Abstract
Background and Purpose
Poor social behaviour and vulnerability to stress are major clinical features of stimulant use disorders. The corticotropin‐releasing factor (CRF) system mediates stress responses and might underlie substance use disorders; however, its involvement in social impairment induced by stimulant substances remains unknown. CRF signalling is mediated by two receptor types, CRF1 and CRF2. In the present study we investigated the role of the CRF2 receptor in social behaviour deficits, vulnerability to stress and related brain alterations induced by cocaine administration and withdrawal.
Experimental Approach
CRF2 receptor‐deficient (CRF2−/−) and littermate wild‐type mice were repeatedly tested in the three‐chamber task for sociability (i.e. preference for an unfamiliar conspecific vs. an object) and social novelty preference (SNP; i.e. preference for a novel vs. a familiar conspecific) before and after chronic cocaine administration. An in situ hybridization assay was used to assess gene expression of the stress‐responsive arginine vasopressin (AVP) and oxytocin (OT) neuropeptides in the hypothalamus.
Key Results
CRF2 receptor deficiency eliminated the sociability deficit induced by cocaine withdrawal. Moreover, CRF2−/− mice did not show either the stress‐induced sociability deficit or the increased AVP and OT expression associated with long‐term cocaine withdrawal, indicating resilience to stress. Throughout, wild‐type and CRF2−/− mice displayed SNP, suggesting that cocaine withdrawal‐induced sociability deficits were not due to impaired detection of social stimuli.
Conclusions and Implications
These findings demonstrate a central role for the CRF2 receptor in social behaviour deficits and biomarkers of vulnerability induced by cocaine withdrawal, suggesting new therapeutic strategies for stimulant use disorders.
Abbreviations
- AVP
arginine vasopressin
- CRF
corticotropin‐releasing factor
- EPS
elevated platform stressor
- ITI
intertrial time interval
- OT
oxytocin
- PVN
paraventricular nucleus of the hypothalamus
- SNP
social novelty preference
- SON
supraoptic nucleus of the hypothalamus
- SSC
saline–sodium citrate
Introduction
Stimulant use disorders are among the most prevalent substance use disorders, carrying a heavy burden of disability and death worldwide (http://www.unodc.org/wdr2016). These conditions are characterized by enhanced processing of substance‐induced reward and reduced valuation of non‐substance rewards, including social stimuli (Volkow et al., 2011). As a result, stimulant users often display dysfunctional social behaviour (APA, 2013; Preller et al., 2014). Furthermore, stressful life events and social ‘breakdown’ are hypothesized to contribute to the high rate of relapse to substance‐seeking and ‐taking behaviour (Sinha, 2001; Preston and Epstein, 2011; Volkow et al., 2011). However, the brain alterations underlying social dysfunction and vulnerability induced by stimulant substances remain largely unknown.
The http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=912 (CRF) system might be involved in substance use disorders. Indeed, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2286 withdrawal increases CRF activity in the amygdala and the frontal cortex, brain regions relevant to the behavioural effects of substances of abuse (Richter and Weiss, 1999; Zorrilla et al., 2001; Noel et al., 2013). Accordingly, CRF antagonism attenuates negative affective‐like states and stress‐induced substance‐seeking behaviour in cocaine‐withdrawn rats (Sarnyai et al., 1995; Basso et al., 1999; Shaham et al., 2003; Koob, 2008). CRF signalling is mediated by two receptor types, named http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=212 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=213 (Hauger et al., 2003). There is compelling evidence indicating that they fulfil distinct roles in the effects of substances of abuse. For instance, CRF1 or CRF2 receptor deficiency increases or decreases, respectively, the somatic signs and the recognition memory deficits induced by morphine withdrawal in mice (Papaleo et al., 2007; 2008; Morisot and Contarino, 2016). Moreover, CRF2 receptor deficiency reduces the negative affective‐like states of early morphine withdrawal phases and eliminates the stress‐induced re‐emergence of recognition memory deficits and motivational states in long‐term morphine‐ or cocaine‐withdrawn mice (Ingallinesi et al., 2012; Rouibi and Contarino, 2013; Morisot et al., 2014; 2015; Morisot and Contarino, 2016). Studies also suggest a complex role for the CRF system in social behaviour. For instance, i.c.v. administration of CRF facilitates partner preference in male prairie voles (DeVries et al., 2002). Moreover, transgenic CRF overexpression, urocortin 3 or CRF2 receptor deficiency enhance social investigation or social memory, as assessed in social discrimination tasks (Deussing et al., 2010; Kasahara et al., 2011). However, CRF responses to stressors are differently modulated by the two major ‘social’ hormones http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2174 (OT) and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2168 (AVP) (Stoop, 2012). Indeed, AVP is co‐released with CRF from the paraventricular nucleus of the hypothalamus (PVN) to potentiate stress responses, whereas OT reduces the stress‐induced PVN‐CRF transcription (Bulbul et al., 2011; Jurek et al., 2015; Herman and Tasker, 2016). Interestingly, CRF2 receptors are expressed both in the PVN and the supraoptic nucleus of the hypothalamus (SON), main sources of OT and AVP production in the brain (Van Pett et al., 2000; Stoop, 2012). Moreover, other studies have suggested the OT and AVP systems have a role in the actions of cocaine. For instance, cocaine administration decreased OT and vasopressin peptide levels in the hypothalamus and hippocampus, reduced vasopressin peptide levels in the amygdala and increased OT receptor binding in the lateral septum and the amygdala (Sarnyai et al., 1992; Georgiou et al., 2016b). Moreover, cocaine withdrawal increased the expression of PVN‐AVP, OT receptor binding in the medial and lateral septum and the amygdala, decreased novel object recognition memory and increased anxiety‐like behaviour in mice and rats (Zhou et al., 2011; Georgiou et al., 2016b). Interestingly, studies have also shown that administration of vasopressin or OT decreases cocaine self‐administration or cue‐induced reinstatement of cocaine seeking in rats respectively (van Ree et al., 1988; Leong et al., 2017). Nevertheless, the role of the CRF/CRF2 receptor system in social behaviour deficits and brain AVP/OT changes induced by stimulant substances remains poorly understood.
