Abstract
Background
Topical intranasal corticosteroid sprays (INCSs) are standard treatment for nasal polyps (NPs), but their efficacy is reduced by poor patient compliance and impaired access of drug to the sinus mucosa. A corticosteroid‐eluting sinus implant was designed to address these limitations in patients with recurrent polyposis after sinus surgery by delivering 1350 μg of mometasone furoate (MF) directly to the ethmoid sinus mucosa over approximately 90 days.
Methods
A randomized, sham‐controlled, double‐blind trial was undertaken in 300 adults with refractory chronic rhinosinusitis with NPs (CRSwNP), who were candidates for repeat surgery. Eligible patients were randomized (2:1) and underwent in‐office bilateral placement of 2 implants or a sham procedure. All patients used the MF INCS 200 μg once daily. Co‐primary efficacy endpoints were the change from baseline in nasal obstruction/congestion score and bilateral polyp grade, as determined by an independent panel based on centralized, blinded videoendoscopy review.
Results
Patients treated with implants experienced significant reductions in both nasal obstruction/congestion score (p = 0.0074) and bilateral polyp grade (p = 0.0073) compared to controls. At day 90, implants were also associated with significant reductions in 4 of 5 prespecified secondary endpoints compared to control: proportion of patients still indicated for repeat sinus surgery (p = 0.0004), percent ethmoid sinus obstruction (p = 0.0007), nasal obstruction/congestion (p = 0.0248), and decreased sense of smell (p = 0.0470), but not facial pain/pressure (p = 0.9130). One patient experienced an implant‐related serious adverse event (epistaxis).
Conclusion
Significant improvements over a range of subjective and objective endpoints, including a reduction in the need for sinus surgery by 61%, suggest that MF sinus implants may play an important role in management of recurrent NP.
Keywords: sinus surgery, corticosteroid use, chronic rhinosinusitis, ESS, mometasone furoate implant, polyposis
Chronic rhinosinusitis (CRS) is a broad, inflammatory syndrome affecting over 10% of Western populations, characterized by persistent nasal obstruction, drainage, facial pressure, and loss of smell.1, 2, 3 CRS is often divided into 2 phenotypes based on nasal endoscopy, CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP), but there is significant clinical overlap. First‐line management consists of saline nasal rinses and standard, topical intranasal corticosteroid sprays (INCSs) for both phenotypes.4 Symptoms fluctuate, and patients are subject to frequent viral, allergic, and bacterial exacerbations.5 In resistant cases, extended courses of systemic corticosteroids are used to reduce symptom burden and avoid sinus surgery.4, 6 Nevertheless, more than 250,000 endoscopic sinus surgeries (ESSs) are performed in the United States annually, with an average of 20% requiring revision surgery within 5 years.7 The estimated direct and indirect costs of CRS in the United States exceed $30 billion, not including the hidden cost of antibiotic resistance, as CRS accounts for 7% of all antibiotic prescriptions in the United States.8, 9 Alternative treatment options are particularly needed for CRSwNP, which is associated with higher symptom burden, increased medication use, greater revision rate, and higher costs compared to CRSsNP.4, 8
CRSwNP is most commonly characterized by type 2 eosinophilic inflammation in Western countries.10 The etiology and pathogenesis are unclear, but severe mucosal inflammation in the ethmoid sinuses results in polyps that project into the nasal cavity, obstructing the airway.11, 12 Corticosteroids diminish eosinophil infiltrates, reverse type 2 inflammation and remain the standard medical treatment for CRSwNP.13, 14 Systemic corticosteroids are effective, acutely shrinking polyps, but the efficacy is transient and limited by dose‐dependent side effects.13, 15, 16 INCSs have a high margin of safety, but efficacy is blunted by patient noncompliance and the limited access of these drugs to the ethmoid sinus mucosa.17 Bioabsorbable sinus implants, which elute corticosteroids, were designed to address these limitations and improve surgical outcomes for CRSwNP when used in the immediate postoperative period.18 Larger, second‐generation steroid implants with nearly 4‐fold higher corticosteroid content (1350 μg vs 370 μg) released over a 3‐times longer period of time (90 days vs 30 days) have been developed for in‐office treatment of recurrent NP as an alternative to revision surgery. These implants are inserted in the ethmoid sinuses under local anesthesia. Early trials with this implant have shown positive trends for treatment of recurrent nasal polyps (NPs) with an excellent safety profile.19, 20, 21, 22 Here, we present the results of a phase 3 trial in adults with CRSwNP, who were indicated for repeat surgery to treat moderate‐to‐severe, medically‐refractory symptoms of nasal obstruction/congestion with recurrent NPs in both ethmoid sinuses.
Patients and methods
Trial design and oversight
We conducted a prospective, randomized, double‐blind, parallel‐group, sham‐controlled trial to evaluate the safety and efficacy of a bioabsorbable corticosteroid‐eluting sinus implant (SINUVA™ Sinus Implant, Intersect ENT, Inc., Menlo Park, CA), containing 1350 μg of mometasone furoate (MF). The implant is a drug/device combination product that was not approved by the U.S. Food and Drug Administration (FDA) at the time the study was conducted. The trial was registered on http://ClinicalTrials.gov (NCT02291549) and conducted in accordance with the principles of the Declaration of Helsinki and applicable regulatory requirements. Patients were enrolled from December 2014 to May 2016 at 34 clinical sites across the United States. The institutional review board of each participating site approved the protocol and provided trial oversight. Each patient provided written informed consent prior to undergoing any study‐related screening procedure. A 14‐day run‐in screening period using INCS was followed by the baseline procedure (implant placement or sham) and 90‐day follow‐up. Eligibility was confirmed based on an electronic diary of nasal obstruction/congestion symptoms over the first 7 days of the 14‐day run‐in screening period using INCS and grading of videoendoscopies by an independent reviewer. Eligible patients were randomized (2:1) to the treatment or control group using an electronic data capture system and underwent either bilateral implant placement or sham procedure. During 90‐day follow‐up both treatment and control groups were required to self‐administer MF nasal spray (MFNS) 200 μg once daily (Nasonex Nasal Spray; Merck & Co., Inc., Whitehouse Station, NJ). Assessments at baseline and each follow‐up visit at days 14, 30, 60, and 90 consisted of subjective patient‐reported and objective endoscopic grading. Patients were masked during the baseline procedure and each follow‐up endoscopy. The implants are made from bioabsorbable polymers designed to gradually soften over time and may be left in the sinuses to gradually release the corticosteroid over 90 days. The study protocol required that all implants were removed by day 60 to ensure blinded assessment by the independent panel at day 90 (co‐primary endpoint).
