Abstract
Objectives
To investigate a range of possible predictors of nocebo responses to medicines.
Design
Prospective cohort study.
Methods
In total, 203 healthy adult volunteers completed measures concerning demographics, psychological factors, medicine‐related beliefs, baseline symptoms, and symptom expectations before taking a sham pill, described as ‘a well‐known tablet available without prescription’ that was known to be associated with several side effects. Associations between these measures and subsequent attribution of symptoms to the tablet were assessed using a hurdle model consisting of a joint logistic and truncated negative binomial regression.
Results
Men had an increased odds of attributing symptoms to the tablet OR = 1.52, and older participants had decreased odds, OR = 0.97. Medicine‐related beliefs were important, with modern health worries, belief that medicines cause harm and perceived sensitivity to medicines associated with increased odds of symptom attribution, OR = 1.02, 1.10, 1.09, respectively. Trust in medicines and pharmaceutical companies decreased the odds of symptom attribution, OR = 0.91, 0.88, respectively. The number of symptoms at baseline and the expected likelihood of symptoms were associated with an increased odds of attributing symptoms to the tablet, OR = 1.07, 1.06, respectively. Anxiety, previous symptom experience, symptom expectations, and modern health worries were also important in predicting the number of symptoms participants attributed to the tablet.
Conclusion
It is hard to predict who is at risk of developing nocebo responses to medicines from demographic or personality characteristics. Context‐specific factors such as beliefs about and trust in medicines, current symptoms and symptom expectations are more useful as predictors. More work is needed to investigate this in a patient sample.
Statement of contribution.
What is already known on this subject?
Many patients report non‐specific side effects to their medication which may arise through a nocebo effect.
Whether some people are particularly predisposed to experience nocebo effects remains unclear.
What does this study add?
Demographic and personality characteristics are poor predictors of symptom attribution to a sham medicine.
Instead, context‐specific factors that concern people's beliefs surrounding medicines, their current symptoms, and symptom expectations are more useful as predictors of symptom attribution.
Keywords: nocebo effect, placebo, predictors, medicine beliefs, symptom attribution
Background
Patients often experience symptoms that they attribute to their medication. However, not all side effects are the result of the pharmacological action of a medication. Indeed, it has been suggested that anywhere between 38% and 100% of apparent side effects are caused by other factors (Mahr et al., 2017). The nocebo effect, defined as the experience of unpleasant or noxious symptoms in response to an inert exposure (Kennedy, 1961), is believed to explain many of these non‐specific side effects (Barsky, Saintfort, Rogers, & Borus, 2002). As such, the nocebo effect can also be operationalized as the non‐specific symptoms that occur after taking a medication which are attributed to the medication (Faasse & Petrie, 2013).
A good example of this has been the recent controversy in the media over the muscle symptoms that some patients experience to their statin medication. High rates of side effects have been attributed to statins among primary care patients (Saxon & Eckel, 2016); however, clinical trials of statins have found side effect rates to be roughly similar in both patients allocated statins and those allocated a sham tablet (Collins et al., 2016; Tobert & Newman, 2016) ‘Statin intolerance’ may therefore be wholly or partly mediated by a nocebo effect, exacerbated by adverse media coverage (Horton, 2016). Similar effects have been proposed for other surprisingly high rates of side effect reporting for medications, such as for oseltamivir during the swine flu outbreak (Kitching, Roche, Balasegaram, Heathcock, & Maguire, 2009) and ciprofloxacin for anthrax exposure (Rubin & Dickmann, 2010; Stein et al., 2004).
Whether related to the pharmacological action of a medication or not, side effects can reduce patient well‐being and be a cause of patient non‐adherence (Ammassari et al., 2001; Kardas, Lewek, & Matyjaszczyk, 2013), both of which can lead to a significant cost to health services (National Institute For Health and Clinical Excellence, 2009). To reduce the negative impact of side effects, it is important to understand more about their causes. Previous research has suggested that expectations (Bingel et al., 2011; Hahn, 1997), learning (Van den Bergh, Kempynck, van de Woestijne, Baeyens, & Eelen, 1995; Vogtle, Barke, & Kroner‐Herwig, 2013), and misattribution of coincidental symptoms (Petrie, Moss‐Morris, Grey, & Shaw, 2004; Petrie et al., 2005) may increase the risk of a nocebo effect occurring. Whether some people are particularly predisposed to experiencing a nocebo effect remains unclear. Several potential predisposing factors have been suggested including gender (Liccardi et al., 2004), anxiety (Nevelsteen, Legros, & Crasson, 2007), somatization (Szemerszky, Koteles, Lihi, & Bardos, 2010), somatosensory amplification (Witthöft & Rubin, 2013), low optimism (Geers, Helfer, Kosbab, Weiland, & Landry, 2005), and baseline symptoms (de la Cruz, Hui, Parsons, & Bruera, 2010). However, in a systematic review, Webster, Weinman, and Rubin (2016) highlighted inconsistent findings with regard to the importance of these factors.
Although dispositional predictors of nocebo effects are not consistent across the literature, there is an important literature concerning peoples’ beliefs about medicines, which have been consistently shown to influence not only peoples’ use of treatment but also their reporting of side effects. Medicine‐related beliefs include people's beliefs about the necessity, harm and use of medicines, their perceptions of their own sensitivity to medicines, and their worries about the health effects of modern medicine. These can all work to increase peoples’ expectations of side effects (Faasse & Petrie, 2013), one of main mechanisms of nocebo effects (Webster et al., 2016). Medicine‐related beliefs have previously been shown to be associated with patients’ choice of medicine (e.g., complementary vs. conventional, or generic vs. branded) (Andersson Sundell & Jönsson, 2016; Figueiras et al., 2010; Petrie et al., 2001), their adherence to their medication (Horne, Chapman, et al., 2013; Menckeberg et al., 2008; Phatak & Thomas, 2006), their information seeking behaviour (Faasse, Grey, Horne, & Petrie, 2015), symptom attribution to a hypothetical medication (Heller, Chapman, & Horne, 2015), and side effect reporting to medications and vaccinations (Nestoriuc, Orav, Liang, Horne, & Barsky, 2010; Petrie et al., 2004; Rief et al., 2012). Given how prevalent these beliefs are, and the importance they have for patients’ decisions and experiences in medical settings, it would be useful to evaluate the contribution of these factors in predicting nocebo responses.
Identifying associations between these variables and symptom attribution might enable clinicians to improve their interactions with patients; the way they address potential side effects with their patients; and allow researchers to develop interventions to target those most at risk of developing a nocebo effect.
As part of a randomized controlled trial testing the effect of patient information leaflet (PIL) wording on symptom attribution, we tested whether the following potential predictors were associated with the attribution of symptoms by healthy participants to a sham medicine: (1) demographics, (2) anxiety, (3) optimism, (4) somatization, (5) somatosensory amplification, (6) previous symptoms, (7) expected side effects, (8) modern health worries, (9) beliefs about medicines, (10) perceived sensitivity to medicines, and (11) trust in medicines and pharmaceutical companies.
Method
Design
This brief prospective cohort study formed part of a randomized controlled trial, additional results for which have been reported elsewhere (Webster, Weinman & Rubin, 2018). It took place at the Wellcome Trust King's Clinical Research Facility and was approved by the King's College London Psychiatry, Nursing and Midwifery Research Ethics Subcommittee (PNM 14/15‐62).
