Summary
Background
In 2013, a systematic review and Delphi consensus reported that specific probiotics can benefit adult patients with irritable bowel syndrome (IBS) and other gastrointestinal (GI) problems.
Aim
To update the consensus with new evidence.
Methods
A systematic review identified randomised, placebo‐controlled trials published between January 2012 and June 2017. Evidence was graded, previously developed statements were reassessed by an 8‐expert panel, and agreement was reached via Delphi consensus.
Results
A total of 70 studies were included (IBS, 34; diarrhoea associated with antibiotics, 13; diarrhoea associated with Helicobacter pylori eradication therapy, 7; other conditions, 16). Of 15 studies that examined global IBS symptoms as a primary endpoint, 8 reported significant benefits of probiotics vs placebo. Consensus statements with 100% agreement and “high” evidence level indicated that specific probiotics help reduce overall symptom burden and abdominal pain in some patients with IBS and duration/intensity of diarrhoea in patients prescribed antibiotics or H. pylori eradication therapy, and have favourable safety. Statements with 70%‐100% agreement and “moderate” evidence indicated that, in some patients with IBS, specific probiotics help reduce bloating/distension and improve bowel movement frequency/consistency.
Conclusions
This updated review indicates that specific probiotics are beneficial in certain lower GI problems, although many of the new publications did not report benefits of probiotics, possibly due to inclusion of new, less efficacious preparations. Specific probiotics can relieve lower GI symptoms in IBS, prevent diarrhoea associated with antibiotics and H. pylori eradication therapy, and show favourable safety. This study will help clinicians recommend/prescribe probiotics for specific symptoms.
1. INTRODUCTION
In 2013, the European Society for Primary Care Gastroenterology (ESPCG) published an evidence‐based international guide for the use of probiotics in the management of specific lower gastrointestinal (GI) symptoms.1 This guide was based on the results of a systematic review of evidence regarding the use of probiotics vs placebo in randomised controlled trials (RCTs). A Delphi panel assessed this evidence and developed a number of consensus statements. Since the publication of these statements, numerous relevant clinical studies of probiotics in the management of lower GI symptoms have been published. In the light of the new evidence available in this rapidly evolving field, the objectives of this publication are to update the systematic review and Delphi consensus, and to incorporate the new findings into the guidelines.
The importance of gut microbiota in health and disease is becoming increasingly evident, and there is a growing body of literature on the therapeutic potential of probiotics in GI disorders2, 3 like irritable bowel syndrome (IBS) and many other conditions. The proposed mechanisms of action for the beneficial effects of probiotics include competitive exclusion of pathogenic microorganisms, inhibition of pathogen adhesion, production of anti‐microbial substances and modulation of the immune system.4, 5, 6 Studies in several animal models have indicated positive therapeutic results for probiotics in a range of conditions, such as asthma,7 obesity,8, 9 diabetes mellitus,10 hypertension,11, 12 and depression and anxiety;13 however, definitive data from human studies are relatively sparse. There is some evidence for a beneficial effect of probiotics in humans in the prevention of hypertension14 and improvement of the symptoms of schizophrenia,15 depression16 and Alzheimer's disease,17 although further studies are needed to confirm these findings. Evaluation of the effect of probiotics in humans is complex due to differences in strains, patient populations and dosing. In addition, many clinical trials report conflicting findings, and results of meta‐analyses have been published that compare non‐identical probiotic strains, making the evidence difficult to interpret. A transparent and rigorous methodology is needed when evaluating the evidence because this topic remains complex.
Lower GI symptoms commonly require a visit to a physician, but the heterogeneity of symptoms presented and their underlying causes may limit the pharmacological treatment options offered because no single dominant drug therapy would be effective in all cases. Although new pharmacological treatments are emerging, challenges remain in terms of their ability to improve symptoms without incurring side effects.18, 19, 20, 21 Current evidence suggests that probiotics in the diet may play a role in reducing uncomfortable lower GI symptoms in adults. Therefore, as before, the emphasis of the ESPCG updated evidence‐based guidelines is on the potential role of probiotics in the management of lower GI symptoms in clinical practice.
2. METHODS
2.1. Design
The repeated consensus procedure was based on an updated systematic literature review and re‐rating of statements by an expert panel.
2.2. Systematic literature search
Placebo‐controlled RCTs evaluating the effects of probiotics on lower GI symptoms were identified through a systematic literature review (based on Appraisal of Guidelines, Research and Evaluation [AGREE] II criteria22) capturing studies published since the original searches were conducted in January 2012.1 The same search terms were used as in the original review to search Embase, MEDLINE In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE (1946‐present). A search was performed in July 2016 to identify publications from 1 January 2012 to 28 July 2016. To keep this publication as current as possible, an updated database search was performed in June 2017.
2.3. Citation screening and full‐text review
Identified publications were screened manually based on the title and abstract in accordance with 2009 Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines23 against predefined eligibility criteria (Table 1). Full‐text versions of all publications meeting the eligibility criteria at initial screening were reviewed to confirm eligibility.
Table 1.
Eligibility criteria for inclusion of publications examining probiotics in the management of lower GI symptoms
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Population |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Study design |
|
|
| Date restrictions | January 2012‐June 2017 | |
| Language restrictions | English language and foreign language publications with an English abstract | |
| Country | Not restricted by country |
FGID, functional gastrointestinal disorders; GI, gastrointestinal; IBS, irritable bowel syndrome; RCT, randomised controlled trial.
2.4. Data items collected and quality assessment
The same data items were collected and tabulated as in the original systematic review, including patient demographics, sample size, strain of probiotic, setting, primary and secondary endpoints, and results. Of note, the term “probiotics” has been used throughout this publication to refer to products that contain probiotics, regardless of whether these are single or multiple strains. The additional step of a quality assessment was performed for each publication (in both the original and the updated review) using a modified version of the Critical Appraisal Skills Programme (CASP) Checklist for Randomised Controlled Trials,24 as recommended by the National Institute for Health and Care Excellence.25
2.5. Delphi consensus
A modified Delphi process was used to review the original consensus statements in the light of the new evidence identified in the current updated systematic review. The Delphi process uses anonymous and iterative feedback and voting to achieve consensus among a panel of independent experts by means of stepwise refinement of responses. The Consensus Group consisted of 10 primary care physicians with an interest in gastroenterology drawn from the ESPCG, with the addition of 2 members from secondary care; 7 of these individuals had taken part in the original consensus. The Group was advised by a nonvoting Chair (APSH) who, in common with the members of the Consensus Group, has experience of systematic reviews and guideline development. For this update, the Steering Committee (APSH, CRM, PW and NdW) reviewed the original statements in the light of the new evidence, and agreed to keep them unchanged for the voting.
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) system26 was used by the Chair and Steering Committee to rate the level of supporting evidence and the strength of each statement. Using the GRADE system, each statement was rated as follows: high—further research is unlikely to change our confidence in the estimate of effect; moderate—further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low—further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; and very low—any estimate of effect is very uncertain.
The Consensus Group members reviewed both the original and new evidence on the use of probiotics in the management of lower GI symptoms. It was anticipated that 3 rounds of anonymous voting would be required to achieve consensus. Votes were cast using an online platform (Google Forms) and the results were analysed by the nonvoting Chair. For each statement, voters indicated their level of agreement on a scale from 1 to 6 (1 = strongly disagree; 2 = disagree with major reservation; 3 = disagree with minor reservation; 4 = agree with major reservation; 5 = agree with minor reservation; and 6 = strongly agree). Consensus was defined a priori as agreement by at least 67% of respondents. In some cases, the consensus statement is indication‐specific; however, studies in other indications that provide relevant data are also described for completeness. In the following discussion, “significant” refers to a statistically significant result (P < 0.05).
After the updated consensus was completed, 3 consensus statements covering general considerations related to probiotic use in daily practice which referenced no specific studies individually (Statements 14, 15 and 16 in the original consensus) were moved to Section 4.
3. RESULTS
The updated database searches identified 3176 articles (January 2012‐July 2016) and 1090 articles (July 2016‐June 2017; Figure 1). After applying inclusion and exclusion criteria, 33 RCTs that reported on the effects of probiotics in the management of lower GI symptoms in clinical practice published since January 2012 were identified, and considered in conjunction with 37 RCTs included in the original systematic review. Of the 33, 6 publications were found in the June 2017 update.27, 28, 29, 30, 31, 32 These could not be included in the consensus voting process; however, they were reviewed by the Steering Committee, which decided that the new evidence provided in these studies would not alter the results of the Delphi consensus and so could be included in our publication.
Figure 1.
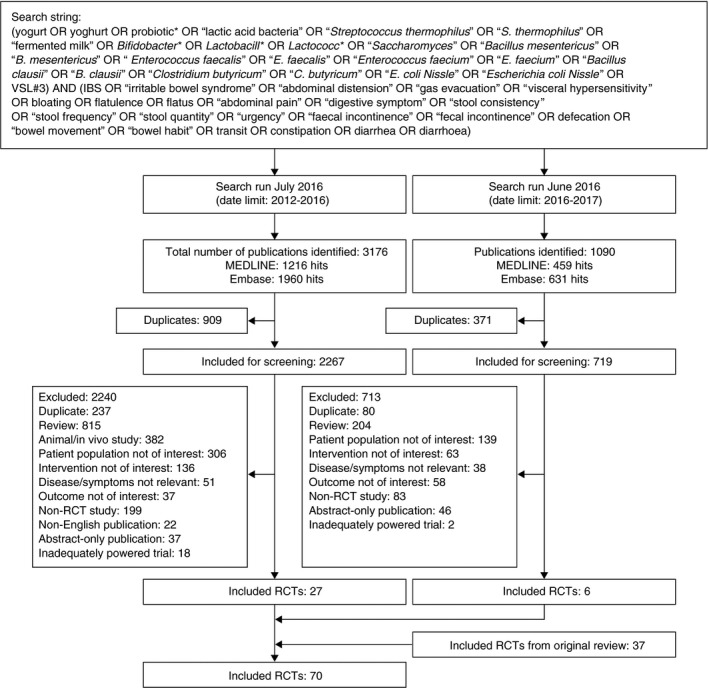
Flow diagram of literature searches. MEDLINE and Embase searches were performed in July 2016 and June 2017. RCT, randomised controlled trial
Collectively, the 70 studies investigated a total of 54 different probiotic products (containing 108 strains either alone or in combination) at doses ranging from 1 × 106 to 4.5 × 1011 colony‐forming units (CFU) per day, administered as 1, 2 or 3 doses. They predominantly contained bacteria (mostly lactobacilli and/or bifidobacteria); a few contained the yeast Saccharomyces. Of the 54 probiotic products, 28 were included in studies published since the original consensus, and the majority of these (22 of 28; 79%) were new probiotics that had not been evaluated in the original consensus. Table 2 provides a summary of the symptoms and indications examined in the 70 studies.
