Abstract
Background
Appropriate goal‐oriented treatment strategies are important for optimal treatment outcomes and may prevent under‐treatment. As treatment goals vary by patient, a study to examine treatment goals is more meaningful when patients and their physicians are paired. There has not been any study that examines alignment between paired psoriasis patients and physicians in real‐world clinical practice using skin clearance as a treatment goal indicator.
Objectives
To evaluate treatment goal alignment between psoriasis patients and their paired physicians, and to quantitatively identify factors associated with goal misalignment.
Methods
The study was a nationwide multicenter cross‐sectional observational study. Subjects were physician‐reported moderate‐to‐severe psoriasis patients with a history of systemic treatments, directly paired with their treating physicians. Subjects completed surveys independently. Treatment goals included seven categories, and patient–physician pairs were grouped as ‘aligned’ or ‘misaligned’ when the answers were the same or different, respectively.
Results
A total of 425 pairs (mean response rate, 94.7%) of responses were collected from 54 sites (64.8% general practitioners or clinics; 35.2% university or large hospitals). Treatment goal misalignment was found in 67.9% of the patient–physician pairs. The misalignment was mainly ‘patient predominant’ (60.9%) indicating that patients had higher goals (‘complete clearance’) than physicians. In the multivariate logistic regression analyses, patients’ treatment expectation for ‘complete clearance’ [odds ratio (OR): 1.927; 95% confidential interval (CI): 1.232–3.016] and physician rating of ‘level of understanding on treatment options’ being low (OR: 1.552, 95% CI; 1.082–2.227) were significant factors for treatment goal misalignment.
Conclusions
The majority of treatment goal misalignment was found between paired psoriasis patients and their treating physicians in Japan. The most important contributing factors to misalignment were patients’ treatment expectation for ‘complete clearance’ and physicians’ rating of their patients’ ‘level of understanding on treatment options’ being low.
Introduction
The prevalence of psoriasis in Japan is approximately 0.3%,1, 2 which is lower than that (2–4%) in Europe and the United States.3, 4, 5 Based on the proportion of the affected area relative to the total body surface area (BSA), the severity of psoriasis is rated on a three‐grade scale (mild, moderate or severe). Psoriasis Area and Severity Index (PASI) scores and static Physician Global Assessment (sPGA) are the most frequently employed clinical tools to evaluate the severity of this disease.
Psoriasis often reduces the physical, mental and social functions of patients, resulting in decrease in quality of life (QOL). According to a QOL survey of 228 patients with psoriasis using the Japanese version of the Psoriasis Disability Index (PDI, a psoriasis‐specific QOL scale) and Short Form‐36 (SF‐36, a comprehensive health‐associated QOL scale), severity rating by PASI correlates with each domain of QOL.6, 7
Setting specific psoriasis treatment goals has gained increasing attention recently, although the definition of treatment success varies among physicians and patients.8 Treatment goals are affected by several factors such as treatment satisfaction, disease severity, treatment efficacy, adverse events, convenience of the current treatment, expectation for the treatment, and improvements in QOL. When treatment goals are set after considering multiple factors, they need to be well communicated bilaterally. Treatment goals should be a shared decision between patients and their physicians. Misalignment (discrepancy) of treatment goals between psoriasis patients and treating physicians may adversely affect treatment outcomes.8, 9 On the other hand, appropriate goal‐oriented treatment strategies may improve patient adherence to drug therapy,8 improve patient's and physician's treatment satisfaction, and reduce the chance of patients being undertreated. In Europe, there was a consensus to adopt PASI 75 as a treatment goal indicator for moderate‐to‐severe psoriasis, and complete remission is viewed as the final goal of treatment.9 Recently, Torres10 and Puig,11 in an opinion and a review article, respectively, suggested that PASI 90 could be the new standard of care. In the same year, Strohal et al.12 released an expert consensus in which they suggested a patient‐centred approach to better align mutual expectation and goals of biological treatments and outcomes. More recently, in the United States, the National Psoriasis Foundation (NPF) indicated that treatment goals need to be BSA 1% or less, both 3 months after the start of a new treatment and every 6 months during the maintenance treatment period.13 In Japan, there is currently no clear definition for using an indicator to set treatment goals. To date, there are no published Japanese guidelines on setting numerical treatment goals.
Studies evaluating treatment goal alignment in the field of psoriasis are limited. Moreover, there has not been any study that examines alignment between paired psoriasis patients and physicians in real‐world clinical practice using skin clearance as a treatment goal indicator. The aim of this study was to evaluate treatment goal alignment between paired psoriasis patients and their treating physicians and to quantitatively identify factors associated with goal misalignment.
Methods
Study design
Our study was a nationwide multicenter cross‐sectional observational study. Subjects were physician‐reported moderate‐to‐severe psoriasis patients with a history of systemic treatments, including biological, and their treating physicians. Customized and standardized questionnaires were used for the survey. Patients participating in or who had completed a clinical trial less than 6 months before, and patients with pustular psoriasis, erythrodermic psoriasis or psoriatic arthritis were excluded. Dermatologists with experience in oral or biological treatments for psoriasis patients were included.