Participants
To be included in the study, participants had to be healthy, aged 18 or over, and fluent in English. This was assessed through a screening questionnaire. Participants with a condition currently causing symptoms such as a chronic or acute illness or those who were pregnant or breast feeding were excluded to prevent any interference with symptom reporting. To enhance the appearance of a genuine drug trial, participants were asked to list any allergies to medicines and/or the inactive ingredients often found in them. Examples of the potential inactive ingredients were given, and these covered all the ingredients included in our sham tablet. Participants who listed allergies to any of the substances in our tablet were excluded. On the day of participation, participants who had taken any pain killers within 4 hrs before taking part or who had been drinking alcohol were rescheduled.
Sample size
The sample size calculation was based on identifying an effect on symptom attribution from the intervention tested in our RCT, the results of which are reported elsewhere (Webster et al., 2018). The associations tested here are therefore exploratory.
Measures
Demographics
Participants were asked their age, gender, ethnicity, highest level of education, and employment status.
Psychological factors
The following measures were included at different phases of the study (see Figure 1).
Figure 1.
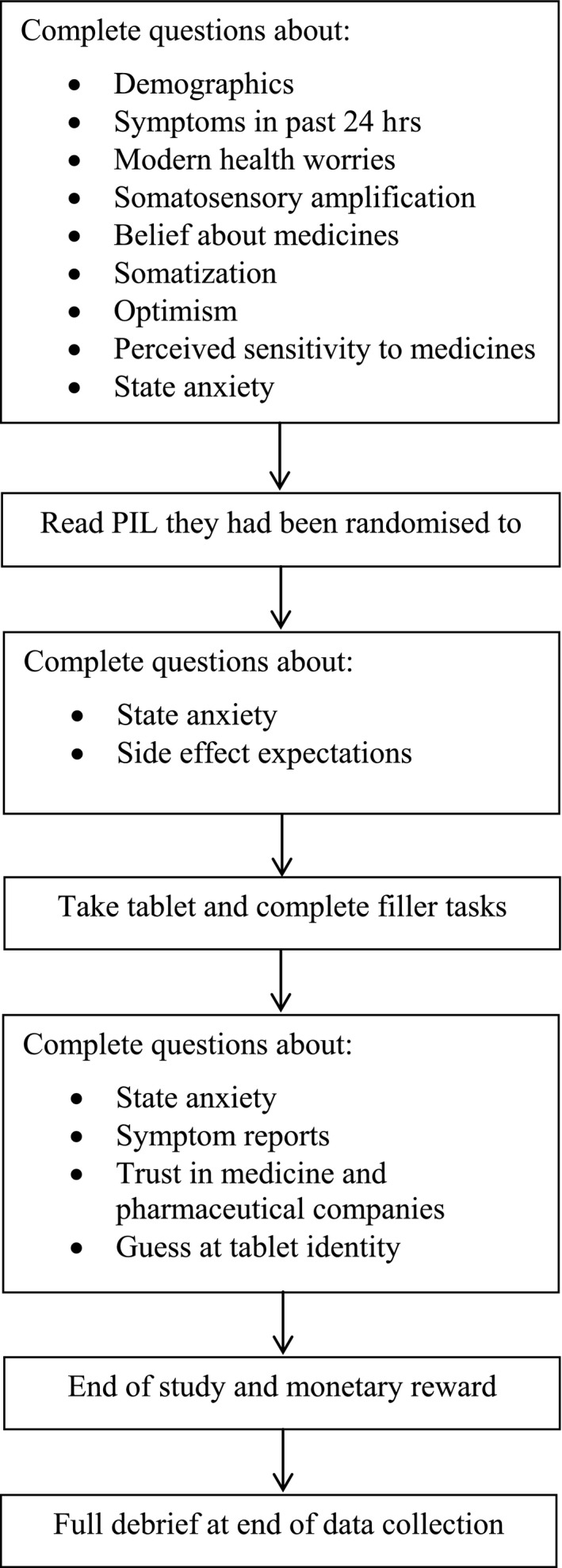
Study procedure.
The State Anxiety Inventory – short version (Marteau & Bekker, 1992) – was used to assess levels of anxiety experienced at the time of measurement. This includes six items which participants rated on a 4‐point scale ranging from 1 ‘not at all’ to 4 ‘very much’. The scores range from 6 to 24 with higher scores indicating greater anxiety.
The Somatosensory Amplification Scale (Barsky, Goodson, Lane, & Cleary, 1988) was used to assess participants’ tendency to experience a somatic sensation as intense, noxious, or disturbing. This 10‐item scale measures the tendency to experience somatic sensations as intense, noxious, and disturbing. Participants rated the degree to which each statement is characteristic of them in general on a 5‐point scale ranging from 1 to 5. The scores range from 10 to 50 with higher scores indicating greater somatosensory amplification.
The Patient Health Questionnaire Somatic Symptom Severity Scale (Kroenke, Spitzer, & Williams, 2002) was used to assess somatization. This is a 15‐item scale designed to measure the prevalence of the most common bodily symptoms (e.g., headache, nausea) experienced in the last 4 weeks. Participants rated each item on a 3‐point scale ranging from 0 ‘not bothered at all’ to 2 ‘bothered a lot’. The scores range from 0 to 30 with higher scores indicating greater somatization.
The Revised Life Orientation Test (Scheier, Carver, & Bridges, 1994) was used to assess dispositional optimism. This consists of six items (plus four filler items) which measures where participants lie on the pessimism‐optimism scale. Participants rated each item on a 5‐point scale ranging from 1 ‘strongly disagree’ to 5 ‘strongly agree’. The scores range from 6 to 30, with higher scores indicating greater optimism.
Symptoms
Participants’ symptom experience in the previous 24 hrs was assessed using a modified version of the Generic Assessment of Side Effects Scale (GASE; Rief, Glombiewski, & Barsky, 2009). The GASE is used to assess side effects in clinical trials and supports the early detection of drug‐induced adverse events. The side effects listed in our modified version were those commonly reported during a nocebo response (14 items) (Wells & Kaptchuk, 2012) or which were already listed on the GASE, detectable within an hour of taking the tablet, and not too serious (e.g., we removed ‘hair loss’ from the list) (nine items). Participants rated each symptom on a 4‐point scale ranging from 0 ‘not present’ to 3 ‘severe’. Scores range from 0 to 23 for the number of symptoms and 0 to 69 for the severity of symptoms.
Symptom expectations were also assessed with a modified version of the GASE (Rief et al., 2009) as used similarly by Nestoriuc et al. (2016). The side effects listed were the same; however, in this case, participants were asked to state how likely they thought they were to experience the symptom in the hour after taking the tablet. Each symptom was rated on a 4‐point scale ranging from 0 ‘not very likely’ to 3 ‘very likely’. Scores range from 0 to 69 with higher scores indicating greater expectations of symptoms.
