen et al.81
Fava et al.82
Grover et al.83
Psychotic illness
Women with psychotic illness experience a variety of dysfunctions, including impaired arousal, delayed or absent orgasm, low frequency of sexual activity and decreased sexual satisfaction.13,17,22,84,85 In contrast to previous assumptions, recent research suggests that both partnered and individual desire may be similar in women with psychotic illness and age-matched healthy women.86
The pathophysiology of sexual dysfunction in psychotic illness remains poorly understood, particularly with regards to psychosocial factors. Potential etiologies of sexual dysfunction include antipsychotic medications, positive and negative symptoms of psychosis, interpersonal difficulties, stigmatization, institutionalization, sexual trauma and somatic concerns. Most studies examining sexual function in psychosis have limited their focus to the effects of anti-psychotic medications. Studies have also largely been conducted using male patients, despite evidence that women with psychosis experience a higher prevalence of sexual dysfunction than men.14,16 Given the differences between male and female sexuality, these under-studied psychosocial factors likely play an even greater role in women’s dysfunctions than in men’s.
A number of investigators have found higher than average levels of sexual dysfunction in un-medicated psychotic patients,21,87 signifying that the causes of sexual dysfunction extend beyond those related to medication. Marques et al.88 found a high prevalence of sexual dysfunction among prodromal, un-medicated psychotic patients, as well as a correlation between symptom severity and the degree of sexual dysfunction. Several studies have corroborated this association between higher scores on the Positive and Negative Symptoms Scale (PANSS) and decreased interest in and enjoyment of sex.85,89 These studies provide further evidence that the symptoms of psychotic illness itself may play a role in the pathophysiology of sexual dysfunction: low motivation, cognitive impairment, poor judgment and hallucinations and delusions, may all play a role in preventing women from forming intimate relationships. Multiple studies have found that women with psychotic illness site “lack of a partner” as a cause for low sexual satisfaction.18,23 Rates of marriage are lower among women with schizophrenia than the general population.15
Societal stigmatization and hospitalization may also play roles in preventing women with psychosis from forming intimate relationships and attaining sexual satisfaction. Stigmatization can be a major source of poor self-esteem and negative self-concept. Research by Segalovitch et al.90 found a correlation between internalized stigma and decreased capacity to form intimate relationships among outpatients with schizophrenia. Several small studies have also shown the impact that stigmatization can have on sexual self-concept and perceptions of sexual competence.23,91,92 In their study of women with psychotic illness, Huguelet et al.86 found that 65% of women had impaired sexual self-esteem (vs 29% of healthy controls). This same study found that desire for solitary sexual activity was intact for women with psychosis. Research also suggests that rates of masturbation are higher among women with schizophrenia than controls, indicating that solitary sexual activity may have modest potential to offset the difficulties posed by having multiple barriers to intimacy.93,94
Women with psychotic illness are more likely than members of the general population to have experienced childhood sexual abuse and intimate partner violence.95–97 Childhood sexual abuse is a well-recognized risk factor for later re-victimization. Due to social and cognitive impairments, psychotic patients may also be more likely to engage in high-risk sexual behaviors. The potential link between sexual trauma and sexual dysfunction in patients with psychotic illness has not been well studied.
Somatic concerns associated with both antipsychotic medications and psychotic illness, such as metabolic syndrome, acne, extrapyramidal symptoms (e.g. abnormal movements, muscle stiffness, restlessness), excessive salivation and abnormal leakage of breast milk almost certainly contribute to impaired sexual function. This topic has not been well documented in the literature, although an association has been found between certain extrapyramidal symptoms and decreased sexual desire and arousal.98,99 In women without psychotic illness, metabolic syndrome is a well-recognized risk factor for sexual dysfunction.100
Antipsychotic induced sexual dysfunction
As is the case with antidepressant medications, it is difficult to evaluate the prevalence of antipsychotic induced sexual dysfunction due to the high rates of sexual dysfunction seen in non-medicated patients with psychotic illness. Sexual dysfunctions associated with antipsychotic medications include impairment of libido, arousal and orgasm. These side effects do not appear to subside over time.89,101
The pathophysiology of antipsychotic induced sexual dysfunction is poorly understood, but appears to be mediated by a combination of the effects of dopamine, prolactin, serotonin, acetylcholine, histamine and the action of noradrenaline at the alpha-1 adrenergic receptor. Dopamine blockade likely affects sexual function by modifying the reward circuitry, thereby negatively impacting sexual motivation and desire. Dopamine (D2) blockade may also have an indirect effect by causing a sustained elevation of serum prolactin. Hyperprolactinemia has been well-documented to cause menstrual irregularities and galactorrhea. Some authors classify these iatrogenic reproductive disturbances as sexual dysfunctions, which has led to confounded results.102,103 With regard to the effects of hyperprolactinemia on female libido, arousal and orgasm, there have been conflicting results, with several studies suggesting an association89,104,105 and others finding no link.16,98,106 Antipsychotics variably cause alpha-1 blockade, which has been associated with impairment of erection and ejaculation in men, as well as potential impairment of lubrication in women.84 Agonism at the 5-HT2 serotonin receptors causes delay of orgasm. The anticholinergic effects of antipsychotic medications may cause decreased lubrication and the antihistaminic effects are theorized to indirectly impact sexual function through sedation. The extent to which each of these neurotransmitters is implicated in sexual dysfunction is not clear.107
The research comparing the adverse sexual effects of different antipsychotic medications has been plagued by conflicting results and methodological problems. Many studies use different surveys to assess sexual function, making it difficult to compare data. Taking into account this paucity of robust evidence, it appears that all antipsychotics cause some degree of sexual dysfunction, with the worst offenders being first generation antipsychotics and risperidone, followed by olanzapine and clozapine. The superior antipsychotics are ziprasidone, quetiapine and aripiprazole.101,107,108 The largest study to date on this topic, which surveyed 3838 schizophrenic patients from 27 countries, found lower rates of self-reported sexual dysfunction for olanzapine (56%) and quetiapine (60%), as compared to risperidone (68%) and haloperidol (71%), although these results were not statistically significant.102 A Japanese study of 352 patients also found no significant difference between haloperidol, risperidone, olanzapine and aripiprazole.109 The EUFEST study, which followed 498 patients with first episode psychosis for one year of treatment found no significant difference in sexual side effects between haloperidol, amisulpride, olanzapine, quetiapine and ziprasidone.89 The most consistently replicated finding in this literature is that aripiprazole, a partial dopamine agonist with agonism at 5-HT1A, is the most sexually neutral antipsychotic.110 The conflicting results in this area of research are due in part to variations in methodology, although they also highlight the important role of psychosis itself in the pathogenesis of sexual dysfunction.