Each patient and the treating physician completed the surveys independently. To avoid selection biases by physicians, patients were enrolled consecutively. The survey was conducted between October 2015 and May 2016.
The survey consisted of 52 questions for patients and 31 questions for physicians. The questions were categorized into (i) background variables of patients and physicians, (ii) disease severity, (iii) treatment goals, and (iv) treatment satisfaction and QOL (Appendix 1).
Treatment goals included seven categories (1 representing the highest goal, 7 representing the lowest goal): 1. complete clearance (PASI 100), 2. almost complete clearance (PASI 90‐100), 3. complete clearance of specific sites (nails, head, genitals, others) but without complete or almost clearance for the remaining lesions, 4. improvement from previous treatment but without complete or almost clearance, 5. relief of itchiness, 6. others and 7. no particular goal set. Subject groups were ‘treatment goal aligned’ when the answers were the same between the patient–physician pair, and ‘treatment goal misaligned’ when any of the answers differed between the pair.
This study was performed in compliance with the Declaration of Helsinki, the Good Pharmacoepidemiology Practices (GPPs), the ‘Ethical Guidelines Concerning Medical Studies in Human Subjects’14 and the ethical principles based on the relevant statutes/standards in the country concerned. It was reviewed and approved in advance by the Ethics Committee of Jichi Medical University, the Central IRB of Medical Corporation Ganka‐Koseikai (Central IRB) and the Ethics Committees organized as needed at each participating hospital.
Statistical analysis
The chi‐squared test was used to evaluate alignment of each patient–physician pair. In treatment goal misaligned pairs, when patients’ goals were higher than the physician's, they were grouped as ‘patient predominant’, and if the physicians’ treatment goal was higher, they were grouped as ‘physician predominant’.
Logistic regression analysis by dichotomy of aligned (denoted as 0) and misaligned (denoted as 1) in the patient–physician pairs was applied. Analyses were conducted by the following steps: Step 1: statistical evaluation of variables between ‘aligned’ and ‘misaligned’ group (P < 0.25); Step 2: variables selected from Step 1 were re‐evaluated for co‐linearity and clinical validity. Step 3: Variables selected in Step 2 were applied into a stepwise logistic regression model.
Normality was evaluated using the Shapiro–Wilk test, and if normality was rejected, the Wilcoxon rank sum test (for two groups) or Kruskal–Wallis test (for three groups or over) was employed. The t‐test (for two groups), chi‐squared test or analyses of variance (anova) (for three groups or over) was applied to test for differences. All statistical analyses were performed on SAS ver. 9.4. (SAS Institute Inc., Cary, North Carolina, USA) P < 0.05 was regarded as significant.
Results
Study population
A total of 425 patient–physician pairs of 449 registered were collected (response rate, 94.7%). Because matched physicians were asked to complete the survey only after the patients had completed their survey, the response rate of patient and physician was 94.7% and 100%, respectively. The investigation was conducted at 54 facilities. Of which, 35 (64.8%) were general practitioners (GP) or clinics, and 19 (35.2%) were university hospitals, private hospitals or public hospitals (HP). Patient distribution included 57.4% from GP/clinics and 42.6% from HP. Overall, the patients were mostly treated by dermatologists (99.8%) followed by generalist (4.2%) and ‘others’ (1.5%). Among the ‘others’, it included plastic surgeon (n = 2), psychologist (n = 1), orthopaedic surgeon (n = 2) and haematologist (n = 1).
A total of 414 pairs were included in the final analyses. Demographic and background characteristics for patients (n = 414) and physicians (n = 70) are shown in Table 1a,b. The patients were 56.2 years old on average and were mostly men (74.9%). Patients had fairly long disease duration (mean 18.8 years), and 16.5% of patients reported BSA of more than 10% for their disease state with the mean score for Patient Global Assessment (patient GA; 0–5 scale) at 2.54 and Physician Global Assessment (sPGA; 0–5 scale) at 2.51. The mean age of physicians was 50.6 years and most of them were men (64.3%). Of all physicians, 86.8% had 10‐year or longer careers in treating psoriasis patients. Treatment satisfaction on a 0–10 scale for both patients and physicians was similar, 6.75 and 6.46, respectively.
Table 1.
