Short abstract
Chronic migraine is a common chronic daily headache featured by frequent headache attacks with at least 15 headache days per month, which brings great disease burden to both the sufferers and the society. Transformed from episodic migraine, the pathophysiology of chronic migraine is not fully understood, even though several risk factors have been associated with migraine progression. Recent studies have identified both structural and functional alterations in some brain regions of chronic migraine patients indicating that maladaptation of the top-down pain modulation and subsequent sensitization of trigeminal system may be important in the pathogenesis of chronic migraine. Moreover, biochemical analysis has confirmed several molecules related to chronic migraine, which may serve as biomarkers and potential therapeutic targets. Chronic migraine is undertreated because of its poor treatment response and limited therapy options. In this article, we reviewed the latest data to outline the clinical feature, pathophysiological mechanism, and management of chronic migraine, in the expectation to provide direction for future research and finally to take good care of chronic migraine patients.
Keywords: Chronic migraine, top-down modulation, central sensitization, biomarker
Introduction
As one of the most common chronic daily headache (CDH) disorders, chronic migraine (CM) is featured by frequent headache attacks with at least 15 headache days per month.1 Sufferers of CM usually have a history of episodic migraine (EM) and their headache frequencies increase with time. It is estimated that approximately 3% EM patients evolve to CM per year,2 and similar result was obtained in a three-month follow-up.3 Besides, this transformation is bidirectional with about 26% of CM patients remitting to EM in a two-year follow-up,4 which makes it difficult to confirm the accurate prevalence of CM. With the increasing headache frequency, CM becomes less intense and more featureless, but is associated with worse treatment response. Both the undertreated headache and associated comorbidities cause greater disease burden for CM compared with EM.5–7
Although regarded as the same spectrum illness with EM,8 the detailed pathophysiology of CM is not fully understood. The latest data have recognized several predisposing factors, such as medication overuse, insufficient migraine prophylactic treatment, low socioeconomic status, stressful events, and so on.4,9 Moreover, recent neurophysiological and imaging studies have indicated that CM may be associated with both structural and functional alterations in some brain regions, especially the cortical hyperexcitability and brainstem dysfunction.10–12 Sensitization of the trigeminal system also play a vital role, as allodynia is quite common in CM patients.13 Besides, several molecular mechanisms have been indicated to be involved in the pathogenesis of CM, such as calcitonin gene-related peptide (CGRP), serotonin (5-HT) system, pituitary adenylate cyclase activating polypeptide (PACAP), and so on.14–16
With the high disability rates and confusing pathogenic process of CM, management of the disease is still a big challenge for clinicians up to now. The treatment choices are quite limited and only few is well evidence-based. The most accepted options include topiramate, onabotulinumtoxin A, and some neuromodulation therapy patterns.17–20 In this article, we reviewed the latest data to outline the clinical feature, pathophysiological mechanism, and management of CM, in the expectation to provide direction for future research and finally to take good care of CM patients.
Clinical aspects of CM
The diagnostic criteria of CM have evolved during the last two decades. The concept of CM was first defined in the International Classification of Headache Disorders second edition as “patients suffering from at least 15 migraine days per month for at least 3 months without medication overuse.”21 Then, in 2006, the diagnosis of CM was broadened as “headache (tension-type and/or migraine) on ≥15 days per month for at least 3 months without medication overuse, of which at least 8 days are migraine.”22 It was further broadened in the International Classification of Headache Disorders third beta version, as medication overuse headache (MOH) is no longer exclusive to CM.1 The diagnosis of CM mainly depends on history taking, but it is usually difficult for patients to recall of past headache attacks especially for those with headache for years. Therefore, the clinical diagnosis of CM is challenging, and objective auxiliary diagnosis measures are in need such as the biomarkers, imaging diagnosis model, and so on.23
The accurate prevalence of CM is unknown as different diagnostic criteria were used in previous studies, and the dynamic transformation between CM and EM also make it difficult. As estimated, the prevalence of CM is about 2% in general population, which is lower in Asia population.24,25 CM is associated with greater disease burden compared with EM, as illustrated in its higher disability rates, more disease cost, and increased rates of psychiatric comorbidities.26–29 In two large-scale longitudinal cohort studies about CM, the Chronic Migraine Epidemiology and Outcomes Study and American Migraine Prevalence and Prevention Study, CM patients got higher scores in both the Migraine Disability Assessment Scale and the Headache Impact Test-6, indicating that CM is more disable than EM.6,30 CM is also more likely to be associated with both psychiatric and somatic comorbidities, which further enlarges its impact. According to the American Migraine Prevalence and Prevention study, more CM patients met the diagnostic criteria for depression or anxiety disorder than those of EM.31 In addition, other chronic pain conditions, respiratory diseases, and cardiovascular events were more common among CM patients.7