Table 2.
Indications and symptoms examined in included studies
| Number of studies | Indication | ||||||
|---|---|---|---|---|---|---|---|
| Symptom | IBS | Functional GI disorders | Antibiotic treatment | Helicobacter pylori eradication | Lactose intolerance | Healthy/minor GI symptoms | Total |
| IBS (global symptoms) | 30 | 0 | 0 | 0 | 0 | 0 | 30 |
| Abdominal pain | 30 | 2 | 0 | 0 | 2 | 4 | 38 |
| Bloating/distension | 27 | 1 | 0 | 0 | 1 | 4 | 33 |
| Flatus | 15 | 2 | 0 | 0 | 2 | 3 | 22 |
| Diarrhoea (treatment) | 4 | 2 | 0 | 0 | 2 | 2 | 10 |
| Diarrhoea (prevention) | 0 | 0 | 13 | 7 | 0 | 0 | 20 |
| Constipation | 4 | 3 | 0 | 0 | 0 | 4 | 11 |
| Bowel habit | 25 | 2 | 0 | 0 | 1 | 8 | 36 |
| Health‐related quality of life | 20 | 1 | 0 | 0 | 0 | 4 | 25 |
| Total | 34 | 3 | 13 | 7 | 2 | 11 | 70 |
GI, gastrointestinal; IBS, irritable bowel syndrome.
Product adherence was addressed in 49 of the included studies. In 42 studies, adherence to the intervention was assessed by counting empty containers or unused test substance returned at the end of the study and/or by participant self‐reporting (in treatment diaries or during investigator visits). Three of these studies used faecal recovery of probiotic strains to measure adherence, and publications from 4 studies did not report the method of assessing adherence. Where adherence data were reported (38 studies), the level of adherence was generally high. In the probiotic intervention groups, the proportion of participants who were adherent to treatment (taking >80% of doses) was >75%.
The majority of the 70 studies (Table S1)27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96 focused on IBS (based on Rome I, II or III criteria or physician diagnosis; 34 studies), antibiotic‐associated diarrhoea (13 studies) or diarrhoea‐associated Helicobacter pylori eradication therapy (7 studies). Other conditions were investigated in 16 studies. Sixty‐four studies provided evidence for the Delphi consensus33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96 (6 were identified after voting was completed).27, 28, 29, 30, 31, 32 The evidence level was graded as “high” for 5 statements, “moderate” for 2, “low” for 4 and “very low” for 2. Table S2 summarises the studies and specific probiotics with evidence for each consensus statement, together with an indication of whether the result was a primary or secondary endpoint. A table stating probiotic availability by country in Europe, the USA and China is available at http://espcg.eu/. For consistency with the original consensus, 10 experts were invited to participate in the Delphi consensus voting in the update but for logistical reasons only 8 voted. Consensus was reached for all of the 16 statements developed in the original Delphi consensus in the first round of voting (see Figure 2). For each consensus statement, the result of the first (final) vote and the grade of supporting evidence are given, followed by a discussion of the evidence. Sometimes, a particular probiotic yielded conflicting results for a symptom/problem when it was investigated in different studies (see Table S2).
Figure 2.
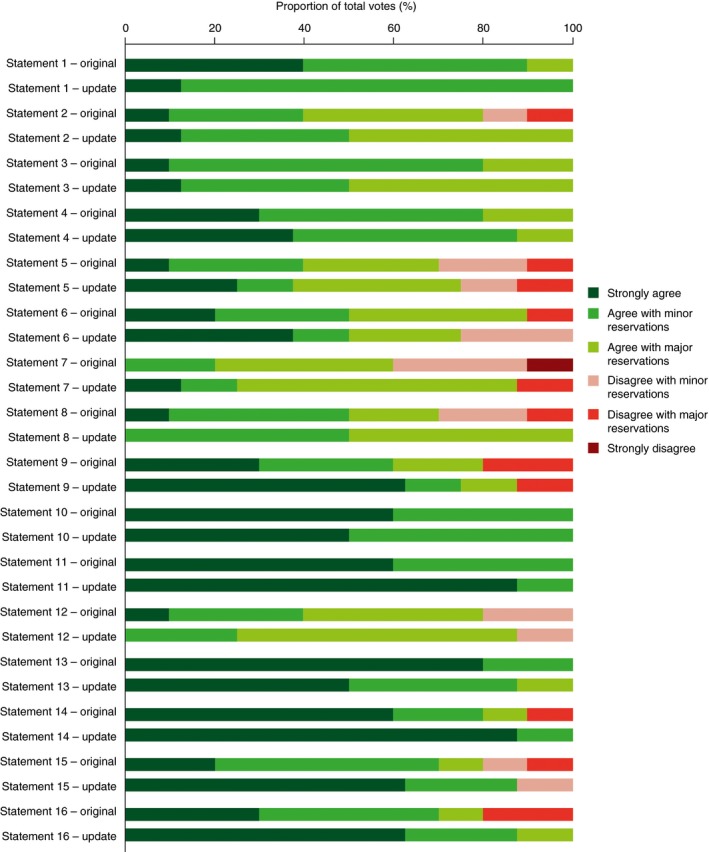
Breakdown of voting agreement for each individual statement in the original and updated Delphi consensus
3.1. IBS (global symptom assessment)
Statement 1: specific probiotics help relieve overall symptom burden in some patients with IBS. Agreement: 100% (6, 12.5%; 5, 87.5%; grade of evidence for effect: high).
Supportive evidence: Twenty‐three studies of 19 different probiotics evaluated overall symptoms in 3112 patients with IBS. Of these studies, 15 evaluated overall IBS symptoms as a primary endpoint, of which 8 reported a significant beneficial effect of 8 different probiotic products (dosed at 3.4 × 107 to 2.5 × 1010 CFU per day) compared with placebo,32, 46, 49, 56, 57, 82, 93, 95 5 reported no significant differences between 2 specific probiotic treatments and placebo,30, 36, 43, 80, 83 and 2 reported mixed results.54, 88 Of the 15 studies, 6 had been published since the original consensus. Two of the new studies (33%) reported no significant difference, compared with 3 of the 9 studies (33%) in the original consensus.
Eight studies of 7 different probiotics evaluated overall IBS symptoms as a secondary endpoint only. Of these, 2 studies found a significant beneficial effect of 2 different probiotic treatments compared with placebo.44, 55 One further dose‐ranging study reported a beneficial effect of the specific probiotic treatment at the 1 × 108 CFU dose, but not at the lower and higher doses tested (1 × 106 and 1 × 1010 CFU).92 Four studies reported no significant differences between 4 different probiotic treatments and placebo37, 66, 85, 94 and 1 study reported a negative effect of a probiotic treatment compared with placebo.64 Of the 8 studies, 6 had been published since the original consensus. Four of the new studies (67%) reported no significant difference or a negative effect, compared with 1 of the 2 studies (50%) in the original consensus.
Statement 2: specific probiotics may help relieve overall symptom burden in some patients with IBS‐C. Agreement: 100% (6, 12.5%; 5, 37.5%; 4, 50%; grade of evidence for effect: very low).
Supportive evidence: Five studies of 4 different probiotics evaluated overall IBS symptoms as a secondary endpoint in 577 patients with constipation‐predominant IBS (IBS‐C). Of these, 1 study reported a beneficial effect of the specific probiotic treatment (dosed at 2.5 × 1010 at CFU per day) vs placebo.33 Another study of the same probiotic found a significant improvement from baseline in the probiotic group but not in the placebo group in a subanalysis of patients with fewer than 3 bowel movements per week.50 In a third study with a different probiotic treatment, an improvement was observed in the composite score of IBS symptoms in the probiotic group vs the placebo group, but this just failed to reach statistical significance. However, the total area under the curve of the composite score of IBS symptoms over 12 weeks was significantly lower in the probiotic group than in the placebo group (P = 0.03).85 Two studies examining 2 different probiotics reported no significant improvement in symptoms vs placebo.86, 92 Of the 5 studies, 2 had been published since the original consensus. Of the 2 new studies, 1 reported no significant improvement, compared with 1 of the 3 studies in the original consensus.
Statement 3: specific probiotics help to relieve overall symptom burden in some patients with IBS‐D. Agreement: 100% (6, 12.5%; 5, 37.5%; 4, 50%; grade of evidence for effect: low).
Supportive evidence: Seven studies examining different probiotics evaluated overall IBS symptoms in 495 patients with diarrhoea‐predominant IBS (IBS‐D). Two studies (both included in the original consensus) evaluated overall IBS symptoms as a primary endpoint, with 1 study reporting a significant beneficial effect of the specific probiotic treatment (dose of 1 × 1010 CFU per day) compared with placebo59 and the other reporting no significant difference.60 Five studies evaluated overall IBS symptoms as a secondary endpoint only. Of these, 2 (both included in the original consensus) found a significant beneficial effect of the specific probiotic treatments,92, 96 and 3 (all published since the original consensus) found no significant difference in the composite IBS symptom score between the probiotic group and the placebo group.65, 85, 86
3.2. Abdominal pain
Statement 4: specific probiotics help reduce abdominal pain in some patients with IBS. Agreement: 100% (6, 37.5%; 5, 50%; 4, 12.5%; grade of evidence for effect: high).
Supportive evidence: Thirty studies examining 23 different probiotics evaluated abdominal pain in 3771 patients with IBS. Of these studies, 9 (examining 8 probiotic treatments dosed at 1 × 106 to 1 × 1010 CFU per day) evaluated abdominal pain as a primary endpoint, of which 7 (4 from the original consensus and 3 new studies) showed a significant beneficial effect of specific probiotic treatments compared with placebo.44, 46, 54, 74, 81, 85, 92 One of these found no statistically significant difference in abdominal pain/discomfort between probiotic and placebo in the overall population but a significantly greater improvement in the subgroup of patients with IBS‐C.85 Two studies (both included in the original consensus) had mixed results: 1 showed a trend towards a beneficial effect in the weekly symptom score for abdominal pain (and, in a secondary analysis, abdominal pain was reduced in a significantly greater proportion of the probiotic group than of the placebo group)56 and the other showed no significant increase in the proportion of patients reporting symptom relief, but a significantly greater decrease in the abdominal pain score in the probiotic group than in the placebo group.43
Abdominal pain was evaluated as a secondary endpoint only in 21 studies. Of these, 5 reported a significant beneficial effect of 5 different probiotics33, 49, 55, 82, 96 (1 of which33 also showed no significant effect in another study50), 15 (examining 11 different probiotics) reported no significant effect30, 36, 37, 50, 57, 59, 60, 61, 66, 80, 83, 88, 93, 94, 95 (of which 1 reported a nonsignificant trend in favour of probiotics vs placebo),95 and another reported a negative effect of the specific probiotic treatment.64 Of the 21 studies, 9 had been published since the original consensus. Seven of the new studies (78%) reported no significant difference or a negative effect, compared with 9 of the 12 studies (75%) in the original consensus.