(a) Background characteristics of psoriasis patients. (b) Background characteristics of treating physicians
| (a) Patient characteristics | |
|---|---|
| Number of patients (males, %) | 414 (310, 74.9%) |
| Age | 56.2 ± 13.9 years (20.0–93.0) |
| BMI | 24.3 ± 4.6 kg/m2 (16.0–54.9) |
| Age at onset/age at diagnosis | 37.2 ± 16.2 (0.0–81.0)/40.0 ± 16.2 (4.0–81.0) years |
| Disease duration from onset | 18.8 ± 11.7 years (0.0–65.0) |
| Affected sites (3 most frequent sites)a | Leg (78.0%), head (70.8%), back (67.1%) |
| BSA (one palm size assumed to be 1%) | <1% (24.4%), 1–2% (22.0%), 3–10% (37.0%), >10% (16.5%) |
| Current treatmenta | Topical‐dose drugs (82.4%), oral‐dose drugs (53.6%), phototherapy (19.1%), biological agents (25.6%), others (1.4%) |
| Treatment satisfaction (0–10 scale)b | 6.75 ± 2.27 |
| Patient rating of severityc (Patient GA: 0–5 scale) | 2.54 ± 1.26 |
| (b) Physician characteristics | |
|---|---|
| Number of responding physicians (males, %) | 70 (45, 64.3%) |
| Age | 50.6 ± 11.7 years (30.0–80.0) |
| Specialtya | Psoriasis (69.6%), Allergy (40.6%), Others (41.8%) |
| Career in psoriasis treatment | <2 years (0.0%), 2 year to <4 years (2.9%), 4 years to <6 years (5.9%), 6 years to <8 years (4.4%), 8 years to <10 years (0.0%), ≥10 years (86.8%) |
| Number of patients managed/month | <5 (1.5%), 5–9 (5.9%), 10–14 (10.3%), 15–19 (7.4%), ≥20 (75.0%) |
| Treatment satisfaction (0–10 scale)b | 6.46 ± 2.08 |
| Severity rating by physiciansc (Physician GA: 0–5 scale) | 2.51 ± 1.15 |
Multiple answers acceptable.
Scale represents treatment satisfaction with 0 indicating the lowest level of satisfaction and 10 indicating the highest level of satisfaction.
Scale represents severity of disease with 0 indicating the lowest level of severity and 5 indicating the highest level of severity.
Values are shown as mean ± SD or %; range (min, max).
Physicians who reported inconsistent background information with patients were excluded.
BMI = (body weight [kg])/(height [m])2.
GA, Global Assessment (severity assessment).
Treatment goal alignment
Between patients and physicians, treatment goal misalignment was found in 67.9% of the pairs. The misalignment was similar between institutions using biological agents (67.4%) vs. non‐biological agents (68.6%) (P = 0.789). The misalignment was also identical in GP (69.7%) vs. HP (66.5%). The misalignment was more frequently ‘patient predominant’ (60.9%), meaning patients had higher goals than physicians (Fig. 1). The ‘patient‐predominant’ misalignment was higher in GP/clinics (65.1%) compared to HP (55.8%), but the difference was not significant (P = 0.142).
Figure 1.
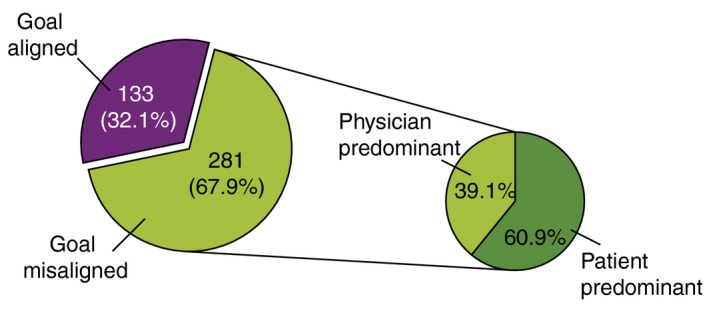
Treatment alignment between psoriasis patients and their physicians. Values are shown as proportion (pie chart on right; response of 256 cases). Treatment goal misalignment: (patient treatment goals) ≠ (physician treatment goals); treatment goal alignment: (patient treatment goals) = (physician treatment goals); patient predominant: when (patient treatment goals) − (physician treatment goals) was negative meaning, higher goals by patients; physician predominant: when (patient treatment goals) − (physician treatment goals) was positive meaning, higher goals by physicians. The number excludes cases that have unknown treatment goals such as the answers were ‘others’ or ‘goals not set’.
Within the misalignment group, the patients who had higher goals tended to have more severe illness (P < 0.0001) were younger (P = 0.008) and had an occupation (P = 0.043)
Although more patients (17.9%) than physicians (8.7%) chose ‘complete clearance’ as their treatment goal, the most frequently chosen treatment goal was ‘almost complete clearance’ in both the patient and physician groups (Fig. 2). When asked about the reasons for the selection of treatment goals, the most frequently selected response of patients was treatment expectation of ‘complete clearance’ (43.5%) (Fig. 3a), while the response of physicians was ‘difficulty in complete clearance’ (40.2%) (Fig. 3b).
Figure 2.