Medicine‐related beliefs
Participants’ general beliefs about medicines were assessed using the overuse and harm general subscales of the beliefs about medicines questionnaire (BMQ) which has been shown to be both reliable and valid (Horne, Weinman, & Hankins, 1999). This included eight items (four relating to harm and four relating to overuse) which participants’ measured on a 5‐point scale, ranging from 1 ‘strongly disagree’ to 5 ‘strongly agree’. The scores range from 4 to 20 with higher scores indicating greater perceived harm or overuse.
The Modern Health Worries Scale (Petrie et al., 2001) assessed the extent to which people had worries or concerns about different aspects of modern life (e.g., over use of antibiotics, pesticide spraying etc.). Participants rated each item on a 5‐point scale, ranging from 0 ‘no concern’ to 4 ‘extreme concern’. The scores range from 0 to 128 with higher scores indication greater worries about modernity.
The Perceived Sensitivity to Medicines Scale (Horne, Faasse, et al., 2013) assessed the extent to which people felt that they were sensitive to different aspects of medication. The scale includes five items assessing the extent to which people felt that they were sensitive to different aspects of medication rated on a 5‐point scale ranging from 1 ‘strongly disagree’ to 5 ‘strongly agree’. The scores range from 5 to 25 with higher scores indicating greater perceived sensitivity.
Trust in medicine was assessed using three bespoke items created for this study regarding how much participants trusted the current process in which medicines were developed, tested and approved for use, rating each statement on a 5‐point scale from 1 ‘strongly disagree’ to 5 ‘strongly agree’. The scores range from 3 to 15 with higher scores indicating greater trust in medicine. Trust in pharmaceutical companies was also assessed using two bespoke items created for this study which concerned whether participants believed pharmaceutical companies acted in patients’ best interests and if they are only interested in making money (reverse scored). Participants rated each statement on a 5‐point scale from 1 ‘strongly disagree’ to 5 ‘strongly agree’. The scores ranged from 2 to 10 with higher scores indication greater trust in pharmaceutical companies.
Symptom reports
Participants’ symptom reports after taking the tablet were again assessed with our modified version of the GASE (Rief et al., 2009). This time, however, participants rated each symptom on a 4‐point scale ranging from 0 ‘not present’ to 3 ‘severe’ and were asked whether any symptom they experienced was related to taking the tablet –’yes’ or ‘no’.
Guess at tablet identity
Participants were asked to give their best guess at what the tablet was and how confident they were from 1 ‘not at all confident’ to 5 ‘extremely confident’ about their answer.
Procedure
Participants were recruited between 1 December 2015 to 5 December 2016 through adverts on university circular emails and posts on the widely used online classifieds and community website, GumTree. Interested people were emailed an information sheet and a screening questionnaire to complete. Before providing consent, potential participants were told the study would assess the severity of short‐term side effects to a well‐known tablet and that we would not tell them what the tablet was till after data collection for the whole study was finished, in order not to bias their views about the tablet. This procedure minimized the amount of deception required in this study and was used in accordance with the principles of ‘authorized deception’ (Miller, Wendler, & Swartzman, 2005).
The researcher let participants know whether they were eligible and arranged a time for them to participate. On the day, participants booked in at the reception of the clinical research facility and were led to a fully equipped testing room. Before starting, the researcher double‐checked the participants’ screening questionnaire and went through the consent form. After providing consent, participants answered questions about their demographic characteristics, recent symptoms, modern health worries, tendency for somatosensory amplification, belief about medicines, somatization, optimism, perceived sensitivity to medicines, and anxiety. As part of the RCT, participants were randomized to receive one of two leaflets about the tablet. Both contained information about the same potential side effects, but framed positively (e.g., ‘90% of people will not be affected’) or negatively (e.g., ‘one in 10 people will be affected’). See [Link], [Link] for a copy of the leaflets. After reading the leaflet, participants completed questions about their anxiety and expectation of side effects. They then took the tablet with water. The tablet was manufactured by Guy's and St Thomas Pharmacy and contained the inactive ingredients lactose, microcrystalline cellulose, and magnesium stearate. It was round, white, and had a breakline through the centre.
Over the next hour, participants completed a variety of vigilance and cognitive tasks to enhance the appearance of a formal clinical trial – data from these tasks were discarded. Participants then completed questions about their anxiety, symptom experience during the past hour, and trust in medicine and pharmaceutical companies. Trust in medicine and pharmaceutical companies was assessed after participants had taken the tablet to prevent any concerns about taking the tablet that answering these questions could cause. All participants received £40 for taking part via shopping vouchers or bank transfer. After all participants had been tested, participants were emailed a debrief explaining the aims of the study and revealing the tablet was a sham (placebo). A summary of the procedure can be been seen in Figure 1.
Analysis
Our primary outcomes were (1) whether participants attributed one or more symptoms to the sham medicine and (2) the number of symptoms participants attributed to the sham medicine.
To analyse these outcomes, we used a hurdle model (Hu, Pavlicova, & Nunes, 2011; Ridout, Demétrio, & Hinde, 1998) fitted to the dependent variable of the number of symptoms attributed to the tablet. This consists of a joint logistic and truncated negative binomial regression, providing us with information on the odds of participants attributing one or more symptoms to the tablet, but also allowing us to identify the effect of the predictors on the number of symptoms participants go on to attribute to the tablet. This is a more powerful analysis to use as our data contained an excess number of zeros than would be expected by a normal negative binomial regression. A truncated negative binomial regression was favoured to a truncated Poisson regression due to over‐dispersion in the data. Single regressions for each predictor were conducted while controlling for gender, age, ethnicity, employment status, highest level of education, and condition participants were randomized to. These variables were controlled for due to suggestions in the literature that they can influence nocebo responses (Bavbek, Aydin, Sözener, & Yüksel, 2015; Hahn, 1997; Papadopoulos & Mitsikostas, 2012).
As a post hoc analysis, we also carried out a multivariate analysis in which all variables (including all predictor and control variables) were entered at the same time to see whether any of the predictors had an effect on our primary outcomes while controlling for all other variables.
Analyses were carried out using Stata 15 (StataCorp LLC, College Station, TX, USA).
Results
Participant characteristics
The final sample contained 65 men and 138 women, with most of the sample being of White ethnicity (59.6%). The mean age of the sample was 27.15 years. The full baseline characteristics of the sample are shown in Table 1. For a diagram of participant flow through the study, see Figure 2.
Table 1.
Baseline characteristics of the sample
| Variable | Total sample (N = 203) |
|---|---|
| Age | 27.15 (8.63) |
| Number of baseline symptoms | 2.60 (2.70) |
| Severity of baseline symptoms | 2.95 (3.29) |
| Baseline anxiety | 9.60 (2.71) |
| Gender | |
| Female | 138 (68.0) |
| Ethnicity | |
| White | 121 (59.6) |
| Education | |
| Higher education | 132 (65.0) |
| Employment | |
| Not working | 125 (61.6) |
Data are Mean (SD), or n (%).
Figure 2.