Management of sexual dysfunction associated with psychiatric illness
Given that disturbed mental health is the major risk factor for women’s sexual dysfunction, the initial essential step is to ensure remission of the psychiatric condition. This is applicable to women with depression, anxiety and psychosis. If pharmacotherapy is required to achieve remission, then every effort should be made to use the lowest possible dose and to choose sexually neutral drugs. Once medications have been optimized, psychological therapies can address the distracting thoughts, low self-image and the fear of the physical sensations of increased sympathetic nervous activity found in patients with depression and anxiety. Both cognitive behavioral therapy (CBT) and mindfulness-based cognitive therapy (MBCT) have proven benefit on depression and anxiety disorders and are basic to the treatment for women’s sexual dysfunctions of arousal and desire. As well, cognitive therapy, especially MBCT, has recently been shown to benefit many types of chronic pain111 including sexual pain.112 Patients with psychotic illness, who are more likely to have cognitive impairment, may not be able to participate in CBT or sex therapy for their sexual dysfunction, requiring instead a more supportive therapeutic approach. Recent qualitative research in sexually active mid-life women who mostly reported sexual problems, found that the participants considered behavioral and psychological treatments more likely than medication to be of benefit for both their physical and emotional sexual concerns.113
Psychological therapies
CBT
CBT has been the mainstay of treatment for women’s concerns with low sexual desire/arousal and was recommended by the 2015 International Consultation on Sexual Medicine: the evidence of benefit was graded as moderate—there being a number of small studies but further research needed.114 CBT can target women’s biased thoughts both during and outside of sexual activity including inaccurate negative self-critical thoughts about their sexuality, teach relaxation skills, address avoidance behavior and to then improve or restore sexual functioning. Recent meta-analyses have reviewed the benefit of CBT for women with low sexual desire/arousal: findings included a large effect size on sexual desire and also a moderate effect on improving sexual satisfaction. Including the male partner in CBT treatment for low desire led to better outcome.115,116
There is evidence of benefit from CBT for sexual pain. PVD is likely the end result of a number of different pathophysiological mechanisms, and there are no official guidelines for optimal therapy. As with other chronic pain conditions, cognitive therapy can be recommended.117,118 When women hear about the complex brain activity during pain, the ability of thoughts and emotions to modulate the physical sensation of pain becomes understandable. Brain areas activated during a painful stimulus include areas involved in regulating emotions (prefrontal cortex-PFC), motivation (anterior cingulate cortex), thoughts (dorso lateral and ventro lateral PFC) and areas processing the sensory aspects of pain (posterior insula).119 With cognitive therapies, catastrophic thinking, so common in women with PVD, can be usefully targeted. Reduction of allodynia as detected by using a non-noxious touch stimulus from a cotton swab, and increased frequency of intercourse has been documented for up to 2.5 years after 10 weeks of CBT.120
MBCT
Mindfulness-based therapies are now increasingly included in Western medicine, notably for depression, anxiety disorders, chronic pain, attention deficit disorder and cognitive decline. In addition to improved attention and focus and ability not to follow distracting thoughts, mindfulness skills include acceptance, non-criticism and non-reaction to the sensations (and thoughts and emotions), of the present moment. Thus, as mindfulness skills increase, the cognitive distractions have less effect, women’s awareness and acceptance of their physical sensations increases and the negative judgments that they harbor, are no longer believed and ruminated upon. Benefit against a waitlist control and against pre-treatment levels of sexual function has been recently shown for women with low desire and arousal.121 Reduced avoidance of sexual interaction and a new focus on the sexual sensory experience rather than on any goal has been observed in a number of sexual dysfunctions including reduced sexual interest and arousal.122
Sexual pain has recently been shown to benefit from MBCT. Mindfulness has been described as “uncoupling” the physical sensation from the emotional and cognitive experience of pain,123 leading to a reduction in catastrophizing.124 To investigate meditation’s means of diminishing pain intensity and its associated unpleasantness, research is beginning to clarify the multiple brain networks involved when interactions between meditation and pain-related brain activation are studied.111 Studies are also suggesting that meditation may alter brain morphology in meditation practitioners.125 In a study comparing MBCT to a wait-list control group in 85 women, researchers found significant reductions in genital pain intensity, rumination, helplessness, magnification, hypervigilance, sexual distress and negative mood, and an increase in feelings of self-efficacy for managing pain.112 Qualitative study identified acceptance to be a major means of benefit to mood and anxiety. Women spoke of feeling less abnormal, developed a stronger sense of self-efficacy and expressed appreciation for their newly acquired mindful skills.126 Recently submitted for publication is an 8-week program to compare 8 weeks CBT versus 8 weeks of MBCT with follow-up for a year.
Sex therapy
Sex therapy typically involves sensate focus exercises127,128 —a series of planned progressive non-demand pleasuring exercises whereby each partner takes turns giving and receiving sensual and later on, sexual touches, caresses, and kisses. Guiding verbally and non-verbally as to what feels most pleasurable is encouraged. Women learn to slow down instead of rushing through a sexual experience sometimes “to get it over with.” Initially, genital areas and breasts are off-limits. The idea of any goal or expectation of arousal or orgasm is discouraged. Usually, each session lasts 15–20 min and two or preferably three sessions occur each week for 3–6 weeks. The clinician and the couple decide when to include breasts and genital areas. As sessions are planned, touches may often be sensual rather than specifically sexual. However, when sexual touches are wanted, oral and manual genital stimulation or the use of a vibrator can be considered. Ultimately, the act of intercourse (or vaginal penetration with dildo) may be included but not be the major focus. Focusing away from intercourse can alleviate anxiety, decrease self-monitoring and cognitive distractions considered to promote women’s sexual dysfunction.129 More low-key sensual pleasuring may be more acceptable to women with poor mental health. Encouragement of exploration of erotica is often a component of sex therapy as are skills to improve couple communication.128
Medical therapies
Vaginal dehydroepiandrosterone (DHEA): initially trialed to confirm benefit for dryness and discomfort post menopause, research in mentally healthy women confirms generalized sexual benefit in terms of easier orgasm and increased sexual desire without any increase in mass spectrometry measured serum levels of either testosterone or estrogen.130 Serum DHEA levels increase modestly, staying in the normal range for age-matched women. Recently Food and Drug Administration (FDA)-approved, 6.5 mg DHEA vaginally is an alternative to local estrogen for menopausal vaginal dryness and pain and has the advantage of more general sexual benefit—at least in women recruited primarily for their vaginal symptoms. Thus, menopausal women reporting antidepressant-associated muting of sexual response who also require local vaginal estrogen may benefit more from using estrogen’s precursor i.e. vaginal DHEA.
Medications for sexual pain
PVD. The medications used for chronic pain including tricyclic antidepressants and anti-seizure drugs have not shown benefit in the few small randomized controlled trials conducted for PVD. However, individual women may nevertheless benefit particularly from the TCA’s or the serotonin-norepinephrine reuptake inhibitor (SNRI) duloxetine.
