Abstract
Background
While the prevalence of Stenotrophomonas maltophilia lung infection in cystic fibrosis (CF) patients has increased in the last decades, its pathogenicity remains controversial. The aim of this study was to investigate the effects of S. maltophilia initial infection on the progression of lung disease in CF children.
Methods
This case‐control retrospective study took place in a pediatric CF center. A total of 23 cases defined by at least one sputum culture positive for S. maltophilia, were matched for age, sex, and CFTR mutations to 23 never infected CF controls. The clinical data were collected for 2 years before and after S. maltophilia initial infection and comprised lung function analyses, rates of exacerbations and of antibiotic courses.
Results
Compared with controls, cases had lower lung function (P = 0.05), more frequent pulmonary exacerbations (P = 0.01), hospitalizations (P = 0.02), and intravenous antibiotic courses (P = 0.04) before S. maltophilia acquisition. In the year following S. maltophilia initial infection, lung function decline was similar in cases and controls but cases remained more severe, with more frequent pulmonary exacerbations (P = 0.01), hospitalizations (P = 0.02) and intravenous antibiotic courses (P = 0.02).
Conclusions
S. maltophilia seems to be a marker of CF lung disease severity and international recommendations to reduce lung infection by this pathogen should rapidly emerge.
Keywords: children, cystic fibrosis, lung function, pulmonary exacerbation Stenotrophomonas maltophilia
1. INTRODUCTION
Cystic fibrosis (CF) is the most common severe autosomal recessive genetic disease in Caucasians. CF is caused by mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR), a chloride channel expressed in the epithelial cells throughout the body.1 The disease affects many organs including the pancreas, the liver, the intestine, and, most critically, the lungs.2 Lung disease remains the major cause of morbidity and mortality in CF, with a progressive decline of the lung function due to a vicious cycle of airway infections and inflammation.3, 4 Despite improvement in treatments with better survival since a few decades, poly‐bacterial and chronic respiratory infections contribute to the lung disease, leading to a respiratory insufficiency.
Although the prevalence of Stenotrophomonas maltophilia (S. maltophilia) lung infection in patients with CF has increased in the last decades, its pathogenicity is still controversial.5, 6, 7, 8 While its prevalence varies worldwide and according to the CF centers, it reached in 2015 almost 14% in the US and 10.4% in France.9, 10 There is no recommendation concerning the management of S. maltophilia infection as it is actually unclear whether antimicrobial treatments directed against S. maltophilia alter its course in CF.11, 12, 13, 14 Therefore, we conducted a retrospective study to investigate the effects of S. maltophilia initial infection on the progression of lung disease in CF children.
2. METHODS
2.1. Patients
This retrospective study took place in a pediatric CF center in Paris where 213 CF patients are registered. The cases were defined as CF patients with at least one positive sputum culture with S. maltophilia during the follow‐up. These cases were matched for age, sex, and CFTR mutations with CF patients for whom S. maltophilia had never been identified (CF controls).
The clinical data were retrospectively collected from electronic patient records, supplemented when necessary with data from the paper patient records. The database and data collection have been approved by French national data protection authorities (CNIL n°908324 and CCTIRS n°08.015bis) and each patient and/or his legal guardians were informed prior to entering their data into the database. For each case, the date of initial infection by S. maltophilia defined time T0. Clinical data were subsequently collected over 2 years before and after T0, defining time T‐24, T‐12, T12, and T24. Measurements of the Forced Expiratory Volume in one second (FEV1) expressed as percent‐predicted values using the modified Knudson equations,15 rates of pulmonary exacerbations, of hospitalizations for pulmonary exacerbations and of antibiotic courses, and changes in the airways’ bacterial colonization were obtained.
2.2. Statistical analysis
The data were expressed as means ± SD for continuous variables and numbers (%) for categorical variables. The paired Wilcoxon rank sum test was used to compare quantitative data and Fisher's exact test for categorical data. A random effects linear regression was used for longitudinal data. The differences were considered significant for P‐values less than 0.05.
