Summary
Objective
Genetic alterations have been identified in the CACNA1H gene, encoding the CaV3.2 T‐type calcium channel in patients with absence epilepsy, yet the precise mechanisms relating to seizure propagation and spike‐wave‐discharge (SWD) pacemaking remain unknown. Neurons of the thalamic reticular nucleus (TRN) express high levels of CaV3.2 calcium channels, and we investigated whether a gain‐of‐function mutation in the Cacna1h gene in Genetic Absence Epilepsy Rats from Strasbourg (GAERS) contributes to seizure propagation and pacemaking in the TRN.
Methods
Pathophysiological contributions of CaV3.2 calcium channels to burst firing and absence seizures were assessed in vitro using acute brain slice electrophysiology and quantitative real‐time polymerase chain reaction (PCR) and in vivo using free‐moving electrocorticography recordings.
Results
TRN neurons from GAERS display sustained oscillatory burst‐firing that is both age‐ and frequency‐dependent, occurring only in the frequencies overlapping with GAERS SWDs and correlating with the expression of a CaV3.2 mutation‐sensitive splice variant. In vivo knock‐down of CaV3.2 using direct thalamic injection of lipid nanoparticles containing CaV3.2 dicer small interfering (Dsi) RNA normalized TRN burst‐firing, and in free‐moving GAERS significantly shortened seizures.
Significance
This supports a role for TRN CaV3.2 T‐type channels in propagating thalamocortical network seizures and setting the pacemaking frequency of SWDs.
Keywords: absence epilepsy, low threshold spike, thalamocortical, T‐type calcium channel
Key points.
Burst‐firing in reticular thalamic neurons is enhanced in the GAERS absence epilepsy model
The frequency range of enhanced burst‐firing correlates with SWDs
A developmental increase occurs in the mutation‐sensitive splice variant of CaV3.2
Genetic knock‐down of CaV3.2 shortens absence seizure duration but not seizure number in GAERS
1. INTRODUCTION
Burst‐firing of thalamic neurons is a characteristic cellular electrophysiological feature accompanying the spike‐and‐wave discharges (SWDs) seen on electroencephalography (EEG) recordings during absence seizures, and can also be observed in other generalized and focal epilepsies.1 Thalamic neurons display similar firing patterns during the physiological thalamocortical oscillatory rhythms observed in non–rapid eye movement (REM) sleep, thus burst‐firing per se is not a pathophysiological marker.2, 3 However, during absence seizures, there is an inappropriate switching during wakefulness of thalamic neuronal firing from tonic to oscillatory burst‐firing, and that is believed to play a key role in the impairment of consciousness during seizures by interrupting the normal relay of incoming sensory information.4, 5 The ability of the thalamus to generate intrinsic oscillations, and the correlation between burst activity in thalamic reticular nucleus (TRN) neurons with spikes in SWDs suggest that the TRN may act as a thalamocortical pacemaker, and is therefore a strong candidate for initiating and/or propagating absence seizures.2, 6, 7 In rat models of absence epilepsy, a region of the primary somatosensory (S1Cx) and S2/insular cortex appears to be the focal point of seizure generation.8, 9
T‐type calcium channels underlie the low threshold calcium spike (LTS) required to generate neuronal burst‐firing in TRN and thalamocortical neurons. The magnitude of the LTS is directly related to the number of action potentials per burst in TRN, but not in cortex‐projecting thalamocortical neurons such as those of the ventrobasal (VB) complex.2, 10, 11, 12 Increased T‐type messenger RNA (mRNA) expression and whole cell currents have been observed in TRN and thalamocortical neurons in genetic rat models of generalized epilepsy.13, 14, 15
We reported previously that the Genetic Absence Epilepsy Rats from Strasbourg (GAERS) model of genetic generalized epilepsy possess an arginine to proline missense (R1584P) mutation in the Cacna1h gene encoding the CaV3.2 T‐type calcium channel.16 The point mutation co‐segregates with SWD expression when outcrossed with Non‐Epileptic Control (NEC) rats, whereby outcrossed offspring homozygous for the R1584P mutation display significantly enhanced seizure activity compared to offspring null for the mutation. Studies in exogenous expression systems indicate that mutation induces a gain‐of‐function phenotype in CaV3.2 channels, selectively in splice variants containing exon 25 (CaV3.2(+25)) found downstream of the mutation. The R1584P mutation increases the rate at which CaV3.2(+25) variant channels recover from inactivation and further results in increased calcium charge transference during mock bursting. The CaV3.2(+25) and exon 25 lacking (CaV3.2(−25)) splice variants display unique biophysical properties and are expressed in approximately equal abundance in the thalamus of NEC rats.16, 17
Here we investigate the role of the TRN in absence seizure generation in GAERS by assessing the firing properties and T‐type currents in intact TRN neurons. To achieve this, we elucidate the neurophysiological impact of the R1584P mutation in TRN neurons of GAERS and correlate it with developmental expression of the CaV3.2(+25) splice variant. Furthermore, we show that selective knock‐down of CaV3.2 channel expression in the GAERS TRN in vivo reduces the duration of seizures. This study demonstrates that CaV3.2 channels in the GAERS TRN play a key role in sustaining burst‐firing in TRN neurons and that this is required for propagation of absence seizures.
2. MATERIALS AND METHODS
All experiments were undertaken according to Canadian Council for Animal Care Guidelines. See Data S1 for detailed Materials and Methods.
3. RESULTS
3.1. Individual bursts in either epileptic or preepileptic GAERS do not display hyperexcitability
GAERS begin to express emergent SWDs around postnatal day (P) 30, and the epileptic phenotype is fully developed in all animals by P120.18 The excitability of NEC (n = 28) and GAERS (n = 20) TRN neurons was first assessed by analyzing single bursts using whole‐cell current clamp in slices from adult rats (P120‐P150). Individual bursts were induced in TRN neurons at their intrinsic Vm by incremental increases in depolarizing DC current injection for 1 second until the threshold for burst‐firing was reached (Figure S1A). There were no differences detected for any single burst parameter in either fully epileptic GAERS or NECs (current required for threshold bursting, number of action potentials per burst, resting membrane potential, input resistance, burst inflection point or latency to first action potential; Figure S1B‐G; NEC n = 28 cells, n = 13 animals; GAERS n = 21 cells, n = 12 animals). We next examined single burst properties in P15‐P20 TRN neurons to establish whether a hyperexcitable neuronal phenotype was present in the TRN neurons of GAERS prior to the expression of seizures. Like for adult rats, we found no differences between NEC (n = 111 cells, n = 52 animals) and GAERS (n = 70 cells, n = 36 animals) concerning single bursts induced by DC depolarization of TRN neurons from their intrinsic Vm or passive membrane properties (Figure S1B‐G).
3.2. Fully epileptic GAERS TRN neurons display hyperexcitable burst‐firing in the GAERS SWD frequency range
During absence seizures, thalamic neurons display a rhythmic firing pattern of multiple bursts, sustained for the duration of the seizure.6, 19 As such, TRN neurons were assessed over a series of multiple bursts at various frequencies (5‐10 Hz) for a period of 2 seconds. This frequency range corresponds to spike frequencies that are at and just above or below those observed during SWDs in GAERS (~6‐9 Hz).1 To induce multiple bursting, a sinusoidal wave of hyperpolarizing/depolarizing current was injected at incrementally increasing amplitudes from each neuron's intrinsic Vm until burst‐firing (>3 action potentials, >100 Hz) was observed in the initial 3 bursts of the series. As the series progressed, the oscillatory bursts induced by this protocol decreased in the number of action potentials per burst, in both NEC and GAERS (Figure 1A), likely a result of attenuated T‐type currents across the series as channels accumulate in the inactivated state.12, 16 To examine the degree of burst‐firing sustained during the sinusoidal stimulation, a percentage was calculated by dividing the number of action potentials on the last burst by the number of action potentials on the first burst. In TRN neurons from fully epileptic GAERS (P120‐P150), the number of action potentials on the last burst displayed no significant difference compared to NEC neurons at 5 Hz or 10 Hz (Figure 1B). However, within the GAERS SWD frequency band of 6‐9 Hz, the number of action potentials per burst on the last burst in GAERS TRN neurons was significantly increased in comparison to NEC (Figure 1B). Together, these data indicate that fully epileptic GAERS TRN neurons possess an inherent ability to sustain burst‐firing over a range of frequencies corresponding to the cycle frequency of SWDs recorded by EEG, compared to those from age‐matched nonepileptic animals.
