Abstract
Cyclotron‐produced carbon‐11 is a highly valuable radionuclide for the production of positron emission tomography (PET) radiotracers. It is typically produced as relatively unreactive carbon‐11 carbon dioxide ([11C]CO2), which is most commonly converted into a more reactive precursor for synthesis of PET radiotracers.
The development of [11C]CO2 fixation methods has more recently enabled the direct radiolabelling of a diverse array of structures directly from [11C]CO2, and the advantages afforded by the use of a loop‐based system used in 11C‐methylation and 11C‐carboxylation reactions inspired us to apply the [11C]CO2 fixation “in‐loop.” In this work, we developed and investigated a new ethylene tetrafluoroethylene (ETFE) loop‐based [11C]CO2 fixation method, enabling the fast and efficient, direct‐from‐cyclotron, in‐loop trapping of [11C]CO2 using mixed DBU/amine solutions. An optimised protocol was integrated into a proof‐of‐concept in‐loop flow radiosynthesis of N,N′‐[11C]dibenzylurea. This reaction exhibited an average 78% trapping efficiency and a crude radiochemical purity of 83% (determined by radio‐HPLC), giving an overall nonisolated radiochemical yield of 72% (decay‐corrected) within just 3 minutes from end of bombardment.
This proof‐of‐concept reaction has demonstrated that efficient [11C]CO2 fixation can be achieved in a low‐volume (150 μL) ETFE loop and that this can be easily integrated into a rapid in‐loop flow radiosynthesis of carbon‐11–labelled products.
This new in‐loop methodology will allow fast radiolabelling reactions to be performed using cheap/disposable ETFE tubing setup (ideal for good manufacturing practice production) thereby contributing to the widespread usage of [11C]CO2 trapping/fixation reactions for the production of PET radiotracers.
1. INTRODUCTION
One of the most prevalent and important radionuclides used in positron emission tomography (PET) is carbon‐11 (11C, t1/2 = 20.4 min); it has been incorporated into a wide variety of exogenous and endogenous ligands, used for both diagnostic and research purposes.1, 2, 3, 4, 5 The short half‐life potentially allows the administration and scanning of multiple PET radiotracers in the same patient on the same day, thereby combining the advantages of imaging multiple biochemical pathways; in addition, it enables the labelling of biologically relevant molecules, without changing their pharmacodynamic and pharmacokinetic properties, by virtue of its isotopology with carbon‐12.
Carbon‐11 is produced as [11C]CO2 (primary precursor), by the cyclotron proton‐bombardment of nitrogen‐14 via the 14N(p,α)11C nuclear reaction. The relatively low reactivity and solubility of [11C]CO2 leads to its rapid conversion to more reactive secondary precursors.1 The most prevalent of these secondary precursors is [11C]CH3I for 11C‐methylation reactions.1 While these reactions are used to produce the vast majority of carbon‐11 radiotracers, in the time taken to convert [11C]CO2 to [11C]CH3I, significant amounts of the starting radioactivity can be lost through multistep synthesis and radioactive decay.6 In addition, 11C‐methylation can somewhat limit the chemical space available for radiolabelling. As such, there have been a variety of alternative secondary and tertiary 11C‐precursors developed to convert the poorly reactive [11C]CO2 into a more versatile toolbox for the 11C‐radiochemist: [11C]CO, [11C]HCN, [11C]CS2, [11C]CH3OTf, and [11C]COCl2 are just a few examples.1
In an effort to avoid this time‐consuming conversion to more reactive precursors, [11C]CO2 has been reacted directly with Grignard reagents (alkyl magnesium bromide) to synthesise 11C‐carboxylic acid derivatives such as [11C]acetate, [11C]palmitic acid, and 2‐[11C]octynoic acid.7, 8, 9 In an effort to easily automate these syntheses, these reactions have been performed “in‐loop” via a captive solvent system; whereby the walls of a small tubing loop are coated with the highly reactive Grignard reagents, then [11C]CO2 is flowed through the loop, where it reacts in‐loop to form the 11C‐labelled carboxylic acid product.10, 11 This in‐loop synthetic approach has also been used in 11C‐methylation reactions using gaseous [11C]CH3I, with the synthesis of [11C]raclopride as a notable example.12, 13, 14, 15, 16 This synthetic methodology lends itself particularly well to the specific demands of 11C‐radiochemistry, and it has a number of advantages over the standard vial‐based methods. For these mixed gas‐liquid phase reactions, the high surface area provided by the walls of the loop maximises gas‐liquid contact, increasing the efficiency and thus decreasing the reaction times required versus the standard vial‐based setup. In addition, since loop synthesis eliminates reaction vials, transfer losses are kept to a minimum; and since this, in effect, miniaturises these reactions, precursor quantities can be greatly reduced. Finally, these reactions are easily automated, which is advantageous when considering the translational potential of a tracer.12, 13
More recently, there has been a resurgence of interest into the direct use of [11C]CO2 in radiolabelling using a technique called [11C]CO2 fixation.6, 17 This new chemistry is a by‐product of the “green‐chemistry” movement and the corresponding interest in new chemical agents for carbon‐capture;6, 17, 18, 19 the most commonly used compounds being the amidine base 1,8‐diazabicyclo[5.4.0]undec‐7‐ene (DBU),20 and the phosphazene base, 2‐tert‐butylimino‐2‐diethylamino‐1,3‐dimethylperhydro‐1,3,2‐diazaphosphorine (BEMP).21 While there is still some uncertainty regarding their trapping/fixation mechanisms,22, 23 solutions of these bases with an amine substrate exhibit efficient [11C]CO2 trapping and transfer to form amine carbamate intermediates.20 This [11C]CO2 fixation has therefore enabled many new 11C‐carbonylation reactions starting from [11C]CO2, and many new syntheses have been developed based on this fixation strategy (11C‐carbamates, 11C‐ureas, 11C‐amides, and 11C‐oxazolidinones, among others).20, 24, 25, 26, 27, 28, 29 Within our group, we have developed a Mitsunobu‐based method for the synthesis of 11C‐ureas.26, 27 This reaction involves the fixation of [11C]CO2 by DBU and amines to form the 11C‐amine carbamate intermediates; Mitsunobu reagents (PBu3/DBAD) are added to form an 11C‐isocyanate intermediate, which reacts with another amine molecule to form an 11C‐labelled urea product, in just 5‐minute total reaction time.26, 27
The recent interest in [11C]CO2 fixation chemistry has the potential to revolutionise the field of carbon‐11 radiochemistry, and the advantages afforded by the use of a loop‐based system used in 11C‐methylation and 11C‐carboxylation reactions inspired us to apply the [11C]CO2 fixation in‐loop.