3. RESULTS
3.1. Analyses of the cases infected with S. maltophilia
The demographic data for the cases included are described in Table 1. Briefly, 34 CF patients (19 girls and 15 boys) were included. Of these, 15 (44%) were homozygotes for the CFTR F508 del mutation, and all were pancreatic insufficient. At T0, the median age was 10.1 ± 5.2 years, and the median BMI z‐score was −1.08 ± 1.43. During the 2 years following S. maltophilia initial infection, from T‐24 to T24, the FEV1 decreased by 3% per year from 81 ± 19% to 70 ± 22% (P < 0.0001, Figure 1A). The annual rate of respiratory exacerbations and hospitalizations did not change substantially (+0.23/year, P = 0.24; and +0.14/year, P = 0.11, respectively) (Figures 1B and 1C).
Table 1.
Baseline characteristics of the cases before S. maltophilia initial infection
| Total (n) | 34 |
|---|---|
| Sex (male/female) | 15/19 |
| CFTR F508 del homozygous, % (n) | 44% (15) |
| FEV1pp, mean (SD) | |
| 2 years before S. maltophilia infection | 81 (19) |
| 1 year before S. maltophilia infection | 74 (22) |
| At S. maltophilia infection | 72 (21) |
| Average decrease/yr | 4.3 (1.4) |
| BMI (Z‐score), mean (SD) | −1.08 (1.43) |
| Annual rates of respiratory exacerbations, mean (SD) | 3.3 (1.7) |
| Annual rates of hospitalizations, mean (SD) | 1.1 (1.2) |
| Number of patients with intravenous antibiotic courses, % (n) | 52% (15) |
| P. aeruginosa colonization, % (n) | 8.8% (3) |
| MRSA colonization, % (n) | 8.8% (3) |
CFTR, cystic fibrosis transmembrane regulator; FEV1pp, forced expiratory volume in one second percent predicted; BMI, body mass index; P. aeruginosa, Pseudomonas aeruginosa; MRSA, methicillin‐resistant Staphyloccocus aureus.
The data are the means (SD) or numbers (%) unless otherwise indicated.
Figure 1.
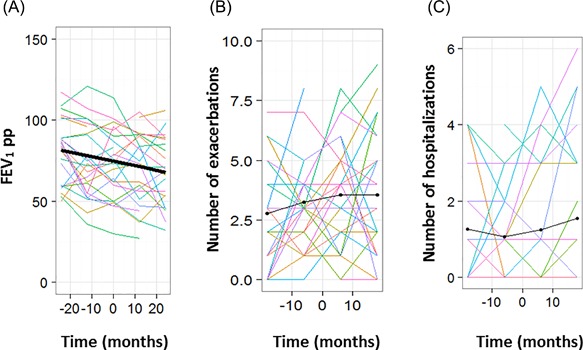
Following S. maltophilia respiratory infection in the cases, evolution of (A) the forced expiratory volume in one second percent predicted (FEV1pp), (B) number of exacerbations and (C) number of hospitalizations. Each colored line represents one patient, the thick line represents the mean
3.2. Case‐control comparison analyses
We found age, sex and CFTR matched controls for 23 of the 34 cases (Table 2). Compared with the controls, the cases already had more frequent pulmonary exacerbations (P = 0.01), hospitalizations (P = 0.02) and intravenous antibiotic courses (P = 0.04) before S. maltophilia acquisition (Table 2). Lung function was lower at S. maltophilia infection onset (FEV1 71 ± 20% vs 90 ± 21%, respectively, P = 0.05). Moreover, colonization by P. aeruginosa and methicillin‐resistant Staphyloccocus aureus (MRSA) was only present in the cases (incidence 13% in the cases vs 0% in the controls for both bacteria, NS).
Table 2.