Figure 1.
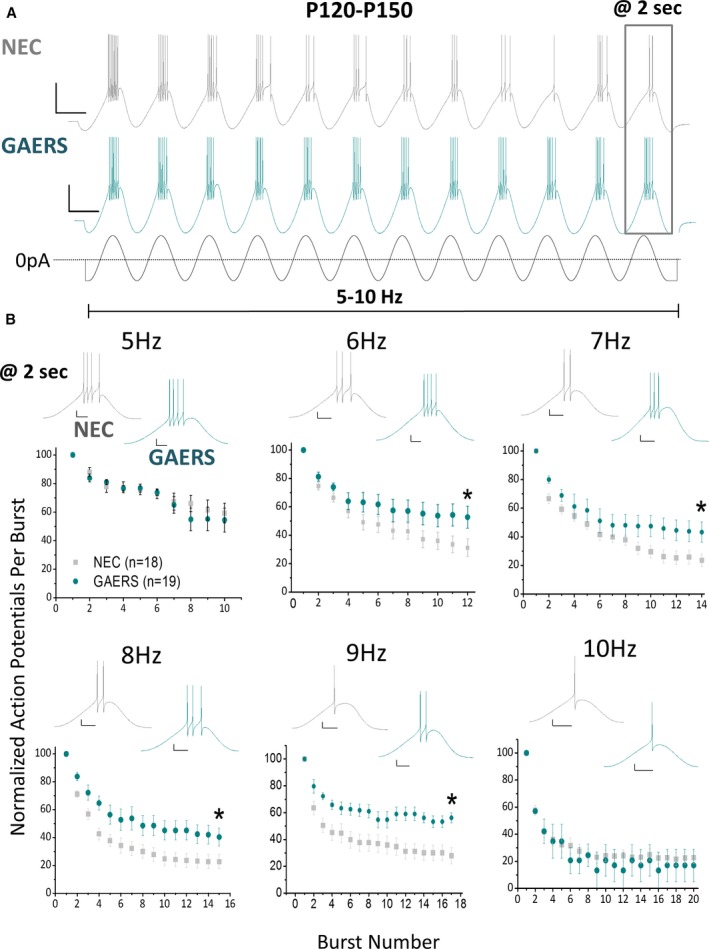
Adult GAERS TRN neurons display sustained oscillatory burst‐firing at 6‐9 Hz. A, Representative traces showing response of adult NEC (A—upper panel) and GAERS (A—middle panel) TRN neurons to a 2 second sinusoidal current injection stimulus (A—lower panel). Stimulus magnitude was incrementally increased until bursting (>3 action potentials) occurred on 3 initial bursts. Scale bars = 30 mV, 100 msec. B, The sinusoidal stimulus was applied at variable frequencies (5‐10 Hz), with the number of action potentials per burst decreasing with time. Insets show representative voltage traces of the last burst in a series. 6 Hz: NEC = 31.2 ± 6.2%, GAERS = 52.8 ± 7.8, P = .034 t test; 7 Hz: NEC = 23.6 ± 4.4%, GAERS = 43.3 ± 7.2%, P = .044 t test; 8 Hz: NEC = 22.7 ± 5.1%, GAERS = 40.3 ± 6.4%, P = .047 t test; 9 Hz: NEC = 30.1 ± 5.9%, GAERS = 56.2 ± 3.7%, P = .005 t test. NEC (n = 20 cells, n = 11 animals), GAERS (n = 15 cells, n = 10 animals). Scale bars represent 20 msec and 10 mV. *P < .05
3.3. Preepileptic GAERS TRN neurons display hyperexcitable burst‐firing in a narrow frequency range
Burst‐firing over a series of multiple bursts was next examined in TRN neurons from preepileptic (P15‐P20) GAERS versus NEC rats (Figure 2). As in adults, the number of action potentials per burst decreased across the series of bursts in both NEC and GAERS preepileptic TRN neurons at all frequencies (Figure 2A). Unexpectedly, given that these experiments were undertaken in preepileptic animals, there was a significant increase in the number of action potentials on the last burst at 8‐9 Hz in GAERS (Figure 2B). However, at lower frequencies (5‐7 Hz) and at the higher frequency of 10 Hz, no difference in the number of action potentials on the last burst was observed. This indicates that TRN neurons in preepileptic GAERS (Figure 2B) can generate hyperexcitable burst‐firing, albeit only at a comparatively narrow frequency range.
Figure 2.
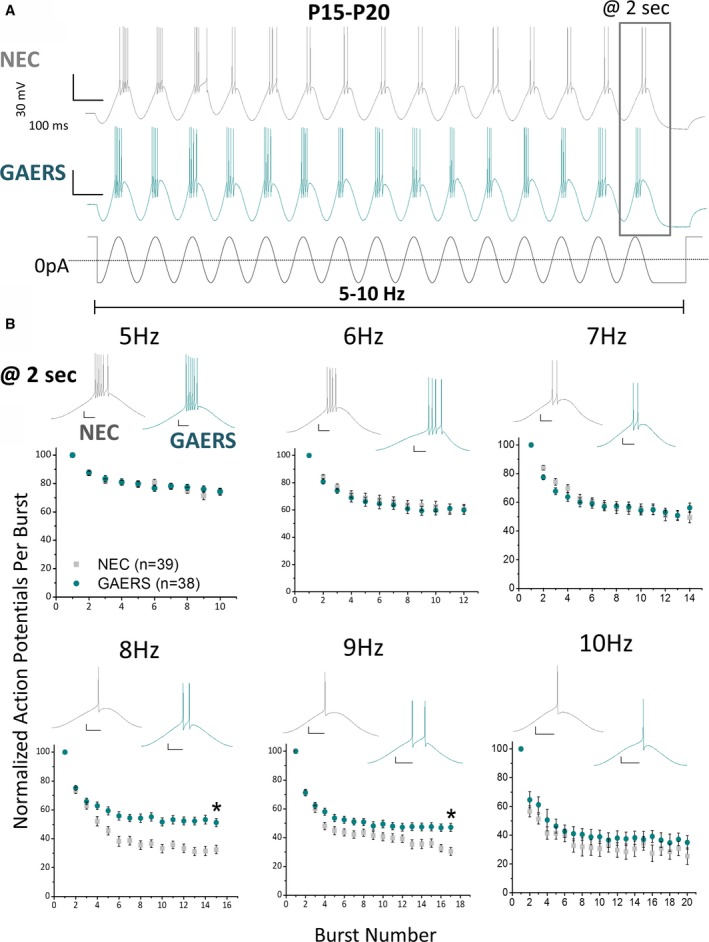
P15‐P20 GAERS TRN neurons display sustained oscillatory burst‐firing at 8‐9 Hz. A, Representative traces showing response of P15‐P20 NEC (A—upper panel) and GAERS (A—middle panel) TRN neurons to a 2‐second sinusoidal current injection stimulus (A—lower panel). Stimulus magnitude was incrementally increased until bursting (>3 action potentials) occurred on 3 initial bursts. Scale bars = 30 mV, 100 msec. B, The sinusoidal stimulus was applied at variable frequencies (5‐10 Hz), with the number of action potentials per burst decreasing with time. Insets show representative voltage traces of the last burst in a series. 8 Hz: NEC = 32.5 ± 2.9%, GAERS = 51.2 ± 2.8%, P = .00001 t test; 9 Hz: NEC = 30.5 ± 2.7%, GAERS = 47.2 ± 2.8%, P = .00006 t test; NEC (n = 32 cells, n = 20 animals), GAERS (n = 32 cells, n = 16 animals). Scale bars = 20 msec and 10 mV. *P < .05
3.4. T‐type currents in GAERS TRN neurons display gain‐of‐function properties
TRN neurons express both the Cav3.2 and Cav3.3 T‐type calcium channel subtypes at approximately equal levels.20, 21 Upon exogenous expression, the GAERS CaV3.2 R1584P mutation selectively increases the rate of recovery from inactivation of CaV3.2(+25) variant channels.16 We hypothesized that native GAERS TRN neurons would also display gain‐of‐function T‐type current biophysical properties. Whole‐cell voltage‐clamp was undertaken in TRN neurons, although limited by space‐clamp considerations in acute brain slices from older animals, T‐type calcium currents were recorded from acute brain slices from neonatal (P7‐P9) NEC and GAERS rats (in the presence of sodium, potassium and HVA calcium channel blockers; TTX [600 nM], 4‐AP [5 mM], TEA [10 mM], Cs+ [120 mM], conotoxin MVIIC [3 μM], SNX‐482 [100 nM], Cd2+ [10 μM], and nimodipine [5 μM]). GAERS TRN neurons displayed a modest but significant increase in T‐type current density in the range of −55 to −40 mV (NEC [n = 6], GAERS [n = 6], P = .03‐.04 t test; Figure S2A), with no apparent differences in the voltage dependences of activation or inactivation between the 2 strains (Figure S2B). The rate of recovery from inactivation was also significantly increased in GAERS TRN neurons, displaying greater fractional recovery from 160 to 640 msec interpulse intervals (Figure S2C) and a significant decrease in the fast time constant of inactivation when fitted individually with a decaying double exponential (NEC = 232.7 ± 14.6 [n = 11 cells, n = 5 animals], GAERS = 182.7 ± 12.9 msec [n = 9 cells, n = 4 animals]; P = .01 t test).