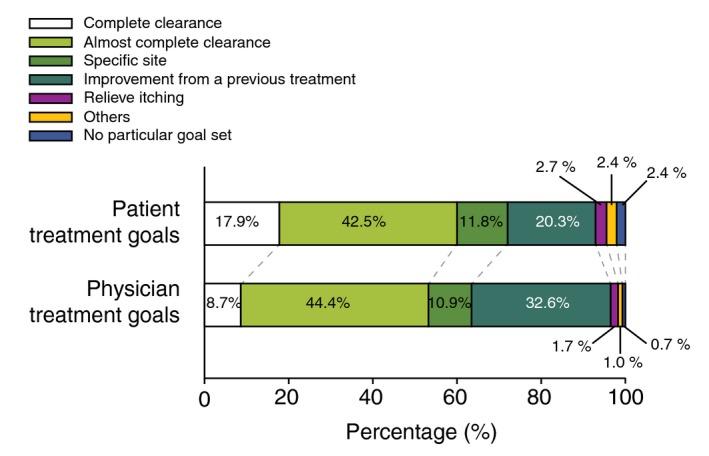
Treatment goals of psoriasis patients and their physicians. Values are shown as proportion. Subjects who did not indicate treatment goal (two physicians, nine patients) were excluded from this analysis.
Figure 3.
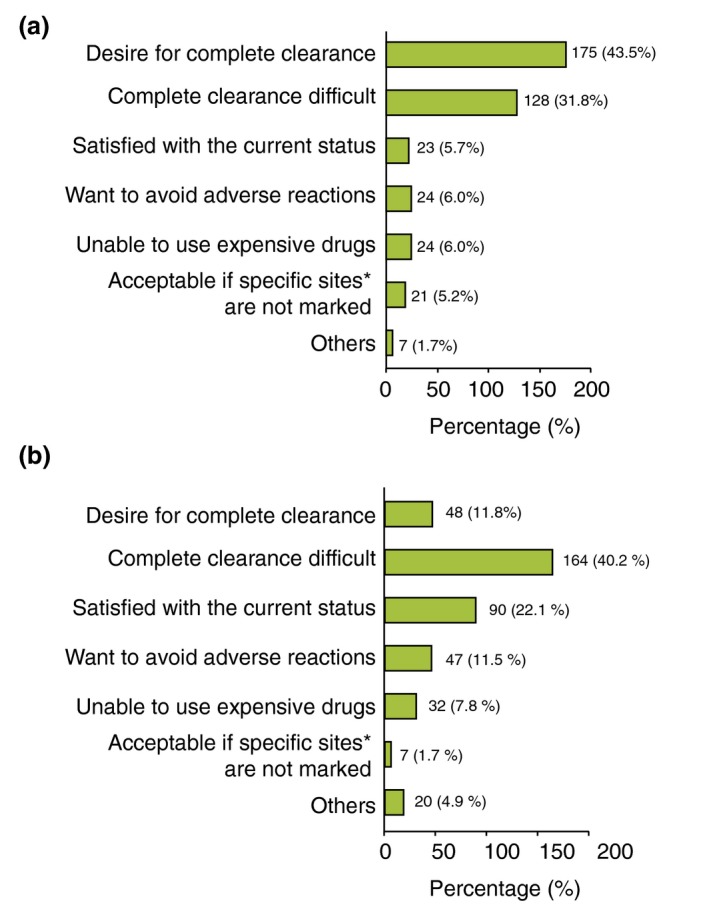
(a) Patient's most important reasons in setting treatment goal. Values are shown as proportion (response: 402 cases). Ten subjects having answered ‘goals not set’ to the question about treatment goal were excluded. Two subjects who did not indicate the most important reason for treatment goal were excluded. (b) Physician's most important reasons in setting treatment goal. Values are shown as proportion (response: 408 cases). Three subjects who indicated ‘goals not set’ to the question about treatment goal were excluded. Three subjects who did not indicate the most important reason for treatment goal were excluded. *Specific site: nails, head, genitals, others.