Abdominal pain was examined in indications other than IBS in 8 studies, each investigating a different probiotic. Of these, 1 study (included in the original consensus) investigated abdominal pain as a primary endpoint in individuals with symptoms related to post‐prandial intestinal gas, and found a significant improvement in the probiotic group compared with the placebo group.58 Seven studies examined abdominal pain as a secondary endpoint only, with 4 of these reporting no significant difference among 4 different probiotic treatments and placebo.47, 51, 62, 69 Another 3 studies (2 examining 2 different probiotics in lactose‐intolerant individuals71, 73 and another reporting on a different probiotic in patients with functional GI symptoms90) found significantly improved abdominal pain from baseline in the probiotic group, but not in the placebo group. Of the 7 studies, 3 had been published since the original consensus. Of the 3 new studies, 2 (67%) reported no significant difference, compared with 2 of the 4 studies (50%) in the original consensus.
3.3. Bloating/distension
Statement 5: specific probiotics help reduce bloating/distension in some patients with IBS. Agreement: 75% (6, 25%; 5, 12.5%; 4, 37.5%; 3, 12.5%; 2, 12.5%; grade of evidence for effect: moderate).
Supportive evidence: The treatment of bloating/distension in 3561 patients with IBS was evaluated in 27 studies examining 20 different probiotics. Of these studies, 4 (examining 4 different probiotics, dosed between 1 × 106 and 2.5 × 1010 CFU per day) evaluated bloating/distension as a primary endpoint. Two studies (including 1 published since the original consensus) reported a significant beneficial effect of the specific probiotic treatment vs placebo,33, 55 whereas a further 2 (both included in the original consensus) reported no significant differences.56, 61 Bloating/distension was evaluated as a secondary endpoint only in 23 studies. Of these, 8 reported a significant beneficial effect of 8 different probiotic treatments44, 46, 49, 50, 57, 85, 92, 93 (1 of which50 also showed a beneficial effect as a primary endpoint in another study33). Of these studies, some found a significant effect only at one time point,50 after a single dose92 or only in patients with IBS‐C.85, 92 Fifteen studies reported no significant difference between 12 different probiotic treatments and placebo30, 36, 37, 59, 60, 64, 66, 74, 80, 82, 83, 88, 94, 95, 96 (1 of these probiotics60 also showed no significant effect on the primary endpoint).61 Of the 23 studies, 11 had been published since the original consensus. Of the 11 new studies, 9 (82%) reported no significant difference, compared with 6 of the 12 studies (50%) in the original consensus.
Six studies investigated the effect of 6 different probiotics on distension/bloating in indications other than IBS. One of these studies (included in the original consensus) evaluated symptoms related to post‐prandial intestinal gas as a primary endpoint in healthy individuals and reported no significant differences between the probiotic and placebo groups.58 The remaining 5 studies (examining 5 different probiotics) evaluated distension/bloating as a secondary endpoint. Single studies reported no significant differences between the probiotic and control groups in women with mild digestive symptoms,51 patients with functional GI disorders (FGID),62 healthy individuals with low defecation frequency and abdominal discomfort,47 and healthy patients with hard or lumpy stools in the past 2 years.69 The fifth study, in individuals with lactose intolerance undergoing a hydrogen breath test, reported significantly reduced bloating in the group receiving the specific probiotic treatment but no significant improvement in the placebo group.71 Of the 5 studies, 2 had been published since the original consensus; both of the new studies reported no significant difference between probiotic and control groups.
3.4. Flatus
Statement 6: probiotics tested to date do not help reduce flatus in patients with IBS. Agreement: 75% (6, 37.5%; 5, 12.5%; 4, 25%; 3, 25%; grade of evidence for effect: low).
Supportive evidence: Overall, 15 studies examining 12 different probiotics evaluated flatus in 1478 patients with IBS. All 3 studies that examined flatus as a primary endpoint (all included in the original consensus) showed no significant difference between 3 specific probiotic treatments and control.54, 56, 79 Twelve studies examined flatus as a secondary endpoint only. Of these, 8 showed no significant difference between 8 specific probiotic treatments and control.33, 37, 57, 59, 60, 66, 85, 94 Four studies reported a significant beneficial effect of 4 different probiotic treatments61, 88, 92, 96 (1 of which61 also showed no significant effect in another study60); the significant effect was seen at 1 dose only in 1 of these studies,92 and at week 16 (after follow‐up) but not week 8 (end of treatment) in another study.88 Of the 12 studies, 5 had been published since the original consensus. Of the 5 new studies, 4 (80%) reported no significant difference, compared with 4 of the 7 studies (57%) in the original consensus.
Seven studies examined the effect of 7 different probiotics on flatus in indications other than IBS. Four of these studies reported no significant effects on flatus (primary endpoint for 1 probiotic58 and secondary endpoint for 3 other probiotics62, 69, 73). Three studies reported a significant benefit of 3 different probiotic treatments on flatus (secondary endpoint) in women with mild digestive symptoms,51 patients with functional GI symptoms90 and individuals with lactose intolerance undergoing a lactose breath test.71 Of the 7 studies, 2 had been published since the original consensus; both of the new studies reported no significant effect on flatus as a secondary endpoint.
3.5. Constipation
Statement 7: specific probiotics may help reduce constipation in some patients with IBS. Agreement: 87.5% (6, 12.5%; 5, 12.5%; 4, 62.5%; 2, 12.5%; grade of evidence for effect: low).
Supportive evidence: Four studies of 4 different probiotics examined the treatment of constipation as a secondary endpoint in 487 patients with IBS. One study (in patients with IBS‐C) reported significant improvements with the specific probiotic treatment (administered at 1.25 × 1010 CFU twice daily) vs control for some of the endpoints (orocaecal transit time, colonic transit time and urgency), but not others (stool frequency and consistency, straining during evacuation and feelings of incomplete evacuation).33 The 3 remaining studies did not detect any statistically significant effects of 3 different probiotic treatments on the relief of constipation.36, 49, 74 Of the 4 studies, 2 had been published since the original consensus; both of the new studies reported no significant effect on constipation.
Seven studies of 7 different probiotics examined constipation in patients with broader FGID. Of these, 3 studies reported significant improvements in the relief of constipation, with 1 reporting an increase in defecation frequency47 and another reporting a significant effect of the probiotic on stool consistency vs placebo (although this study did not show a significant effect of the probiotic on stool frequency vs placebo).89 The third study did not provide a between‐group statistical analysis; however, the decrease in constipation frequency score was approximately twofold greater in the probiotic groups than in the placebo group.90 Four studies reported no significant effect of 4 different probiotic treatments;31, 62, 68, 69 however, 1 of these studies showed a nonsignificant trend in favour of probiotics.68 Of the 7 studies, 5 had been published since the original consensus. Of the 5 new studies, 3 (60%) reported no significant effect, compared with 1 of the 2 studies (50%) in the original consensus.
3.6. Bowel habit
Statement 8: specific probiotics help improve frequency and/or consistency of bowel movements in some patients with IBS. Agreement: 100% (5, 50%; 4, 50%; grade of evidence for effect: moderate).
Supportive evidence: Twenty‐five studies examining 20 different probiotics evaluated bowel habit in 3069 patients with IBS. Two studies of 2 different probiotics (administered at doses of between 1.3 × 108 and 9 × 109 CFU per day) evaluated bowel habit as a primary endpoint, with 1 study (included in the original consensus) reporting no significant difference in weekly defecation frequency between the probiotic and placebo groups, although a significant positive effect of the specific probiotic treatment vs placebo was observed on the secondary endpoints of urgency and feelings of incomplete evacuation.56 The second study (published since the original consensus) found that the number of bowel movements changed favourably in the probiotic group compared with the placebo group.32 Of the 25 studies in patients with IBS, 22 evaluated bowel habit as a secondary endpoint only. Seventeen studies used 1 or more of 3 main endpoints: stool frequency, stool consistency and satisfaction with bowel habits. Eleven reported significant beneficial effects of 11 different probiotics;43, 44, 46, 49, 50, 55, 59, 81, 82, 92, 93 1 of these studies reported a significant improvement in the feeling of incomplete defecation on completion of 4 weeks of treatment that was not significant 1 month later.55 Nine studies reported no significant effects of 7 different probiotics37, 54, 57, 61, 74, 80, 83, 85, 95 (1 of which61 showed no significant benefit on the primary endpoint in another study60). One found a trend to normalisation of stool consistency (P = 0.058); however, no significant effects on straining and feelings of incomplete evacuation were observed.33 Another study reported a significant negative effect of the specific probiotic treatment.64 Of the 22 studies, 7 had been published since the original consensus. Of the 7 new studies, 4 (57%) reported no significant effect or a negative effect, compared with 6 of the 15 studies (40%) in the original consensus.
Two studies (both included in the original consensus) examined the effects of probiotics on GI transit times. This was assessed as a primary endpoint in 1 study that reported no difference in GI transit times between the probiotic and placebo groups.60 A second study showed significant improvements in the secondary endpoints of colonic and small bowel transit times in the probiotics vs placebo groups.33
Eleven studies examining 10 different probiotic treatments assessed bowel habit in indications other than IBS. Of these, 8 studies of 8 different probiotics reported that probiotics produced significant improvements in measures of bowel habit vs placebo,31, 47, 51, 63, 75, 87, 89, 90 whereas 3 reported no difference between 3 different probiotics and placebo.68, 69, 73 Of the 11 studies, 7 had been published since the original consensus. Of the 7 new studies, 3 (43%) reported no significant difference, compared with none of the 4 studies in the original consensus.
3.7. Diarrhoea
Statement 9: probiotics tested to date do not reduce diarrhoea in patients with IBS. Agreement: 87.5% (6, 62.5%; 5, 12.5%; 4, 12.5%; 2, 12.5%; grade of evidence for effect: very low).
Supportive evidence: Four studies of 4 different probiotics (dosed at between 4 × 107 and 5.2 × 1010 CFU per day) evaluated the treatment of diarrhoea as a secondary endpoint in 283 patients with IBS. Of these, 3 studies (including 1 published since the previous consensus) reported no difference between specific probiotic treatments and placebo,36, 57, 59 and another found significant worsening of diarrhoea with the specific probiotic treatment compared with placebo.64 Six studies of 6 different probiotics (including 2 studies published since the previous consensus) evaluated diarrhoea as a secondary endpoint in indications other than IBS. Specific probiotic treatment had no significant effect on diarrhoea in elderly nursing home residents,75 patients with a functional bowel disorder,62 individuals with functional GI symptoms90 or individuals with hard or lumpy stools in the past 2 years.69 Two studies (including 1 published since the previous consensus) showed a beneficial effect of 2 different probiotics vs placebo on symptoms of diarrhoea, both of which were in patients with lactose intolerance.71, 73
Statement 10: in patients receiving antibiotic therapy, specific probiotics are helpful as adjuvant therapy to prevent or reduce the duration of associated diarrhoea. Agreement: 100% (6, 50%; 5, 50%; grade of evidence for effect: high).