To further understand the frequency‐dependent contribution of T‐type conductance to burst‐firing, isolated T‐type calcium currents were recorded in voltage‐clamp while burst‐firing waveforms were applied that were previously recorded from an adult GAERS TRN neuron in current clamp at 5 and 8 Hz (Figure S2D). The charge transference (taken from the integral of the current vs time per burst) was normalized to the whole‐cell capacitance to account for variability in neuron size and provide a value of charge transference density, similar to the method of normalizing whole‐cell currents for variability in cell size. Charge transference density was significantly increased for the last burst at 2 seconds in GAERS compared to NEC TRN neurons at 8 Hz (Figure S2E), but not at 5 Hz (Figure S2F). This confirms the presence in GAERS of a frequency‐dependent, T‐type calcium current gain‐of‐function using voltage‐clamp in a physiological milieu at an early developmental stage.
3.5. CaV3.2(+25) expression increases with development
The GAERS genome possesses a gain‐of‐function missense mutation in the CaV3.2 gene.16 To assess whether this mutation affects the thalamic expression of calcium channel α1 subunits between GAERS and NEC during postnatal development quantitative real‐time polymerase chain reaction (qRT‐PCR) analyses were undertaken in samples of whole thalamus excised from preepileptic P10 and P120 epileptic rats. In these stages, thalamic expression of 8 of the 10 calcium channel α1 subunits was detected, including all 3 T‐types (CaV3.1, CaV3.2, CaV3.3). Of note, within each subunit type there were no significant differences in overall relative expression between NEC and GAERS at the developmental stages examined (Figure 3A). Previously, we showed that the GAERS R1584P mutation produced a selective gain‐of‐function phenotype in CaV3.2(+25) splice variant channels with a concomitant modest loss‐of‐function in CaV3.2(−25) variant channels.16 The developmental expression of the CaV3.2(+25) and CaV3.2(−25) splice variants were assessed in samples of whole thalamus from P10, P20, and P120 animals using qRT‐PCR and corrected to display splice‐variant transcript copy number (see Data S1). Figure 3B shows that within each stage, the NEC and GAERS strains express equal amounts of CaV3.2(+25) transcripts. This was accompanied by a significant developmental increase in the overall copy number expression of CaV3.2(+25) transcripts in both NEC and GAERS between preepileptic (P10 and P20) thalamic samples compared to fully epileptic (P120) thalamus (Figure 3B). Contrastingly, thalamic expression of the CaV3.2(−25) variant was significantly lower in GAERS compared to NEC at all 3 stages examined. In addition, NEC animals displayed a developmental decrease in CaV3.2(−25) expression at P20 and P120 compared to P10, whereas in GAERS, a decrease was observed at P20 compared to P10 and P120. Together, the differences in relative splice‐variant expression gives rise to a consistently greater ratio of CaV3.2(+25)/CaV3.2(25) transcripts in GAERS versus NEC animals through the course of development (Figure 3C). Furthermore, the CaV3.2(+25)/CaV3.2(−25) transcript expression ratio is significantly different between all developmental stages in both NEC and GAERS (for clarity, significance between developmental stages for each strain is not shown in Figure 3C). The developmental increase in the mutation‐sensitive CaV3.2(+25) splice variant, together with hyperexcitable, sustained burst‐firing in older GAERS provides a mechanism for the emergence of seizures in this model.
Figure 3.
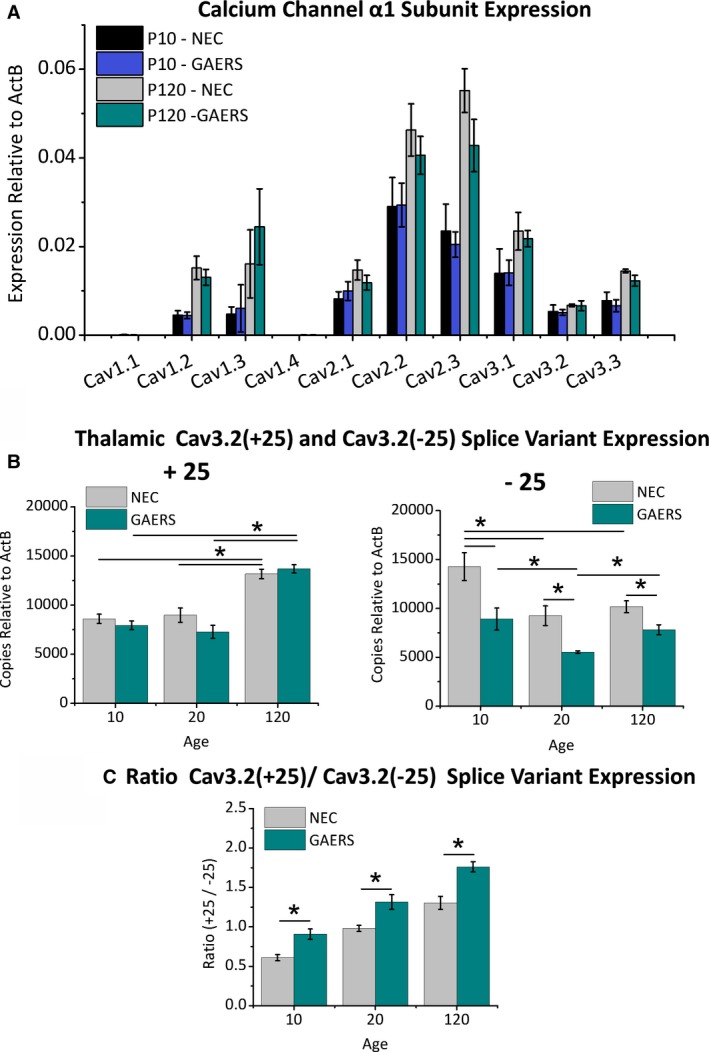
GAERS show a developmental increase in the expression of the Cav3.2(+25) splice variant. A, The relative mRNA expression of the T‐type Cav3.1‐Cav3.3 and the high‐voltage activated Cav1.1‐Cav1.4 and Cav2.1‐Cav2.3 Ca2+ channels was determined using qRT‐PCR, comparing isoform expression between P10 and P120 in thalamic tissue from GAERS and NEC animals. Amounts are relative to Actin‐B as a control. B, Using splice variant–specific probes for Cav3.2(+25) and Cav3.2(−25), the relative number of transcript copies of Cav3.2(+25) and Cav3.2(−25) splice variants was determined across development in GAERS and NEC animals (n = 3 animals per strain): Cav3.2(+25): P10 NEC = 8601.2 ± 475.7, P10 GAERS = 7944.6 ± 443.8; P20 NEC = 8985.0 ± 726.4, P20 GAERS = 7278.1 ± 654.4; P120 NEC = 13177.8 ± 487.5, P120 GAERS = 13692.3 ± 424.0; [P10 NEC vs P120 NEC] P = .003, [P20 NEC vs P120 NEC] P = .005 ANOVA; [P10 GAERS vs P120 GAERS] P = .0006, [P20 GAERS vs P120 GAERS] P = .0003 ANOVA. Cav3.2(−25): P10 NEC = 14279.6 ± 1421.0, P10 GAERS = 8910.9 ± 1125.8, P = .04 t test; P20 NEC = 9246.1 ± 1006.7, P20 GAERS = 5524.7 ± 214.0, P = .02 t test; P120 NEC = 10170.6 ± 599.2, P120 GAERS = 7814.7 ± 513.8, P = .04 t test; [P10 NEC vs P20 NEC] P = .035, [P10 NEC vs P120 NEC] P = .045 ANOVA; [P10 GAERS vs P20 GAERS] P = .036, [P20 GAERS vs P120 GAERS] P = .04 ANOVA. C, The ratio of Cav3.2(+25) to Cav3.2(−25) was determined from copy number analysis for each of the developmental time points from both GAERS and NEC animals. All experiments were performed using thalamus samples from 3 rats for each age group (P10, P20, or P120) and from each of GAERS or NEC animals: P10 NEC = 0.61 ± 0.04, P10 GAERS = 0.91 ± 0.07, P = .02 t test; P20 NEC = 0.98 ±0.04, P20 GAERS = 1.31 ± 0.09, P = .03 t test; P120 NEC = 1.30 ± 0.08, P120 GAERS = 1.76 ± 0.06, P = .01 t test; [P10 NEC vs P20 NEC] P = .009, [P10 NEC vs P120 NEC] P = .0004, [P20 NEC vs P120 NEC] P = .017; [P10 GAERS vs P20 GAERS] P = .02, [P10 GAERS vs P120 GAERS] P = .0005, [P20 GAERS vs P120 GAERS] P = .014 ANOVA. *P < .05
3.6. Pharmacological blockade of the CaV3.2 T‐type calcium channel abolishes sustained burst‐firing in GAERS TRN neurons
To confirm whether the GAERS CaV3.2 channel was responsible for the increased TRN burst‐firing properties, we removed the contribution of CaV3.2‐mediated calcium conductance by pharmacological blockade. Oscillatory current clamp experiments using 8‐Hz sinusoidal stimuli were performed in slices from P15‐P20 NEC and GAERS in the presence of nickel (Ni2+; 100 μM) to block CaV3.2, while allowing the majority of the CaV3.3 channels to remain unblocked and contribute to bursting (Figure S3).22 In the presence of Ni2+, no significant effect was seen on passive membrane properties, such as resting Vm or input resistance (not shown); however, burst‐firing required significantly greater current injection to achieve threshold in both NEC and GAERS TRN neurons (Figure 4A‐C) as the contribution of CaV3.2 was removed. Oscillatory burst‐firing at 8 Hz was then assessed by comparing the number of action potentials on the last burst of a 2‐second oscillatory stimulation, relative to the first burst. It is notable that in the presence of Ni2+ there was no significant difference in sustained burst firing in GAERS TRN neurons compared to NEC, thereby abolishing the hyperexcitable phenotype (Figure 4A,D).
Figure 4.
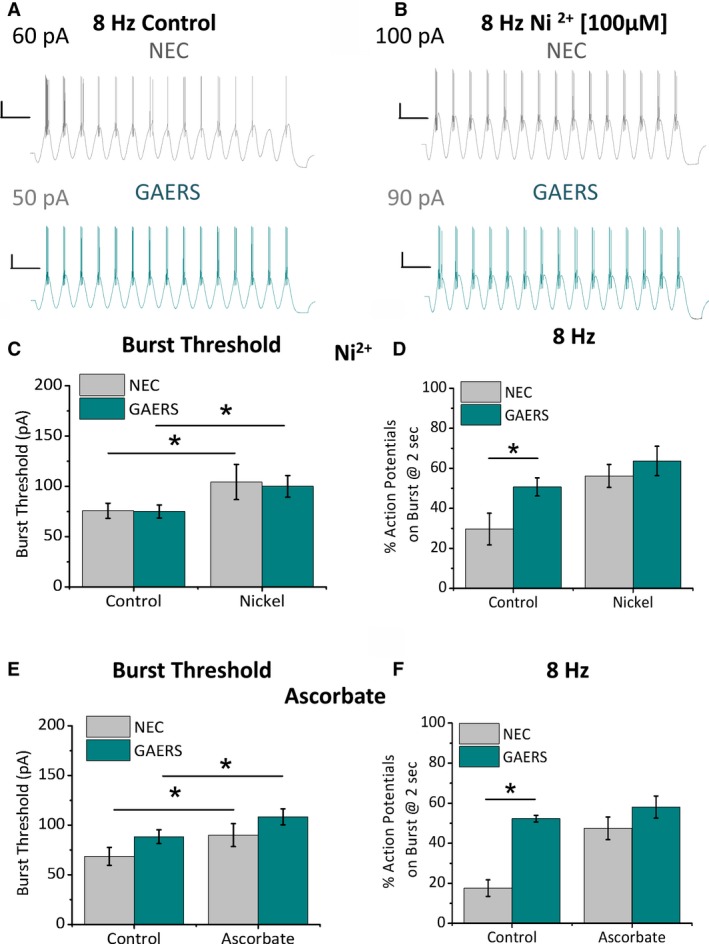
Sustained oscillatory burst‐firing in P15‐P20 GAERS TRN neurons is normalized by pharmacological CaV3.2 blockade. Representative traces show an oscillatory burst series in response to a 2‐second sinusoidal current injection at 8 Hz in P15‐P20 NEC (A, B—upper panels) and GAERS (A, B—lower panels) TRN neurons. Traces show burst‐firing before (control; A) and after 10‐minute application of Ni2+ (100 μM, B) via the perfusate to block CaV3.2. Histograms display mean data for burst threshold (C, E) and number of action potentials on the last burst of a 2‐second stimulation, relative to the first burst (D, F) before (control) and after application of Ni2+ (100 μM, C, D; Control: NEC = 29.7 ± 7.9%, GAERS = 50.7 ± 4.5% [n = 8], P = .03 t test, Ni2+: NEC = 56.2 ± 5.7%, GAERS = 63.7 ± 7.3%, P = .15 t test, NEC [n = 8 cell, n = 3 animals], GAERS [n = 8 cells, n = 3 animals]) and ascorbate (300 μM, E, F; Control: NEC = 17.6 ± 15.3%, GAERS = 52.3 ± 1.7%, P = .001 t test), Asc: NEC = 47.5 ± 5.7%, GAERS = 58.1 ± 5.5%, P = .22 t test, NEC (n = 8 cells, n = 3 animals), GAERS (n = 6 cells, n = 3 animals) to block CaV3.2 in NEC and GAERS TRN neurons. *P < .05
CaV2.3 (R‐type) calcium channels are also sensitive to Ni2+; therefore, we sought to confirm the result with a second CaV3.2 channel inhibitor. Ascorbate (Asc; 300 μM) inhibits CaV3.2 channels in TRN neurons with little effect on CaV3.3, thought to occur via oxidation of a redox sensitive amino acid specific to CaV3.2.21, 23 Asc was efficacious at attenuating burst threshold in most, but not all, TRN neurons (n = 7 of 10 NEC and n = 6 of 7 GAERS). The “non‐responding” neurons were not included in further analysis, and we speculate that CaV3.2 channels in these neurons existed in a fully oxidized state prior to Asc application. In the remaining TRN neurons, Asc increased the current injection threshold required to achieve burst‐firing in both NEC and GAERS (Figure 4E). Furthermore, in the presence of Asc, GAERS TRN neurons did not show sustained oscillatory burst‐firing at 8 Hz compared to NEC (Figure 4F).
