Abstract
Background and Purpose
Inflammatory bowel disease (IBD) is characterized by pain, bleeding, cramping and altered gastrointestinal (GI) function. Changes in mucosal 5‐HT (serotonin) signalling occur in animal models of colitis and in humans suffering from IBD. Melatonin is co‐released with 5‐HT from the mucosa and has a wide variety of actions in the GI tract. Here, we examined how melatonin signalling is affected by colitis and determined how this relates to 5‐HT signalling.
Experimental Approach
Using electroanalytical approaches, we investigated how 5‐HT release, reuptake and availability as well as melatonin availability are altered in dextran sodium sulfate (DSS)‐induced colitis in mice. Studies were conducted to explore if melatonin treatment during active colitis could reduce the severity of colitis.
Key Results
We observed an increase in 5‐HT and a decrease in melatonin availability in DSS‐induced colitis. A significant reduction in 5‐HT reuptake was observed in DSS‐induced colitis animals. A reduction in the content of 5‐HT was observed, but no difference in tryptophan levels were observed. A reduction in deoxycholic acid‐stimulated 5‐HT availability and a significant reduction in mechanically‐stimulated 5‐HT and melatonin availability were observed in DSS‐induced colitis. Orally or rectally administered melatonin once colitis was established did not significantly suppress inflammation.
Conclusion and Implications
Our data suggest that DSS‐induced colitis results in a reduction in melatonin availability and an increase in 5‐HT availability, due to a reduction/loss of tryptophan hydroxylase 1 enzyme, 5‐HT content and 5‐HT transporters. Mechanosensory release was more susceptible to inflammation when compared with chemosensory release.
Abbreviations
- 5‐HIAA
5‐hydroxyindoleacetic acid
- 5‐HTP
5‐hydroxytryptophan
- BDD
boron‐doped diamond
- DSS
dextran sodium sulfate
- GI
gastrointestinal
- HPLC
high performance liquid chromatography
- IBD
inflammatory bowel disease
- NAT
N‐acetyltransferase
- SERT
5‐HT transporter
- DCA
sodium deoxycholic acid
- TNBS
trinitrobenzene sulfonic acid
- TpH1
tryptophan hydroxylase 1
Introduction
Ulcerative colitis is one of the two major forms of inflammatory bowel disease (IBD) and is characterized by inflammatory damage of the intestinal tissue, which results in symptoms of pain, bleeding, cramping and altered gastrointestinal (GI) motility and secretion (Bassotti et al., 2014). The inflammatory damage in ulcerative colitis is largely restricted to the mucosal epithelium of the colonic wall. The mucosal epithelium contains enterochromaffin (EC) cells, which release transmitters following either chemical or mechanical stimulation (Racke et al., 1995; Racke and Schworer, 1991). EC cells serve as epithelial transducers between the lumen and the underlying neuronal and glial network of the enteric nervous system (Bertrand and Bertrand, 2010; Mawe and Hoffman, 2013). Various studies have shown that EC cells act as a key regulator of propulsive motility patterns (Heredia et al., 2013; Lavoie et al., 2015; Morris et al., 2016; Patel, 2016). The EC cell has been shown to release a range of chemical transmitters, of which most studies have focused on http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5 (serotonin) (Costedio et al., 2007; Mawe and Hoffman, 2013). However, few studies have focused on http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=224 (Bubenik, 2001; Raikhlin, 1976), which has been shown to be expressed and released from the mucosa (Bubenik et al., 1977; Patel, 2008; Raikhlin et al., 1975, 1976) and is a metabolite of 5‐HT.
Human and animal studies have shown that inflammation results in changes to various aspects of mucosal 5‐HT signalling (Costedio et al., 2007). Using the trinitrobenzene sulfonic acid (TNBS)‐induced model of colitis, changes in 5‐HT content, the number of EC cells, the amount of 5‐HT released and expression of the 5‐HT (serotonin) transporter (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=928) have been observed (Linden et al., 2003; 2005; O'Hara et al., 2004). Dextran sodium sulfate (DSS) has also been utilized to induce colitis. DSS colitis is a mixed Th1/Th2 cytokine mediated colitis and is generally less severe than TNBS colitis. In murine DSS colitis, 5‐HT availability increased primarily by an increase in the numbers of EC cells and/or content of 5‐HT in these cells (Bertrand et al., 2010a). In humans with ulcerative colitis, there were decreases in the storage of 5‐HT and EC cells, no change in 5‐HT release and a loss in SERT function (Coates et al., 2004). The observations in animal models and in humans with colitis are somewhat inconsistent, due in part to the differences between IBD and chemical models; however, in virtually all conditions and models examined to date, there is an increased 5‐HT availability in the inflamed gut (Costedio et al., 2007).
Melatonin has been shown to have a wide range of effects on GI function (Bubenik, 1999; 2002). In particular, melatonin is believed to function as a physiological antagonist of the actions of 5‐HT (Bubenik and Pang, 1994). Melatonin release has not been examined in colitis; however, increased expression of the melatonin synthesis enzyme hydroxyindole‐O‐methyltransferase has been observed in patients with ulcerative colitis (Chojnacki et al., 2013). Changes in melatonin signalling have been observed with ageing in mice (Diss et al., 2013). Of interest, melatonin treatment has been shown to reduce markers of inflammation in mouse colon and the severity of DSS colitis in mice, due to its action as an antioxidant (Bertrand et al., 2010b; Pascua et al., 2011; Pozo et al., 2010). A study has also indicated that adjuvant melatonin treatment may help in sustaining remission in patients with ulcerative colitis (Chojnacki et al., 2011; Mozaffari and Abdollahi, 2011).
Here, we have examined changes in 5‐HT and melatonin mucosal signalling following DSS colitis in mice. We have utilized electrochemical sensors as they provide temporally and spatially precise measurements of changes in release, availability and reuptake from live tissue. Using such approaches, we have monitored how the 5‐HT and melatonin signalling mechanism are altered following chemical or mechanical stimulation. We also utilized chromatography to monitor the changes in precursors and metabolites to provide insights into key enzymes involved in the mucosal signalling processes. Finally, we explored if oral or rectal administration of melatonin during active colitis could reduce the severity of the disease.
Methods
Animals
Wild‐type male CD1 mice (6–8 weeks; 28 ± 2 g, Charles River, Montreal, QC) were used. Animal protocols were approved by the University of Calgary Animal Care Committee and were carried out in accordance with the guidelines of the Canadian Council on Animal Care. Animal studies were also reported in compliance with ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015). Animals were housed in polystyrene cages with free access to food and tap water and maintained on a 12 h light–dark cycle in a temperature and humidity controlled room. All animals were killed by cervical dislocation under deep isofluorane anaesthesia. The group sizes for untreated and treated animals were not identical due to the natural variation in the severity of colitis, and therefore, a greater number of colitis animals were utilized to see correlations between signalling and tissue damage.