Recent studies have reported some risk factors for migraineurs progressing to CM, such as female gender, low socioeconomic status, obesity, baseline headache frequency, medication overuse (MO), insufficient headache relief and prophylaxis, stressful events, comorbid pain, and so on.3,9 More than half of CM were reported to overuse medication, and use of barbiturates and opiates has been especially associated with increased risk of CM.25 However, the causal relationship between CM and MO is still in debate.32 Meanwhile, inadequate acute headache treatment was also found to contribute to new onset CM.2 Therefore, appropriate acute therapeutic choice and sufficient prophylaxis is very important for preventing migraine chronification. The comorbidity of depression/anxiety also promotes transformation of migraine, indicating the bidirectional relationship between depression/anxiety and migraine.31
Pathophysiology of CM
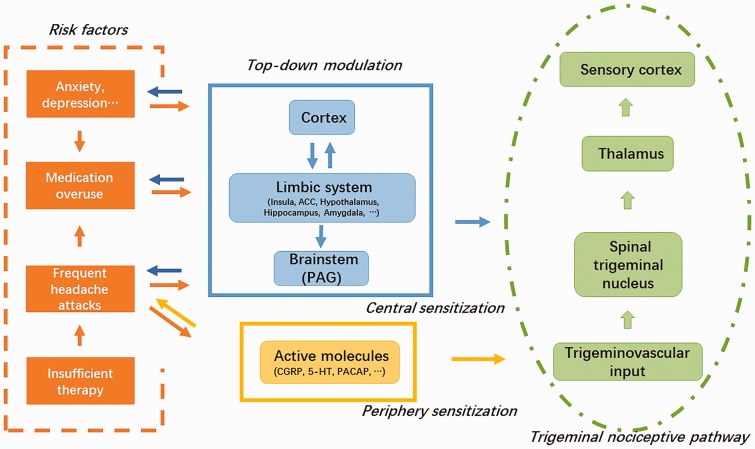
Up to now, the pathophysiological process of migraine is not fully understood, and the same to CM. However, all recent data indicate that migraine is a disorder of brain dysfunction with both the genetic background and environment triggering.33 Transformed from EM, the pathogenesis of CM is also related to the brain. And recent evidence has proved both structural and functional alterations in brain, especially the cortical hyperexcitability and abnormities in brainstem.12 Larger proportion of CM patients reported cutaneous allodynia than EM ones,34 illustrating that sensitization of trigeminal system involves in the development of the disease. In addition, several molecules, such as CGRP and 5-HT,14,15 have been reported to be correlated with migraine chronification. In this article, the latest data were reviewed and the pathophysiological model was outlined (Figure 1). In brief, both recurring headache attacks and the comorbid conditions (medication-overuse, anxiety, and depression) promote the derangement of top-down pain modulation and also atypical release of nociceptive molecules, which aggravates trigeminal sensitization induced by repeated nociceptive inputs. With this hypersensitive state, the EM finally progresses to a “never-ending” condition, namely, CM. To be noted, the neural plasticity induced by the risk factors of CM may influence themselves in turn.
Figure 1.
The proposed pathophysiological process of chronic migraine (CM). Both recurring headache attacks and other risk factors for migraine transformation (medication-overuse, anxiety, and depression) promote the derangement of top-down pain modulation and also atypical release of nociceptive molecules, which aggravates trigeminal sensitization induced by repeated nociceptive inputs. With this hypersensitive state, the episodic migraine finally progresses to a “never-ending” condition, namely, CM. To be noted, the neural plasticity induced by the risk factors of CM may influences themselves in turn. ACC: anterior cingulate cortex; PAG: periaqueductal gray; CGRP: calcitonin gene-related peptide; 5-HT: serotonin; PACAP: pituitary adenylate cyclase activating polypeptide.
Dysfunction in top-down pain modulation
Cortical hyperexcitability
With the development of electrophysiological and neuroimaging technique, lots of recent studies have focused on the responsivity in CM brain. Generally, studies of cortical responsivity tend to indicate an increase in excitability, in particular of somatosensory and visual cortices.35
In a transcranial magnetic stimulation study, excitability of the cortex of CM patients was assessed by magnetic suppression of perceptual accuracy profiles.10 And CM sufferers exhibited reduced visual suppression than both EM patients and normal controls, indicating the existence of cortical hyperexcitability. Subsequent positron emission test scan of part of the same cohort found increased metabolism in brainstem and decreased metabolism in the medial frontal and parietal as well as the somatosensory cortex.10 This brainstem activation and inhibition in certain cortical areas of the cortex maybe interpreted as a potential dysfunction in the inhibitory pathways, which induces increase in cortical excitability and finally the migraine transformation.
CM patients also demonstrated a persistent ictal-like excitability pattern of the visual cortex between migraine attacks according to visual evoked potential assessment,36 suggesting that evolution of CM is consistent with alteration of central excitability and that CM is a status of “never-ending” migraine. In a positron emission test analysis of CM combined MO patients, altered metabolism were also found in similar pain-related cortical regions, and these changes remitted to normal after successful analgesic withdraw therapy.37 To be noted, functional and metabolic changes in the prefrontal cortex are especially correlated with MOH, which may be a newfound brain region in pain-modulation in MOH.38 All these data support the claim that these pain-related changes are related with both frequent headache attacks and medication overuse and may contribute to migraine chronification.
Also, the structural change of cortex has been studied among CM patients, including the gray matter volume and cortical thickness.11,39–42 However, relatively small sample sizes were included in these researches and no consistent results were obtained. The mechanisms underlying the structural alterations remain to be elucidated. In addition, further longitudinal data are needed to determine the causal-consequence relationship between these functional/structural alterations and migraine chronification.
