Abstract
Background and Purpose
Although the antinociceptive efficacies of imidazoline I2 receptor agonists have been established, the exact post‐receptor mechanisms remain unknown. This study tested the hypothesis that monoaminergic transmission is critical for I2 receptor agonist‐induced antinociception.
Experimental Approach
von Frey filaments were used to assess antinociceptive effects of two I2 receptor agonists, 2‐BFI and CR4056 on chronic constriction injury (CCI)‐induced neuropathic pain or complete Freund's adjuvant (CFA)‐induced inflammatory pain in rats. Rectal temperature was measured to assess hypothermic effects of 2‐BFI. A two‐lever drug discrimination paradigm in which rats were trained to discriminate 5.6 mg·kg−1 2‐BFI (i.p.) from its vehicle was used to examine the discriminative stimulus effects of 2‐BFI. In each experiment, pharmacological mechanisms were investigated by combining 2‐BFI or CR4056 with various pharmacological manipulations of the monoaminergic system including selective reuptake inhibition, monoamine depletion and monoamine receptor antagonism.
Key Results
In the CCI model, selective reuptake inhibitors of 5‐HT (fluoxetine) or noradrenaline (desipramine), but not dopamine (GBR12909), enhanced 2‐BFI‐induced antinociception. Selective depletion of 5‐HT or noradrenaline almost abolished 2‐BFI‐induced antinociception. 5‐HT1A, 5‐HT2A and α1‐adrenoceptor antagonists, but not other monoaminergic antagonists, attenuated 2‐BFI and CR4056‐induced antinociception in CCI and/or CFA models. However, none of these monoamine receptor antagonists significantly altered 2‐BFI‐induced hypothermia or discriminative stimulus effects.
Conclusions and Implications
Antinociception induced by I2 receptor agonists was mediated by serotonergic and noradrenergic mechanisms with 5‐HT1A, 5‐HT2A and α1‐adrenoceptor being particularly important. In contrast, the hypothermic and discriminative stimulus effects of I2 receptor agonists were mediated by distinct, independent mechanisms.
Abbreviations
- 2‐BFI
2‐(2‐benzofuranyl)‐2‐imidazoline
- CCI
chronic constriction injury
- CFA
complete Freund's adjuvant
- CL
confidence limit
- CR4056
2‐phenyl‐6‐(1H‐imidazol‐1yl) quinazoline
- MPE
maximal possible effect
- PWT
paw withdrawal threshold
Introduction
Despite efforts by researchers and funding agencies in recent years to address the issue of chronic pain, it remains one of the United States' largest healthcare problems, affecting almost one‐third of Americans (NIH, 2013). While opioids have been relied upon as first‐line pain therapies, they are ill‐suited to extended pain management because of unwanted effects such as analgesic tolerance, dependence, constipation and addiction. When viewed in light of the current opioid epidemic, the lack of mechanistically novel painkillers entering the market in the past 50 years (Kissin, 2010) highlights the urgent need for the development of novel, safer and more effective analgesics.
Recent preclinical investigations have established the http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=4 as a promising target for the development of such analgesics. Effective in several rodent chronic pain models when administered alone, I2 receptor agonists also enhance opioid antinociception in an additive to synergistic manner while preventing some opioid use‐related side effects such as tolerance and dependence (Thorn et al., 2015; Siemian et al., 2016; Thorn, Zhang, and Li, 2016b; Thorn et al., 2017). However, despite many investigations on I2 receptors since their discovery decades ago, they have not yet been cloned or had their structure elucidated. As such, their functional mechanisms are still poorly understood. The most widely accepted theory regarding I2 receptor function stems from the localization of I2 receptors to allosteric inhibitory binding sites on the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2489 (Jones et al., 2007) and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2490 (McDonald et al., 2010), which implies the involvement of monoaminergic systems. Indeed, acute systemic administration of I2 receptor agonists in rats increases http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=484, and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=940 in several areas of the CNS, including the striatum, frontal cortex, hippocampus, dorsal raphe and spinal cord (Nutt et al., 1995; Ugedo et al., 1999; Ferrari et al., 2011). Although I2 receptors appear to have dense populations in several brain areas important for monoaminergic function such as dorsal raphe and locus coeruleus (MacKinnon et al., 1995; Lione et al., 1998), no study has systematically examined monoaminergic involvement in any I2 receptor‐mediated behaviours. MacInnes and Handley (2003) proposed the potential involvement of 5‐HT and noradrenaline in the discriminative stimulus effects of one I2 receptor agonist, 2‐BFI, but more recent studies have failed to replicate these findings (Qiu et al., 2014).
This study has systematically examined the antinociceptive effects of the selective I2 receptor agonist 2‐BFI alone and in combination with various pharmacological manipulations of the monoamine system in adult male rats with chronic constriction injury (CCI)‐induced neuropathic pain. Similar tests were conducted which used either another selective I2 receptor agonist , CR4056, or another pain model, complete Freund's adjuvant (CFA)‐induced inflammatory pain. In a separate set of experiments, the hypothermic effects of 2‐BFI alone and in combination with several selective monoaminergic receptor antagonists were examined. In a final experiment, rats were trained to discriminate 5.6 mg·kg−1 2‐BFI from its vehicle. The discriminative stimulus effects of 2‐BFI alone and in combination with either selective monoaminergic reuptake inhibitors or selective monoaminergic receptor antagonists were examined.
Methods
Animals
All animal care and experimental protocols complied with the 2011 Guide for the Care and Use of Laboratory Animals (National Research Council, 2011) and the guidelines of the International Association for the Study of Pain (Zimmermann, 1983), and were approved by the Institutional Animal Care and Use Committee, University at Buffalo, the State University of New York (Buffalo, NY, USA). Care was taken to minimize animal suffering and any animals showing clear signs of discomfort or illness were killed. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015).
Male (n = 192 rats) Sprague–Dawley rats (Envigo, Indianapolis, IN, USA) approximately 12 weeks old and weighing approximately 250 g at experiment onset were housed individually in standard housing conditions on a 12/12 h light/dark cycle with behavioural experiments conducted during the light period. All rats had free access to water, except during test sessions. Rats used in nociception (n = 5–6 per group) and body temperature (n = 6–7 per group) tests had free access to standard rodent chow in their home cages and were randomly assigned to different study groups. Group size was determined by previous studies from our laboratory to ensure sufficient statistical power. Rats used in drug discrimination studies (n = 7) were provided with restricted access to food after their daily sessions, such that their bodyweights were maintained at 85% of their free‐feeding counterparts. Specific group numbers are given in the figure legends.
Experimental procedures
Validation of models
The CCI and CFA‐induced pain models are well‐described and widely used animal models of chronic neuropathic and inflammatory pain respectively. In rodents, each manipulation induces persistent hypersensitivity to a range of stimuli (Bennett and Xie, 1988; Nagakura et al., 2003; Li et al., 2014), making them appropriate models with which to study potential analgesics. Hypothermia was studied as a well‐characterized physiological endpoint of I2 receptor activation (Thorn et al., 2012). Drug discrimination is a well‐validated model first described in 1951 (Conger, 1951) and has been used to characterize the discriminative properties of many drugs since; I2 receptor agonists have been previously characterize to produce discriminative stimulus effects (Qiu et al., 2014; Qiu et al., 2015).
Induction of inflammatory pain and neuropathic pain
Inflammatory pain was induced by CFA, as previously described (Li et al., 2014). Briefly, rats were anesthetized in an induction chamber with 2% isoflurane mixed with 100% oxygen. Adequate anaesthesia was determined by loss of righting and toe‐pinch reflexes. The right hind paw was cleaned and disinfected with a gauze pad saturated with 70% isopropyl alcohol; 0.1 mL of CFA (Sigma‐Aldrich, St. Louis, MO, USA) containing approximately 0.05 mg of Mycobacterium butyricum dissolved in paraffin oil was injected s.c. into the plantar surface of the right hind paw. Rats were then returned to their home cages.
Neuropathic pain was induced by CCI, using the procedure described earlier by Bennett and Xie, (1988) and Li et al., (2014). Rats were anesthetized with a mixture of ketamine (60 mg·kg−1) and xylazine (15 mg·kg−1) i.p. prior to surgery. Sufficient anaesthesia was determined by loss of righting and toe‐pinch reflexes. Under aseptic technique, the common sciatic nerve was exposed at the level of the middle of the thigh by blunt dissection through the biceps femoris. Proximal to the sciatic nerve's trifurcation, approximately 7 mm of nerve was freed of adhering tissue, and four ligatures (4.0 chromic gut suture; Patterson Veterinary, Devens, MA, USA) were tied loosely around it with approximately 1 mm spacing. Care was taken such that the ligatures just barely constricted the nerve so that circulation through the superficial epineural vasculature was uninterrupted. The incisions were closed with surgical clips. Rats were given post‐operative saline and antibiotics and allowed to recover on heating pads until righting reflex was regained.
Mechanical nociception
A total of 147 rats were used for these studies. Mechanical hyperalgesia was measured by the von Frey filament test using equipment and procedures as described in detail previously (Siemian et al., 2016). Briefly, rats (n = 5–6 per group) were placed in transparent test chambers on top of a wire mesh platform through which filaments were applied perpendicularly to the medial plantar surface of the hind paw from below the mesh floor to determine the paw withdrawal threshold (PWT), defined as the lowest strength filament that elicited a behavioural response in at least two out of three applications. Behavioural testing began 1 day after CFA treatment or 5 days after CCI surgery as described previously (Li et al., 2014). By design, all groups contained six rats each; some rats failed to develop mechanical hypersensitivity following CCI or CFA treatment, leading to five rats in certain groups; specific group numbers are given in the figure legends. For all nociception tests, a cumulative dosing procedure was used to establish dose‐effect curves with a 20 min (2‐BFI) or 30 min (CR4056 and reuptake inhibitors) inter‐injection interval as described previously (Siemian et al., 2016b) with the cumulative dose increasing by 0.25 log unit per injection, except for the reuptake inhibitors for which the doses increased by 0.5 log unit, up to doses that could be safely studied. For the investigations of 2‐BFI or CR4056 with monoamine receptor antagonists or reuptake inhibitors, each group of rats was tested on three or four occasions. The first test was always with an I2 receptor agonist alone, and subsequent tests were with a study compound in combination with the I2 receptor agonist. Monoamine receptor antagonists and reuptake inhibitors were administered 20 and 30 min prior to the first injection of the I2 receptor agonist respectively. Each group of rats was assigned to one study compound, and the test order for study compounds was arranged in an ascending dose order. A final test of monoamine receptor antagonist or reuptake inhibitor alone was conducted following combination studies. All tests were separated by at least 3 days.