In this work, our aim was therefore to (1) establish a novel in‐loop trapping/fixation of [11C]CO2 for the investigation and optimisation of different trapping solutions and (2) implement the setup in an in‐loop flow‐radiosynthesis of 11C‐labelled urea functional groups from [11C]CO2.
2. RESULTS AND DISCUSSION
2.1. Three‐component [11C]CO2 loop trapping
To assess the potential for in‐loop trapping/fixation of [11C]CO2, we designed a novel prototype 3‐component loop trapping apparatus (Figure 1). In designing the system, good manufacturing practice (GMP) requirements led us to opt for a single‐use disposable ethylene tetrafluoroethylene (ETFE)–based loop system since it obviates the need for rigorous cleaning etc. The system consists of 3 easily separable components: a trapping loop (1/16″ O.D. ETFE tubing, 150‐μL volume), the walls of which will be coated with a trapping solution; a crimped glass waste vial; and a trapping cartridge able to fix all unreacted [11C]CO2 (ascarite trap, Figure 1). On passing [11C]CO2 through this system, the [11C]CO2 is totally trapped within these 3 components. The components are then simply separated, and the radioactivity levels of the loop, waste vial, and ascarite trap (Rloop, Rwaste, and Rtrap, respectively) are measured. Comparison of these values allows the calculation of trapping efficiencies for a given solution.
Figure 1.
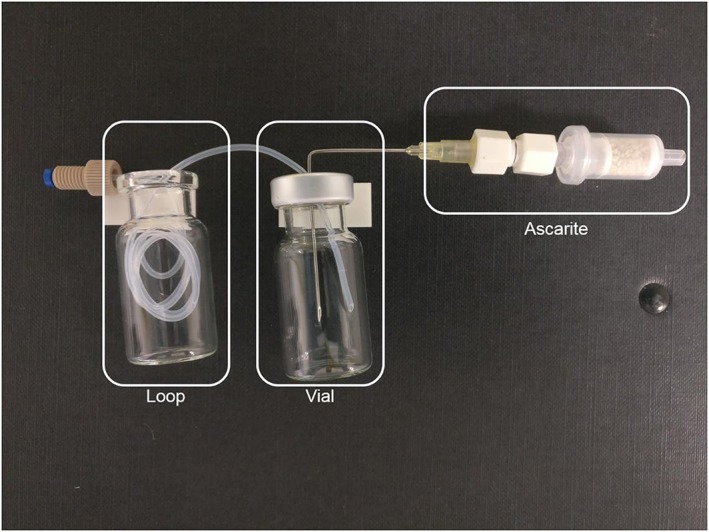
Representative 3‐component [11C]CO2 trapping: trapping loop, vial, and [11C]CO2 trapping cartridge (ascarite)
To precoat the walls of the loop, it is half filled with 75‐μL trapping solution, then it is connected to the cyclotron delivery line and flushed with helium gas through to the waste vial. The waste vial contains the trapping solution that was not retained by the loop. Therefore, when [11C]CO2 is passed through the 3‐component system, it is trapped within the loop and any untrapped [11C]CO2 will be fixed in the solution within the waste vial and the trapping cartridge.
To assess a solution's overall trapping efficiency as well as its suitability for our applications, we extracted 2 values from the acquired data: These are termed total‐solution trapping (Tsol) and loop‐trapping (Tloop).
Tsol (Tsol = (Rloop + Rwaste)/(Rloop + Rwaste + Rtrap)) gives an insight into a solution's ability to trap [11C]CO2 in bulk and is a proxy measure for the chemical trapping efficiency of a given solution. While a low Tsol indicates that a solvent mixture is unsuitable for application within our in‐loop [11C]CO2 trapping/fixation method, a high Tsol does not conversely guarantee success for our work. Since a highly efficient trapping solution may still have poor loop‐retention, the degree to which the trapping solution is retained on the walls of the loop, and thus, [11C]CO2 trapping/fixation will not occur in‐loop but in the waste vial.
Instead Tloop (Tloop = Rloop/(Rloop + Rwaste + Rtrap)) accounts both for the chemical trapping ability of a solution, but also the degree to which it is retained within our 150‐μL ETFE loop. High values of Tsol and Tloop should guarantee success in developing an in‐loop [11C]CO2 fixation methodology.
Our model trapping solutions contained varying concentrations of benzylamine and DBU dissolved in acetonitrile (MeCN). The DBU was chosen instead of BEMP, since in previous work, using these Mitsunobu reagents, both compounds gave good [11C]CO2 trapping but only DBU led to 11C‐urea formation.27
To ensure MeCN does not exhibit any [11C]CO2 trapping itself, pure MeCN trapping experiments were attempted and – as expected considering the low solubility of [11C]CO2 in MeCN – negligible trapping was seen for this experiment (Tsol = 6.5 ± 0.1%, Tloop = 0.2%, n = 2).