Comparison between aligned and misaligned groups
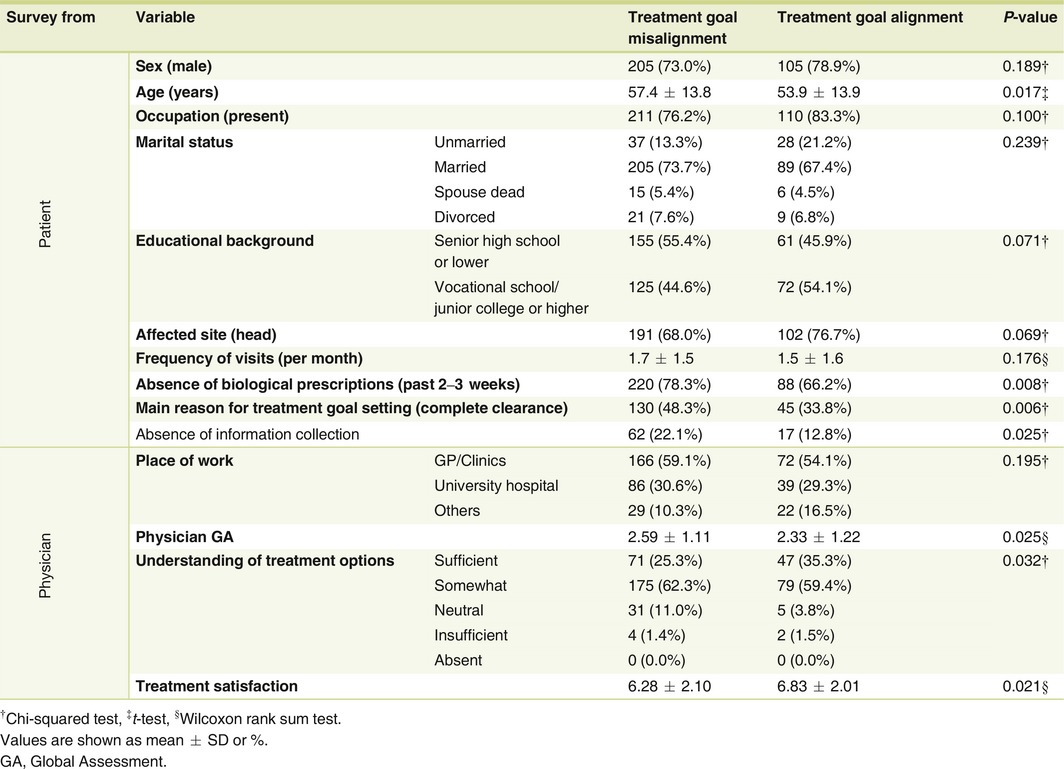
Comparison of the aligned and misaligned groups (Table 2) revealed that the misaligned group had patients who were older (P = 0.017), had not received biological prescription during the past 2–3 weeks (P = 0.008), chose ‘complete clearance’ as the most important reason for treatment goal setting (P = 0.006), and who had never collected information about psoriasis (P = 0.025). On the other hand, the physicians in the misaligned group had rated patients as more severe in disease (P = 0.025) and were less satisfied with treatments (P = 0.021). Furthermore, the percentage of physicians who chose ‘patients sufficiently understanding treatments options’ was lower in the misaligned group than in the aligned group (P = 0.032).
Table 2.
Comparison between treatment goal aligned and misaligned groups in psoriasis patients and their treating physicians
Evaluation of factors affecting treatment goal misalignment
Stepwise logistic regression demonstrated that the two contributing factors significantly leading to treatment goal misalignment were as follows: (i) patients’ ‘complete clearance’ as the most important reason for treatment goal [odds ratio (OR): 1.927; 95% confidential interval (CI): 1.232–3.016], and (ii) physician rating of patients’ ‘level of understanding on treatment options’ being low (OR: 1.552; 95% CI: 1.082–2.227) (Table 3).
Table 3.
Factors affecting treatment goal misalignment (N = 380a)
| Factor | Odds ratio Exp (β) | Odds ratio 95% CI | P‐value | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| Patient's leading reason for the treatment goal (‘complete clearance’) | 1.927 | 1.232 | 3.016 | 0.004 |
| Physician rating of patient's understanding of treatment options | 1.552 | 1.082 | 2.227 | 0.017 |
Goodness‐of‐fit test (Hosmer and Lemeshow test): P = 0.156.
Accurate discrimination rate (cut‐off level = 0.50): 66.9%.
Pairs with incomplete answers about physician or patient treatment goals (11 pairs) were excluded from the analysis. Pairs defective for any of the variables applied to the model (34 pairs) were excluded from the analysis.
GA, Global Assessment.
Subjects with the two contributing factors were further evaluated, and we found that the group of patients desiring complete clearance were characterized by a shorter disease duration than the group of patients not desiring complete clearance (P = 0.002). In addition, more patients in the group judged by physicians as ‘sufficiently understanding treatment options’ were characterized as being younger (P = 0.026), had searched for information about psoriasis (P = 0.001) and had discussed the current treatment goals with their physicians (P < 0.001).
Discussion
Our study investigated treatment goal alignment among 425 moderate‐to‐severe psoriasis patients paired with their treating physicians in real‐world clinical practice. Our study revealed approximately 70% treatment goal misalignment in paired patient–physicians. The treatment expectation of ‘complete clearance’ by patients and lack of understanding on treatment options were two significant contributing factors to treatment goal misalignment.
To the best of knowledge, our study is the first to find treatment goal misalignment between paired psoriasis patient–physician using dermatological symptoms as an indicator. Non‐Japanese studies have examined misalignment of disease severity between paired patients and physicians in various disease areas such as atopic dermatitis,15 rheumatoid arthritis,16 pain17 and other disease areas. For psoriasis18 and psoriatic arthritis,19, 20, 21 studies on misalignment were mostly on perception of disease severity or treatment satisfaction in paired subjects but the objectives were not on treatment goal. A survey on paired psoriasis patients and physicians reported a treatment satisfaction discrepancy rate of 18.3%.22 On the other hand, in Japan, the study by Torii et al.23 noted a 27.8% discrepancy in the satisfaction with the treatment between patients and physicians, but the subjects were not paired. On studies related to treatment goals among psoriasis, there were two studies performed between paired patients and physicians. Zweegers et al.,24 in BioCAPTURE registry, showed that treatment goal algorithm was well followed in real‐world where 64% (253 or 395 visits) of physicians followed the treatment goal algorithm in most visits. In their study, PASI was analysed as treatment effectiveness and Dermatology Life Quality Index (DLQI) was used to assess patient perspective. However, there was no mentioning of whether patients were aligned with the treatment goals in terms of skin clearance set by the clinicians. Radtke et al.25 conducted a study and showed that most patients experience having treatment goals in real‐world clinical settings. In the questionnaire they developed and used for the study, patients were asked of their treatment expectations or treatment goals. The result showed that 63.9% of treatment goals were defined together between paired clinicians and patients. However, specific treatment goal was not defined in the study.
