Abstract
Background
Fatigue is commonly experienced in end‐stage kidney disease (ESKD) patients. In order to develop patient‐centred psychosocial interventions to help patients manage fatigue symptoms, a more in‐depth understanding regarding the experience of fatigue is needed.
Objective
The objective of this study was to explore renal patients’ experiences of fatigue, across renal replacement therapy (RRT) modalities.
Methods
Twenty‐five in‐depth semi‐structured interviews were conducted. Interviews were audio‐taped, transcribed, and analysed using inductive thematic analysis.
Results
Main themes included the strong role of the illness and treatment in the aetiology of fatigue. Two contrasting streams of illness–fatigue interpretations emerged: catastrophizing versus normalizing. Participants emphasized the importance of having a sense of purpose in facilitating active management of fatigue. Many participants described the consequences of fatigue on their functioning. Low mood, frustration, and anger were common emotional consequences of fatigue. Three dominant fatigue management strategies emerged: one related to accommodation of activities around fatigue, another on increasing activities to counteract fatigue, and the third one revolved around self‐compassion. Social support emerged as an important aspect of the fatigue experience, serving as a source of motivation, yet participants were wary of becoming a burden to others.
Conclusion
Findings identify casual attributions, behavioural and emotional reactions, management strategies, and facilitators of active management of fatigue in ESKD. Untying fatigue from the illness and treatment may help patients to develop alternative less catastrophic perceptions of fatigue, increase their perception of control over fatigue, and facilitate active fatigue management.
Statement of contribution.
What is already known on this subject?
Fatigue is persistent and debilitating in end‐stage kidney disease (ESKD), with no consistent treatment model.
Promising evidence is available for psychological fatigue interventions in other chronic conditions.
There is a gap in studies looking at the fatigue experiences of patients with ESKD across renal replacement therapies.
What does this study add?
Fatigue is not inherently negative, but shaped by patients’ beliefs and behaviours.
Findings provide novel insights, for example, on the important role social support seems to play in fatigue.
An in‐depth understanding of fatigue may help to inform a future patient‐centred intervention in ESKD.
Keywords: kidney disease, haemodialysis, fatigue, vitality, depression, anxiety, sleep quality, qualitative study, thematic analysis, coping
Background
End‐stage kidney disease (ESKD) is a complex and serious illness, characterized by insufficient renal functioning with renal replacement therapy (RRT) necessary to sustain life. Fatigue is one of the most prominent and debilitating symptoms among renal patients affecting between 42% and 89% of patients, depending on treatment modality and fatigue assessment used (Artom, Moss‐Morris, Caskey, & Chilcot, 2014; Bossola, Vulpio, & Tazza, 2011). Fatigue is a complex and subjective symptom that has been described as extreme and persistent tiredness that cannot be alleviated by rest (Dittner, Wessely, & Brown, 2004). There is extensive evidence on its detrimental consequences on functioning and clinical outcomes (Artom et al., 2014; Bonner, Caltabiano, & Berlund, 2013; Bossola et al., 2015; Davison & Jhangri, 2010; Jhamb et al., 2009, 2011; Koyama et al., 2010).
There is increasing recognition regarding the importance of psychological factors in the perpetuation and maintenance of fatigue in chronic conditions, above and beyond the role of demographic, social‐situational, and clinical factors (Donovan, Small, Andrykowski, Munster, & Jacobsen, 2007; Irving, Matcham, Ali, & Chalder, 2015; Van Kessel & Moss‐Morris, 2006). An understanding of the contribution of these factors to fatigue has translated into successful psychological interventions, particularly Cognitive Behavioural Therapy (CBT) that focuses on changing negative beliefs and unhelpful behaviours (Cramp et al., 2013; Kangas, Bovbjerg, & Montgomery, 2008; van den Akker et al., 2016). Although limited, there is also evidence in haemodialysis (HD) on the important role of distress, negative beliefs about fatigue, and unhelpful behavioural patterns in fatigue (Chilcot, Moss‐Morris, et al., 2016). Qualitative methods can provide a more in‐depth understanding of these and other factors in fatigue, particularly the content and context of the negative fatigue beliefs patients hold, which may guide the development of patient‐centred fatigue management interventions.
Although fatigue has emerged as a dominant theme across several qualitative studies on the experience of living with ESKD (e.g., Hagren, Pettersen, Severinsson, Lützén, & Clyne, 2001; Heiwe, Clyne, & Dahlgren, 2003), at present only four fatigue‐specific qualitative studies are available among dialysis patients (Horigan & Barroso, 2016; Horigan, Schneider, Docherty, & Barroso, 2013; Lee, Lin, Chaboyer, Chiang, & Hung, 2007; Yngman‐Uhlin, Friedrichsen, Gustavsson, Fernström, & Edéll‐Gustafsson, 2010). These studies offer valuable insights into the dominance of biomedical causal attributions for fatigue, the role of sleep quality in further exacerbating fatigue, and pervasiveness of passive fatigue management strategies (Horigan et al., 2013; Lee et al., 2007; Yngman‐Uhlin et al., 2010). Horigan and Barroso (2016) explored in more depth temporal patterns of fatigue in HD patients, some patients reported continuous severe fatigue, whilst others only suffered from post‐dialysis‐specific fatigue. In our study, we build on the findings of the aforementioned studies, in a larger sample across RRT modalities, exploring fatigue in kidney transplant recipients and patients in pre‐dialysis care. Given different RRT modalities involve different treatment demands and stressors, it is important to understand the experiences of fatigue across the spectrum of kidney failure. In order to address the current gaps in our knowledge, we will explore in more depth patients’ perceptions of general tiredness, illness‐related fatigue, and post‐dialysis‐specific fatigue and the ways in which they manage fatigue.
Study aims
The aim of this study was to explore renal patients’ experiences of fatigue across the full spectrum of ESKD, with subquestions exploring:
What is the nature of fatigue?
How do patients describe general tiredness, illness‐related fatigue, and post‐dialysis‐specific fatigue?
What fatigue‐specific beliefs patients hold?
What are the consequences of fatigue on functioning?
What strategies patients use to cope with fatigue?
Are there differences in fatigue experiences between treatment modalities?
t
Method
Design
A qualitative study of fatigue in ESKD, adopting a pluralist methodological approach through an exploration of patterns between the qualitative data and the comprehensive quantitative characteristics of the sample.
Sample
Patients were recruited from a renal outpatient unit in England. Participants were selected for the interview using purposive maximum variation to ensure variability of the sample (Patton, 2002): in demographic characteristics, RRT, dialysis vintage, fatigue severity, and exercise.
Inclusion/exclusion criteria
The inclusion criteria were as follows: (1) adults with a confirmed ESKD diagnosis, (2) receiving any RRT or in pre‐dialysis care, and (3) able and willing to provide informed consent. Patients were excluded if they had any psychological comorbidity, such as a depression diagnosis or severe mental health disorder (e.g., psychosis), or were receiving psychotherapy, had any known cognitive impairments (based on medical record), or had been diagnosed with cancer, or did not have full verbal and written proficiency in English. Patients not suitable to participate in the study according to the clinical care team, particularly considerations around the clinical stability of patients post‐transplant surgery or during change in RRT modality, were also excluded.
Psychological measure
Chalder Fatigue Questionnaire (CFQ; Chalder et al., 1993)
This instrument measures fatigue severity via 11 items scored against a four‐point Likert‐type response scale. Scores are assigned for each response, using either continuous scoring from 0 to 3 or bimodal scoring. Higher scores represent greater fatigue severity. This scale displays excellent psychometric properties (Chalder, Tong, & Deary, 2002; Chalder et al., 1993) and has been validated in HD (Picariello, Moss‐Morris, Macdougall, & Chilcot, 2016). A cut‐off of 4 using the bimodal scoring was used to define a fatigue case (Chalder et al., 1993). Mean scores were presented using the sum of the continuous scores of mental and physical fatigue items based on recent psychometric evidence (Chilcot, Norton, Kelly, & Moss‐Morris, 2016; Picariello et al., 2016).
Data collection
Potential participants were invited to complete a screening questionnaire, consisting of demographic and illness‐related questions and the CFQ (Chalder et al., 1993). Thirty‐nine returned the screening questionnaire, and 25 participants were interviewed, as data saturation was reached, defined as the point at which no new information or themes emerge from the data (Guest, Bunce, & Johnson, 2006).