Firstly, we explored the effect of varying benzylamine concentration (1%, 5%, and 10%) in the presence of a fixed content of DBU (10% v/v) (Figure 2). A solution of 10% DBU in MeCN without adding benzylamine (Figure 2) showed good Tsol (62.7 ± 2.3%), but very low Tloop (2.1 ± 0.1%). While the solution can trap [11C]CO2 reasonably well, it is poorly retained in the loop. Any solutions to be used within this loop trapping/fixation methodology must therefore exhibit high Tloop. Increasing benzylamine content up to 10%, both Tsol and Tloop increased. Notably, adding 1%, 5%, and 10% benzylamine, Tsol showed near‐quantitative trapping of total [11C]CO2 (93.8 ± 2.5%, 97.6 ± 0.8%, and 97.5 ± 0.4%, respectively). However, despite the comparable Tsol seen for these solutions, there was a marked difference in Tloop values (Figure 2). Using 1% and 5% benzylamine content showed a two‐fold increase of Tloop (10.9 ± 4.4% and 24.4 ± 8.0%, respectively); however, increasing further the content of benzylamine up to 10% Tloop did not significantly improve (27.0 ± 3.2%).
Figure 2.
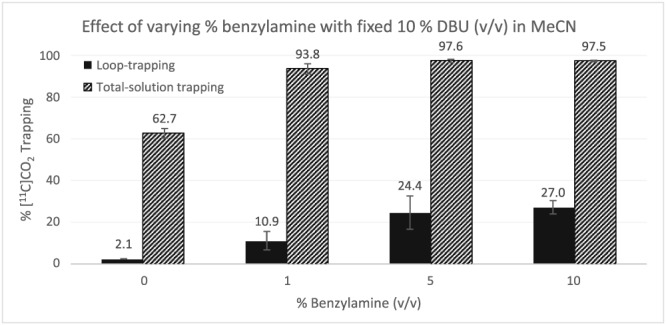
Effect of varying % benzylamine with fixed 10% DBU (v/v) in MeCN
These results show that—chemically—these mixed benzylamine/DBU solutions are exquisitely fine [11C]CO2 trapping/fixation solutions, even at relatively low concentrations of benzylamine (1%, Tsol > 90%). However, for their usage in loop trapping/fixation, only the solutions with high Tloop values are suitable (10% benzylamine, Tsol = 97%, Tloop = 27%). We suspected that the increased benzylamine content of the solutions with the highest Tloop values resulted in the increased viscosity of these solutions, replacing less‐viscous MeCN30 (0.343 mPa.s) with more viscous benzylamine31 (1.492 mPa.s), which therefore gave an increased retention of the solution on the walls of the loop.
Since we observed no significant improvement of Tloop using 5% or 10% of benzylamine, we decided to fix the content of benzylamine to 10% in the next experiments. We then explored the effect of varying DBU concentration in our solutions (Figure 3). In MeCN, 10% benzylamine (no DBU added) showed a reasonable Tsol (44.1 ± 15.4%), but very low Tloop (1.6 ± 1.1%). These results are very similar to those seen for a solution of 10% DBU in MeCN (Figure 2); indeed, while both solutions (10% DBU in MeCN or 10% benzylamine in MeCN) can trap [11C]CO2 fairly well, their poor loop‐retention hampers the Tloop. Adding DBU at 10%, 50%, and 90% led to near‐quantitative Tsol (97.5 ± 0.4%, 96.5 ± 1.8%, and 89.9 ± 4.0%, respectively), again demonstrating that these are highly powerful [11C]CO2 trapping/fixation solutions (Figure 3). However Tloop increased by adding 10%, 50%, and 90% DBU (27.0 ± 3.2%, 26.8 ± 15.6%, and 41.8 ± 7.1, respectively). The highest Tloop value (42%) for this 3‐component trapping setup was obtained using an MeCN‐free system containing 90% DBU and 10% benzylamine (v/v) mixture. This again supports the suggestion that solution viscosity dictates loop‐retention and therefore affects Tloop, since we are increasing the content of more viscous DBU32 (11.76 mPa.s) and decreasing the content of less viscous MeCN31 (0.343 mPa.s).
Figure 3.
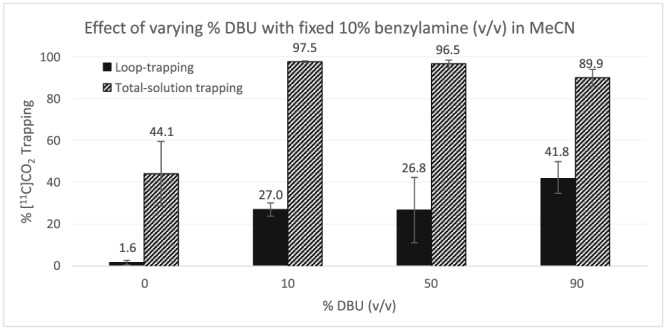
Effect of varying % DBU with fixed 10% benzylamine (v/v) in MeCN
This section of work demonstrated the successful trapping and fixation of [11C]CO2 using a mixture of benzylamine/DBU solutions coating the walls of a 150‐μL ETFE loop. The modular 3‐component experimental setup was easy to set up as well as to disconnect for radioactivity measurements and so allows the rapid screening of a number of different trapping solutions. We next wanted to explore the potential integration of this in‐loop [11C]CO2 trapping into more complex flow syntheses of 11C‐labelled compounds. To investigate this, we performed a proof‐of‐concept flow synthesis of N,N′‐[11C]dibenzylurea using a more complex setup and using the optimal trapping solution developed above: 90% DBU and 10% benzylamine (v/v) mixture.
2.2. In‐loop flow radiosynthesis of N,N′‐[11C]dibenzylurea
The apparatus setup for the flow‐synthesis section of this work was based on the simple trapping apparatus described above. The [11C]CO2 trapping/fixation in‐loop was initially performed in a similar manner, before passing a solution of Mitsunobu reagents (PBu3 and DBAD in MeCN) through the trapping loop, through a second 150‐μL reaction loop (to ensure adequate mixing), and into a product vial (Figure 4). The [11C]CO2 is trapped initially as an N‐[11C]benzyl carbamate intermediate, which is converted by the Mitsunobu reagents to a highly reactive 11C‐isocyanate intermediate. This then undergoes attack from another molecule of benzylamine, to form the desired N,N′‐[11C]dibenzylurea product (Figure 4).
Figure 4.