For the 5‐HT depletion experiment, one group of rats was used. One 2‐BFI dose‐effect curve was established prior to a 3 day treatment consisting of an injection of 200 mg·kg−1 i.p. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5240 on each of the first and second days and an injection of 10 mg·kg−1 i.p. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4613 on the third day, which depletes brain 5‐HT levels by approximately 93% (Prinssen et al., 2002; Li et al., 2009a, 2009b). On the subsequent day (day 4), a second 2‐BFI dose‐effect curve was established. For the noradrenaline depletion experiment, one group of rats was used. One 2‐BFI dose‐effect curve was established, and on the subsequent day, an injection of 50 mg·kg−1 N‐(2‐chloroethyl)‐N‐ethyl‐2‐bromobenzylamine hydrochloride (DSP‐4) was administered (Jaim‐Etcheverry and Zieher, 1980), which depletes brain noradrenaline by approximately 60% after 10 days (Kudo et al., 2011). On day 10 after the DSP‐4 injection, a second 2‐BFI dose‐effect curve was established. In all experiments, experimenters were blind to the treatments, and they received extensive training with the von Frey procedure to ensure accurate judgement of paw withdrawal responses and minimize experimenter bias.
Body temperature
A total of 38 rats were used in body temperature tests which were performed as previously described (Li et al., 2009a). Briefly, rats were habituated to a quiet procedure room for at least 30 min before each test. Body temperature was measured by gently inserting a lubricated probe approximately 5.0 cm into the rectum and recording the temperature from the digital thermometer (BAT7001H, Physitemp Instruments Inc., Clifton, NJ, USA). A cumulative dosing procedure was used with rats receiving vehicle prior to the first 30 min cycle followed by increasing doses of 2‐BFI every 30 min with the cumulative dose increasing by 0.25 log unit per injection. Body temperature was measured in the last minute of each 30 min cycle. Rats were handled and habituated to the procedure for 3 days before the testing began. Each group of rats (n = 6–7 per group, specific group numbers are given in the figure legend) was tested on three or four occasions. The first test was always with 2‐BFI alone, and subsequent tests were with a monoamine receptor antagonist in combination with 2‐BFI. Each group of rats was assigned to one antagonist, and the antagonists were always tested in an ascending dose order. All tests were separated by at least 3 days.
Drug discrimination
Drug discrimination studies were performed using previously described chambers (Coulbourn Instruments Inc., Allentown, PA, USA) and software (Graphic State 3.03, Coulbourn Instruments Inc., Whitehall, PA, USA) (Qiu et al., 2014; Qiu et al., 2015). A modification of previously described training protocols was used (Siemian et al., 2017). Seven rats were trained to discriminate 5.6 mg·kg−1 2‐BFI injected i.p. from saline in a multiple cycle, cumulative‐dosing procedure. Each cycle consisted of a 10 min timeout during which the chamber was dark and responses had no programmed consequence, followed by a 5 min response period, during which a house light and a cue light above each lever were illuminated and signalled availability of reinforcers. Ten consecutive responses (fixed ratio 10) on the correct lever resulted in food delivery (45 mg; BioServ Inc., Frenchtown, NJ, USA). The correct lever was predetermined by an injection (e.g. right, saline; left, 2‐BFI). Response periods ended after 5 min or after delivery of 10 food pellets, whichever occurred first. Training began with single‐cycle sessions, in which saline or 5.6 mg·kg−1 2‐BFI was administered i.p. 10 min before the start of the session. Sessions were conducted 7 days·week−1 according to a roughly double alternation schedule (e.g. saline, saline, drug and drug). Rats had to achieve at least 90% of the total active responses on the correct lever for five consecutive or six out of seven consecutive sessions to progress to multiple cycle training. For multiple cycle training, a saline or 5.6 mg·kg−1 2‐BFI injection was given 10 min before the start of a two‐ to five‐cycle session. Some training days consisted of two cycles in which either the saline lever or the 2‐BFI lever was active during both cycles. On other training days, one to three saline training cycles preceded the administration of 2‐BFI. These protocols were varied non‐systematically; rats needed to pass two consecutive sessions (one saline training session and one 2‐BFI training session) by responding at least 90% on the correct lever during each active period before each test. Test sessions lasted up to five cycles and were identical to training sessions except that 10 consecutive responses on either lever delivered a food pellet. During test sessions, vehicle, monoamine receptor antagonists or reuptake inhibitors were administered 10 min before the start of the first cycle followed by increasing cumulative doses of 2‐BFI, with the cumulative dose increasing by 0.25 log unit per injection, during the first minute of each subsequent cycle up to doses that occasioned at least 80% responding on the 2‐BFI‐appropriate lever or suppressed the rate of responding. In tests examining whether reuptake inhibitors substituted for the discriminative stimulus of 2‐BFI, the inter‐injection time was increased from 15 to 30 min.
Randomization and blinding
Laboratory rats were acquired from Envigo and randomly assigned to experiments and treatment conditions therein. The treatments for all experiments in this study were blinded to minimize experimenter bias with the exception of drug discrimination, for which the data were collected by a computer program.
Data analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). To control for subject‐to‐subject variation, the antinociceptive effects were normalized and quantified for each rat as % maximal possible effect (MPE) according to the following formula: % MPE = (post‐drug PWT − pre‐drug PWT)/(pre‐CFA PWT − pre‐drug PWT) × 100%. These percentages were averaged (±SEM) across rats and plotted as a function of dose. Log(ED50) [±95% confidence limits (CLs)] values were individually calculated from the % MPE values of each rat and averaged within the group to determine the group ED50 values of 2‐BFI or CR4056 in each nociception test. Effects were considered significant if 95% CL values from tests with vehicle or test compounds did not overlap. In certain cases, one‐way repeated‐measures ANOVA was used, and P < 0.05 was considered statistically significant. Specific group numbers are given in the figure legends. Raw group mean PWTs are shown in Supporting Information Tables S2–S5.
For body temperature studies, two‐way repeated measures ANOVA (treatment dose × 2‐BFI dose) was used, and P < 0.05 was considered statistically significant. Specific group numbers are given in the figure legends.
For drug discrimination studies, two sets of data were collected for each test: (a) the percentage of responses on the 2‐BFI‐associated lever, calculated by dividing the number of responses on the 2‐BFI‐associated lever by the number of total responses on either lever within a cycle and multiplying by 100; and (b) response rate, calculated by dividing the total number of responses made on both levers by the duration of the response period in seconds. When a rat responded at a rate less than 20% of the vehicle control rate (the rate recorded after saline was administered), the percentage of responses on the 2‐BFI‐associated lever for that rat at that dose was not included in further analyses. The response rate data were still included. 2‐BFI ED50 values (±95% CL) were calculated for 2‐BFI‐associated lever responding (dataset a). Effects were considered significant if 95% CL values from tests with vehicle or test compounds did not overlap. Paired t‐tests or one‐ or two‐way ANOVA were used to compare response rate data, and P < 0.05 was considered statistically significant.
Materials
2‐BFI and CR4056 were synthesized according to standard procedures (Ishihara and Togo, 2007) and were provided by Dr. Yanan Zhang. The pharmacological properties of these drugs have been detailed previously (Nutt et al., 1995; Ozaita et al., 1997; McDonald et al., 2010; Ferrari et al., 2011). http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=185, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=80 maleate, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=499 hydrochloride, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=102 methyl ester hydrochloride, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2399 hydrochloride, pCPA, fenfluramine hydrochloride and DSP‐4 hydrochloride were purchased from Sigma (Sigma‐Aldrich). http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=193 dihydrochloride, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=943 hydrochloride, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=943, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=357 hydrochloride, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=203 hydrochloride and GBR12909 hydrochloride were purchased from Cayman (Cayman Chemical, Ann Arbor, MI, USA). All drugs were dissolved in 0.9% saline except for SB242084, MDL100907 and CR4056 which were dissolved in 10%, 20% and 20% DMSO in saline respectively. Doses of drugs are expressed in terms of their salt form, and all drugs were administered i.p., except for SCH23390 which was administered s.c., in a volume of 1–2 mL·kg−1. The pharmacological target and property of each drug is given in Supporting Information Table S1.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a, 2017b, 2017c).