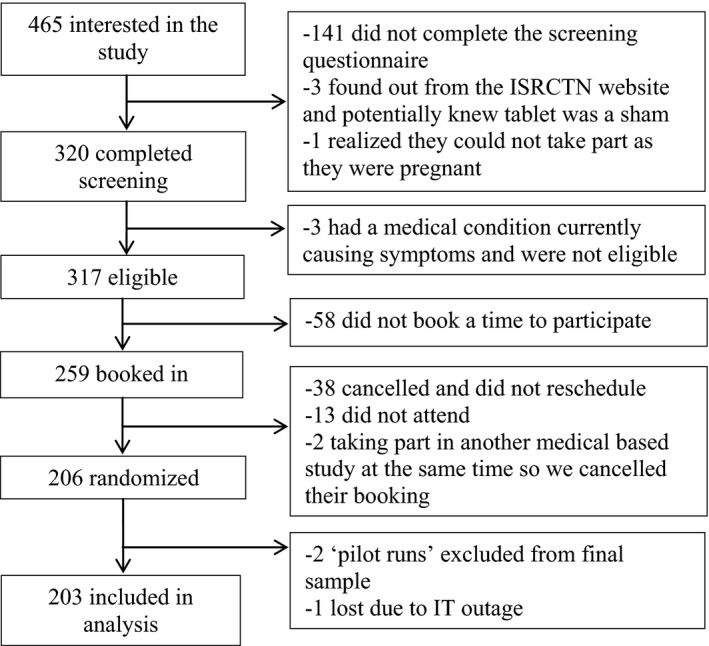
Participant flow through the study.
Symptom attribution
The mean number of symptoms attributed to the tablet was 1.03 (SD = 1.49). Almost half of the participants (n = 95, 46.8%) attributed one or more symptoms to the tablet. Of the 108 who did not attribute symptoms to the tablet, 37 (34.3%) experienced no symptoms at all, and 71 (65.7%) experienced one or more symptoms but did not attribute them to the tablet.
Predictors
Table 2 shows the association between the measures and whether participants attributed one or more symptoms to the tablet, and the number of symptoms they go onto attribute.
Table 2.
Predictors of attributing one or more symptoms to the sham medicine and the number of symptoms which are subsequently attributed
| Variable | No (%) or Mean (SD) | Symptom attribution | Number of attributed symptoms | ||
|---|---|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusted ORa (95% CI) | Unadjusted RR (95% CI) | Adjusted RRa (95% CI) | ||
| Demographics | |||||
| Gender | |||||
| Male | 65 (32.0) | 1.37 (0.94–1.99) | 1.52 (1.02–2.25) | 0.90 (0.71–1.16) | 0.85 (0.66–1.09) |
| Female | 138 (68.0) | Reference | Reference | Reference | Reference |
| Age | 27.15 (8.63) | 0.98 (0.96–1.00) | 0.97 (0.95–0.99) | 1.00 (0.99–1.02) | 1.01 (0.99–1.02) |
| Ethnicity | |||||
| Other | 82 (40.4) | 0.93 (0.66–1.32) | 0.91 (0.63–1.31) | 0.89 (0.70–1.14) | 0.89 (0.69–1.13) |
| White | 121 (59.6) | Reference | Reference | Reference | Reference |
| Employment | |||||
| Working | 78 (38.4) | 0.97 (0.68–1.39) | 1.16 (0.77–1.76) | 1.11 (0.86–1.42) | 1.16 (0.89–1.52) |
| Not working | 125 (61.6) | Reference | Reference | Reference | Reference |
| Education | |||||
| School qualifications | 71 (35.0) | 1.23 (0.85–1.76) | 1.11 (0.76–1.63) | 1.05 (0.83–1.35) | 1.15 (0.88–1.49) |
| University degree | 132 (65.0) | Reference | Reference | Reference | Reference |
| Experimental condition | |||||
| Control leaflet | 101 (49.8) | 1.47 (1.04–2.08) | 1.47 (1.03–2.10) | 0.82 (0.64–1.01) | 0.76 (0.60–0.97) |
| Positively framed leaflet | 102 (50.2) | Reference | Reference | Reference | Reference |
| Psychological factors | |||||
| Anxiety before leaflet | 9.60 (2.71) | 1.01 (0.94–1.07) | 1.01 (0.95–1.08) | 1.03 (0.99–1.08) | 1.03 (0.98–1.08) |
| Anxiety after leaflet | 9.57 (2.51) | 1.04 (0.97–1.12) | 1.06 (0.99–1.14) | 1.06 (1.02–1.11) | 1.06 (1.01–1.11) |
| Anxiety 1 hr after taking tablet | 9.14 (2.62) | 1.04 (0.97–1.11) | 1.05 (0.98–1.12) | 1.03 (0.99–1.08) | 1.03 (0.99–1.08) |
| Change in anxiety | −0.46 (2.34) | 1.04 (0.96–1.12) | 1.04 (0.96–1.12) | 1.00 (0.96–1.05) | 1.01 (0.97–1.05) |
| Optimism | 14.80 (3.68) | 1.02 (0.97–1.07) | 1.02 (0.97–1.07) | 0.99 (0.96–1.03) | 1.00 (0.96–1.03) |
| Somatization | 4.18 (3.25) | 1.04 (0.98–1.09) | 1.05 (0.99–1.11) | 1.03 (0.99–1.07) | 1.02 (0.99–1.07) |
| Somatosensory amplification | 23.52 (6.16) | 1.02 (0.99–1.04) | 1.03 (1.00–1.06) | 0.99 (0.98–1.01) | 0.99 (0.97–1.01) |
| Symptoms | |||||
| Number of symptoms in previous 24 hrs | 2.60 (2.70) | 1.06 (0.99–1.13) | 1.07 (1.00–1.15) | 1.05 (1.01–1.10) | 1.04 (1.00–1.09) |
| Severity of symptoms in previous 24 hrs | 2.95 (3.29) | 1.04 (0.98–1.09) | 1.05 (0.99–1.11) | 1.05 (1.01–1.08) | 1.04 (1.00–1.08) |
| Expected likelihood of symptoms | 5.42 (6.03) | 1.06 (1.03–1.10) | 1.06 (1.02–1.09) | 1.03 (1.01–1.05) | 1.03 (1.01–1.05) |
| Medicine‐related beliefs | |||||
| Modern health worries | 25.82 (20.37) | 1.01 (1.00–1.02) | 1.02 (1.01–1.03) | 1.01 (1.00–1.01) | 1.01 (1.00–1.01) |
| BMQ overuse | 10.91 (3.28) | 1.01 (0.96–1.07) | 1.03 (0.97–1.09) | 1.02 (0.98–1.06) | 1.01 (0.98–1.05) |
| BMQ harm | 7.94 (2.68) | 1.06 (0.99–1.07) | 1.10 (1.02–1.08) | 1.01 (0.97–1.05) | 1.00 (0.95–1.05) |
| Perceived sensitivity to medicines | 7.26 (2.81) | 1.07 (1.00–1.14) | 1.09 (1.02–1.17) | 1.01 0.97–1.04) | 0.99 (0.96–1.03) |
| Trust in medicine development | 11.72 (2.10) | 0.92 (0.85–1.00) | 0.91 (0.83–0.99) | 0.96 (0.91–1.01) | 0.97 (0.92–1.02) |
| Trust in pharmaceutical companies | 5.42 (1.63) | 0.87 (0.78–0.98) | 0.88 (0.79–0.98) | 0.92 (0.85–1.00) | 0.93 (0.86–1.01) |
Bold = significant at p < .05.