Baseline characteristics of the cases and their sex, age, and CFTR matched controls before S. maltophilia initial infection
| Cases (n = 23) | Controls (n = 23) | P‐value a | |
|---|---|---|---|
| Age (yrs), mean (SD) | 8.8 (5.3) | 8.9 (5.0) | 1 |
| Sex (male/female) | 8/15 | 8/15 | 1 |
| CFTR F508 del homozygous, % (n) | 48% (11) | 52% (12) | 1 |
| FEV1pp, mean (SD) | |||
| 2 years before S. maltophilia infection | 83 (17) | 91 (18) | 0.25 |
| 1 year before S. maltophilia infection | 72 (21) | 89 (20) | 0.13 |
| At S. maltophilia infection | 71 (20) | 90 (21) | 0.05* |
| BMI (Z‐score), mean (SD) | −0.88 (1.01) | −0.15 (0.73) | 0.09 |
| Annual rates of respiratory exacerbations, mean (SD) | 3.0 (1.6) | 1.6 (1.5) | 0.01* |
| Annual rates of hospitalizations, mean (SD) | 0.71 (1.01) | 0.11 (0.32) | 0.02* |
| Number of patients with intravenous antibiotic courses, % (n) | 34.7% (8) | 8.6% (2) | 0.04* |
| P. aeruginosa colonization, % (n) | 13% (3) | 0% (0) | 0.25 |
| MRSA colonization, % (n) | 13% (3) | 0% (0) | 0.25 |
The data are the means (SD) or % (numbers) unless otherwise indicated.
FEV1pp, forced expiratory volume in one second percent predicted; BMI, body mass index; P. aeruginosa, Pseudomonas aeruginosa; MRSA, methicillin‐resistant Staphyloccocus aureus.
For the comparison of sex, age, and CFTR matched cases and controls.
*P < 0.05.
In the year following S. maltophilia initial infection, FEV1 rate of decline was similar in the cases and the controls (P = 0.8, Table 3 & Figure 2A). The cases remained more severe, with more frequent pulmonary exacerbations (P = 0.015, Figure 2B), hospitalizations (P = 0.018, Figure 2C) and intravenous antibiotic courses (P = 0.024) (Table 3 ). New P. aeruginosa airway colonization was more frequent in the cases, although not significantly (incidence 43% in the cases vs 4.3% in the controls, P = 0.18, Table 3).
Table 3.
Case‐control comparison in the year following S. maltophilia initial infection
| Cases (n = 23) | Controls (n = 23) | P‐value a | |
|---|---|---|---|
| Delta‐FEV1, mean (SD) | −1.3 (9.5) | −2.3 (11.9) | 0.8 |
| Delta‐BMI (Z‐score), mean (SD) | 0.12 (0.78) | 0.10 (0.51) | 0.98 |
| Annual rates of respiratory exacerbations, mean (SD) | 3.6 (1.8) | 2.1 (1.8) | 0.015 * |
| Annual rates of hospitalizations, mean (SD) | 1.14 (1.71) | 0.29 (0.90) | 0.018 * |
| Number of patients with intravenous antibiotic courses, % (n) | 39% (9) | 9% (2) | 0.024 * |
| Number of patients with incident colonization by P. aeruginosa, % (n) | 29% (5) | 6% (1) | 0.18 |
| Number of patients with incident colonization by MRSA, % (n) | 12% (2) | 6% (1) | 1.0 |
The data are the means (SD) or % (numbers) unless otherwise indicated.
FEV1, forced expiratory volume in one second; BMI, body mass index, P. aeruginosa, Pseudomonas aeruginosa; MRSA, methicillin‐resistant Staphyloccocus aureus.
For the comparison of sex, age, and CFTR matched cases and controls.
P < 0.05.
Figure 2.
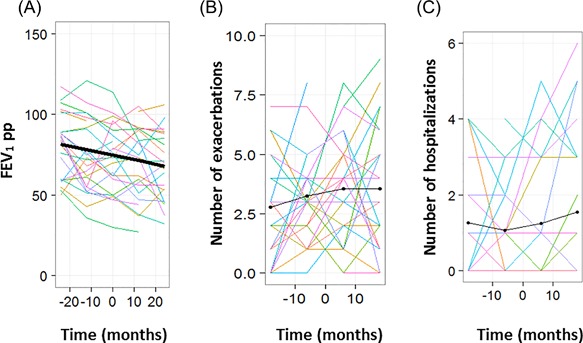
In the matched cases and controls, evolution of (A) the forced expiratory volume in one second percent predicted (FEV1pp), (B) number of exacerbations and (C) number of hospitalizations. Each colored line represents one patient, the thick line represents the mean
4. DISCUSSION
In age, sex and CFTR matched CF children, we have shown that initial infection by S. maltophilia was more frequently found in those with the more severe lung disease. Indeed, before the initial infection and in comparison with age, sex and CFTR matched CF controls, these children had a lower lung function, experienced more frequent respiratory exacerbations and required more frequent hospitalizations and intravenous antibiotics. Besides, after the initial infection, the cases remained more severe.