Genitourinary syndrome of menopause (GSM). A woman whose depression is associated with menopause may also be dealing with GSM. Often the symptoms require local estrogen in pill, cream or sialastic ring formulation to restore vaginal cell health, decreasing pH, and increasing vulvar and vaginal blood flow. When systemic estrogen is used for nonsexual reasons, additional topical vaginal estrogen may still be required.131 Vaginal hyaluronic acid has been shown to be non-inferior to 0.5 mg estriol twice weekly with both treatments showing benefit within 2 weeks.132 For women with both depression and GSM associated with past breast cancer treatment, topical lidocaine applied to the vestibule for 10 min before penetration is another non-estrogen product and has been shown to significantly lessen dyspareunia.133
Physical therapy
Pelvic muscle physiotherapy is often helpful to lessen sexual pain both by means of addressing concomitant hypertonicity of pelvic muscles which in itself can be painful134 and also as a form of desensitization135 as physiotherapy-associated pain becomes more familiar and non-threatening. Incorporating a mindfulness approach to the physical therapy has recently been encouraged.136
Management of antidepressant induced sexual dysfunction
Although sometimes dose reduction can continue to benefit mood and lessen sexual side effects, and for some 10% sexual side effects may lessen with time,76 other interventions are often necessary to address antidepressant–associated sexual dysfunction. A number of specific strategies will be outlined, but the overall stance is often to accept the sexual side effects and use evidence-based psychotherapy. Both CBT- and MBCT-based therapies are effective for women with sexual dysfunction caused at least in part by antidepressants.115,116,121 Unlike most trials of pharmacological agents, studies of psychological treatments tend not to exclude women with mood and anxiety disorders currently in remission, and thus a number of women in those studies of cognitive therapy–based programs would have been taking antidepressants. Outlining the sexual response cycle and explaining to women that because of the drug’s effects, more attention is needed on the sexual circumstances (not unduly fatigued, surroundings private, conducive to sensual feelings, positive feelings for the partner in that moment), the sexual stimuli- that they are optimal for her (e.g. that there is sufficient non-genital and non-breast caressing, sufficient non-penetrative genital sex and time to enjoy subsequent emotional closeness) and to focus on pleasure not performance can be helpful.137
In addition one or more of the following interventions can be chosen:
Switching antidepressants. With the choice of some medications with fewer sexual side effects, switching medication is an option. Thus, switching to vortioxetine, vilazodone, moclobemide, bupropion or desvenlafaxine can all be considered see Table 1
Additional psychotropic agent. A number of “antidotes” have been suggested, but only three have evidence in the form of randomized control trials using approved medications: adding bupropion can reverse SSRI induced dysfunction,138 as can the addition of aripiprazole.139 Recently, vortioxetine has been shown to improve sexual dysfunction from SSRIs in patients in remission from depression, to a greater degree than did escitalopram.140
One study suggests the use of transdermal testosterone for treating SSRI-/SNRI-induced loss of sexual desire in women showed some benefit.141 However, testosterone supplementation in women is controversial given the need for supplementing estrogen also, the lack of benefit in premenopausal women, of long-term safety data, of FDA approval and of any formulation for women.
There is also some evidence of improved lubrication and desire in women with the use of acupuncture.142
Two small recent placebo-controlled trials have shown improvement in antidepressant-associated dysfunction in women first from saffron143 and second from maca root.144
In contrast to clinical experience with investigational use of sildenafil in women, one study with strict recruitment criteria and extended recruitment period, reported benefit to orgasm dysfunction from the addition of sildenafil to treat antidepressant-associated dysfunction.145
Acute exercise. There is some evidence that exercise, by increasing sympathetic nervous system drive might combat the sexual side effects of serotonergic antidepressants given serotonin has an inhibitory effect on noradrenaline and women’s genital sexual arousal is sympathetically-driven. Laboratory studies of women reporting SSRI or SNRI-induced sexual dysfunction have been conducted.146 Women watched an erotic film while their genital congestion was measured, their subjective sexual arousal recorded and their sympathetic nervous system activity recorded by means of heart rate variation. Women using SSRIs were found to have increased genital arousal when they exercised for 20 min either 5 or 15 min prior to the films. However, there was no increase in their subjective arousal and thus the usefulness of this intervention (in addition to its relative impracticality), limits its use.
Management of antipsychotic induced sexual dysfunction
Options for the pharmacologic management of antipsychotic induced sexual dysfunction in women are limited. Recommended treatment approaches include reducing the dose of medication or changing to a more sexually neutral antipsychotic, such as quetiapine or aripiprazole.101,107 In a study of 27 patients with psychosis, Mir et al.147 found that libido was significantly improved after switching to aripiprazole or adding on aripiprazole. There is little evidence to support waiting for spontaneous remission of sexual symptoms or taking a drug holiday.148 In addition to optimizing the antipsychotic regimen, it is important to target modifiable risk factors that may be impacting sexual function, such as metabolic syndrome, hyperprolactinemia and substance use.
Many women with psychosis will be on antipsychotic medications lifelong and even patients on optimal medical management experience significant sexual dysfunction, thus it is important to incorporate non-pharmacologic strategies to alleviate this distressing persistent side effect. Regular, non-judgmental inquiry into patient’s sexual function in order to reduce stigma and understand the particular circumstances of each woman’s difficulties is advocated. Multiple studies have demonstrated that patients wish to speak more openly about sexuality and intimate relationships with their psychiatrists.22,84,92 Based on the barrier(s) present and the level of functioning of the patient, clinicians may then find it helpful to pursue an individualized approach, providing either psychoeducation, relationship counseling or more cognitively challenging therapies such as MBCT or sex therapy. A small qualitative study by Östman and Bjorkman149 found that patients with psychotic illness who are in relationships would like to have their partners participate more actively in clinical discussions of sexual function. Given that many women with psychosis cite lack of intimate relationships as a source of sexual impairment, this population would also likely benefit from interventions aimed at reducing hospitalization, improving social skills, decreasing stigma and enhancing integration into society.
Directions for future research
The prevalence of DSM-5 defined Sexual Disorders in psychiatric conditions using validated assessment tools needs to be identified. When researching the sexual effects of antidepressant and antipsychotic medications, greater care needs to be taken to compare pre-treatment sexual dysfunction with treatment-emergent sexual dysfunction, as the problem of causal attribution of sexual dysfunction among patients taking psychotropic medications is a major problem within the literature.
Second, when new treatments, including pharmacological choices, are trialed, women with psychiatric conditions (in remission) need to be included since they are the main cohort requiring treatment. To date, pharmacological studies in particular have excluded women with treated psychiatric conditions. This is true for recent studies of controversial medications:
Transdermal testosterone. Neither accurately measured (by mass spectrometry) serum levels of testosterone nor total androgen metabolites to reflect ovarian and adrenal sources of androgens are linked to women’s sexual desire disorder.150 Nevertheless, there is a long history of using off-label often supra-physiological testosterone supplementation. Using lower hormonal dosage via a transdermal patch releasing 300 μg testosterone daily, a series of studies beginning in 2005 showed modest benefit, whereas a second series using an equivalent gel, failed to show benefit. The latter has only been published in abstract form.151 Prior to the failed gel studies, the “patch” was approved in Europe but due to low sales is no longer available. It was not approved in the United States. Importantly neither series recruited women with Sexual Disorders as currently defined. Recruited women reported 2–3 sexually satisfactory experiences at baseline, that is, the participants did not have sexual interest arousal disorder since absence of arousal and pleasure are two key criteria for the diagnosis.28 The long-term safety of testosterone supplementation is unknown.152
Flibanserin. Women with treated psychiatric conditions were again excluded in trials of flibanserin—an agent initially studied as an antidepressant and inconsistently found to have very mild benefit to desire, albeit with major risk factors. Two meta-analyses based on both published and unpublished randomized controlled trials showed that flibanserin led to a mean increase of 0.49 satisfying sexual events per month.153 There was an increase of 0.3 (range 1.2—6.0) on the desire subscale of the validated questionnaire: the analysis concluded that the magnitude of that increase did not differ from placebo.154 As with the testosterone studies, the women in the trials reported 2–3 rewarding sexual experiences each month at baseline. Although approved for limited prescription in the United States sales are extremely low given the serious risks, doubtful benefit, daily dosage and contraindications, including alcohol and hormonal contraceptives.
