Abstract
An insufficiency of accessible allograft tissue for corneal transplantation leaves many impaired by untreated corneal disease. There is promise in the field of regenerative medicine for the development of autologous corneal tissue grafts or collagen-based scaffolds. Another approach is to create a suitable corneal implant that meets the refractive needs of the cornea and is integrated into the surrounding tissue but does not attempt to perfectly mimic the native cornea on a cellular level. Materials that have been investigated for use in the latter concept include natural polymers such as gelatin, semisynthetic polymers like gelatin methacrylate, and synthetic polymers. There are advantages and disadvantages inherent in natural and synthetic polymers: natural polymers are generally more biodegradable and biocompatible, while synthetic polymers typically provide greater control over the characteristics or property adjustment of the materials. Additive manufacturing could aid in the precision production of keratoprostheses and the personalization of implants.
Keywords: Cornea, keratoprosthesis, additive manufacturing, polymer, hydrogel
Introduction
The cornea has important protective and refractive roles which are supported by its transparent, avascular structure. Its anterior position allows it to transmit and refract light entering the eye and also protect the eye from mechanical damage, infection, and ultraviolet (UV) radiation.1 Because of this, its dysfunction can lead to significant visual impairment. Diseases of the cornea have a significant impact on visual health globally. Collectively, they are the fourth leading cause of bilateral blindness worldwide, following cataracts, glaucoma, and age-related macular degeneration. Corneal diseases are estimated to affect between four and eight million individuals, the majority of whom live in developing countries.2,3 The leading causes of corneal dysfunction include trachoma (involving scarring and vascularization of the cornea), ocular trauma, corneal ulceration, and infections, such as those due to herpes simplex virus.3
Due to the lack of effective medical treatments for corneal disease, keratoplasty (corneal transplant) remains the definitive treatment for corneal blindness. In 2016, 82,994 corneal grafts were performed using tissue supplied by US eye banks.4 This includes penetrating keratoplasties (PKs), anterior lamellar keratoplasties (ALKs), endothelial keratoplasties (EKs), keratolimbal allografts, and keratoprosthesis (K-Pro) placement. In recent years, the performance of EKs has increased, while that of PKs has decreased; since 2012, EKs have outnumbered PKs in the United States.4
The use of living tissue from donors is the mainstay of current corneal transplant technique and these vision-restoring procedures benefit many individuals. There are some drawbacks to the use of donor tissue in keratoplasties, although they are significantly reduced in comparison with other allografts due to the relative immune privilege of the cornea and anterior chamber. Despite this, Stulting et al.5 found that 23% of keratoplasty patients experienced at least one rejection event within 5 years post-op, with pseudophakic or aphakic corneal edema and female gender being risk factors for rejection. In addition, it is reported that the tissue available is only sufficient for approximately 1 in 70 patients in need of corneal transplant worldwide, and that about 53% of the global population has no access to corneal transplantation.6 Other difficulties with corneal allografts include their relatively high cost and inconveniences surrounding the safe extraction, storage, and transportation of living tissue.
While the use of biomaterials and the incorporation of a transplant recipient’s own cells in tissue engineering could resolve the problem of rejection, it may not address the issues of cost and inaccessibility; however, such investigations remain of paramount importance and will likely contribute to the advancement of keratoplasty technique and improve the options available to patients. Thus, there is significant potential for the development of artificial corneas from synthetic/non-living materials. The most commonly used synthetic cornea is the Boston Keratoprosthesis with more than 4500 having been placed worldwide, which individually retailed as of 2010, at US$5000.7,8 The Boston Keratoprosthesis is generally reserved for use in cases that are refractory to treatment with allograft placement, exhibiting multiple graft rejections. The efficient production of synthetic corneas could reduce the expenses associated with allografts and currently available prostheses. Synthetic corneas would also have the distinct advantage, from a global health perspective, of requiring little accommodation during transportation as compared to living grafts. This article will explore the synthetic materials that have been investigated, and the potential utilization of three-dimensional (3D) printing in streamlining and personalizing the production of synthetic keratoprostheses.
Methodology
All articles and resources referenced herein were accessed between 1 November 2017 and 5 January 2018 and located through PubMed/MEDLINE database and Google searches using the listed keywords.
Materials
Various materials are currently being studied that have the potential to serve either as corneal prostheses or scaffolds on which cells can attach. Chen et al.1 recently reviewed these materials and their applicability to corneal transplants. Their investigation covered both natural and synthetic polymers, namely, collagen, silk, gelatin, chitosan, decellularized cornea, thermal responsive polymers, and other synthetic polymer hydrogels. Our intent is to explore those materials which have the greatest potential to be used to 3D print complete, non-cell-based keratoprostheses for utilization in PK.
Natural polymers
Gelatin is an inexpensive, natural polymer derived through hydrolysis denaturation of collagen. While modifiable to an extent, gelatin maintains its cell-binding motifs such as arginyl-glycyl-aspartic acid (RGD) and matrix metalloproteinase (MMP)-sensitive degradation sites.9–12 The primary methods of cross-linking gelatin include dehydrothermal and chemical processes. Dehydrothermal cross-linking results in improved transparency, elastic modulus, and albumin permeability compared to collagen.1 The gelatin produced in this manner also accommodates normal expression of ZO-1, Na+/K+-ATPase, and N-cadherin by seeded primary human corneal endothelial (HCEn) cells.1,13 Chemical cross-linking using glutaraldehyde has been shown to produce gelatin that is conducive to the adherence of fibroblasts and their precursors and extracellular matrix (ECM) deposition in rabbits.14
Cross-linking of EDC (1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide) and NHS (N-hydroxysuccinimide) in gelatin formation allows for variability in particular characteristics of the polymer, such as porosity, Young’s modulus, and swelling ratios.1,15 EDC cross-linking has also been shown to allow for the incorporation of other components such as heparin which increases the absorption and release of basic fibroblast growth factors (bFGF).16 Niu et al.16 demonstrated that with the scaffold formed using EDC cross-linking, HCEn cells can be effectively grown, morphology and function are maintained, and the scaffold fuses with the native stroma. Chondroitin sulfate (CS) is another structural component that has been incorporated into gelatin, with the effect of enhanced water content, glucose permeation, fibronectin absorption, and stimulation of cultivated keratocyte biosynthetic activity.17
Semisynthetic polymers
Gelatin methacrylate (GelMA) has potential as an implantable tissue material due to its combination of many of the advantageous qualities of natural and synthetic polymers such as the retained cell-binding motifs and degradation sites of gelatin and the stability and modifiable physical characteristics of synthetic biomaterials.1 Production of GelMA involves radical polymerization with a photoinitiator and has been described as a 1- to 2-week process.18 VA-086 (2,2ʹ-azobis[2-methyl-N-(2-hydroxyethyl)propionamide]) has been specifically noted to be an appropriate photoinitiator in the presence of a UV LED source for the cross-linking of cell-laden GelMA; increased concentrations of gelatin resulted in shorter polymerization times and enhanced rheological properties.19 A composite hydrogel made up of poly(ethylene glycol) dimethacrylate (PEGDMA) and GelMA was synthesized in varying concentrations by Kim et al.20 and may represent a suitable material for tissue graft procedures due to the tunability and reproducibility conferred by the synthetic polymer PEGDMA and the capability to endothelialize inherent in the semisynthetic GelMA.