Colitis
Colitis was induced in 5‐ to 6‐week‐old male CD1 using dextran sodium sulfate (DSS; molecular weight 36 000–50 000; MP Biomedical, Solon, OH; 5% w.v‐1 in water) dissolved in drinking water for 5 days, followed by 2 days of normal drinking water. DSS‐treated animals were killed 7 days after initiation of treatment. No adverse events were observed. Body weight, stool consistency and the presence of faecal blood was monitored throughout. The extent of damage induced by intestinal inflammation was assessed using a modified version of an established macroscopic damage score (Cluny et al., 2010; MacEachern et al., 2015). Briefly, the total colonic damage score consisted of the sum of a score for the change in body weight (percentage body weight loss from initial where 1 = 0–5%, 2 = 5.1–10%, 3 = 10.1–15% and 4 > 15%), a score for the change in colon length (% of control colon length where 1 = 75–85%, 2 = 65–74.9% and 3 < 64.9%; control colon length = 9.2 cm), the percentage of the colon inflamed, summed with scores for the presence or absence of erythema (0 or 1), diarrhoea (0 or 1; defined as unformed faecal pellets) and faecal blood (0 or 1).
Dosing studies
Treatment of mice began 4 days following initiation of DSS during active colitis. Mice received either vehicle (0.5% DMSO in water) or melatonin (8 mg·kg−1; Sigma M5250) once daily by gavage via a 30 g feeding needle (3 cm; Fine Science Tools, Vancouver, BC, Canada) or by enema via a 5 French flexible feeding tube (Covidien, Mansfield, MA, USA) inserted 4.5 cm intrarecatally, under isofluorane anaesthesia. A volume of 200 μL.30 g‐1 body weight was administered. In order to determine if any effects of melatonin were specific to its action at the http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=39, a group of mice receiving both oral and rectal melatonin were also treated with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1363, a specific melatonin receptor antagonist. Mice received either vehicle (0.5% DMSO) or luzindole (5 mg·kg−1; Tocris 0877) ip 15 min prior to the gavage or enema treatment in a volume of 200μL.30 g‐1 body weight. Mice were killed 7 days after initiation of DSS. We observed one death in the oral melatonin, oral melatonin plus luzindole and rectal melatonin groups during the study. The extent of damage induced by intestinal inflammation was assessed by an individual blinded to the treatment group.
Amperometric approach curve profiling for measuring 5‐HT release and reuptake
For approach curve profiling measurements, constant potential amperometry was utilized. A platinum wire was used as the auxiliary electrode and a ‘no leak’ Ag/AgCl electrode (3 M KCl, Cypress Systems Inc., USA) served as the reference electrode in a three‐system configuration. The working electrode consisted of a 76 μm boron‐doped diamond (BDD) microelectrode. All the electrodes were placed in a flow bath that was continuously perfused with warm (37 °C) oxygenated (95% O2 and 5% CO2) Krebs' buffer solution, pH 7.4 (117 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM MgCl2, 1.2 mM NaH2PO4, 25 mM NaHCO3 and 11 mM glucose) at a flow rate of 4 mL·min−1; 1 cm2 colon segments taken 2 cm proximal to the rectum were placed into the Krebs' buffer solution prior to measurements. The segments were bisected along the mesenteric border to open up the preparations, and the tissue was pinned down with the mucosal layer upmost. Tissues were allowed to rest for 15 min prior to commencing recordings.
For measurements, the BDD microelectrode was affixed to a micromanipulator (Fine Scientific Tools, USA) and placed >5 mm over the centre of a tissue piece in the bulk of the media. The BDD electrode was held at a potential of +650 mV versus Ag|AgCl. Measurements were carried out using a previously published protocol (Marcelli and Patel, 2010; Zhao et al., 2010). Briefly, the electrode was carefully positioned over the tissue for a duration of 40 s for each of the following electrode – tissue distances: 1000, 800, 600, 400 and 200 μm (Supporting Information Figure S1A). Measurements were repeated two times on each tissue segment. Following this, the tissue was perfused with 1 μM http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=203 for 30 min before the measurement protocol was repeated.
Constant‐potential amperometry detection of 5‐HT and melatonin availability during mechanical and chemical stimulation
For the detection of 5‐HT and melatonin, a three‐electrode system was utilized where the BDD electrode served as the working electrode. Isolated colonic tissue segments were mounted in the flow bath and constantly perfused with oxygenated warm Krebs buffer. Measurement of 5‐HT and melatonin availability was conducted as previously described (Patel, 2008). Briefly, the BDD electrode was placed in bulk media and then placed over the tissue at an electrode tissue distance of 500 μm using a manipulator for a duration of 40 s. Following this, the electrode was retracted back to bulk media. Sequential measurements from the same tissue location were carried out at +650 mV for detection of 5‐HT and +800 mV for the detection of melatonin and 5‐HT. These measurements were repeated five times over various regions of the tissue section.
The protocol was slightly modified for mechanical stimulation, where the BDD electrode was taken from bulk media to an electrode‐tissue distance of 500 μm. For the first 40 s, the sensor was utilized to monitor basal levels of the transmitters. Following this without any movement of the BDD electrode, a glass capillary was utilized to gently touch the mucosal villi located 200 μm adjacent to the BDD electrode for 20 s. After the mechanical stimulation was removed, a further 40 s recording was carried out prior to retraction of the BDD electrode into bulk media (Supporting Information Figure S1B). To identify the effect of chemical stimulation, the tissue was perfused with 10 μM sodium http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=610 for 20 min and the same protocol as above was conducted.
High‐performance liquid chromatography measurements
High performance liquid chromatography (HPLC) was utilized to monitor the levels of 5‐HT precursors and metabolites, as previously described (Chau and Patel, 2009; Parmar et al., 2011). Briefly, a 1 cm2 segment of the distal colon was isolated, and using a sharp scalpel, the mucosal layer of the tissue was scraped. The mucosal tissue was placed in 500 μL of ice cold 0.1 M perchloric acid. Samples were homogenized and centrifuged at 14 680 g for 10 min at 4 °C. Prior to chromatographic analysis, samples were filtered through a 0.2 μm Phenex RC membrane syringe filter.
The HPLC apparatus consisted of a Jasco HPLC pump (Model: PU‐980) and Rheodyne manual injector equipped with a 20 μL loop. A Kinetic® ODS 2.6 μm, 100 mm × 2.1 mm i.d., analytical column with a guard column (Phenomenex®, Macclesfield, UK) was employed. The HPLC system was run at a flow rate of 0.1 mL·min−1. A CHI630B potentiostat (CH Instruments, Austin, TX, USA) was used to control the detector voltage and record the current. A 3 mm glassy carbon electrode (flow cell, BAS) served as the working electrode and was used with a Ag/AgCl reference electrode and a stainless‐steel block as the auxiliary electrode. Amperometric recordings were carried out, where the working electrode was set at a potential of +850 mV versus Ag/AgCl reference electrode. Control and data collection/processing were handled through the CHI630B software. The stock buffer for the mobile phase was composed of the following: 0.1 M sodium acetate, 0.1 M citric acid and 27 μM disodium EDTA. This was then buffered to pH 3.0. The mobile phase was prepared with the stock buffer mixed with methanol in the ratio of 8:2 (v.v‐1) and degassed after mixing.
Standard solutions were prepared from 1 mM stock standards of each analyte and were made up in 0.1 M perchloric acid (BDH). Each of the standard solutions was prepared on the day of analysis and stored at 4 °C prior to injection. A calibration plot was obtained for each 5‐HT precursor and metabolite. The peak areas obtained from chromatographic analysis of all injected samples were converted to concentrations utilizing the calibration responses. The concentration of each 5‐HT precursor and metabolite was normalized by protein content using the Bradford method to remove any variation in amount of tissue sample. To obtain the activity of key intracellular enzymes, the ratios of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4671 : http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=717 and 5‐HT : 5‐HTP were obtained.