Results
Prior to CCI surgery, the average PWT was 24.3 ± 0.4 g, which decreased to 5.8 ± 0.2 g following CCI surgery. According to one‐way repeated measures ANOVA, fluoxetine (Figure 1A), desipramine (Figure 1B) and GBR12909 (Figure 1C) all produced a significant main effect on PWT [F(3, 20) = 6.25 for fluoxetine, F(3, 16) = 5.21 for desipramine and F(3, 16) = 6.48 for GBR12909]. Bonferroni's post tests revealed that PWTs at 32 mg·kg−1 fluoxetine, 10 mg·kg−1 desipramine and 3.2 and 10 mg·kg−1 GBR12909 were all significantly different as compared to their respective vehicle controls. However, the highest %MPE among these drugs did not exceed 12.1%. When administered prior to 2‐BFI, 3.2 and 10 mg·kg−1 fluoxetine produced 1.66‐ and 2.98‐fold leftward shifts of the 2‐BFI dose‐effect curve (Figure 1D). Likewise, 3.2 and 10 mg·kg−1 desipramine produced 1.56‐ and 3.05‐fold leftward shifts of the 2‐BFI dose–response curve (Figure 1E). In contrast, GBR12909 did not significantly affect the 2‐BFI dose‐effect curve (Figure 1F; see Table 1 for ED50 values). Prior to 5‐HT depletion, the ED50 (±95% CL) of 2‐BFI was 7.43 (5.80, 9.53) mg·kg−1. On the day following the 3 day treatment regimen of pCPA and fenfluramine, the ED50 of 2‐BFI was >17.8 mg·kg−1 (Figure 2A). The baseline nociceptive threshold of this group was not significantly different before (6.33 ± 1.09 g) and after depletion (6.66 ± 0.66 g). Likewise, before depletion of noradrenaline, the ED50 (±95% CL) of 2‐BFI was 6.63 (4.49, 9.80) mg·kg−1. On the 10th day following DSP‐4 treatment, the ED50 of 2‐BFI was >17.8 mg·kg−1 (Figure 2B). The baseline nociceptive threshold of this group was not significantly different before (5.66 ± 0.95 g) and after depletion (6.33 ± 0.95 g).
Figure 1.
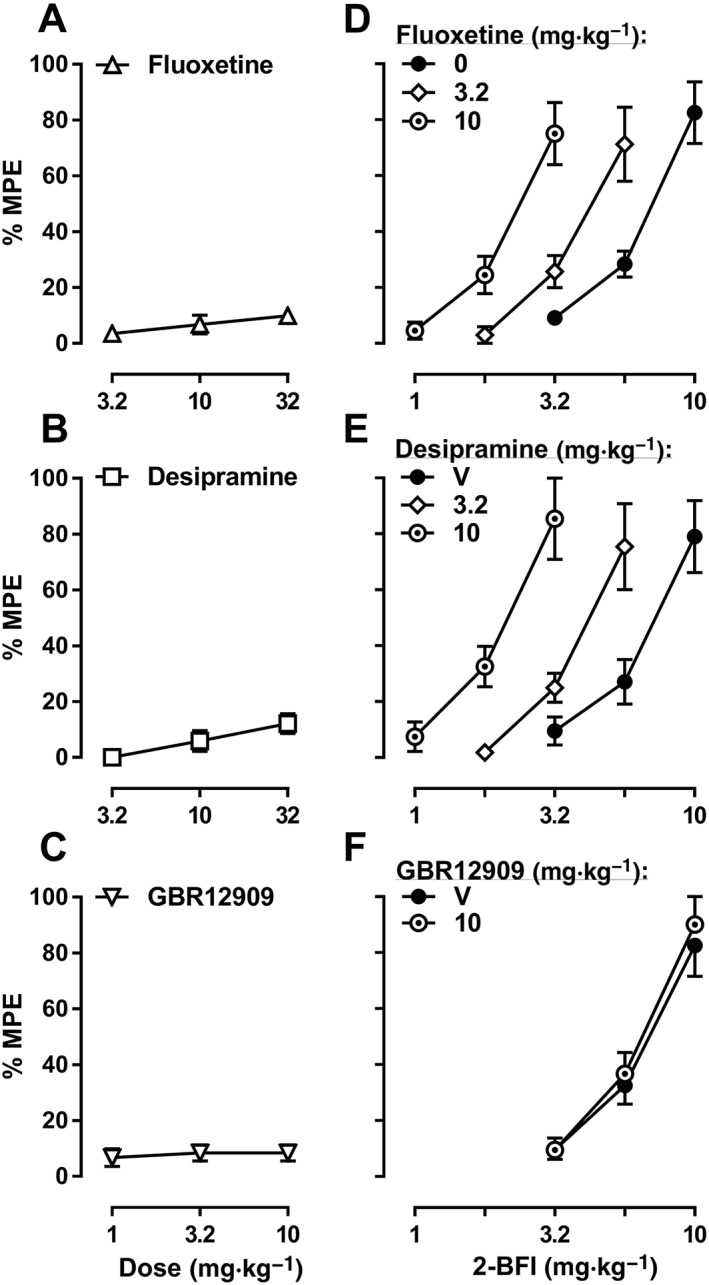
Percentage of the maximum possible effects (%MPE) of fluoxetine (A, n = 6), desipramine (B, n = 5) or GBR12909 (C, n = 5) or 2‐BFI alone and in combination with fluoxetine (D), desipramine (E) or GBR12909 (F), on CCI‐induced mechanical nociception. Data shown are means ± SEM.
Table 1.
ED50 values (with 95% CL) of 2‐BFI or CR4056 alone or in combination with pharmacological manipulations of monoaminergic mechanisms
| Dose | V | 0.01 | 0.032 | 0.1 | 0.32 | 1.0 | 3.2 | 10 |
|---|---|---|---|---|---|---|---|---|
| BFI/CCI | ||||||||
| Fluoxetine | 7.06 (5.92, 8.44) | – | – | – | – | – | 4.25 (3.43, 5.30)a | 2.37 (1.93, 2.91)a |
| Desipramine | 7.05 (5.36, 9.26) | – | – | – | – | – | 4.53 (3.29, 6.25) | 2.31 (1.48, 3.59)a |
| GBR12909 | 6.68 (5.37, 8.31) | – | – | – | – | – | – | 6.27 (5.57, 7.06) |
| WAY100635 | 7.64 (6.17, 9.48) | 8.08 (7.19, 9.07) | 15.62 (11.62, 21.00)b | 19.24 (13.38, 27.68)b | – | – | – | – |
| MDL100907 | 5.90 (4.40, 7.92) | 5.88 (4.58, 7.55) | 12.27 (9.11, 16.53)b | 19.18 (13.53, 27.20)b | – | – | – | – |
| SB242084 | 6.53 (4.91, 8.66) | – | – | – | 6.34 (5.09, 7.90) | 6.18 (4.64, 8.24) | – | – |
| WB4101 | 8.86 (7.27, 10.80) | – | – | – | 7.43 (5.46, 10.13) | 13.72 (11.17, 16.86)b | 22.21 (18.63, 26.49)b | – |
| Yohimbine | 6.84 (5.82, 8.05) | – | – | – | – | 6.65 (5.23, 8.45) | 6.63 (4.98, 8.84) | – |
| SCH23390 | 6.43 (4.46, 9.29) | – | 6.47 (4.51, 9.30) | 5.85 (4.16, 8.21) | – | – | – | – |
| Raclopride | 6.50 (4.77, 8.86) | – | – | – | 6.93 (4.77, 10.07) | 6.09 (4.28, 8.68) | – | – |
| CR4056/CCI | ||||||||
| WAY100635 | 10.33 (8.80, 12.12) | 11.47 (9.27, 14.19) | 22.16 (18.42, 26.66)b | 40.25 (32.47, 49.88)b | – | – | – | – |
| MDL100907 | 10.49 (7.72, 14.25) | 13.81 (10.45, 18.23) | 25.19 (17.70, 35.86)b | 45.45 (31.12, 66.37)b | – | – | – | – |
| SB242084 | 10.85 (7.69, 15.30) | – | – | – | 10.94 (8.34, 14.35) | 12.45(8.89, 17.42) | – | – |
| WB4101 | 11.71 (9.12, 15.04) | – | – | – | 12.67 (10.26, 15.66) | 24.44 (18.34, 32.57)b | 43.72 (35.36, 54.07)b | – |
| Yohimbine | 12.81 (9.91, 16.57) | – | – | – | – | 11.11 (9.11, 13.54) | 11.47 (8.09, 16.29) | – |
| SCH23390 | 11.10 (8.29, 14.96) | – | 10.88 (7.18, 16.49) | 11.00 (8.10, 14.96) | – | – | – | – |
| Raclopride | 12.65 (9.50, 16.84) | – | – | – | 12.95 (9.50, 17.66) | 9.27 (6.79, 12.67) | – | – |
| BFI/CFA | ||||||||
| WAY100635 | 8.46 (7.29, 9.81) | 8.46 (7.05, 10.15) | 15.34 (13.63, 17.27)b | 24.99 (21.15, 29.52)b | – | – | – | – |
| MDL100907 | 8.35 (7.11, 9.82) | 8.23 (6.75, 10.04) | 14.69 (11.51, 18.74)b | 19.99 (14.91, 26.81)b | – | – | – | – |
| SB242084 | 8.33 (7.13, 9.74) | – | – | – | 8.49 (7.31, 9.87) | 8.52 (6.84, 10.60) | – | – |
| WB4101 | 8.59 (6.80, 10.86) | – | – | – | 8.40 (6.93, 10.18) | 12.47 (10.85, 14.34) | 21.68 (17.98, 26.14)b | – |
| Yohimbine | 7.78 (6.76, 8.96) | – | – | – | – | 6.75 (5.14, 8.88) | 7.86 (6.88, 8.97) | – |
| SCH23390 | 7.79 (6.72, 9.02) | – | 6.38 (4.44, 9.16) | 8.29 (7.19, 9.55) | – | – | – | – |
| Raclopride | 7.68 (6.40, 9.20) | – | – | – | 8.64 (7.57, 9.86) | 7.37 (6.03, 9.00) | – | – |
Combination ED50 lower than control.
Combination ED50 higher than control.
The above indicates that CLs do not overlap with those of the control values (2‐BFI or CR4056 alone).
Figure 2.
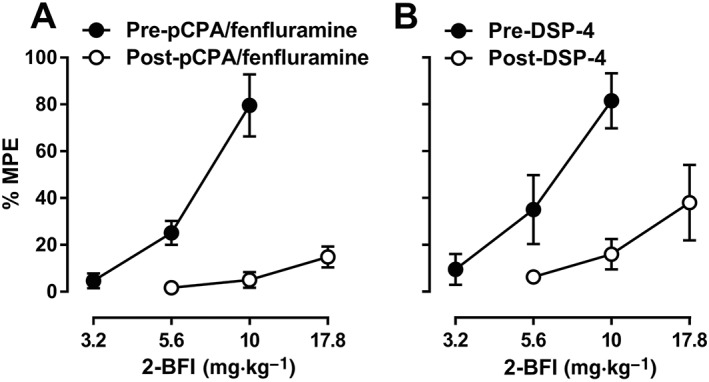
Percentage of the maximum possible effects (%MPE) of 2‐BFI on CCI‐induced mechanical nociception before and after treatment with pCPA and fenfluramine (A, n = 6) or DSP‐4 (B, n = 6). Data shown are means ± SEM.