Semi‐structured interviews (Appendix S1 for the interview schedule) were conducted face to face in a private environment, either at the participant's home or at the university, or over the phone, according to participants’ preferences. Interviews with in‐centre HD patients were conducted between dialysis treatments, to allow patients to recover from dialysis. The interview questions were broad and open‐ended, such as: ‘Can you tell me about the fatigue you experience since your kidney disease?’ and ‘Tell me what you usually do when you experience fatigue?’ Non‐directive prompts were used to follow up leads from patients’ responses. There were more interviews over the phone (N = 17) than face to face (N = 8). The length of the interviews ranged from 18 to 88 min (mean = 44.46; SD = 17.12). The interviews were audio‐recorded with a digital device and transcribed verbatim.
Clinical information was extracted from participants’ medical notes after the qualitative interview and interpreted based on the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (K/DOQI) clinical guidelines. Additionally, HD patients (home or in‐centre) also completed the CFQ on a second occasion, on a dialysis day.
Analysis
Sample characteristics and fatigue scores on the CFQ were summarized with descriptive statistics, computed in SPSS version 23 (IBM Corp., Armonk, NY, USA).
Transcripts were analysed using inductive thematic analysis, a qualitative method used for ‘identifying, analysing and reporting patterns (themes) within data … without trying to fit it into a pre‐existing coding frame, or the researcher's analytic preconceptions’ (Braun & Clarke, 2006). Charting and diagramming techniques were used to aid the development and organization of the clustering of themes. The analysis was founded on a Critical Realist approach, a combination of a Realist Ontology and an Interpretivist Epistemology, that assumes that there may be an objective truth, yet meaning is subjectively constructed (Olsen, 2007; Sayer, 2000), allowing, through the thematic analysis, to infer the multiple fatigue‐related motivations, experiences, and meanings of patients.
NVivo software (QSR International, Melbourne, Australia) was used for the qualitative analysis. Braun and Clarke (2006)'s six‐phase thematic analysis approach was used (Appendix S2 for further detail). Analysis consisted of initial open coding, proceeding to second phase coding, including pattern, focused and axial coding (Saldaña, 2015), conducted by FP.
The coding manual was developed and revised through discussion with the team (Appendix S3). Anonymized quotes are presented in the Findings section and they were selected for typicality in illustrating the themes.
Findings
Tables 1, 2, 3 summarize the sociodemographic (Table 1), clinical (Table 2), and biochemical (Table 3) characteristics of the sample. Based on the continuous scoring, the mean fatigue severity score was M = 17.10 (SD = 6.18, range = 10–29) across RRTs. Fatigue severity scores were comparable between HD and non‐HD days (mean = 18.70, SD = 7.29, range = 11–29; mean = 18.10, SD = 8.31, range = 10–29, respectively). Fatigue scores by RRT modality are presented in Figure 1. According to the bimodal scoring, 60% of the sample (N = 15) could be deemed clinically fatigued. Graphic representations of the total fatigue scores, based on continuous and bimodal scoring, are available in Appendix S4.
Table 1.
Summary of the sociodemographic characteristics of the sample (N = 25)
| Variable | Statistic |
|---|---|
| Female (n, %) | 10 (40) |
| Age (mean, SD, range) | 60.84 (13.92; 33–83) |
| Ethnicity (n, %) | |
| White | 16 (64) |
| Black | 7 (28) |
| Asian | 2 (8) |
| Marital status (n, %) | |
| Single | 7 (28) |
| Married | 12 (48) |
| Widowed | 4 (16) |
| Separated/divorced | 1 (4) |
| Co‐habiting | 1 (4) |
| Living arrangements (n, %) | |
| With spouse/partner/relatives/friends | 14 (56) |
| Alone | 11 (44) |
| Years of education (mean, SD, range) | 13.84 (3.79, 5–25) |
| Employment status (n, %) | |
| Working full‐time | 6 (24) |
| Working part‐time | 3 (12) |
| Homemaker | 1 (4) |
| Retired | 9 (36) |
| Unemployed | 3 (12) |
| Other | 3 (12) |
| Smoker (n, %) | |
| Current or ex‐smoker | 6 (24) |
| Non‐smoker | 19 (76) |
| Exercise status (n, %) | |
| Exercise more than three times per week | 8 (32) |
| Exercise less than three times per week | 6 (24) |
| No exercise | 11 (44) |
n = total number; SD = standard deviation.
Table 2.
Summary of the clinical characteristics of the sample (N = 25)
| Variable | Statistic |
|---|---|
| Renal replacement therapy (n, %) | |
| In pre‐dialysis care | 5 (20) |
| Hospital‐based HD | 7 (28) |
| Home‐based HD | 3 (12) |
| CAPD | 2 (8) |
| APD | 3 (12) |
| Transplant recipient (Tx) | 5 (20) |
| Transplant status (n, %) | |
| Active | 2 (8) |
| Working up | 7 (28) |
| Unfit reconsider/Unfit permanent | 9 (36) |
| Off by patient request | 2 (8) |
| N/A – currently transplanted | 5 (20) |
| Previous episodes of graft rejection (n, %) | |
| Yes | 5 (20) |
| No | 20 (80) |
| Time since CKD diagnosis in yrs (mean, SD, range, n) | 8.70 (7.23, 1.24–35.49, 20) |
| Time since progression to ESKD in yrs (mean, SD, range, n) | 5.64 (6.41, 0.11–26.49, 25) |
| Primary renal diagnosis (n, %)a | |
| Renal vascular disease due to hypertension | 10 (40) |
| Type 2 diabetes | 7 (28) |
| Glomerulonephritis | 4 (16) |
| Polycystic kidney disease | 2 (8) |
| Congenital absence of second kidney | 2 (8) |
| Other | 4 (16) |
| Renal function eGFR (mean, SD, range) | 17.64 (17.27, 4–67) |
| Comorbidities (n, %) | |
| Yes | 23 (92) |
| No | 2 (8) |
| Dialysis vintage (yrs) (mean, SD, range) | 2.70 (2.99, 0.24–12.46) |
| Time since Tx (yrs) (mean, SD, range) | 5.28 (4.39, 0.46–9.42) |
| HD adequacy via URR (%) (mean, SD, range) | 67.20 (7.67, 58–83) |
| IDWL (kg) (mean, SD, range, n) | −1.45 (0.90, −3.0 to −0.5, 10) |
| BMI (mean, SD, range, n) | 28.53 (6.83, 18.6–49.8) |
| Blood pressure (mean, SD, range, n) | |
| Systolic | 132.24 (22.70, 90–171) |
| Diastolic | 74.12 (15.13, 47–98) |
| Number of medications prescribed (mean, SD, range, n) | 9.36 (3.55, 2–16) |
| Receipt of ESA (n, %) | |
| Yes | 14 (56) |
| No | 11 (44) |
n = total number; SD = standard deviation; HD = haemodialysis; CAPD = continuous ambulatory peritoneal dialysis; APD = automated peritoneal dialysis; Tx = transplant; yrs = years; eGFR = glomerular filtration rate; URR = urea reduction ratio; IDWL = intra‐dialytic weight loss, BMI = body mass index; ESA = erythropoiesis‐stimulating agent.
Exceeding 100% because some participants reported multiple precursors of CKD.
Table 3.
Summary of the biochemical characteristics of the sample (N = 25)
| Variable | Statistic (mean, SD, range) |
|---|---|
| Haemoglobin (g/L) | 114.56 (17.57, 74–151) |
| Ferritin (ng/mL) | 278.48 (285.25, 22–1,215) |
| Serum albumin (g/L) | 38.44 (2.57, 34–42) |
| Cholesterol (mmol/L) | 4.40 (1.20, 2.1–7.3) |
| Total protein (g/L) | 66.96 (4.76, 59–76) |
| Creatinine (mmol/L) | 521.08 (276.29, 105–972) |
| Urea (mg/dL) | 18.97 (6.89, 7.3–33.4) |
| C‐reactive protein (mg/L) | 16.92 (24.32, 2.0–102.4) |
| PTH (pg/mL) | 293.28 (263.71, 0–1,086) |
| Calcium (mmol/L) | 2.29 (0.15, 1.84–2.55) |
| Potassium (mmol/L) | 5.00 (0.66, 3.8–6.00) |
| Phosphate (mmol/L) | 1.44 (0.51, 0.38–2.47) |
| Magnesium (mmol/L) | 0.85 (0.10, 0.67–1.06) |
| Sodium (mmol/L) | 142 (12.07, 135–198) |
SD = standard deviation; g/L = gram per litre; ng/mL = nanogram per millilitre; mmol/L = millimole per litre; mg/dL = milligram per decilitre; mg/L = milligram per litre; pg/mL = picograms per millilitre; PTH = parathyroid hormone.