Patients
Eligible patients were 18 years old or older, had a confirmed diagnosis of CRSwNP23 based on symptoms and endoscopic examination, had undergone prior ESS, including bilateral total ethmoidectomy, and were currently indicated for repeat ESS. To be indicated for repeat ESS, a patient had to: (1) be using INCS daily for at least 14 days prior to eligibility; (2) receive at least 1 course of high‐dose steroid therapy (eg, oral steroids, parenteral steroid injections, budesonide drops/irrigations, nebulized steroids) or refused such therapy due to side effects within the past 1 year; (3) continue to have moderate‐to‐severe symptoms of nasal obstruction/congestion (score ≥ 2 on a scale from 0 to 3 on at least 5 of 7 days, as determined based on an electronic daily diary); and (4) have endoscopic evidence of bilateral ethmoid sinus obstruction due to polyposis (grade ≥ 2 on a scale from 0 to 4 on each side, as determined by an independent reviewer based on centralized videoendoscopy review). Patients were excluded if they had NPs grade 4 on at least 1 side; extensive adhesions/synechiae that could preclude access to either ethmoid sinus; allergy or intolerance to corticosteroids; concurrent condition requiring active chemotherapy, immunotherapy, or oral steroids; clinical evidence of acute bacterial sinusitis, invasive fungal sinusitis, diabetes mellitus; or known history or diagnosis of glaucoma, ocular hypertension, or subcapsular cataract.
Interventions
Patients assigned to the treatment group underwent in‐office bilateral placement of 2 implants into the ethmoid sinuses under local anesthesia and continuous endoscopic visualization. Patients assigned to the control group underwent an in‐office bilateral sham procedure, consisting of insertion of the implants into the ethmoid sinuses followed by immediate withdrawal without placement. None of the polyp tissue was removed before or during the baseline procedure. Patient compliance with daily dosing of 200 μg of MFNS once daily was carefully monitored by research center staff and was reinforced by use of the electronic diary, which reminded patients to score their symptoms of nasal obstruction/congestion prior to dosing.
Leading up to the baseline procedure, there was a 30‐day restriction for use of parenteral injection of steroids and a 14‐day restriction for use of oral steroids, budesonide drops/irrigations, and nebulized steroids. Following the baseline procedure, prohibited concomitant medications included systemic steroids, budesonide drops/irrigations, and nebulized steroids. Preexisting asthma and allergy regimens, including inhaled corticosteroids, leukotriene receptor antagonists, and immunotherapies, were maintained throughout day 90 if patients were currently on such regimens. Rescue treatments with antibiotics, oral steroids, or revision surgery were provided if medically necessary. Patients who received prohibited steroids or surgery could continue in the study and were analyzed according to their assigned treatment group, and their most recent scores and videos prior to intervention were used for analysis of subsequent time points.
Endpoints
The 2 co‐primary efficacy endpoints were the change from baseline to day 30 in nasal obstruction/congestion score, as determined by patients, and change from baseline to day 90 in bilateral polyp grade, as determined by the independent, blinded panel. Patients scored nasal obstruction/congestion symptoms on a scale of 0 (no symptoms) to 3 (severe symptoms) for 7 days preceding each visit using an electronic diary in the morning before dosing with MFNS. Bilateral polyp grade represents a sum of left and right polyp grades, each scored using an 8‐point scale, which included 3 intermediate grades (1.5, 2.5, and 3.5) that were added to the 5‐point scale from 0 (no polyps) to 4 (polyps completely obstructing the nasal cavity) validated by Meltzer et al.24 to allow more sensitivity in quantifying the burden of polyposis in post‐ESS patients with altered anatomy and varied amount of obstruction by polypoid edema (≥ 25%, 50%, or 75% of the ethmoid sinus/middle meatus). NP grading was performed by the panel of 3 sinus surgeons based on a centralized, independent, blinded review of videoendoscopies at baseline and day 90 (co‐primary endpoint) and by unblinded clinical investigators during endoscopy at all time points (secondary endpoints), resulting in a total grade on a scale from 0 to 8, with higher grade representing greater severity.
Five secondary efficacy endpoints were evaluated and analyzed according to a prespecified plan to control familywise type 1 error rate (FWER, see Statistical Analysis). These endpoints were as follows: (1) nasal obstruction/congestion score change from baseline to day 90; (2) change in percent ethmoid sinus obstruction at day 90, as determined by the independent, blinded panel on a 100‐mm visual analogue scale (VAS); (3) decreased sense of smell score change from baseline to day 90, as determined by patients on a 6‐point Likert scale of 0 (absent) to 5 (very severe); (4) facial pain/pressure score change from baseline to day 90, as determined by patients on a 6‐point Likert scale of 0 (absent) to 5 (very severe); and (5) proportion of patients still indicated for repeat ESS at day 90 despite ongoing INCS use based on clinical investigator assessment using study‐specific criteria. To be still indicated, the patient had to: (1) complain of CRS symptoms including nasal obstruction/congestion and postnasal discharge, facial pain/pressure, or altered sense of smell/taste; (2) have endoscopic evidence of persisting NPs (grade ≥ 2 on each side); and (3) have received or need a systemic steroid as noted during endoscopy.
To further assess the clinical benefits of the sinus implants, additional exploratory endpoints included the proportion of responders, defined as reduction from baseline to day 30 in nasal obstruction/congestion score by ≥ 0.5 and ≥ 1.0 point and reduction from baseline to day 90 by ≥ 1.0 and ≥ 2.0 point in bilateral polyp grade. In the absence of established minimally clinically important differences (MCIDs) for nasal obstruction/congestion score and bilateral polyp grade, the above improvement thresholds have been considered clinically meaningful changes for treatment of NPs based on the criteria used in prior MFNS studies.25, 26
We evaluated safety by monitoring adverse events, including severity of the event and relationship to implants, which was categorized as either related to the study drug, study device, or implant procedure.
Statistical analysis
For the primary and continuous secondary endpoints, we used the analysis of covariance (ANCOVA) model with baseline value as a covariate and site and treatment group as fixed effects. Between treatment differences were estimated from the same ANCOVA model and reported as least squares means with 95% confidence intervals (CIs) and p values. For categorical secondary endpoints, we used the Cochran‐Mantel‐Haenszel test with site as the stratification variable. For the study to be successful, both co‐primary efficacy endpoints required statistical significance. After meeting the co‐primary endpoints, the 5 prespecified secondary endpoints were adjusted for multiplicity using the Holm's step‐down method to control for FWER. Other endpoints were considered exploratory. Analyses were adjusted for any steroid and surgical interventions by imputing that consisted of carrying forward the most recent values and videos prior to receiving or initiating such intervention. Statistical analyses were performed by independent biostatisticians using an intent‐to‐treat (ITT) population, which included all patients who underwent randomization and in whom sinus implant placement or sham procedure was attempted. The bilateral polyp grades from the 3 reviewers for the same patient were averaged. The results for a given sinus were set as missing if 2 of the 3 independent reviewers could not provide assessments. The results for a given patient were set as missing if the value for one sinus was missing. No imputations for missing data were performed. All reported p values are 2‐sided.