We found a high prevalence of airway infections by S. maltophilia, reaching almost 16% in children with CF. The 2015 French CF Registry reports a 10.4% prevalence in the overall French CF cohort with a higher prevalence in the younger patients, ie, 13% for the 10‐14 years old and 18.5% for the 15‐19 years old.10 Similarly, a large US cohort also shows a recent increase in the prevalence.16 This recent increasing prevalence may be secondary to the improvement of S. maltophilia detection that requires expert laboratories as evidenced by the study of Burns et al.17
Our results are in accordance to those of Goss et al who evaluated the effect of S. maltophilia on the lung function of CF patients from the US registry.6 They observed indeed that patients with S. maltophilia had a worse lung function at the time of positivity but no modification in the lung function rate of decline. Talmaciu et al further questioned whether long‐term antibiotics may have predisposed the patients to acquisition of this bacterium.14 We also observed in this pediatric cohort that only the cases were colonized by P. aeruginosa and MRSA, two pathogens known to be associated with an accelerated lung function decline in CF patients.18 Moreover, Junge et al recently showed that S. maltophila co‐infection was a risk factor of a worse lung function in CF patients with persistent S. aureus airway colonization.19 Besides, Waters et al showed that chronic S. maltophilia infection was associated with an almost three‐fold increased risk of death or lung transplant in CF patients.20 It is however still unclear whether S. maltophilia infection is only a marker of CF lung disease severity or whether it leads to an acceleration of the disease progression.20 In a large cohort of young CF patients, a recent study identified that a respiratory culture positive for S. maltophilia was one of the risk factors identified associated with a larger annual FEV1 rate of decline.21 Altogether, these studies along with ours, agree that S. maltophilia infection is associated with a severe CF lung disease.
To conclude, we have shown that S. maltophilia may be a marker of severity of the CF respiratory disease. While S. maltophilia was considered as a classical but infrequent bacterium infecting the airways of CF patients until the 2000's, its incidence appears to be increasing in the recent decades.9, 10 The effectiveness of antibiotic treatments for this multi‐drug resistant organism is still unclear, and despite several Cochrane reviews, there are no treatment recommendations.11, 22, 23 As this bacterium should be considered as a severe airway pathogen, international recommendations to reduce S. maltophilia lung infection should rapidly emerge.
CONFLICT OF INTEREST
None of the authors have any commercial or other associations that might pose a conflict of interest.
ACKNOWLEDGMENTS
The authors would like to thank the nurses from Trousseau Pediatric CF Center for their help in the patients’ data collection. This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Berdah L, Taytard J, Leyronnas S, Clement A, Boelle P‐Y, Corvol H. Stenotrophomonas maltophilia: A marker of lung disease severity. Pediatric Pulmonology. 2018;53: 426–430. https://doi.org/10.1002/ppul.23943
REFERENCES
- 1. Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989; 245:1066–1073. [DOI] [PubMed] [Google Scholar]
- 2. Corvol H, Thompson KE, Tabary O, le Rouzic P, Guillot L. Translating the genetics of cystic fibrosis to personalized medicine. Transl Res. 2016; 168:40–49. [DOI] [PubMed] [Google Scholar]
- 3. Ratjen F, Doring G. Cystic fibrosis. Lancet. 2003; 361:681–689. [DOI] [PubMed] [Google Scholar]
- 4. Robinson P. Cystic fibrosis. Thorax. 2001; 56:237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003; 168:918–951. [DOI] [PubMed] [Google Scholar]
- 6. Goss CH, Mayer‐Hamblett N, Aitken ML, Rubenfeld GD, Ramsey BW. Association between Stenotrophomonas maltophilia and lung function in cystic fibrosis. Thorax. 2004; 59:955–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goss CH, Otto K, Aitken ML, Rubenfeld GD. Detecting Stenotrophomonas maltophilia does not reduce survival of patients with cystic fibrosis. Am J Respir Crit Care Med. 2002; 166:356–361. [DOI] [PubMed] [Google Scholar]
- 8. Waters V, Atenafu EG, Salazar JG, et al. Chronic Stenotrophomonas maltophilia infection and exacerbation outcomes in cystic fibrosis. J Cyst Fibros. 2011; 11:8–13. [DOI] [PubMed] [Google Scholar]
- 9.Cystic Fibrosis Foundation Patient Registry − 2015 Annual Data Report. 2016.