Supportive evidence: Thirteen studies of 10 different probiotics examined the prevention of antibiotic‐associated diarrhoea and/or reduction in antibiotic‐associated diarrhoea in 6091 patients who received antibiotics (although they were initiated in a hospital setting, these studies were included because of the relevance of antibiotic‐associated diarrhoea to primary care). Of these, 11 studies examined antibiotic‐associated diarrhoea as a primary endpoint. Six studies of 4 different probiotics administered at doses of 2 × 109 to 5 × 1010 CFU per day35, 38, 48, 53, 72, 78 (3 of which tested the same probiotic treatment)35, 48, 78 showed a significant reduction in antibiotic‐associated diarrhoea compared with placebo. One study reported a significant benefit of the probiotic vs placebo on the duration of antibiotic‐associated diarrhoea only.28 In contrast, 4 studies of 4 other probiotics reported no evidence that probiotics were effective in the prevention of antibiotic‐associated diarrhoea vs placebo,34, 45, 77, 84 although 1 of these (an underpowered study) showed a nonsignificant reduction in antibiotic‐associated diarrhoea.84 Of the 11 studies, 6 had been published since the original consensus. Of the 6 new studies, 3 (43%) reported no significant effect compared with 1 of the 5 studies (20%) in the original consensus. The 2 studies that assessed antibiotic‐associated diarrhoea as only a secondary endpoint (1 study from the original consensus and 1 new study) found no difference between the probiotic and placebo groups.29, 76
Statement 11: in patients receiving H. pylori eradication therapy, specific probiotics are helpful as adjuvant therapy to prevent or reduce the duration/intensity of associated diarrhoea. Agreement: 100% (6, 87.5%; 5, 12.5%; grade of evidence for effect: high).
Supportive evidence: Seven studies evaluated the effect of 9 different probiotics (at doses of between 2 × 106 and 2 × 1010 CFU per day) on diarrhoea as a side effect of H. pylori eradication therapy in 1480 patients. All the 5 studies examining H. pylori eradication therapy‐associated diarrhoea as a primary endpoint (including 1 study published since the original consensus) reported a significant benefit of specific probiotic treatments compared with placebo.39, 40, 41, 42, 70 However, the results for 2 of the studies were mixed, with a significant benefit of the specific probiotic treatment seen after 1 week, but not 2 weeks, in 1 study,70 and significantly fewer days with diarrhoea and a shorter mean duration of diarrhoea episodes, but no significant difference in the frequency of diarrhoea episodes, in the probiotic group compared with the placebo group in another study.42 Two studies (both published since the original consensus) were identified that assessed the occurrence of diarrhoea as a secondary endpoint, and both reported that the addition of probiotics to H. pylori eradication therapy significantly decreased diarrhoea as a side effect of treatment.27, 52
3.8. Health‐related quality of life
Statement 12: with specific probiotics, improvement of symptoms has been shown to lead to improvement in some aspects of health‐related quality of life. Agreement: 87.5% (5, 25%; 4, 62.5%; 3, 12.5%; grade of evidence for effect: low).
Supportive evidence: Health‐related quality of life (HRQoL) was assessed as a primary endpoint in 6 studies with 4 different probiotics (administered at doses of between 5 × 107 and 3 × 1010 CFU per day). Two studies of 2 different probiotics reported a significantly greater improvement in HRQoL with probiotics, as measured by an improvement in GI well‐being in women with minor GI symptoms51 and improvements in scores using the Irritable Bowel Syndrome Quality of Life (IBS‐QOL) instrument in patients with IBS,65 compared with placebo. One study in patients with IBS‐C reported no significant difference between the probiotic and placebo groups for the change from baseline in the discomfort dimension score of the Functional Digestive Disorders Quality of Life (FDDQL) questionnaire after 3 and 6 weeks of treatment; however, the probiotic group had a significantly greater proportion of responders for the discomfort dimension score than the placebo group at week 3.50 Another study assessed 2 different probiotics in patients with FGID and found no significant differences between the probiotic and control groups for the Gastrointestinal Quality of Life Index (GIQLI) total score and well‐being subscales (physical, social and mental; primary endpoint); however, use of the 36‐item Short‐Form Health Survey (SF‐36; secondary endpoint) revealed significant improvements in physical functioning and/or “role‐physical” domains with probiotics, but no significant changes in the control groups.62 In 1 study, a significant reduction in “health‐related worry” was observed in patients with IBS receiving the probiotic treatment vs placebo, but not in other domains of the IBS‐QOL.32 The remaining study in women with minor GI symptoms found no significant difference in the percentage of women reporting an improvement in GI well‐being with probiotics vs placebo.67 Nineteen studies assessed aspects of HRQoL as secondary endpoints only. Fourteen of these (evaluating 12 different probiotics) found no difference between treatment groups in measures of HRQoL,30, 31, 36, 54, 56, 57, 58, 80, 82, 83, 85, 86, 88, 92 whereas 5 studies (all in patients with IBS) reported significant benefits of 5 different probiotic treatments for some aspects of HRQoL.43, 46, 49, 59, 93
3.9. Adverse events
Statement 13: probiotics have a favourable safety profile in patients with a range of lower GI symptoms typically managed in primary care or general practice. Agreement: 100% (6, 50%; 5, 37.5%; 4, 12.5%; grade of evidence for effect: high).
Supportive evidence: Safety data were reported in 50 studies. The majority of studies revealed no meaningful treatment‐emergent adverse events that were attributed to probiotic use. Forty‐three studies found no relevant differences in safety between 37 specific probiotic treatments and placebo.30, 31, 34, 35, 37, 39, 40, 41, 42, 44, 45, 47, 48, 49, 50, 53, 54, 55, 56, 57, 59, 60, 61, 62, 63, 65, 68, 69, 72, 74, 77, 78, 80, 81, 82, 83, 84, 90, 92, 93, 94, 95, 96 Findings of the remaining 7 studies (examining 7 probiotic strains) are summarised below.
In 1 study of patients with IBS, 2 patients in the probiotic group discontinued involvement because of adverse events (moderate nausea and severe exanthema). However, the most frequent adverse events (fatigue, pruritus and diarrhoea) occurred equally often in the probiotic and placebo groups.46 In another study of patients with IBS, 1 participant had a short stay in hospital for cervicobrachialgia 2 weeks after the end of the specific probiotic treatment; however, there was no organic explanation for this, and the patient continued in the trial.64 Two patients with IBS treated with probiotics in a third study reported an itching rash, causing one patient to drop out.36 A study of patients with IBS‐C reported 16 adverse events, which were judged to be possibly linked to the research or to the study product by the investigators (10 events were reported in the active comparator group and 4 in the placebo group).85 The dropout rate was significantly higher in the probiotic group than in the placebo group in the final study in patients with IBS (P = 0.048); however, most of the dropouts were due to noncompliance (n = 5), the requirement for an antibiotic (n = 5) or worsening of IBS symptoms (n = 2).86 In a study of healthy athletes, there was a twofold increase in the number and duration of mild GI symptoms in the probiotic group compared with the placebo group, although the severity of these symptoms tended to be lower in the probiotic group than in the placebo group.91 In a study examining the effects of probiotics on antibiotic‐associated diarrhoea, the incidence of nonserious adverse events in the probiotic group was 2.0% compared with 0% in the placebo group. A causality assessment was carried out for all adverse events, and all were found to be of either probable or possible association.38
4. DISCUSSION
This is an update to the evidence‐based ESPCG consensus published in 2013 on the role of probiotics in the management of lower GI symptoms in adults consulting in primary care. It aims to provide an overview of the role of probiotics in dealing with patients with a variety of abdominal problems, and the practical implications of consensus statements for physicians are shown in Table 3.
Table 3.
Practical implications of consensus statements for physicians
| Grade of evidence for effect | Symptoms/indications | Meaning for physicians |
|---|---|---|
| High |
Overall symptoms and abdominal pain in IBS Prevention or reduction of diarrhoea in patients receiving antibiotics Prevention or reduction of diarrhoea in patients receiving Helicobacter pylori eradication therapy |
Probiotics with supportive evidence for benefit should be tried |
| Moderate | Bowel movements and bloating/distension in IBS | Probiotics with supportive evidence for benefit could be tried |
| Low |
Overall symptoms in IBS‐D Flatus in IBS Constipation in IBS |
Probiotics with supportive evidence for benefit could be considered |
| Very low |
Overall symptoms in IBS‐C Diarrhoea in IBS |
Currently no evidence to support use of probiotics |
IBS, irritable bowel syndrome; IBS‐C, constipation‐predominant IBS; IBS‐D, diarrhoea‐predominant IBS.
Data from the 33 newly identified publications, in addition to those in the original 37, significantly strengthened the evidence base on the role of probiotics in GI care in just over 5 years. In addition to information from new RCTs performed in patients with IBS, more data were identified in patient populations with other GI conditions (healthy individuals with minor GI complaints, patients with lactose intolerance and those receiving antibiotics or undergoing H. pylori eradication therapy). The number of probiotics included in the consensus increased from 32 to 54. After assessment of the evidence, no new statements were developed, and the wording of the original statements remained unaltered. The strength of evidence assessed using the GRADE system was graded as “high” for 5 statements, “moderate” for 2, “low” for 4 and “very low” for 2. It was maintained for all 5 statements previously rated as having a high level of evidence and 100% agreement, but reduced for 2 of the 4 statements previously rated as having moderate evidence and 70%‐100% agreement, and 1 of the 3 statements previously rated as having low evidence and 60%‐90% agreement reflecting the more heterogeneous evidence among the studies identified in the updated review.
There may be several explanations for the more diverse results reflected in the reduced grading of evidence in this review. The field is challenging owing to the varied nature of bowel symptoms and abdominal complaints, combined with the relatively undefined mode of action of the wide range of individual probiotic strains studied. As well as including a wider range of patients, different probiotics were assessed in the original and the updated review; of the 32 probiotics evaluated in the original manuscript, only 6 were assessed in publications from 2012 onwards. It may be that the 22 new probiotics evaluated in the updated review were less efficacious in producing a beneficial response than those assessed in the original manuscript. In addition, the more recent studies in this review included a larger number of secondary endpoints than the older studies. Because the studies were not powered to detect statistical significance in these endpoints, they may show false negative results.