Results
Of the 531 patients enrolled and screened, 300 were randomized and underwent a baseline procedure (Fig. 1). The ITT population comprised 201 treatment and 99 control patients. Implant delivery success rate was 99.0%. One treatment patient (0.5%) was lost to follow‐up, and 1 control patient (1.0%) withdrew from the study. Patient demographics and baseline clinical characteristics were well balanced between the treatment groups (Table 1). The majority of patients complained of nasal obstruction/blockage, postnasal discharge, and altered sense of smell/taste, underwent 2 or more prior revision ESS, and had history of allergic rhinitis and asthma.
Figure 1.
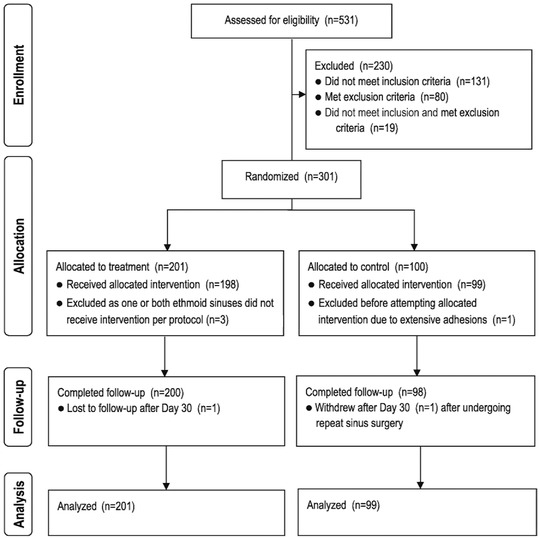
RESOLVE II patient disposition (CONSORT diagram). CONSORT = Consolidated Standards Of Reporting Trials.
Table 1.
Patient baseline demographic and clinical characteristics*
| Variable | Treatment (n = 201) | Control (n = 99) |
|---|---|---|
| Age (years) | 50.5 ± 12.9 | 47.9 ± 12.4 |
| Male subjects, n (%) | 127 (63.2) | 56 (56.6) |
| Race, n (%) | ||
| White | 164 (81.6) | 80 (80.8) |
| Black | 27 (13.4) | 13 (13.1) |
| Asian | 4 (2.0) | 4 (4.0) |
| Other | 6 (3.0) | 2 (2.0) |
| CRS symptoms despite ongoing use of INCS, n (%) | ||
| Nasal obstruction/blockage | 185 (92.0) | 90 (90.9) |
| Postnasal discharge | 182 (90.5) | 83 (83.8) |
| Altered sense of smell/taste | 174 (86.6) | 89 (89.9) |
| Facial pain/pressure/fullness | 77 (38.3) | 44 (44.4) |
| ESS history, n (%) | ||
| Number of prior ESS | ||
| 1 | 83 (41.3) | 41 (41.4) |
| 2 | 57 (28.4) | 36 (36.4) |
| 3 | 32 (15.9) | 7 (7.1) |
| ≥4 | 29 (14.4) | 15 (15.2) |
| Indicated for repeat ESS | 201 (100) | 99 (100) |
| Medical history, n (%)a | ||
| Allergic rhinitis | 155 (77.1) | 79 (79.8) |
| Mild | 52 (25.9) | 23 (23.2) |
| Moderate | 78 (38.8) | 43 (43.4) |
| Severe | 25 (12.4) | 13 (13.1) |
| Asthma | 148 (73.6) | 61 (61.6) |
| Mild | 84 (41.8) | 31 (31.3) |
| Moderate | 56 (27.9) | 26 (26.3) |
| Severe | 8 (4.0) | 4 (4.0) |
| AERD | 30 (14.9) | 17 (17.2) |
| Smoking | ||
| Never smoked | 130 (64.7) | 69 (69.7) |
| Former smoker | 65 (32.3) | 24 (24.2) |
| Current smoker | 6 (3.0) | 6 (6.1) |
| Symptomatic findings | ||
| Nasal obstruction/congestion score ≥2 on 5 of 7 days, n (%) | 201 (100) | 99 (100) |
| Nasal obstruction/congestion score (scale 0 to 3) | 2.4 ± 0.5 | 2.4 ± 0.5 |
| Decreased sense of smell score (scale 0 to 5) | 4.1 ± 1.4 | 4.1 ± 1.4 |
| Facial pain/pressure score (scale 0 to 5) | 1.9 ± 1.4 | 2.2 ± 1.4 |
| Endoscopic findings by an independent reviewer | ||
| Polyp grade ≥2 on each side (scale 0 to 4), n (%) | 201 (100) | 99 (100) |
| Bilateral polyp grade (scale 0 to 8) | 5.95 ± 0.94 | 5.87 ± 1.00 |
*Values are means ± SD or as indicated. Only incidence of asthma (mainly mild) differed significantly between the groups (p = 0.0336).
aMedical history based on physician diagnosis as recorded in patient medical records.
AERD = aspirin exacerbated respiratory disease; CRS = chronic rhinosinusitis; ESS = endoscopic sinus surgery; INCS = intranasal corticosteroid sprays; SD = standard deviation.
Patients receiving implants demonstrated significant reductions in both nasal obstruction/congestion score (p = 0.0074) and bilateral polyp grade (p = 0.0073) compared to control (Table 2). Therefore, both co‐primary efficacy objectives were met.
Table 2.