In studying GelMA microparticles with a wide range of cross-linking densities (15%–90%), Nguyen et al.21 observed that less methacrylation of microparticles corresponded with decreased elastic moduli and increased mesh sizes, while increased methacrylation correlated with greater moduli and smaller mesh sizes. Shirahama et al.22 noted that a low feed ratio of methacrylic acid (MAA) to gelatin enabled optimal GelMA synthesis in a single pot. Cross-linking density was inversely related to degradation rate, and lower densities enabled greater growth factor holding and release of bone morphogenic protein 4 (BMP4) and bFGF upon collagenase treatment.21 Bertassoni et al.23 used direct-write bioprinting of photolabile, cell-laden GelMA hydrogels at concentrations ranging from 7% to 15% and noted that the printability of the polymer bores a direct relationship to the mechanical properties of the hydrogel. Successful bioprinting of methacrylate ethanolamide gelatin and methacrylated hyaluronan has also been reported.24
Hybrid hydrogel systems can also be formed with desired properties and characteristics through the combination of GelMA with nanoparticles such as graphene oxide and carbon nanotubes.25 The production of hybrid cross-linked GelMA hydrogel (GelMA+) with an eightfold improvement on GelMA in mechanical strength due to decreased pore size and greater uniformity, as well as good support of HCEn cells, has been reported.26 GelMA+ is the result of physical cross-linking, through incubation of the prepolymer solution at 4°C for 1 h, followed by UV cross-linking. GelMA+ films seeded with HCEn cells and transplanted into rabbit models demonstrated good cell viability.1,26 GelMA has been combined with a variety of different polymers and in conjunction with various carbon-based composites in attempts to confer specific qualities on the resulting mixtures. Such efforts may enable the efficient use of semisynthetic polymers in many biomedical applications.27–35
Synthetic polymer hydrogels
Many synthetic polymers have been employed in the study and production of keratoprostheses. One of the primary difficulties encountered in the synthesis and implantation of fully synthetic artificial corneas is their inability to integrate properly with native cells. Due to this complication, most keratoprostheses employ the “core and skirt” design (Figure 1) which involves a transparent core that is surrounded by porous material that enables host cellular association and the anchoring of the prosthesis through fibroblast in-growth.36 Poly(methyl methacrylate) (PMMA) was the first synthetic polymer to be used in artificial cornea construction, and the introduction of the “core and skirt” model slightly improved the rate of successful prosthokeratoplasties. However, insufficient host tissue integration into the prosthetic skirts remains one of the significant barriers to the creation of a scalable keratoprosthetic design successful in the majority of cases.37 A variety of porous skirt designs have been developed with varying levels of success reported. In general transplantation, hydrophilic materials and/or those of high porosity were associated with improved host cell integration; in the context of artificial corneas, materials with small pores and without long, oriented attachment surfaces are most accommodating of an advantageous healing process without angiogenesis or fibrous capsule formation.37–41 It is notable that keratoprostheses can fail due to retroprosthetic membrane formation and calcification in addition to the more general keratoplasty complications of infection, glaucoma, and retinal detachment.36 Synthetic keratoprostheses have historically carried increased risk of microbial infection or prosthesis protrusion due to their inability to support intact epithelium establishment.42 Extrusion of the keratoprosthesis is largely due to necrosis around the edge of the prosthesis and consequent dislodging.37
Figure 1.
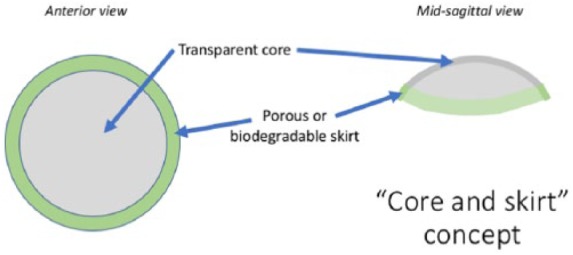
The core is to remain indefinitely clear and to exhibit structural stability throughout the life of the prosthesis. The skirt is to enable the implant to integrate into the host tissue and allow host cells to associate with it either through surface modification or use of a highly biocompatible/biodegradable material.
Legeais et al.43–48 developed two distinct generations of artificial corneas with porous skirts, of which one is marketed in Europe.37 This second generation of keratoprosthesis consisted of a polyvinylpyrrolidone (PVP)-coated polydimethylsiloxane core fused to an expanded polytetrafluoroethylene (PTFE) skirt.48 This artificial cornea was reportedly successful in human clinical trials, although the authors did note that a lack of epithelial formation over the optical interface likely contributed to a lack of long-term stability of the implant.37,48
Poly(vinyl alcohol) (PVA) has been explored for its potential use in keratoprostheses. In efforts to enable epithelial association, type I collagen (Col I) has been immobilized onto PVA to form a PVA-Col I scaffold. Miyashita et al.42 found that such a scaffold supports epithelial growth and that the resulting prosthesis is suturable in the rabbit cornea. The use of electrospinning and surface modification in PVA nanofiber production process is associated with improved mechanical anchoring of the epithelial cell layer.49 The transmissibility of light through PVA-based artificial corneas has not yet been shown to be sufficient for optimal vision.