Data interpretation and statistics
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). During the generation of data, the severity of the colitis score was blinded from the experimental data. For electrochemistry measurement, it was not possible to blind the untreated to treated animals for colitis due to the visual nature of the tissue damage; however, this was randomised and blinded for HPLC analysis. For analysis of the amperometric approach curve profiling traces, the current was recorded at each of the electrode‐tissue distances and plotted against the electrode‐tissue distance. This resultant plot generally shows the response of an exponential decay. The natural log of the current in whole numbers (pA scale) was calculated and plotted against the electrode‐tissue distance, and a linear regression line was fitted to the data enabling the slope and intercept of the vertical axis to be derived. From the slope and intercept, information on the reuptake and release of 5‐HT can be obtained, respectively, as previously shown (Marcelli and Patel, 2010). For amperometric measurement of 5‐HT and melatonin, the difference in the current over the tissue compared with baseline was obtained. During mechanical stimulation, the current difference over the tissue to that during mechanical stimulation was measured. To obtain the current response for melatonin, the response obtained at +800 mV was subtracted from that obtained at +650 mV as previously shown (Diss et al., 2013; Patel, 2008). In both amperometry approach curve profiling and basic amperometry measurements, the current for 5‐HT and melatonin was converted to concentration using calibration plots. For comparison between control measurements and those in the presence of colitis, data are presented as mean ± 95% confidence interval and were compared using two‐way ANOVA followed by post hoc pair‐wise comparisons with Bonferroni's test when F achieved P < 0.05, and there was no significant inhomogeneity. For all data, P < 0.05 was accepted as a level of statistically significant difference (analysed using Graphpad Prism 6.07, GraphPad Software Inc.). Correlation plots were also obtained by plotting individual animal responses of the various parameters investigated against the macroscopic damage score.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b,c).
Results
Changes in the availability of 5‐HT and melatonin in DSS colitis
There was a significantly greater amount of melatonin availability when compared with 5‐HT availability (P < 0.05, Figure 1A). A significant increase in 5‐HT availability and a significant decrease in melatonin availability were observed from DSS‐treated animals when compared with untreated mice (P < 0.05, Figure 1A). The ratio of melatonin : 5‐HT significantly decreased from 2.1 ± 0.1 in untreated animals to 0.3 ± 0.2 in DSS‐treated animals (P < 0.05, Mann–Whitney test, Figure 1B). Correlations of the macroscopic damage score with 5‐HT availability were obtained, where no meaningful relationship was observed (Figure 1C). A significant decrease in melatonin availability was associated with increasing macroscopic damage scores (P < 0.05, Figure 1D).
Figure 1.
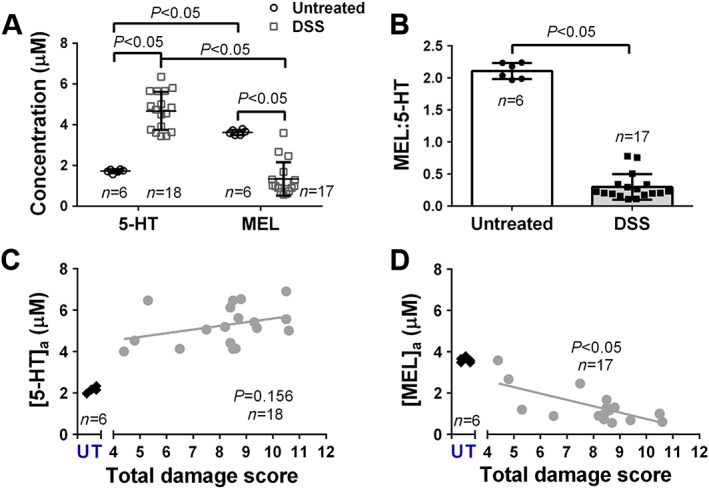
Alterations in 5‐HT and melatonin availability in animals with DSS‐induced colitis. (A) Current responses of measured 5‐HT and melatonin availability in wild‐type and DSS inflamed isolated colonic segments. (B) Ratio of melatonin : 5‐HT. (C) Correlations of 5‐HT and (D) melatonin availability versus total damage score. The mean damage score in DSS‐treated mice was 8.2 ± 0.9. Data shown as mean ± 95% CI.
Alterations in 5‐HT release and reuptake
Measurements at varying distances between the tissue and electrode provide the means to understand changes in release and reuptake. Figure 2A shows the natural log (Ln) current obtained from wild‐type animals in the presence and absence of 1 μM fluoxetine, which is known to inhibit SERT. The slope, which is a marker of reuptake is less steep in presence of 1 μM fluoxetine, suggesting inhibited reuptake. Ln current responses from wild‐type and DSS‐treated mice are shown in Figure 2B, where changes in the slope can be observed.
Figure 2.
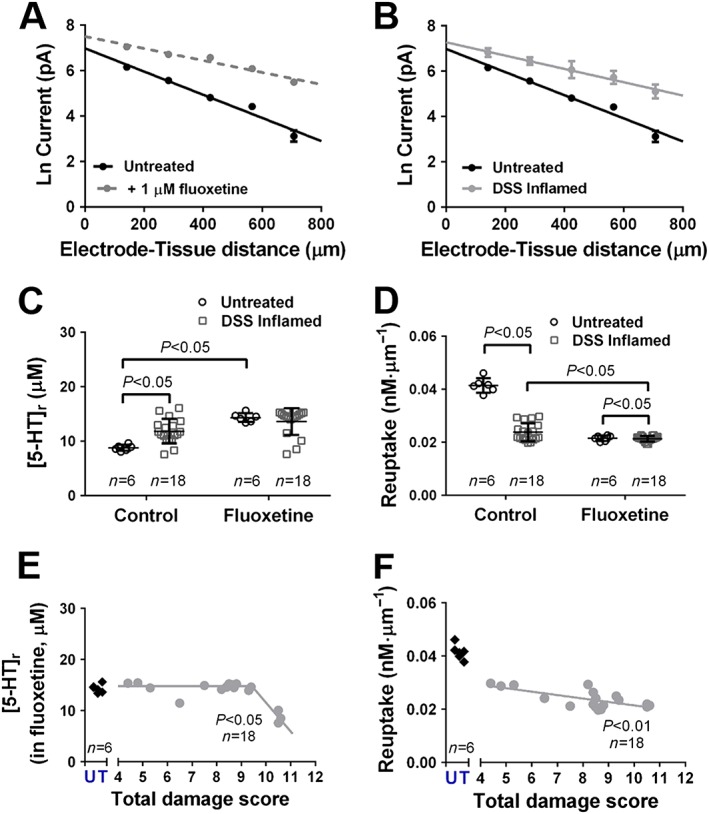
Changes in 5‐HT release and reuptake following inflammation. Ln current response at varying electrode‐tissue distances obtained from amperometry approach curve profiling when investigating the (A) influence of fluoxetine on wild‐type colonic tissue and (B) effect of DSS‐induced inflammation. (C) Release of 5‐HT. (D) Reuptake of 5‐HT. Correlation of (E) 5‐HT release and (F) 5‐HT reuptake against total damage score. Data shown as mean ± 95% CI.