Co‐primary and key secondary efficacy outcomes
| Endpoint | Treatment (n = 201) | Control (n = 99) | Between group difference (95% CI) or odds ratio (95% CI) | p |
|---|---|---|---|---|
| Co‐primary efficacy endpoints | ||||
| Nasal obstruction/congestion score change from baseline to day 30 (scale 0–3) | ||||
| n (%) | 199 (99.0) | 97 (98.0) | ||
| Mean ± SD | −0.80 ± 0.73 | −0.56 ± 0.62 | −0.23 (−0.39, −0.06) | 0.0074 |
| Bilateral polyp grade change from baseline to day 90 by an independent, blinded panel (scale 0–8) | ||||
| n (%) | 195 (97.0) | 97 (98.0) | ||
| Mean ± SD | −0.56 ± 1.06 | −0.15 ± 0.91 | −0.35 (−0.60, −0.09) | 0.0073 |
| Secondary efficacy endpoints adjusted for multiplicity | ||||
| Patients still indicated for repeat sinus surgery at day 90, n/n total (%) | 78/200 (39.0) | 62/98 (63.3) | ||
| Odds ratio (95% CI) | 2.69 (1.63, 4.44) | 0.0004 | ||
| Percent ethmoid sinus obstruction change from baseline to day 90 by an independent panel (scale 0–100) | ||||
| n (%) | 195 (97.0) | 97 (98.0) | ||
| Mean ± SD | −11.3 ± 18.1 | −1.9 ± 14.4 | −7.96 (−12.10, −3.83) | 0.0007 |
| Nasal obstruction/congestion score change from baseline to day 90 (scale 0–3) | ||||
| n (%) | 177 (88.1) | 89 (89.9) | ||
| Mean ± SD | −0.93 ± 0.80 | −0.69 ± 0.79 | −0.27 (−0.48, −0.07) | 0.0248 |
| Decreased sense of smell score change from baseline to day 90 (scale 0–5) | ||||
| n (%) | 198 (98.5) | 97 (98.0) | ||
| Mean ± SD | −1.20 ± 1.66 | −0.76 ± 1.60 | −0.46 (−0.85 −0.06) | 0.0470 |
| Facial pain/pressure score change from baseline to day 90 (scale 0–5) | ||||
| n (%) | 197 (98.0) | 96 (97.0) | ||
| Mean ± SD | −0.77 ± 1.21 | −0.90 ± 1.27 | 0.01 (−0.24, 0.27) | 0.9130 |
CI = confidence interval; SD = standard deviation.
At day 90, implants were associated with significant reductions in 4 of the 5 prespecified secondary endpoints compared to control (Table 2). Significantly fewer patients receiving implants than sham remained indicated for repeat ESS based on the prespecified study criteria (39.0% vs 63.3%, p = 0.0004). Patients treated with implants also had significantly greater decrease in percent ethmoid sinus obstruction (p = 0.0007) and experienced sustained symptomatic improvements in nasal obstruction/congestion (p = 0.0248) and sense of smell (p = 0.0470), but not in facial pain/pressure (p = 0.9130).
Significant improvements favoring implants were also observed in symptoms of nasal obstruction/congestion at day 60 and endoscopic outcomes by investigators through day 90 (Fig. 2). The improvement in endoscopic outcomes is illustrated in Video 1 that contains endoscopic images from a treatment patient at the baseline and through follow‐up. Significantly more patients receiving implants than sham experienced clinically meaningful improvement in nasal obstruction/congestion score and bilateral polyp grade (Table 3).
Figure 2.
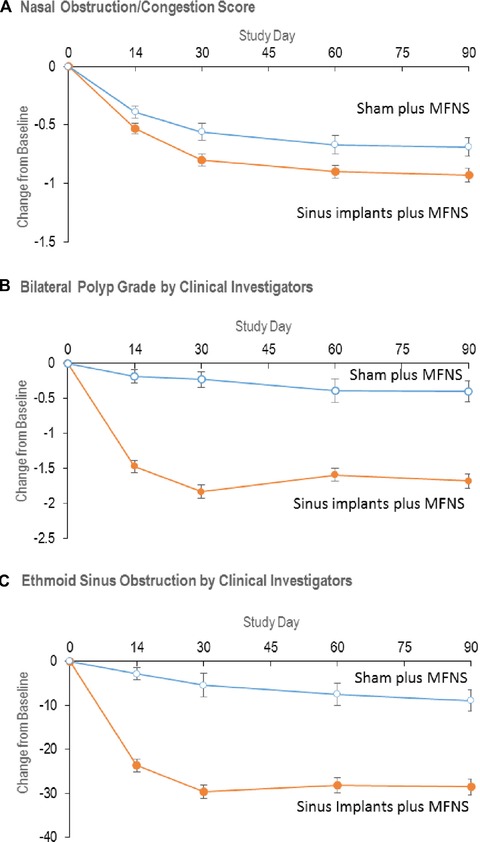
Secondary endpoints. (A) Change in mean nasal obstruction/congestion score (scale 0 to 3), as determined by patients using an electronic diary over 7 days immediately preceding each visit. p = 0.0556 for change from baseline to day 14, p = 0.0074 to day 30 (co‐primary efficacy endpoint, Table 2), p = 0.0129 to day 60, and p = 0.0083 to day 90 (p = 0.0248 when adjusted for multiplicity, Table 2). (B) Change in mean bilateral polyp grade as determined by clinical investigators on a scale from 0 to 8, with higher scores indicating greater severity. p < 0.0001 for change from baseline to each time point. (C) Change in percent ethmoid sinus obstruction as determined by clinical investigators using a 100‐mm visual analogue scale. p < 0.0001 for change from baseline to each time point. All values are means with 2‐sided standard error bars calculated based on intent‐to‐treat population. Data from patients who received surgical or medical interventions were imputed using most recent values prior to initiating or receiving intervention and represent intervention‐adjusted values. MFNS = mometasone furoate nasal spray.
Table 3.
Proportion of responders
| Outcome | Treatment (n = 201) | Control (n = 99) | p | Odds ratio (95% CI) |
|---|---|---|---|---|
| Nasal obstruction/congestion score change from baseline to day 30 | ||||
| ≥0.5‐point reduction, n/n total (%) | 125/199 (62.8) | 49/97 (50.5) | 0.0440 | 1.24 (0.99–1.56) |
| ≥1.0‐point reduction, n/n total (%) | 88/199 (44.2) | 25/97 (25.8) | 0.0022 | 1.72 (1.18–2.49) |
| Bilateral polyp grade change from baseline to day 90 by clinical investigators | ||||
| ≥1.0‐point reduction, n/n total (%) | 144/200 (72.0) | 36/98 (36.7) | <0.0001 | 1.96 (1.49–2.58) |
| ≥2.0‐point reduction , n/n total (%) | 95/200 (47.5) | 16/98 (16.3) | <0.0001 | 2.91 (1.82–4.66) |
CI = confidence interval.
Fewer patients in the treatment group than control received rescue treatments through day 90 (Table 4). Oral steroids for ethmoid sinus obstruction were used by 13.9% of treatment patients, compared to 18.2% of controls (p = 0.4080), with the majority of treatment patients not requiring oral steroids until day 90. There was similar proportion of patients in the treatment and control groups that remained on montelukast for the treatment of asthma throughout the study (35.3.% vs 32.2%). Three (3.0%) control patients underwent repeat ESS prior to day 90 compared to none of treatment group.
Table 4.