- 10. Bellis G, Delhillotte C, Lemonnier L. Registre Français de la mucoviscidose – Bilan des données. 2015. 2016.
- 11. Amin R, Waters V. Antibiotic treatment for Stenotrophomonas maltophilia in people with cystic fibrosis. Cochrane Database Syst Rev. 2016; 7:CD009249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Denton M, Todd NJ, Littlewood JM. Role of anti‐pseudomonal antibiotics in the emergence of Stenotrophomonas maltophilia in cystic fibrosis patients. Eur J Clin Microbiol Infect Dis. 1996; 15:402–405. [DOI] [PubMed] [Google Scholar]
- 13. Marchac V, Equi A, Le Bihan‐Benjamin C, Hodson M, Bush A. Case‐control study of Stenotrophomonas maltophilia acquisition in cystic fibrosis patients. Eur Respir J. 2004; 23:98–102. [DOI] [PubMed] [Google Scholar]
- 14. Talmaciu I, Varlotta L, Mortensen J, Schidlow DV. Risk factors for emergence of Stenotrophomonas maltophilia in cystic fibrosis. Pediatr Pulmonol. 2000; 30:10–15. [DOI] [PubMed] [Google Scholar]
- 15. Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow‐volume curve with growth and aging. Am Rev Respir Dis. 1983; 127:725–734. [DOI] [PubMed] [Google Scholar]
- 16. Salsgiver EL, Fink AK, Knapp EA, et al. Changing epidemiology of the respiratory bacteriology of patients with cystic fibrosis. Chest. 2016; 149:390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burns JL, Emerson J, Stapp JR, et al. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis. 1998; 27:158–163. [DOI] [PubMed] [Google Scholar]
- 18. Hubert D, Reglier‐Poupet H, Sermet‐Gaudelus I, et al. Association between Staphylococcus aureus alone or combined with Pseudomonas aeruginosa and the clinical condition of patients with cystic fibrosis. J Cyst Fibros. 2013; 12:497–503. [DOI] [PubMed] [Google Scholar]
- 19. Junge S, Gorlich D, den Reijer M, et al. Factors associated with worse lung function in cystic fibrosis patients with persistent Staphylococcus aureus . PLoS ONE. 2016; 11:e0166220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Waters V, Atenafu EG, Lu A, Yau Y, Tullis E, Ratjen F. Chronic Stenotrophomonas maltophilia infection and mortality or lung transplantation in cystic fibrosis patients. J Cyst Fibros. 2013; 12:482–486. [DOI] [PubMed] [Google Scholar]
- 21. Cogen J, Emerson J, Sanders DB, et al. Risk factors for lung function decline in a large cohort of young cystic fibrosis patients. Pediatr Pulmonol. 2015; 50:763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Amin R, Waters V. Antibiotic treatment for Stenotrophomonas maltophilia in people with cystic fibrosis. Cochrane Database Syst Rev. 2012; 5:CD009249. [DOI] [PubMed] [Google Scholar]
- 23. Amin R, Waters V. Antibiotic treatment for Stenotrophomonas maltophilia in people with cystic fibrosis. Cochrane Database Syst Rev. 2014; 4:CD009249. [DOI] [PubMed] [Google Scholar]