Another synthetic polymer that has received some attention in artificial cornea research is poly(2-hydroxyethyl methacrylate) (PHEMA). Early use of PHEMA in artificial cornea construction involved the glow discharge polymerization of PHEMA onto a silicon membrane, with good corneal epithelial cell migration onto the resulting prosthesis noted.50 Chirila and colleagues37,51–54 reported good results with a keratoprosthesis comprised entirely of PHEMA of varying texture: an opaque sponge for the porous skirt and a transparent gel for the core. The PHEMA sponge component was synthesized through phase-contrast polymerization in aqueous solution, and a permanent joint was established between the differing components. The greatest success was observed with the slightly smaller (7 mm diameter, 0.5 mm thickness) “type II” implant in which the skirt material passed through the entire depth of the prosthesis rather than only sitting on the anterior aspect as in the “type I” design. In the initial human trial, 9 of the 10 implants were still functional and intact during the reported follow-up period which ranged from 1 to 23 months; the rejected implant was reversed to a graft due to recurrent inflammation of the cornea.37 Calcification of PHEMA sponges is a potentially concerning complication; it appears that calcium-chelated plasma cell adsorption onto the sponge surface, establishment of nucleation sites for calcium phosphate crystallization, and spontaneous calcium phosphate precipitation due to low water content all contribute to the calcification of PHEMA implants.55 Sinha and Gupte56 recently reported promising results with a PHEMA graft that incorporated graphite into the skirt into which pores were introduced through a salt leaching process using sodium chloride. The optimal water content of the polymeric film was determined to be 50% at which a refractive index of 1.4 was achieved. Optimal porosity through optical and scanning electron microscopy and corresponding adjustment of salt concentration enabled production of keratoprostheses with suitable mechanical strength, water vapor transmission rate, and degradation behavior. Cell adhesion appeared to be encouraged by the incorporation of graphite into the skirt, with cell adhesion being observed in the skirt region and not on the optical core.56 Grafting of phosphate groups onto PHEMA hydrogels using atom transfer radical polymerization (ATRP) has also been shown to support the attachment and growth of human corneal epithelial cells (HCECs).57
Yanez-Soto and colleagues58,59 have investigated the potential of biochemically and topographically engineered poly(ethylene glycol) diacrylate (PEGDA) hydrogels in keratoprostheses. They observed that corneal epithelial wound healing was increased by 50% in hydrogel substrates containing specific bioligands in comparison with those without.59 An example of such topographic surface functionalization is the incorporation of the RGD integrin-binding peptide.58
The importance of surface modification of synthetic polymers as substrates was echoed by Kim et al.60 who coated poly(lactic-co-glycolic acid) (PLGA) with type I collagen (Col I). By analyzing the material using field emission scanning electron microscopy (FESEM), atomic force microscopy (AFM), Fourier-transform infrared spectroscopy (FTIR), and contact angle analysis, it was determined that the Col I-PLGA film is of suitable structural and morphological construction for corneal implantation: exhibiting transparency, appropriate hydrophilicity, stability, and good water uptake. In addition, the Col I-PLGA films demonstrated higher Ra (nm) values (indicating increased roughness or improved surface texture) as compared to plain PLGA films. Rabbit corneal endothelial (RCEn) cells cultured on the Col I-PLGA films exhibited enhanced initial attachment, proliferation, and expression of messenger RNAs (mRNAs) as compared to plain PLGA films in addition to adopting the desired polygonal shape characteristic of their cell type.60
Poly(ethylene glycol)/poly(acrylic acid) (PEG/PAA)-based hydrogels have also shown promise for use in keratoprostheses as highly refractive materials. PEG/PAA-interpenetrating polymer network (IPN) hydrogels have been created that exhibit glucose permeability almost identical to that of the human cornea (~2.5 × 10−6 cm2/s) which were reported to be well tolerated when implanted in rabbits in 9 out of 10 cases up to 14 days.61 Hartmann et al.62 reported inflammation and corneal hazing related to the implanting of inlays based on a PEG-diacrylate precursor. However, PEG/PAA IPN hydrogel implants with the acrylate groups modified to stable acrylamide functionalities exhibited significantly improved biocompatibility under hydrolytic conditions with implanted rabbits exhibiting clear eyes and lack of inflammation up to 6 months post-op. An IPN hydrogel containing zinc sulfide (ZnS) nanoparticles covalently linked to PHEMA combined with PAA is reported to exhibit desirable qualities for keratoprostheses with high refractive indexes of 1.65 (dry) and 1.49 (hydrated) with observed variation due to water content, an equilibrium water content of 60.2%, and minimal cytotoxicity toward primary epidermal keratinocytes.63 The same group that reported on the ZnS/PHEMA/PAA hydrogel also developed hydrogel nanocomposites comprising ZnS, PVP, and poly-dimethylacrylamide (PDMAA) with similar characteristics.64
Regardless of the synthetic polymer that is used in corneal prostheses, the literature indicates that some sort of surface or structural modification appears to be necessary to enable appropriate integration of the host tissue (Table 1).
Table 1.
Summary of materials/polymers, their key strengths and weaknesses, and commercial availability.