When studied in combination with 2‐BFI in the CCI‐induced pain model, the 5‐HT1A receptor antagonist WAY100635, the 5‐HT2A receptor antagonist MDL100907 and the α1‐adrenoceptor antagonist WB4101 all produced dose‐dependent rightward shifts of the 2‐BFI dose‐effect curve: 0.01, 0.032 and 0.1 mg·kg−1 WAY100635 produced 1.06‐, 2.04‐ and 2.52‐fold rightward shifts respectively (Figure 3A); 0.01, 0.032 and 0.1 mg·kg−1 MDL100907 produced 1.00‐, 2.08‐ and 3.25‐fold rightward shifts respectively (Figure 3B); and 0.32, 1.0 and 3.2 mg·kg−1 WB4101 produced 0.84‐, 1.55‐ and 2.51‐fold rightward shifts respectively (Figure 3D). In contrast, the 5‐HT2C receptor antagonist SB242084, the α2‐adrenoceptor antagonist yohimbine, the dopamine D1 receptor antagonist SCH23390 and the dopamine D2 receptor antagonist raclopride did not significantly alter the 2‐BFI dose‐effect curve (Figure 3C, E–G; see Table 1 for ED50 values). Similar results were found with another I2 receptor agonist, CR4056, in the CCI‐induced pain model: 0.01, 0.032 and 0.1 mg·kg−1 WAY100635 produced 1.11‐, 2.15‐ and 3.90‐fold rightward shifts of the CR4056 dose‐effect curve respectively (Figure 4A); 0.01, 0.032 and 0.1 mg·kg−1 MDL100907 produced 1.32‐, 2.40‐ and 4.33‐fold rightward shifts respectively (Figure 4B); and 0.32, 1.0 and 3.2 mg·kg−1 WB4101 produced 1.08‐, 2.09‐ and 3.73‐fold rightward shifts respectively (Figure 4D). In contrast, none of the other antagonists tested significantly altered the CR4056 dose‐effect curve (Figure 4C, E–G; see Table 1 for ED50 values). Similar results were also found when 2‐BFI was tested in rats with CFA‐induced inflammatory pain: 0.01, 0.032 and 0.1 mg·kg−1 WAY100635 produced 1.00‐, 1.81‐ and 2.95‐fold rightward shifts of the 2‐BFI dose‐effect curve respectively (Figure 5A); 0.01, 0.032 and 0.1 mg·kg−1 MDL100907 produced 0.99‐, 1.76‐ and 2.39‐fold rightward shifts respectively (Figure 5B); and 0.32, 1.0 and 3.2 mg·kg−1 WB4101 produced 0.98‐, 1.45‐ and 2.52‐fold rightward shifts respectively (Figure 5D). In contrast, none of the other antagonists tested significantly altered the 2‐BFI dose‐effect curve (Figure 5C, E–G; see Table 1 for ED50 values). When tested alone, no antagonist significantly altered PWT over a 90 min period (data not shown).
Figure 3.
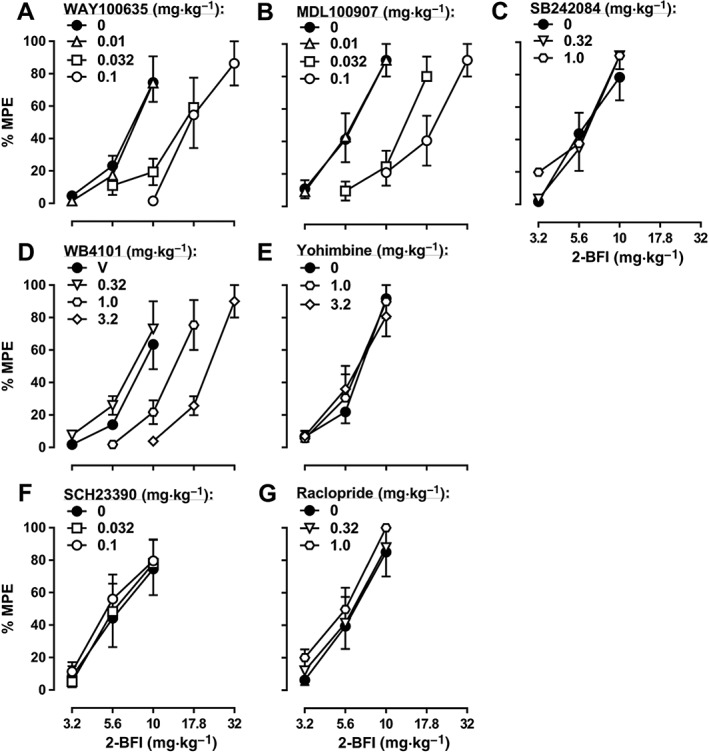
Percentage of the maximum possible effects (%MPE) of 2‐BFI alone and in combination with WAY100635 (A, n = 6), MDL100907 (B, n = 5), SB242084 (C, n = 6), WB4101 (D, n = 5), yohimbine (E, n = 6), SCH23390 (F, n = 6) or raclopride (G, n = 6) on CCI‐induced mechanical nociception. Data shown are means ± SEM.
Figure 4.
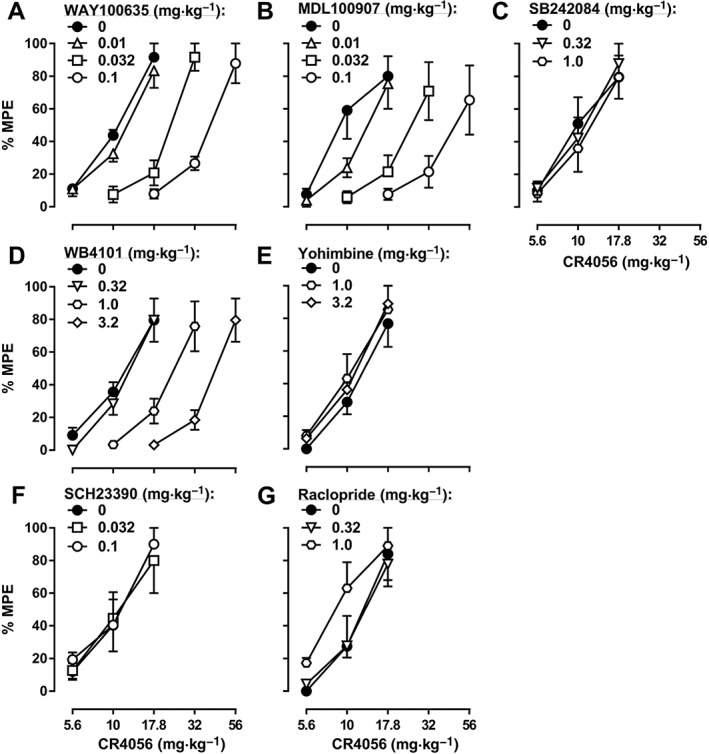
Percentage of the maximum possible effects (%MPE) of CR4056 alone and in combination with WAY100635 (A, n = 6), MDL100907 (B, n = 5), SB242084 (C, n = 6), WB4101 (D, n = 6), yohimbine (E, n = 5), SCH23390 (F, n = 5) or raclopride (G, n = 5) on CCI‐induced mechanical nociception. Data shown are means ± SEM.
Figure 5.
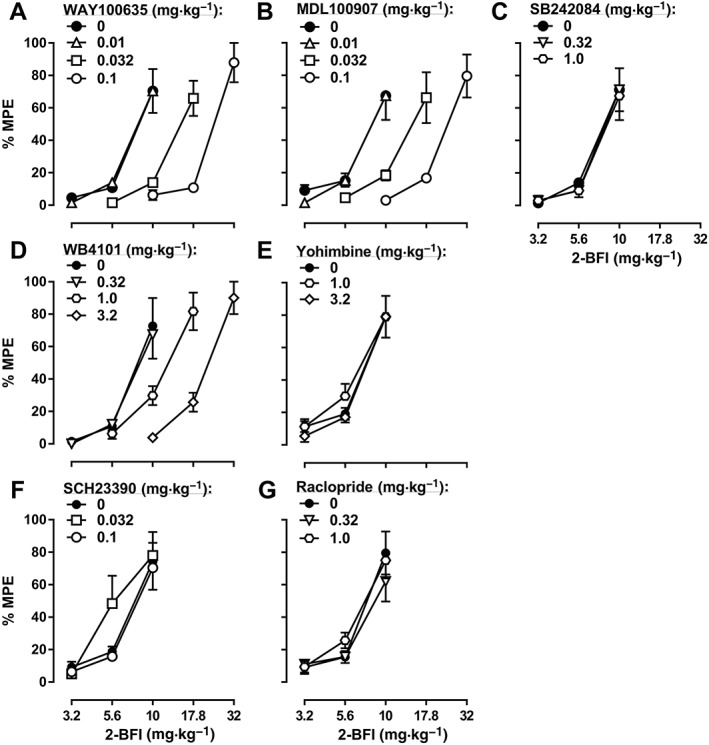
Percentage of the maximum possible effects (%MPE) of 2‐BFI alone and in combination with WAY100635 (A, n = 6), MDL100907 (B, n = 6), SB242084 (C, n = 6), WB4101 (D, n = 6), yohimbine (E, n = 5), SCH23390 (F, n = 6) or raclopride (G, n = 6) on CFA‐induced mechanical nociception. Data shown are means ± SEM.