Finally, more research needs to be conducted to understand the complex pathophysiology of the sexual dysfunction seen among women with psychosis. It is likely multifactorial, with contribution from medications, the symptoms of psychosis, somatic illness and the sociocultural effects of severe mental illness; however, until we understand the etiology of the dysfunction, we will be limited in our ability to develop effective treatment strategies. Were this knowledge more readily available, clinicians would also feel more comfortable making the needed inquiries and treatment recommendations: currently, this remains a documented but unmet need.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Lutfey KE, Link CL, Rosen RC, et al. Prevalence and correlates of sexual activity and function in women: results from the Boston Area Community Health (BACH) survey. Arch Sex Behav 2009; 38: 514–527. [DOI] [PubMed] [Google Scholar]
- 2. Wåhlin-Jacobsen S, Pedersen AT, Kristensen E, et al. Androgens and psychosocial factors related to sexual dysfunctions in premenopausal women. J Sex Med 2017; 14: 366–379. [DOI] [PubMed] [Google Scholar]
- 3. Avis NE, Zhao X, Johannes CB, et al. Correlates of sexual function among multi ethnic middle aged women: results from the study of women’s health across the nation (SWAN). Menopause 2005; 12: 385–398. [DOI] [PubMed] [Google Scholar]
- 4. Mitchell KR, Mercer CH, Ploubidis GB, et al. Sexual function in Britain: findings from the third national survey of sexual attitudes and lifestyles (Natsal-3). Lancet 2013; 26: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maiorino MI, Bellastella G, Castaldo F, et al. Sexual function in young women with type 1 diabetes: the METRO study. J Endocrinol Invest 2017; 40: 169–177. [DOI] [PubMed] [Google Scholar]
- 6. Zivadinov R, Zorzon M, Bosco A, et al. Sexual dysfunction in multiple sclerosis: correlation analysis. Mult Scler 1999; 5: 428–431. [DOI] [PubMed] [Google Scholar]
- 7. Peng YS, Chiang CK, Kao TW, et al. Sexual dysfunction in female hemodialysis patients: a multicenter study. Kidney Int 2005; 68: 760–765. [DOI] [PubMed] [Google Scholar]
- 8. Anyfanti P, Pyrpasopoulou A, Triantafyllou A, et al. Association between mental health disorders and sexual dysfunction in patients suffering from rheumatic diseases. J Sex Med 2014; 11: 2653–2660. [DOI] [PubMed] [Google Scholar]
- 9. Dunlop BW, Hill E, Johnson BN, et al. Mediators of sexual functioning and marital quality in chronically depressed adults with and without a history of childhood sexual abuse. J Sex Med 2015; 12: 813–823. [DOI] [PubMed] [Google Scholar]
- 10. Wang V, Depp CA, Ceglowski J, et al. Sexual health and function in later life: a population-based study of 606 older adults with a partner. J Geriatr Psychiatry 2015; 23: 227–233. [DOI] [PubMed] [Google Scholar]
- 11. Flynn KE, Lin MSL, Bruner DW, et al. Sexual satisfaction and the importance of sexual health to quality of life throughout the life course of U.S. adults. J Sex Med 2016; 13: 1642–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Forbes MK, Baillie AJ, Schniering CA. A structural equation modeling analysis of the relationships between depression, anxiety, and sexual problems over time. J Sex Res 2016; 53: 942–954. [DOI] [PubMed] [Google Scholar]
- 13. Harley EWY, Boardman J, Craig T. Sexual problems in schizophrenia: prevalence and characteristics: a cross sectional survey. Soc Psychiatry Psychiatr Epidemiol 2010; 45(7): 759–766. [DOI] [PubMed] [Google Scholar]
- 14. Hou CL, Zang Y, Rosen RC, et al. Sexual dysfunction and its impact on quality of life in Chinese patients with schizophrenia treated in primary care. Compr Psychiatry 2016; 65: 116–121. [DOI] [PubMed] [Google Scholar]
- 15. İncedere A, Küçük L. Sexual life and associated factors in psychiatric patients. Sex Disabil 2017; 35(1): 89–106. [Google Scholar]
- 16. Kikuchi T, Iwamoto K, Sasada K, et al. Sexual dysfunction and hyperprolactinemia in japanese schizophrenic patients taking antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry 2012; 37(1): 26–32. [DOI] [PubMed] [Google Scholar]
- 17. Mahmoud SB, Zouari L, Dammak M, et al. Evaluation of sexuality in 61 subjects suffering from chronic psychosis. Sexologies 2013; 22(2): e59–e63. [Google Scholar]
- 18. Tharoor H, Kaliappan SGA. Sexual dysfunctions in schizophrenia: professionals and patients perspectives. Indian J Psychiatry 2015; 57(1): 85–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nnaji R, Friedman T. Sexual dysfunctions and schizophrenia: psychiatrists’ attitudes and training needs. Psychiatrist 2008; 32: 208–210. [Google Scholar]
- 20. Bushong ME, Nakonezny PA, Byerly MJ. Subjective quality of life and sexual dysfunction in outpatients with schizophrenia or schizoaffective disorder. J Sex Marital Ther 2011; 39: 336–346. [DOI] [PubMed] [Google Scholar]
- 21. Olfson M, Uttaro T, Carson WH, et al. Male sexual dysfunction and quality of life in schizophrenia. J Clin Psychiatry 2005; 66: 331–338. [DOI] [PubMed] [Google Scholar]
- 22. Östman M. Low satisfaction with sex life among people with severe mental illness living in a community. Psychiatry Res 2014; 216(3): 340–345. [DOI] [PubMed] [Google Scholar]
- 23. Wasow M. Sexuality and the institutionalized mentally ill. Sex Disabil 1980; 3: 3–16. [Google Scholar]
- 24. Meston CM, Buss DM. Why humans have sex. Arch Sex Behav 2007; 36: 477–507. [DOI] [PubMed] [Google Scholar]
- 25. Both S, Everaerd W, Laan E. Desire emerges from excitement: a psychophysiological perspective on sexual motivation. In: Janssen E. (ed.) The psychophysiology of sex. Bloomington, IA: Indiana University Press, 2007, pp. 327–339. [Google Scholar]
- 26. Laan E, Both S. What makes women experience desire? Fem Psych 2008; 18(4): 505–514. [Google Scholar]
- 27. Basson R. The female sexual response: the role of drugs in the management of sexual dysfunction. Obstet Gynecol 2001; 98: 350–352. [DOI] [PubMed] [Google Scholar]
- 28. DSM-5 American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Arlington, TX: American Psychiatric Publishing, 2013. [Google Scholar]
- 29. Brotto LA. The DSM diagnostic criteria for hypoactive sexual desire disorder in women. Arch Sex Behav 2010; 39: 221–239. [DOI] [PubMed] [Google Scholar]
- 30. Harlow BL, Wise LA, Stewart EG. Prevalence and predictors of chronic lower genital tract discomfort. Am J Obset Gynecol 2001; 185: 545–550. [DOI] [PubMed] [Google Scholar]