Measurement of the intercept provided insight into the changes with 5‐HT release. There was a significant increase in 5‐HT release in wild‐type animals in the presence of fluoxetine (P < 0.05) and in DSS‐induced mice under control conditions (P < 0.05, Figure 2C). There was no difference in 5‐HT release in wild‐type tissues in the presence of fluoxetine when compared with DSS‐treated animals in either absence or presence of fluoxetine (Figure 2C). Measurement of the slope provided insight into the changes in reuptake between untreated and DSS‐treated animals. When studies were conducted in fluoxetine, there was significant decrease in the rate of reuptake in wild type animals (P < 0.05) when compared with control. There was a significant decrease in the rate of reuptake in DSS‐treated animals when compared with wild‐type animals under control conditions (P < 0.05, Figure 2D). In the presence of fluoxetine, there was a significant decrease in the rate of reuptake in DSS‐treated animal colonic tissues (P < 0.05, Figure 2D) when compared with control.
Correlations of 5‐HT release levels measured in the presence of fluoxetine against macroscopic damage score from individual animals treated with DSS are shown in Figure 2E. The correlation was conducted using data where release was monitored in the presence of fluoxetine to remove the bias of release due to reuptake at the epithelium surface. There was a significant decrease in 5‐HT release in the presence of fluoxetine with increased macroscopic damage score (P < 0.05, Figure 2E). The changes in 5‐HT release at macroscopic damage scores between 5 and 8 were minor; however, significant reductions in 5‐HT release were observed with macroscopic damage score ≥8.5. There was a significant decrease in the rate of 5‐HT reuptake with increasing macroscopic damage score (P < 0.05, Figure 2F).
Chromatographic measurements of 5‐HT precursors and metabolites
Chromatographic measurements of colonic mucosal tissue were conducted, where no significant differences in the levels of tryptophan were observed between untreated and DSS‐treated animals (Figure 3A). Significant decreases in 5‐HTP (P < 0.05, Figure 3B), 5‐HT (P < 0.05, Figure 3C) and 5‐hydroxyindoleacetic acid (5‐HIAA, P < 0.05, Figure 3D) were observed in DSS‐induced colitis mice when compared with untreated mice. Decreases in the ratio of 5‐HTP : tryptophan were observed in colonic tissues of animals treated with DSS when compared to untreated animals (P < 0.05, Mann–Whitney test, Figure 3E). No significant changes in the 5‐HT : 5‐HTP ratio were observed in DSS‐treated animals when compared with untreated animals (Figure 3F). Using the amperometric and chromatographic data, the ratio of intracellular to extracellular 5‐HT can be obtained. A significant decrease in the intracellular to extracellular 5‐HT was observed in DSS‐treated animals (P < 0.05, Supporting Information Figure S2).
Figure 3.
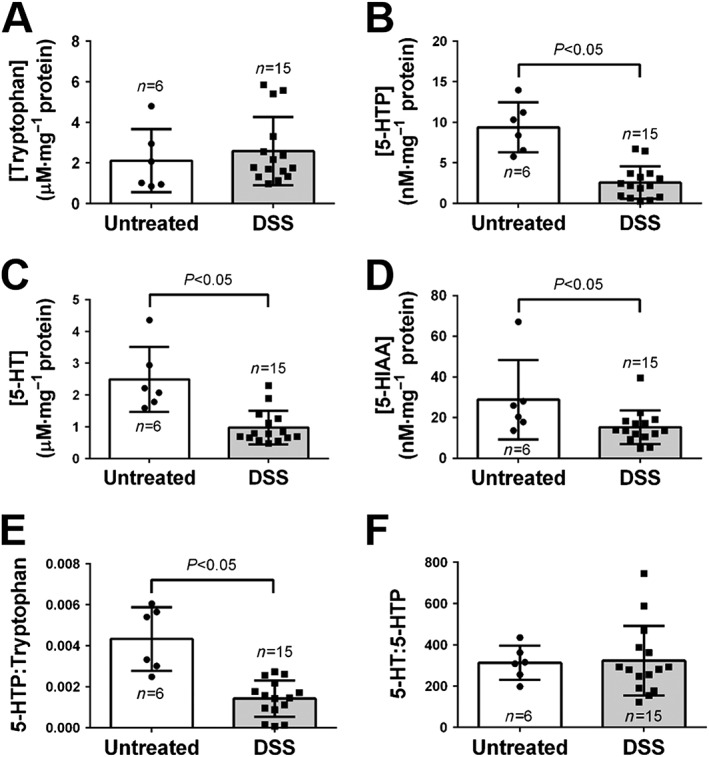
Changes in precursors and metabolites involved in 5‐HT signalling process. Changes in levels of (A) tryptophan, (B) 5‐HTP, (C) 5‐HT and (D) 5‐HT before and after DSS‐induced inflammation. Ratios of (E) 5‐HTP : tryptophan and (F) 5‐HT : 5‐HTP. Data shown as mean ± 95% CI.
Influence of mechanical and chemical stimulation on 5‐HT and melatonin availability in DSS colitis
Studies were conducted to determine the ability of DCA to stimulate 5‐HT release in DSS colitis. Figure 4A shows the basal release of 5‐HT, due to low level mechanical stimulation by the flow of the physiological media within the bath, where there is a significant increase in 5‐HT availability in DSS‐treated animals (P < 0.05, n = 9–16). The contribution of DCA to drive 5‐HT availability was shown to be of the same capacity of that observed at basal stimulation. This 5‐HT availability contribution induced by DCA was significantly lower in DSS‐inflamed colonic tissue when compared with wild‐ type tissue (P < 0.05, Figure 4A).
Figure 4.

Contribution of 5‐HT and melatonin availability following DSS‐induced inflammation. Contribution of DCA and additional mechanical stimulation on (A) 5‐HT and (D) melatonin availability. Correlations of 5‐HT availability due to (B) chemical and (C) additional mechanical stimulation against total damage score. Correlations of melatonin availability due to (E) chemical and (F) additional mechanical stimulation against macroscopic damage score. Data shown as mean ± 95% CI.
A glass capillary was utilized to evoke an additional mechanical response above the basal stimulation. Mechanical stimulation in wild type animals results in approximately half the response under basal stimulation. In untreated and DSS‐induced inflammation in tissue, the component of additional 5‐HT availability was greater due to DCA stimulation when compared with mechanical stimulation (P < 0.05). There was a reduction in the 5‐HT availability contribution by mechanical stimulation (P < 0.05) and combined mechanical‐DCA stimulation (P < 0.05) in DSS‐treated animals when compared with wild‐type (Figure 4A).
There was a significant reduction in the DCA‐stimulated 5‐HT availability with increasing macroscopic damage score (P < 0.05, Figure 4B). No correlation was observed with mechanically stimulated 5‐HT availability and macroscopic damage score (Figure 4C).
Similar studies were conducted with melatonin, where under basal stimulation, there was a significant reduction in melatonin availability in DSS‐treated animals (P < 0.05, Figure 4D). Under chemical stimulation with DCA, there was no additional contribution to the underlying basal response. No differences in the levels of melatonin availability due to DCA activation were observed between untreated and DSS‐treated animals. In untreated tissue, the component of additional melatonin availability was greater due to mechanical stimulation when compared with DCA stimulation (P < 0.05). Additional mechanical stimulation elevated the melatonin availability by half the response of the basal measurement (Figure 4D). A significant reduction in mechanically stimulated melatonin availability was observed in DSS‐treated animals when compared with wild‐type (P < 0.05), which resulted in a significant reduction in melatonin availability in DSS‐treated animals when a combined DCA and mechanical stimulation was utilized (P < 0.05, Figure 4D).