Rescue treatments*
| Rescue therapy | Treatment (n = 201) | Control (n = 99) |
|---|---|---|
| MFNS, 200 μg once daily (required by protocol), n (%) | ||
| Compliance at each visit | ||
| Day 14 | 197 (98.0) | 99 (100) |
| Day 30 | 201 (100) | 97 (98.0) |
| Day 60 | 197 (98.0) | 97 (98.0) |
| Day 90 | 186 (92.5) | 90 (90.9) |
| Any steroid or surgical interventions requiring imputation, n (%) | 31 (15.4) | 16 (16.2) |
| Oral steroids for ethmoid sinus obstruction, n (%) | 28 (13.9) | 18 (18.2) |
| First instance | ||
| Day 14 | 1 | 3 |
| Day 30 | 1 | 5 |
| Day 60 | 7 | 3 |
| Day 90 | 19 | 7 |
| Oral steroids for other reasons, n (%)a | 35 (17.4) | 11 (11.1) |
| First instance | ||
| Day 14 | 4 | 0 |
| Day 30 | 6 | 3 |
| Day 60 | 6 | 3 |
| Day 90 | 19 | 5 |
| Polypectomy, n (%) | 2 (1.0) | 1 (1.0) |
| First instance | ||
| Day 14 | 0 | 1 |
| Day 30 | 0 | 0 |
| Day 60 | 2 | 0 |
| Day 90 | 0 | 0 |
| Repeat ESS, n (%) | 0 (0) | 3 (3.0) |
| First instance | ||
| Day 14 | 0 | 1 |
| Day 30 | 0 | 1 |
| Day 60 | 0 | 1 |
| Day 90 | 0 | 0 |
*Values are counts and percentages of patients who received any steroid or surgical interventions through 90 days. First instance was when the medical or surgical intervention was reported. Percentage is calculated based on the intent‐to‐treat population. Any medical or surgical intervention received before the day 90 follow‐up that was considered as confounding was imputed (see Statistical analysis). Patients who received medical interventions that commenced after the day 90 follow‐up visit were not included.
Key indications for oral steroids received at day 90 by the treatment patients for other reasons were: 4 (2.0%) asthma exacerbation, 4 (2.0%) acute sinusitis in frontal, maxillary and/or sphinoid sinuses, and 3 (1.5%) upper respiratory tract infection.
ESS = endoscopic sinus surgery; MFNS = mometasone furoate nasal spray.
The overall incidence of adverse events was similar in both groups, and the most common was sinusitis, which occurred less frequently in the treatment group (Table 5). Five treatment patients experienced a device‐related adverse event (epistaxis, nasal discomfort, rhinalgia, parosmia). Four serious adverse events (asthma, streptococcal asthmatic bronchitis, epistaxis, pneumonia) occurred in 3 patients, but only epistaxis requiring surgical cautery was judged to be related to the sinus implant. It occurred after day 30, and the patient recovered without sequelae.
Table 5.
AEs*
| Event System organ class Preferred term | Treatment (n = 201) | Control (n = 99) |
|---|---|---|
| Patients with any AE | 91 (45.3) | 37 (37.4) |
| Patients with common AEs (>2% incidence rate) by system organ class | ||
| Infections and infestations | 61 (30.3) | 25 (25.3) |
| Acute sinusitis | 22 (10.9) | 12 (12.1) |
| Chronic sinusitis | 10 (5.0) | 8 (8.1) |
| Upper respiratory tract infection | 11 (5.5) | 4 (4.0) |
| Bronchitis | 5 (2.5) | 2 (2.0) |
| Ear infection | 5 (2.5) | 0 |
| Respiratory, thoracic and mediastinal disorders | 24 (11.9) | 9 (9.1) |
| Asthma | 10 (5.0) | 4 (4.0) |
| Nasal congestion | 5 (2.5) | 3 (3.0) |
| Patients with any study drug‐related AE | 0 | – |
| Patients with any study device‐related AE | 5 (2.5) | – |
| Respiratory, thoracic, and mediastinal disorders | ||
| Nasal discomfort | ||
| Mild | 1 (0.5) | – |
| Moderate | 1 (0.5) | – |
| Epistaxis (severe) | 1 (0.5) | – |
| Parosmia (mild) | 1 (0.5) | – |
| Rhinalgia (mild) | 1 (0.5) | – |
| Patients with any implant procedure‐ related AE | 8 (4.0) | – |
| General disorders and administration site conditions | ||
| Facial pain (mild) | 1 (0.5) | – |
| Nervous system disorders | ||
| Presyncope (mild) | 2 (1.0) | – |
| Dizziness (mild) | 1 (0.5) | – |
| Respiratory, thoracic and mediastinal disorders | ||
| Epistaxis (moderate) | 1 (0.5) | – |
| Nasal congestion (moderate) | 1 (0.5) | – |
| Nasal discomfort (moderate) | 1 (0.5) | – |
| Parosmia (mild) | 1 (0.5) | – |
| Patients with any AE with indeterminaterelationship | ||
| Study drug | 2 (1.0) | – |
| Study device | 13 (6.5) | – |
| Implant procedure | 10 (5.0) | – |
| Patients with SAE | 2 (1.0) | 1 (1.0) |
| Study drug related SAE | 0 | – |
| Study device related SAE (epistaxis) | 1 (0.5) | – |
| AE leading to study discontinuation | 0 | – |
*Values given in the table are patient counts and percentages. AEs coded using the MedDRA dictionary, version 17.0. At each level of summation, patients are counted only once. Patients experiencing AEs of more than 1 severity are summarized according to the maximum severity experienced over all episodes of an adverse event. Since the mometasone furoate sinus implant is a drug/device combination product, each AE and SAE was evaluated in terms of its relationship to the implant drug, the implant device component, and the implant placement procedure.
AE = adverse event; SAE = serious adverse event.
Discussion
In this phase 3 trial, sinus implants were superior to sham with once‐daily MFNS for the treatment of recurrent NPs in patients with CRS across multiple subjective and objective outcomes. The observed magnitude of improvements with sinus implants is compelling, especially because the study population comprised CRSwNP patients with high prevalence of allergic rhinitis, asthma, and AERD, and those who failed several prior surgeries. All patients were candidates for repeat ESS because of moderate‐to‐severe CRS symptoms and recurrent NPs despite ongoing use of INCS and 1 or more courses of high‐dose corticosteroids in the prior year.
Nasal obstruction/congestion is the most common subjective complaint associated with NP. The treatment group demonstrated rapid superiority to controls in reducing nasal obstruction/congestion scores by day 30 that was sustained throughout the whole duration of the study, with maximal symptomatic improvement in the treatment group at day 90, a full month after implant removal. The observed improvement in the sham group speaks to the high level of compliance with daily MFNS in this study.