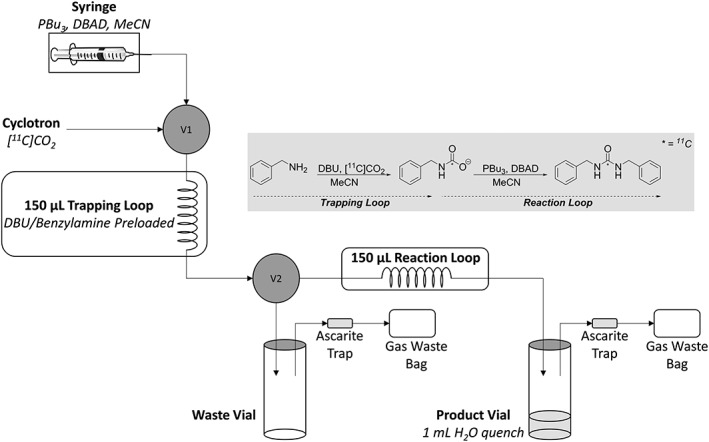
Schematic of in‐loop flow radiosynthetic setup showing the [11C]CO2 trapping loop as well as the additional reaction loop to allow Mitsunobu reaction to form N,N′‐[11C]dibenzylurea
The setup (Figure 4) includes 2 switching valves (V1 and V2) on an E&Z Modular Lab automated synthesis system. The trapping loop is connected to these 2 valves, the inlet is connected to V1, and the outlet is connected to V2. V1 can be switched to either the cyclotron outlet (for helium flush and [11C]CO2 delivery) or a syringe containing the Mitsunobu reagents. V2 can divert the flow either towards the waste vial or through the reaction loop and into the product vial (see Section 4.1 for step‐by‐step procedure). To ascertain the quantity of [11C]CO2 delivered through the system per experiment, the system was washed with MeCN and the radioactivity of all the washings and components (lines, fittings, and needles) were measured.
An additional feature of this setup is the 1‐mL H2O added as a quenching solution in the final product vial. This ensures that any 11C‐urea derivatives detected in the crude HPLC are products formed exclusively in the loop. Without this simple addition, the reaction could feasibly be simply occurring in the product vial that receives the reaction mixture, during the time taken to measure product radioactivity and prepare the sample for HPLC analysis. The active Mitsunobu intermediate formed is a Morrison‐Brunn‐Huisgen betaine, which will react with protic substrates: alcohols, amines, and carboxylic acids.33, 34, 35, 36, 37 Our own non‐radioactive experiments have confirmed that this betaine reacts with water forming the tri‐n‐butylphosphine oxide (PBu3O) and di‐tert‐butyl hydrazodiformate (DBAD‐H2). Therefore, in the 11C‐urea synthesis, any remaining unreacted urea‐forming betaine will react with the excess water (in the product vial) upon leaving the reaction loop, ensuring that the crude‐HPLC is representative of the 11C‐labelled species formed in‐loop, not in‐vial. In addition, this serves to predilute the crude product ready for HPLC injection, which helps to streamline the synthetic process.
One notable observation during these 11C‐urea syntheses was that we saw a significantly increased Tloop (78.3 ± 3.6%) compared to that seen within the trapping experiments (41.8 ± 7.1%), indicating an increase in loop‐retention of our optimised trapping solution. This variance in Tloop value might be due to the difference between the 2 setups; in the flow synthesis of N,N′‐[11C]dibenzylurea, the loops are routed via the E&Z switching valves (V1 and V2). We speculate that the use of these valves increases the backpressure in the system and correspondingly increases the loop retention of the trapping solution.
Because of the demanding time‐constraints placed upon carbon‐11 radiochemistry in the development of this method, we attempted to minimise all process times, avoiding any product losses because of radioactive decay. Since we avoided pretrapping or concentration of [11C]CO2 (instead delivered diluted in the helium carrier gas), and the rate of delivery was not slowed from the cyclotron's 70 mL/min, the [11C]CO2 is delivered through the system and trapped in the loop within 105 seconds of the end of bombardment (EOB). V1 and V2 are instantly switched, and the trapping loop is filled with Mitsunobu reagents within 30 seconds (Figure 4). V1 is then switched back to a helium flush, and the reagents are pushed through the reaction loop and into the product vial at 70 mL/min. Therefore, the process is complete within just 3 minutes from the EOB and 1 minute from the end of delivery.
In the synthesis of our model substrate, N,N′‐[11C]dibenzylurea, we had to consider the concentration and stoichiometry of our Mitsunobu reagents added to the 150‐μL trapping loop. Based on the assumption of 7‐ to 8‐μL trapping solution retention (based on preliminary non‐radioactive flushing experiments filling the loop with 75 μL of solution) and considering the optimal conditions found in our previous radiosynthesis of 11C‐symmetrical ureas,27 the reagent concentration was selected to ensure a 2:1 stoichiometric ratio of Mitsunobu reagents (PBu3/DBAD) to benzylamine.
Using the in‐loop flow radiosynthesis setup, the radio‐HPLC of the crude solution showed a radiochemical purity of 82.6 ± 3.3%. This coupled with the overall Tloop of 78.3 ± 3.6% led to a decay‐corrected nonisolated radiochemical yield (RCY) of 72.3 ± 5.1% (n = 3) for the synthesis of N,N′‐[11C]dibenzylurea, in 3 minutes from EOB, with a molar radioactivity of 0.72 ± 0.17 GBq/μmol (for 300‐350 MBq initial [11C]CO2; other work in the group has demonstrated that this molar radioactivity would be expected to increase towards 60 to 70 GBq/μmol for an initial 30 GBq [11C]CO2 production,28 which is consistent with the molar radioactivities obtained for other clinical 11C‐labelled radiotracers within our institution).38 This therefore presents a reproducible, rapid, and high‐yielding synthesis of a carbon‐11–labelled urea product directly from [11C]CO2.