| Materials | Key strengths/weaknesses | Available commercially? |
|---|---|---|
| Gelatin | Strengths: inexpensive; maintains cell-binding motifs (e.g. RGD) and matrix metalloproteinase-sensitive degradation sites; can be cross-linked to improve transparency, elastic modulus, albumin permeability, porosity, swelling ratios, and cell adherence Weaknesses: less relative modifiability compared to synthetic polymers; relative lack of stability as a corneal implant |
Yes |
| Gelatin methacrylate | Strengths: retains cell-binding motifs and degradation sites like gelatin; improved stability and modifiability of physical characteristics compared to gelatin; can be synthesized as a composite material with a synthetic polymer such as PEGDMA for improved tunability and reproducibility; modifiable through combination with nanoparticles or hybrid hydrogel synthesis Weaknesses: expensive; production requires a 1- to 2-week process involving radical polymerization with a photoinitiator |
Yes (photoinitiation in presence of UV light required) |
| Poly(methyl methacrylate) | Strengths: moderately inexpensive; extensive clinical use in keratoprostheses compared to other materials; extensive use in 3DP; modifiability of physical characteristics such as transparency Weaknesses: rigid material; lacks ability to integrate well into host tissue; associated with retroprosthetic membrane formation, calcification, glaucoma, retinal detachment, infection, and prosthesis extrusion |
Yes |
| Poly(vinyl alcohol) | Strengths: inexpensive; already used extensively in 3DP; nontoxic, water-soluble, and biodegrades slowly; can support cell growth when combined with collagen Weaknesses: relative instability; inadequate transmissibility of light |
Yes |
| Poly(2-hydroxyethyl methacrylate) | Strengths: extensive clinical experience in ocular applications (in contact lenses and some keratoprostheses); can support cell migration and integration into the host tissue; relatively good results as keratoprostheses in limited human clinical experience Weaknesses: moderately expensive; prone to calcification |
Yes |
| Poly(ethylene glycol) diacrylate | Strengths: flexible; modifiable; can be engineered to contain specific bioligands for improved integration Weaknesses: expensive, limited in vivo experience |
Yes (photoinitiation in presence of UV light required) |
| Poly(lactic-co-glycolic acid) coated with type 1 collagen | Strengths: appropriate transparency, hydrophilicity, water uptake, stability, and surface texture; can support cell attachment, proliferation, and mRNA expression; has FDA approval for many applications; biodegradable and biocompatible Weaknesses: expensive; limited experience in ocular applications |
Yes (must be coated with Col I separately) |
| Poly(ethylene glycol)/poly(acrylic acid) | Strengths: highly refractive; modifiable; limited experience in rabbits for 14 days yielded few rejection events Weaknesses: limited experience |
Components available separately |
RGD: arginyl-glycyl-aspartic acid; PEGDMA: poly(ethylene glycol) dimethacrylate; 3DP: three-dimensional printing; UV: ultraviolet; PLGA: poly(lactic-co-glycolic acid); FDA: Food and Drug Administration; Col I: type 1 collagen; mRNA: messenger RNA.
3D printing
Inkjet, extrusion-based, and laser-assisted printing are three commonly used techniques in additive manufacturing.65 Inkjet and extrusion printing may have particular efficacy in the printing of corneal implants or tissues. Inkjet printing involves the deposition of small droplets of material in precise locations. Inkjet printing may be useful in cell-based efforts: in investigating the possibility of printing retinal cells, Lorber et al.66 successfully printed retinal ganglion cells and glia from rats using a piezoelectric printer. An extruder system involves cold and hot components: the cold end feeds the material into the printer, while the hot end melts the material and forces it through the nozzle to attach to the surface onto which it is applied. Micro-extrusion printing is relatively inexpensive and fast, but reports indicate that cell viability may be lower than with inkjet systems.67,68 Alginate hydrogel is another commonly used material in bioprinting. Wu et al.69 used an extrusion system to print a hydrogel consisting of HCECs, collagen, gelatin, and alginate and incubated it in sodium citrate-containing medium which enabled a higher HCEC proliferation rate and increased cytokeratin 3 (CK 3) expression as compared to alginate control. Alteration of the mole ratio of sodium citrate to sodium alginate allowed for control of degradation of the constructs. Laser-assisted bioprinting, in which reflected lasers are used to direct the deposition of bioink onto a surface, should also be considered as a potential system for corneal prosthesis or tissue creation, particularly for its accuracy and maintenance of cell viability.70,71
In 3D printing, materials are deposited in sequential layers until a 3D object is formed. 3D printing has traditionally been used to produce engineering prototypes, but there has been increasing application of 3D bioprinting in medical applications (Figure 2).72
Figure 2.

A concept map outlining the potential process involved in 3D printing an artificial cornea. Ultrasound (US) and optical coherence tomography (OCT) would be used to acquire the images upon which the model or artificial cornea is to be based; in cases of severely damaged or distorted corneas, US and OCT may primarily be of importance in gathering measurements relating to the surrounding structures. A 3D model is created from the acquired images through segmentation. The model is converted to standard tessellation language (STL) and sliced. Depending on which printing modality is chosen or developed for artificial corneal manufacturing, the model would be printed, likely through either an inkjet or extrusion-based system. The implant would then be placed by a qualified ocular surgeon in the same manner as a standard allograft.
Collagen is a popular material of investigation in 3D bioprinting. Collagen derived from fish scales, a waste product in the fishing industry, has been shown to have efficacy for replacement cornea scaffold formation.73–78 While there have been reports in the popular media of groups 3D printing corneas using fish scale–derived collagen, the scalability and refractive efficacy of pure-collagen implants have not yet been demonstrated in the literature. It is reasonable to expect a necessity for cell seeding in some form to populate the graft in a manner compliant with refractive requirements. Collagen has many desirable qualities such as lack of cytotoxicity, accommodation of corneal epithelial cells, and lack of inflammation inducement that may make it a suitable material for cornea creation using additive manufacturing techniques.77–79 However, further investigation, particularly into the refractive properties of collagen scaffold-based corneas and their long-term stability, is needed.
De Miguel et al. 80–84 and other groups have been investigating the use of autologous, adipose-derived stem cells for the treatment of keratoconus. Stem cells or autologous corneal cells are promising candidates for corneal bioprinting due to their adaptability and the minimal infection and rejection risks associated with their implantation. While the development of corneal 3D printing processes involving autologous cells could be of tremendous value to those with access to the technology, it is unlikely that such a development will significantly affect the accessibility of keratoplasty to those in developing countries due to its expensive and time-consuming nature. Decellularized ECM shows potential as a bioink upon which cell-laden construction may occur due to its ability to mimic natural ECM characteristics such as the flexibility and the ability to provide cues for cell engraftment, survival, and function.85,86
Ruzza et al.87 demonstrated the usefulness of 3D printing technology in keratoplasty procedure by 3D printing a smart storage glide for the storage and delivery of posterior lenticules in Descemet stripping automated EK.