OR = odds ratio; RR = rate ratio; BMQ = belief about medicines questionnaire.
Controlling for gender, age, ethnicity, employment, education and experimental condition.
Demographics
Men had a 52% increase in the odds of attributing symptoms to the tablet compared to women. Older participants were less likely to attribute symptoms, with each increase of 1 year in age resulting in a 3% decrease in the odds of participants attributing symptoms to the tablet. There was no association between ethnicity, employment status or education level, and attributing symptoms to the tablet. There was no association between any of the demographic variables measured and the number of symptoms attributed to the tablet.
Psychological factors
There was no effect of anxiety, optimism, somatization, or somatosensory amplification on the odds of symptom attribution. With regard to the number of symptoms attributed, only anxiety scores after reading the PIL showed a significant effect with each increase in anxiety score being associated with a 6% increase in the rate of symptoms attributed to the tablet.
Symptoms
For baseline symptoms, each increase in the number of symptoms in the previous 24 hrs was associated with a 7% increase in the odds of attributing symptoms to the tablet. There was no effect of the severity of baseline symptoms on the odds of symptom attribution. For each increase in participants’ likelihood of expected symptoms, there was a 6% increase in the odds of attributing symptoms to the tablet. Both number and severity of previous symptoms, and expected likelihood of symptoms were associated with the number of symptoms participants go on to attribute to the tablet. Each one‐point increase in these variables was associated with a 4%, 4%, and 3% increase in the rate of symptoms attributed to the tablet, respectively.
Medicine‐related beliefs
Each increase in modern health worries, belief that medicines cause harm and perceived sensitivity to medicine score was associated with a 2%, 10%, and 9% increase in the odds, respectively, of participants attributing symptoms to the tablet. For each increase in participants’ level of trust in medicine development and pharmaceutical companies, there was a 9% and 12% decrease in the odds of participants attributing symptoms to the tablet, respectively. There was no effect of these variables on the number of symptoms participants go on to attribute to the tablet, apart from modern health worries, which was associated with a 1% increase in the rate of symptoms attributed to the tablet.
Guess at tablet identity and sensitivity analysis
Only nine of the 203 participants guessed that the tablet was a placebo, and the mean confidence of participants who guessed this was 2.22 of 5. All other participants identified it as a form of active medication or simply stated that they did not know what it was. Re‐running the primary analysis without the participants who guessed the tablet was a placebo did not change any of the adjusted results, see [Link], [Link] for full results.
Post hoc analyses
Adjusting for all other variables, being male increased odds of symptom attribution, OR = 1.92, 95% CI (1.23–2.99), and each 1‐year increase in age was associated with a decrease in the odds of symptom attribution, OR = 0.97, 95% CI (0.94–0.99): All other predictors for the odds of symptom attribution were non‐significant. For the number of symptoms attributed to the tablet, increases in somatosensory amplification were associated with a decrease in the rate of symptoms attributed to the tablet, RR = 0.97, 95% CI (0.96–0.99), while expected likelihood of symptoms and modern health worries were associated with an increase in the rate of symptoms attributed to the tablet, RR = 1.02, 95% CI (1.00–1.04) and RR = 1.01 95% CI (1.00–1.01). No other predictors were significant.
Discussion
Summary of main results
This study suggests that several factors may predispose people towards experiencing a nocebo effect after taking a new medication. In terms of demographic predictors, men were more likely to attribute symptoms to the tablet than women, even after controlling for all variables measured. This is despite previous findings from a systematic review suggesting no robust evidence for an effect of gender on nocebo responses (Webster et al., 2016). This might have been due to the way that nocebo effects were induced. It may be that men are specifically susceptible to nocebo effects induced through information which alters expectancies, something which has been shown previously by Klosterhalfen et al. (2009). In addition, despite previous evidence showing no effect of age in predicting nocebo effects (Webster et al., 2016), we found older participants had a decreased odds of experiencing nocebo effects than younger participants. It is unclear why this should be so, particularly as the age range was not very large (18–64 years, SD = 8.63). One reason could be due to the fact that in the previous studies reviewed by Webster et al. (2016), the age spread of participants studied was different to the age spread in this study. Interestingly, gender and age did not affect the number of symptoms participants go on to attribute to the tablet, suggesting they play more of a ‘predisposing’ role rather than affecting the intensity of a nocebo response. Other demographic factors such as education or employment status showed no effect, supporting the results from the review (Webster et al., 2016).
In terms of psychological characteristics, there was only an association between anxiety scores after reading the PIL and the number of attributed symptoms, similar to previous findings showing a weak effect of anxiety (Webster et al., 2016). The fact that only anxiety scores after reading the leaflet had an effect on symptom attribution suggests that the effect of anxiety on nocebo responding is situational. Only anxiety immediately after thinking about what symptoms to expect had an effect, as opposed to anxiety at the beginning or end of the study. It is surprising that anxiety levels were only related to the number of symptoms attributed and not the odds of participants attributing symptoms to the tablet. It is possible, however, that in this case, the lack of an association with anxiety and the odds of symptom attribution reflected the generally low levels of state anxiety seen in this sample, which in turn probably reflected the voluntary nature of the experiment. Higher levels of anxiety, and a different pattern of results, might be seen among patients for whom a medication is necessary, rather than voluntary.
More general personality characteristics such as optimism, somatization, and somatosensory amplification did not show any significant associations supporting the inconsistent effects seen for these factors in a recent systematic review (Webster et al., 2016). However, beliefs that directly concerned medication, such as a belief that medicines cause harm, modern health worries, perceived sensitivity to medicines, trust in medicine development, and trust in pharmaceutical companies were all associated with the odds of symptom attribution. Attempts to predict and prevent nocebo responses might therefore be better directed at attitudes related to medicine rather than on more dispositional factors. It is interesting that these factors (apart from modern health worries) did not affect the number of symptoms attributed. This may be because they do not have much weight in affecting the intensity of a nocebo response, or it could be because our sample consisted of healthy individuals who are unlike most people that take medications. This reduces our ability to detect the influence of potential psychological predictors which may show more variation in less healthy samples.
The fact that participants with more symptoms at baseline were more likely to attribute symptoms to the tablet, and then go on to attribute a higher number of symptoms to the tablet adds support to the theory that many nocebo effects could be due to misattribution of coincidental symptoms (Faasse & Petrie, 2013; Petrie et al., 2004). This suggestion is supported by the apparently similar nature of the symptoms reported at baseline and post‐exposure. The more symptoms a participant has at baseline, the more opportunity there is for one of these symptoms to be misattributed as a symptom caused by the tablet. In addition participants with higher expectations of developing side effects from the tablet were more likely to attribute symptoms to the tablet and then attribute a higher number of symptoms to the tablet (even after controlling for all other variables), supporting the role that negative expectations play in generating nocebo effects (Barsky et al., 2002; Hahn, 1997).
Limitations
One limitation inherent in our design was that participants might have engaged in symptom monitoring more than they would have in daily life after taking a tablet. We attempted to reduce this by occupying participants with cognitive tasks after taking the tablet; however, this is still likely to have resulted in raised levels of symptom reporting. On the other hand, it is also likely that those who volunteered for our study were people who were generally trusting of medications or medical science, as shown by the low mean scores in baseline anxiety, perceived sensitivity to medicine, and modern health worries, and high mean scores for trust in medicine development and pharmaceutical companies. Given that these factors may reduce the likelihood of nocebo effects occurring, the rate of symptom attribution in our study could also be an underrepresentation compared to the general public.