- 31. Mitchell KR, Jones KG, Wellings K, et al. Estimating the prevalence of sexual function problems: the impact of morbidity criteria. J Sex Res 2016; 53: 955–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. West SL, D’Aloisio AA, Agans RP, et al. Prevalence of low sexual desire and hypoactive sexual desire disorder in a nationally representative sample of US women. Arch Intern Med 2008; 168: 1441–1449. [DOI] [PubMed] [Google Scholar]
- 33. Hartmann U, Philippsohn S, Heiser K, et al. Low sexual desire in midlife and older women: personality factors, psychosocial development, present sexuality. Menopause 2004; 11(6): 726–740. [DOI] [PubMed] [Google Scholar]
- 34. Dennerstein L, Guthrie JR, Hayes RD, et al. Sexual function, dysfunction, and sexual distress in a prospective, population-based sample of mid-aged, Australian-born women. J Sex Med 2008; 5: 2291–2299. [DOI] [PubMed] [Google Scholar]
- 35. Kokcu A, Kurtoglu E, Bildircin D, et al. Does surgical menopause affect sexual performance differently from natural menopause? J Sex Med 2015; 12: 1407–1414. [DOI] [PubMed] [Google Scholar]
- 36. Hendrickx L, Gijs L, Enzlin P. Age-related prevalence rates of sexual difficulties, sexual dysfunctions, and sexual distress in heterosexual women: results from an online survey in Flanders. J Sex Med 2015; 12(2): 424–435. [DOI] [PubMed] [Google Scholar]
- 37. Leiblum SR, Hayes RD, Wanser RA, et al. Vaginal dryness: a comparison of prevalence and interventions in 11 countries. J Sex Med 2009; 6: 2425–2433. [DOI] [PubMed] [Google Scholar]
- 38. De Pierrepont C, Polomeno V, Bouchard L, et al. What do we know about perinatal sexuality? A scoping review on sexoperinatality–part 1. J Gynecol Obstet Biol Reprod 2016; 45(8): 796–808. [DOI] [PubMed] [Google Scholar]
- 39. De Pierrepont C, Polomeno V, Bouchard L, et al. What do we know about perinatal sexuality? A scoping review on sexoperinatality–part 2. J Gynecol Obstet Biol Reprod 2016; 45(8): 809–820. [DOI] [PubMed] [Google Scholar]
- 40. Rosen C, Brown J, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther 2000; 26(2): 191–208. [DOI] [PubMed] [Google Scholar]
- 41. Meyer-Bahlburg HFL, Dolezal C. The female sexual function index: a methodological critique and suggestions for improvement. J Sex Marital Ther 2007; 33: 217–224. [DOI] [PubMed] [Google Scholar]
- 42. Leeners B, Hengartner MP, Rössler W, et al. The role of psychopathological and personality covariates in orgasmic difficulties: a prospective longitudinal evaluation in a cohort of women from age 30 to 50. J Sex Med 2014; 11: 2928–2937. [DOI] [PubMed] [Google Scholar]
- 43. Field N, Prah P, Mercer CH, et al. Are depression and poor sexual health neglected comorbidities? Evidence from a population sample. BMJ Open 2016; 6: e010521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kalmbach DA, Ciesla JA, Janata JW, et al. Specificity of anhedonic depression and anxious arousal with sexual problems among sexually healthy young adults. J Sex Med 2012; 9: 501–513. [DOI] [PubMed] [Google Scholar]
- 45. Khandker M, Brady SS, Vitonis AF, et al. The influence of depression and anxiety on risk of adult onset vulvodynia. J Womens Health 2011; 20: 1445–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kalmbach DA, Kingsberg SA, Ciesla JA. How changes in depression and anxiety symptoms correspond to variations in female sexual response in a nonclinical sample of young women: a daily diary study. J Sex Med 2014; 11: 2915–2927. [DOI] [PubMed] [Google Scholar]
- 47. Kalmbach DA, Pillai V. Daily affect and female sexual function. J Sex Med 2014; 11: 2938–2954. [DOI] [PubMed] [Google Scholar]
- 48. Cyranowski JM, Bromberger J, Youk A, et al. Lifetime depression history and sexual function in women at midlife. Arch Sex Behav 2004; 33: 539–548. [DOI] [PubMed] [Google Scholar]
- 49. Dell’Osso L, Carmassi C, Carlini M, et al. Sexual dysfunctions and suicidality in patients with bipolar disorder and unipolar depression. J Sex Med 2009; 6: 3063–3070. [DOI] [PubMed] [Google Scholar]
- 50. Bradford A, Meston CM. The impact of anxiety on sexual arousal in women. Behav Res Ther 2006; 44: 1067–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Van Minnen A, Kampman M. The interaction between anxiety and sexual functioning: a controlled study of sexual functioning in women with anxiety disorders. Sex Rel Ther 2000; 15: 47–57. [Google Scholar]
- 52. Aksaray G, Yelken B, Kaptanoglu C, et al. Sexuality in women with obsessive compulsive disorder. J Sex Marital Ther 2001; 27: 273–277. [DOI] [PubMed] [Google Scholar]
- 53. Bodinger L, Hermesh H, Aizenberg D, et al. Sexual function and behavior in social phobia. J Clin Psychiatry 2002; 63: 874–879. [DOI] [PubMed] [Google Scholar]
- 54. Figueira I, Possidente E, Marques C, et al. Sexual dysfunction: a neglected complication of panic disorder and social phobia. Arch Sex Behav 2001; 30: 369–377. [DOI] [PubMed] [Google Scholar]
- 55. Palace EM, Gorzalka BB. The enhancing effects of anxiety on arousal in sexually dysfunctional and functional women. J Abnorm Psychol 1990; 99: 403–411. [DOI] [PubMed] [Google Scholar]
- 56. Chivers ML, Seto MC, Lalumière ML, et al. Agreement of self-reported and genital measures of sexual arousal in men and women: a meta-analysis. Arch Sex Behav 2010; 39: 5–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nobre P, Pinto-Gouveia J. Emotions during sexual activity: differences between sexually functional and dysfunctional men and women. Arch Sex Behav 2006; 35: 491–499. [DOI] [PubMed] [Google Scholar]
- 58. Stoléru S, Fonteille V, Cornélis C, et al. Functional neuroimaging studies of sexual arousal and orgasm in healthy men and women: a review and meta-analysis. Neurosci Biobehav Rev 2012; 36: 1481–1509. [DOI] [PubMed] [Google Scholar]
- 59. Fontenelle LF, de Souza WF, de Menezes GB, et al. Sexual function and dysfunction in Brazilian patients with obsessive compulsive disorder and social anxiety disorder. J Nerv Ment Dis 2007; 195: 254–257. [DOI] [PubMed] [Google Scholar]
- 60. Burri A, Ogata S, Williams F. Female sexual pain: epidemiology and genetic overlap with chronic widespread pain. Eur J Pain 2017; 21: 1408–1416. [DOI] [PubMed] [Google Scholar]
- 61. Desrochers G, Bergeron S, Landry T, et al. Do psychosexual factors play a role in the etiology of provoked vestibulodynia? A critical review. J Sex Marital Ther 2008; 34: 198–226. [DOI] [PubMed] [Google Scholar]