Figure 1.
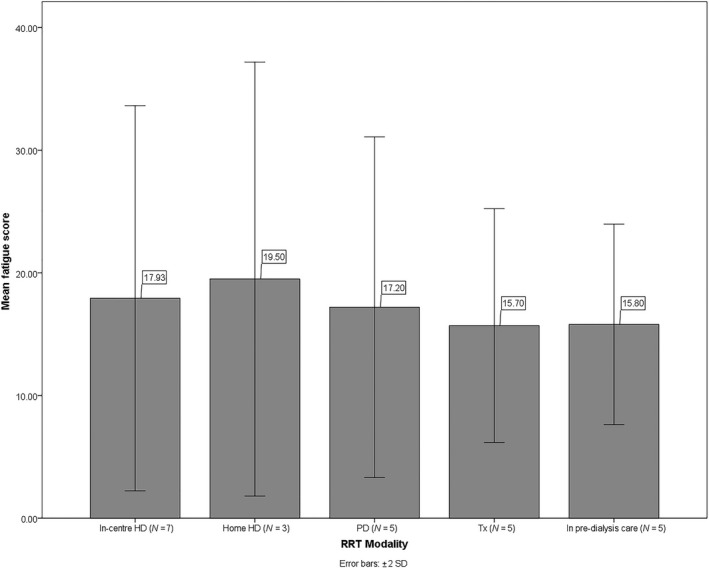
Bar chart of fatigue scores by renal replacement therapy (RRT) modality. The following are the descriptive statistics of fatigue scores by RRT: in‐centre haemodialysis (HD) mean = 17.93 (SD = 7.85, range = 10.50–29); home HD mean = 19.50 (SD = 8.85, range = 11.50–29); PD mean = 17.20 (SD = 6.94, range = 11–29); Tx mean = 15.70 (SD = 4.76, range = 10–22); and in pre‐dialysis care mean = 15.80 (SD = 4.09, range = 11–22). Scores of HD patients on HD and non‐HD days were averaged together.
Findings from the thematic analysis
Six main themes emerged through the thematic analysis (Table 4) and are discussed in more detail below. Figure 2 is a thematic diagram of the identified themes and subthemes.
Table 4.
Summary of the recurrent themes and subthemes across the narratives
| Themes | Subthemes |
|---|---|
| 1. Causes of fatigue | 1.1 Biomedical explanations of fatigue |
| 1.2 Other aetiologies of fatigue | |
| 2. Double‐edged fatigue | 2.1 A bit more than ordinary tiredness, but not a big deal |
| 2.2 Negative view of fatigue | |
| 3. Finding motivation to manage fatigue | 3.1 It takes effort |
| 3.2 Having a sense of purpose helps | |
| 4. Fatigue management | 4.1 Accommodating activity around fatigue |
| 4.2 When busy and active fatigue doesn't exist | |
| 4.3 At own pace, self‐compassion | |
| 4.4 To talk or not to talk about fatigue? | |
| 5. Impact of fatigue | 5.1 Emotional consequences of fatigue |
| 5.2 Fatigue is inhibiting | |
| 5.3 Persevering despite fatigue | |
| 5.4 Getting used to tiredness | |
| 6. Social support | 6.1 Social support as a source of motivation |
| 6.2 Seeking understanding from others | |
| 6.3 Don't want to be a burden |
Figure 2.
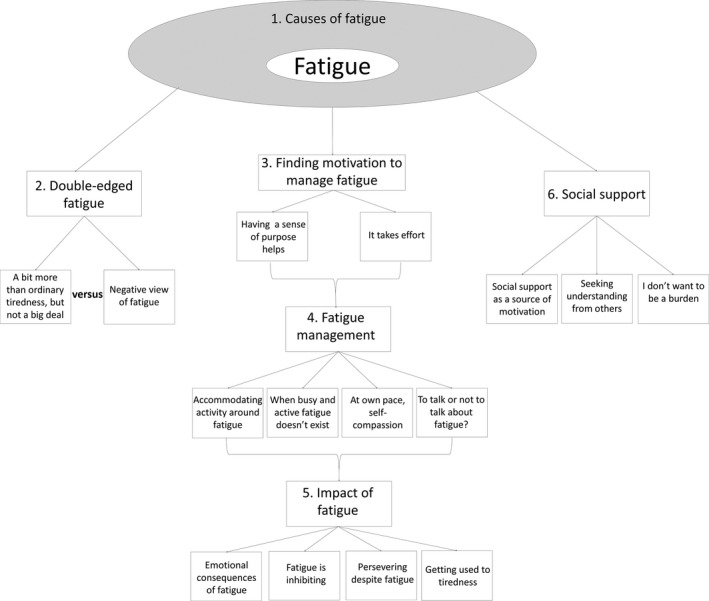
Thematic diagram of the themes and subthemes identified.
1. Causes of fatigue
This theme encompassed participants’ causal beliefs around fatigue, with biomedical explanations of fatigue prevailing.
1.1 Biomedical explanations of fatigue
Most participants attributed their fatigue to the illness: ‘As the kidneys have got worse, I get more and more tired’ (pt89), and dialysis:
there is a fatigue that I do associate with dialysis, and that is when the machine goes on … suddenly, I'm exhausted.
(pt31)
On the other hand, almost all participants expected their energy levels to improve with a kidney transplant. The role of anaemia and biochemical factors in the initiation of fatigue also emerged: ‘I think the fatigue is in fact all about the iron’ (pt41).
The strong dominance of biomedical fatigue aetiologies reflected itself in the general uncertainty and scepticism towards psychological treatments for fatigue, particularly prevalent among male and older participants:
I don't think it [a psychological therapy] can help. Not with the fatigue anyway … I can't see how talking can help it?
(pt89)
There was inconsistency between participants’ experience of fatigue and their biochemical profiles. For example, pt43 reported low levels of fatigue, yet was biochemically poorly controlled, with below normal haemoglobin (Hb), inadequate urea reduction ratio (URR), a marker of dialysis adequacy, and abnormal C‐reactive protein (CRP), a measure of general levels of inflammation in the body, and other biochemical values outside of the recommended ranges. This is in contrast to some participants, who had generally acceptable clinical values, yet reported higher fatigue (e.g., pt25 and pt36).
1.2 Other aetiologies of fatigue
Despite the dominance of biomedical fatigue explanations, the majority of participants also offered alternative explanations, including the role of ageing: ‘I'm over 60 now so I suppose age takes it out of me as well’ (pt3) and food being of ‘essence, that's what gives you energy’ (pt31).
Many participants placed a lot of emphasis on the role of sleep quality in fatigue. The accumulation of insufficient/fragmented sleep seemed particularly problematic: ‘I can go a couple of days with an interrupted sleep pattern. But it does, I think, draw on your reserves’ (pt90).
Only a handful of participants attributed their fatigue to stress: ‘I just know that this [fatigue] is stress‐related … Sometimes at work I'm very very stressed so with that I automatically know’ (pt6).
2. Double‐Edged Fatigue
This theme encapsulated participants’ contrasting interpretations of fatigue.
2.1 A bit more than ordinary tiredness, but not a big deal
General tiredness was consistently described in positive terms and perceived as a normal consequence of any activity or exercise:
I always associate tiredness with happiness … you've had a good day. So, ordinary tiredness is quite acceptable, because you've fulfilled whatever you wanted to do that day and it's a positive thing.
(pt90)
For some participants, the positive sense of general tiredness was seen as a contrast to illness‐related fatigue:
When I have a long day at work or I exercise or something, I know the difference because I know that I've done something to make me tired.
(pt6)
However, other participants normalized their experience of illness‐related fatigue, minimizing its presence and severity:
It's not like I'm feeling unwell, I'm just like I feel a bit tired … it wasn't like a disaster in terms of fatigue, but the fatigue was noticeably more there [after transplant rejection episode].
(pt15)
They also did not expect fatigue to be permanent, but rather a temporary experience:
You can go and sit down and wait for a bus, by the time you've got to Dartford any tiredness has passed.