It is also possible that some of the symptoms participants experienced could have been due to natural variation, and were not triggered by the sham pill. Future studies should address this limitation by including a control group who do not receive a pill. As trust in medicine and pharmaceutical companies was measured after participants had taken the pill, readers should also be cautious in interpreting these results.
Other limitations concern the representativeness of our participants who were particularly well educated, with 65% having a higher education qualification, and young, with a mean age of 27. In addition, participants were only given a relatively short duration to report any symptoms, it is likely that more symptoms would have been reported if a longer duration was given. The sample size calculation for this study was based on the requirements of our linked RCT, rather than the ability to assess associations between baseline measures and symptom attribution. As such, the results reported here should be interpreted with caution. This is, however, one of the largest studies to date to have examined these effects on symptom attribution (Webster et al., 2016).
Finally, in our study, we chose to minimize the amount of deception required by informing participants that we would not tell them the identity of the tablet until after the study was completed, rather than providing them with a false cover story about the identity of the tablet. This procedure aligns with the principles of authorized deception (Miller et al., 2005) and, as our results demonstrate, is an effective way of triggering the nocebo response. One additional positive feature of this procedure is that our results were not influenced by participants’ idiosyncratic preconceived perceptions about the risks and benefits of any one specific medicine, while symptom expectations were kept uniform across participants by providing them with a PIL containing the essential details about their tablet. In real life, of course, participants usually are aware of the medication they have taken, and the impact of the variables we assessed may not be as clear‐cut.
Implications for clinical practice
Although many predictors only showed small effect sizes, given how common nocebo‐induced side effects can be, any way to reduce them may result in a large impact at a population level. Patients with high expectations of side effects, negative beliefs about medicines, low trust in pharmaceutical companies or the way in which medicines are developed, or high perceived sensitivity to medicines may benefit from reassurance by clinical practitioners or interventions aimed at correcting any unrealistic beliefs or expectations they may have about their medication.
Future research
To allow clinicians to identify patients at risk of a nocebo response, it is important for future research to replicate the investigation of baseline predictors of nocebo responses, preferably in a patient sample to confirm if the factors found in this study are important in predicting the attribution of non‐specific symptoms to a real medication. This would also be useful to shed more light on whether there are different factors that affect someone being a nocebo responder or not in a given situation, or the intensity of a nocebo response. It is also important for future research to try and establish what the role of gender is in nocebo effects. Due to the extensive literature on gender differences in symptom reporting (van Wijk & Kolk, 1997), it has often been assumed that women are more susceptible to nocebo effects than men. However, a previous review found no evidence for this, while this trial showed men to be more susceptible. Further research is needed to decipher this relationship.
Conclusions
The results from this study suggest that it is hard to predict who is at risk of developing nocebo responses to their medicines just from their general demographics or personality. Instead, the results suggest we should be looking at more specific factors relating to their beliefs about the supposed exposure. In addition, we should also take into account the number of symptoms they are experiencing before they are exposed and their side effect expectations. Future work is needed to investigate these factors in a patient sample and to decipher the relationship between gender and nocebo effects.
Conflict of interest
All authors declare no conflict of interests.
Supporting information
Table S1. Re‐running main analyses without participants who guessed the tablet was a placebo.
Data S1. Copy of patient information leaflets.
Acknowledgements
The research was funded by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Emergency Preparedness and Response at King's College London in partnership with Public Health England (PHE), in collaboration with the University of East Anglia and Newcastle University. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, the Department of Health, or PHE.
References
- Ammassari, A. , Murri, R. , Pezzotti, P. , Trotta, M. P. , Ravasio, L. , De Longis, P. , … Antinori, A. (2001). Self‐reported symptoms and medication side effects influence adherence to highly active antiretroviral therapy in persons with HIV infection. Journal of Acquired Immune Deficiency Syndromes, 28, 445–449. https://doi.org/10.1097/00042560-200112150-00006 [DOI] [PubMed] [Google Scholar]
- Andersson Sundell, K. , & Jönsson, A. K. (2016). Beliefs about medicines are strongly associated with medicine‐use patterns among the general population. International Journal of Clinical Practice, 70, 277–285. https://doi.org/10.1111/ijcp.12781 [DOI] [PubMed] [Google Scholar]
- Barsky, A. J. , Goodson, J. D. , Lane, R. S. , & Cleary, P. D. (1988). The amplification of somatic symptoms. Psychosomatic Medicine, 50, 510–519. https://doi.org/10.1097/00006842-198809000-00007 [DOI] [PubMed] [Google Scholar]
- Barsky, A. J. , Saintfort, R. , Rogers, M. P. , & Borus, J. F. (2002). Nonspecific medication side effects and the nocebo phenomenon. JAMA, 287, 622–627. https://doi.org/10.1001/jama.287.5.622 [DOI] [PubMed] [Google Scholar]
- Bavbek, S. , Aydin, Ö. , Sözener, Z. Ç. , & Yüksel, S. (2015). Determinants of nocebo effect during oral drug provocation tests. Allergologia et Immunopathologia, 43, 339–345. https://doi.org/10.1016/j.aller.2014.04.008 [DOI] [PubMed] [Google Scholar]
- Bingel, U. , Wanigasekera, V. , Wiech, K. , Ni Mhuircheartaigh, R. , Lee, M. C. , Ploner, M. , & Tracey, I. (2011). The effect of treatment expectation on drug efficacy: Imaging the analgesic benefit of the opioid remifentanil. Science Translational Medicine, 3(70), 70ra14 https://doi.org/10.1126/scitranslmed.3001244 [DOI] [PubMed] [Google Scholar]
- Collins, R. , Reith, C. , Emberson, J. , Armitage, J. , Baigent, C. , Blackwell, L. , … Peto, R. (2016). Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet, 388, 2532–2561. https://doi.org/10.1016/s0140-6736(16)31357-5 [DOI] [PubMed] [Google Scholar]
- de la Cruz, M. , Hui, D. , Parsons, H. A. , & Bruera, E. (2010). Placebo and nocebo effects in randomized double‐blind clinical trials of agents for the therapy for fatigue in patients with advanced cancer. Cancer, 116, 766–774. https://doi.org/10.1002/cncr.24751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faasse, K. , Grey, A. , Horne, R. , & Petrie, K. J. (2015). High perceived sensitivity to medicines is associated with higher medical care utilisation, increased symptom reporting and greater information‐seeking about medication. Pharmacoepidemiology and Drug Safety, 24, 592–599. https://doi.org/10.1002/pds.3751 [DOI] [PubMed] [Google Scholar]
- Faasse, K. , & Petrie, K. J. (2013). The nocebo effect: Patient expectations and medication side effects. Postgraduate Medical Journal, 89, 540–546. https://doi.org/10.1136/postgradmedj-2012-131730 [DOI] [PubMed] [Google Scholar]
- Figueiras, M. , Marcelino, D. S. , Claudino, A. , Cortes, M. A. , Maroco, J. , & Weinman, J. (2010). Patients’ illness schemata of hypertension: The role of beliefs for the choice of treatment. Psychology & Health, 25, 507–517. https://doi.org/10.1080/08870440802578961 [DOI] [PubMed] [Google Scholar]