All of the materials discussed in this review have been 3D printed in one conformation or another either individually or in combination with other polymers, and they have potential for use in vivo.33,88–96 PVA is one of the materials with the most extensive 3D printing experience as it is commonly used as a support material in multi-extrusion printing due to its water solubility, ease of use, and inexpensiveness.97 The printing of materials requiring a photoinitiator such as GelMA is of special interest due to the additional step needed for their polymerization. Such a process requires the balanced application of not only biological and chemical principles but also engineering expertise in designing a printer that employs extrusion heads and additional UV and/or visible light curing capabilities.88 This process necessitates careful adjustment to get a product with the desired physical characteristics; this is further complicated if cells, which must remain viable, are being printed onto the scaffold. Depending on the process and materials employed, support material may or may not be necessary for proper shaping of the material.94 The various characteristics of each material can have a great impact on its suitability for 3D printing and implantation, and the advantages and disadvantages associated with each should be investigated further.
Future directions
The concept of 3D bioprinting stem cells or collagen into a corneal scaffold is promising, and investigation into these areas would be prudent. The ability to regenerate the cornea with autologous cells could dramatically improve outcomes in patients. Potential advantages to the use of cell-based corneal replacements such as tissue-engineered matrices include relative resistance to immune rejection, inflammation, and infection, as wellas extensive integration into the host tissue and formation of well-differentiated epithelia.98,99 Continued research into materials that can be used in keratoprostheses and their surface modulation is also warranted.
The most overt ways in which 3D printing could be utilized in corneal transplant, outside of the printing of instruments and procedural items, are in the printing of a scaffold upon which cells are seeded (either in vitro or in vivo) and the printing of a synthetic or semisynthetic keratoprosthesis employing a “core and skirt”–like model with either different materials or the same material of differing porosities for the respective core and skirt components. In the authors’ estimation, the former process would be inherently limited in its scalability due to the expensive and personalized nature of autologous cell use, and its ability to permeate remote regions would be limited consequently. In pursuing the latter, difficulties are likely to arise in attaining adequate integration not only of the host tissue into the skirt but also of the skirt and core components if different polymers are employed. It would be interesting to investigate the feasibility of printing a prosthesis comprising a synthetic core material and a semisynthetic skirt material (such as GelMA or its variant).
Bioengineering and 3D printing are likely to have a significant impact on the lives of the millions of individuals affected by corneal disease. While it remains to be seen whether the use of natural polymers, synthetic polymers, cell-based designs, or a combination of multiple components will prevail as suitable and preferable alternatives to allograft corneas, the development of improved technology and techniques and an increased understanding of ocular physiology will undoubtedly improve patient outcomes.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Chen Z, You J, Liu X, et al. Biomaterials for corneal bioengineering. Biomed Mater 2018; 13: 032002. [DOI] [PubMed] [Google Scholar]
- 2. Akpek EK, Alkharashi M, Hwang FS, et al. Artificial corneas versus donor corneas for repeat corneal transplants. Cochrane Database Syst Rev 2014; 11: CD009561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ 2001; 79: 214–221. [PMC free article] [PubMed] [Google Scholar]
- 4.2016 Eye Banking Statistical Report, http://restoresight.org/wp-content/uploads/2017/04/2016_Statistical_Report-Final-040717.pdf (2017, accessed 13 November 2017).
- 5. Stulting RD, Sugar A, Beck R, et al. Effect of donor and recipient factors on corneal graft rejection. Cornea 2012; 31: 1141–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gain P, Jullienne R, He Z, et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol 2016; 134: 167–173. [DOI] [PubMed] [Google Scholar]
- 7.Boston Keratoprosthesis (KPro)—EyeWiki, http://eyewiki.aao.org/Boston_Keratoprosthesis_(KPro) (accessed 18 November 2017).
- 8. Walcott-Harris R and, Dohlman C. (eds). Boston Keratoprosthesis update, newsletter VII, https://www.masseyeandear.org/~/media/testupload/files/2010-kpro-newsletter-1.pdf?la=en (2010, accessed 17 November 2017).
- 9. Nichol JW, Koshy S, Bae H, et al. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010; 31: 5536–5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Den Bulcke AI, Bogdanov B, De Rooze N, et al. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000; 1: 31–38. [DOI] [PubMed] [Google Scholar]
- 11. Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res 2002; 90: 251–262. [PubMed] [Google Scholar]
- 12. Van den Steen PE, Dubois B, Nelissen I, et al. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Crit Rev Biochem Mol Biol 2002; 37: 375–536. [DOI] [PubMed] [Google Scholar]
- 13. Watanabe R, Hayashi R, Kimura Y, et al. A novel gelatin hydrogel carrier sheet for corneal endothelial transplantation. Tissue Eng Part A 2011; 17: 2213–2219. [DOI] [PubMed] [Google Scholar]
- 14. Mimura T, Amano S, Yokoo S, et al. Tissue engineering of corneal stroma with rabbit fibroblast precursors and gelatin hydrogels. Mol Vis 2008; 14: 1819–1828. [PMC free article] [PubMed] [Google Scholar]