(pt78)
Most participants downplaying their fatigue reported lower levels of fatigue on the CFQ, despite a range of abnormal clinical data (e.g., pt43 and pt91).
Similarly, positive beliefs about the illness and treatment also emerged. In particular, some participants were not defeated by their illness and treatment:
People used to moan about going to dialysis … I always called it my pop in parlour … This is one of those things that you've got and … don't let it get on top of you. You can't feel sorry for yourself. You've just gotta get on with it.
(pt12)
Although the impact of the illness and treatment was undeniable, many participants, particularly female and older, expressed gratitude:
Prior to that, I'd never ailed a thing. I'd never lost any work at all. I was really good. So I have to be thankful,
(pt88)
And younger participants valued positive thinking: ‘just stay positive all the time, I think that's the best thing to do’ (pt76).
2.2 Negative view of fatigue
Illness‐related fatigue was often perceived as abnormal and excessive tiredness, disproportionate and unrelated to activity/exertion:
It's [illness] tiredness that's caused by inactivity, when your brain's muddled and your legs are not working properly, and you've got cramps.
(pt90)
Many participants found that illness–fatigue would persist despite rest: ‘the illness tiredness is a tiredness that it doesn't obey even if you have long periods of rest and sleep, and recuperation’ (pt43) and some participants felt that their energy levels could ‘only [get] worse’ (pt36).
Frequently participants provided vivid descriptions of fatigue they experienced after dialysis, such as:
I've got no energy whatsoever and it's a bit like having a big, heavy weight on you … I feel like I've got a big, heavy load on me and I'm just so tired.
(pt89)
Negative views of the illness and treatment were also evident across the interviews, such as the inescapable and burdensome nature of CKD: ‘I don't think my kidneys are going to get better because they said that's never the case. There's only one way so I know it's downhill all the way’ (pt36) and dialysis being perceived as a ‘necessary evil’ (pt84). The disruptive nature of dialysis was particularly apparent with in‐centre HD.
3. Finding motivation to manage fatigue
Another major theme that emerged was related to the motivation necessary in order to self‐manage fatigue.
3.1 It takes effort
An underlying aspect of the fatigue experience was a loss in motivation: ‘I can't be bothered”‐type fatigue’ (pt35):
Whether it's the fact that I've unintentionally got lazy because I thought “oh, well I can't do this or I can't do that,” so I haven't tried to do other things … it might be do as much as I want to do, not do as much as I can, I think, some of the time.
(pt25)
Across the narratives, participants emphasized the importance of intrinsic motivation: ‘it's down to you … you have to conquer it, internally, nobody can help you except you’ (pt58). Some participants with markedly abnormal clinical profiles spoke of the beneficial effects of intrinsic motivation (e.g., pt43 and pt91). In contrast, other participants with higher fatigue scores on the CFQ, yet satisfactory clinical profiles, reported a feeling of helplessness with regard to fatigue (e.g., pt25 and pt36).
An enabling factor for motivation was the notion of control over fatigue, especially evident among those living alone, but less frequent among those with higher fatigue scores:
Choices are all our own – how we're sleeping, how we are eating, how we are breathing, how we are moving – I would say that we have ultimately a huge control over that.
(pt15)
3.2 Having a sense of purpose helps
Participants recognized the importance of having faith, goals, and a sense of intentionality in enabling motivation:
I think having a sense of purpose actually, that's another thing that's very important in terms of fatigue … If you have a sense of purpose you're more likely to overcome things because you think I'm here to do something meaningful … It doesn't have to be a huge grand thing. It could be I'm here to do the ironing today.
(pt15)
The sense of purpose was personal, for some it was continuing work for financial stability, for others work gave ‘purpose to your body or your mind’ (pt84), and for others it was family: ‘because of my children, I have to do it [keep going] … Because they are young’ (pt58).
Many participants felt that achieving targets provided them with a sense of accomplishment:
But I wasn't tired so I don't know if it was because I achieved so much, that's why I didn't feel that way.
(pt6)
This notion was parallel to the ‘good tiredness’ previously discussed (see Subtheme 2.1 A bit more than ordinary tiredness, but not a big deal).
The desire to retain one's identity was also coupled with discomfort towards asking for help:
I'd struggle with that [asking for help] … ‘cause I'm a fully grown male who likes to think that people should be looking to you for help and guidance, and, strength.
(pt43)
Therefore, there was a strong preference for wanting to have a security net of support: ‘it's nice to know that there is someone there who will do it [help] for you’ (pt68), yet, not wanting to relinquish one's independence, particularly among those living alone.
4. Fatigue management
This theme encompassed comments around different strategies participants selected to cope with fatigue.
4.1 Accommodating activity around fatigue
For many participants, fatigue dictated their lifestyle and activities, adjusting the timing and intensity of their activities to accommodate their fatigue from day to day:
I just have to do it and do it when I can … If I'm gonna do anything, like housework … I just have to do it when I can … And if it's 7 o'clock at night, or 8 o'clock at night, then that's when I do it.
(pt12)
Participants also learnt to avoid potentially fatigue inducing activities and keeping things to a minimum:
I used to spend an hour a day at a gym and that all stopped, I just didn't go any more … If I do this it might make me feel worse and in the end you end up, you just don't do anything.
(pt25)
This fatigue relief strategy was particularly evident following HD, and dialysis days were frequently perceived as days for rest: ‘I don't do much or anything those [dialysis] days’ (pt25).
Feeling that nothing can be done was dominant in participants reporting higher fatigue severity scores on the CFQ, even in clinically well‐controlled participants (e.g., pt25 and pt36).
4.2 When busy and active fatigue doesn't exist
In contrast, some participants saw activity as positive for fatigue: ‘if I feel a bit tired I will force myself to do something’ (pt3).
Participants valued exercise: ‘Once I was back to full fitness, I was okay. I didn't really get much fatigue at all’ (pt3).
Overall, keeping busy and active emerged as a particularly helpful relief strategy for many participants, ‘jerk[ing] them out’ of fatigue (pt3):
If I sit here it will only get worse won't it? … They [symptoms] will get worse if you just lay around and vegetate.
(pt36)
4.3 At own pace, self‐compassion
Many participants mentioned the importance of maintaining activities, yet learning to pace themselves:
I've learnt to pace myself really … For example, sometimes with big finish productions, they would like me to do two recordings in one week, record on the Tuesday, and on the Thursday. I would definitely say no to that now. I would only do it one day a week.
(pt31)
Although remaining active seemed important, overdoing things could lead to fatigue, emphasizing on the importance of self‐compassion and setting limits:
It's acknowledging maybe doing too much, maybe needing to take things down a bit, looking at how much you're taking on.
(pt15)
Remaining active, yet with self‐compassion was evident among participants reporting lower levels of fatigue, again regardless of their abnormal clinical profiles (pt43 and pt91).
4.4 To talk or not to talk about fatigue?
Mixed feelings emerged with regard to fatigue communication, with some participants perceiving disclosure as helpful, particularly female participants and those living alone:
It could be helpful to talk things out … I think it's because sometimes people bottle things up, and that can be fatigue‐ing … and it could help talking out, sharing your concerns and worries, it can help you to relax and it might reduce fatigue.
(pt49)
However, others, especially male participants, felt that discussing fatigue, and the illness more generally, would only ‘make [things] worse’ (pt35).
Talking about fatigue as matter of fact was many participants’ preference: ‘I may mention it passingly, like the person may say “we want to do such and such” and I will say “I'm not feeling up to it”’ (pt43).
Across the narratives, selective disclosure was evident, with participants selecting one person to share their experiences with: ‘I do [talk] to my wife a bit. “I've been really tired this week” or whatever, but about the only person I would say … Maybe my son because he goes to the gym as well’ (pt3).
5 Impact of fatigue
Fatigue was often described through its impact on participants’ lives rather than the sensation of fatigue itself.
5.1 Emotional consequences of fatigue
Participants’ feelings of anger and frustration because of the physical and social limitations placed on them by fatigue were prevalent across the narratives, especially among transplant recipients and home HD patients:
This is getting on my nerves … It's just the tiredness that I don't like … But it's just so frustrating now, ‘cause the girls want to go out sometimes, and I can't do it, because I just don't have the energy to do it.
(pt89)
Frustration and impatience with one's functioning seemed to act as barriers to self‐compassion (see Subtheme 4.3 At own pace, self‐compassion).