Brainstem alterations, especially the periaqueductal gray
As an important part of the top-down regulation of pain, the brainstem exerts descending modulation of the trigeminal nuclei and associated sensory and motor response in migraine, and also interacts with other cortical and subcortical areas. To date, migraine has been associated with this altered endogenous descending pain-modulation in brainstem by a number of studies, of which the periaqueductal gray (PAG) attracted the most attention.8,10,12 Receiving inputs from frontal cortex, hypothalamus, and other supraspinal structures, PAG is the center of the descending pain-modulation network via projections to the rostral ventromedial medulla, which can either inhibit or facilitate pain transmission through direct projections to the spinal and medullary dorsal horn.43
Several clinical observations have indicated the possible role of PAG in migraine generation, and the dysfunction of this brain region may also be associated with migraine transformation.44,45 A resting-state fMRI has confirmed that migraineurs showed stronger connectivity between PAG and several brain areas within nociceptive and somatosensory processing pathways with the increasing headache frequency. In contrast, the strength of the connectivity between PAG and pain modulation regions (prefrontal cortex, anterior cingulate, and amygdala) was weaker.46 These data reveal an impairment of the descending pain modulatory circuits in the process of migraine transformation, leading to loss of pain inhibition and hyperexcitability in nociceptive areas. Similar atypical functional connectivity of the PAG with brain regions involved in nociception, somatosensory processing, emotional processing, and pain modulation has been revealed in rats induced by repeated meningeal inflammation,47 indicating that this brainstem dysfunction may be the consequence of recurring headache attacks. Meanwhile, medication-overuse also aggravates the maladaptation of descending pain-modulation.48 Chronic morphine exposure has been proved to induce both loss of diffuse noxious inhibitory controls49 and increase in descending pain-facilitation.50
Aside these functional alterations, involvement of PAG in migraine chronification has also been demonstrated through iron homeostasis impairment44 and gray matter density alteration.51 Increased tissue iron represents a disturbed functional state of neuron. And by high-solution MRI, significant increase in tissue iron levels has been found among both EM and CDH patients compared with control subjects.44 The iron homeostasis in the PAG was persistently and progressively in EM and CDH, manifesting that iron accumulation in PAG may have resulted from repeated attacks, possibly caused by free radicals generated during repeated migraine attacks.52 It is also worth noting that tissue iron levels tend to be higher than normal at the outset of migraine-susceptible,44 indicating the causal role of iron homeostasis impairment in migraine and its transformation. Furthermore, in a recent volume analysis of PAG in migraine, EM patients showed a larger PAG volume than healthy controls, with that of CM in between, which may be considered as a diagnostic and evaluated imaging biomarker for migraine.53
Other brain regions
Besides the somatosensory processing, migraine also includes emotional, autonomic, and cognitive aspects. From this perspective, some studies have focused on anterior cingulate cortex (ACC), hypothalamus, insula, hippocampus, amygdala, and other brain regions related to the limbic system.54–56
By resting-state functional connectivity analysis, CM was associated with interictal atypical functional connectivity with affective pain regions which included regions in anterior insula, amygdala, pulvinar, mediodorsal thalamus, middle temporal cortex, and PAG, which significantly correlated with disease duration.57 This atypical functional connectivity may relate to aberrant affective pain processing and atypical affective responses to painful stimuli in CM. Structurally, CM (including MOH) patients showed a focal gray matter decrease in the bilateral ACC, left amygdala, and bilateral insula than these of EM.23 In particular, there was a significant correlation between headache frequency and ACC alteration. ACC has always been acknowledged to involve in the affective dimension of pain, while recent evidences also support its role in the descending modulation of spinal nociception.58,59 Recently, an abnormal pattern of hypothalamic hormonal secretion has been discovered in CM patients as shown by a chronobiological dysregulation and a possible hyperdopaminergic state,60 supporting the involvement of the hypothalamus in the pathophysiology of CM.
Although no significant difference in the volumetric analysis of hippocampus and amygdala were found between migraineurs and healthy controls, a bidirectional correlation between headache frequency and volume of these two regions was confirmed among patients with migraine, with a peak at moderate-frequency (about 5–7 days/month).55 This structural plasticity linked to headache frequency may represent the process from adaption to maladaptation, with respect to both the painful and emotional aspects of migraine. Abnormal functional connectivity of bilateral amygdala has also been observed in CM patients compared to that of EM, which is correlated with the score of sleep quality,61 indicating the possible role of neurolimbic pain-modulating in the migraine chronicization.
The above-mentioned brain regions are related to pain-modulation, but are more important in the regulation of mood, sleep, visceral activities, learning, and so on. Meanwhile, CM is a complex syndrome with many associated conditions including acute medication overuse, anxiety disorder, depression, insomnia, and so on.7 Furthermore, significant change of regional cerebral blood flow in the dorsal rostral pons, ACC, cuneus, and left pulvinar has been found among CM, which can be modified by suboccipital stimulation, an effective therapeutic choice for CM patients,54 suggesting a role for these structures in the pathophysiology of CM and also possible targets for CM therapy. Thus, based on above evidence, we can speculate that frequent headache attacks cause maladaptation of these regions of the limbic system, which in turn exacerbates headache and headache-related comorbidities, such as anxiety, depression, sleep disorder, and so on.
Sensitization of trigeminal system
A series of clinical observations have indicated that CM patients are hypersensitive. On one hand, cutaneous allodynia is more common and more severe in CM compared to EM and other primary headaches, which is a risk factor for migraine progression.34 One the other hand, CM patients have lower pain thresholds than EM patients, as measured by quantitative sensory testing.62 Cutaneous allodynia, a condition featured by feeling of pain elicited by ordinary nonpainful stimulation to skin, is regarded as the result of trigeminal sensitization.63 Therefore, it is obvious that sensitization of the trigeminal system involves in the development of CM.