Polyp grade is a commonly accepted objective measure of severity in CRSwNP. The implant group demonstrated greater polyp shrinkage compared to sham at day 90, which was also supported by the greater decrease in ethmoid sinus obstruction. Both reductions were based on videoendoscopy grading by an independent panel of 3 sinus surgeons who were masked to both treatment assignment and patient symptoms. The polyp shrinkage was further supported by the onsite clinical investigators who reported the rapid onset of action of implants compared to sham. Although an MCID for NP has not yet been established, a ≥ 1.0‐point reduction in polyp grade has been defined as a meaningful response.26, 27 Based on the clinical investigator scoring, 72% of patients who received implants achieved at least 1.0‐grade reduction and 48% at least 2.0‐grade reduction by day 90, compared to 37% and 16% of sham, respectively. The 2‐fold higher responder rate among treatment patients than control further supports the notion that the treatment with the corticosteroid‐eluting implants provides clinically meaningful benefits to patients with recurrent NP.
Olfactory loss affects quality of life of patients with CRSwNP and represents 1 of the main reasons to seek medical or surgical treatment.28, 29, 30, 31 The magnitude of improvement in olfaction with implants in this study exceeded that seen in prior MFNS trials for NPs and was similar to that seen with surgery, indicating clinical significance.25, 26, 32 The failure to improve facial pain/pressure scores is not surprising because this symptom is more closely associated with CRSsNP, and its association with CRS in general has been questioned.33
A key parameter determining the clinical relevance of any treatment for recurrent CRSwNP would be a reduction in the need for surgery. In the clinical setting, this is largely a decision driven by patient preference, and no standard subjective or objective outcome criteria have not yet been established. For this trial, we utilized prespecified study criteria for indication for repeat surgery, and there was a significantly greater reduction in the need for repeat ESS at day 90 for the treatment group compared to control. Specifically, there was a 61% reduction (from 100% at the onset to 39%) among patients receiving implants, compared to 37% reduction (from 100% to 63.3%) among sham patients. The observed reduction is clinically meaningful given that only patients who experienced significant improvements in both symptoms and NP were considered to be no longer indicated for repeat surgery. Furthermore, the results from an earlier trial suggests that the significant reduction in the need for repeat surgery extends through 6 months.19
The efficacy of corticosteroids for the treatment of nasal polyps is generally dose‐dependent, particularly for oral corticosteroids, which access the ethmoid polyps via the bloodstream. For INCS, doubling the standard dose of MF from 200 μg once daily (QD) to 200 μg twice daily (BID) also results in significantly greater reduction in nasal obstruction. Added reduction in NP size, however, was not clearly demonstrated.9, 25 Although the reason is unclear, this may reflect the fact that 70% of MFNS is swallowed, accessing only the superficial surface of the NPs present in the nasal airway.34 In this trial, the sinus implant plus MFNS significantly reduced both bilateral polyp grade and nasal obstruction/congestion scores when compared to MFNS alone. This likely reflects the ability of the implant to more effectively deliver corticosteroids to the ethmoid mucosa via direct elution onto the roots of the polyps. Unpublished, preclinical animal studies with the implant indicate high tissue concentrations of MF for over 60 days postremoval. Furthermore, the implant may shrink the polyps, permitting superior access and, therefore, increased efficacy of continued daily MFNS. This is analogous to the sustained improvement sometimes observed with INCS following a burst of oral corticosteroids or with surgery.
Limitations of the current study include the absence of a defined medical regimen prior to enrollment. To ensure generalizability, we relied on real‐world management by the treating rhinologists in concert with patient preferences. A second limitation was that the clinical investigators performing endoscopic grading and assessment of indication for repeat ESS at day 90 were not blinded to the treatment assignment. This was mitigated by removing all implants by day 60, providing the day 90 videoendoscopies to the panel for centralized, independent, blinded grading of the co‐primary endpoint and by requiring real‐time grading by clinical investigators without reviewing prior grading and following the prespecified and standardized set of criteria. Although the 2 endoscopic grading methodologies correlated well, the magnitude of polyp shrinkage was greater when evaluated by the unblinded investigators than by the independent, blinded panel. The reasons are unclear but may reflect limitations of videoendoscopy review without other clinical information to fully appraise the magnitude of change. It is noteworthy that the sham arm receiving MFNS only also demonstrated greater polyp shrinkage when evaluated by onsite investigators than when evaluated the blinded panel, which suggests that the lack of investigator blinding may not be a major factor in the rating differences. Specifically, the onsite investigator performing the endoscopy may be better able to assess the magnitude of change in polyp score than someone merely viewing an endoscopy performed by a third party. A third limitation was that the length of the trial was also relatively short (90 days), reflecting the time course of drug release from the implant.
The morbidity and cost of CRSwNP remains substantial, which has led to trials of new treatment options designed to shrink NPs.27, 35, 36, 37 In this regard NPs are now generally viewed as a group of clinical entities driven by distinct but likely overlapping molecular pathways.10 Absent biomarkers, it remains unclear which specific treatment is best suited for these endotypes.38, 39 Furthermore, although initial results are promising, the cost of biologic agents will be high and the durability of response unclear. Corticosteroids on the other hand, are inexpensive and broadly effective, limited primarily by drug induced morbidity and patient compliance. Corticosteroid‐eluting sinus implants are drug/device combination products designed to deliver drug directly to the sinus mucosa in a controlled fashion.22 Our current results suggest that the improvements with the sinus implants are maintained on INCS after implant removal, supporting prior studies indicating efficacy at 6 months postinsertion.19 Moreover, the high degree of safety suggests that repeat placement may be an option for patients still indicated for revision surgery.
Conclusion
Significant improvements over a range of subjective and objective endpoints, including a reduction in the need for sinus surgery by 61%, suggest that MF sinus implants may play an important role in management of CRS patients recurrent NPs.