- 62. Arpana G, Rapkin AJ, Gill Z, et al. Disease-related differences in resting state networks: a comparison between localized provoked vulvodynia, irritable bowel syndrome, and healthy control subjects. Pain 2015; 156(5): 809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Donaldson RL, Meana M. Early dyspareunia experience in young women: confusion, consequences, and help-seeking barriers. J Sex Med 2011; 8: 814–823. [DOI] [PubMed] [Google Scholar]
- 64. Basson R. The recurrent pain and sexual sequelae of provoked vestibulodynia: a perpetuating cycle. J Sex Med 2012; 9: 2077–2092. [DOI] [PubMed] [Google Scholar]
- 65. Mago R. Adverse effects of psychotropic medications A call to action. Psychiatric Clin N Am 2016; 39(3): 361–361. [DOI] [PubMed] [Google Scholar]
- 66. Lorenz T, Rullo J, Faubion S. Antidepressant-induced female sexual dysfunction. Mayo Clin Proc 2016; 91: 1280–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Reichenpfader U, Gartlehner G, Morgan LC, et al. Sexual dysfunction associated with second-generation antidepressants in patients with major depressive disorder: results from a systematic review with network meta-analysis. Drug Saf 2014; 37: 19–31. [DOI] [PubMed] [Google Scholar]
- 68. Cartwright C, Gibson K, Read J, et al. Long-term antidepressant use: patient perspectives of benefits and adverse effects. Patient Prefer Adherence 2016; 10: 1401–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. La Torre A, Giupponi G, Duffy D, et al. Sexual dysfunction related to psychotropic drugs: a critical review—part 1: antidepressants. Pharmacopsychiatry 2013; 46: 191–199. [DOI] [PubMed] [Google Scholar]
- 70. Bijlsma EY, Chan JS, Olivier B, et al. Sexual side effects of serotonergic antidepressants: mediated by inhibition of serotonin on central dopamine release? Pharmacol Biochem Behav 2014; 121: 88–101. [DOI] [PubMed] [Google Scholar]
- 71. Segraves RT, Balon R. Antidepressant-induced sexual dysfunction in men. Pharmacol Biochem Behav 2014; 121: 132–137. [DOI] [PubMed] [Google Scholar]
- 72. Ellison JM. Exercise-induced orgasms associated with fluoxetine treatment of depression. J Clin Psychiatry 199; 57(12): 596–597. [DOI] [PubMed] [Google Scholar]
- 73. Clayton A, Kornstein S, Prakash A. Changes in sexual functioning associated with duloxetine, escitalopram, and placebo in the treatment of patients with major depressive disorder. J Sex Med 2007; 4: 917–929. [DOI] [PubMed] [Google Scholar]
- 74. Culpepper L, Kovalick LJ. A review of the literature on the selegiline transdermal system: an effective and well-tolerated monoamine oxidase inhibitor for the treatment of depression. Prim Care Companion J Clin Psychiatry 2008; 10: 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Clayton AH, Locklear JC, Svedsater H, et al. Sexual functioning in patients with major depressive disorder in randomized placebo-controlled studies of extended release quetiapine fumarate. CNS Spectr 2014; 19: 182–196. [DOI] [PubMed] [Google Scholar]
- 76. Montejo AL, Llorca G, Izquierdo JA, et al. Incidence of sexual dysfunction associated with antidepressant agents: a prospective multicenter study of 1022 outpatients. Spanish Working Group for the study of Psychotropic-Related Sexual Dysfunction. J Clin Psychiatry 2001; 62(3): 10–21. [PubMed] [Google Scholar]
- 77. Pereira VM, Arias-Carrion O, Machado S, et al. Bupropion in the depression-related sexual dysfunction: a systematic review. CNS Neurol Disord Drug Targets 2014; 13: 1079–1088. [DOI] [PubMed] [Google Scholar]
- 78. Boyarsky BK, Hirshfeld RM. The management of medication-induced sexual dysfunction. Essent Psychopharmacol 2000; 3: 39–58. [Google Scholar]
- 79. Baldwin DS. Sexual dysfunction associated with antidepressant drugs. Expert Opin Drug Saf 2004; 3: 457–470. [DOI] [PubMed] [Google Scholar]
- 80. Clayton AH, Durgam S, Li D, et al. Effects of vilazodone on sexual functioning in healthy adults: results from a randomized, double-blind, placebo-controlled, and active-controlled study. Int Clin Psychopharmacol 2017; 32: 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jacobsen PL, Mahableshwarkar AR, Palo WA, et al. Treatment-emergent sexual dysfunction in randomized trials of vortioxetine for major depressive disorder or generalized anxiety disorder: a pooled analysis. CNS Spectr 2016; 21: 367–378. [DOI] [PubMed] [Google Scholar]
- 82. Fava M, Dording CM, Baker RA. Effects of adjunctive aripiprazole on sexual functioning in patients with major depressive disorder and an inadequate response to standard antidepressant monotherapy: a post hoc analysis of 3 randomized, double-blind, placebo-controlled studies. Prim Care Companion CNS Disord 2011; 13: e1–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Grover S, Ghosh A, Sarkar S, et al. Sexual dysfunction in clinically stable patients with bipolar disorder receiving lithium. J Clin Psychopharmacol 2014; 34: 475–482. [DOI] [PubMed] [Google Scholar]
- 84. De Boer MK, Castelein S, Wiersma D, et al. The facts about sexual (Dys) function in schizophrenia: an overview of clinically relevant findings. Schizophr Bull 2015; 41(3): 674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Macdonald S, Halliday J, Mac Ewan T, et al. Nithsdale schizophrenia surveys 24: sexual dysfunction: case-control study. Br J Psychiatry 2003; 182: 50–56. [DOI] [PubMed] [Google Scholar]
- 86. Huguelet P, Mohr S, Miserez C, et al. An exploration of sexual desire and sexual activities of women with psychosis. Community Ment Health J 2015; 51(2): 229–238. [DOI] [PubMed] [Google Scholar]
- 87. Bitter I, Basson BR, Dossenbach MR. Antipsychotic treatment and sexual functioning in first-time neuroleptic-treated schizophrenic patients. Int Clin Psychopharmacol 2005; 20(1): 19–21. [DOI] [PubMed] [Google Scholar]
- 88. Marques TR, Smith S, Bonaccorso S, et al. Sexual dysfunction in people with prodromal or first-episode psychosis. Br J Psychiatry 2012; 201: 131–136. [DOI] [PubMed] [Google Scholar]
- 89. Malik P, Kemmler G, Hummer M, et al. EUFEST Study Group. Sexual dysfunction in first-episode schizophrenia patients results from European first episode schizophrenia trial. J Clin Psychopharmacol 2011; 31(3): 274–280. [DOI] [PubMed] [Google Scholar]
- 90. Segalovich J, Doron A, Behrbalk P, et al. Internalization of stigma and self-esteem as it affects the capacity for intimacy among patients with schizophrenia. Arch Psychiatr Nurs 2013; 27(5): 231–234. [DOI] [PubMed] [Google Scholar]
- 91. Peitl M, Rubesa G, Peitl V, et al. Aspects of sexual self-perception in schizophrenic patients. Eur J Psychiatry 2009; 23: 37–46. [Google Scholar]
- 92. McCann E. Investigating mental health service user views regarding sexual and relationship issues. J Psychiatr Ment Health Nurs 2010; 17(3): 251–259. [DOI] [PubMed] [Google Scholar]