Patients desiring complete clearance were those with shorter disease duration which may suggests that the goal for aggressive treatment is gradually lowered as disease progresses. Our result seems to suggest that treatment goal misalignment is less likely to occur in patients who actively collected information on treatments and those having good communication with the physician. Shared decisions and favourable communication between patients and physicians are keys to treatment satisfaction and optimal treatment. Factors contributing to treatment goals by individuals are diverse.8 For example, gender, marital status and communication competency may be factors associated with treatment goal alignment. Therefore, treatment goals fit for each individual would need to be established and communicated.
Our results demonstrated that more patients set higher goals than physicians. The percentage of ‘patient‐predominant’ misalignment was higher than that of ‘physician‐predominant’ misalignment. This result was supported by the finding that most patients desire for ‘complete clearance’ as their most important reason for treatment goal.
When looking at misalignment by institutions, we found the misalignment almost identical between GP (69.7%) and HP (66.5%). Within the misaligned group, ‘patient‐predominant’ misalignment in GP was 65.1% and in HP was 55.8%. The difference between GP and HP was not large. The small ‘patient‐predominant’ difference between GP and HP in Japan may be explained by its healthcare system and the relatively effective referral system for psoriasis care in Japan.26 The universal healthcare system allows most patients to have free access to small or large hospitals. Although only limited GPs are licensed to initiate biologicals and aggressive treatments are generally carried out in larger hospitals, moderate‐to‐severe psoriasis patients may be referred to larger specialized hospitals at a fairly early disease stage, resulting in small difference of ‘patient‐predominant’ misalignment between GP and HP. This phenomenon could be a different picture in other countries. To confirm this implication, a larger study and further examination of referral system in Japan would be needed.
The validity to use PASI scores for treatment goal evaluation may be challenged. Although the achievement of PASI 75 has been regarded as a treatment goal in Europe,9 more recent articles tend to suggest setting higher goals11, 13 In a preliminary pilot survey prior to the present study, we evaluated the feasibility of patients to answer questions based on these skin clearance categories referencing PASI as an indicator. From the pilot survey, we concluded that using PASI or skin clearance categories as a treatment goal was feasible. There may be indicators for treatment goal evaluation other than PASI and DLQI. Other investigators suggested the applicability of BSA and physician GA as more practical and easier tools of evaluation.8 Further studies are needed to examine the validity of the respective indicators for treatment goal evaluations. It would be also important to examine the value of skin clearance (PASI score cut‐off) as a measure of treatment success independent of other clinical factors. In addition, further studies would need to validate how clinically meaningful are the PASI treatment goals to patient outcomes.
Our study presents several limitations. First, although we attempted to reduce biases in patient selection by adopting the consecutive enrolment method, selection bias in observational studies is not completely eliminated. Second, the sample size of our study may be limited. Due to limited preceding studies, our study was exploratory and the final target number of subjects was set as 300 pairs for practicality (425 pairs enrolled). A clinical study design taking into account the sample size with our results may be needed in the future to verify the outcomes.
Bridging the gap of treatment goals misalignment, and setting patient‐oriented realistic goals may contribute to better management of psoriasis treatment in Japan. Our study may provide preliminary evidence towards a goal‐oriented psoriasis treatment strategy in Japan.
Acknowledgements
We would like to thank all the patients and physicians from the clinics and hospitals (Appendix 2), who generously shared their precious time for this study. The study was funded by Eli Lilly Japan.
Appendix 1.
Major items included in the questionnaires of patients and physicians
| Category | Patient variable | Physician variable |
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
Treatment goal: 1. complete clearance, 2. almost complete clearance, 3. complete clearance of specific sites (nails, head, genitals, others) but without complete or almost clearance for the remaining lesions, 4. improvement from previous treatment but without complete or almost clearance, 5. to relieve itching, 6. others and 7. no particular goal set.
BSA, Body surface area; DLQI, Dermatology Life Quality Index (skin disease‐specific QOL scale); GA, Global Assessment (severity assessment); TSQM, Treatment Satisfaction Questionnaire for Medication (Treatment Satisfaction Scale).
Appendix 2.