- Geers, A. L. , Helfer, S. G. , Kosbab, K. , Weiland, P. E. , & Landry, S. J. (2005). Reconsidering the role of personality in placebo effects: Dispositional optimism, situational expectations, and the placebo response. Journal of Psychosomatic Research, 58, 121–127. https://doi.org/10.1016/j.jpsychores.2004.08.011 [DOI] [PubMed] [Google Scholar]
- Hahn, R. (1997). The nocebo phenomenon: Concept, evidence, and implications for public health. Preventive Medicine, 26, 607–611. https://doi.org/10.1006/pmed.1996.0124 [DOI] [PubMed] [Google Scholar]
- Heller, M. K. , Chapman, S. C. E. , & Horne, R. (2015). Beliefs about medication predict the misattribution of a common symptom as a medication side effect—Evidence from an analogue online study. Journal of Psychosomatic Research, 79, 519–529. https://doi.org/10.1016/j.jpsychores.2015.10.003 [DOI] [PubMed] [Google Scholar]
- Horne, R. , Chapman, S. C. E. , Parham, R. , Freemantle, N. , Forbes, A. , & Cooper, V. (2013). Understanding patients’ adherence‐related beliefs about medicines prescribed for long‐term conditions: A meta‐analytic review of the necessity‐concerns framework. PLoS ONE, 8, e80633 https://doi.org/10.1371/journal.pone.0080633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne, R. , Faasse, K. , Cooper, V. , Diefenbach, M. A. , Leventhal, H. , Leventhal, E. , & Petrie, K. J. (2013). The perceived sensitivity to medicines (PSM) scale: An evaluation of validity and reliability. British Journal of Health Psychology, 18, 18–30. https://doi.org/10.1111/j.2044-8287.2012.02071.x [DOI] [PubMed] [Google Scholar]
- Horne, R. , Weinman, J. , & Hankins, M. (1999). The beliefs about medicines questionnaire: The development and evaluation of a new method for assessing the cognitive representation of medication. Psychology & Health, 14(1), 1–24. https://doi.org/10.1080/08870449908407311 [Google Scholar]
- Horton, R. (2016). Offline: Lessons from the controversy over statins. Lancet, 388, 1040 https://doi.org/10.1016/s0140-6736(16)31583-5 [DOI] [PubMed] [Google Scholar]
- Hu, M.‐C. , Pavlicova, M. , & Nunes, E. V. (2011). Zero‐inflated and hurdle models of count data with extra zeros: Examples from an HIV‐risk reduction intervention trial. The American Journal of Drug and Alcohol Abuse, 37, 367–375. https://doi.org/10.3109/00952990.2011.597280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardas, P. , Lewek, P. , & Matyjaszczyk, M. (2013). Determinants of patient adherence: A review of systematic reviews. Frontiers in Pharmacology, 4, 91 https://doi.org/10.3389/fphar.2013.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, W. P. (1961). The nocebo reaction. Medicina experimentalis. International Journal of Clinical and Experimental Medicine, 95, 203–205. [Google Scholar]
- Kitching, A. , Roche, A. , Balasegaram, S. , Heathcock, R. , & Maguire, H. (2009). Oseltamivir adherence and side effects among children in three London schools affected by influenza A(H1N1)v, May 2009 – an internet‐based cross‐sectional survey. Eurosurveillance, 14, 19287 https://doi.org/10.2807/ese.14.30.19287-en [DOI] [PubMed] [Google Scholar]
- Klosterhalfen, S. , Kellermann, S. , Braun, S. , Kowalski, A. , Schrauth, M. , Zipfel, S. , & Enck, P. (2009). Gender and the nocebo response following conditioning and expectancy. Journal of Psychosomatic Research, 66, 323–328. https://doi.org/10.1016/j.jpsychores.2008.09.019 [DOI] [PubMed] [Google Scholar]
- Kroenke, K. , Spitzer, R. L. , & Williams, J. B. (2002). The PHQ‐15: Validity of a new measure for evaluating the severity of somatic symptoms. Psychosomatic Medicine, 64, 258–266. https://doi.org/10.1097/00006842-200203000-00008 [DOI] [PubMed] [Google Scholar]
- Liccardi, G. , Senna, G. , Russo, M. , Bonadonna, P. , Crivellaro, M. , Dama, A. , … Passalacqua, G. (2004). Evaluation of the nocebo effect during oral challenge in patients with adverse drug reactions. Journal of Investigational Allergology and Clinical Immunology, 14, 104–107. [PubMed] [Google Scholar]
- Mahr, A. , Golmard, C. , Pham, E. , Iordache, L. , Deville, L. , & Faure, P. (2017). Types, frequencies, and burden of nonspecific adverse events of drugs: Analysis of randomized placebo‐controlled clinical trials. Pharmacoepidemiology and Drug Safety, 26, 731–741. https://doi.org/10.1002/pds.4169 [DOI] [PubMed] [Google Scholar]
- Marteau, M. , & Bekker, H. (1992). The development of a six‐item short‐form of the state scale of the Spielberger State‐Trait Anxiety Inventory (STAI). British Journal of Clinical Psychology, 32, 301–306. https://doi.org/10.1111/j.2044-8260.1992.tb00997.x [DOI] [PubMed] [Google Scholar]
- Menckeberg, T. T. , Bouvy, M. L. , Bracke, M. , Kaptein, A. A. , Leufkens, H. G. , Raaijmakers, J. A. M. , & Horne, R. (2008). Beliefs about medicines predict refill adherence to inhaled corticosteroids. Journal of Psychosomatic Research, 64(1), 47–54. https://doi.org/10.1016/j.jpsychores.2007.07.016 [DOI] [PubMed] [Google Scholar]
- Miller, F. G. , Wendler, D. , & Swartzman, L. C. (2005). Deception in research on the placebo effect. PLoS Medicine, 2, e262 https://doi.org/10.1371/journal.pmed.0020262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute For Health and Clinical Excellence . (2009). Medicines adherence: Involving patients in decisions about prescribed medicines and supporting adherence. Retrieved from http://www.nice.org.uk/guidance/cg76/resources/guidance-medicines-adherence-pdf [PubMed]
- Nestoriuc, Y. , Orav, E. J. , Liang, M. H. , Horne, R. , & Barsky, A. J. (2010). Prediction of nonspecific side effects in rheumatoid arthritis patients by beliefs about medicines. Arthritis Care & Research, 62, 791–799. https://doi.org/10.1002/acr.20160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestoriuc, Y. , von Blanckenburg, P. , Schuricht, F. , Barsky, A. J. , Hadji, P. , Albert, U. S. , & Rief, W. (2016). Is it best to expect the worst? Influence of patients’ side‐effect expectations on endocrine treatment outcome in a 2‐year prospective clinical cohort study. Annals of Oncology, 27, 1909–1915. https://doi.org/10.1093/annonc/mdw266 [DOI] [PubMed] [Google Scholar]
- Nevelsteen, S. , Legros, J. J. , & Crasson, M. (2007). Effects of information and 50 Hz magnetic fields on cognitive performance and reported symptoms. Bioelectromagnetics, 28(1), 53–63. https://doi.org/10.1002/(ISSN)1521-186X [DOI] [PubMed] [Google Scholar]
- Papadopoulos, D. , & Mitsikostas, D. D. (2012). A meta‐analytic approach to estimating nocebo effects in neuropathic pain trials. Journal of Neurology, 259, 436–447. https://doi.org/10.1007/s00415-011-6197-4 [DOI] [PubMed] [Google Scholar]
- Petrie, K. J. , Broadbent, E. , Kley, N. , Moss‐Morris, R. , Horne, R. , & Rief, W. (2005). Worries about modernity predict symptom complaints after environmental pesticide spraying. Psychosomatic Medicine, 67, 778–782. https://doi.org/10.1097/01.psy.0000181277.48575.a4 [DOI] [PubMed] [Google Scholar]
- Petrie, K. J. , Moss‐Morris, R. , Grey, C. , & Shaw, M. (2004). The relationship of negative affect and perceived sensitivity to symptom reporting following vaccination. British Journal of Health Psychology, 9, 101–111. https://doi.org/10.1348/135910704322778759 [DOI] [PubMed] [Google Scholar]
- Petrie, K. J. , Sivertsen, B. , Hysing, M. , Broadbent, E. , Moss‐Morris, R. , Eriksen, H. R. , & Ursin, H. (2001). Thoroughly modern worries – The relationship of worries about modernity to reported symptoms, health and medical care utilization. Journal of Psychosomatic Research, 51(1), 395–401. https://doi.org/10.1016/S0022-3999(01)00219-7 [DOI] [PubMed] [Google Scholar]
- Phatak, H. , & Thomas, J. (2006). Relationships between beliefs about medications and nonadherence to prescribed chronic medications. Annals of Pharmacotherapy, 40, 1737–1742. https://doi.org/10.1345/aph.1h153 [DOI] [PubMed] [Google Scholar]
- Ridout, M. , Demétrio, C. G. , & Hinde, J. (1998). Models for count data with many zeros. Paper presented at the Proceedings of the XIXth international biometric conference, Cape Town.