Supporting information
Supporting Video
Acknowledgements
We thank the patients who participated in RESOLVE II and the study investigators and clinical research coordinators for their contributions. The national co‐principal investigators of the RESOLVE II study were Robert C. Kern, MD (Department of Otolaryngology–Head and Neck Surgery at Northwestern University, Feinberg School of Medicine, Chicago, IL) and J. Pablo Stolovitzky, MD (ENT of Georgia, Atlanta, GA). The RESOLVE II study principal investigators and their affiliations are listed below in descending order of patient enrollment: Stacey L. Silvers, MD (Madison ENT & Facial Plastic Surgery, New York, NY); Andrew R. Gould, MD (Advanced ENT & Allergy Louisville, KY); David M. Yen, MD, FACS (Bethlehem ENT Associates, Bethlehem, PA); Ameet Singh, MD (George Washington Medical Faculty Associates, Washington DC); Jivianne T. Lee, MD (Department of Head and Neck Surgery, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA); David B. Keschner, MD, JD (Orange County Sinus Institute, Southern California Permanente Medical Group, Irvine, CA); Randall Ow, MD (Sacramento Ear, Nose, & Throat Surgical and Medical Group, Inc., Sacramento, CA); Nithin D. Adappa, MD (University of Pennsylvania, Philadelphia, PA); Simon K. Wright, MD, PhD (Iowa ENT Center, PLLC, West Des Moines, IA); Roland Z. Gerencer, MD (BreatheAmerica Albuquerque, NM); Francis P. J. Langford, MD (Charlotte Eye Ear Nose & Throat Associates, P.A., Concord, NC); Alfred M.C. Iloreta, MD (Mount Sinai Hospital, New York, NY); Kevin E. McLaughlin, MD (Associated Surgical Specialists, Covington, LA); Feodor Ung, MD (DuPage Medical Group, Naperville, IL); Jeremy B. Rogers, MD (Florida ENT and Allergy, Riverview, FL); Richard P. Manes, MD (Yale School of Medicine, Yale Otolaryngology, New Haven, CT); Bruce K.H. Tan, MD (Department of Otolaryngology–Head and Neck Surgery, Northwestern University, Chicago, IL); Robert J. Brager, MD (Virginia Ear Nose & Throat Associates, Richmond, VA); Edwin J. Lee, MD (Reston ENT, PC, Reston, VA); Steven K. Miller, MD (Intermountain Ear, Nose & Throat Center, Salt Lake City, UT); Daniel A. Rontal, MD (The Rontal‐Akervall Clinic, Royal Oak, MI); Joseph K. Han, MD (Department of Otolaryngology, Eastern Virginia Medical School, Norfolk, VA); Rakesh K. Chandra, MD (Vanderbilt University Medical Center, Nashville, TN); Jeffrey G. Bennion, MD (Cache Valley Ear Nose & Throat, Bridgerland Clinical Research, North Logan, UT); Donald D. Beahm, MD (University of Kansas Medical Center, Kansas City, KS); Jeffrey D. LeBenger, MD (Summit Medical Group, Berkeley Heights, NJ); David M. Poetker, MD (Medical College of Wisconsin, Milwaukee, WI); Andrew P. Lane, MD (Johns Hopkins University [Outpatient Center], Baltimore, MD); Do‐Yeon Cho, MD (The Kirklin Clinic of UAB Hospital, Birmingham, AL); Robert T. Adelson, MD (Albany ENT & Allergy Services, PC, Albany, NY); Joseph L. Romett, MD (Colorado ENT & Allergy, Colorado Springs, CO); Amber U. Luong, MD, PhD (McGovern Medical School at the University of Texas Health Science Center, Houston, TX); Rodney J. Schlosser, MD (Department of Otolaryngology–Head and Neck Surgery, Medical University of South Carolina, Charleston, SC); and William J. Brown, MD (South Florida ENT, Miami, FL).
How to Cite this Article: Kern RC, Stolovitzky JP, Silvers SL, et al. A phase 3 trial of mometasone furoate sinus implants for chronic sinusitis with recurrent nasal polyps. Int Forum Allergy Rhinol. 2018;8:471–481.
All principal investigators participating in the RESOLVE II study are listed in the Acknowledgments.
Funding sources for the study: Intersect ENT, Inc.
Public clinical trial registration: http://clinicaltrials.gov/show/NCT02291549. RESOLVE II: A Clinical Evaluation of the Safety and Efficacy of the Steroid‐Releasing S8 Sinus Implant in Chronic Sinusitis Patients With Recurrent Sinus Obstruction.
Potential conflict of interest: The study sponsor (Intersect ENT, Inc.) provided the investigational product, funding and administrative and logistical support to the participating clinical sites. The sponsor was involved in the design and conduct of the RESOLVE II trial, as it was an U.S. Food and Drug Administration (FDA)‐regulated study, and assisted in monitoring and collection of data. The sponsor participated in the interpretation of the data and preparation of the manuscript. All authors reviewed and critiqued the draft manuscript and approved the final manuscript prior to submission for publication. R.L.K. has no conflict of interest to report. J.P.S. received consulting fees from Intersect ENT, Acclarent, and Spirox, as well as research grants from Acclarent, Spirox, and Otonomy. S.L.S. has no conflict of interest to report. A.S. received consulting fees from Intersect ENT. J.T.L. received consulting fees from Intersect ENT. D.M.Y. received consulting fees from Intersect ENT. A.M.C.I. has no conflict of interest to report. F.P.J.L. has no conflict of interest to report; B.K. received consulting fees from Intersect ENT. K.E.M. received consulting fees from Intersect ENT, Acclarent, Aerin Medical, ENTVantageDx, Olympus, and Stryker. J.W.S. and A.K.G. are employees of and hold stock in Intersect ENT. Andy Mugglin, PhD, from Paradigm Biostatistics, Inc. provided biostatistical advice. The statistical analyses were performed by independent biostatisticians (Saling Huang, PhD, and I‐Ling Hsiue). SINUVA™ (mometasone furoate) Sinus Implant was approved by the U.S. FDA on December 8, 2017, for the treatment of nasal polyps, in patients ≥ 18 years of age who have had ethmoid sinus surgery.