- 93. El Kissi Y, Laaroussi M, Gaabout S, et al. Sexual activity and marital relationships in Tunisian schizophrenic patients: comparison with healthy controls. Europ Neuropsychopharmacol 2010; 20: S511. [Google Scholar]
- 94. Kim CK, Kim HG, Kim DJ, et al. Sexual life and the view of marriage in the unmarried outpatients with schizophrenia. Europ Neuropsychopharmacol 2009; 19: S482. [Google Scholar]
- 95. Meade CS, Kershaw TS, Hansen NB, et al. Long-term correlates of childhood abuse among adults with severe mental illness: adult victimization, substance abuse, and HIV sexual risk behavior. AIDS Behav 2009; 13: 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Murphy J, Shevlin M, Houston JE, et al. Modelling the co-occurrence of psychosis-like experiences and childhood sexual abuse. Soc Psychiatry Psychiatr Epid 2014; 49(7): 1037–1044. [DOI] [PubMed] [Google Scholar]
- 97. Shevlin M, O’Neill T, Houston JE, et al. Patterns of lifetime female victimization and psychotic experiences: a study based on the adult psychiatric morbidity survey. Soc Psychiatry Psychiatr Epid 2012; 48: 15–24. [DOI] [PubMed] [Google Scholar]
- 98. Lee JY, Kim SW, Lee YH, et al. Factors associated with self-rated sexual function in Korean patients with schizophrenia receiving risperidone monotherapy. Hum Psychopharmacol 2015; 30(6): 416–424. [DOI] [PubMed] [Google Scholar]
- 99. Tenback DE, van Harten PN, Slooff CJ, et al. Tardive dyskinesia in schizophrenia is associated with prolactin-related sexual disturbances. Neuropsychopharmacol 2006; 31: 1832–1837. [DOI] [PubMed] [Google Scholar]
- 100. Otunctemur A, Dursun M, Ozbek E, et al. Effect of metabolic syndrome on sexual function in pre- and postmenopausal women. J Sex Marital Ther 2014; 41(4): 440–449. [DOI] [PubMed] [Google Scholar]
- 101. Chiesa A, Leucci V, Serretti A, et al. Antipsychotics and sexual dysfunction: epidemiology, mechanisms and management. Clin Neuropsychiatry 2013; 10(1): 31. [Google Scholar]
- 102. Dossenbach M, Dyachkova Y, Pirildar S, et al. Effects of atypical and typical antipsychotic treatments on sexual function in patients with schizophrenia: 12- month results from the Intercontinental Schizophrenia Outpatient Health Outcomes (IC-SOHO) study. Eur Psychiatry 2006; 21(4): 251–258. [DOI] [PubMed] [Google Scholar]
- 103. Eberhard J, Lindström E, Holstad M, et al. Prolactin level during 5 years of risperidone treatment in patients with psychotic disorders. Acta Psychiatr Scand 2007; 115(4): 268–276. [DOI] [PubMed] [Google Scholar]
- 104. Rettenbache MA, Hofer A, Ebenbichler C, et al. Prolactin levels and sexual adverse effects in patients with schizophrenia during antipsychotic treatment. J Clin Psychopharmacol 2010; 30(6): 711–715. [DOI] [PubMed] [Google Scholar]
- 105. Rubio-Abadal E, Del Cacho N, Saenz-Navarrete G, et al. How hyperprolactinemia affects sexual function in patients under antipsychotic treatment. J Clin Psychopharmacol 2016; 36(5): 422–428. [DOI] [PubMed] [Google Scholar]
- 106. Howes OD, Wheeler MJ, Pilowsky LS, et al. Sexual function and gonadal hormones in patients taking antipsychotic treatment for schizophrenia or schizoaffective disorder. J Clin Psychiatry 2007; 68: 361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. La Torre A, Conca A, Duffy D, et al. Sexual dysfunction related to psychotropic drugs: a critical review part II: antipsychotics. Pharmacopsychiatry 2013; 46(6): 201–208. [DOI] [PubMed] [Google Scholar]
- 108. Baggaley M. Sexual dysfunction in schizophrenia: focus on recent evidence. Hum Psychopharmacol 2008; 23(3): 201–209. [DOI] [PubMed] [Google Scholar]
- 109. Fujii A, Yasui-Furukori N, Sugawara N, et al. Sexual dysfunction in Japanese patients with schizophrenia treated with antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34: 288–293. [DOI] [PubMed] [Google Scholar]
- 110. Hanssens L, L’Italien G, Loze J, et al. The effect of antipsychotic medication on sexual function and serum prolactin levels in community-treated schizophrenic patients: results from the schizophrenia trial of aripiprazole (STAR) study (NCT00237913). BMC Psychiatry 2008; 8(1): 95–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zeidan F, Martucci KT, Kraft RA, et al. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci 2011; 31: 5540–5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Brotto LA, Basson R, Smith K, et al. Mindfulness-based group therapy for women with provoked vestibulodynia. Mindfulness 2014; 6: 417–432. [Google Scholar]
- 113. Thomas HN, Hamm M, Hess R, et al. Patient-centred outcomes and treatment preferences regarding sexual problems: a qualitative study among midlife women. J Sex Med 2017; 14: 1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Brotto L, Atallah S, Johnson- Agbakwu C, et al. Psychological and interpersonal dimensions of sexual function and dysfunction. J Sex Med 2016; 13: 538–571. [DOI] [PubMed] [Google Scholar]
- 115. Gunzler C, Berner MM. Efficacy of psychosocial interventions in men and women with sexual dysfunctions—a systematic review of controlled clinical trials: part 2. J Sex Med 2012; 9: 3108–3125. [DOI] [PubMed] [Google Scholar]
- 116. Frühauf S, Gerger H, Schmidt HM. Efficacy of psychological interventions for sexual dysfunction: a systematic review and meta-analysis. Arch Sex Behav 2013; 42: 915–933. [DOI] [PubMed] [Google Scholar]
- 117. Landry T, Bergeron S, Dupuis MJ, et al. The treatment of provoked vestibulodynia: a critical review. Clin J Pain 2008; 24: 155–171. [DOI] [PubMed] [Google Scholar]
- 118. Masheb RM, Kerns RD, Lozano C, et al. A randomized clinical trial for women with vulvodynia: cognitive-behavioral therapy vs. supportive therapy. Pain 2009; 141: 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron 2007; 55: 377–391. [DOI] [PubMed] [Google Scholar]
- 120. Bergeron S, Khalifé S, Glazer HI, et al. Surgical and behavioral treatments for vestibulodynia: two-and-one-half year follow-up and predictors of outcome. Obstet Gynecol 2008; 111: 159–166. [DOI] [PubMed] [Google Scholar]
- 121. Brotto LA, Basson R. Group mindfulness-based therapy significantly improves sexual desire in women. Behav Res Ther 2014; 57: 43–54. [DOI] [PubMed] [Google Scholar]
- 122. Kocsis A, Newbury-Helps J. Mindfulness in sex therapy and intimate relationships (MSIR): clinical protocol and theory development. Mindfulness 2016; 7: 7690–7699. [Google Scholar]
- 123. Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation. Gen Hosp Psychiatry 1982; 4: 33–47. [DOI] [PubMed] [Google Scholar]