The RCYs for the synthesis of N,N′‐[11C]dibenzylurea using this in‐loop method (72% decay‐corrected) are slightly lower than those for the traditional in‐vial method (82% decay‐corrected), because of a lower [11C]CO2 trapping efficiency (78% vs 96%), but with a comparable radiochemical purity (83% vs 85%, by crude radio‐HPLC).27 However, this in‐loop method uses smaller quantities of reagents compared to the in‐vial method (75 μL vs 400 μL), which should simplify purification, and will minimise most of the transfer losses associated with in‐vial synthesis, and the use of cheap, disposable, ETFE loops means that this method is particularly well suited to GMP production. These factors combined therefore mean that this in‐loop method presents an appealing alternative to in‐vial [11C]CO2 fixation reactions.
3. CONCLUSIONS
In this work, we demonstrated that cyclotron‐produced [11C]CO2 can be fixed in a low‐volume (150 μL) ETFE loop. We showed that while amine/DBU solutions are chemically efficient [11C]CO2 fixation agents in‐vial, both the chemical and physical properties (primarily viscosity) of these solutions determine their degree of loop trapping efficiency (Tloop). This optimised direct‐from‐cyclotron [11C]CO2 fixation methodology avoids the need for cryogenic preconcentration commonly used in carbon‐11 procedures. This setup was implemented in a proof‐of‐concept in‐loop flow radiosynthesis of N,N′‐[11C]dibenzylurea by passing Mitsunobu reagents through the loop containing a trapped N‐[11C]benzyl carbamate intermediate and benzylamine. N,N′‐[11C]dibenzylurea was obtained with high nonisolated RCYs (approximately 72%), comparable to those previously reported in‐vial (approximately 82%), within 3 minutes from EOB (recently presented in abstract form at the International Symposium on Radiopharmaceutical Sciences, ISRS, Dresden, 14‐19 May 2017).39 This novel methodology has demonstrated the potential for direct‐from‐cyclotron in‐loop [11C]CO2 fixation and has demonstrated that this can be used as part of a more complex synthesis (eg, amides and carbamates).28 The speed of the reaction (3 min from EOB) and the cheap/disposable ETFE tubing setup (ideal for GMP production) mean that this method should be suitable for further applications in the direct trapping/fixation reactions of [11C]CO2. We anticipate that this new methodology will facilitate a more widespread uptake and application of these powerful reactions, for the radiolabelling of a diverse array of structures directly from [11C]CO2.
4. EXPERIMENTAL
4.1. Materials and general methods
Anhydrous acetonitrile (MeCN, 99.8%), ascarite, benzylamine (99%), di‐tert‐butyl‐azodicarboxylate (DBAD, 98%), triethylamine (Et3N, ≥99.5%), and tri‐n‐butyl phosphine (PBu3, 99%) were purchased from Sigma‐Aldrich. Ethyl acetate (EtOAc, ≥99.5%) was purchased from Fisher Scientific. Anhydrous magnesium sulphate (MgSO4, 98%) was purchased from Fluka. 1,8‐Diazabicyclo[5.4.0]undec‐7‐ene (DBU, 99%) and benzyl isocyanate (98%) were purchased from Alfa Aesar. Carbon dioxide (CO2) was purchased from BOC Gases.
The ETFE tubing (1/16″ O.D. × 0.75 mm I.D., 25 m/pkg) was obtained from VICI Jours. The ascarite traps were constructed from empty SPE‐ED cartridges, obtained from Biosys Solutions Ltd: Fritted Empty MiniSPE‐ED Cartridges, part # 2447. All fluidic connections were obtained from Upchurch Scientific; the product codes are as follows: fingertight flangeless fitting short, PEEK, XP‐235X; female to male quick‐connect Luer adapter, P‐675‐01.
[11C]CO2 was produced using a Siemens RDS112 cyclotron in a 14N(p,α)11C reaction, by the 11‐MeV proton bombardment of nitrogen (+1% O2) gas. The cyclotron produced [11C]CO2 was transferred in a stream of helium gas at 70 mL/min directly into a switching valve of an E&Z Modular Lab automated synthesis unit. Unless otherwise specified, all radioactive experiments used a 5‐μA bombardment for 1 minute, giving on average 300 to 350 MBq [11C]CO2 at EOB. The RCYs reported are calculated as a percentage of the total radioactivity delivered from the cyclotron. Unless otherwise stated, all experiments were repeated 3 times (n = 3).
1H and 13C{1H} NMR spectra were recorded on a Bruker Avance DRX 400 MHz spectrometer at 294 K. Chemical shifts are reported in parts per million (ppm) relative to the residual solvent proton impurities (1H) or the residual solvent carbon impurities (13C), as internal standards.
The HPLC analysis was performed on an Agilent 1200 system, with a variable wavelength UV detector and a LabLogic Flow‐RAM β+ detector equipped in series. Analytical reverse‐phase column: Agilent XDB‐C18, 5 μm, 4.6 × 150 mm. Gradient used: 95% H2O, 5% MeCN; to 5% H2O, 95% MeCN; over 9 minutes. Identity of radioactive products was confirmed by coelution with the non‐radioactive standard compounds. The HPLC was used to determine molar radioactivities, by reference to a variable dilution calibration curve. Previous experiments within our laboratory have shown that scaling reactions to higher starting radioactivities, with all other factors kept constant, lead to correspondingly higher molar radioactivities. From these results, we assume that a 100‐fold increase from approximately 300 MBq (preliminary experiments) to 30 GBq initial [11C]CO2 (clinical production) will give a roughly 100‐fold increase in molar radioactivity.
Mitsunobu solutions were prepared by dissolving DBAD (21.1 mg, 91.6 μmol, 3 eq.) in 1‐mL MeCN (anhydrous). PBu3 (22.9 μL, 91.6 μmol, 3 eq.) was added, and the mixture was briefly shaken. A colour change from pale yellow to colourless was observed on successful formation of the active Mitsunobu intermediate. Trapping solutions were prepared as 1‐mL solutions by diluting the corresponding percentages (v/v) of DBU and benzylamine in MeCN. All trapping (benzylamine/DBU/MeCN) and Mitsunobu (PBu3/DBAD/MeCN) solutions were prepared under an inert argon atmosphere, using anhydrous MeCN.