In the body temperature experiment, when WAY100635 and 2‐BFI were studied in combination, both 2‐BFI [F(4, 20) = 52.59] and WAY100635 [F(3, 15) = 4.82] produced significant main effects according to two‐way repeated‐measures ANOVA, but no significant 2‐BFI × WAY100635 interaction was found [F(12, 60) = 0.51] (Figure 6A). When MDL100907 and 2‐BFI were studied in combination, both 2‐BFI [F(4, 20) = 38.31] and MDL100907 [F(3, 15) = 7.20] produced significant main effects according to two‐way repeated‐measures ANOVA, but no significant 2‐BFI × MDL100907 interaction was found [F(12, 60) = 0.68] (Figure 6B). When SB242084 and 2‐BFI were studied in combination, both 2‐BFI [F(4, 20) = 88.70] and SB242084 [F(2, 10) = 7.28] produced significant main effects according to two‐way repeated‐measures ANOVA, but no significant 2‐BFI × SB242084 interaction was found [F(8, 40) = 1.17] (Figure 6C). When WB4101 and 2‐BFI were studied in combination, both 2‐BFI [F(4, 24) = 99.81] and WB4101 [F(3, 18) = 3.99] produced significant main effects according to two‐way repeated‐measures ANOVA, but no significant 2‐BFI × WB4101 interaction was found [F(12, 72) = 0.82] (Figure 6D). When SCH23390 and 2‐BFI were studied in combination, 2‐BFI [F(4, 24) = 64.41] produced a significant main effect according to two‐way repeated‐measures ANOVA, but no significant main effect of SCH23390 [F(2, 12) = 1.13] or 2‐BFI × SCH23390 interaction was found [F(8, 48) = 0.77] (Figure 6E). When raclopride and 2‐BFI were studied in combination, 2‐BFI [F(4, 20) = 49.18] produced a significant main effect according to two‐way repeated‐measures ANOVA, but no significant main effect of raclopride [F(2, 10) = 2.50] or 2‐BFI × raclopride interaction was found [F(8, 40) = 1.48] (Figure 6F). Combinations of yohimbine and 2‐BFI were not tested in this study since this had been investigated in a previous study (Thorn et al., 2012).
Figure 6.
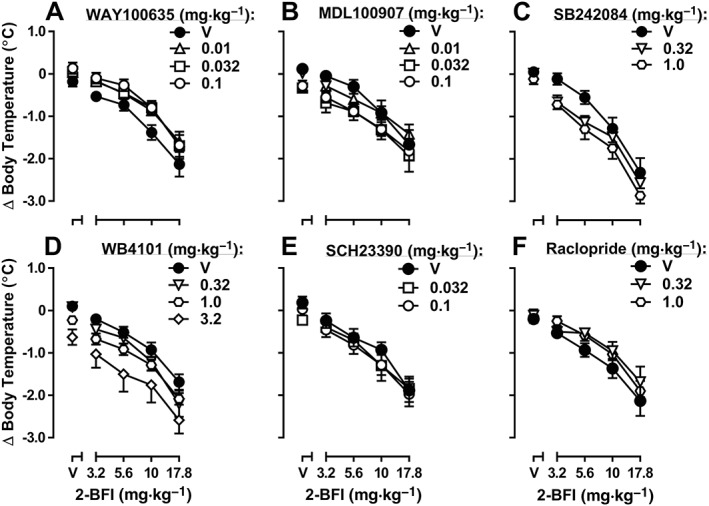
Hypothermic effects of 2‐BFI alone and in combination with WAY100635 (A, n = 6), MDL100907 (B, n = 6), SB242084 (C, n = 6), WB4101 (D, n = 7), SCH23390 (E, n = 7) or raclopride (F, n = 6). Data shown are means ± SEM of body temperature change from baseline.
In the drug discrimination study, rats met test criteria after an average of 41 training sessions (range = 39–44). Saline produced 1.5% 2‐BFI‐associated lever responding, whereas 2‐BFI dose‐dependently increased responding on the 2‐BFI‐associated lever up to a maximum of 98.4% at a dose of 5.6 mg·kg−1. The ED50 value (±95% CL) for 2‐BFI substitution was 1.80 (1.35, 2.38) mg·kg−1 (Figure 7A). When the highest dose of each monoamine receptor antagonist used in the nociception studies (or that did significantly affect operant responding) was administered as pretreatments, the ED50 value for 2‐BFI substitution did not significantly change. The ED50 values (±95% CL) following antagonist administration are as follows: 0.1 mg·kg−1 WAY100635, 2.14 (1.29, 3.54) mg·kg−1 2‐BFI; 0.1 mg·kg−1 MDL100907, 1.71 (1.01, 2.87) mg·kg−1 2‐BFI; 1.0 mg·kg−1 SB242084, 3.67 (1.57, 8.65) mg·kg−1 2‐BFI; 3.2 mg·kg−1 WB4101, 2.34 (1.41, 3.90) mg·kg−1 2‐BFI; 3.2 mg·kg−1 yohimbine, 1.99 (1.24, 3.21) mg·kg−1 2‐BFI; 0.01 mg·kg−1 SCH23390, 2.96 (1.67, 5.27) mg·kg−1 2‐BFI; and 0.1 mg·kg−1 raclopride, 2.70 (1.67, 4.35) mg·kg−1 2‐BFI (Figure 7A–G). The response rates for all treatments are shown in Figure 7H–N. Two‐way ANOVAs revealed that the following treatments produced significant main effects on response rate: 0.1 mg·kg−1 WAY100635 [F(1, 58) = 4.50], 1.0 mg·kg−1 SB242084 [F(1, 54) = 4.30], 3.2 mg·kg−1 WB4101 [F(1, 54) = 8.40], 3.2 mg·kg−1 yohimbine [F(1, 58) = 4.66] and 0.01 mg·kg−1 SCH23390 [F(1, 54) = 13.81]. However, in none of these tests was there a significant treatment × 2‐BFI interaction. Paired t‐tests showed that 0.032 mg·kg−1 SCH23390 [t(6) = 10.90] and 0.32 mg·kg−1 raclopride [t(6) = 2.38] significantly reduced response rate when administered alone.
Figure 7.
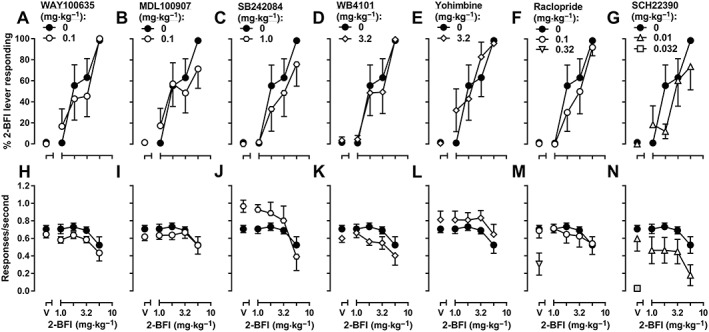
Discriminative stimulus effects of 2‐BFI alone or in combination with WAY100635 (A), MDL100907 (B), SB242084 (C), WB4101 (D), yohimbine (E), raclopride (F) or SCH23390 (G) in rats discriminating 5.6 mg·kg−1 2‐BFI from its vehicle (n = 7). Upper graphs: substitution profiles of 2‐BFI or drug combinations. Data shown are means ± SEM of percentage of 2‐BFI‐appropriate lever responding. Lower graphs: response rate. Data shown are means ± SEM of the response rate (responses·s−1). Shaded symbols are significantly different (P < 0.05) from 2‐BFI alone.
Following these tests, the 2‐BFI substitution dose‐effect curve was reestablished. Saline produced 0% 2‐BFI‐associated lever responding, whereas 2‐BFI dose‐dependently increased responding on the 2‐BFI‐associated lever up to a maximum of 98.7% at a dose of 5.6 mg·kg−1; the ED50 value (±95% CL) for 2‐BFI substitution was 3.17 (2.45, 4.11) mg·kg−1 (Figure 8B). None of the monoamine reuptake inhibitors, when administered alone, induced significant 2‐BFI‐associated lever responding (Figure 8A); the highest substitution induced by any of these drugs was 33.3% 2‐BFI‐associated lever responding, produced by 10 mg·kg−1 GBR12909. One‐way ANOVAs revealed that fluoxetine [F(3, 18) = 8.79], desipramine [F(3, 20) = 13.04], imipramine [F(3, 19) = 6.33] and GBR12909 [F(3, 24) = 7.64] all produced significant main effects on response rate (Figure 8F). When these reuptake inhibitors were administered as pretreatments, the ED50 values for 2‐BFI substitution did not significantly change. The ED50 values (±95% CL) following reuptake inhibitor administration are as follows: 1.0 mg·kg−1 fluoxetine, 2.51 (1.49, 4.24) mg·kg−1 2‐BFI; 3.2 mg·kg−1 fluoxetine, 2.37 (1.36, 4.14) mg·kg−1 2‐BFI; 3.2 mg·kg−1 desipramine, 3.34 (1.95, 5.70) mg·kg−1 2‐BFI; 3.2 imipramine, 2.69 (1.75, 4.13) mg·kg−1 2‐BFI; 3.2 GBR12909, 3.19 (2.23, 4.56) mg·kg−1 2‐BFI (Figure 8B–E). Two‐way ANOVA revealed that the following treatments produced significant main effects on response rate: fluoxetine [F(2, 88) = 8.84] and 3.2 mg·kg−1 desipramine [F(1, 59) = 5.57] (Figure 8G–H). Paired t‐tests showed that 10 mg·kg−1 fluoxetine [t(6) = 6.91], 10 mg·kg−1 desipramine [t(6) = 17.66], 10 mg·kg−1 imipramine [t(6) = 17.66] and 10 mg·kg−1 GBR12909 [t(6) = 7.04] significantly reduced response rate when administered alone (Figure 8G–J).
Figure 8.