Across the narratives, there was a strong bidirectional link between low mood and fatigue. According to some participants, low mood and depression were a consequence of the fatigue‐related limitations on functioning:
There was definitely an effect of fatigue … so that restrained me from doing my tasks and whatever I wanted to do, so that was more depressing than anything else.
(pt91)
However, other participants felt that ‘the more unhappy you are the more it wears you out mentally’ (pt84).
5.2 Fatigue is inhibiting
Across the narratives, participants offered examples of how fatigue had impacted on their daily lives and functioning, describing it as inhibiting: ‘can't get done as much as I would like to’ (pt49), providing contrasting descriptions of functioning before and after:
I used to go to work. I used to play bowls … this morning I got up and done the washing, put that out, and that's about it. I haven't done anything else.
(pt12)
To convey the impact of fatigue, participants referred to simple actions of daily living that they could no longer accomplish, and impact on hobbies and ‘fun’ (pt68), and their ability to work:
I wouldn't be able to drive or anything like that … even just writing letters or something like that I just haven't got the energy to get up and do it.
(pt36)
Fatigue‐related restrictions on functioning were further amplified on dialysis. For some, this meant ‘hav[ing] only three [days] at the most’ out of the week (pt20).
The perception of a greater impact of fatigue on functioning was evident among those with higher fatigue scores, irrespective of their clinical data (e.g., pt25 and pt36).
Overall, higher scores on the CFQ could be observed among participants who perceived the illness and fatigue more negatively and engaged in avoidance or boom‐bust behaviours, coupled with poor clinical profiles, such as inadequate URR, abnormal albumin – a marker of nutritional status, and other biochemical imbalances (e.g., pt89 and pt20), suggesting a possibly cumulative effect of psychological and physiological factors on fatigue among some patients. These relationships are likely to be bidirectional, with greater fatigue also leading to more negative fatigue‐related thoughts and maladaptive coping behaviours, resulting in a vicious cycle.
5.3 Persevering despite fatigue
On the other hand, some participants refused to succumb to fatigue and continued with their normal daily lives and routines:
I am not inclined to let circumstances dictate to me … tiredness is something that isn't, you can't always say “oh its 7 o'clock I am going to be tired so I won't go out.” … I try to act as normal as possible. And by doing so, I don't have any effects of fatigue.
(pt78)
This was particularly evident with chores, with some participants disclosing renunciation of personal time:
The house doesn't clean itself … it has got to be done, so you make it part of normal life.
(pt78)
Some participants displayed a similar determination with regard to post‐dialysis fatigue:
Especially on Tuesdays and Thursdays, from dialysis sessions, I will maybe do a couple of things, like go to the bank or do some shopping, then from there go to work … but I don't really come home to sleep, I have to go and do some other activities.
(pt8)
Perseverance sometimes resulted in overdoing things, linking to Subtheme 4.3 At own pace, self‐compassion.
6. Social support
This theme revolved around the role of the social context in fatigue.
6.1 Social support as a source of motivation
Frequently, family and friends subtly or directly encouraged physical activity and provided participants with a sense of role and belonging, particularly for those living with their partners:
My wife says “Let's go out for a walk” and I'll say “No,” she accepts it and she'll say “Let's just try” and now and again I will accept.
(pt25)
Participants often engaged in social comparisons, which was a source of intrinsic motivation for some, facilitating positive thinking and gratitude (see Subtheme 1.2 Other aetiologies of fatigue):
I look at others, and that really does encourage me to not be anything like that … there are others who have been given a prognosis, and then that's it … they laid down and thought “this is it, this is it for me, this is my life from there on end”.
(pt43)
However, a minority of participants felt that there may be drawbacks to social comparisons, whereby ‘if you are next to people who don't take a positive attitude, it can drag you down’ (pt76).
6.2 Seeking understanding from others
Peer support was valued by participants and provided validation: ‘I have spoken with many patients at [satellite unit] and often they disregard the day after. They are all having the same thing’ (pt20) and was potentially a source of relevant self‐management tips: ‘if someone is talking to another patient who has already been through the same thing, and who's found a certain solution for energy levels, or a positive mental attitude, they are more likely to listen to them because they have experienced it themselves’ (pt84).
Yet, mixed feelings were evident with regard to feeling understood by family and friends. Some participants felt that understanding was limited: ‘I don't think they really know the inside of the fatigue … I don't think that emotionally‐wise and mentally‐wise they know how I feel’ (pt58), whilst others perceived full understanding and suitable support from family and friends: ‘when I come home [from dialysis], nobody is going to bother me, because they know that I'm completely out of everything, my body is completely fatigued, so they don't trouble me at all’ (pt42).
6.3 Don't want to be a burden
Frequently participants, particularly older, female, and married ones, articulated concern around becoming a burden to family and friends:
They [sons] just have to make sure I'm alright before they do anything … I don't really want to be a burden to them any more than what I am.
(pt12)
Discussion
This study provides a unique insight into the experience of fatigue across the spectrum of advance kidney disease. Throughout the interviews, tiredness was not inherently negative, in fact a dichotomy was evident between descriptions of illness‐related fatigue, as dramatic and uncontrollable, and general tiredness as a positive experience. Perceptions of fatigue appeared to shape participants’ fatigue management strategies and the impact of fatigue on mental and physical functioning. Although there was a general consensus around the importance of keeping active, some participants felt that self‐compassion was also necessary. Social support was a dominant theme across the interviews, for many participants, encouragement from family and friends as well as social comparisons appeared to be sources of intrinsic motivation, whilst peer support provided validation. Yet, a downside to social support was the commonly disclosed apprehension of being a burden to others.
The findings of this study provide an in‐depth account of the experience of fatigue in advanced kidney disease. A number of novel insights were apparent, for example, some participants normalized their experience of fatigue. The emotional impact of fatigue has been previously documented in the literature (Hewlett et al., 2005; Magnusson, Möller, Ekman, & Wallgren, 1999). An interesting finding was evident with regard to the bidirectional relationship between low mood and fatigue, where low mood could act as both a consequence and a cause of fatigue. There is extensive evidence from quantitative studies on the strong association between depression and fatigue in ESKD (Picariello, Moss‐Morris, Macdougall, & Chilcot, 2017); however, given the prevalence of cross‐sectional studies, the causality and direction of this association are unclear. The role of social support in renal fatigue was greatly accentuated throughout the narratives here, in contrast to some quantitative studies which reported negative findings on the role of social support in fatigue (Picariello et al., 2017). Therefore, loneliness and isolation may only further exacerbate fatigue (Abrahams et al., 2016; Wu & McSweeney, 2007).
The findings here also complement previous quantitative and qualitative research of fatigue in other chronic conditions. For example, the strong biomedical causal attribution of fatigue has previously emerged in other qualitative studies in rheumatoid arthritis (RA) and multiple sclerosis (Magnusson et al., 1999; Mills & Young, 2008; Tack, 1990), and HD (Horigan et al., 2013). Although biomedical explanations of fatigue prevailed, age, sleep quality, and nutrition were factors frequently mentioned by participants. There is extensive qualitative and quantitative evidence in support of the relationship between sleep quality and fatigue across chronic conditions (Kaminska et al., 2012; Lee et al., 2007; Menting et al., 2016; Rongen‐van Dartel et al., 2016; Scott, Lasch, Barsevick, & Piault‐Louis, 2011; Stuifbergen & Rogers, 1997; Wolfe, Hawley, & Wilson, 1996). In a recent study, poor sleep quality was directly associated with higher fatigue severity, and also mediated the association between lower mood and fatigue severity in RA (Rongen‐van Dartel et al., 2016). In fact, trials of CBT interventions aimed at sleep quality in dialysis patients found significant improvements in fatigue (Chen et al., 2008, 2011).
The themes identified here resonate with the themes of qualitative studies of fatigue in dialysis (Horigan & Barroso, 2016; Horigan et al., 2013; Lee et al., 2007; Yngman‐Uhlin et al., 2010) and in other chronic conditions, providing further validation of the previous findings and also highlighting the shared elements of the fatigue experience across conditions with the potential of a trans‐diagnostic approach to the treatment of fatigue. Clear overlaps in findings were also evident with regard to the description of fatigue through its impact on functioning (Magnusson et al., 1999; Scott et al., 2011), the distinction between tiredness caused by activity versus tiredness disproportionate to or unrelated to activity or exertion (Adamsen et al., 2004; Glaus, Crow, & Hammond, 1996), the role of motivation in the experience of fatigue (Bennett, Goldstein, Friedlander, Hickie, & Lloyd, 2007; Hewlett et al., 2005; Lee et al., 2007; Mills & Young, 2008; Yngman‐Uhlin et al., 2010), and the contribution of social comparisons to fatigue (Repping‐Wuts, Uitterhoeve, van Riel, & van Achterberg, 2008; Tack, 1990).