4.2. Three‐component [11C]CO2 trapping apparatus: design and setup
The trapping apparatus setup is shown in Figure 1. One end of a 35‐cm length of ETFE tubing (trapping loop, 1/16″ O.D., 0.75 mm I.D., 150‐μL volume) was fitted with a fingertight screw fitting, and the other end was cleanly cut at a 45° taper. The loop was tightly coiled and placed inside a 10‐mL glass vial for ease of handling. The tapered end was inserted through the rubber septum of a sealed crimped 10‐mL waste vial. This vial was vented via a needle through an ascarite trap into a gas waste bag. The loop was half filled with 75‐μL trapping solution, using a 100‐μL syringe, and connected via a Luer slip fitting to the [11C]CO2 outlet line from the E&Z Modular Lab, providing a simple, modular, and easy to disconnect setup, which traps all [11C]CO2 passed through.
4.3. 11C‐urea derivative flow synthesis apparatus: design and setup
The 11C‐urea synthesis apparatus builds upon the trapping apparatus proof‐of‐concept and is shown in Figure 4. The cyclotron [11C]CO2 outlet line was connected to switching valve 1 (V1) as was a syringe containing Mitsunobu reagents (PBu3 and DBAD in MeCN). Both ends of a 35‐cm length of ETFE tubing (trapping loop, 1/16″ O.D., 0.75 mm I.D., 150‐μL volume) were fitted with fingertight screw fittings, and the loop was half filled with 75‐μL trapping solution. One end was attached to the outlet of V1 and the other to the inlet of switching valve 2 (V2). To one outlet of V2, a short length of ETFE tubing was connected to a waste vial, vented via an ascarite trap. To the other outlet is connected a second 35‐cm length of coiled ETFE tubing (reaction loop, 1/16″ O.D., 0.75 mm I.D., 150 μL) running into a sealed crimped product vial (containing 1‐mL water), vented via an ascarite trap.
4.4. Generic procedure: [11C]CO2 trapping
The preloaded loop was connected to the [11C]CO2 delivery line from the cyclotron, and helium was flushed through the system for 3 minutes at 70 mL/min, leaving a just a small, residual amount of trapping solution coating the walls of the trapping loop, and flushing the bulk of the trapping solution into the waste vial. The cyclotron produced [11C]CO2 was then directly delivered diluted in carrier helium gas (without prior trapping and concentration) at 70 mL/min into the E&Z Modular Lab and subsequently through the 3‐component trapping apparatus. Since all [11C]CO2 is trapped within either the trapping loop, the waste vial, or the ascarite trap, these 3 components are quickly separated and their radioactivities measured within a Capintec dose calibrator. Comparison of the distribution of radioactivity within this apparatus allowed calculation of different solvent trapping efficiencies.
4.5. Generic procedure: 11C‐urea derivative synthesis
(1) Helium was flushed through the preloaded trapping loop to the waste vial for 3 minutes at 70 mL/min. (2) Cyclotron produced [11C]CO2 was then directly delivered, diluted in carrier helium gas (without prior trapping and concentration) at 70 mL/min, through the trapping loop, waste vial, and ascarite trap. (3) V1 and V2 are switched, and the trapping loop was filled with 150‐μL Mitsunobu solution (PBu3/DBAD in MeCN). (4) V1 was switched, and a 70‐mL/min helium flush from the cyclotron transferred the contents of the trapping loop through the reaction loop and into the product vial (containing 1‐mL water as a quench). (5) The crude products were analysed by radio‐HPLC and the system was washed with MeCN, and all washings and component radioactivities were measured to determine the total [11C]CO2 radioactivity delivered from the cyclotron.
4.6. Synthesis of N,N′‐dibenzylurea
Benzylamine (1.2 mmol, 131 μL, 3 eq.) was dissolved in 2‐mL EtOAc with stirring at room temperature. To this was added Et3N (1.2 mmol, 167 μL, 3 eq.) and benzyl isocyanate (400 μmol, 49.4 μL, 1 eq.). Solution was stirred for 2 hours under ambient conditions. 1 M HCl was added, and the solution was extracted 3 times with EtOAc. The combined organic extracts were washed with brine and dried over MgSO4. The solvent was removed under reduced pressure to yield a white solid (83.5 mg, 87% yield); 1H NMR (400 MHz, CDCl3) δ 7.25‐7.1 (m, 10H, CH), 4.75 (br s, 2H, NH), 4.26 (d, 4H, CH2); 13C NMR (100 MHz, CDCl3) δ 158.1 (CO), 139.0 (C), 128.6 (CH), 127.4 (CH), 127.3 (CH), 44.5 (CH2).
ACKNOWLEDGEMENTS
This work was supported by the Medical Research Council (MRC, MR/K022733/1) and the King's College London and Imperial College London EPSRC Centre for Doctoral Training in Medical Imaging (EP/L015226/1). The authors acknowledge financial support from the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy's and St Thomas' NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust and the Centre of Excellence in Medical Engineering funded by the Wellcome Trust and EPSRC under grant WT088641/Z/09/Z. This work was supported by the Wellcome EPSRC Centre for Medical Engineering at King's College London (WT 203148/Z/16/Z).
Downey J, Bongarzone S, Hader S, Gee AD. In‐loop flow [11C]CO2 fixation and radiosynthesis of N,N′‐[11C]dibenzylurea. J Label Compd Radiopharm. 2018;61:263–271. https://doi.org/10.1002/jlcr.3568
Joseph Downey and Salvatore Bongarzone contributed equally to this work.