3.7. Selective thalamic knock‐down of CaV3.2 in vivo effectively shortens seizures
The specific contributions of T‐type calcium currents in TRN neurons toward absence seizures remain a subject of considerable investigation, and there have been no reports examining this issue in the GAERS model. As such, we next sought to assess the contribution of TRN CaV3.2 currents to seizure activity in vivo. The approach combined dicer small interfering (Dsi) RNAs targeted to CaV3.2 transcripts together with a recently developed methodology involving packaged lipid nanoparticles (LNPs) previously demonstrated as an efficacious vector for in vivo knock‐down.24, 25 Initially, several distinct DsiRNAs were screened for their ability to attenuate exogenous CaV3.2 expression in transfected HEK cells (not shown) and subsequently on native CaV3.2 expression in cultured rat cortical neurons, with the best performing DsiRNA selected for packaging into LNPs containing DiI as a marker (Figure 5A,B). Control LNPs were generated by packaging DsiRNA for luciferase (Luc). CaV3.2 and Luc DsiRNA‐containing LNPs were separately stereotaxically injected bilaterally into the thalamus of P120‐P150 GAERS (Figure 5C, see Experimental Procedures). Eight days to 9 days postinjection, TRN samples were isolated by dissecting from 3, 300‐μm sequential, horizontal thalamic sections, and T‐type calcium channel expression was analyzed by qRT‐PCR. TRN slices from GAERS injected with CaV3.2 DsiRNA‐LNPs displayed a selective knock‐down of both CaV3.2(+25) and CaV3.2(−25) splice variants, without significantly affecting the expression of the CaV3.1 or CaV3.3 T‐type isoforms (Figure 5E). Expression of the control gene CASC3 relative to glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) was not significantly affected in TRN tissue from Luc versus CaV3.2 DsiRNA‐injected GAERS (Luc DsiRNA = 0.0083 ± 0.0005, CaV3.2 DsiRNA = 0.0086 ± 0.0002, P = .56 t test).
Figure 5.
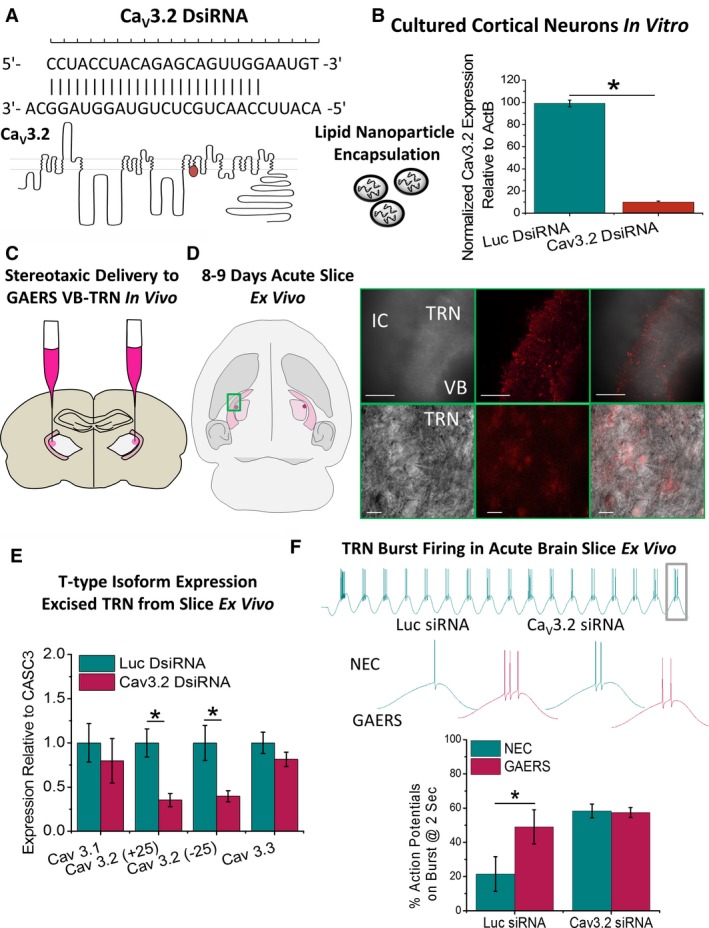
Selective knock‐down of thalamic Cav3.2 in vivo. A, Dicer siRNA (DsiRNA) was designed to selectively anneal to the Cav3.2 channel and target for degradation. DsiRNA was packaged with DiI into LNPs for ApoE‐mediated delivery to brain cells. B, Selective Cav3.2 knock‐down was first confirmed in vitro by applying to cultured cortical neurons for 48 hours and expression levels assessed with qPCR. C, For in vivo analyses, P120‐P150 GAERS rats were stereotaxically injected with luciferase control (Luc) or Cav3.2 DsiRNA LNPs bilaterally into the ventroposterior lateral thalamus to deliver DsiRNA to TRN neurons without damaging or lesioning the TRN. D, Following a 7‐ to 8‐day recovery period, animals were euthanized, the brain removed, and LNP invasion of TRN was confirmed by DiI fluorescence ex vivo (scale bars: top panels = 400 μm, bottom panels = 20 μm). E, Cav3.1‐Cav3.3 T‐type isoform expression in TRN tissue was assayed using qPCR relative to the control gene CASC3. Histograms display mean data for expression of T‐type calcium channel isoforms in TRN tissue dissected from 3 sequential 300‐μm‐thick horizontal brain slices for GAERS injected with Luc (n = 6 animals) and Cav3.2 DsiRNA (n = 8 animals); CaV3.1 isoform: Luc DsiRNA = 1.00 ± 0.22, CaV3.2 DsiRNA = 0.79 ± 0.25, P = .55 t test; CaV3.2(+25) splice variant: Luc DsiRNA = 1.00 ± 0.16, CaV3.2 DsiRNA = 0.35 ± 0.07, P = .0017 t test; CaV3.2(−25) splice variant: Luc DsiRNA = 1.00 ± 0.19, CaV3.2 DsiRNA = 0.39 ± 0.06, P = .0067 t test; CaV3.3 isoform: Luc DsiRNA = 1.00 ± 0.12, CaV3.2 DsiRNA = 0.81 ± 0.08, P = .21 t test. F, Oscillatory burst‐firing was assessed in TRN neurons from acute thalamic brain slices in P19‐P20 NEC and GAERS, 6‐8 d following injection with either Luc (n = 3 animals, n = 6 cells) or Cav3.2 (n = 3 animals, n = 7 cells) siRNA. Bar charts show percentage number of action potentials on the last burst relative to the first burst from a 2‐s oscillating wave stimulation at 8 Hz. *P < .05
In a separate set of experiments, P11‐P12 NEC and GAERS pups were stereotaxically injected with LNPs containing CaV3.2 or Luc DsiRNA into the thalamus (Figure S4). Following an 8‐ to 9‐day period, acute brain slices were prepared, and neuronal transfection verified by the presence of DiI in cell bodies (Figure 5D). Oscillatory burst‐firing at 8 Hz was again assessed by comparing the number of action potentials on the last burst of a 2‐second oscillatory stimulation relative to the first burst. This confirmed that TRN neurons from GAERS injected with Luc displayed enhanced oscillatory burst‐firing compared to NEC (as observed in Figures 3 and 6), seen as a significantly greater number of action potentials on the last burst (Figure 5F). Conversely, the hyperexcitable phenotype was not observed GAERS TRN neurons compared to NEC from animals injected with CaV3.2 DsiRNA. We did not perform voltage‐clamp experiments examining the effect of DsiRNA on T‐type currents due to space‐clamp limitations in TRN neurons from P15‐P20 brain slices.