Different attitudes towards management of fatigue were evident across the interviews, as has been previously identified in the literature, such as avoidance and accommodation of activity according to fatigue (Graydon, Bubela, Irvine, & Vincent, 1995; Heiwe & Dahlgren, 2004; Horigan et al., 2013; Johnson et al., 2004; Magnusson et al., 1999; Mills & Young, 2008; Rhodes, Watson, & Hanson, 1988; Scott et al., 2011; Stuifbergen & Rogers, 1997; Yngman‐Uhlin et al., 2010), or restoring to various activities to cope with fatigue (Magnusson et al., 1999; Mills & Young, 2008; Scott et al., 2011; Wu & McSweeney, 2007; Yngman‐Uhlin et al., 2010). According to a quantitative study, avoidance coping was an independent predictor of greater fatigue over time in RA (Scharloo et al., 1999). However, the notion of self‐compassion was acknowledged by participants here and in other qualitative studies (Hewlett et al., 2005; Mills & Young, 2008; Repping‐Wuts et al., 2008; Scott et al., 2011; Wu & McSweeney, 2007).
Clinical implications
Psychological treatments, like CBT and mindfulness‐based interventions, have been found effective for fatigue across different chronic conditions (Ulrichsen et al., 2016; van den Akker et al., 2016). Findings from this study identify several areas that could be targeted in a psychological treatment of fatigue, for example, addressing negative beliefs about fatigue and differentiating fatigue from the illness and treatment, in order to bridge fatigue closer to the “good tiredness” experience. The detrimental role of negative beliefs about fatigue has been previously documented in HD (Chilcot, Moss‐Morris, et al., 2016). Separating fatigue from a solely biomedical understanding would also be important to facilitate acceptance of and engagement with a psychological treatment (e.g., Sharpe, Hawton, Seagroatt, & Pasvol, 1992). Additionally, to reduce scepticism towards psychological treatments for fatigue, within a CBT‐based approach, therapy could first concentrate on the behavioural components of treatment (e.g., activity, sleep) to trigger some initial reduction in fatigue, before proceeding to cognitive methods to explore unhelpful thoughts in relation to fatigue.
One of the thoughts to target is perception of control over fatigue. Across chronic conditions, including one study among dialysis patients (Baak, 2015), there is evidence for the association between lower self‐efficacy concerning fatigue and greater fatigue severity (Menting et al., 2016; Rongen‐van Dartel et al., 2016; Treharne et al., 2008). In view of the effort necessary to actively manage fatigue, for those patients lacking purpose and self‐motivation, this could also be addressed in treatment. It may also be valuable during therapy to address unhelpful behaviours to help patients establish balance between activities and rest, and develop self‐compassion, as has been previously advocated (Chilcot, Moss‐Morris, et al., 2016). Preliminary evidence is available to suggest that self‐compassion can be developed within mindfulness‐based interventions, thereby improving outcomes, like fatigue (Gu, Strauss, Bond, & Cavanagh, 2015). Given the potentially bidirectional link between mood and fatigue, management of negative emotional responses would also be necessary. Finally, given the important role of social support, selective disclosure may be utilized in treatment and it would be valuable to address the apprehension of becoming a burden.
Limitations of the current study and future directions
There were several limitations with this study. A single data‐gathering period cannot elucidate the variable and ever‐changing presentation of renal fatigue. It would be useful for a future qualitative study to conduct interviews at multiple time points. Additionally, only one researcher (FP) was involved in the coding of the data. Participants were not screened for fatigue in order to capture a whole range of fatigue experiences because attitudes towards fatigue and its management, in particular normalizing the experience of fatigue, may influence scores on the fatigue scale. The exclusion of non‐English speakers may mean findings do not extend to renal patients from different cultural backgrounds.
Conclusion
Fatigue is a prevalent and debilitating symptom of kidney failure. Our data indicate that the experience of fatigue is complex with wide‐ranging consequences on functioning. Participants in this study reported a strong belief in the biomedical roots of fatigue. In contrast to illness–fatigue, general tiredness was described in positive terms. Unhelpful fatigue beliefs may lead to ineffective management strategies and further perpetuation of a vicious cycle between fatigue, negative emotions, and reduced functioning. A comprehensive understanding of fatigue may help to inform a future patient‐centred intervention for fatigue in this patient population.
Funding
This work was funded by a Biomedical Research Studentship to Miss Federica Picariello from the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
Ethical approval was granted by the South East Scotland Research Ethics Committee 02 (IRAS Ref: 194204; REC Reference Number: 16/SS/0037).
Supporting information
Appendix S1. Interview schedule.
Appendix S2. Qualitative analysis: further detail.
Appendix S3. Coding manual.
Appendix S4. Distribution of fatigue scores.
Acknowledgements
We would like to thank the patients, involved in this study, and renal team at King's College Hospital for assistance with recruitment and data collection.
References
- Abrahams, H. , Gielissen, M. , Schmits, I. , Verhagen, C. , Rovers, M. , & Knoop, H. (2016). Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: A meta‐analysis involving 12 327 breast cancer survivors. Annals of Oncology, 27, 965–974. https://doi.org/10.1093/annonc/mdw099 [DOI] [PubMed] [Google Scholar]
- Adamsen, L. , Midtgaard, J. , Andersen, C. , Quist, M. , Moeller, T. , & Roerth, M. (2004). Transforming the nature of fatigue through exercise: Qualitative findings from a multidimensional exercise programme in cancer patients undergoing chemotherapy. European Journal of Cancer Care, 13, 362–370. https://doi.org/10.1111/j.1365-2354.2004.00502.x [DOI] [PubMed] [Google Scholar]
- Artom, M. , Moss‐Morris, R. , Caskey, F. , & Chilcot, J. (2014). Fatigue in advanced kidney disease. Kidney International, 86, 497–505. https://doi.org/10.1038/ki.2014.86 [DOI] [PubMed] [Google Scholar]
- Baak, A. (2015). The association between worrying, self‐efficacy and quality of life in renal patients on dialysis (Psychology MSc). Leiden University. [Google Scholar]
- Bennett, B. , Goldstein, D. , Friedlander, M. , Hickie, I. , & Lloyd, A. (2007). The experience of cancer‐related fatigue and chronic fatigue syndrome: A qualitative and comparative study. Journal of Pain and Symptom Management, 34, 126–135. https://doi.org/10.1016/j.jpainsymman.2006.10.014 [DOI] [PubMed] [Google Scholar]
- Bonner, A. , Caltabiano, M. , & Berlund, L. (2013). Quality of life, fatigue, and activity in Australians with chronic kidney disease: A longitudinal study. Nursing & Health Sciences, 15, 360–367. https://doi.org/10.1111/nhs.12038 [DOI] [PubMed] [Google Scholar]
- Bossola, M. , Di Stasio, E. , Antocicco, M. , Panico, L. , Pepe, G. , & Tazza, L. (2015). Fatigue is associated with increased risk of mortality in patients on chronic hemodialysis. Nephron, 130, 113–118. https://doi.org/10.1159/000430827 [DOI] [PubMed] [Google Scholar]