References
- 1. Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: national health interview survey, 2012. Vital Health Stat 10. 2014;260:1–161. [PubMed] [Google Scholar]
- 2. Hastan D, Fokkens WJ, Bachert C, et al. Chronic rhinosinusitis in Europe—an underestimated disease. A GA(2)LEN study. Allergy. 2011;66:1216–1223. [DOI] [PubMed] [Google Scholar]
- 3. Hirsch AG, Stewart WF, Sundaresan AS, et al. Nasal and sinus symptoms and chronic rhinosinusitis in a population‐based sample. Allergy. 2017;72:274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Orlandi RR, Kingdom TT, Hwang PH, et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(Suppl 1):S22–S209. [DOI] [PubMed] [Google Scholar]
- 5. Tan BK, Chandra RK, Pollak J, et al. Incidence and associated premorbid diagnoses of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;131:1350–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ghogomu N, Kern R. Chronic rhinosinusitis: the rationale for current treatments. Expert Rev Clin Immunol. 2017;13:259–270. [DOI] [PubMed] [Google Scholar]
- 7. Hopkins C, Slack R, Lund V, et al. Long‐term outcomes from the English national comparative audit of surgery for nasal polyposis and chronic rhinosinusitis. Laryngoscope. 2009;119:2459–2465. [DOI] [PubMed] [Google Scholar]
- 8. Rudmik L. Economics of chronic rhinosinusitis. Curr Allergy Asthma Rep. 2017;17:20. [DOI] [PubMed] [Google Scholar]
- 9. Smith SS, Evans CT, Tan BK, et al. National burden of antibiotic use for adult rhinosinusitis. J Allergy Clin Immunol. 2013;132:1230–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang X, Zhang N, Bo M, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016;138:1344–1353. [DOI] [PubMed] [Google Scholar]
- 11. Lam K, Schleimer R, Kern RC. The etiology and pathogenesis of chronic rhinosinusitis: a review of current hypotheses. Curr Allergy Asthma Rep. 2015;15:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schleimer RP. Immunopathogenesis of chronic rhinosinusitis and nasal polyposis. Annu Rev Pathol. 2017;12:331–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Head K, Chong LY, Piromchai P, et al. Systemic and topical antibiotics for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016;4:CD011994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kalish L, Snidvongs K, Sivasubramaniam R, et al. Topical steroids for nasal polyps. Cochrane Database Syst Rev. 2012;12:CD006549. [DOI] [PubMed] [Google Scholar]
- 15. Leung RM, Chandra RK, Kern RC, et al. Primary care and upfront computed tomography scanning in the diagnosis of chronic rhinosinusitis: a cost‐based decision analysis. Laryngoscope. 2014;124:12–18. [DOI] [PubMed] [Google Scholar]
- 16. Poetker DM. Oral corticosteroids in the management of adult chronic rhinosinusitis with and without nasal polyps: an evidence‐based review with recommendations. Int Forum Allergy Rhinol. 2013;3:104–120. [DOI] [PubMed] [Google Scholar]
- 17. Sanan A, Rabinowitz M, Rosen M, Nyquist G. Topical therapies for refractory chronic rhinosinusitis. Otolaryngol Clin North Am. 2017;50:129–141. [DOI] [PubMed] [Google Scholar]
- 18. Han JK, Marple BF, Smith TL, et al. Effect of steroid‐releasing sinus implants on postoperative medical and surgical interventions: an efficacy meta‐analysis. Int Forum Allergy Rhinol. 2012;2:271–279. [DOI] [PubMed] [Google Scholar]
- 19. Forwith KD, Han JK, Stolovitzky JP, et al. RESOLVE: bioabsorbable steroid‐eluting sinus implants for in‐office treatment of recurrent sinonasal polyposis after sinus surgery: 6‐month outcomes from a randomized, controlled, blinded study. Int Forum Allergy Rhinol. 2016;6:573–581. [DOI] [PubMed] [Google Scholar]
- 20. Han JK, Forwith KD, Smith TL, et al. RESOLVE: a randomized, controlled, blinded study of bioabsorbable steroid‐eluting sinus implants for in‐office treatment of recurrent sinonasal polyposis. Int Forum Allergy Rhinol. 2014;4:861–870. [DOI] [PubMed] [Google Scholar]
- 21. Lavigne F, Miller SK, Gould AR, et al. Steroid‐eluting sinus implant for in‐office treatment of recurrent nasal polyposis: a prospective, multicenter study. Int Forum Allergy Rhinol. 2014;4:381–389. [DOI] [PubMed] [Google Scholar]
- 22. Ow R, Groppo E, Clutter D, Gawlicka AK. Steroid‐eluting sinus implant for in‐office treatment of recurrent polyposis: a pharmacokinetic study. Int Forum Allergy Rhinol. 2014;4:816–822. [DOI] [PubMed] [Google Scholar]
- 23. Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50:1–12. [DOI] [PubMed] [Google Scholar]
- 24. Meltzer EO, Hamilos DL, Hadley JA, et al. Rhinosinusitis: developing guidance for clinical trials. J Allergy Clin Immunol. 2006;118:S17–S61. [DOI] [PubMed] [Google Scholar]
- 25. Small C, Hernandez J, Reyes A, et al. Efficacy and safety of mometasone furoate nasal spray in nasal polyposis. J Allergy Clin Immunol. 2005;116:1275–1281. [DOI] [PubMed] [Google Scholar]
- 26. Stjarne P, Mosges R, Jorissen M, et al. A randomized controlled trial of mometasone furoate nasal spray for the treatment of nasal polyposis. Arch Otolaryngol Head Neck Surg. 2006;132:179–185. [DOI] [PubMed] [Google Scholar]
- 27. Bachert C, Mannent L, Naclerio RM, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. 2016;315:469–479. [DOI] [PubMed] [Google Scholar]
- 28. Alobid I, Mullol J. Role of medical therapy in the management of nasal polyps. Curr Allergy Asthma Rep. 2012;12:144–153. [DOI] [PubMed] [Google Scholar]
- 29. Blomqvist E, Lundblad L, Anggards A, et al. A randomized controlled study evaluating medical treatment versus surgical treatment in addition to medical treatment of nasal polyposis. J Allergy Clin Immunol. 2001;107:224–228. [DOI] [PubMed] [Google Scholar]
- 30. Hox V, Bobic S, Callebaux I, et al. Nasal obstruction and smell impairment in nasal polyp disease: correlation between objective and subjective parameters. Rhinology. 2010;48:426–432. [DOI] [PubMed] [Google Scholar]
- 31. Rudmik L, Smith TL. Olfactory improvement after endoscopic sinus surgery. Curr Opin Otolaryngol Head Neck Surg. 2012;20:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bhattacharyya N. Clinical and symptom criteria for the accurate diagnosis of chronic rhinosinusitis. Laryngoscope. 2006;116:1–22. [DOI] [PubMed] [Google Scholar]
- 33. Falco JJ, Thomas AJ, Quin X, et al. Lack of correlation between patient reported location and severity of facial pain and radiographic burden of disease in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016;6:1173–1181. [DOI] [PubMed] [Google Scholar]
- 34. Lipworth BJ, Jackson CM. Safety of inhaled and intranasal corticosteroids: lessons for the new millennium. Drug Saf. 2000;23:11–33. [DOI] [PubMed] [Google Scholar]
- 35. Bachert C, Sousa AR, Lund VJ, et al. Reduced need for surgery in severe nasal polyposis with mepolizumab: randomized trial. J Allergy Clin Immunol. 2017;140:1024–1031.e14. [DOI] [PubMed] [Google Scholar]
- 36. Gevaert P, Calus L, Van Zele T, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013;131:110–116.e1. [DOI] [PubMed] [Google Scholar]
- 37. Gevaert P, Van Bruaene N, Cattaert T , et al. Mepolizumab, a humanized anti‐IL‐5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011;128:989–995.e8. [DOI] [PubMed] [Google Scholar]
- 38. Bachert C, Zhang L, Gevaert P. Current and future treatment options for adult chronic rhinosinusitis: focus on nasal polyposis. J Allergy Clin Immunol. 2015;136:1431–1440; quiz 1441. [DOI] [PubMed] [Google Scholar]
- 39. Lam K, Kern RC, Luong A. Is there a future for biologics in the management of chronic rhinosinusitis? Int Forum Allergy Rhinol. 2016;6:935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Video