Thus, in the present study we investigated the involvement of the CRF2 receptor in social dysfunction and stress vulnerability induced by repeated administration of cocaine and withdrawal from cocaine. For this purpose, we used the widely employed and validated three‐chamber task for social behaviour in mice, which is thought to reliably measure sociability and social novelty preference (SNP), independently of emotional‐like states (Silverman et al., 2010; Moy et al., 2013). Social behaviour was first assessed in substance‐naïve wild‐type and CRF2 receptor‐deficient (CRF2−/−) mice. Then, mice were treated with escalating doses of cocaine, and social behaviour examined immediately and throughout a relatively long period after the last substance administration. We also assessed the role of the CRF2 receptor in social behaviour vulnerability to an environmental ethological stressor long time after cocaine discontinuation. To further search for brain alterations associated with the long‐lasting CRF2 receptor‐dependent vulnerability induced by cocaine withdrawal, OT and AVP gene expression were measured in the PVN and the SON, brain circuits implicated in social behaviour and stress responses (McCall and Singer, 2012; Neumann and Landgraf, 2012).
Methods
Subjects
Knockout mouse models are essential to evaluate the contribution of specific signalling pathways in key behavioural phenotypes relevant to addiction research. Thus, herein, CRF2 receptor null mutant (CRF2−/−) and wild‐type mice were used to assess the role of the CRF2 receptor in cocaine‐induced social behaviour deficits. Littermate wild‐type (n = 32), CRF2 receptor heterozygous (CRF2+/−; n = 53) and CRF2−/− (n = 34) male mice were obtained from mating of CRF2+/− mice using a trio breeding programme (one male was mated with two female mice). The specific pathogen‐free mouse colony was on a C57BL/6J × 129 background and originated from Bale et al. (2000). The colony room (22 ± 2°C, relative humidity: 50–60%) was maintained on a 12 h light/dark cycle (lights on at 0800 h). The genotype of the mice was determined by PCR analysis of tail DNA. Mice were housed in groups of 2–4 in polycarbonate cages (29.5 × 11.5 × 13 cm; L × W × H) containing bedding and a cotton nestlet (SAFE, Augy, France). They had ad libitum access to standard laboratory food (3.3 kcal·g−1; SAFE) and water. The mice were 3–7 months old at the beginning of the experiment; such a relatively large age range did not influence social behaviour in cocaine‐naïve or ‐treated mice (data not shown). At the beginning of the experiment, body weight of the mice was 24–44 g (32.5 ± 0.5 g; mean ± SEM). Animals were monitored daily for the presence of adverse effects of the experimental treatment, and veterinary advice was sought if animals displayed signs of distress. All studies were conducted in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and were approved by the local Animal Care and Use Committee. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015).
Three‐chamber apparatus
The three‐chamber apparatus was a rectangular box (60 × 40 × 20 cm, L × W × H) divided in three equal chambers and made of transparent Plexiglas. Dividing walls had small square doors (8 cm) that could be manually opened and closed. The central chamber was empty, and each side chamber contained a round wire cage (12 cm diameter and 14 cm high with bars spaced 1 cm apart) in which an object or a mouse could be placed.