The reduced strength of evidence for the beneficial effect of probiotics was reflected in the levels of agreement reached for several statements during the wider Delphi voting process. For example, although consensus was achieved for Statement 1 (“Specific probiotics help to relieve overall symptom burden in some patients with IBS”), the individual levels of agreement were lower in the updated consensus (“strongly agree”, 12.5%; “agree with minor reservation”, 87.5%) than in the original consensus (“strongly agree”, 40%; “agree with minor reservation”, 50%; “agree with major reservation”, 10%). There was a reduction in the number of voters choosing to “strongly agree” with Statement 13 (“Probiotics have a favourable safety profile in patients with a range of lower GI symptoms typically managed in primary care or general practice”), despite the updated review providing no additional evidence for an adverse safety profile of probiotics. This may reflect an awareness of the limited data on long‐term safety in the GI community, despite a lack of published evidence suggesting safety issues in general populations.
There are, however, statements for which the new evidence appears to have improved the experts’ confidence in the statements (Figure 2). For example, the proportion of the voting panel that “strongly agreed” with Statement 11 (“In patients receiving H. pylori eradication therapy, specific probiotics are helpful as adjuvant therapy to prevent or to reduce the duration/intensity of associated diarrhoea”) increased from 60% in the original Delphi consensus to 87.5% in the update. This reflects the results of 2 additional RCTs identified in the updated systematic review that increased to 7 the number of RCTs showing a significant beneficial effect of a probiotic on treatment‐induced diarrhoea vs placebo. For Statement 9 (“Probiotics tested to date do not reduce diarrhoea in patients with IBS”), confidence increased for a negligible effect of the probiotic vs placebo.
When focusing on primary endpoint data, the overall evidence for the beneficial effect of probiotics was strong. For example, 30 publications reported the effects of specific probiotics vs placebo on overall lower GI symptoms as a primary or secondary endpoint (23 in patients with IBS in general [included in Statement 1]); 14 of these studies reported a significant improvement with probiotics vs placebo (61%). When publications which reported overall lower GI symptoms in patients with IBS as a primary endpoint were assessed (15 studies), 10 found a significant benefit of probiotics vs placebo (67%). A similar pattern was observed for other symptoms, with a significant benefit of probiotics compared with placebo being observed in a greater proportion of studies evaluating the symptom as a primary endpoint than as any endpoint (abdominal pain: 40% any endpoint, 78% primary endpoint; constipation: 36% any endpoint, 50% primary endpoint; bowel habit: 56% any endpoint, 63% primary endpoint; HRQoL: 40% any endpoint, 83% primary endpoint).
Although there is an abundance of data supporting the use of multiple strains of probiotics for the relief of lower GI symptoms, large meta‐analyses are difficult to carry out owing to the lack of comparable data available on single specific probiotic strains. Strictly speaking, they should only be performed on data for the same organism at comparable doses. There are inherent problems with meta‐analyses that compare combinations of multiple probiotic strains because they make it difficult to establish the exact role of individual strains in the management of IBS symptoms and the extent of their contribution to the efficacy of the composite probiotics. Comparisons of different strains may also dilute any positive effect of individual probiotics; however, many of these analyses have shown a beneficial effect of probiotic products.97, 98, 99, 100, 101, 102
For meta‐analysis, it is more scientifically valid to include RCTs in which participants are allocated the same single‐strain probiotic and compared with a placebo. Several recent small meta‐analyses have been performed that examine the effects of specific individual probiotics on lower GI symptoms using data from the studies identified in our systematic review, with results showing a beneficial effect of probiotics over placebo. A systematic review and meta‐analysis evaluated the effects of fermented milk with Bifidobacterium animalis subsp. lactis CNCM I‐2494 (DN‐173 010) and lactic acid bacteria on GI discomfort in the general adult population.103 The systematic review identified 3 RCTs (2 of which were eligible for inclusion in our review).51, 67 Individual data from 598 participants were evaluated in meta‐analyses using random‐effects models. Results from the analyses showed that consumption of the specific probiotic was associated with a significant improvement in overall GI discomfort (based on responder/nonresponder status) compared with placebo (odds ratio [OR]: 1.48; 95% confidence interval [CI]: 1.07‐2.05). The study also found that the probiotic was superior to placebo in terms of reducing digestive symptoms, as measured using a composite score. A meta‐analysis of the effect of Saccharomyces cerevisiae CNCM I‐3856 on GI symptoms in patients with IBS used data from 2 trials74, 85 (both of which are included in our review). The authors reported that patients consuming the probiotic had a significantly higher chance of reduction in abdominal pain/discomfort (P = 0.0134) and improvement in stool consistency (P = 0.0003) than those consuming placebo.104 Another recent meta‐analysis examined the effects of the probiotic Bifidobacterium longum subsp. infantis 35624 in patients with IBS.105 Analysis of data from the 5 studies that met the inclusion criteria (3 of which are included in our review)37, 61, 92 showed that consumption of single probiotic B. infantis did not impact on GI symptoms, whereas patients who received composite probiotics containing B. infantis had significantly reduced abdominal pain and bloating/distention. Other recently published meta‐analyses have examined whether probiotics are of benefit in the prevention of Clostridium difficile infection, reporting both positive106 and negative107, 108 results.
The current systematic review found strong evidence for the beneficial effect of probiotics in the prevention of diarrhoea in H. pylori eradication therapy. All the 6 identified studies showed a reduction in diarrhoea with probiotic consumption vs placebo. This is supported by the results of 2 recent meta‐analyses that each examined the effects of a variety of probiotics on H. pylori eradication rates (primary endpoint) and diarrhoea associated with H. pylori eradication therapy (secondary endpoint).109, 110 The first, a meta‐analysis of 13 studies (1 of which is included in the current review)52 involving a total of 2306 patients, found a reduced risk of diarrhoea in the probiotic group compared with the placebo group (risk ratio [RR]: 0.51; 95% confidence interval [CI]: 0.31‐0.84; P = 0.008).110 Lactobacillus alone (RR: 1.24; 95% CI: 1.12‐1.38; P < 0.0001) and multistrain probiotics (RR: 1.12; 95% CI: 1.07‐1.18; P < 0.00001) were effective at improving H. pylori eradication rates compared with placebo. Similarly, the second meta‐analysis, which involved 4515 patients from 30 RCTs (5 of which are included in this systematic review),40, 41, 52, 70, 84 found a significant reduction in the risk of diarrhoea with the addition of probiotics to standard triple therapy compared with triple therapy alone (RR: 0.549; 95% CI: 0.391‐0.771; P = 0.001). The addition of probiotics to standard triple therapy also significantly increased H. pylori eradication rates compared with triple therapy alone (P < 0.001).109
Our expert consensus panel made 3 general recommendations for practising clinicians. We recommend that specific probiotics have a role in the management of some IBS symptoms and can also be used as an adjunct to conventional treatment. We also recommend that probiotic strains should be selected based on the patient's symptoms, the clinical indication and the available evidence; no probiotic alleviates the full range of symptoms in IBS. Finally, we recommend that, when trying a probiotic therapy for a chronic GI problem, the product should be taken for 1 month; dose selection should be based on available evidence and manufacturers’ recommendations. These general, pragmatic recommendations for daily practice were included in the original consensus as Statements 14, 15 and 16, respectively; when the updated evidence was presented to the voting panel for the current consensus, the level of agreement with these 3 statements increased from that obtained in the original consensus, in terms of both overall agreement (which reached 100% for Statements 14 and 16) and the proportion of respondents voting to “strongly agree” with the statements (Figure 2 and Table S2).
To enable the current publication to be as up to date as possible and to avoid a time lag, 6 publications identified in the updated June 2017 database search did not undergo the Delphi consensus process. This could have had an impact on the levels of agreement of the voting panel. However, these publications were reviewed by the Steering Committee, members of which judged that the new evidence was in line with that previously reported and concluded that exclusion of the more recent publications would make little or no difference to the levels of agreement within the Delphi consensus. As in the original systematic review, only studies that were randomised, placebo‐controlled clinical trials of probiotics with suitable follow‐up periods were included in the analysis in an attempt to obtain the highest quality data. Publications included in both the original and updated systematic reviews were subjected to quality assessment using the CASP checklist for RCTs.24 This was carried out at the suggestion of the Steering Committee to allow the wider Delphi voting panel to judge the quality of the presented evidence and to use this to aid their decision‐making. The majority of the publications (67%) were classified as being of “high quality” or above. Despite the inclusion of adequately powered, high‐quality studies, the results remain diverse. Variations in probiotic strain(s), doses and modes of administration, the health status of patients, and diet and concomitant medications (eg antibiotics and antacids) make comparisons between probiotics difficult.
Studies that did not strictly fit into the statement categories were excluded from those statements. For example, 1 study reported that B. lactis CNCM I‐2494 (DN‐173 010) produced a significant reduction in the “composite score of digestive symptoms” when it was administered to healthy women reporting minor digestive symptoms compared with those receiving a control dairy product (P < 0.05; secondary endpoint).67 However, this study was not included in the evidence base for Statement 1 because the statement focused on patients with IBS only. Only a small subset of the studies identified in the systematic review examined probiotics in healthy individuals, or patients with lactose malabsorption, other functional GI problems or mild lower GI symptoms; hence, specific statements were not prepared for these groups.
Other limitations of the current update that also applied to the original consensus1 are as follows: the potential for publication bias; the potential for chance findings in secondary endpoints; the focus on adults (statements cannot be extended to children); and the presentation of physicians’ rather than patients’ perspectives. Overall, the studies identified in this systematic review, which were powered for specific primary endpoints, reported evidence supporting the effectiveness of probiotics for the relief of lower GI symptoms (especially overall GI symptom score, abdominal pain and bowel habit), improvement of HRQoL, and prevention of both antibiotic‐associated diarrhoea and diarrhoea associated with H. pylori eradication therapy. For safety outcomes, probiotics were comparable to placebo. When this evidence was presented to clinical experts, the panel reached consensus.
In the past 5 years, since the original review, the evidence for the effects of probiotics on lower GI symptoms has doubled. After evaluation of this evidence using the same rigorous methodology as before, the statements remain the same, and consensus was reached. This demonstrates that clinicians can remain confident that specific probiotics have a role in the management of lower GI symptoms.