REFERENCES
- 1. Miller PW, Long NJ, Vilar R, Gee AD. Synthesis of 11C, 18F, 15O, and 13N radiolabels for positron emission tomography. Angew Chemie Int Ed. 2008;47(47):8998‐9033. https://doi.org/10.1002/anie.200800222 [DOI] [PubMed] [Google Scholar]
- 2. Antoni G. Development of carbon‐11 labelled PET tracers‐radiochemical and technological challenges in a historic perspective. J Labelled Comp Radiopharm. 2015;58(3):65‐72. https://doi.org/10.1002/jlcr.3258 [DOI] [PubMed] [Google Scholar]
- 3. Tu Z, Mach RH. C‐11 radiochemistry in cancer imaging applications. Curr Top Med Chem (Sharjah, United Arab Emirates). 2010;10(11):1060‐1095. https://doi.org/10.2174/156802610791384261 [DOI] [PubMed] [Google Scholar]
- 4. Ametamey SM, Honer M, Schubiger PA. Molecular imaging with PET. Chem Rev. 2008;108(5):1501‐1516. https://doi.org/10.1021/cr0782426 [DOI] [PubMed] [Google Scholar]
- 5. Piel M, Vernaleken I, Rösch F. Positron emission tomography in CNS drug discovery and drug monitoring. J Med Chem. 2014;57(22):9232‐9258. https://doi.org/10.1021/jm5001858 [DOI] [PubMed] [Google Scholar]
- 6. Rotstein BH, Liang SH, Holland JP, et al. 11CO2 fixation: a renaissance in PET radiochemistry. Chem Commun. 2013;49(50):5621 https://doi.org/10.1039/c3cc42236d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pike VW, Eakins MN, Allan RM, Selwyn AP. Preparation of carbon‐11 labelled acetate and palmitic acid for the study of myocardial metabolism by emission‐computerised axial tomography. J Radioanal Chem. 1981;64(1‐2):291‐297. https://doi.org/10.1007/BF02518360 [Google Scholar]
- 8. Kawashima H, Yajima K, Kuge Y, Hashimoto N, Miyake Y. Synthesis of [1‐11C]‐2‐octynoic acid, [1‐11C]‐2‐decynoic acid and [1‐11C]‐3‐(R,S)‐methyloctanoic acid as potential markers for PET studies of fatty acid metabolism. J Label Compd Radiopharm. 1997;39(3):181‐193. https://doi.org/10.1002/(SICI)1099-1344(199703)39:3%3C181::AID-JLCR962%3E3.0.CO;2-W [Google Scholar]
- 9. Runkle AC, Shao X, Tluczek LJM, Henderson BD, Hockley BG, Scott PJH. Automated production of [11C]acetate and [11C]palmitate using a modified GE Tracerlab FXC‐Pro. Appl Radiat Isot. 2011;69(4):691‐698. https://doi.org/10.1016/j.apradiso.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 10. Davenport RJ, Dowsett K, Pike VW. A simple technique for the automated production of no‐carrier‐added [1‐ 11C]acetate. Appl Radiat Isot. 1997;48(8):1117‐1120. https://doi.org/10.1016/S0969-8043(97)00118-8 [DOI] [PubMed] [Google Scholar]
- 11. Rami‐Mark C, Ungersboeck J, Haeusler D, et al. Reliable set‐up for in‐loop 11C‐carboxylations using Grignard reactions for the preparation of [carbonyl‐11C]WAY‐100635 and [11C]‐(+)‐PHNO. Appl Radiat Isot. 2013;82:75‐80. https://doi.org/10.1016/j.apradiso.2013.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilson AA, Garcia A, Jin L, Houle S. Radiotracer synthesis from [11C]‐iodomethane: a remarkably simple captive solvent method. Nucl Med Biol. 2000;27(6):529‐532. https://doi.org/10.1016/S0969-8051(00)00132-3 [DOI] [PubMed] [Google Scholar]
- 13. Wilson AA, Garcia A, Houle S, Vasdev N. Utility of commercial radiosynthetic modules in captive solvent [11C]‐methylation reactions. J Label Compd Radiopharm. 2009;52(11):490‐492. https://doi.org/10.1002/jlcr.1618 [Google Scholar]
- 14. Iwata R, Pascali C, Bogni A, Miyake Y, Yanai K, Ido T. A simple loop method for the automated preparation of [11C]raclopride from [11C]methyl triflate. Appl Radiat Isot. 2001;55(1):17‐22. https://doi.org/10.1016/S0969-8043(00)00368-7 [DOI] [PubMed] [Google Scholar]
- 15. Shao X, Kilbourn MR. A simple modification of GE tracerlab FX C Pro for rapid sequential preparation of [11C]carfentanil and [11C]raclopride. Appl Radiat Isot. 2009;67(4):602‐605. https://doi.org/10.1016/j.apradiso.2008.12.013 [DOI] [PubMed] [Google Scholar]
- 16. Shao X, Schnau PL, Fawaz MV, Scott PJH. Enhanced radiosyntheses of [11C]raclopride and [11C]DASB using ethanolic loop chemistry. Nucl Med Biol. 2013;40(1):109‐116. https://doi.org/10.1016/j.nucmedbio.2012.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rotstein BH, Liang SH, Placzek MS, et al. 11C=O bonds made easily for positron emission tomography radiopharmaceuticals. Chem Soc Rev. 2016;45(17):4708‐4726. https://doi.org/10.1039/C6CS00310A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jessop PG, Heldebrant DJ, Li X, Eckert CA, Liotta CL. Green chemistry: reversible nonpolar‐to‐polar solvent. Nature. 2005;436(7054):1102‐1102. https://doi.org/10.1038/4361102a [DOI] [PubMed] [Google Scholar]
- 19. Heldebrant DJ, Yonker CR, Jessop PG, Phan L. Organic liquid CO2 capture agents with high gravimetric CO2 capacity. Energ Environ Sci. 2008;124(July):487‐493. https://doi.org/10.1039/b809533g [Google Scholar]