- Bossola, M. , Vulpio, C. , & Tazza, L. (2011). Fatigue in chronic dialysis patients. Seminars in Dialysis, 24, 550–555. https://doi.org/10.1111/j.1525-139X.2011.00956.x [DOI] [PubMed] [Google Scholar]
- Braun, V. , & Clarke, V. (2006). Using thematic analysis in psychology. Qualitative Research in Psychology, 3(2), 77–101. https://doi.org/10.1191/1478088706qp063oa [Google Scholar]
- Chalder, T. , Berelowitz, G. , Pawlikowska, T. , Watts, L. , Wessely, S. , Wright, D. , & Wallace, E. (1993). Development of a fatigue scale. Journal of Psychosomatic Research, 37, 147–153. https://doi.org/10.1016/0022-3999(93)90081-P [DOI] [PubMed] [Google Scholar]
- Chalder, T. , Tong, J. , & Deary, V. (2002). Family cognitive behaviour therapy for chronic fatigue syndrome: An uncontrolled study. Archives of Disease in Childhood, 86(2), 95–97. https://doi.org/10.1136/adc.86.2.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H.‐Y. , Cheng, I.‐C. , Pan, Y.‐J. , Chiu, Y.‐L. , Hsu, S.‐P. , Pai, M.‐F. , … Wu, K.‐D. (2011). Cognitive‐behavioral therapy for sleep disturbance decreases inflammatory cytokines and oxidative stress in hemodialysis patients. Kidney International, 80, 415–422. https://doi.org/10.1038/ki.2011.151 [DOI] [PubMed] [Google Scholar]
- Chen, H.‐Y. , Chiang, C.‐K. , Wang, H.‐H. , Hung, K.‐Y. , Lee, Y.‐J. , Peng, Y.‐S. , … Tsai, T.‐J. (2008). Cognitive‐behavioral therapy for sleep disturbance in patients undergoing peritoneal dialysis: A pilot randomized controlled trial. American Journal of Kidney Diseases, 52, 314–323. https://doi.org/10.1053/j.ajkd.2008.03.012 [DOI] [PubMed] [Google Scholar]
- Chilcot, J. , Moss‐Morris, R. , Artom, M. , Harden, L. , Picariello, F. , Hughes, H. , … Macdougall, I. C. (2016). Psychosocial and clinical correlates of fatigue in haemodialysis patients: The importance of patients’ illness cognitions and behaviours. International Journal of Behavioral Medicine, 23, 271–281. https://doi.org/10.1007/s12529-015-9525-8 [DOI] [PubMed] [Google Scholar]
- Chilcot, J. , Norton, S. , Kelly, M. E. , & Moss‐Morris, R. (2016). The Chalder Fatigue Questionnaire is a valid and reliable measure of perceived fatigue severity in multiple sclerosis. Multiple Sclerosis Journal, 22, 677–684. https://doi.org/10.1177/1352458515598019 [DOI] [PubMed] [Google Scholar]
- Cramp, F. , Hewlett, S. , Almeida, C. , Kirwan, J. R. , Choy, E. H. , Chalder, T. , … Christensen, R. (2013). Non‐pharmacological interventions for fatigue in rheumatoid arthritis. Cochrane Database Systematic Review, 8, CD008322 https://doi.org/10.1002/14651858.CD008322.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison, S. N. , & Jhangri, G. S. (2010). Impact of pain and symptom burden on the health‐related quality of life of hemodialysis patients. Journal of Pain and Symptom Management, 39, 477–485. https://doi.org/10.1016/j.jpainsymman.2009.08.008 [DOI] [PubMed] [Google Scholar]
- Dittner, A. J. , Wessely, S. C. , & Brown, R. G. (2004). The assessment of fatigue: A practical guide for clinicians and researchers. Journal of Psychosomatic Research, 56, 157–170. https://doi.org/10.1016/S0022-3999(03)00371-4 [DOI] [PubMed] [Google Scholar]
- Donovan, K. A. , Small, B. J. , Andrykowski, M. A. , Munster, P. , & Jacobsen, P. B. (2007). Utility of a cognitive‐behavioral model to predict fatigue following breast cancer treatment. Health Psychology, 26, 464 https://doi.org/10.1037/0278-6133.26.4.464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaus, A. , Crow, R. , & Hammond, S. (1996). A qualitative study to explore the concept of fatigue/tiredness in cancer patients and in healthy individuals. European Journal of Cancer Care, 5(Suppl. 2), 8–23. https://doi.org/10.1111/j.1365-2354.1996.tb00247.x [DOI] [PubMed] [Google Scholar]
- Graydon, J. E. , Bubela, N. , Irvine, D. , & Vincent, L. (1995). Fatigue‐reducing strategies used by patients receiving treatment for cancer. Cancer Nursing, 18(1), 23–28. https://doi.org/10.1097/00002820-199502000-00004 [PubMed] [Google Scholar]
- Gu, J. , Strauss, C. , Bond, R. , & Cavanagh, K. (2015). How do mindfulness‐based cognitive therapy and mindfulness‐based stress reduction improve mental health and wellbeing? A systematic review and meta‐analysis of mediation studies. Clinical Psychology Review, 37, 1–12. https://doi.org/10.1016/j.cpr.2015.01.006 [DOI] [PubMed] [Google Scholar]
- Guest, G. , Bunce, A. , & Johnson, L. (2006). How many interviews are enough? An experiment with data saturation and variability. Field Methods, 18(1), 59–82. https://doi.org/10.1177/1525822X05279903 [Google Scholar]
- Hagren, B. , Pettersen, I. M. , Severinsson, E. , Lützén, K. , & Clyne, N. (2001). The haemodialysis machine as a lifeline: Experiences of suffering from end‐stage renal disease. Journal of Advanced Nursing, 34, 196–202. https://doi.org/10.1046/j.1365-2648.2001.01745.x [DOI] [PubMed] [Google Scholar]
- Heiwe, S. , Clyne, N. , & Dahlgren, M. A. (2003). Living with chronic renal failure: Patients’ experiences of their physical and functional capacity. Physiotherapy Research International, 8(4), 167–177. https://doi.org/10.1002/(ISSN)1471-2865 [DOI] [PubMed] [Google Scholar]
- Heiwe, S. , & Dahlgren, M. A. (2004). Living with chronic renal failure: Coping with physical activities of daily living. Advances in Physiotherapy, 6(4), 147–157. https://doi.org/10.1080/14038190410019540 [Google Scholar]
- Hewlett, S. , Cockshott, Z. , Byron, M. , Kitchen, K. , Tipler, S. , Pope, D. , & Hehir, M. (2005). Patients’ perceptions of fatigue in rheumatoid arthritis: Overwhelming, uncontrollable, ignored. Arthritis Care & Research, 53, 697–702. https://doi.org/10.1002/(ISSN)1529-0131 [DOI] [PubMed] [Google Scholar]
- Horigan, A. E. , & Barroso, J. V. (2016). A comparison of temporal patterns of fatigue in patients on hemodialysis. Nephrology Nursing Journal: Journal of the American Nephrology Nurses’ Association, 43, 129. [PMC free article] [PubMed] [Google Scholar]
- Horigan, A. E. , Schneider, S. M. , Docherty, S. , & Barroso, J. (2013). The experience and self‐management of fatigue in hemodialysis patients. Nephrology Nursing Journal: Journal of the American Nephrology Nurses’ Association, 40, 113. [PMC free article] [PubMed] [Google Scholar]
- Irving, K. , Matcham, F. , Ali, S. , & Chalder, T. (2015). 085. Fatigue and functional disability in rheumatoid arthritis: Evidence for a cognitive behavioural model. Rheumatology, 54(Suppl. 1), i83–i84. https://doi.org/10.1093/rheumatology/kev088.083 [Google Scholar]
- Jhamb, M. , Argyropoulos, C. , Steel, J. L. , Plantinga, L. , Wu, A. W. , Fink, N. E. , … Unruh, M. L. (2009). Correlates and outcomes of fatigue among incident dialysis patients. Clinical Journal of the American Society of Nephrology, 4, 1779–1786. https://doi.org/10.2215/CJN.00190109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhamb, M. , Pike, F. , Ramer, S. , Argyropoulos, C. , Steel, J. , Dew, M. A. , … Unruh, M. (2011). Impact of fatigue on outcomes in the hemodialysis (hemo) study. American Journal of Nephrology, 33, 515–523. https://doi.org/10.1159/000328004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, K. L. , Yorkston, K. M. , Klasner, E. R. , Kuehn, C. M. , Johnson, E. , & Amtmann, D. (2004). The cost and benefits of employment: A qualitative study of experiences of persons with multiple sclerosis. Archives of Physical Medicine and Rehabilitation, 85, 201–209. https://doi.org/10.1016/S0003-9993(03)00614-2 [DOI] [PubMed] [Google Scholar]