List of participating institutions
| Department of Dermatology, Asahikawa Medical University |
| Department of Dermatology, Jichi Medical University |
| Department of Dermatology, Tokyo Woman's Medical University |
| Department of Dermatology, School of Medicine, Teikyo University |
| Department of Dermatology, Tokyo Medical University |
| Department of Dermatology, School of Medicine, Tokai University |
| Department of Dermatology, Graduate School of Medicine, Gifu University |
| Graduate School of Medical Sciences, Nagoya City University |
| Department of Dermatology, School of Medicine, Kindai University |
| Department of Dermatology, Graduate School of Medicine, Osaka City University |
| Department of Dermatology, Kawasaki Medical School |
| Department of Dermatology, Faculty of Medicine, Fukuoka University |
| Department of Dermatology, Tokyo Teishin Hospital |
| Department of Dermatology, St Luke's International Hospital |
| Department of Dermatology, Yokohama Chuo Hospital |
| Public Interest Incorporated Foundation Jiai‐kai, Branch of Imamura Hospital |
| Department of Dermatology, Ina Central Hospital |
| Department of Dermatology, Iida Municipal Hospital |
| Department of Dermatology, Osaka Kaisei Hospital |
| Medical Corporation Kojin‐kai, Sapporo Dermatology Clinic |
| Medical Corporation Kojin‐kai, Fukuzumi Dermatology Clinic |
| Kobayashi Skin Clinic |
| Department of Dermatology, EST Clinic |
| Sugawara Dermatology Clinic |
| Medical Corporation Subaru‐kai, Sugai Dermatology Park Side Clinic |
| Hattori Dermatology Clinic |
| Medical Corporation Kouten‐kai, Iidabashi Clinic, Iidabashi Clinic |
| Medical Corporation Shohei‐kai, Futaki Skin Care Clinic |
| Hihuno Clinic Ningyocho, Ningyocho |
| Dr. Mariko Skin & Dermatology Clinic |
| Tsujimoto Skincare Clinic |
| Shirosaki Dermatology & Neurology Clinic |
| Kato Dermatology |
| Hou Dermatology Clinic |
| Machino Skin Clinique |
| Yasumoto Dermatology Clinic |
| Takagi Dermatology Clinic |
| Fushimi Skin Clinic |
| Omorimachi Dermatology |
| Hayashibe Derma Clinic |
| Hasegawa Dermatology Clinic |
| Medical Corporation Kojin‐kai, Ario Sapporo Dermatology Clinic |
| Atago Dermatology |
| Medical Corporation Syotoku‐kai, Hino Clinic |
| Nomura Dermatology Clinic |
| Zoshiki Dermatology Clinic |
| Nakatsu Dermatology Clinic |
| Saruwatari Dermatology Clinic |
| Kusuhara Dermatology Clinic |
| Medical Corporation Shimizu Dermatology Clinic |
| Kokubu Clinic, Abashiri Dermatology Clinic |
| Nishide Skin Clinic |
| Kazama Skin Clinic |
| Shimizu Skin Clinic |
Conflicts of interest
There is no product being studied in this manuscript. A.C. Tang, H. Torisu‐Itakura and T. Hanada are an employee of Eli Lilly K.K. Japan. Y. Okubo has been a consultant, scientific advisor and/or investigator for Eli Lilly Japan K. K., Kyowa Hakko Kirin Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Maruho Co., Ltd., Celgene K. K., Janssen Pharmaceutical K.K., AbbVie GK, Eisai Co., Ltd., Torii Pharmaceutical Co., Ltd., Leo Pharma, MSD K. K. and Boehringer Ingelheim Japan, Inc. S. Inoue is an employee of CRECON Medical Assessment Inc.; CRECON Medical Assessment Inc. was paid to conduct analyses for this article. M. Otsuki has been paid as a consultant to Abbvie, Boeringer‐Ingelheim, Celgene, Eisai, Janssen, Kyowa‐kirin, LEO pharm, Eli lilly and company, Maruho, Novartis, Pfizer, and Tanabe‐Mitsubishi. The remaining author has no conflicts of interest.
Funding sources
The study was funded by Eli Lilly Japan.