- 15. Lai J-Y, Ma DH-K, Lai M-H, et al. Characterization of cross-linked porous gelatin carriers and their interaction with corneal endothelium: biopolymer concentration effect. PLoS ONE 2013; 8: e54058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Niu G, Choi J-S, Wang Z, et al. Heparin-modified gelatin scaffolds for human corneal endothelial cell transplantation. Biomaterials 2014; 35: 4005–4014. [DOI] [PubMed] [Google Scholar]
- 17. Lai J-Y. Corneal stromal cell growth on gelatin/chondroitin sulfate scaffolds modified at different NHS/EDC molar ratios. Int J Mol Sci 2013; 14: 2036–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khademhosseini A, Meinert C, Loessner D, et al. Functionalization, preparation and use of cell-laden gelatin methacryloyl–based hydrogels as modular tissue culture platforms. Nat Protoc 2016; 11: 727. [DOI] [PubMed] [Google Scholar]
- 19. Occhetta P, Visone R, Russo L, et al. VA-086 methacrylate gelatine photopolymerizable hydrogels: a parametric study for highly biocompatible 3D cell embedding. J Biomed Mater Res A 2015; 103: 2109–2117. [DOI] [PubMed] [Google Scholar]
- 20. Kim P, Yuan A, Nam K-H, et al. Fabrication of poly(ethylene glycol): gelatin methacrylate composite nanostructures with tunable stiffness and degradation for vascular tissue engineering. Biofabrication 2014; 6: 024112. [DOI] [PubMed] [Google Scholar]
- 21. Nguyen AH, McKinney J, Miller T, et al. Gelatin methacrylate microspheres for controlled growth factor release. Acta Biomater 2015; 13: 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shirahama H, Lee BH, Tan LP, et al. Precise tuning of facile one-pot gelatin methacryloyl (GelMA) synthesis. Sci Rep 2016; 6: 31036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bertassoni LE, Cardoso JC, Manoharan V, et al. Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication 2014; 6: 024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Skardal A, Zhang J, McCoard L, et al. Photocrosslinkable hyaluronan-gelatin hydrogels for two-step bioprinting. Tissue Eng Part A 2010; 16: 2675–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yue K, Trujillo-de Santiago G, Alvarez MM, et al. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015; 73: 254–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rizwan M, Peh GSL, Ang H-P, et al. Sequentially-crosslinked bioactive hydrogels as nano-patterned substrates with customizable stiffness and degradation for corneal tissue engineering applications. Biomaterials 2017; 120: 139–154. [DOI] [PubMed] [Google Scholar]
- 27. Gao G, Schilling AF, Hubbell K, et al. Improved properties of bone and cartilage tissue from 3D inkjet-bioprinted human mesenchymal stem cells by simultaneous deposition and photocrosslinking in PEG-GelMA. Biotechnol Lett 2015; 37: 2349–2355. [DOI] [PubMed] [Google Scholar]
- 28. Nemeth CL, Janebodin K, Yuan AE, et al. Enhanced chondrogenic differentiation of dental pulp stem cells using nanopatterned PEG-GelMA-HA hydrogels. Tissue Eng Part A 2014; 20: 2817–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang H, Zhou L, Liao J, et al. Cell-laden photocrosslinked GelMA-DexMA copolymer hydrogels with tunable mechanical properties for tissue engineering. J Mater Sci Mater Med 2014; 25: 2173–2183. [DOI] [PubMed] [Google Scholar]
- 30. Shin SR, Zihlmann C, Akbari M, et al. Reduced graphene oxide-GelMA hybrid hydrogels as scaffolds for cardiac tissue engineering. Small Weinh Bergstr Ger 2016; 12: 3677–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu Y, Chen YX, Yan J, et al. Fabrication of conductive gelatin methacrylate-polyaniline hydrogels. Acta Biomater 2016; 33: 122–130. [DOI] [PubMed] [Google Scholar]
- 32. Chen X, Bai S, Li B, et al. Fabrication of gelatin methacrylate/nanohydroxyapatite microgel arrays for periodontal tissue regeneration. Int J Nanomedicine 2016; 11: 4707–4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wei Zhu, Harris BT, Zhang LG. Gelatin methacrylamide hydrogel with graphene nanoplatelets for neural cell-laden 3D bioprinting. In: 2016 IEEE 38th annual international conference of the Engineering in Medicine and Biology Society (EMBC), Orlando, FL, 16–20 August 2016, pp. 4185–4188. New York: IEEE. [DOI] [PubMed] [Google Scholar]
- 34. Modaresifar K, Hadjizadeh A, Niknejad H. Design and fabrication of GelMA/chitosan nanoparticles composite hydrogel for angiogenic growth factor delivery. Artif Cells Nanomed Biotechnol 2017; 24: 1–10. [DOI] [PubMed] [Google Scholar]
- 35. Stratesteffen H, Köpf M, Kreimendahl F, et al. GelMA-collagen blends enable drop-on-demand 3D printability and promote angiogenesis. Biofabrication 2017; 9: 045002. [DOI] [PubMed] [Google Scholar]
- 36. Griffith M, Fagerholm P, Lagali N, et al. Chapter 49—regenerative medicine in the cornea. In: Atala A, Lanza R, Thomson JA, et al. (eds) Principles of regenerative medicine. 2nd ed. San Diego, CA: Academic Press, pp. 911–924. [Google Scholar]
- 37. Chirila TV. An overview of the development of artificial corneas with porous skirts and the use of PHEMA for such an application. Biomaterials 2001; 22: 3311–3317. [DOI] [PubMed] [Google Scholar]
- 38. Clowes AW, Zacharias RK, Kirkman TR. Early endothelial coverage of synthetic arterial grafts: porosity revisited. Am J Surg 1987; 153: 501–504. [DOI] [PubMed] [Google Scholar]
- 39. Guénard V, Valentini RF, Aebischer P. Influence of surface texture of polymeric sheets on peripheral nerve regeneration in a two-compartment guidance system. Biomaterials 1991; 12: 259–263. [DOI] [PubMed] [Google Scholar]
- 40. Brauker JH, Carr-Brendel VE, Martinson LA, et al. Neovascularization of synthetic membranes directed by membrane microarchitecture. J Biomed Mater Res 1995; 29: 1517–1524. [DOI] [PubMed] [Google Scholar]