- Kaminska, M. , Kimoff, R. , Benedetti, A. , Robinson, A. , Bar‐Or, A. , Lapierre, Y. , … Trojan, D. (2012). Obstructive sleep apnea is associated with fatigue in multiple sclerosis. Multiple Sclerosis Journal, 18, 1159–1169. https://doi.org/10.1177/1352458511432328 [DOI] [PubMed] [Google Scholar]
- Kangas, M. , Bovbjerg, D. H. , & Montgomery, G. H. (2008). Cancer‐related fatigue: A systematic and meta‐analytic review of non‐pharmacological therapies for cancer patients. Psychological Bulletin, 134, 700 https://doi.org/10.1037/a0012825 [DOI] [PubMed] [Google Scholar]
- Koyama, H. , Fukuda, S. , Shoji, T. , Inaba, M. , Tsujimoto, Y. , Tabata, T. , … Okamura, M. (2010). Fatigue is a predictor for cardiovascular outcomes in patients undergoing hemodialysis. Clinical Journal of the American Society of Nephrology, 5, 659–666. https://doi.org/10.2215/CJN.08151109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, B. O. , Lin, C. C. , Chaboyer, W. , Chiang, C. L. , & Hung, C. C. (2007). The fatigue experience of haemodialysis patients in Taiwan. Journal of Clinical Nursing, 16, 407–413. https://doi.org/10.1111/j.1365-2702.2005.01409.x [DOI] [PubMed] [Google Scholar]
- Magnusson, K. , Möller, A. , Ekman, T. , & Wallgren, A. (1999). A qualitative study to explore the experience of fatigue in cancer patients. European Journal of Cancer Care, 8, 224–232. https://doi.org/10.1046/j.1365-2354.1999.00168.x [DOI] [PubMed] [Google Scholar]
- Menting, J. , Nikolaus, S. , van der Veld, W. M. , Goedendorp, M. M. , Tack, C. J. , & Knoop, H. (2016). Severe fatigue in type 1 diabetes: Exploring its course, predictors and relationship with hba 1c in a prospective study. Diabetes Research and Clinical Practice, 121, 127–134. https://doi.org/10.1016/j.diabres.2016.09.011 [DOI] [PubMed] [Google Scholar]
- Mills, R. , & Young, C. (2008). A medical definition of fatigue in multiple sclerosis. QJM, 101(1), 49–60. https://doi.org/10.1093/qjmed/hcm122 [DOI] [PubMed] [Google Scholar]
- Olsen, W. (2007). Critical realist explorations in methodology. Methodological Innovations Online, 2(2), 1–5. https://doi.org/10.4256/mio [Google Scholar]
- Patton, M. Q. (2002). Two decades of developments in qualitative inquiry a personal, experiential perspective. Qualitative Social Work, 1, 261–283. https://doi.org/10.1177/1473325002001003636 [Google Scholar]
- Picariello, F. , Moss‐Morris, R. , Macdougall, I. C. , & Chilcot, J. (2016). Measuring fatigue in haemodialysis patients: The factor structure of the Chalder Fatigue Questionnaire (CFQ). Journal of Psychosomatic Research, 84, 81–83. https://doi.org/10.1016/j.jpsychores.2016.03.124 [DOI] [PubMed] [Google Scholar]
- Picariello, F. , Moss‐Morris, R. , Macdougall, I. C. , & Chilcot, J. (2017). The role of psychological factors in fatigue among end‐stage kidney disease patients: A critical review. Clinical Kidney Journal, 10(1), 79–88. https://doi.org/10.1093/ckj/sfw113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repping‐Wuts, H. , Uitterhoeve, R. , van Riel, P. , & van Achterberg, T. (2008). Fatigue as experienced by patients with rheumatoid arthritis (RA): A qualitative study. International Journal of Nursing Studies, 45, 995–1002. https://doi.org/10.1016/j.ijnurstu.2007.06.007 [DOI] [PubMed] [Google Scholar]
- Rhodes, V. A. , Watson, P. M. , & Hanson, B. M. (1988). Patients’ descriptions of the influence of tiredness and weakness on self‐care abilities. Cancer Nursing, 11, 186–194. https://doi.org/10.1097/00002820-198806000-00009 [PubMed] [Google Scholar]
- Rongen‐van Dartel, S. , Repping‐Wuts, H. , Donders, R. , van Hoogmoed, D. , Knoop, H. , Bleijenberg, G. , … Fransen, J. (2016). A multidimensional ‘path analysis’ model of factors explaining fatigue in rheumatoid arthritis. PDF hosted at the Radboud Repository of the Radboud University Nijmegen, 79. [PubMed]
- Saldaña, J. (2015). The coding manual for qualitative researchers. London, UK: Sage. [Google Scholar]
- Sayer, A. (2000). Realism and social science. London, UK: Sage. [Google Scholar]
- Scharloo, M. , Kaptein, A. , Weinman, J. , Hazes, J. , Breedveld, F. , & Rooijmans, H. (1999). Predicting functional status in patients with rheumatoid arthritis. The Journal of Rheumatology, 26, 1686–1693. [PubMed] [Google Scholar]
- Scott, J. A. , Lasch, K. E. , Barsevick, A. M. , & Piault‐Louis, E. (2011). Patients’ experiences with cancer‐related fatigue: A review and synthesis of qualitative research. Oncology Nursing Forum, 38(3), E191–E203. [DOI] [PubMed] [Google Scholar]
- Sharpe, M. , Hawton, K. , Seagroatt, V. , & Pasvol, G. (1992). Follow up of patients presenting with fatigue to an infectious diseases clinic. BMJ, 305(6846), 147–152. https://doi.org/10.1136/bmj.305.6846.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuifbergen, A. K. , & Rogers, S. (1997). The experience of fatigue and strategies of self‐care among persons with multiple sclerosis. Applied Nursing Research, 10(1), 2–10. https://doi.org/10.1016/S0897-1897(97)80023-7 [DOI] [PubMed] [Google Scholar]
- Tack, B. B. (1990). Fatigue in rheumatoid arthritis: Conditions, strategies, and consequences. Arthritis & Rheumatism, 3(2), 65–70. https://doi.org/10.1002/art.1790030203 [PubMed] [Google Scholar]
- Treharne, G. , Lyons, A. , Hale, E. , Goodchild, C. , Booth, D. , & Kitas, G. (2008). Predictors of fatigue over 1 year among people with rheumatoid arthritis. Psychology, Health and Medicine, 13, 494–504. https://doi.org/10.1080/13548500701796931 [DOI] [PubMed] [Google Scholar]
- Ulrichsen, K. M. , Kaufmann, T. , Dørum, E. S. , Kolskår, K. K. , Richard, G. , Alnæs, D. , … Nordvik, J. E. (2016). Clinical utility of mindfulness training in the treatment of fatigue after stroke, traumatic brain injury and multiple sclerosis: A systematic literature review and meta‐analysis. Frontiers in Psychology, 7, 912 https://doi.org/10.3389/fpsyg.2016.00912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Akker, L. E. , Beckerman, H. , Collette, E. H. , Eijssen, I. C. J. M. , Dekker, J. , & de Groot, V. (2016). Effectiveness of cognitive behavioral therapy for the treatment of fatigue in patients with multiple sclerosis: A systematic review and meta‐analysis. Journal of Psychosomatic Research, 90, 33–42. https://doi.org/10.1016/j.jpsychores.2016.09.002 [DOI] [PubMed] [Google Scholar]
- Van Kessel, K. , & Moss‐Morris, R. (2006). Understanding multiple sclerosis fatigue: A synthesis of biological and psychological factors. Journal of Psychosomatic Research, 61, 583–585. https://doi.org/10.1016/j.jpsychores.2006.03.006 [DOI] [PubMed] [Google Scholar]
- Wolfe, F. , Hawley, D. J. , & Wilson, K. (1996). The prevalence and meaning of fatigue in rheumatic disease. The Journal of Rheumatology, 23, 1407–1417. [PubMed] [Google Scholar]
- Wu, H.‐S. , & McSweeney, M. (2007). Cancer‐related fatigue: “It's so much more than just being tired”. European Journal of Oncology Nursing, 11, 117–125. https://doi.org/10.1016/j.ejon.2006.04.037 [DOI] [PubMed] [Google Scholar]
- Yngman‐Uhlin, P. , Friedrichsen, M. , Gustavsson, M. , Fernström, A. , & Edéll‐Gustafsson, U. (2010). Circling around in tiredness: Perspectives of patients on peritoneal dialysis. Nephrology Nursing Journal, 37, 407–413. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Interview schedule.
Appendix S2. Qualitative analysis: further detail.
Appendix S3. Coding manual.
Appendix S4. Distribution of fatigue scores.