References
- 1. Ohkawara A, Yasuda H, Kobayashi H et al Generalized pustular psoriasis in Japan: two distinct groups formed by differences in symptoms and genetic background. Acta Derm Venereol 1996; 76: 68–71. [DOI] [PubMed] [Google Scholar]
- 2. Kubota K, Kamijima Y, Sato T et al Epidemiology of psoriasis and palmoplantar pustulosis: a nationwide study using the Japanese national claims database. BMJ Open 2015; 5: e006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gelfand JM, Weinsten R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: a population‐based study. Arch Dermatol 2005; 141: 1537–1541. [DOI] [PubMed] [Google Scholar]
- 4. Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003‐2004. J Am Acad Dermatol 2009; 60: 218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parisi R, Symmons DP, Griffiths CE, Ashcroft DM, Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team . Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013; 133: 377–385. [DOI] [PubMed] [Google Scholar]
- 6. Hirabe M, Hasegawa T, Fujishiro Y, Kigawa M, Fukuchi O, Nakagawa H. Factors associated with quality of life among patients with psoriasis – comparison between psoriasis‐specific QOL measures and generic QOL measures. Nihon Koshu Eisei Zasshi 2008; 55: 65–74. [PubMed] [Google Scholar]
- 7. Okubo Y, Arai K, Fujiwara S, Amaya M, Tsuboi R. Assessment of the quality of life of patients with psoriasis using skindex‐16 and GHQ‐28. Jpn J Dermatol 2007; 117: 2495–2505. [Google Scholar]
- 8. Brezinski EA, Armstrong AW. Strategies to maximize treatment success in moderate to severe psoriasis: establishing treatment goals and tailoring of biologic therapies. Semin Cutan Med Surg 2014; 33: 91–97. [DOI] [PubMed] [Google Scholar]
- 9. Mrowietz U, Kragballe K, Reich K et al Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res 2011; 303: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Torres T, Puig L. Treatment goals for psoriasis: should PASI 90 become the standard of care? Actas Dermosifiliogr 2015; 106: 155–157. [DOI] [PubMed] [Google Scholar]
- 11. Puig L. PASI90 response: the new standard in therapeutic efficacy for psoriasis. J Eur Acad Dermatol Venereol 2014; 29: 645–648. [DOI] [PubMed] [Google Scholar]
- 12. Strohal R, Prinz JC, Girolomoni G et al A patient‐centred approach to biological treatment decision making for psoriasis: an expert consensus. J Eur Acad Dermatol Venereol 2015; 29: 2390–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Armstrong AW, Siegel MP, Bagel J et al From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol 2017; 76: 290–298. [DOI] [PubMed] [Google Scholar]
- 14. Ministry of Education, Culture, Sports, Science and Technology . Ethical guidelines concerning medical studies in human subjects. [WWW document] 2015. URL http://www.lifescience.mext.go.jp/files/pdf/n1500_02.pdf (last accessed: 12 June 2017).
- 15. Wei W, Anderson P, Gadkari A et al Discordance between physician‐ and patient‐reported disease severity in adults with atopic dermatitis: a US cross‐sectional survey. Am J Clin Dermatol 2017. http://doi.org/10.1007/s40257-017-0284-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Desthieux C, Hermet A, Granger B et al Patient‐physician discordance in global assessment in rheumatoid arthritis: a systematic literature review with meta‐analysis. Arthritis Care Res (Hoboken) 2016; 68: 1767–1773. [DOI] [PubMed] [Google Scholar]
- 17. Calpin P, Imran A, Harmon D. A comparison of expectations of physicians and patients with chronic pain for pain clinic visits. Pain Pract 2017; 17: 305–311. [DOI] [PubMed] [Google Scholar]
- 18. Feldman S, Bushnell D, Viswanathan HN et al Differences in patient‐reported psoriasis symptom severity between patients rated as clear versus almost clear based on physician global assessment. J Am Acad Dermatol 2015; 72: AB230. [Google Scholar]
- 19. Eder L, Thavaneswaran A, Chandran V et al Factors explaining the discrepancy between physician and patient global assessment of joint and skin disease activity in psoriatic arthritis patients. Arthritis Care Res 2015; 67: 264–272. (ahead of print). [DOI] [PubMed] [Google Scholar]
- 20. Furst DE, Tran M, Sullivan E et al Misalignment between physicians and patient satisfaction with psoriatic arthritis disease control. Clin Rheumatol 2017; 36: 2045–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Desthieux C, Granger B, Balanescu AR et al Determinants of patient‐physician discordance in global assessment in psoriatic arthritis: a multicenter European study. Arthritis Care Res 2017; 69: 1606–1611. [DOI] [PubMed] [Google Scholar]
- 22. Korman NJ, Zhao Y, Pike J, Roberts J, Sullivan E, Kemhus M. Satisfaction with current psoriasis treatment: misalignment between physician and patient perceptions. Dermatol Online J 2016; 22: 1. [PubMed] [Google Scholar]
- 23. Torii H, Nakagawa H. Questionnaire survey of perceived satisfaction with treatment of patients with psoriasis. Jpn J Dermatol 2013; 123: 1935–1944. [Google Scholar]
- 24. Zweegers J, van den Reek JM, van de Kerkhof PC et al Comparing treatment goals for psoriasis with treatment decisions in daily practice: results from a prospective cohort of patients with psoriasis treated with biologics: BioCAPTURE. Br J Dermatol 2014; 171: 1091–1098. [DOI] [PubMed] [Google Scholar]
- 25. Radtke MA, Reich K, Spehr C et al Treatment goals in psoriasis routine care. Arch Dermatol Res 2015; 307: 445–449. [DOI] [PubMed] [Google Scholar]
- 26. Kouro O, Usuda T, Takeoka K et al Health care system examination committee chairman advisory report: a field survey of dermatology in community health care. J Commun Dis 2014; 31: 520–541. (in Japanese). [Google Scholar]