ACKNOWLEDGEMENTS
Declaration of personal interests: APSH, LA, PF, CL, JM, BF, K‐AW and NdW are committee members of the ESPCG. ASPH has received financial support for the literature review and consensus development activities from Danone, and medical writing assistance from Oxford PharmaGenesis Ltd funded by Danone, for the submitted study. APSH has served as a speaker, a consultant and an advisory board member for Allergan, Danone and Reckitt Benckiser, and has received research funding from Reckitt Benckiser. APSH is an employee of Durham University and a committee member of the Rome Foundation for functional GI disorders. CRM is an employee of Oxford PharmaGenesis Ltd, which has received project funding from Danone (for the submitted study), Amgen, Ardelyx, AstraZeneca, Bayer, Endostim, Isofol Medical, Shionogi, Shire, Takeda, Zealand Pharma, the Canadian Association of Gastroenterology, the University of Bologna and the University of Durham. PW has acted as a consultant for, or received research grant support from, the following pharmaceutical companies in the last 5 years: Almirall Pharma, Boehringer‐Ingelheim, Chr. Hansen, Danone Research, Ironwood Pharmaceuticals, Salix, Shire UK, Sucampo Pharmaceuticals and Allergan. CM is a Director and shareholder of Beacon Medical Communications Ltd (Brighton, UK), which has received project funding from Oxford PharmaGenesis Ltd. OC is an employee of Oxford PharmaGenesis Ltd, which has received project funding from Danone (for the submitted study). LA is an employee of the Karolinska Institutet, and stockowner in Praktikertjänst AB. PF has no conflict of interest. CL has received funding for Development of Functional Food Products with Encapsulated Active Ingredients (national project) and payment for participation in advisory panels from APR‐Ibuprofen, MSD, Vianex, UCB, Leo and Janssen. CL also holds the following patents: “Compositions of botanical extracts and their use” and “A water olive extract with metabolic and cardioprotective properties”. JM has no conflict of interest. J‐MPdF served as consultant and speaker for Metagenics Europe (food supplement) until 1 January 2015. BS has no conflict of interest. CW is an employee of Oxford PharmaGenesis Ltd, which has received project funding from Danone (for the submitted study), has shares in Oxford PharmaGenesis Holdings Ltd, and is Director of several Oxford PharmaGenesis companies and trustee of a charity. NdW's institution has received a grant from the ESPCG for the submitted study.
Declaration of funding interests: This consensus was supported and facilitated by the ESPCG, which received an unrestricted grant from Danone (Paris, France). At the request of the ESPCG, support for the consensus was provided by Dr Catherine Mitchell, Oliver Cole and Dr Chris Winchester (Research Evaluation Unit, Oxford PharmaGenesis Ltd, Oxford, UK). The systematic literature searches and initial title/abstract screening were conducted by Dr Catherine Mitchell (Oxford PharmaGenesis Ltd). Oxford PharmaGenesis Ltd received project funding from Danone. The funding body had no input into the screening or analysis of retrieved references, the content or conduct of the workshop, the grading of the evidence, voting on the statements or the decision to submit the manuscript for publication.
AUTHORSHIP
Guarantor of the article: APSH initiated the project.
Author contributions: APSH, CRM, CM, OC, PW, CW and NdW performed the updated literature searches. APSH, BF, CM, CRM, OC, PW, LA, PF, CL, JM, J‐MPdF, CW, K‐AW and NdW took part in the screening and analysis of the retrieved references. APSH, CRM, PW and NdW reviewed the original wording of statements in the light of new evidence. BF, PW, PF, CL, JM, J‐MPdF, K‐AW and NdW voted on the statements. CRM and OC were involved in the execution and collation of results from the Delphi voting process. CRM and CW prepared an outline and revised subsequent drafts of the paper. APSH, BF, CM, CRM, OC, PW, LA, PF, CL, JM, J‐MPdF, CW, K‐AW and NdW revised the outline and subsequent drafts of the paper. All authors have approved the final version of the article, including the authorship list.
Supporting information
APPENDIX 1. THE AUTHORS AND THEIR COMPLETE AFFILIATIONS
1.1.
A. P. S. Hungin, Institute of Health and Society, Newcastle University, Newcastle upon Tyne, UK; C. R. Mitchell, O. Cole and C. Winchester, Research Evaluation Unit, Oxford PharmaGenesis, Oxford, UK; P. Whorwell, Centre for Gastrointestinal Sciences, University of Manchester, Manchester, UK; C. Mulligan, Beacon Medical Communications Ltd, Brighton, UK; L. Agréus, Division of Family Medicine and Primary Care, Karolinska Institute, Stockholm, Sweden; P. Fracasso, Don Bosco Outpatient Clinic, Rome, Italy; C. Lionis, Clinic of Social and Family Medicine, School of Medicine, University of Crete, Heraklion, Greece; J. Mendive, La Mina Primary Care Centre, Barcelona, Spain; J.‐M. Philippart de Foy, Nutrition Committee of the Scientific Society of General Practice (SSMG, Belgium), Brussels, Belgium; B. Seifert, Department of General Practice, First Faculty of Medicine, Charles University, Prague, Czech Republic; K.‐A. Wensaas, Research Unit for General Practice, Uni Research Health, Bergen, Norway; N. de Wit, Julius Centre for Health Sciences and Primary Care, UMC Utrecht, Utrecht, The Netherlands
Hungin APS, Mitchell CR, Whorwell P, et al. Systematic review: probiotics in the management of lower gastrointestinal symptoms – an updated evidence‐based international consensus. Aliment Pharmacol Ther. 2018;47:1054–1070. https://doi.org/10.1111/apt.14539
The Handling Editor for this article was Professor Alexander Ford, and this uncommissioned review was accepted for publication after full peer‐review.
Authors' complete affiliations are listed in Appendix 1.
REFERENCES
- 1. Hungin AP, Mulligan C, Pot B, et al. Systematic review: probiotics in the management of lower gastrointestinal symptoms in clinical practice – an evidence‐based international guide. Aliment Pharmacol Ther. 2013;38:864‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee HJ, Choi JK, Ryu HS, et al. Therapeutic modulation of gut microbiota in functional bowel disorders. J Neurogastroenterol Motil. 2017;23:9‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simren M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bron PA, Kleerebezem M, Brummer RJ, et al. Can probiotics modulate human disease by impacting intestinal barrier function? Br J Nutr. 2017;117:93‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bermudez‐Brito M, Plaza‐Diaz J, Munoz‐Quezada S, Gomez‐Llorente C, Gil A. Probiotic mechanisms of action. Ann Nutr Metab. 2012;61:160‐174. [DOI] [PubMed] [Google Scholar]
- 6. Sarkar A, Mandal S. Bifidobacteria – insight into clinical outcomes and mechanisms of its probiotic action. Microbiol Res. 2016;192:159‐171. [DOI] [PubMed] [Google Scholar]
- 7. Fonseca VM, Milani TM, Prado R, et al. Oral administration of Saccharomyces cerevisiae UFMG A‐905 prevents allergic asthma in mice. Respirology. 2017;22:905‐912. [DOI] [PubMed] [Google Scholar]
- 8. Li Z, Jin H, Oh SY, Ji GE. Anti‐obese effects of two Lactobacilli and two Bifidobacteria on ICR mice fed on a high fat diet. Biochem Biophys Res Commun. 2016;480:222‐227. [DOI] [PubMed] [Google Scholar]
- 9. Park S, Ji Y, Jung HY, et al. Lactobacillus plantarum HAC01 regulates gut microbiota and adipose tissue accumulation in a diet‐induced obesity murine model. Appl Microbiol Biotechnol. 2017;101:1605‐1614. [DOI] [PubMed] [Google Scholar]
- 10. Balakumar M, Prabhu D, Sathishkumar C, et al. Improvement in glucose tolerance and insulin sensitivity by probiotic strains of Indian gut origin in high‐fat diet‐fed C57BL/6J mice. Eur J Nutr 2016; https://doi.org/10.1007/s00394-016-1317-7 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 11. Santisteban MM, Qi Y, Zubcevic J, et al. Hypertension‐linked pathophysiological alterations in the gut. Circ Res. 2017;120:312‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yap WB, Ahmad FM, Lim YC, Zainalabidin S. Lactobacillus casei strain C1 attenuates vascular changes in spontaneously hypertensive rats. Korean J Physiol Pharmacol. 2016;20:621‐628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu WH, Chuang HL, Huang YT, et al. Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ‐free mice. Behav Brain Res. 2016;298:202‐209. [DOI] [PubMed] [Google Scholar]
- 14. Aoyagi Y, Park S, Matsubara S, et al. Habitual intake of fermented milk products containing Lactobacillus casei strain Shirota and a reduced risk of hypertension in older people. Benef Microbes. 2017;8:23‐29. [DOI] [PubMed] [Google Scholar]
- 15. Severance EG, Gressitt KL, Stallings CR, et al. Probiotic normalization of Candida albicans in schizophrenia: a randomized, placebo‐controlled, longitudinal pilot study. Brain Behav Immun. 2016;62:41‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wallace CJ, Milev R. The effects of probiotics on depressive symptoms in humans: a systematic review. Ann Gen Psychiatry. 2017;16:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Akbari E, Asemi Z, Daneshvar Kakhaki R, et al. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer's disease: a randomized, double‐blind and controlled trial. Front Aging Neurosci. 2016;8:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. American College of Gastroenterology Task Force on Irritable Bowel Syndrome , Brandt LJ, Chey WD, Foxx‐Orenstein AE, et al. An evidence‐based position statement on the management of irritable bowel syndrome. Am J Gastroenterol 2009;104 (Suppl. 1):S1‐S35. [DOI] [PubMed] [Google Scholar]
- 19. Trinkley KE, Nahata MC. Medication management of irritable bowel syndrome. Digestion. 2014;89:253‐267. [DOI] [PubMed] [Google Scholar]
- 20. Barboza JL, Talley NJ, Moshiree B. Current and emerging pharmacotherapeutic options for irritable bowel syndrome. Drugs. 2014;74:1849‐1870. [DOI] [PubMed] [Google Scholar]
- 21. Foxx‐Orenstein AE. New and emerging therapies for the treatment of irritable bowel syndrome: an update for gastroenterologists. Therap Adv Gastroenterol. 2016;9:354‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting, and evaluation in health care. Prev Med. 2010;51:421‐424. [DOI] [PubMed] [Google Scholar]
- 23. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. The Critical Appraisal Skills Programme (CASP) . CASP randomised controlled trial checklist Oxford, 2014. http://media.wix.com/ugd/dded87_40b9ff0bf53840478331915a8ed8b2fb.pdf (accessed 2017).