Figure 6.
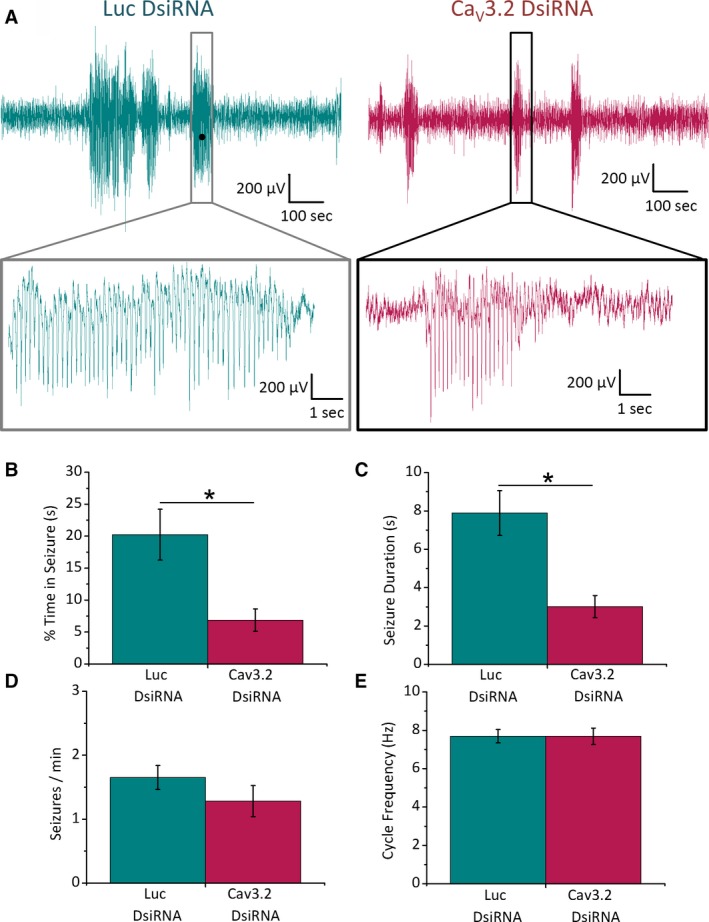
Thalamic Cav3.2 knock‐down reduces seizure duration in GAERS. Adult GAERS rats were stereotaxically injected with Cav3.2 or Luc DsiRNA LNPs bilaterally into the VB thalamus, transfecting the TRN and implanted with skull screw electrodes on the somatosensory cortex connected to a custom EEG interface. A, Representative ECoG recordings from GAERS injected with Luc (left panel) or Cav3.2 (right panel) DsiRNA LNPs. Lower panels show ECoG data with expanded time resolution. Histograms show mean data with (B) % of total time spent in seizure state (Luc = 20.2 ± 4.0%, CaV3.2 = 6.9 ± 1.8%, P = .005), (C) seizure duration (Luc = 7.9 ± 1.1 s, CaV3.2 = 3.0 ± 0.6 s, P = .002), (D) number of seizures per minute (Luc = 1.65 ± 0.19, CaV3.2 = 1.28 ± 0.24%, P = .27), and (E) SWD spike cycle frequency (Luc = 7.7 ± 0.3 Hz, CaV3.2 = 7.7 ± 0.4 Hz, P = .99) for GAERS rats injected with Luc (n = 6 animals) and Cav3.2 DsiRNA (n = 8 animals). *P < .05
Seizure activity was also evaluated in GAERS P120‐P150 animals injected with either Luc or CaV3.2 DsiRNA into the thalamus (Figure 6). Following a 7‐ to 8‐day recovery period (the day before tissue extraction for mRNA analysis) free‐moving electrocorticography (ECoG) recordings were performed using a wireless transmitter connected to a custom interface relaying signals from electrodes surgically implanted on the somatosensory cortex. Spontaneous SWDs were observed throughout the 60‐minute recording period in both treatment groups (Figure 6A). However, compared to Luc DsiRNA‐injected animals (n = 6), GAERS injected with CaV3.2 DsiRNA (n = 8) exhibited a significantly lower percentage time spent in the seizure state (Figure 6B) as well as significantly shorter seizure duration (Figure 6C). Conversely, the number of seizures occurring during the recording period was not significantly altered by CaV3.2 DsiRNA compared to Luc DsiRNA injection (Figure 6D). Furthermore, CaV3.2 and Luc DsiRNA‐injected animals displayed no significant difference in SWD cycle frequency (Figure 6E).
4. DISCUSSION
Although both increased T‐type currents and an upregulation in CaV3.2 mRNA have been observed in GAERS TRN neurons,14, 15 to our knowledge altered TRN burst‐firing has not been thus far reported. GAERS TRN neurons have been shown to burst fire in synchrony with SWDs,6, 19 and lesioning the TRN completely abolishes seizures,26 confirming this region as an integral nucleus in the absence seizure‐generating thalamocortical system. Here, we demonstrate that GAERS TRN neurons display a hyperexcitable oscillatory firing pattern, predicted to result from the gain‐of‐function induced by the CaV3.2 R1584P missense mutation.16 The hyperexcitability is progressively exacerbated during development and parallels an increased expression of the CaV3.2(+25) splice variant sensitive to the R1584P mutation. We conclude that the developmental appearance of TRN hyperexcitability contributes to the age‐dependent emergence of seizures in GAERS, due to a combined effect of the mutation's selective effect on CaV3.2(+25) splice variant properties and the developmental increase in the relative abundance of this variant.
4.1. GAERS TRN neurons display enhanced oscillatory burst‐firing
To establish whether a gain‐of‐function in GAERS TRN T‐type currents could induce a pathophysiologically relevant alteration to excitability we investigated TRN burst‐firing properties. Although single burst characteristics were virtually identical between NEC and GAERS, we found enhanced oscillatory multiple burst‐firing properties in GAERS TRN neurons. During multiple bursting in GAERS, the number of action potentials per burst attenuated to a significantly lesser degree compared to NEC animals. Furthermore, this occurred in a frequency and age‐dependent manner with sustained burst‐firing observed at a wide range of frequencies (6‐9 Hz) in GAERS adults and corresponding to the frequency range of SWDs during GAERS seizures.27 Contrastingly, preepileptic GAERS exhibited sustained burst‐firing over a narrower frequency range (8‐9 Hz). Together, these results demonstrate a developmental broadening of the input stimulation frequencies required to induce pathophysiological burst‐firing that correlate with the emergence of absence seizures.