CM patient exhibits both cephalic and extracephalic allodynia, corresponding to respective sensitization of the second-order neurons in medullary horn or third-order thalamic neurons.8 Central sensitization has been long accepted as a pathophysiological feature and process chronic pain conditions, manifesting as a prolonged but reversible increase in the excitability of neurons in central nociceptive pathways triggered by repeated nociceptive inputs.63 The mechanisms underlying central sensitization include synaptic plasticity, imbalance between excitatory and inhibitory neurotransmitters (glutamate/GABA), and derangement of monoamine neurotransmitters (5-HT, norepinephrine, and dopamine).64 In addition to neural plasticity, recent studies have implicated glia–neuron interaction in chronic pain; future studies can focus on the role of glia in migraine and its progression.65
Increase in cortical spreading depression-evoked Fos expression and upregulation of 5-HT2A receptor in spinal trigeminal nucleus was observed in rats with chronic acetaminophen exposure.66 Sustained morphine in rats induced lower electrical and mechanical activation thresholds in medullary dorsal horn neurons.49 Moreover, both chronic dural inflammatory stimulation and triptan overuse caused mechanical allodynia and trigeminal sensitization in rats.67 All these preclinical studies indicate that both frequent attacks and medication overuse promote the development of central sensitization. In addition, comorbid mood disorder also can influence the formation of hyperalgesia, as depression models induced by olfactory bulbectomy or unpredictable chronic mild stress both exhibited severer nociceptive behavior and hyperalgesia state.68,69
Peripheral sensitization also plays a role in development of CM. A significant increase of TRPV1 (transient receptor potential vanilloid type-1 receptor) immunoreactive nerve fibers in the arterial wall has been found in CM patients compared with control patients.70 Expressed in small sensory neurons, TRPV1 receptors evoke release of CGRP and substance P, causing a higher sensitivity to algogenic agents. TRPV1-positive neurons can be decreased by onabotulinumtoxin A in the rat trigeminal ganglion, suggesting that TRPV1 may be a potential therapy target for CM.71
Molecular mechanisms
The clinical heterogeneity of migraine and the “featureless” of CM bring great diagnostic and therapeutic challenges to clinicians. And lack of appropriate biomarkers delays accurate diagnosis and impedes development of more effective therapeutic methods for migraine. Recent studies have identified several molecules which involve in the development and progression of migraine.72
CGRP
Peripherally secreted from trigeminal afferents, CGRP mediates vasodilation and inflammatory events within the dura as well as trigeminal ganglion, which is important in triggering and amplification of a migraine attack.73 Significant elevations of saliva CGRP have been noted in the premonitory and headache phase of migraine compared with baseline (interictal) levels, which is predictive of responsiveness to rizatriptan.74 Interictal increase of CGRP levels in peripheral blood has been found among CM patients compared with both EM patients and non-headache controls,14 supporting its role as a reliable biomarker for CM. Also, the probability of being a responder to onabotulinumtoxin A was much higher for CM patients with an elevated CGRP level.75 Moreover, TEV-48125, a monoclonal anti-CGRP antibody, has been proved to be tolerable and effective for the therapy of CM in a phase 2b clinical trial.76 The CGRP receptors also widely express in the central nervous system and may exert pain-modulation effects;77 future work can focus on this aspect of CGRP.
5-HT
Mainly released from the brainstem, 5-HT has long been implicated in the pathophysiology of migraine, especially in the descending pain modulation.33 Peripherally, serotonin exerts vasoconstrictive and anti-inflammatory effects via 5-HT1B and 5-HT1D receptor, respectively, which is therapeutic target for triptans.78 Besides the pain modulation, 5-HT also plays an important role in sleep pathophysiology and the genesis of mood disorders,79 making these problems common in migraine, especially CM. According to clinical and preclinical studies, both the frequent headache attacks and medication overuse can induce decrease of 5-HT and upregulation of serotonin receptors,67,80 which enhances hyperalgesia and promotes headache chronicity. Furthermore, abnormality of serotonin-related metabolism is present in CM and MOH sufferes.81,82,83
PACAP
Recent studies have reported low levels of interictal PACAP in migraine patients,16,84 suggesting a possible role for PACAP in the pathogenesis of migraine. Particularly, the interictal PACAP plasma levels negatively correlated with attack duration in the CM cohort.16 In addition, decreased PACAP content in plasma and trigeminal ganglia and increased PACAP related receptor expression in the trigeminal ganglia have been found in rats after repetitive chronic dural inflammatory stimulation.85 This decrease of PACAP induced by frequent headache attacks and the subsequent upregulation of related receptors maybe important in migraine progression and may serve as a novel target for migraine treatment. However, in another analysis of CM patients, interictal serum PACAP levels were not increased or decreased in CM women when compared to matched controls.86 Further studies are needed to confirm the precise role of this neuropeptide in migraine and migraine chronicity, as the sample sizes are quite limited in current researches.
Others
Elevation levels of tumor necrosis factor α were found in cerebrospinal fluid of CM patients,87 indicating the role of inflammation and endothelial dysfunction in the progression of migraine. Both CM and MOH have been associated with higher levels of cerebrospinal fluid orexin-A,56 which maybe an expression of hypothalamic response to stress due to chronic pain. The brain-derived neurotrophic factor has also been linked to CM,88 indicating the role of glia-neuron interaction in CM. Both CM and MOH patients have endocannabinoid system dysfunction, which may be related to serotonin system and then contribute to the chronification of both diseases.15
Management of CM
With confusion in the pathogenesis, CM is undertreated currently. Once the chronification is established, the treatment response becomes poorer. Therefore, in the management of CM, the first and most important step is to avoid the formation of CM by rigorous control of risk factors, including sufficient pain relief, timely prophylactic treatment of migraine, effective management of mood disorder, and other comorbidities.
Therapeutic options for CM are quite limited, and evidence-based effective treatment includes topiramate, onabotulinumtoxin A, and some neuromodulation therapy patterns. The efficacy and safety of topiramate for treatment of CM has been proved by double-blind RCTs, and a daily dose of 100 mg is generally effective and tolerable.17 A standard injection pattern of Onabotulinumtoxin A is effective and well-tolerated for prophylaxis of headache in CM patients as shown in the PREEMPT (two phase three studies: 24-week, double-blind, placebo-controlled, parallel-group phase, followed by 32-week, open-label phase) study.89 Some other preventive medications, including amitriptyline, valproate, gabapentin, and pregabalin, have also been shown to be effective in CM therapy in limited studies, which remains to be further investigated.90 For pharmacologically intractable CM patients, neuromodulatory methods targeting at peripheral or central modulation may offer help through both invasive and noninvasive patterns, such as blockade of the greater occipital nerve, occipital nerve stimulation, vagal nerve stimulation, and transcranial magnetic stimulation.