- 124. Schütze R, Rees C, Preece M, et al. Low mindfulness predicts pain catastrophizing in a fear-avoidance model of chronic pain. Pain 2010; 148: 120–127. [DOI] [PubMed] [Google Scholar]
- 125. Fox KCR, Nijeboer S, Dixon ML, et al. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci Biobehav Rev 2014; 43: 48–73. [DOI] [PubMed] [Google Scholar]
- 126. Brotto LA, Basson R, Carlson M, et al. Impact of a mindfulness-based cognitive behavioural treatment for Provoked Vestibulodynia: a qualitative study. Sex Rel Ther 2013; 28: 3–19. [Google Scholar]
- 127. Masters WH, Johnson VE. Human sexual inadequacy. Boston, MA: Ishi Press, 1970. [Google Scholar]
- 128. Pereira VM, Arias- Carrión O, Machado S, et al. Sex therapy for female sexual dysfunction. Int Arch Med 2013; 6: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Peixoto MM, Nobre P. Cognitive schemas activated in sexual context: a comparative study with homosexual and heterosexual men and women, with and without sexual problems. Cognitive Ther Res 2015; 39(3): 390–402. [Google Scholar]
- 130. Labrie F, Derogatis L, Archer DF, et al. Effect of intravaginal prasterone on sexual dysfunction in postmenopausal women with vulvovaginal atrophy. J Sex Med 2015; 12: 2401–2412. [DOI] [PubMed] [Google Scholar]
- 131. Van Geelen JM, van de Weijer PH, Arnolds HT. Urogenital symptoms and resulting discomfort in non-institutionalized Dutch women aged 50-75 years. Int Urogynecol J Pelvic Floor Dysfunct 2000; 11: 9–14. [DOI] [PubMed] [Google Scholar]
- 132. Chen J, Geng L, Song X, et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med 2013; 10: 1575–1584. [DOI] [PubMed] [Google Scholar]
- 133. Goetsch MF, Lim JY, Caughey AB. A practical solution for dyspareunia in breast cancer survivors: a randomized controlled trial. J Clin Oncol 2015; 33: 3394–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Prendergast SA. Pelvic floor physical therapy for vulvodynia. Obstet Gynecol Clin N Am 2017; 44: 509–522. [DOI] [PubMed] [Google Scholar]
- 135. Morin M, Carroll M-S, Bergeron S. Systematic review of the effectiveness of physical therapy modalities in women with provoked vestibulodynia. Sex Med Rev 2017; 5: 295–322. [DOI] [PubMed] [Google Scholar]
- 136. Rosenbaum TY. An integrated mindfulness-based approach to the treatment of women with sexual pain and anxiety: promoting autonomy and mind/body connection. Sex Rel Ther 2013; 28: 20–28. [Google Scholar]
- 137. Basson RJ. Using a different model for female sexual response to address women’s problematic low sexual desire. J Sex Marital Ther 2001; 27: 395–403. [DOI] [PubMed] [Google Scholar]
- 138. Safarinejad MR, Hosseini SY, Asgari MA. A randomized, double-blind, placebo-controlled study of the efficacy and safety of bupropion for treating hypoactive sexual desire disorder in ovulating women. BJU Int 2010; 106: 832–839. [DOI] [PubMed] [Google Scholar]
- 139. Clayton AH, Baker RA, Sheehan JJ. Comparison of adjunctive use of aripiprazole with bupropion or selective serotonin reuptake inhibitors/serotonin-norepinephrine reuptake inhibitors: analysis of patients beginning adjunctive treatment in a 52-week, open-label study. BMC Res Notes 2014; 7: 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Jacobsen PL, Mahableshwarkar AR, Chen Y, et al. Effect of vortioxetine vs. escitalopram on sexual functioning in adults with well-treated major depressive disorder experiencing SSRI-induced sexual dysfunction. J Sex Med 2015; 12: 2036–2048. [DOI] [PubMed] [Google Scholar]
- 141. Fooladi E, Bell RJ, Jane F, et al. Testosterone improves antidepressant-emergent loss of libido in women: findings from a randomized, double-blind, placebo-controlled trial. J Sex Med 2014; 11: 831–839. [DOI] [PubMed] [Google Scholar]
- 142. Khamba B, Aucoin M, Lytle M. Efficacy of acupuncture treatment of sexual dysfunction secondary to antidepressants. J Altern Compl Med 2013; 19: 862–869. [DOI] [PubMed] [Google Scholar]
- 143. Kashani L, Raisi F, Saroukhani S, et al. Saffron for treatment of fluoxetine-induced sexual dysfunction in women: randomized double-blind placebo-controlled study. Hum Psychopharmacol 2013; 28: 54–60. [DOI] [PubMed] [Google Scholar]
- 144. Dording CM, Schettler PJ, Dalton ET. A double-blind placebo-controlled trial of maca root as treatment for antidepressant-induced sexual dysfunction in women. Evid Based Compl Altern Med 2015; 2015: 949036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Nurnberg HG, Hensley PL, Heiman JR, et al. Sildenafil treatment of women with antidepressant-associated sexual dysfunction. JAMA 2008; 300: 395–404. [DOI] [PubMed] [Google Scholar]
- 146. Lorenz TA, Meston CM. Acute exercise improves physical sexual arousal in women taking antidepressants. Ann Behav Med 2012; 43: 352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Mir A, Shivakumar K, Williamson RJ, et al. Change in sexual dysfunction with aripiprazole: a switching or add-on study. J Psychopharmacol 2008; 22(3): 244–253. [DOI] [PubMed] [Google Scholar]
- 148. Clayton AH, Alkis AR, Parikh NB, et al. Sexual dysfunction due to psychotropic medications. Psychiatric Clinics 2016; 39(3): 427–463. [DOI] [PubMed] [Google Scholar]
- 149. Östman M, Björkman AC. Schizophrenia and relationships: the effect of mental illness on sexuality. Clin Schizophr Relat Psychoses 2013; 7: 20–24. [PubMed] [Google Scholar]
- 150. Basson R, Brotto LA, Petkau J, et al. Role of androgens in women’s sexual dysfunction. Menopause 2010; 17(5): 962–971. [DOI] [PubMed] [Google Scholar]
- 151. Snabes MC, Zborowski J, Simes S. Libigel (testosterone gel) does not differentiate from placebo therapy in the treatment of hypoactive sexual desire in postmenopausal women. 11th Annual Meeting of the International Society for the Study of Women’s Sexual Health; Jerusalem Il. J Sex Med 2012; 9: 171. [Google Scholar]
- 152. Davis SR, Moreau M, Kroll R, et al. Testosterone for low libido in postmenopausal women not taking estrogen. N Engl J Med 2008; 359: 2005–2017. [DOI] [PubMed] [Google Scholar]
- 153. Jaspers L, Feys F, Bramer WM, et al. Efficacy and safety of flibanserin for the treatment of hypoactive sexual desire disorder in women: a systematic review and meta-analysis. JAMA Intern Med 2016; 176: 453–462. [DOI] [PubMed] [Google Scholar]
- 154. Sahebkar A, Saadat SH, Panahi Y, et al. Systematic review and meta-analysis of flibanserin’s effects and adverse events in women with hypoactive sexual desire disorder. Curr Drug Metab 2016; 18: 78–85. [DOI] [PubMed] [Google Scholar]