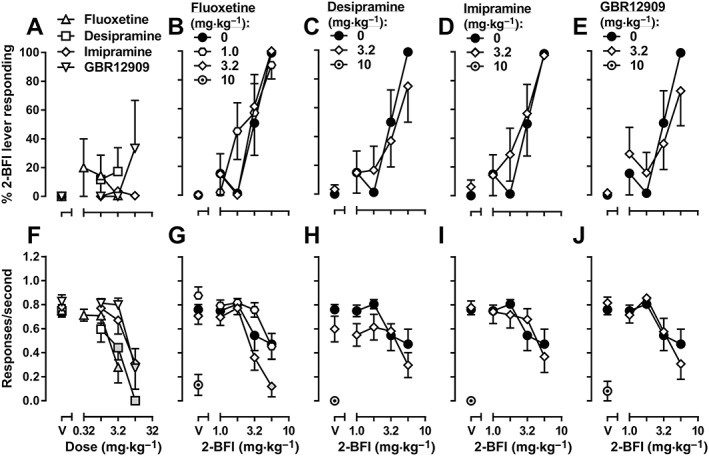
Discriminative stimulus effects of fluoxetine, desipramine, imipramine or GBR12909 alone (A) or 2‐BFI alone or in combination with fluoxetine (B), desipramine (C), imipramine (D) or GBR12909 (E) in rats discriminating 5.6 mg·kg−1 2‐BFI from its vehicle (n = 7). Upper graphs: substitution profiles of drugs or drug combinations. Data shown are means ± SEM of percentage of 2‐BFI‐appropriate lever responding. Lower graphs: response rate. Data shown are means ± SEM response rate (responses·s−1). Shaded symbols are significantly different (P < 0.05) from vehicle (A) or 2‐BFI alone (B–E).
Discussion
The primary findings of this study were that selective 5‐HT and noradrenaline reuptake inhibitors each induced significant leftward shifts of the antinociceptive dose‐effect curve of the I2 receptor agonist 2‐BFI, whereas specific depletions of either 5‐HT or noradrenaline abolished the antinociceptive effect of 2‐BFI. Specific antagonists of the 5‐HT1A, 5‐HT2A and α1‐adrenoceptors each induced significant, dose‐dependent rightward shifts of the antinociceptive dose‐effect curves of 2‐BFI and another I2 receptor agonist, CR4056. In contrast, none of the specific antagonists used in this study produced a significant interaction with 2‐BFI in an assay measuring 2‐BFI‐induced hypothermia. Likewise, none of the specific antagonists or monoamine reuptake inhibitors significantly altered the 2‐BFI substitution dose‐effect curve in rats trained to discriminate 5.6 mg·kg−1 2‐BFI from saline. These results suggest that the antinociceptive effects produced by I2 receptor agonists involve serotonergic and noradrenergic components but that the hypothermic and discriminative stimulus effects of these drugs are likely to involve different mediators. This finding will increase the understanding of I2 receptor pharmacology and may help guide the development of novel I2 receptor ligands towards specifically producing therapeutically important (e.g. analgesic) effects without producing other, unwanted effects.
CCI‐ and CFA‐induced hyperalgesia are well‐described and widely used animal models of chronic neuropathic and inflammatory pain respectively. CCI surgery or CFA hind paw injection each induces persistent hypersensitivity to a range of stimuli (Bennett and Xie, 1988; Nagakura et al., 2003; Li et al., 2014), making them appropriate models with which to study potential analgesics. Because the I2 receptors exist as inhibitory sites on MAO A and B (Jones et al., 2007; McDonald et al., 2010) and because administration of I2 receptor agonists increases spinal and supraspinal CNS levels of 5‐HT, noradrenaline and dopamine (Nutt et al., 1995; Ugedo et al., 1999; Ferrari et al., 2011), we examined whether manipulations of these monoamine systems altered the anti‐nociception induced by I2 receptor agonists, in rats with CCI‐induced neuropathic pain. We initially studied fluoxetine, desipramine and GBR12909, selective reuptake inhibitors of 5‐HT, noradrenaline and dopamine respectively. While none of these three drugs produced antinociception when administered alone, pretreatments with either fluoxetine or desipramine produced dose‐dependent leftward shifts of the 2‐BFI dose‐effect curve. To verify the importance of 5‐HT and noradrenaline in the antinociceptive effects of 2‐BFI, we used well‐established methods to deplete 5‐HT or noradrenaline in separate groups of rats (Jaim‐Etcheverry and Zieher, 1980; Prinssen et al., 2002). As compared to dose‐effect curves established beforehand, the dose‐effect curves of 2‐BFI following either 5‐HT or noradrenaline depletion were nearly flat, as though the anti‐nociceptive effects of 2‐BFI had been almost fully abolished. To understand whether particular 5‐HT receptors or adrenoceptors were involved in the anti‐nociceptive effects of 2‐BFI, we investigated a series of specific monoamine receptor antagonists in combination with 2‐BFI. The 5‐HT1A receptor antagonist WAY100635, the 5‐HT2A receptor antagonist MDL100907 and the α1‐adrenoceptor antagonist WB4101, each dose‐dependently shifted the dose‐effect curve of 2‐BFI rightward, whereas antagonists of other 5‐HT, noradrenaline or dopamine receptors had no significant effect, demonstrating pharmacological specificity of these serotonergic and noradrenergic components. This profile of results was reproduced with CR4056 in the CCI model and with 2‐BFI in the CFA‐induced inflammatory pain model. Importantly, both 2‐BFI and CR4056 are remarkably specific for I2 receptors and do not display appreciable affinity for monoaminergic receptors or transporters (Nutt et al., 1995; Ferrari et al., 2011).
The I2 receptor agonists produce several behavioural and physiological effects: antinociception (Li et al., 2014; Li, 2017), hypothermia (Thorn et al., 2012) and discriminative stimulus effects (Jordan et al., 1996; MacInnes and Handley, 2002; MacInnes and Handley, 2003; Qiu et al., 2014; Qiu et al., 2015). In order to test the generality of the monoaminergic component observed above, we next tested the role of monoaminergic transmission in the hypothermic effects of 2‐BFI. Consistent with the literature, 2‐BFI consistently induced hypothermia over a dose range of 3.2–17.8 mg·kg−1. Interestingly, none of the specific monoamine receptor antagonists tested above produced a significant interaction with 2‐BFI on hypothermia. This study did not investigate combinations of yohimbine and 2‐BFI as data showing that yohimbine did not alter 2‐BFI‐induced hypothermia had been published previously (Thorn et al., 2012). These data suggest that the hypothermic effects of 2‐BFI are not likely to involve serotonergic, noradrenergic or dopaminergic transmission and are instead mediated via mechanisms involving other mediators.
We finally tested the role of monoaminergic mechanisms in mediating the discriminative stimulus effects of 2‐BFI. Several I2 receptor agonists have been previously studied as discriminative stimuli including 2‐BFI (Jordan et al., 1996; Qiu et al., 2014; Qiu et al., 2015; Siemian et al., 2017). Interestingly, MacInnes and Handley (2002; 2003) reported that serotonergic and noradrenergic components were involved in the discriminative stimulus effects of 2‐BFI. For example, monoamine releasers (d‐http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2147, fenfluramine), MAO inhibitors (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7428, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7262), as well as 5‐HT (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2398, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7547) and noradrenaline (desipramine, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4808) reuptake inhibitors all at least partly substituted for 2‐BFI. Further, α1‐adrenoceptor antagonism disrupted the discriminative cue produced by 2‐BFI. In the present study, all rats acquired 5.6 mg·kg−1 2‐BFI discrimination at a similar rate as in previous studies where, for example, rats had acquired 7 mg·kg−1 2‐BFI discrimination after 44–64 training sessions (Jordan et al., 1996). However, in contrast to the monoaminergic components suggested by previous studies, none of the monoamine receptor antagonists tested significantly altered the 2‐BFI substitution dose‐effect curve when administered at the highest dose tested in the nociception studies or at the highest possible dose which did not suppress response rates to the point of obscuring substitution data interpretation (SCH23390 and raclopride). Additionally, among four monoamine reuptake inhibitors tested – fluoxetine (5‐HT), desipramine (noradrenaline), imipramine (5‐HT/ noradrenaline) and GBR12909 (dopamine) – no drug significantly substituted for 2‐BFI or significantly altered the dose‐effect curve for 2‐BFI substitution when given as a pretreatment. Thus, these results from the current study oppose previous findings that monoaminergic components are important for the discriminative stimulus effects of 2‐BFI and suggest that these effects are instead likely to be due to a distinct, independent mechanism. Support for this also comes from a recent finding that the 5‐HT2A receptor agonist http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?tab=summary&ligandId=164 did not substitute for 2‐BFI (Siemian et al., 2017). Moreover, that the same treatments which significantly altered 2‐BFI‐indued antinociception did not have a significant effect on the discriminative stimulus effects of 2‐BFI suggests that these behavioural endpoints are mediated via distinct post‐ I2 receptor signalling pathways or I2 receptor populations. Similar divergence was found in a recent study, which showed that I2 receptor‐mediated antinociception and discriminative stimulus effects were differentially sensitive to inhibitors of calcium influx or signalling mechanisms (Siemian et al., 2017), which also lends credence to the present findings, as calcium influx is necessary for vesicular monoamine release (Matthews, 1996).