No correlations were observed between DCA (Figure 4E) and mechanically (Figure 4F) stimulated melatonin availability and macroscopic damage score.
Investigation of the impact of orally and rectally delivered melatonin on DSS‐induced colitis in mice
Studies were conducted to explore if treatment with melatonin could reduce the severity of inflammation in DSS‐induced colitis in mice. Animals were treated both orally and rectally. No differences in body weight during the 7 days post DSS initiation were observed when treated with melatonin or luzindole plus melatonin orally compared with vehicle control (Figure 5A). No significant difference in the total damage score between the melatonin and melatonin plus luzindole treatment groups were observed when compared with the vehicle control group when treated orally (Figure 5B). No differences in body weight during the 7 days post DSS initiation were observed when treated with melatonin or luzindole plus melatonin rectally when compared with vehicle control (Figure 5C). When treated rectally, no significant differences in the total damage score between the melatonin and melatonin plus luzindole treatment groups were observed when compared with the vehicle control group (Figure 5D).
Figure 5.

Effect of orally and rectally delivered melatonin on parameters of colitis. (A) Change in body weight during progression of colitis using DSS, where oral (p.o.) treatment was initiated on day 4. (B) The resultant total damage score for orally dosed melatonin (MEL) against the vehicle (V) and melatonin plus luzindole (MEL + LUZ). (C) Change in body weight during induction of colitis using DSS, where rectal (p.r.) treatment was initiated on day 4. (B) The resultant total damage score for rectally administered melatonin (MEL) against the vehicle (V) and melatonin plus luzindole (MEL + LUZ). Data shown as mean ± 95% CI.
Discussion
The main findings of this study are that 5‐HT availability is increased and melatonin availability is decreased in a mouse model of DSS‐induced colitis. Such increases in 5‐HT availability are supportive of a previous study that utilized electrochemical methods to monitor 5‐HT in DSS‐induced colitis in mice (Bertrand et al., 2010a).
How does 5‐HT availability increase and melatonin availability decrease in DSS‐colitis?
The increased 5‐HT availability was evaluated using an electrochemical approach that monitored levels at various distances from the tissue surface. This provides the ability to gain insight into 5‐HT release and reuptake (Marcelli and Patel, 2010). Our findings showed that reuptake was significantly reduced in DSS‐induced animals to a similar extent that could be achieved in untreated tissues that were exposed to 1 μM fluoxetine. This suggests that SERT activity is lost and/or impaired secondary to inflammation induced by DSS. This was supported by reduced levels of 5‐HIAA observed in mucosal tissue using chromatography. Loss and/or impairment of SERT is a consistent finding in all animal and human studies that have investigated changes in the 5‐HT signalling process during inflammation (Costedio et al., 2007).
In measurements in physiological buffer, we observed a significant increase in 5‐HT release in DSS‐treated animals when compared with wild‐type; however, this does not provide a true reflection of release, as in wild‐type animals, the reuptake process by SERT is active, which can reduce the signal observed at the electrode, providing an underestimation of release. Therefore, it is most logical to determine how release is altered in the presence of the SERT inhibitor fluoxetine, so a reflective contribution of release can be monitored. Our study indicates that release is not altered in either untreated or DSS‐treated animal tissues that were exposed to fluoxetine. To further support this, no differences were observed in the release of 5‐HT in tissue from DSS‐treated animals when compared with wild‐type in the presence of fluoxetine. This finding supports observations obtained in TNBS‐induced colitis in mice and in human patients with ulcerative colitis (Coates et al., 2004; Linden et al., 2005).
We observed no changes in tryptophan levels, but significant decreases in 5‐HTP and 5‐HT levels in mucosal tissue of DSS‐treated animals when compared with untreated animals. These changes suggest that 5‐HT content or the number of EC cells are reduced following inflammation. A significant reduction in the 5‐HTP : tryptophan ratio was observed, suggesting that the http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=249#1241 (TpH1) enzyme is impaired and/or lost as a result of inflammation. No changes were observed in 5‐HT : 5‐HTP, suggested that the aromatic L‐amino acid decarboxylase (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1271) enzyme is not altered during inflammation. Our findings are contradictory to other published findings, which in both TBNS and DSS‐induced inflammation in animal models increases in either 5‐HT content or number of EC cells have been observed (Bertrand et al., 2010a; Linden et al., 2003; 2005; O'Hara et al., 2004). This may be due to the nature of the studies conducted as increased content was thought to be due to increases in the number of EC cells monitored extracellularly, whereas we have monitored intracellular 5‐HT levels. However, our findings agree with observations in human patients with ulcerative colitis, where a reduction in 5‐HT content was seen (Coates et al., 2004).
Our study is the first to show that melatonin levels were significantly reduced following inflammation. Melatonin has been shown to be synthesized on demand from 5‐HT by the enzymes N‐acetyltransferase (NAT) and hydroxyindole‐O‐methyltransferase (Bubenik, 2001). Therefore, the reduction in the amount of melatonin produced is directly associated with a reduction in 5‐HT.
Relationship between 5‐HT and melatonin changes with inflammation
Our study is the first to show that the ratio of melatonin : 5‐HT availability decreases from approximately 2 to 0.5 following inflammation. This is most likely due to the loss in SERT function and a reduction in 5‐HT content. This reduction in the melatonin : 5‐HT content is most likely to have implications to the function of the colon, as the relationship between these two molecules has been shown to direct motility (Bubenik, 1986; Bubenik and Dhanvantari, 1989; Thor et al., 2007). However, these molecules also have an interesting ying‐yang relationship with regard to the regulation of inflammation. Various studies have shown that elevated 5‐HT can induce inflammation and exacerbate colitis (Bischoff et al., 2009; Ghia et al., 2009). By contrast, melatonin is well known to act as an antioxidant and reduce markers of inflammation (Pascua et al., 2011; Pentney and Bubenik, 1995; Talero et al., 2014). Our findings provide an important insight as they are suggestive that in wild‐type animals, a greater amount of melatonin compared with 5‐HT availability is present, which may be responsible for providing resilience of the colon to react to inflammation. Following DSS treatment, this trend is reversed; and therefore, this will most likely exacerbate the inflammation, as 5‐HT acts to induce this and melatonin cannot serve to reduce this. Preserving the balance in the ratio of 5‐HT and melatonin may be key to reducing the severity of the inflammation in colitis. These changes are suggestive that adjuvant therapy using melatonin may be of benefit in restoring the ratio of 5‐HT and melatonin, which may explain observations in studies that have shown sustained remission following melatonin treatment (Chojnacki et al., 2011).
Alterations in chemo‐ and mechano‐stimulated 5‐HT and melatonin release
It is well known that 5‐HT is released following mechanical and chemical stimulation of the mucosal epithelium. However, little is known about what drives melatonin production other than that it is produced on demand. Within our study, we utilized DCA as a chemical stimulant to drive 5‐HT release. DCA has been shown to increase 5‐HT secretion through transport into the EC cell to induce ERK phosphorylation (Braun et al., 2007). A glass capillary was utilized to distort the villus and therefore activate mechanosensory ion channels, such as transient receptor potential channels to drive 5‐HT release (Nozawa et al., 2009; Patel, 2016).