4.2. CaV3.2 knock‐down in GAERS TRN selectively affects seizure duration
We observed CaV3.2‐dependent enhanced oscillatory burst‐firing in GAERS TRN neurons and predicted that the selective knock‐down of CaV3.2 would alter absence seizure activity. Using intrathalamic injection of LNPs containing CaV3.2 DsiRNA, the expression of CaV3.2 transcripts in the TRN region was found to be reduced to approximately 35%‐40% compared to that for Luc control–injected animals. Selectively decreasing CaV3.2 expression in the TRN significantly shortened seizure duration without significantly altering the number of seizures in GAERS. It is possible that a more pronounced degree of CaV3.2 knock‐down in the TRN would result in a further decrease in seizure duration to the point where seizures cannot be sustained. Because no effect was observed following CaV3.2 knockdown on the number of seizures, the current results support the notion that absence seizures are initiated outside of the thalamus but are propagated by the TRN. In support, the initiating locus for absence seizures in both GAERS and WAG/Rij rats appears to be a cortical region involving the primary somatosensory cortex (S1Cx)28, 29, 30, 31 and S2/insular cortex.9 Our results support the theory, in that epileptic GAERS TRN neurons are shown to exhibit hyperexcitability (enhanced burst‐firing) and therefore can propagate the oscillatory burst discharges that underlie absence seizures. Consistent with this notion, in vivo single‐cell recordings have shown that physiological theta frequency oscillations (5‐9 Hz) originating in the S1Cx of NEC rats briefly engage TRN neurons in oscillatory burst‐firing, but that this is nonsustained, lasting <1 second.32 In contrast, GAERS cortical oscillations trigger sustained oscillatory burst‐firing in TRN neurons, which is accompanied by SWDs on cortical EEG recordings and behavioral arrest in the animals.
Also to consider, the S1Cx region expresses all 3 T‐type calcium channels, albeit only the layer V neurons intrinsically involved in thalamocortical network oscillations exhibit relatively higher expression of the CaV3.2 subtype.33 As such, the gain‐of‐function effects of the GAERS R1584P mutation on CaV3.2 may also be reflected in the ability of CaV3.2 channels in the cortex to initiate or propagate oscillations in GAERS cortical pyramidal and/or interneurons. Putative epileptogenic burst‐firing neurons have been observed in the GAERS somatosensory cortex,31 and global blockade of all 3 T‐type calcium channels attenuates both the duration and number of seizures in addition to reducing cycle frequency.34 Together with our data this provides strong evidence that seizures are initiated in the cortex and that the TRN acts to propagate seizures, thereby prolonging seizure duration.
4.3. Altered CaV3.2 activity as a trigger for epileptic seizures
Absence seizures occur spontaneously in genetic animal models wherein T‐type calcium channel activity is enhanced in the thalamus.35 The case for involvement of the CaV3.2 subtype in the pathogenesis of epileptic seizures is increasingly strong. Within subpopulations of genetic generalized and childhood absence epilepsy patients a number of gain‐of‐function mutations have been identified in the CACNA1H gene encoding CaV3.2.36 In addition, increased burst‐firing in CA1 hippocampal pyramidal neurons during the spontaneous seizure development phase of the pilocarpine model of temporal lobe epilepsy appears to be due primarily to increased dendritic CaV3.2 expression.35 In the electrical kindling model of temporal lobe epilepsy, increased T‐type currents are also observed in CA1 neurons37, and the development of seizures is prevented by administration of a high‐affinity T‐type calcium channel blocker during the kindling phase.38
It should be stated that selective genetic deletion of CaV3.2 and CaV3.3 channels in mouse TRN neurons abolishes burst‐firing and has little effect on, or even exacerbates, pharmacological absence seizures generated by systemic administration of γ‐butyrolactone (GBL).39 We note, however that the GBL model does not induce typical absence seizures in mice.40 Rather than displaying SWDs, the hallmark of absence seizures, mice injected with GBL and the neuroactive drug into which it is converted (γ‐hydroxybutyric acid; GHB) demonstrate slow oscillating waves, lacking spikes and that are more comparable to a hypnotic state. Furthermore, GHB is a known γ‐aminobutyric acid (GABA) agonist and mimics the native contribution of the GABAergic TRN neurons by hyperpolarizing thalamocortical neurons, thus a functional TRN is intrinsically redundant in this pharmacological model. Accordingly, it must be considered that the effects we observe in GAERS as related to CaV3.2 and the TRN may be specific to the GAERS model. Indeed, we speculate that absence seizures may be generated by hyperexcitability in any locus in the thalamocortical system by a number of means that enhance burst‐firing. The mechanistic spread observed in different animal models most likely reflects a variety of polygenic, underlying causes in patients.
In summary, the current study demonstrates that the splice variant‐sensitive and larger/faster recovering CaV3.2 currents induced by the R1584P mutation in GAERS are linked to abnormally sustained oscillatory burst‐firing properties in TRN neurons. This allows TRN neurons to more readily engage in the pathological thalamocortical network oscillations that underlie absence seizures through corticoreticular hyperexcitability. Furthermore, the epileptic phenotype in GAERS becomes apparent as the natural expression of the R1584P mutation‐sensitive CaV3.2(+25) splice variant increases developmentally, thereby increasing in severity as animals age. In addition, we validate LNP‐mediated delivery of DsiRNA as an effective methodology to selectively manipulate pathophysiological network activity in the brain. Finally, the study highlights the need for the development of selective T‐type calcium channel blockers for the treatment of seizures in epilepsy.
AUTHOR CONTRIBUTIONS
S.M.C. designed experiments, performed in vivo and in vitro electrophysiology recordings, sampled brain tissue for mRNA analysis, performed animal surgeries, designed seizure analysis software, and co‐wrote the manuscript. J.R.T. performed mRNA and qPCR analyses and designed DsiRNA constructs. L.Y. performed animal surgeries. J.M.L. designed seizure analysis software. P.C.L. and P.R.C. provided the DsiRNA packaged into LNPs, and H.B.C., R.K., L.P.B. and R.L.R. assisted with in vitro testing of LNPs. K.L.P. and T.O.B. provided NEC and GAERS animals and edited the manuscript. B.A.M. assisted with editing of the manuscript. T.P.S. designed experiments, analyzed data, and co‐wrote the manuscript. All authors participated in editing of the manuscript.
DISCLOSURE
The authors declare no competing financial interests. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
ACKNOWLEDGMENTS
T.P. Snutch is supported by an operating grant from the Canadian Institutes of Health Research (CIHR; #10677), the Canada Research Chair (CRC) in Biotechnology and Genomics‐Neurobiology, and a Brain Canada Multi‐Investigator Research Initiative Grant with matching support from Genome British Columbia, the Michael Smith Foundation for Health Research, and the Koerner Foundation. PRC acknowledges support from CIHR (FRN148469). B.A. MacVicar is supported by the CRC in Neuroscience, CIHR operating grants (#148397, #8545, #115121 and TCE‐117869 ERA‐NET NEURON), and Fondation Leducq. S.M. Cain was supported by a postdoctoral fellowship from the Michael Smith Foundation for Health Research and the BC Epilepsy Society, a research grant from the B.C. Epilepsy Society, and by a CURE‐Taking Flight Award.
Cain SM, Tyson JR, Choi H‐B, et al. CaV3.2 drives sustained burst‐firing, which is critical for absence seizure propagation in reticular thalamic neurons. Epilepsia. 2018;59:778–791. https://doi.org/10.1111/epi.14018
Cain and Tyson equally contributed to this work.