In treatment of CM, the modification of comorbidities such as sleep and mood disorders is as important as pain relief. And for anxiety/depression disorder in CM patients, tricyclic antidepressants (amitriptyline) seem to be more effective than SNRIs (selective serotonin/norepinephrine reuptake inhibitor) and SSRIs (selective serotonin reuptake inhibitor),91 but large-scale clinical trials are needed to verify the best option.
Summary
Transformed from EM, CM is featured by higher headache frequency, lager ratio of comorbidities, and severer disease burden. Some risk factors associated with migraine transformation have been identified, of which medication overuse, insufficient pain relief, and mood disorders need to be concerned in the attempt to avoid migraine chronification. The pathophysiology of CM is not fully understood, and recent advances in electrophysiology and neuroimaging have indicated that cortical hyperexcitability, brainstem dysfunction, and central sensitization are important in the development of CM. Taken together, CM may differ from EM in central excitability which links to headache progression toward a nearly daily basis. Alterations in much brain regions have been identified in CM patients, but future longitudinal studies are required to determine whether these plastic changes are causes or consequences of migraine chronification and whether they can serve as a “brain signature” for migraine phenotypes, evolution, and prognosis. Several molecules have also been identified in CM patients by biochemical analysis, which may serve as biomarkers of CM and also potential therapeutic targets. For management of CM, rigorous control of risk factors is most important, and the most established preventive therapies for CM include topiramate, onabotulinumtoxin A injections, and some neuromodulatory methods.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Beijing Science and Technology Project (grant Z161100002616013).
References
- 1.Headache Classification Committee of International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 33: 629–808. [DOI] [PubMed] [Google Scholar]
- 2.Lipton RB Fanning KM Serrano D Reed ML Cady R andBuse DC.. Ineffective acute treatment of episodic migraine is associated with new-onset chronic migraine. Neurology 2015; 84: 688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scher AI Buse DC Fanning KM Kelly AM Franznick DA Adams AM andLipton RB.. Comorbid pain and migraine chronicity: the chronic migraine epidemiology and outcomes study. Neurology 2017; 89: 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manack A Buse DC Serrano D Turkel CC andLipton RB.. Rates, predictors, and consequences of remission from chronic migraine to episodic migraine. Neurology 2011; 76: 711–718. [DOI] [PubMed] [Google Scholar]
- 5.Adams AM Serrano D Buse DC Reed ML Marske V Fanning KM andLipton RB.. The impact of chronic migraine: the chronic migraine epidemiology and outcomes (CaMEO) study methods and baseline results. Cephalalgia 2015; 35: 563–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipton RB Adams AM Buse DC Fanning KM andReed ML.. A comparison of the chronic migraine epidemiology and outcomes (CaMEO) study and American migraine prevalence and prevention (AMPP) study: demographics and headache‐related disability. Headache 2016; 56: 1280–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buse DC Manack A Serrano D Turkel C andLipton RB.. Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. J Neurol Neurosurg Psychiatr 2010; 81: 428–432. [DOI] [PubMed] [Google Scholar]
- 8.Aurora SK. Spectrum of illness: understanding biological patterns and relationships in chronic migraine. Neurology 2009; 72: 8–13. [DOI] [PubMed] [Google Scholar]
- 9.Cho SJ andChu MK.. Risk factors of chronic daily headache or chronic migraine. Curr Pain Headache Rep 2015; 19: 465. [DOI] [PubMed] [Google Scholar]
- 10.Aurora SK Barrodale PM Tipton RL andKhodavirdi A.. Brainstem dysfunction in chronic migraine as evidenced by neurophysiological and positron emission tomography studies. Headache 2007; 47: 996–1003; discussion 1004-7. [DOI] [PubMed] [Google Scholar]
- 11.Lai TH Chou KH Fuh JL Lee PL Kung YC Lin CP andWang SJ.. Gray matter changes related to medication overuse in patients with chronic migraine. Cephalalgia 2016; 36: 1324–1333. [DOI] [PubMed] [Google Scholar]
- 12.Mathew NT. Pathophysiology of chronic migraine and mode of action of preventive medications. Headache 2011; 51: 84–92. [DOI] [PubMed] [Google Scholar]
- 13.Schwedt TJ Larsonprior L Coalson RS Nolan T Mar S Ances BM Benzinger T andSchlaggar BL.. Allodynia and descending pain modulation in migraine: a resting state functional connectivity analysis. Pain Med 2014; 15: 154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cernuda-Morollón E Larrosa D Ramón C Vega J Martínez-Camblor P andPascual J.. Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology 2013; 81: 1191–1196. [DOI] [PubMed] [Google Scholar]
- 15.Rossi C Pini LA Cupini ML Calabresi P andSarchielli P.. Endocannabinoids in platelets of chronic migraine patients and medication-overuse headache patients: relation with serotonin levels. Eur J Clin Pharmacol 2008; 64: 1–8. [DOI] [PubMed] [Google Scholar]
- 16.Han X Dong Z Hou L Wan D Chen M Tang W andYu S.. Interictal plasma pituitary adenylate cyclase-activating polypeptide levels are decreased in migraineurs but remain unchanged in patients with tension-type headache. Clin Chim Acta 2015; 450: 151–154. [DOI] [PubMed] [Google Scholar]