- 41. Ratner BD. New ideas in biomaterials science—a path to engineered biomaterials. J Biomed Mater Res 1993; 27: 837–850. [DOI] [PubMed] [Google Scholar]
- 42. Miyashita H, Shimmura S, Kobayashi H, et al. Collagen-immobilized poly(vinyl alcohol) as an artificial cornea scaffold that supports a stratified corneal epithelium. J Biomed Mater Res B Appl Biomater 2006; 76: 56–63. [DOI] [PubMed] [Google Scholar]
- 43. Legeais JM, Renard G, Parel JM, et al. Keratoprosthesis with biocolonizable microporous fluorocarbon haptic. Preliminary results in a 24-patient study. Arch Ophthalmol 1995; 113: 757–763. [DOI] [PubMed] [Google Scholar]
- 44. Legeais JM, Renard G, Pouliquen Y. [Keratoprosthesis with biocolonizable support]. J Fr Ophtalmol 1993; 16: 577–583. [PubMed] [Google Scholar]
- 45. Legeais JM, Parel JM, Savoldelli M, et al. [Reticulated polyethylene oxide for gel injection adjustable keratoplasty. Biocompatibility in critical situation]. J Fr Ophtalmol 1997; 20: 31–36. [PubMed] [Google Scholar]
- 46. Legeais JM, Drubaix I, Briat B, et al. [2nd generation bio-integrated keratoprosthesis. Implantation in animals]. J Fr Ophtalmol 1997; 20: 42–48. [PubMed] [Google Scholar]
- 47. Legeais JM, Werner LP, Legeay G, et al. In vivo study of a fluorocarbon polymer-coated intraocular lens in a rabbit model. J Cataract Refract Surg 1998; 24: 371–379. [DOI] [PubMed] [Google Scholar]
- 48. Legeais JM, Renard G. A second generation of artificial cornea (Biokpro II). Biomaterials 1998; 19: 1517–1522. [DOI] [PubMed] [Google Scholar]
- 49. Kobayashi H. Surface modified poly(vinyl alcohol) nanofiber for the artificial corneal stroma. Key Eng Mater 2007; 342–343: 209–212. [Google Scholar]
- 50. Lee SD, Hsiue GH, Kao CY, et al. Artificial cornea: surface modification of silicone rubber membrane by graft polymerization of pHEMA via glow discharge. Biomaterials 1996; 17: 587–595. [DOI] [PubMed] [Google Scholar]
- 51. Hicks CR, Fitton JH, Chirila TV, et al. Keratoprostheses: advancing toward a true artificial cornea. Surv Ophthalmol 1997; 42: 175–189. [DOI] [PubMed] [Google Scholar]
- 52. Chirila TV, Hicks CR, Dalton PD, et al. Artificial cornea. Prog Polym Sci 1998; 23: 447–473. [Google Scholar]
- 53. Chirila TV, Vijayasekaran S, Horne R, et al. Interpenetrating polymer network (IPN) as a permanent joint between the elements of a new type of artificial cornea. J Biomed Mater Res 1994; 28: 745–753. [DOI] [PubMed] [Google Scholar]
- 54. Chirila TV, Lou X, Vijayasekaran S, et al. Hydrophilic sponges based on 2-hydroxyethyl methacrylate. VI. Effect of phase sequence inversion on the characteristics of IPN between sponges and homogeneous gels. Int J Polym Mater Polym Biomater 1998; 40: 97–104. [Google Scholar]
- 55. Vijayasekaran S, Chirila TV, Robertson TA, et al. Calcification of poly(2-hydroxyethyl methacrylate) hydrogel sponges implanted in the rabbit cornea: a 3-month study. J Biomater Sci Polym Ed 2000; 11: 599–615. [DOI] [PubMed] [Google Scholar]
- 56. Sinha M, Gupte T. Design and evaluation of artificial cornea with core-skirt design using polyhydroxyethyl methacrylate and graphite. Int Ophthalmol. Epub ahead of print 10 June 2017. DOI: 10.1007/s10792-017-0586-3. [DOI] [PubMed] [Google Scholar]
- 57. Zainuddin Barnard Z, Keen I, et al. PHEMA hydrogels modified through the grafting of phosphate groups by ATRP support the attachment and growth of human corneal epithelial cells. J Biomater Appl 2008; 23: 147–168. [DOI] [PubMed] [Google Scholar]
- 58. Yañez-Soto B, Liliensiek SJ, Murphy CJ, et al. Biochemically and topographically engineered poly(ethylene glycol) diacrylate hydrogels with biomimetic characteristics as substrates for human corneal epithelial cells. J Biomed Mater Res A 2013; 101: 1184–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yanez-Soto B, Liliensiek SJ, Gasiorowski JZ, et al. The influence of substrate topography on the migration of corneal epithelial wound borders. Biomaterials 2013; 34: 9244–9251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kim EY, Tripathy N, Cho SA, et al. Collagen type I–PLGA film as an efficient substratum for corneal endothelial cells regeneration. J Tissue Eng Regen Med 2017; 11: 2471–2478. [Google Scholar]
- 61. Myung D, Farooqui N, Waters D, et al. Glucose-permeable interpenetrating polymer network hydrogels for corneal implant applications: a pilot study. Curr Eye Res 2008; 33: 29–43. [DOI] [PubMed] [Google Scholar]
- 62. Hartmann L, Watanabe K, Zheng LL, et al. Toward the development of an artificial cornea: improved stability of interpenetrating polymer networks. J Biomed Mater Res B Appl Biomater 2011; 98: 8–17. [DOI] [PubMed] [Google Scholar]
- 63. Zhang Q, Fang Z, Cao Y, et al. High refractive index inorganic–organic interpenetrating polymer network (IPN) hydrogel nanocomposite toward artificial cornea implants. ACS Macro Lett 2012; 1: 876–881. [DOI] [PubMed] [Google Scholar]
- 64. Zhang Q, Su K, Chan-Park MB, et al. Development of high refractive ZnS/PVP/PDMAA hydrogel nanocomposites for artificial cornea implants. Acta Biomater 2014; 10: 1167–1176. [DOI] [PubMed] [Google Scholar]
- 65. Seol Y-J, Kang H-W, Lee SJ, et al. Bioprinting technology and its applications. Eur J Cardiothorac Surg 2014; 46: 342–348. [DOI] [PubMed] [Google Scholar]
- 66. Lorber B, Hsiao W-K, Hutchings IM, et al. Adult rat retinal ganglion cells and glia can be printed by piezoelectric inkjet printing. Biofabrication 2014; 6: 015001. [DOI] [PubMed] [Google Scholar]
- 67. Lorber B, Hsiao W-K, Martin KR. Three-dimensional printing of the retina. Curr Opin Ophthalmol 2016; 27: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol 2014; 32: 773–785. [DOI] [PubMed] [Google Scholar]
- 69. Wu Z, Su X, Xu Y, et al. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci Rep 2016; 6: 24474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Koch L, Gruene M, Unger C, et al. Laser assisted cell printing. Curr Pharm Biotechnol 2013; 14: 91–97. [PubMed] [Google Scholar]