The populations of I2 receptors are heterogenous, not only existing as binding sites on MAO but also as several other proteins of 30, 45 and 66 kDa sizes (Keller and Garcia‐Sevilla, 2015). Thus far, only the 45 kDa protein has been identified as brain creatine kinase (Kimura et al., 2009). While the contributions of these different proteins to the behavioural effects of I2 receptor agonists are unknown, this heterogeneity supports the concept of divergence between different I2 receptor‐mediated behavioural effects. Still, that this phenomenon has only recently begun to be observed makes the results of the current study important. For the population of I2 receptors that underlie antinociception, the data from the current study support previous theories that I2 receptors are alternative inhibitory binding sites on MAO. Here, we provide a hypothesis to explain the pharmacological role of these I2 receptors based on existing evidence as follows. Upon binding to I2 receptors, the agonists inhibit MAO activity thereby slowing the metabolism of monoamines and increasing their synaptic concentrations. This higher concentration of certain monoamines (e.g. 5‐HT and noradrenaline noradrenaline) causes greater activation of certain corresponding receptors (i.e. 5‐HT1A, 5‐HT2A and α1‐adrenoceptors) which then produces anti‐nociception, presumably through their downstream mechanisms (Figure 9). The involvement of the three receptors implicated in antinociception in this study has previously been demonstrated (Tasker et al., 1992; Bardin, 2011; Valhondo et al., 2013; Wattiez et al., 2013; Sun et al., 2014). Interestingly, the anti‐nociceptive effects of the reversible MAOA inhibitor moclobemide appear to be mediated by α2‐adrenoceptors and not 5‐HT receptors or α1‐adrenoceptors (Schreiber et al., 1998), whereas those of the tricyclic 5‐HT/noradrenaline reuptake inhibitor imipramine seem to be mediated by 5‐HT2A and α1‐adrenoceptors but not 5‐HT1A receptors (Otsuka et al., 2001). That the mechanistic profile of I2 receptor agonists overlaps more with reuptake inhibitors than MAO inhibitors is surprising, given the presumptive mechanism of action (i.e. MAO inhibition) of I2 receptor agonists. However, whether MAO inhibition fully accounts in full for these monoaminergic mechanisms is unknown. Enigmatically, although I2 receptor ligands appear to saturate their receptors at low nanomolar concentrations, they only inhibit MAO with IC50s in the high nanomolar or low micromolar range in vitro (Ozaita et al., 1997). This may result from the competitive inhibition of MAO A, but mixed inhibition of MAO B, wherein a low‐affinity I2 site exists on the enzyme in its active conformation, but a high‐affinity site exists during the enzyme in its inactive conformation which occurs during monoamine oxidation (Jones et al., 2007; McDonald et al., 2010). Thus, these inhibition values may be inflated, relative to those actually occurring in vivo. No pharmacokinetic/pharmacodynamic studies have been performed on I2 receptor ligands, so the CNS dose range achieved by systemic administration is currently unknown. In any case, the dose ranges of 2‐BFI which produce behavioural effects such as antinociception (3.2–17.8 mg·kg−1) are similar to those which increase monoamines (5–20 mg·kg−1 2‐BFI), supporting the idea that these effects are related (Nutt et al., 1995; Ferrari et al., 2011). Additionally, while the antinociceptive effects of I2 receptor agonists are known to be centrally mediated, whether monoaminergic effects are more important to antinociception at the spinal or supraspinal levels needs to be further investigated, as there are reports of roles at both levels of the CNS (Diaz et al., 1997; Ugedo et al., 1999; Ruiz‐Durantez et al., 2003; Thorn, Qiu, et al., 2016a). Future research, which may elucidate the mechanisms underlying the hypothermic or discriminative stimulus effects as well as the contributions from different proteins or signalling pathways to such functions, should allow the refinement of new I2 receptor ligands toward the specific production of antinociception while minimizing unwanted side effects.
Figure 9.
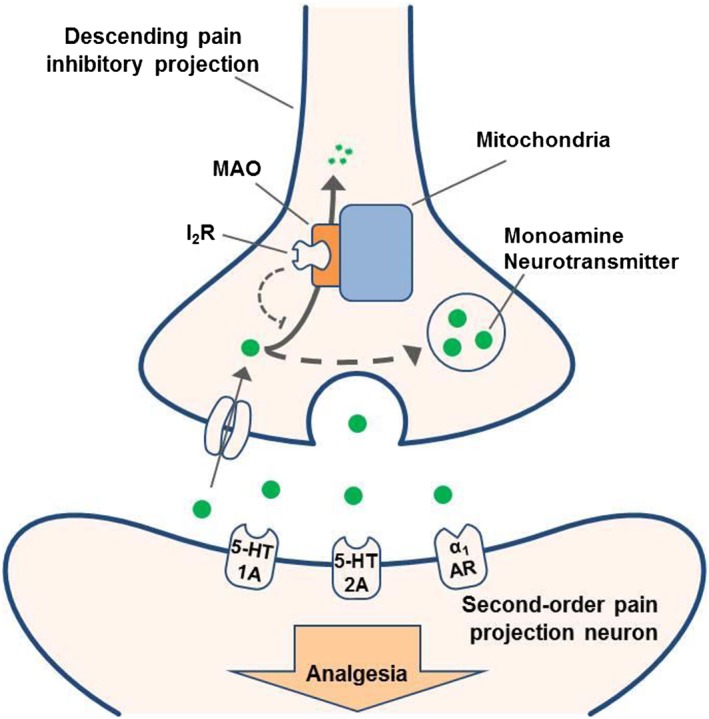
Diagram describing the probable mechanism of action of I2 receptor agonists. Under normal conditions, monoamines such as 5‐HT and noradrenaline undergo reuptake and are metabolized by MAO enzymes (solid arrow). Administration of I2 receptor agonists, and I2 receptor activation, inhibits MAO activity and allows monoamine neurotransmitters to be repackaged, increasing their synaptic concentration (dashed arrows). The corresponding increased activation of certain downstream monoaminergic receptors (i.e. 5‐HT1A, 5‐HT2A and α1‐adrenoceptors) leads to the production of analgesia.
In summary, this is the first study to demonstrate the direct functional involvement of 5‐HT and noradrenaline as well as 5‐HT1A, 5‐HT2A and α1‐adrenoceptors in the antinociceptive effects of I2 receptor agonists. In contrast, the hypothermic and discriminative stimulus effects of 2‐BFI appear to be mediated by distinct, unrelated mechanisms. These results support previous findings regarding dissociation between I2 receptor‐mediated behavioural effects (Siemian et al., 2017) and provide further in vivo evidence that I2 receptor populations and/or post‐receptor signalling mechanisms are heterogeneous. Some of these mechanisms, such as those that underlie antinociceptive effects, involve 5‐HT and noradrenaline and are likely to be related to the presence of I2 receptors on MAO enzymes, whereas other mechanisms, such as those that underlie hypothermic and discriminative stimulus effects, are more likely to be unrelated to MAO.
Author contributions
The main idea of this study was from J.S. and J.‐X.L. J.S. and K.W. conducted the experiments. J.S. and J.‐X.L. designed the study, conducted the data analysis and wrote the first draft of the manuscript. Y.Z. provided 2‐BFI and CR4056. All authors contributed to and approved the final version of the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This http://onlinelibrary.wiley.com/doi/10.1111/bph.13405/abstract acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Table S1 Pharmacological properties of drugs used in this study.
Table S2 Group mean PWTs (in g) for the effects of 2‐BFI alone and in combination with monoaminergic reuptake inhibitors or before and after monoamine depletion on CCI‐induced mechanical nociception.
Table S3 Group mean PWTs (in g) for the effects of 2‐BFI alone and in combination with monoaminergic receptor antagonists on CCI‐induced mechanical nociception.
Table S4 Group mean PWTs (in g) for the effects of CR4056 alone and in combination with monoaminergic receptor antagonists on CCI‐induced mechanical nociception.
Table S5 Group mean PWTs (in g) for the effects of 2‐BFI alone and in combination with monoaminergic receptor antagonists on CFA‐induced mechanical nociception.
Acknowledgements
This work was supported by the National Institute on Drug Abuse of the National Institutes of Health (Award no. R01DA034806). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Siemian, J. N. , Wang, K. , Zhang, Y. , and Li, J.‐X. (2018) Mechanisms of imidazoline I2 receptor agonist‐induced antinociception in rats: involvement of monoaminergic neurotransmission. British Journal of Pharmacology, 175: 1519–1534. doi: 10.1111/bph.14161.