- 20. Hooker JM, Reibel AT, Hill SM, Schueller MJ, Fowler JS. One‐pot, direct incorporation of [11C]CO2 into carbamates. Angew Chem Int Ed. 2009;48(19):3482‐3485. https://doi.org/10.1002/anie.200900112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilson AA, Garcia A, Houle S, Vasdev N. Direct fixation of [11C]‐CO2 by amines: formation of [11C‐carbonyl]‐methylcarbamates. Org Biomol Chem. 2010;8(2):428‐432. https://doi.org/10.1039/B916419G [DOI] [PubMed] [Google Scholar]
- 22. Heldebrant DJ, Jessop PG, Thomas CA, Eckert CA, Liotta CL. The reaction of 1,8‐diazabicyclo[5.4.0]undec‐7‐ene (DBU) with carbon dioxide. J Org Chem. 2005;70(13):5335‐5338. https://doi.org/10.1021/jo0503759 [DOI] [PubMed] [Google Scholar]
- 23. Nicholls R, Kaufhold S, Nguyen BN. Observation of guanidine–carbon dioxide complexation in solution and its role in the reaction of carbon dioxide and propargylamines. Cat Sci Technol. 2014;4(10):3458‐3462. https://doi.org/10.1039/C4CY00480A [Google Scholar]
- 24. Vasdev N, Sadovski O, Garcia A, et al. Radiosynthesis of [11C]SL25.1188 via [11C]CO2 fixation for imaging monoamine oxidase B. J Label Compd Radiopharm. 2011;54(10):678‐680. https://doi.org/10.1002/jlcr.1908 [Google Scholar]
- 25. Wilson AA, Garcia A, Houle S, Sadovski O, Vasdev N. Synthesis and application of isocyanates radiolabeled with Carbon‐11. Chem ‐ A Eur J. 2011;17(1):259‐264. https://doi.org/10.1002/chem.201002345 [DOI] [PubMed] [Google Scholar]
- 26. Haji Dheere AK, Yusuf N, Gee A. Rapid and efficient synthesis of [11C]ureas via the incorporation of [11C]CO2 into aliphatic and aromatic amines. Chem Commun (Camb). 2013;49(74):8193‐8195. https://doi.org/10.1039/c3cc44046j [DOI] [PubMed] [Google Scholar]
- 27. Dheere A, Bongarzone S, Taddei C, Yan R, Gee A. Synthesis of 11C‐labelled symmetrical ureas via the rapid incorporation of [11C]CO2 into aliphatic and aromatic amines. Synlett. 2015;26(16):2257‐2260. https://doi.org/10.1055/s-0034-1381055 [Google Scholar]
- 28. Bongarzone S, Runser A, Taddei C, Haji Dheere AK, Gee A. From [11C]CO2 to [11C]amides: a rapid one‐pot synthesis via the Mitsunobu reaction. Chem Commun. 2017;53(38):5334‐5337. https://doi.org/10.1039/C7CC01407D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mossine AV, Brooks AF, Jackson IM, et al. Synthesis of diverse 11C‐labeled PET radiotracers via direct incorporation of [11C]CO2 . Bioconjug Chem. 2016;27(5):1382‐1389. https://doi.org/10.1021/acs.bioconjchem.6b00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. CHERIC . CHERIC: chemical engineering and materials research information center, Pure Component Properties, Acetonitrile. https%3A%2F%2Fwww.cheric.org%2Fresearch%2Fkdb%2Fhcprop%2Fshowcoef.php%3Fcmpid%3D1386%26amp%3Bprop%3DVSL. Accessed June 14, 2017.
- 31. Weng WL, Chang LT, Shiah IM. Viscosities and densities for binary mixtures of benzylamine with 1‐pentanol, 2‐pentanol, 2‐methyl‐1‐butanol, 2‐methyl‐2‐butanol, 3‐methyl‐1‐butanol, and 3‐methyl‐2‐butanol. J Chem Eng Data. 1999;44(5):994‐997. https://doi.org/10.1021/je990031d [Google Scholar]
- 32. Fisher Scientific . DBU MSDS, Fisher Scientific. https://www.fishersci.com/store/msds?partNumber=AC160610025&productDescription=1+8-DIAZABICYCLO+5.4.0+U+2.5KG&vendorId=VN00032119&countryCode=US&language=en. Published 2014. Accessed June 14, 2017.
- 33. Brunn E, Huisgen R. Structure and reactivity of the betaine derived from triphenylphosphine and dimethyl azodicarboxylate. Angew Chemie Int Ed English. 1969;8(7):513‐515. https://doi.org/10.1002/anie.196905131 [Google Scholar]
- 34. Satish Kumar N, Praveen Kumar K, Pavan Kumar KVP, Kommana P, Vittal JJ, Kumara Swamy KC. Diverse modes of reactivity of dialkyl azodicarboxylates with P(III) compounds: synthesis, structure, and reactivity of products other than the Morrison‐Brunn‐Huisgen intermediate in a Mitsunobu‐type reaction. J Org Chem. 2004;69(6):1880‐1889. https://doi.org/10.1021/jo035634d [DOI] [PubMed] [Google Scholar]
- 35. But TYS, Toy PH. The Mitsunobu reaction: origin, mechanism, improvements, and applications. Chem ‐ An Asian J. 2007;2(11):1340‐1355. https://doi.org/10.1002/asia.200700182 [DOI] [PubMed] [Google Scholar]
- 36. Swamy KCK, Kumar NNB, Balaraman E, Kumar KVPP. Mitsunobu and related reactions: advances and applications. Chem Rev. 2009;109(6):2551‐2651. https://doi.org/10.1021/cr800278z [DOI] [PubMed] [Google Scholar]
- 37. Fletcher S. The Mitsunobu reaction in the 21st century. Org Chem Front. 2015;2(6):739‐752. https://doi.org/10.1039/C5QO00016E [Google Scholar]
- 38. One example of a clinical radiotracer produced at our institution is [11C]methionine, which is obtained by 11C‐methylation using [11C]CH3I produced via the gas‐phase method.
- 39. Downey J, Bongarzone S, Hader S, Gee AD. Oral presentations (O 066). In. J Label Compd Radiopharm. 2017;60:S88‐S89. https://doi.org/10.1002/jlcr.3507 [Google Scholar]