- 17.Diener HC Bussone G Van Oene JC Lahaye M Schwalen S andGoadsby PJ.. Topiramate reduces headache days in chronic migraine: a randomized, double-blind, placebo-controlled study. Cephalalgia 2007; 27: 814–823. [DOI] [PubMed] [Google Scholar]
- 18.Diener HC Dodick DW DeGryse RE andTurkel CC.. OnabotulinumtoxinA for treatment of chronic migraine: PREEMPT 24-week pooled subgroup analysis of patients without medication overuse. J Headache Pain 2013; 14: P204. [DOI] [PubMed] [Google Scholar]
- 19.Dodick DW Silberstein SD Reed KL Deer TR Slavin KV Huh B Sharan AD Narouze S Mogilner AY andTrentman TL.. Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: long-term results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia 2015; 35: 344–358. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y Zhao Wang DR Ran A Xun O Tang HW andYu S.. Migraine prevention using different frequencies of transcutaneous occipital nerve stimulation: a randomized controlled trial. J Pain 2017; 18: 1006–1015. [DOI] [PubMed] [Google Scholar]
- 21.ICHD-II classification: parts 1–3: primary, secondary and other. Cephalalgia 2004; 24: 23–136.14687009 [Google Scholar]
- 22.Committee HC Olesen J Bousser MG Diener HC Dodick D First M Goadsby PJ Göbel H Lainez M andLance JW.. New appendix criteria open for a broader concept of chronic migraine. Cephalalgia 2006; 26: 742–746. [DOI] [PubMed] [Google Scholar]
- 23.Schwedt TJ Chong CD Wu T Gaw N Fu Y andLi J.. Accurate classification of chronic migraine via brain magnetic resonance imaging. Headache 2015; 55: 762–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Natoli JL Manack A Dean B Butler Q Turkel CC Stovner L andLipton RB.. Global prevalence of chronic migraine: a systematic review. Cephalalgia 2010; 30: 599–609. [DOI] [PubMed] [Google Scholar]
- 25.Stark RJ Ravishankar K Siow HC Lee KS Pepperle R andWang SJ.. Chronic migraine and chronic daily headache in the Asia-Pacific region: a systematic review. Cephalalgia 2013; 33: 266–283. [DOI] [PubMed] [Google Scholar]
- 26.Lantéri-Minet M Duru G Mudge M andCottrell S.. Quality of life impairment, disability and economic burden associated with chronic daily headache, focusing on chronic migraine with or without medication overuse: a systematic review. Cephalalgia 2011; 31: 837–850. [DOI] [PubMed] [Google Scholar]
- 27.Linde M Gustavsson A Stovner LJ Steiner TJ Barré J Katsarava Z Lainez JM Lampl C Lantéri-Minet M andRastenyte D.. The cost of headache disorders in Europe: the Eurolight project. Eur J Neurol 2012; 19: 703–711. [DOI] [PubMed] [Google Scholar]
- 28.Yu S Liu R Zhao G Yang X Qiao X Feng J Fang Y Cao X He M andSteiner T.. The prevalence and burden of primary headaches in China: a population-based door-to-door survey. Headache 2012; 52: 582–591. [DOI] [PubMed] [Google Scholar]
- 29.Raggi A Schiavolin S Leonardi M Giovannetti AM Bussone G Curone M Di FP Grazzi L Usai S andD’Amico D.. Chronic migraine with medication overuse: association between disability and quality of life measures, and impact of disease on patients’ lives. J Neurol Sci 2015; 348: 60–66. [DOI] [PubMed] [Google Scholar]
- 30.Buse D Manack A Serrano D Reed M Varon S Turkel C andLipton R.. Headache impact of chronic and episodic migraine: results from the American Migraine Prevalence and Prevention study. Headache 2012; 52: 3–17. [DOI] [PubMed] [Google Scholar]
- 31.Buse DC Silberstein SD Manack AN Papapetropoulos S andLipton RB.. Psychiatric comorbidities of episodic and chronic migraine. J Neurol 2013; 260: 1960–1969. [DOI] [PubMed] [Google Scholar]
- 32.Lipton RB andBigal ME.. Chronic daily headache: is analgesic overuse a cause or a consequence? Neurology 2003; 61: 154–155. [DOI] [PubMed] [Google Scholar]
- 33.Goadsby PJ Holland PR Martinsoliveira M Hoffmann J Schankin C andAkerman S.. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev 2017; 97: 553–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bigal ME Ashina S Burstein R Reed ML Buse D Serrano D andLipton RB.. Prevalence and characteristics of allodynia in headache sufferers: a population study. Neurology 2008; 70: 1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coppola G andSchoenen J.. Cortical excitability in chronic migraine. Curr Pain Headache Rep 2012; 16: 93–100. [DOI] [PubMed] [Google Scholar]
- 36.Chen WT Wang SJ Fuh JL Lin CP Ko YC andLin YY.. Persistent ictal-like visual cortical excitability in chronic migraine. Pain 2011; 152: 254–258. [DOI] [PubMed] [Google Scholar]
- 37.Fumal A Laureys S Di Clemente L Boly M Bohotin V Vandenheede M Coppola G Salmon E Kupers R andSchoenen J.. Orbitofrontal cortex involvement in chronic analgesic-overuse headache evolving from episodic migraine. Brain 2006; 129: 543–550. [DOI] [PubMed] [Google Scholar]
- 38.Chen Z Chen X Liu M Dong Z Ma L andYu S.. Altered functional connectivity architecture of the brain in medication overuse headache using resting state fMRI. J Headache Pain 2017; 18: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt-Wilcke T Gänssbauer S Neuner T Bogdahn U andMay A.. Subtle grey matter changes between migraine patients and healthy controls. Cephalalgia 2008; 28: 1–4. [DOI] [PubMed] [Google Scholar]
- 40.Maleki N Becerra L Brawn J Bigal M Burstein R andBorsook D.. Concurrent functional and structural cortical alterations in migraine. Cephalalgia 2012; 32: 607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim JH Kim JB Suh SI Seo WK Oh K andKoh SB.. Thickening of the somatosensory cortex in migraine without aura. Cephalalgia 2014; 34: 1125–1133. [DOI] [PubMed] [Google Scholar]