Within our studies, we observed that there was a reduction in both DCA and mechanically‐stimulated 5‐HT availability in DSS‐induced colitis animals, of which the reduction was of a greater extent following mechanical stimulation. This would suggest that chemosensory channels are more resilient than mechanosensory ion channels in the inflamed state.
No detectable melatonin availability was observed with DCA, suggesting that DCA does not influence or drive melatonin production. However, like 5‐HT availability, a significant reduction in melatonin availability was observed in DSS‐induced colitis animals following mechanical stimulation. This suggests that activation of mechanosensory ion channels can enhance the production of melatonin, which is most likely through protein kinase A that has been shown to phosphorylate NAT (Rosiak and Zawilska, 2005).
Does oral or rectally delivered melatonin suppress inflammation in DSS‐induced colitis in mice?
Within our study, we utilized both oral and rectal delivery of melatonin, neither of which showed the ability to significantly reduce the total damage score and therefore the severity of colitis. However, we did see a modest but not significant reduction in damage score when melatonin treatment was given as an enema.
Our study is at odds with other studies that have seen improvements in either TNBS‐ or DSS‐induced colitis when treated with melatonin. However, with most of these studies, melatonin was given before the onset of the disease (Cuzzocrea et al., 2001; Liu et al., 2017; Trivedi and Jena, 2013; Zielińska et al., 2016), which likely accounts for the differences observed when compared with our results where treatment was given once symptoms were present. In these studies phenotypic changes, such as shortening of the colon, were also observed to an extent greater that DSS alone (Zielińska et al., 2016), which from our observations is highly correlated with a worsening of colitis.
Although our data were not significant, there are still indications that melatonin could play a role as an adjunctive therapy; however, the route of administration, the duration of treatment and dose need further investigation.
Are the changes in 5‐HT and melatonin correlated with the extent of damage by inflammation?
There is considerable interest in utilizing chemical signalling molecules as potential biomarkers for bowel conditions (Clarke et al., 2009); however, the majority of studies have looked for changes without showing any correlative information on the extent of the condition and changes in mucosal signalling. Our study is the first to show the relationship between 5‐HT and melatonin signalling with the extent of damage in colitis.
We observed that no correlation was present between macroscopic damage score and 5‐HT availability; however, a correlation was present with melatonin availability. When the availability of 5‐HT was investigated for contribution of reuptake and release, there was a significant decrease in reuptake with increased damage score. However, for 5‐HT release, no changes in 5‐HT release were observed until a very high macroscopic damage score of eight of which afterwards there was a significant decline in 5‐HT release. These changes suggest that 5‐HT availability alone may not be a good marker of the extent of damage, but melatonin may be more reflective. It also indicates that 5‐HT reuptake by SERT is less resilient to inflammation than processes that regulate 5‐HT release, which are lost only after much higher macroscopic damage score.
We also noticed that increased 5‐HT availability due to DCA stimulation was lost with increasing macroscopic damage score; however, the mechanically‐stimulated 5‐HT and melatonin availability was lost over the entire range of damage scores. This indicates that mechanosensory ion channels are high susceptible to inflammation when compared with chemosensory receptors. Therefore, chemosensory receptors may be a better target to alter 5‐HT signalling in colitis. However, this study only provides reflection on one chemosensory target, and others may be more or less susceptible during inflammation.
The observed changes with DSS‐induced inflammation on 5‐HT and melatonin signalling are summarized in Figure 6.
Figure 6.
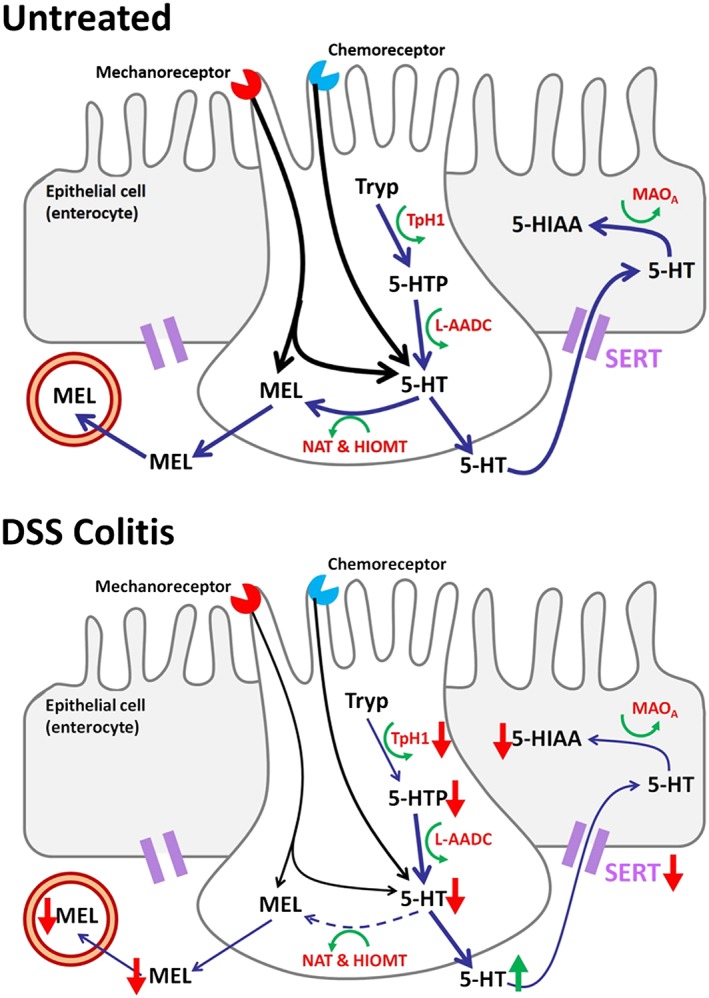
Changes in 5‐HT and melatonin signalling process following DSS‐induced colitis. Where a decrease in intracellular 5‐HT results in a reduction in melatonin availability, whilst an increase in 5‐HT availability is observed due to a loss/impairment in SERT. Tryp is tryptophan; 5‐HTP is 5‐hydroxytryptophan; MEL is melatonin; 5‐HIAA is 5‐hydroxyindoleacetic acid; TpH1 is tryptophan hydroxylase 1; L‐AADC is aromatic L‐amino acid decarboxylase; NAT is N‐acetyltransferase; HIOMT is hydroxyindole‐O‐methyltransferase; MOAA is http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2489; SERT is 5‐HT transporter.
Conclusion
This study provides insight into the changes in 5‐HT and melatonin signalling in DSS‐induced colitis in mice. We observed decreases in 5‐HT content, a reduction in TpH1 activity and a reduction in SERT, which resulted in increased 5‐HT availability. Due to the reduction in 5‐HT content, we observed a reduction in melatonin availability. The loss in melatonin availability correlated with increasing extent of damage. This would suggest that adjuvant therapy with melatonin may be of benefit, but further investigation into the route and timing of treatment is necessary. Mechanosensory ion channels were lost with inflammation; however, DCA‐stimulated 5‐HT availability was lost with increasing extent of inflammation. Overall, these findings provide novel insights into changes in mucosal signalling with inflammation, and such approaches can provide valuable tools in understanding the functional changes observed in inflammatory bowel disease.