- Rief, W. , Glaesmer, H. , Baehr, V. , Broadbent, E. , Braehler, E. , & Petrie, K. J. (2012). The relationship of modern health worries to depression, symptom reporting and quality of life in a general population survey. Journal of Psychosomatic Research, 72, 318–320. https://doi.org/10.1016/j.jpsychores.2011.11.017 [DOI] [PubMed] [Google Scholar]
- Rief, W. , Glombiewski, J. A. , & Barsky, A. J. (2009). Generic assessment of side effects. Retrieved from http://www.gase-scale.com/
- Rubin, G. J. , & Dickmann, P. (2010). How to reduce the impact of “low‐risk patients” following a bioterrorist incident: Lessons from SARS, anthrax, and pneumonic plague. Biosecurity and Bioterrorism, 8(1), 37–43. https://doi.org/10.1089/bsp.2009.0059 [DOI] [PubMed] [Google Scholar]
- Saxon, D. R. , & Eckel, R. H. (2016). Statin intolerance: A literature review and management strategies. Progress in Cardiovascular Diseases, 59, 153–164. https://doi.org/10.1016/j.pcad.2016.07.009 [DOI] [PubMed] [Google Scholar]
- Scheier, M. F. , Carver, C. S. , & Bridges, M. W. (1994). Distinguishing optimism from neuroticism (and trait anxiety, self‐mastery, and self‐esteem: A re‐evaluation of the Life Orientation Test. Journal of Personality and Social Psychology, 67, 1063–1078. https://doi.org/10.1037/0022-3514.67.6.1063 [DOI] [PubMed] [Google Scholar]
- Stein, B. D. , Tanielian, T. L. , Ryan, G. W. , Rhodes, H. J. , Young, S. D. , & Blanchard, J. C. (2004). A bitter pill to swallow: Nonadherence with prophylactic antibiotics during the anthrax attacks and the role of private physicians. Biosecurity and Bioterrorism, 2, 175–185. https://doi.org/10.1089/bsp.2004.2.175 [DOI] [PubMed] [Google Scholar]
- Szemerszky, R. , Koteles, F. , Lihi, R. , & Bardos, G. (2010). Polluted places or polluted minds? An experimental sham‐exposure study on background psychological factors of symptom formation in ‘Idiophatic Environmental Intolerance attributed to electromagnetic fields’. International Journal of Hygiene and Environmental Health, 213, 387–394. https://doi.org/10.1016/j.ijheh.2010.05.001 [DOI] [PubMed] [Google Scholar]
- Tobert, J. A. , & Newman, C. B. (2016). The nocebo effect in the context of statin intolerance. Journal of Clinical Lipidology, 10, 739–747. https://doi.org/10.1016/j.jacl.2016.05.002 [DOI] [PubMed] [Google Scholar]
- Van den Bergh, O. , Kempynck, P. J. , van de Woestijne, K. P. , Baeyens, F. , & Eelen, P. (1995). Respiratory learning and somatic complaints: A conditioning approach using CO2‐enriched air inhalation. Behaviour Research and Therapy, 33, 517–527. https://doi.org/10.1016/0005-7967(94)00080-4 [DOI] [PubMed] [Google Scholar]
- van Wijk, C. M. T. G. , & Kolk, A. M. (1997). Sex differences in physical symptoms: The contribution of symptom perception theory. Social Science & Medicine, 45, 231–246. https://doi.org/10.1016/s0277-9536(96)00340-1 [DOI] [PubMed] [Google Scholar]
- Vogtle, E. , Barke, A. , & Kroner‐Herwig, B. (2013). Nocebo hyperalgesia induced by social observational learning. Pain, 154, 1427–1433. https://doi.org/10.1016/j.pain.2013.04.041 [DOI] [PubMed] [Google Scholar]
- Webster, R. K. , Weinman, J. , & Rubin, G. J. (2016). A systematic review of factors that contribute to nocebo effects. Health Psychology, 35, 1334–1355. https://doi.org/10.1037/hea0000416 [DOI] [PubMed] [Google Scholar]
- Webster, R. K. , Weinman, J. , & Rubin, G. J. (2018). Positively framed risk information in patient information leaflets reduces side effect reporting: A double‐blind randomized controlled trial. Annals of Behavioral Medicine, kax064. https://doi.org/10.1093/abm/kax064 [DOI] [PubMed] [Google Scholar]
- Wells, R. E. , & Kaptchuk, T. J. (2012). To tell the truth, the whole truth, may do patients harm: The problem of the nocebo effect for informed consent. American Journal of Bioethics, 12(3), 22–29. https://doi.org/10.1080/15265161.2011.652798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witthöft, M. , & Rubin, G. J. (2013). Are media warnings about the adverse health effects of modern life self‐fulfilling? An experimental study on idiopathic environmental intolerance attributed to electromagnetic fields (IEI‐EMF). Journal of Psychosomatic Research, 74, 206–212. https://doi.org/10.1016/j.jpsychores.2012.12.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Re‐running main analyses without participants who guessed the tablet was a placebo.
Data S1. Copy of patient information leaflets.