- 25. National Institute for Health and Care Excellence . Developing NICE guidelines: the manual appendix H, 2015. https://www.nice.org.uk/process/pmg20/resources/developing-nice-guidelines-the-manual-appendix-h-pdf-2549711485 (accessed 2017). [PubMed]
- 26. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Çekin AH, Şahintürk Y, Akbay Harmandar F, et al. Use of probiotics as an adjuvant to sequential H. pylori eradication therapy: impact on eradication rates, treatment resistance, treatment‐related side effects, and patient compliance. Turk J Gastroenterol. 2017;28:3‐11. [DOI] [PubMed] [Google Scholar]
- 28. Evans M, Salewski RP, Christman MC, Girard SA, Tompkins TA. Effectiveness of Lactobacillus helveticus and Lactobacillus rhamnosus for the management of antibiotic‐associated diarrhoea in healthy adults: a randomised, double‐blind, placebo‐controlled trial. Br J Nutr. 2016;116:94‐103. [DOI] [PubMed] [Google Scholar]
- 29. Kabbani TA, Pallav K, Dowd SE, et al. Prospective randomized controlled study on the effects of Saccharomyces boulardii CNCM I‐745 and amoxicillin‐clavulanate or the combination on the gut microbiota of healthy volunteers. Gut Microbes. 2017;8:17‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lyra A, Hillila M, Huttunen T, et al. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J Gastroenterol. 2016;22:10631‐10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mirghafourvand M, Homayouni Rad A, Mohammad Alizadeh Charandabi S, Fardiazar Z, Shokri K. The effect of probiotic yogurt on constipation in pregnant women: a randomized controlled clinical trial. Iran Red Crescent Med J. 2016;18:e39870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nobutani K, Sawada D, Fujiwara S, et al. The effects of administration of the Lactobacillus gasseri strain CP2305 on quality of life, clinical symptoms and changes in gene expression in patients with irritable bowel syndrome. J Appl Microbiol. 2017;122:212‐224. [DOI] [PubMed] [Google Scholar]
- 33. Agrawal A, Houghton LA, Morris J, et al. Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN‐173 010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2009;29:104‐114. [DOI] [PubMed] [Google Scholar]
- 34. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic‐associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet. 2013;382:1249‐1257. [DOI] [PubMed] [Google Scholar]
- 35. Beausoleil M, Fortier N, Guenette S, et al. Effect of a fermented milk combining Lactobacillus acidophilus Cl1285 and Lactobacillus casei in the prevention of antibiotic‐associated diarrhea: a randomized, double‐blind, placebo‐controlled trial. Can J Gastroenterol. 2007;21:732‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Begtrup LM, De Muckadell OBS, Kjeldsen J, Christensen RD, Jarbol DE. Long‐term treatment with probiotics in primary care patients with irritable bowel syndrome – a randomised, double‐blind, placebo controlled trial. Scand J Gastroenterol. 2013;48:1127‐1135. [DOI] [PubMed] [Google Scholar]
- 37. Charbonneau D, Gibb RD, Quigley EM. Fecal excretion of Bifidobacterium infantis 35624 and changes in fecal microbiota after eight weeks of oral supplementation with encapsulated probiotic. Gut Microbes. 2013;4:201‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chatterjee S, Kar P, Das T, et al. Randomised placebo‐controlled double blind multicentric trial on efficacy and safety of Lactobacillus acidophilus la‐5 and Bifidobacterium bb‐12 for prevention of antibiotic‐associated diarrhoea. J Assoc Physician India. 2013;61:708‐712. [PubMed] [Google Scholar]
- 39. Chitapanarux T, Thongsawat S, Pisespongsa P, Leerapun A, Kijdamrongthum P. Effect of Bifidobacterium longum on PPI‐based triple therapy for eradication of Helicobacter pylori: a prospective, randomized study. J Gastroenterol Hepatol. 2014;29:23. [Google Scholar]
- 40. Cindoruk M, Erkan G, Karakan T, Dursun A, Unal S. Efficacy and safety of Saccharomyces boulardii in the 14‐day triple anti‐Helicobacter pylori therapy: a prospective randomized placebo‐controlled double‐blind study. Helicobacter. 2007;12:309‐316. [DOI] [PubMed] [Google Scholar]
- 41. Cremonini F, Di Caro S, Covino M, et al. Effect of different probiotic preparations on anti‐Helicobacter pylori therapy‐related side effects: a parallel group, triple blind, placebo‐controlled study. Am J Gastroenterol. 2002;97:2744‐2749. [DOI] [PubMed] [Google Scholar]
- 42. de Vrese M, Kristen H, Rautenberg P, Laue C, Schrezenmeir J. Probiotic lactobacilli and bifidobacteria in a fermented milk product with added fruit preparation reduce antibiotic associated diarrhea and Helicobacter pylori activity. J Dairy Res. 2011;78:396‐403. [DOI] [PubMed] [Google Scholar]
- 43. Drouault‐Holowacz S, Bieuvelet S, Burckel A, et al. A double blind randomized controlled trial of a probiotic combination in 100 patients with irritable bowel syndrome. Gastroenterol Clin Biol. 2008;32:147‐152. [DOI] [PubMed] [Google Scholar]
- 44. Ducrotte P, Sawant P, Jayanthi V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol. 2012;18:4012‐4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ehrhardt S, Guo N, Hinz R, et al. Saccharomyces boulardii to prevent antibiotic‐associated diarrhea: a randomized, double‐masked, placebo‐controlled trial. Open Forum Infect Disease. 2016;3:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Enck P, Zimmermann K, Menke G, Klosterhalfen S. Randomized controlled treatment trial of irritable bowel syndrome with a probiotic E.‐coli preparation (DSM17252) compared to placebo. Z Gastroenterol. 2009;47:209‐214. [DOI] [PubMed] [Google Scholar]
- 47. Eskesen D, Jespersen L, Michelsen B, et al. Effect of the probiotic strain Bifidobacterium animalis subsp. lactis, BB‐12, on defecation frequency in healthy subjects with low defecation frequency and abdominal discomfort: a randomised, double‐blind, placebo‐controlled, parallel‐group trial. Br J Nutr. 2015;114:1638‐1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gao XW, Mubasher M, Fang CY, Reifer C, Miller LE. Dose‐response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic‐associated diarrhea and Clostridium difficile‐associated diarrhea prophylaxis in adult patients. Am J Gastroenterol. 2010;105:1636‐1641. [DOI] [PubMed] [Google Scholar]
- 49. Guglielmetti S, Mora D, Gschwender M, Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life – a double‐blind, placebo‐controlled study. Aliment Pharmacol Ther. 2011;33:1123‐1132. [DOI] [PubMed] [Google Scholar]
- 50. Guyonnet D, Chassany O, Ducrotte P, et al. Effect of a fermented milk containing Bifidobacterium animalis DN‐173 010 on the health‐related quality of life and symptoms in irritable bowel syndrome in adults in primary care: a multicentre, randomized, double‐blind, controlled trial. Aliment Pharmacol Ther. 2007;26:475‐486. [DOI] [PubMed] [Google Scholar]
- 51. Guyonnet D, Schlumberger A, Mhamdi L, Jakob S, Chassany O. Fermented milk containing Bifidobacterium lactis DN‐173 010 improves gastrointestinal well‐being and digestive symptoms in women reporting minor digestive symptoms: a randomised, double‐blind, parallel, controlled study. Br J Nutr. 2009;102:1654‐1662. [DOI] [PubMed] [Google Scholar]
- 52. Hauser G, Salkic N, Vukelic K, JajacKnez A, Stimac D. Probiotics for standard triple Helicobacter pylori eradication: a randomized, double‐blind, placebo‐controlled trial. Medicine. 2015;94:e685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hickson M, D'Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hong KS, Kang HW, Im JP, et al. Effect of probiotics on symptoms in Korean adults with irritable bowel syndrome. Gut Liv. 2009;3:101‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jafari E, Vahedi H, Merat S, Momtahen S, Riahi A. Therapeutic effects, tolerability and safety of a multi‐strain probiotic in Iranian adults with irritable bowel syndrome and bloating. Arch Iran Med. 2014;17:466‐470. [PubMed] [Google Scholar]
- 56. Kajander K, Hatakka K, Poussa T, Farkkila M, Korpela R. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: a controlled 6‐month intervention. Aliment Pharmacol Ther. 2005;22:387‐394. [DOI] [PubMed] [Google Scholar]
- 57. Kajander K, Myllyluoma E, Rajilic‐Stojanovic M, et al. Clinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment Pharmacol Ther. 2008;27:48‐57. [DOI] [PubMed] [Google Scholar]
- 58. Kalman DS, Schwartz HI, Alvarez P, et al. A prospective, randomized, double‐blind, placebo‐controlled parallel‐group dual site trial to evaluate the effects of a Bacillus coagulans‐based product on functional intestinal gas symptoms. BMC Gastroenterol. 2009;9:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ki Cha B, Mun Jung S, Hwan Choi C, et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea‐dominant irritable bowel syndrome: a randomized, double‐blind, placebo‐controlled trial. J Clin Gastroenterol. 2012;46:220‐227. [DOI] [PubMed] [Google Scholar]
- 60. Kim HJ, Camilleri M, McKinzie S, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea‐predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17:895‐904. [DOI] [PubMed] [Google Scholar]
- 61. Kim HJ, Vazquez Roque MI, Camilleri M, et al. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17:687‐696. [DOI] [PubMed] [Google Scholar]
- 62. Kim LS, Hilli L, Orlowski J, et al. Efficacy of probiotics and nutrients in functional gastrointestinal disorders: a preliminary clinical trial. Dig Dis Sci. 2006;51:2134‐2144. [DOI] [PubMed] [Google Scholar]
- 63. Larsen CN, Nielsen S, Kaestel P, et al. Dose‐response study of probiotic bacteria Bifidobacterium animalis subsp lactis BB‐12 and Lactobacillus paracasei subsp paracasei CRL‐341 in healthy young adults. Eur J Clin Nutr. 2006;60:1284‐1293. [DOI] [PubMed] [Google Scholar]
- 64. Ligaarden SC, Axelsson L, Naterstad K, Lydersen S, Farup PG. A candidate probiotic with unfavourable effects in subjects with irritable bowel syndrome: a randomised controlled trial. BMC Gastroenterol. 2010;10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lorenzo‐Zuniga V, Llop E, Suarez C, et al. I.31, a new combination of probiotics, improves irritable bowel syndrome‐related quality of life. World J Gastroenterol 2014;20:8709‐8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ludidi S, Jonkers DM, Koning CJ, et al. Randomized clinical trial on the effect of a multispecies probiotic on visceroperception in hypersensitive IBS patients. Neurogastroenterol Motil. 2014;26:705‐714. [DOI] [PubMed] [Google Scholar]