- 42.Yu S Chen X Chen Z Dong Z andMa L.. EHMTI-0307. Chronification of migraine: a clinical and voxel-based morphometry study. J Headache Pain 2014; 15: E43. [Google Scholar]
- 43.Heinricher MM Tavares I Leith JL andLumb BM.. Descending control of nociception: specificity, recruitment and plasticity. Brain Res Rev 2009; 60: 214–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welch KM Nagesh V Aurora SK andGelman N.. Periaqueductal gray matter dysfunction in migraine: cause or the burden of illness? Headache 2001; 41: 629–637. [DOI] [PubMed] [Google Scholar]
- 45.Chen Z Chen X Liu M Liu S Ma L andYu S.. Disrupted functional connectivity of periaqueductal gray subregions in episodic migraine. J Headache Pain 2017; 18: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mainero C Boshyan J andHadjikhani N.. Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol 2011; 70: 838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jia Z Tang W Zhao D andYu S.. Disrupted functional connectivity between the periaqueductal gray and other brain regions in a rat model of recurrent headache. Sci Rep 2017; 7: 3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perrotta A Serrao M Sandrini G Burstein R Sances G Rossi P Bartolo M Pierelli F andNappi G.. Sensitisation of spinal cord pain processing in medication overuse headache involves supraspinal pain control. Cephalalgia 2010; 30: 272–284. [DOI] [PubMed] [Google Scholar]
- 49.Okada-Ogawa A Porreca F andMeng ID.. Sustained morphine-induced sensitization and loss of diffuse noxious inhibitory controls in dura-sensitive medullary dorsal horn neurons. J Neurosci 2009; 29: 15828–15835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meng ID andHarasawa I.. Chronic morphine exposure increases the proportion of on-cells in the rostral ventromedial medulla in rats. Life Sci 2007; 80: 1915–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Z Chen X Liu M Liu S Ma L andYu S.. Volume gain of periaqueductal gray in medication-overuse headache. J Headache Pain 2017; 18: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Welch KMA, NV, SK, Aurora GN. Periaqueductal gray matter dysfunction in migraine and chronic daily headache may be due to free radical damage. J Headache Pain 2001; 2: s33–s41. [DOI] [PubMed] [Google Scholar]
- 53.Chen Z Chen X Liu M Liu S Ma L andYu S.. Volume expansion of periaqueductal gray in episodic migraine: a pilot MRI structural imaging study. J Headache Pain 2017; 18: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matharu MS Bartsch T Ward N Frackowiak RSJ Weiner R andGoadsby PJ.. Central neuromodulation in chronic migraine patients with suboccipital stimulators: a PET study. Brain 2004; 127: 220–230. [DOI] [PubMed] [Google Scholar]
- 55.Liu HY Chou KH Lee PL Fuh JL Niddam DM Lai KL Hsiao FJ Lin YY Chen WT andWang SJ.. Hippocampus and amygdala volume in relation to migraine frequency and prognosis. Cephalalgia 2017; 37: 1329–1336. [DOI] [PubMed] [Google Scholar]
- 56.Sarchielli P Rainero I Coppola F Rossi C Mancini M Pinessi L andCalabresi P.. Involvement of corticotrophin-releasing factor and orexin-A in chronic migraine and medication-overuse headache: findings from cerebrospinal fluid. Cephalalgia 2008; 28: 714–722. [DOI] [PubMed] [Google Scholar]
- 57.Schwedt TJ Schlaggar BL Mar S Nolan T Coalson RS Nardos B Benzinger T andLarson-Prior LJ.. Atypical resting-state functional connectivity of affective pain regions in chronic migraine. Headache 2013; 53: 737–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calejesan AA Kim SJ andZhuo M.. Descending facilitatory modulation of a behavioral nociceptive response by stimulation in the adult rat anterior cingulate cortex. Eur J Pain 2000; 4: 83–96. [DOI] [PubMed] [Google Scholar]
- 59.Wang G Erpelding N andDavis KD.. Sex differences in connectivity of the subgenual anterior cingulate cortex. Pain 2014; 155: 755–763. [DOI] [PubMed] [Google Scholar]
- 60.Peres MFP Rio MSD Seabra MLV Tufik S Abucham J Cipollaneto J Silberstein SD andZukerman E.. Hypothalamic involvement in chronic migraine. J Neurol Neurosurg Psychiatry 2001; 71: 747–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen Z Chen X Liu M Dong Z Ma L andYu S.. Altered functional connectivity of amygdala underlying the neuromechanism of migraine pathogenesis. J Headache Pain 2017; 18: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kitaj MB andKlink M.. Pain thresholds in daily transformed migraine versus episodic migraine headache patients. Headache 2005; 45: 992–998. [DOI] [PubMed] [Google Scholar]
- 63.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011; 152: S2–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aguggia M Saracco MG Cavallini M Bussone G andCortelli P.. Sensitization and pain. Neurol Sci 2013; 34: S37–S40. [DOI] [PubMed] [Google Scholar]
- 65.Bartley J. Could glial activation be a factor in migraine? Med Hypotheses 2009; 72: 255–257. [DOI] [PubMed] [Google Scholar]
- 66.Supornsilpchai W Le GS andSrikiatkhachorn A.. Involvement of pro-nociceptive 5-HT2A receptor in the pathogenesis of medication-overuse headache. Headache 2010; 50: 185–197. [DOI] [PubMed] [Google Scholar]
- 67.Su M Ran Y Han X Liu Y Zhang X Tan Q Li R andYu S.. Rizatriptan overuse promotes hyperalgesia induced by dural inflammatory stimulation in rats by modulation of the serotonin system. Eur J Neurosci 2016; 44: 2129–2138. [DOI] [PubMed] [Google Scholar]