- 71. Devillard R, Pagès E, Correa MM, et al. Cell patterning by laser-assisted bioprinting. Methods Cell Biol 2014; 119: 159–174. [DOI] [PubMed] [Google Scholar]
- 72. Ozbolat IT, Peng W, Ozbolat V. Application areas of 3D bioprinting. Drug Discov Today 2016; 21: 1257–1271. [DOI] [PubMed] [Google Scholar]
- 73. Krishnan S, Sekar S, Katheem MF, et al. Fish scale collagen—a novel material for corneal tissue engineering. Artif Organs 2012; 36: 829–835. [DOI] [PubMed] [Google Scholar]
- 74. Lin CC, Ritch R, Lin SM, et al. A new fish scale-derived scaffold for corneal regeneration. Eur Cell Mater 2010; 19: 50–57. [PubMed] [Google Scholar]
- 75. van Essen TH, Lin CC, Hussain AK, et al. A fish scale-derived collagen matrix as artificial cornea in rats: properties and potential. Invest Ophthalmol Vis Sci 2013; 54: 3224–3233. [DOI] [PubMed] [Google Scholar]
- 76. Chen S-C, Telinius N, Lin H-T, et al. Use of fish scale-derived BioCornea to seal full-thickness corneal perforations in pig models. PLoS ONE 2015; 10: e0143511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. van Essen TH, van Zijl L, Possemiers T, et al. Biocompatibility of a fish scale-derived artificial cornea: cytotoxicity, cellular adhesion and phenotype, and in vivo immunogenicity. Biomaterials 2016; 81: 36–45. [DOI] [PubMed] [Google Scholar]
- 78. Yuan F, Wang L, Lin C-C, et al. A cornea substitute derived from fish scale: 6-month followup on rabbit model. J Ophthalmol 2014; 2014: 914542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rhee S, Puetzer JL, Mason BN, et al. 3D bioprinting of spatially heterogeneous collagen constructs for cartilage tissue engineering. ACS Biomater Sci Eng 2016; 2: 1800–1805. [DOI] [PubMed] [Google Scholar]
- 80. De Miguel MP, Alio JL, Arnalich-Montiel F, et al. Cornea and ocular surface treatment. Curr Stem Cell Res Ther 2010; 5: 195–204. [DOI] [PubMed] [Google Scholar]
- 81. Alio del Barrio JL, Chiesa M, Garagorri N, et al. Acellular human corneal matrix sheets seeded with human adipose-derived mesenchymal stem cells integrate functionally in an experimental animal model. Exp Eye Res 2015; 132: 91–100. [DOI] [PubMed] [Google Scholar]
- 82. Fuentes-Julián S, Arnalich-Montiel F, Jaumandreu L, et al. Adipose-derived mesenchymal stem cell administration does not improve corneal graft survival outcome. PLoS ONE 2015; 10: e0117945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Alió Del Barrio JL, El Zarif M, de Miguel MP, et al. Cellular therapy with human autologous adipose-derived adult stem cells for advanced keratoconus. Cornea 2017; 36: 952–960. [DOI] [PubMed] [Google Scholar]
- 84. Alió Del Barrio JL, El Zarif M, Azaar A, et al. Corneal stroma enhancement with decellularized stromal laminas with or without stem cell recellularization for advanced keratoconus. Am J Ophthalmol 2018; 186: 47–58. [DOI] [PubMed] [Google Scholar]
- 85. Pati F, Jang J, Ha D-H, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun 2014; 5: 3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kim BS, Kim H, Gao G, et al. Decellularized extracellular matrix: a step towards the next generation source for bioink manufacturing. Biofabrication 2017; 9: 034104. [DOI] [PubMed] [Google Scholar]
- 87. Ruzza A, Parekh M, Ferrari S, et al. Preloaded donor corneal lenticules in a new validated 3D printed smart storage glide for Descemet stripping automated endothelial keratoplasty. Br J Ophthalmol 2015; 99: 1388–1395. [DOI] [PubMed] [Google Scholar]
- 88. Billiet T, Gevaert E, De Schryver T, et al. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials 2014; 35: 49–62. [DOI] [PubMed] [Google Scholar]
- 89. Strong SM. 3D printing, polymethyl methacrylate acrylic, and fully milled zirconia for anterior implant restorations: the brave new world of prosthetic dentistry. Gen Dent 2015; 63: 11–13. [PubMed] [Google Scholar]
- 90. Goyanes A, Buanz ABM, Hatton GB, et al. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur J Pharm Biopharm 2015; 89: 157–162. [DOI] [PubMed] [Google Scholar]
- 91. Badea A, McCracken JM, Tillmaand EG, et al. 3D-printed pHEMA materials for topographical and biochemical modulation of dorsal root ganglion cell response. ACS Appl Mater Interfaces 2017; 9: 30318–30328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hockaday LA, Kang KH, Colangelo NW, et al. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication 2012; 4: 035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pati F, Song T-H, Rijal G, et al. Ornamenting 3D printed scaffolds with cell-laid extracellular matrix for bone tissue regeneration. Biomaterials 2015; 37: 230–241. [DOI] [PubMed] [Google Scholar]
- 94. Hong S, Sycks D, Chan HF, et al. 3D printing of highly stretchable and tough hydrogels into complex, cellularized structures. Adv Mater Deerfield Beach Fla 2015; 27: 4035–4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yin M-J, Yao M, Gao S, et al. Rapid 3D patterning of poly(acrylic acid) ionic hydrogel for miniature pH sensors. Adv Mater 2016; 28: 1394–1399. [DOI] [PubMed] [Google Scholar]
- 96. Hinton TJ, Hudson A, Pusch K, et al. 3D printing PDMS elastomer in a hydrophilic support bath via freeform reversible embedding. ACS Biomater Sci Eng 2016; 2: 1781–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Duran C, Subbian V, Giovanetti MT, et al. Experimental desktop 3D printing using dual extrusion and water-soluble polyvinyl alcohol. Rapid Prototyp J 2015; 21: 528–534. [Google Scholar]
- 98. Griffith M, Jackson WB, Lagali N, et al. Artificial corneas: a regenerative medicine approach. Eye 2009; 23: 1985–1989. [DOI] [PubMed] [Google Scholar]
- 99. McLaughlin CR, Tsai RJ-F, Latorre MA, et al. Bioengineered corneas for transplantation and in vitro toxicology. Front Biosci Landmark Ed 2009; 14: 3326–3337. [DOI] [PubMed] [Google Scholar]