Author contributions
S.J.M. contributed to the study design, acquisition, analysis and interpretation of the data. E.P. contributed to the acquisition and analysis of the HPLC data. C.M.K. was responsible for conducting dosing studies and interpretation of the data. K.A.S. and B.A.P. were responsible for the study design, analysis and interpretation of the data. B.A.P. drafted and reviewed the manuscript alongside S.J.M., C.M.K. and K.A.S. All authors approved the final version of the manuscript for publication and agree to be accountable for all aspects of the study.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This http://onlinelibrary.wiley.com/doi/10.1111/bph.13405/abstract acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1 Experimental traces showing the varying electrochemical approaches utilised. (A) Amperometry approach curve profiling, where the current is monitoring at varying electrode‐tissue distances and utilised to obtain contribution of release and reuptake. (B) Protocol to monitor levels of 5‐HT to study contribution of chemical and mechanical stimulation.
Figure S2 Changes in intracellular to extracellular ratio. Data shown as mean ± 95% C.I.
Acknowledgements
This study was supported by the Canadian Institutes of Health Research (grant to KAS). B.A.P. would like to acknowledge UoB sabbatical fund. K.A.S. is the Crohn's and Colitis Canada Chair in Inflammatory Bowel Disease Research at the University of Calgary.
MacEachern, S. J. , Keenan, C. M. , Papakonstantinou, E. , Sharkey, K. A. , and Patel, B. A. (2018) Alterations in melatonin and 5‐HT signalling in the colonic mucosa of mice with dextran‐sodium sulfate‐induced colitis. British Journal of Pharmacology, 175: 1535–1547. doi: 10.1111/bph.14163.
Contributor Information
Keith A Sharkey, Email: ksharkey@ucalgary.ca.
Bhavik Anil Patel, Email: b.a.patel@brighton.ac.uk.
References
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017a). The concise guide to pharmacology 2017/18: G protein‐coupled receptors. Br J P harmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: Transporters. Br J Pharmacol 174: S360–S446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassotti G, Antonelli E, Villanacci V, Salemme M, Coppola M, Annese V (2014). Gastrointestinal motility disorders in inflammatory bowel diseases. World J Gastroenterol: WJG 20: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand PP, Barajas‐Espinosa A, Neshat S, Bertrand RL, Lomax AE (2010a). Analysis of real‐time serotonin (5‐HT) availability during experimental colitis in mouse. Am J Physiol Gastrointest Liver Physiol 298: G446–G455. [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Bertrand RL (2010). Serotonin release and uptake in the gastrointestinal tract. Auton Neurosci : basic & clinical 153: 47–57. [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Bertrand RL, Camello PJ, Pozo MJ (2010b). Simultaneous measurement of serotonin and melatonin from the intestine of old mice: the effects of daily melatonin supplementation. J Pineal Res 49: 23–34. [DOI] [PubMed] [Google Scholar]
- Bischoff SC, Mailer R, Pabst O, Weier G, Sedlik W, Li Z et al (2009). Role of serotonin in intestinal inflammation: knockout of serotonin reuptake transporter exacerbates 2,4,6‐trinitrobenzene sulfonic acid colitis in mice. Am J Physiol Gastrointest Liver Physiol 296: G685–G695. [DOI] [PubMed] [Google Scholar]
- Braun T, Voland P, Kunz L, Prinz C, Gratzl M (2007). Enterochromaffin cells of the human gut: sensors for spices and odorants. Gastroenterology 132: 1890–1901. [DOI] [PubMed] [Google Scholar]
- Bubenik G (1986). The effect of serotonin, N‐acetylserotonin, and melatonin on spontaneous contractions of isolated rat intestine. J Pineal Res 3: 41–54. [DOI] [PubMed] [Google Scholar]
- Bubenik GA (1999). Localisation and physiological significance of gastrointestinal melatonin In: Watson R. (ed). Melatonin in Health Promotion. CRC Press: Boca Raton, pp. 21–39. [Google Scholar]
- Bubenik GA (2001). Localization and physiological significance and possible clinical implications of gastroinestinal melatonin. Biol Signals Recept 10: 350–366. [DOI] [PubMed] [Google Scholar]
- Bubenik GA (2002). Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci 47: 2336–2348. [DOI] [PubMed] [Google Scholar]
- Bubenik GA, Brown GM, Grota L (1977). Immunohistological localization of melatonin in the rat digestive system. Experientia 33: 662–663. [DOI] [PubMed] [Google Scholar]
- Bubenik GA, Dhanvantari S (1989). Influence of serotonin and melatonin on some parameters of gastrointestinal activity. J Pineal Res 7: 333–344. [DOI] [PubMed] [Google Scholar]
- Bubenik GA, Pang S (1994). The role of serotonin and melatonin in gastrointestinal physiology: ontogeny, regulation of food intake, and mutual serotonin‐melatonin feedback. J Pineal Res 16: 91–99. [DOI] [PubMed] [Google Scholar]
- Chau RMW, Patel BA (2009). Determination of serotonin, melatonin and metabolites in gastrointestinal tissue using high‐performance liquid chromatography with electrochemical detection. Biomed Chromatogr 23: 175–181. [DOI] [PubMed] [Google Scholar]
- Chojnacki C, Wiśniewska‐Jarosińska M, Kulig G, Majsterek I, Reiter RJ, Chojnacki J (2013). Evaluation of enterochromaffin cells and melatonin secretion exponents in ulcerative colitis. World J Gastroenterol : WJG 19: 3602–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chojnacki C, Wisniewska‐Jarosinska M, Walecka‐Kapica E, Klupinska G, Jaworek J, Chojnacki J (2011). Evaluation of melatonin effectiveness in the adjuvant treatment of ulcerative colitis. J Physiol Pharmacol 62: 327. [PubMed] [Google Scholar]
- Clarke G, Quigley EM, Cryan JF, Dinan TG (2009). Irritable bowel syndrome: towards biomarker identification. Trends Mol Med 15: 478–489. [DOI] [PubMed] [Google Scholar]
- Cluny NL, Keenan CM, Duncan M, Fox A, Lutz B, Sharkey KA (2010). Naphthalen‐1‐yl‐(4‐pentyloxynaphthalen‐1‐yl)methanone (SAB378), a peripherally restricted cannabinoid CB1/CB2 receptor agonist, inhibits gastrointestinal motility but has no effect on experimental colitis in mice. J Pharmacol Exp Ther 334: 973–980. [DOI] [PubMed] [Google Scholar]
- Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H et al (2004). Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 126: 1657–1664. [DOI] [PubMed] [Google Scholar]
- Costedio M, Hyman N, Mawe G (2007). Serotonin and its role in colonic function and in gastrointestinal disorders. Dis Colon Rectum 50: 376–388. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SPA, Giembycz MA et al (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzocrea S, Mazzon E, Serraino I, Lepore V, Luisa Terranova M, Ciccolo A et al (2001). Melatonin reduces dinitrobenzene sulfonic acid‐induced colitis. J Pineal Res 30: 1–12. [DOI] [PubMed] [Google Scholar]