- 67. Marteau P, Guyonnet D, Lafaye de Micheaux P, Gelu S. A randomized, double‐blind, controlled study and pooled analysis of two identical trials of fermented milk containing probiotic Bifidobacterium lactis CNCM I‐2494 in healthy women reporting minor digestive symptoms. Neurogastroenterol Motil 2013;25:331‐e252. [DOI] [PubMed] [Google Scholar]
- 68. Mazlyn MM, Nagarajah LH, Fatimah A, Norimah AK, Goh KL. Effects of a probiotic fermented milk on functional constipation: a randomized, double‐blind, placebo‐controlled study [Erratum appears in J Gastroenterol Hepatol. 2013;28:1584]. J Gastroenterol Hepatol. 2013;28:1141‐1147. [DOI] [PubMed] [Google Scholar]
- 69. Merenstein DJ, D'Amico F, Palese C, et al. Short‐term, daily intake of yogurt containing Bifidobacterium animalis ssp. lactis Bf‐6 (LMG 24384) does not affect colonic transit time in women. Br J Nutr. 2014;111:279‐286. [DOI] [PubMed] [Google Scholar]
- 70. Nista EC, Candelli M, Cremonini F, et al. Bacillus clausii therapy to reduce side‐effects of anti‐Helicobacter pylori treatment: randomized, double‐blind, placebo controlled trial. Aliment Pharmacol Ther. 2004;20:1181‐1188. [DOI] [PubMed] [Google Scholar]
- 71. Ojetti V, Gigante G, Gabrielli M, et al. The effect of oral supplementation with Lactobacillus reuteri or tilactase in lactose intolerant patients: randomized trial. Eur Rev Med Pharmacol Sci. 2010;14:163‐170. [PubMed] [Google Scholar]
- 72. Ouwehand AC, ten Bruggencate SJ, Schonewille AJ, et al. Lactobacillus acidophilus supplementation in human subjects and their resistance to enterotoxigenic Escherichia coli infection. Br J Nutr. 2014;111:465‐473. [DOI] [PubMed] [Google Scholar]
- 73. Pakdaman MN, Udani JK, Molina JP, Shahani M. The effects of the DDS‐1 strain of lactobacillus on symptomatic relief for lactose intolerance – a randomized, double‐blind, placebo‐controlled, crossover clinical trial. Nutr J 2016;15:56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pineton de Chambrun G, Neut C, Chau A, et al. A randomized clinical trial of Saccharomyces cerevisiae versus placebo in the irritable bowel syndrome. Digest Liver Dis. 2015;47:119‐124. [DOI] [PubMed] [Google Scholar]
- 75. Pitkala KH, Strandberg TE, Finne Soveri UH, et al. Fermented cereal with specific bifidobacteria normalizes bowel movements in elderly nursing home residents. A randomized, controlled trial. J Nutr Health Aging. 2007;11:305‐311. [PubMed] [Google Scholar]
- 76. Plummer S, Weaver MA, Harris JC, Dee P, Hunter J. Clostridium difficile pilot study: effects of probiotic supplementation on the incidence of C. difficile diarrhoea. Int Microbiol. 2004;7:59‐62. [PubMed] [Google Scholar]
- 77. Pozzoni P, Riva A, Bellatorre AG, et al. Saccharomyces boulardii for the prevention of antibiotic‐associated diarrhea in adult hospitalized patients: a single‐center, randomized, double‐blind, placebo‐controlled trial. Am J Gastroenterol. 2012;107:922‐931. [DOI] [PubMed] [Google Scholar]
- 78. Sampalis J, Psaradellis E, Rampakakis E. Efficacy of BIO K+ CL1285 in the reduction of antibiotic‐associated diarrhea – a placebo controlled double‐blind randomized, multi‐center study. Arch Med Sci. 2010;6:56‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sen S, Mullan MM, Parker TJ, et al. Effect of Lactobacillus plantarum 299v on colonic fermentation and symptoms of irritable bowel syndrome. Dig Dis Sci. 2002;47:2615‐2620. [DOI] [PubMed] [Google Scholar]
- 80. Simrén M, Ohman L, Olsson J, et al. Clinical trial: the effects of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome – a randomized, double‐blind, controlled study. Aliment Pharmacol Ther. 2010;31:218‐227. [DOI] [PubMed] [Google Scholar]
- 81. Sinn DH, Song JH, Kim HJ, et al. Therapeutic effect of Lactobacillus acidophilus‐SDC 2012, 2013 in patients with irritable bowel syndrome. Dig Dis Sci. 2008;53:2714‐2718. [DOI] [PubMed] [Google Scholar]
- 82. Sisson G, Ayis S, Sherwood RA, Bjarnason I. Randomised clinical trial: a liquid multi‐strain probiotic vs. placebo in the irritable bowel syndrome – a 12 week double‐blind study. Aliment Pharmacol Ther. 2014;40:51‐62. [DOI] [PubMed] [Google Scholar]
- 83. Sondergaard B, Olsson J, Ohlson K, et al. Effects of probiotic fermented milk on symptoms and intestinal flora in patients with irritable bowel syndrome: a randomized, placebo‐controlled trial. Scand J Gastroenterol. 2011;46:663‐672. [DOI] [PubMed] [Google Scholar]
- 84. Song HJ, Kim JY, Jung SA, et al. Effect of probiotic Lactobacillus (Lacidofil® cap) for the prevention of antibiotic‐associated diarrhea: a prospective, randomized, double‐blind, multicenter study. J Korean Med Sci. 2010;25:1784‐1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Spiller R, Pelerin F, Cayzeele Decherf A, et al. Randomized double blind placebo‐controlled trial of Saccharomyces cerevisiae CNCM I‐3856 in irritable bowel syndrome: improvement in abdominal pain and bloating in those with predominant constipation. United Eur Gastroenterol J. 2016;4:353‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Stevenson C, Blaauw R, Fredericks E, Visser J, Roux S. Randomized clinical trial: effect of Lactobacillus plantarum 299 v on symptoms of irritable bowel syndrome. Nutrition. 2014;30:1151‐1157. [DOI] [PubMed] [Google Scholar]
- 87. Tanaka Y, Takami K, Nishijima T, et al. Short‐ and long‐term dynamics in the intestinal microbiota following ingestion of Bifidobacterium animalis subsp. lactis GCL2505. Bio Microbiota Food Health. 2015;34:77‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Thijssen AY, Clemens CH, Vankerckhoven V, et al. Efficacy of Lactobacillus casei Shirota for patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2016;28:8‐14. [DOI] [PubMed] [Google Scholar]
- 89. Tilley L, Keppens K, Kushiro A, et al. A probiotic fermented milk drink containing Lactobacillus casei strain shirota improves stool consistency of subjects with hard stools. Int J Probiotics Prebiotics. 2014;9:23‐30. [Google Scholar]
- 90. Waller PA, Gopal PK, Leyer GJ, et al. Dose‐response effect of Bifidobacterium lactis HN019 on whole gut transit time and functional gastrointestinal symptoms in adults. Scand J Gastroenterol. 2011;46:1057‐1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. West NP, Pyne DB, Cripps AW, et al. Lactobacillus fermentum (PCC®) supplementation and gastrointestinal and respiratory‐tract illness symptoms: a randomised control trial in athletes. Nutr J. 2011;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581‐1590. [DOI] [PubMed] [Google Scholar]
- 93. Williams EA, Stimpson J, Wang D, et al. Clinical trial: a multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double‐blind placebo‐controlled study. Aliment Pharmacol Ther. 2009;29:97‐103. [DOI] [PubMed] [Google Scholar]
- 94. Yao CK, Barrett JS, Philpott H, et al. Poor predictive value of breath hydrogen response for probiotic effects in IBS. J Gastroenterol Hepatol. 2015;30:1731‐1739. [DOI] [PubMed] [Google Scholar]
- 95. Yoon JS, Sohn W, Lee OY, et al. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double‐blind, placebo‐controlled trial. J Gastroenterol Hepatol. 2014;29:52‐59. [DOI] [PubMed] [Google Scholar]
- 96. Zeng J, Li YQ, Zuo XL, et al. Clinical trial: effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea‐predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:994‐1002. [DOI] [PubMed] [Google Scholar]
- 97. Ford AC, Brandt LJ, Young C, et al. Efficacy of 5‐HT3 antagonists and 5‐HT4 agonists in irritable bowel syndrome: systematic review and meta‐analysis. Am J Gastroenterol 2009;104:1831‐1843; quiz 44. [DOI] [PubMed] [Google Scholar]
- 98. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325‐332. [DOI] [PubMed] [Google Scholar]
- 99. Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta‐analysis. Am J Gastroenterol 2014;109:1547‐1561; quiz 46, 62. [DOI] [PubMed] [Google Scholar]
- 100. Ortiz‐Lucas M, Tobias A, Saz P, Sebastian JJ. Effect of probiotic species on irritable bowel syndrome symptoms: a bring up to date meta‐analysis. Rev Esp Enferm Dig. 2013;105:19‐36. [DOI] [PubMed] [Google Scholar]
- 101. Hoveyda N, Heneghan C, Mahtani KR, et al. A systematic review and meta‐analysis: probiotics in the treatment of irritable bowel syndrome. BMC Gastroenterol. 2009;9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Brenner DM, Moeller MJ, Chey WD, Schoenfeld PS. The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review. Am J Gastroenterol 2009;104:1033‐1049; quiz 50. [DOI] [PubMed] [Google Scholar]
- 103. Eales J, Gibson P, Whorwell P, et al. Systematic review and meta‐analysis: the effects of fermented milk with Bifidobacterium lactis CNCM I‐2494 and lactic acid bacteria on gastrointestinal discomfort in the general adult population. Therap Adv Gastroenterol. 2017;10:74‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cayzeele‐Decherf A, Pelerin F, Leuillet S, et al. Saccharomyces cerevisiae CNCM I‐3856 in irritable bowel syndrome: an individual subject meta‐analysis. World J Gastroenterol. 2017;23:336‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Yuan F, Ni H, Asche CV, et al. Efficacy of Bifidobacterium infantis 35624 in patients with irritable bowel syndrome: a meta‐analysis. Curr Med Res Opin 2017;33:1191‐1197. [DOI] [PubMed] [Google Scholar]
- 106. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta‐regression analysis. Gastroenterology 2017;152:1889‐1900. [DOI] [PubMed] [Google Scholar]
- 107. Sinclair A, Xie X, Saab L, Dendukuri N. Lactobacillus probiotics in the prevention of diarrhea associated with Clostridium difficile: a systematic review and Bayesian hierarchical meta‐analysis. CMAJ Open. 2016;4:E706‐E718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Vernaya M, McAdam J, Hampton MD. Effectiveness of probiotics in reducing the incidence of Clostridium difficile‐associated diarrhea in elderly patients: a systematic review. JBI Database System Rev Implement Rep. 2017;15:140‐164. [DOI] [PubMed] [Google Scholar]
- 109. Lau CS, Ward A, Chamberlain RS. Probiotics improve the efficacy of standard triple therapy in the eradication of Helicobacter pylori: a meta‐analysis. Infect Drug Resist. 2016;9:275‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lu M, Yu S, Deng J, et al. Efficacy of probiotic supplementation therapy for Helicobacter pylori eradication: a meta‐analysis of randomized controlled trials. PLoS ONE. 2016;11:e0163743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