Contributor Information
Stuart M. Cain, Email: scain@msl.ubc.ca
Terrance P. Snutch, Email: snutch@msl.ubc.ca
REFERENCES
- 1. Danober L, Deransart C, Depaulis A, et al. Pathophysiological mechanisms of genetic absence epilepsy in the rat. Prog Neurobiol. 1998;55:27–57. [DOI] [PubMed] [Google Scholar]
- 2. Contreras D. The role of T‐channels in the generation of thalamocortical rhythms. CNS Neurol Disord Drug Targets. 2006;5:571–85. [DOI] [PubMed] [Google Scholar]
- 3. Crunelli V, Cope DW, Hughes SW. Thalamic T‐type Ca2+ channels and NREM sleep. Cell Calcium. 2006;40:175–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crunelli V, Leresche N. Childhood absence epilepsy: genes, channels, neurons and networks. Nat Rev Neurosci. 2002;3:371–82. [DOI] [PubMed] [Google Scholar]
- 5. Pinault D, O'Brien TJ. Cellular and network mechanisms of genetically‐determined absence seizures. Thalamus Relat Syst. 2007;3:181–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Slaght SJ, Leresche N, Deniau JM, et al. Activity of thalamic reticular neurons during spontaneous genetically determined spike and wave discharges. J Neurosci. 2002;22:2323–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pinault D. Cellular interactions in the rat somatosensory thalamocortical system during normal and epileptic 5–9 Hz oscillations. J Physiol. 2003;552:881–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Polack PO, Mahon S, Chavez M, et al. Inactivation of the somatosensory cortex prevents paroxysmal oscillations in cortical and related thalamic neurons in a genetic model of absence epilepsy. Cereb Cortex. 2009;19:2078–91. [DOI] [PubMed] [Google Scholar]
- 9. Zheng TW, O'Brien TJ, Morris MJ, et al. Rhythmic neuronal activity in S2 somatosensory and insular cortices contribute to the initiation of absence‐related spike‐and‐wave discharges. Epilepsia. 2012;53:1948–58. [DOI] [PubMed] [Google Scholar]
- 10. Chemin J, Monteil A, Perez‐Reyes E, et al. Specific contribution of human T‐type calcium channel isotypes (alpha(1G), alpha(1H) and alpha(1I)) to neuronal excitability. J Physiol. 2002;540:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Broicher T, Kanyshkova T, Meuth P, et al. Correlation of T‐channel coding gene expression, IT, and the low threshold Ca2+ spike in the thalamus of a rat model of absence epilepsy. Mol Cell Neurosci. 2008;39:384–99. [DOI] [PubMed] [Google Scholar]
- 12. Cain SM, Snutch TP. Contributions of T‐type calcium channel isoforms to neuronal firing. Channels. 2010;4:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cain SM, Tyson JR, Jones KL, et al. Thalamocortical neurons display suppressed burst‐firing due to an enhanced Ih current in a genetic model of absence epilepsy. Pflugers Arch. 2015;467:1367–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Talley EM, Solorzano G, Depaulis A, et al. Low‐voltage‐activated calcium channel subunit expression in a genetic model of absence epilepsy in the rat. Brain Res Mol Brain Res. 2000;75:159–65. [DOI] [PubMed] [Google Scholar]
- 15. Tsakiridou E, Bertollini L, de Curtis M, et al. Selective increase in T‐type calcium conductance of reticular thalamic neurons in a rat model of absence epilepsy. J Neurosci. 1995;15:3110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Powell KL, Cain SM, Ng C, et al. A Cav3.2 T‐type calcium channel point mutation has splice‐variant‐specific effects on function and segregates with seizure expression in a polygenic rat model of absence epilepsy. J Neurosci. 2009;29:371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. David LS, Garcia E, Cain SM, et al. Splice‐variant changes of the Ca(V)3.2 T‐type calcium channel mediate voltage‐dependent facilitation and associate with cardiac hypertrophy and development. Channels (Austin) 2010;4:375–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Depaulis A, David O, Charpier S. The genetic absence epilepsy rat from Strasbourg as a model to decipher the neuronal and network mechanisms of generalized idiopathic epilepsies. J Neurosci Methods. 2016;260:159–74. [DOI] [PubMed] [Google Scholar]
- 19. Pinault D, Leresche N, Charpier S, et al. Intracellular recordings in thalamic neurones during spontaneous spike and wave discharges in rats with absence epilepsy. J Physiol. 1998;509:449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Astori S, Wimmer RD, Prosser HM, et al. The Ca(V)3.3 calcium channel is the major sleep spindle pacemaker in thalamus. Proc Natl Acad Sci USA 2011;108:13823–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Joksovic PM, Nelson MT, Jevtovic‐Todorovic V, et al. CaV3.2 is the major molecular substrate for redox regulation of T‐type Ca2+ channels in the rat and mouse thalamus. J Physiol. 2006;574:415–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee JH, Gomora JC, Cribbs LL, et al. Nickel block of three cloned T‐type calcium channels: low concentrations selectively block alpha1H. Biophys J. 1999;77:3034–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Joksovic PM, Doctor A, Gaston B, et al. Functional regulation of T‐type calcium channels by s‐nitrosothiols in the rat thalamus. J Neurophysiol. 2007;97:2712–21. [DOI] [PubMed] [Google Scholar]
- 24. Rungta RL, Choi HB, Tyson JR, et al. The cellular mechanisms of neuronal swelling underlying cytotoxic edema. Cell. 2015;161:610–21. [DOI] [PubMed] [Google Scholar]
- 25. Rungta RL, Choi HB, Lin PJ, et al. Lipid nanoparticle delivery of siRNA to silence neuronal gene expression in the brain. Mol Ther Nucleic Acids. 2013;2:e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vergnes M, Marescaux C. Cortical and thalamic lesions in rats with genetic absence epilepsy. J Neural Transm Suppl. 1992;35:71–83. [DOI] [PubMed] [Google Scholar]
- 27. Akman O, Demiralp T, Ates N, et al. Electroencephalographic differences between WAG/Rij and GAERS rat models of absence epilepsy. Epilepsy Res. 2010;89:185–93. [DOI] [PubMed] [Google Scholar]
- 28. Meeren HK, Pijn JP, Van Luijtelaar EL, et al. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neurosci. 2002;22:1480–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nersesyan H, Hyder F, Rothman DL, et al. Dynamic fMRI and EEG recordings during spike‐wave seizures and generalized tonic–clonic seizures in WAG/Rij rats. J Cereb Blood Flow Metab. 2004;24:589–99. [DOI] [PubMed] [Google Scholar]
- 30. Polack PO, Guillemain I, Hu E, et al. Deep layer somatosensory cortical neurons initiate spike‐and‐wave discharges in a genetic model of absence seizures. J Neurosci. 2007;27:6590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chipaux M, Charpier S, Polack P‐O. Chloride‐mediated inhibition of the ictogenic neurones initiating genetically‐determined absence seizures. Neuroscience. 2011;192:642–51. [DOI] [PubMed] [Google Scholar]
- 32. Pinault D, Slezia A, Acsady L. Corticothalamic 5–9 Hz oscillations are more pro‐epileptogenic than sleep spindles in rats. J Physiol. 2006;574:209–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Talley EM, Cribbs LL, Lee JH, et al. Differential distribution of three members of a gene family encoding low voltage‐activated (T‐type) calcium channels. J Neurosci. 1999;19:1895–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tringham E, Powell KL, Cain SM, et al. T‐type calcium channel blockers that attenuate thalamic burst firing and suppress absence seizures. Sci Transl Med 2012;4:121ra19. [DOI] [PubMed] [Google Scholar]
- 35. Cain SM, Snutch TP. Voltage‐gated calcium channels in epilepsy In: Noebels JL, Avoli M, Rogawski MA, et al., editors. Jasper's basic mechanisms of the epilepsies. 4th ed Bethesda, USA: Oxford University Press, 2012; p. 66–84. [Google Scholar]
- 36. Cain SM, Snutch TP. Voltage‐gated calcium channels and disease. BioFactors. 2011;37:197–205. [DOI] [PubMed] [Google Scholar]
- 37. Faas GC, Vreugdenhil M, Wadman WJ. Calcium currents in pyramidal CA1 neurons in vitro after kindling epileptogenesis in the hippocampus of the rat. Neuroscience. 1996;75:57–67. [DOI] [PubMed] [Google Scholar]
- 38. Casillas‐Espinosa P, Hicks A, Jeffries A, et al. Z944, a novel T‐type calcium channel antagonist delays the progression of epileptogenesis in the amygdala kindling model. PLoS ONE 2015;10:e0130012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee SE, Lee J, Latchoumane C, et al. Rebound burst firing in the reticular thalamus is not essential for pharmacological absence seizures in mice. Proc Natl Acad Sci USA. 2014;111:11828–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Venzi M, Di Giovanni G, Crunelli V. A critical evaluation of the gamma‐hydroxybutyrate (GHB) model of absence seizures. CNS Neurosci Ther. 2015;21:123–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