- Diss LB, Robinson SD, Wu Y, Fidalgo S, Yeoman MS, Patel BA (2013). Age‐related changes in melatonin release in the murine distal colon. ACS Chem Nerosci 4: 879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghia JE, Li N, Wang H, Collins M, Deng Y, El ‐Sharkawy RT, et al (2009). Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology 137 : 1649–1660. [DOI] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia DJ, Gershon MD, Koh SD, Corrigan RD, Okamoto T, Smith TK (2013). Important role of mucosal serotonin in colonic propulsion and peristaltic reflexes: in vitro analyses in mice lacking tryptophan hydroxylase 1. J Physiol 591: 5939–5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie B, Tchitchkan D, Mawe G (2015). Abundant 5‐HT release from EC cells disrupts colonic motility. FASEB J 29: 1002–1010. [Google Scholar]
- Linden DR, Chen J‐X, Gershon MD, Sharkey KA, Mawe GM (2003). Serotonin availability is increased in mucosa of guinea pigs with TNBS‐induced colitis. Am J Physiol Gastrointest Liver Physiol 285: G207–G216. [DOI] [PubMed] [Google Scholar]
- Linden DR, Foley KF, McQuoid C, Simpson J, Sharkey KA, Mawe GM (2005). Serotonin transporter function and expression are reduced in mice with TNBS‐induced colitis. Neurogastroenterol Motil 17: 565–574. [DOI] [PubMed] [Google Scholar]
- Liu G, Jiang Q, Chen S, Fang J, Ren W, Yin J et al (2017). Melatonin alters amino acid metabolism and inflammatory responses in colitis mice. Amino Acids 49: 2065–2071. [DOI] [PubMed] [Google Scholar]
- MacEachern SJ, Patel BA, Keenan CM, Dicay M, Chapman K, McCafferty D‐M et al (2015). Inhibiting inducible nitric oxide synthase in enteric glia restores electrogenic ion transport in mice with colitis. Gastroenterology 149: 445–455.e443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelli G, Patel BA (2010). Understanding changes in uptake and release of serotonin from gastrointestinal tissue using a novel electroanalytical approach. Analyst 135: 2340–2347. [DOI] [PubMed] [Google Scholar]
- Mawe GM, Hoffman JM (2013). Serotonin signalling in the gut functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 10: 473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R, Fagan‐Murphy A, MacEachern SJ, Covill D, Patel BA (2016). Electrochemical fecal pellet sensor for simultaneous real‐time ex vivo detection of colonic serotonin signalling and motility. Sci Rep 6: 23442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffari S, Abdollahi M (2011). Melatonin, a promising supplement in inflammatory bowel disease: a comprehensive review of evidences. Curr Pharm Des 17: 4372–4378. [DOI] [PubMed] [Google Scholar]
- Nozawa K, Kawabata‐Shoda E, Doihara H, Kojima R, Okada H, Mochizuki S et al (2009). TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. PNAS 106: 3408–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara JR, Ho W, Linden DR, Mawe GM, Sharkey KA (2004). Enteroendocrine cells and 5‐HT availability are altered in mucosa of guinea pigs with TNBS ileitis. Am J Physiol Gastrointest Liver Physiol 287: G998–G1007. [DOI] [PubMed] [Google Scholar]
- Parmar L, Morgan LD, Patel BA (2011). Intracellular and extracellular sampling to monitor the neurotransmission process using a chromatographic method. Anal Methods 3: 2770–2776. [Google Scholar]
- Pascua P, Camello‐Almaraz C, Camello PJ, Martin‐Cano FE, Vara E, Fernandez‐Tresguerres JA et al (2011). Melatonin, and to a lesser extent growth hormone, restores colonic smooth muscle physiology in old rats. J Pineal Res 51: 405–415. [DOI] [PubMed] [Google Scholar]
- Patel BA (2008). Continuous amperometric detection of co‐released serotonin and melatonin from the mucosa in the ileum. Analyst 133: 516–524. [DOI] [PubMed] [Google Scholar]
- Patel BA (2016). Mucosal serotonin overflow is associated with colonic stretch rather than phasic contractions. Neurogastroenterol Motil 28: 914–923. [DOI] [PubMed] [Google Scholar]
- Pentney PT, Bubenik GA (1995). Melatonin reduces the severity of dextran‐induced colitis in mice. J Pineal Res 19: 31–39. [DOI] [PubMed] [Google Scholar]
- Pozo MJ, Gomez‐Pinilla PJ, Camello‐Almaraz C, Martin‐Cano FE, Pascua P, Rol MA et al (2010). Melatonin, a potential therapeutic agent for smooth muscle‐related pathological conditions and aging. Curr Med Chem 17: 4150–4165. [DOI] [PubMed] [Google Scholar]
- Racke K, Reimann A, Schworer H, Kilbinger H (1995). Regulation of 5‐HT release from enterochromaffin cells. Behav Brain Res 73: 83–87. [DOI] [PubMed] [Google Scholar]
- Racke K, Schworer H (1991). Regulation of serotonin release from the intestinal mucosa. Pharmacol Res 23: 13–25. [DOI] [PubMed] [Google Scholar]
- Raikhlin N (1976). Melatonin and enterochromaffine cells. Acta histochem Bd 55: 19–24. [DOI] [PubMed] [Google Scholar]
- Raikhlin N, Kvetnoi IM, Kadagidze ZG, Sokolov AV (1976). Immunohistochemical detection of the localization of melatonin and N‐acetylserotonin in enterochromaffin cells. Byullenten' Eksperimental'noi Biologii i Meditsiny 82: 1400–1401. [PubMed] [Google Scholar]
- Raikhlin N, Kvetnoy I, Tolkachev V (1975). Melatonin may be synthesized in enterochromaffin cells. Nature 255: 344–345. [DOI] [PubMed] [Google Scholar]
- Rosiak J, Zawilska J (2005). 14‐3‐3 proteins‐‐a role in the regulation of melatonin biosynthesis. Postepy Biochem 52: 35–41. [PubMed] [Google Scholar]
- Talero E, Garcia‐Maurino S, Motilva V (2014). Melatonin, autophagy and intestinal bowel disease. Curr Pharm Des 20: 4816–4827. [DOI] [PubMed] [Google Scholar]
- Thor P, Krolczyk G, Gil K, Zurowski D, Nowak L (2007). Melatonin and serotonin effects. J Physiol Pharmacol 58: 97–105. [PubMed] [Google Scholar]
- Trivedi P, Jena G (2013). Melatonin reduces ulcerative colitis‐associated local and systemic damage in mice: investigation on possible mechanisms. Dig Dis Sci 58: 3460–3474. [DOI] [PubMed] [Google Scholar]
- Zhao H, Bian X, Galligan JJ, Swain GM (2010). Electrochemical measurements of serotonin (5‐HT) release from the guinea pig mucosa using continuous amperometry with a boron‐doped diamond microelectrode. Diamond Relat Mater 19: 182–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielińska M, Jarmuż A, Sałaga M, Kordek R, Laudon M, Storr M et al (2016). Melatonin, but not melatonin receptor agonists Neu‐P11 and Neu‐P67, attenuates TNBS‐induced colitis in mice. Naunyn Schmiedebergs Arch Pharmacol 389: 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Experimental traces showing the varying electrochemical approaches utilised. (A) Amperometry approach curve profiling, where the current is monitoring at varying electrode‐tissue distances and utilised to obtain contribution of release and reuptake. (B) Protocol to monitor levels of 5‐HT to study contribution of chemical and mechanical stimulation.
Figure S2 Changes in intracellular to extracellular ratio. Data shown as mean ± 95% C.I.