References
- National Research Council (2011). Guide for the Care and Use of Laboratory Animals. edn, 10.17226/12910. National Academy of Sciences: Washington DC. [Google Scholar]
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017a). The Concise Guide to Pharmacology 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: Transporters. Br J Pharmacol 174: S360–S446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin L (2011). The complex role of serotonin and 5‐HT receptors in chronic pain. Behav Pharmacol 22: 390–404. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK (1988). A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33: 87–107. [DOI] [PubMed] [Google Scholar]
- Conger JJ (1951). The effects of alcohol on conflict behavior in the albino rat. Q J Stud Alcohol 12: 1–29. [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz A, Mayet S, Dickenson AH (1997). BU‐224 produces spinal antinociception as an agonist at imidazoline I2 receptors. Eur J Pharmacol 333: 9–15. [DOI] [PubMed] [Google Scholar]
- Ferrari F, Fiorentino S, Mennuni L, Garofalo P, Letari O, Mandelli S et al (2011). Analgesic efficacy of CR4056, a novel imidazoline‐2 receptor ligand, in rat models of inflammatory and neuropathic pain. J Pain Res 4: 111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara M, Togo H (2007). Direct oxidative conversion of aldehydes and alcohols to 2‐imidazolines and 2‐oxazolines using molecular iodine. Tetrahedron 63: 1474–1480. [Google Scholar]
- Jaim‐Etcheverry G, Zieher LM (1980). DSP‐4: a novel compound with neurotoxic effects on noradrenergic neurons of adult and developing rats. Brain Res 188: 513–523. [DOI] [PubMed] [Google Scholar]
- Jones TZ, Giurato L, Guccione S, Ramsay RR (2007). Interactions of imidazoline ligands with the active site of purified monoamine oxidase A. FEBS J 274: 1567–1575. [DOI] [PubMed] [Google Scholar]
- Jordan S, Jackson HC, Nutt DJ, Handley SL (1996). Discriminative stimulus produced by the imidazoline I2 site ligand, 2 ‐BFI. Journal of psychopharmacology (Oxford, England) 10: 273–278. [DOI] [PubMed] [Google Scholar]
- Keller B, Garcia‐Sevilla JA (2015). Immunodetection and subcellular distribution of imidazoline receptor proteins with three antibodies in mouse and human brains: Effects of treatments with I1‐ and I2‐imidazoline drugs. Journal of psychopharmacology (Oxford, England) 29: 996–1012. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A, Tyacke RJ, Robinson JJ, Husbands SM, Minchin MC, Nutt DJ et al (2009). Identification of an imidazoline binding protein: creatine kinase and an imidazoline‐2 binding site. Brain Res 1279: 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissin I (2010). The development of new analgesics over the past 50 years: a lack of real breakthrough drugs. Anesth Analg 110: 780–789. [DOI] [PubMed] [Google Scholar]
- Kudo T, Kushikata T, Kudo M, Kudo T, Hirota K (2011). Antinociceptive effects of neurotropin in a rat model of central neuropathic pain: DSP‐4 induced noradrenergic lesion. Neurosci Lett 503: 20–22. [DOI] [PubMed] [Google Scholar]
- Li JX (2017). Imidazoline I2 receptors: an update. Pharmacol Ther https://doi.org/10.1016/j.pharmthera.2017.03.009 178: 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Koek W, France CP (2009a). Food restriction and streptozotocin differentially modify sensitivity to the hypothermic effects of direct‐ and indirect‐acting serotonin receptor agonists in rats. Eur J Pharmacol 613: 60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Unzeitig A, Javors MA, Rice KC, Koek W, France CP (2009b). Discriminative stimulus effects of 1‐(2,5‐dimethoxy‐4‐methylphenyl)‐2‐aminopropane (DOM), ketanserin, and (R)‐(+)‐{alpha}‐(2,3‐dimethoxyphenyl)‐1‐[2‐(4‐fluorophenyl)ethyl]‐4‐pipidinemetha nol (MDL100907) in rats. J Pharmacol Exp Ther 331: 671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J‐X, Thorn DA, Qiu Y, Peng B‐W, Zhang Y (2014). Antihyperalgesic effects of imidazoline I(2) receptor ligands in rat models of inflammatory and neuropathic pain. Brit J Pharmacol 171: 1580–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lione LA, Nutt DJ, Hudson AL (1998). Characterisation and localisation of [3H]2‐(2‐benzofuranyl)‐2‐imidazoline binding in rat brain: a selective ligand for imidazoline I2 receptors. Eur J Pharmacol 353: 123–135. [DOI] [PubMed] [Google Scholar]
- MacInnes N, Handley SL (2002). Characterization of the discriminable stimulus produced by 2‐BFI: effects of imidazoline I(2)‐site ligands, MAOIs, beta‐carbolines, agmatine and ibogaine. Br J Pharmacol 135: 1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacInnes N, Handley SL (2003). Potential serotonergic and noradrenergic involvement in the discriminative stimulus effects of the selective imidazoline I2‐site ligand 2‐BFI. Pharmacol Biochem Behav 75: 427–433. [DOI] [PubMed] [Google Scholar]
- MacKinnon AC, Redfern WS, Brown CM (1995). [3H]‐RS‐45041‐190: a selective high‐affinity radioligand for I2 imidazoline receptors. Br J Pharmacol 116: 1729–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G (1996). Neurotransmitter release. Annu Rev Neurosci 19: 219–233. [DOI] [PubMed] [Google Scholar]
- McDonald GR, Olivieri A, Ramsay RR, Holt A (2010). On the formation and nature of the imidazoline I2 binding site on human monoamine oxidase‐B. Pharmacol Res 62: 475–488. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagakura Y, Okada M, Kohara A, Kiso T, Toya T, Iwai A et al (2003). Allodynia and hyperalgesia in adjuvant‐induced arthritic rats: time course of progression and efficacy of analgesics. J Pharmacol Exp Ther 306: 490–497. [DOI] [PubMed] [Google Scholar]
- NIH (2013). Pain in America. In: NINDS Chronic Pain Information Page. Bethesda, MD.
- Nutt DJ, French N, Handley S, Hudson A, Husbands S, Jackson H et al (1995). Functional studies of specific imidazoline‐2 receptor ligands. Ann N Y Acad Sci 763: 125–139. [DOI] [PubMed] [Google Scholar]
- Otsuka N, Kiuchi Y, Yokogawa F, Masuda Y, Oguchi K, Hosoyamada A (2001). Antinociceptive efficacy of antidepressants: assessment of five antidepressants and four monoamine receptors in rats. J Anesth 15: 154–158. [DOI] [PubMed] [Google Scholar]
- Ozaita A, Olmos G, Boronat MA, Lizcano JM, Unzeta M, Garcia‐Sevilla JA (1997). Inhibition of monoamine oxidase A and B activities by imidazol(ine)/guanidine drugs, nature of the interaction and distinction from I2‐imidazoline receptors in rat liver. Br J Pharmacol 121: 901–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinssen EP, Assie MB, Koek W, Kleven MS (2002). Depletion of 5‐HT disrupts prepulse inhibition in rats: dependence on the magnitude of depletion, and reversal by a 5‐HT precursor. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 26: 340–347. [DOI] [PubMed] [Google Scholar]
- Qiu Y, He XH, Zhang Y, Li JX (2014). Discriminative stimulus effects of the novel imidazoline I(2) receptor ligand CR4056 in rats. Sci Rep 4: 6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Zhang Y, Li JX (2015). Discriminative stimulus effects of the imidazoline I2 receptor ligands BU224 and phenyzoline in rats. Eur J Pharmacol 749: 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz‐Durantez E, Torrecilla M, Pineda J, Ugedo L (2003). Attenuation of acute and chronic effects of morphine by the imidazoline receptor ligand 2‐(2‐benzofuranyl)‐2‐imidazoline in rat locus coeruleus neurons. Br J Pharmacol 138: 494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S, Getslev V, Weizman A, Pick CG (1998). The antinociceptive effect of moclobemide in mice is mediated by noradrenergic pathways. Neurosci Lett 253: 183–186. [DOI] [PubMed] [Google Scholar]
- Siemian JN, Obeng S, Zhang Y, Zhang Y, Li JX (2016). Antinociceptive interactions between the imidazoline I2 receptor agonist 2‐BFI and opioids in rats: role of efficacy at the mu‐opioid receptor. J Pharmacol Exp Ther 357: 509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemian JN, Qiu Y, Zhang Y, Li J‐X (2017). Role of intracellular Ca2+ signaling in the antinociceptive and discriminative stimulus effects of the imidazoline I2 receptor agonist 2‐BFI in rats. Psychopharmacology (Berl) 234: 3299–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YH, Li HS, Zhu C, Hu W, Yang J, Zhao GL et al (2014). The analgesia effect of duloxetine on post‐operative pain via intrathecal or intraperitoneal administration. Neurosci Lett 568: 6–11. [DOI] [PubMed] [Google Scholar]
- Tasker RA, Connell BJ, Yole MJ (1992). Systemic injections of alpha‐1 adrenergic agonists produce antinociception in the formalin test. Pain 49: 383–391. [DOI] [PubMed] [Google Scholar]
- Thorn DA, An XF, Zhang Y, Pigini M, Li JX (2012). Characterization of the hypothermic effects of imidazoline I(2) receptor agonists in rats. Br J Pharmacol 166: 1936–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn DA, Siemian JN, Zhang Y, Li JX (2015). Anti‐hyperalgesic effects of imidazoline I2 receptor ligands in a rat model of inflammatory pain: interactions with oxycodone. Psychopharmacology (Berl) 232: 3309–3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn DA, Qiu Y, Jia S, Zhang Y, Li JX (2016a). Antinociceptive effects of imidazoline I2 receptor agonists in the formalin test in rats. Behav Pharmacol 27: 377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn DA, Zhang Y, Li JX (2016b). Effects of the imidazoline I2 receptor agonist 2‐BFI on the development of tolerance to and behavioural/physical dependence on morphine in rats. Br J Pharmacol 173: 1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn DA, Zhang Y, Li JX (2017). Tolerance and cross‐tolerance to the antinociceptive effects of oxycodone and the imidazoline I2 receptor agonist phenyzoline in adult male rats. Psychopharmacology (Berl) 234: 1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugedo L, Pineda J, Martin‐Ruiz R, Ruiz‐Ortega JA, Artigas F (1999). Imidazoline‐induced inhibition of firing rate of 5‐HT neurons in rat dorsal raphe by modulation of extracellular 5‐HT levels. Ann N Y Acad Sci 881: 365–368. [DOI] [PubMed] [Google Scholar]
- Valhondo M, Marco I, Martin‐Fontecha M, Vazquez‐Villa H, Ramos JA, Berkels R et al (2013). New serotonin 5‐HT1A receptor agonists endowed with antinociceptive activity in vivo . J Med Chem 56: 7851–7861. [DOI] [PubMed] [Google Scholar]
- Wattiez AS, Pichon X, Dupuis A, Hernandez A, Privat AM, Aissouni Y et al (2013). Disruption of 5‐HT2A receptor‐PDZ protein interactions alleviates mechanical hypersensitivity in carrageenan‐induced inflammation in rats. PloS one 8: e74661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M (1983). Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16: 109–110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Pharmacological properties of drugs used in this study.
Table S2 Group mean PWTs (in g) for the effects of 2‐BFI alone and in combination with monoaminergic reuptake inhibitors or before and after monoamine depletion on CCI‐induced mechanical nociception.
Table S3 Group mean PWTs (in g) for the effects of 2‐BFI alone and in combination with monoaminergic receptor antagonists on CCI‐induced mechanical nociception.
Table S4 Group mean PWTs (in g) for the effects of CR4056 alone and in combination with monoaminergic receptor antagonists on CCI‐induced mechanical nociception.
Table S5 Group mean PWTs (in g) for the effects of 2‐BFI alone and in combination with monoaminergic receptor antagonists on CFA‐induced mechanical nociception.