- 68.Liang J Yu S Dong Z Wang X Liu R Chen X andLi Z.. The effects of OB-induced depression on nociceptive behaviors induced by electrical stimulation of the dura mater surrounding the superior sagittal sinus. Brain Res 2011; 1424: 9–19. [DOI] [PubMed] [Google Scholar]
- 69.Mingjie Z Wei D Jingyao L Xiaoyan C Yueqing H Bingqian C Meiyan P Zhao D andShengyuan Y.. Effects of UCMS-induced depression on nociceptive behaviors induced by electrical stimulation of the dura mater. Neurosci Lett 2013; 551: 1–6. [DOI] [PubMed] [Google Scholar]
- 70.Del FM Quartu M Boi M Serra MP Melis T Boccaletti R Shevel E andCianchetti C.. TRPV1, CGRP and SP in scalp arteries of patients suffering from chronic migraine. J Neurol Neurosurg Psychiatry 2015; 86: 393–397. [DOI] [PubMed] [Google Scholar]
- 71.Shimizu T Shibata M Toriumi H Iwashita T Funakubo M Sato H Kuroi T Ebine T Koizumi K andSuzuki N.. Reduction of TRPV1 expression in the trigeminal system by botulinum neurotoxin type-A. Neurobiol Dis 2012; 48: 367–378. [DOI] [PubMed] [Google Scholar]
- 72.Durham P andPapapetropoulos S.. Biomarkers associated with migraine and their potential role in migraine management. Headache 2013; 53: 1262–1277. [DOI] [PubMed] [Google Scholar]
- 73.Ho TW Edvinsson L andGoadsby PJ.. CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol 2010; 6: 573. [DOI] [PubMed] [Google Scholar]
- 74.Cady RK Vause CV Ho TW Bigal ME andDurham PL.. Elevated saliva calcitonin gene-related peptide levels during acute migraine predict therapeutic response to rizatriptan. Headache 2009; 49: 1258–1266. [DOI] [PubMed] [Google Scholar]
- 75.Cernudamorollón E Martínezcamblor P Serranopertierra E Larrosa D Ramón C andPascual J.. EHMTI-0105. CGRP and VIP levels as predictors of efficacy of onabotulinumtoxin type A in chronic migraine. J Headache Pain 2014; 15: 1–1. [DOI] [PubMed] [Google Scholar]
- 76.Bigal ME Edvinsson L Rapoport AM Lipton RB Spierings ELH Diener HC Burstein R Loupe PS Ma Y andYang R.. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol 2015; 14: 1081–1090. [DOI] [PubMed] [Google Scholar]
- 77.Durham PL. Diverse physiological roles of calcitonin gene-related peptide in migraine pathology: modulation of neuronal-glial-immune cells to promote peripheral and central sensitization. Curr Pain Headache Rep 2016; 20: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Keller DL. Triptan therapy in migraine. New Engl J Med 2010; 363: 63–70. [DOI] [PubMed] [Google Scholar]
- 79.Pakalnis A Splaingard M Splaingard D Kring D andColvin A.. Serotonin effects on sleep and emotional disorders in adolescent migraine. Headache 2009; 49: 1486–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Felice M Ossipov MH andPorreca F.. Persistent medication-induced neural adaptations, descending facilitation, and medication overuse headache. Curr Opin Neurol 2011; 24: 193–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.D’Andrea G D’Amico D Bussone G Bolner A Aguggia M Saracco MG Galloni E De RV Colavito D andLeon A.. The role of tyrosine metabolism in the pathogenesis of chronic migraine. Cephalalgia 2013; 33: 932–937. [DOI] [PubMed] [Google Scholar]
- 82.D’Andrea G D’amico D Bussone G Bolner A Aguggia M Saracco MG Galloni E Riva VD D’arrigo A andColavito D.. Tryptamine levels are low in plasma of chronic migraine and chronic tension-type headache. Neurol Sci 2014; 35: 1941–1945. [DOI] [PubMed] [Google Scholar]
- 83.Ilya A Mark O Kirsten L Leonora F Min-Suk Y Hans-Christoph D andZaza K.. Increased activity of serotonin uptake in platelets in medication overuse headache following regular intake of analgesics and triptans. J Headache Pain 2008; 9: 109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tuka B Helyes Z Markovics A Bagoly T Szolcsányi J Szabó N Tóth E Kincses ZT Vécsei L andTajti J.. Alterations in PACAP-38-like immunoreactivity in the plasma during ictal and interictal periods of migraine patients. Cephalalgia 2013; 33: 1085. [DOI] [PubMed] [Google Scholar]
- 85.Xun H Ye R Min S Liu Y Tang W Zhao D andYu S.. Chronic changes in pituitary adenylate cyclase-activating polypeptide and related receptors in response to repeated chemical dural stimulation in rats. Mol Pain 2017; 13: 1744806917720361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cernuda-Morollón E Riesco N Martínez-Camblor P Serrano-Pertierra E García-Cabo C andPascual J.. No change in interictal PACAP levels in peripheral blood in women with chronic migraine. Headache 2016; 56: 1448–1454. [DOI] [PubMed] [Google Scholar]
- 87.Rozen T andSwidan SZ.. Elevation of CSF tumor necrosis factor α levels in new daily persistent headache and treatment refractory chronic migraine. Headache 2007; 47: 1050–1055. [DOI] [PubMed] [Google Scholar]
- 88.Martins LB, Duarte H, Ferreira AV, Rocha NP, Teixeira AL and, Domingues RB. Migraine is associated with altered levels of neurotrophins. Neurosci Lett 2015; 587: 6. [DOI] [PubMed] [Google Scholar]
- 89.Aurora SK Dodick DW Turkel CC Degryse RE Silberstein SD Lipton RB Diener HC andBrin MF.. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Headache 2013; 68: 921–936. [DOI] [PubMed] [Google Scholar]
- 90.Weatherall MW. The diagnosis and treatment of chronic migraine. Ther Adv Chronic Dis 2015; 6: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Torta R andIeraci V.. Migraine and depression comorbidity: antidepressant options. Neurol Sci 2012; 33: S117–S118. [DOI] [PubMed] [Google Scholar]