Three‐chamber testing in cocaine‐naïve mice
Prior to the beginning of the experiment, during three consecutive days, each animal was handled for 1 min·day−1. The experiments were conducted during the light phase of the 12 h light/dark cycle in a quiet room dimly illuminated (10 lx). The three‐chamber task allowed the study of (i) sociability (i.e. preference for an unfamiliar conspecific vs. an object) and (ii) SNP (i.e. preference for a novel vs. a familiar conspecific) (Silverman et al., 2010), as depicted in Figure 1A. To reduce the number of animals used, mice were repeatedly tested in the three‐chamber task, as depicted in Figure 1B. Due to the limited number of animals that could be tested daily in the three‐chamber paradigm and to the breeding capacity of the mouse colony, behavioural experiments were identically carried out using three independent cohorts of mice. No statistical difference was found between the independent experiments, and therefore, the results were pooled. Within each animal cohort and across test days, all of the experimental conditions (i.e. genotype, treatment and stress) were pseudorandomized. During the habituation phase, the subject mouse was confined to the central chamber for 5 min; then, the doors were opened, so the mouse could freely explore the three chambers and the empty wire cages for 10 min. During the subsequent 10 min sociability phase, the subject mouse was allowed to explore the entire apparatus with one wire cage containing an unfamiliar mouse and the other an object. The unfamiliar mice were substance‐naïve CRF2+/− male mice age matched with the subject mice. They were handled (1 min·day−1) and habituated to the wire cages (10 min·day−1) over the 3 days preceding the first three‐chamber test. The position (left‐ or right‐side of chamber) of the unfamiliar mouse was counterbalanced within each experimental group. Then, a 10 min SNP test was carried out, during which a novel mouse was placed in the wire cage that previously contained the object, and the subject mouse could choose between the already investigated familiar and the novel mouse. To assess social recognition memory, the mice were tested once a week using an intertrial time interval (ITI) between the sociability and the SNP tests (Silverman et al., 2010; Millan and Bales, 2013). In particular, on a given test day, within each experimental group, one‐third of the animals were tested using the 0 h ITI, one‐third using the 2 h ITI and one‐third using the 4 h ITI. Between each test, the apparatus was cleaned with water and the wire cages with 50% ethanol. The experiments were recorded on a video system. The time spent exploring each wire cage and the number of entries made into each side chamber by the subject mouse were scored by a trained observer. Exploration was defined as the animal directing the nose within 0.5 cm of the wire cage. An entry was defined as all four paws in one side of the chamber.
Figure 1.
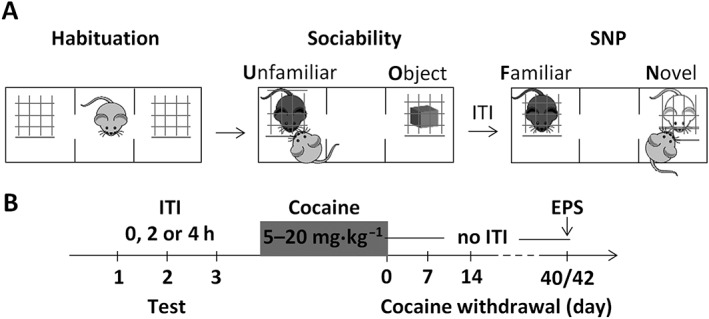
Experimental procedure. (A) Drawing illustrating the habituation, sociability and the SNP phases of the three‐chamber test. (B) Wild‐type and CRF2−/− mice are tested (ticks) once a week in the three‐chamber task using a 0, 2 or 4 h ITI between the sociability and the SNP test. Then, over an 11 day period, cocaine (5–20 mg·kg−1, i.p.) is injected twice a day (0800–2000 h), except for the last day when only one injection is given in the morning, immediately prior to testing (cocaine withdrawal day 0). Following cessation of cocaine administration, the mice are tested weekly in the three‐chamber task throughout a relatively long period using a 0 h ITI between the sociability and the SNP test. Then, using a within‐subject design, on cocaine withdrawal day 40 or 42, the mice are exposed or not to an EPS 1 h prior to the three‐chamber test.
Cocaine administration paradigm
Following the initial three‐chamber tests, within genotype mice were pseudorandomly assigned to two treatment groups (saline or cocaine) having similar age and three‐chamber performance, as assessed with the 2 h ITI (data not shown). For the biochemical experiment, within genotype mice were pseudorandomly assigned to two groups (saline or cocaine) having a similar age. Mice received i.p. injections (10 mL·kg−1) of physiological saline or cocaine hydrochloride (Coopération Pharmaceutique Française, Melun, France) every 12 h (0800–2000 h) for 11 consecutive days, as follows: day 1: 5 mg·kg−1; days 2 and 3: 10 mg·kg−1; days 4–6: 15 mg·kg−1; days 7–10: 20 mg·kg−1; and day 11: 20 mg·kg−1, only one injection in the morning. The latter treatment was chosen because it produced cognitive deficits and vulnerability to stress (Morisot et al., 2014). The mice were weighed immediately before each injection and body weight changes calculated as a percentage of the body weight recorded just prior to the first injection.
Three‐chamber testing after cocaine administration and withdrawal
The three‐chamber tests were carried out immediately and once a week after the last injection, up to cocaine withdrawal day 42. To control for the ability to detect social stimuli without loading on cognitive function, no ITI was applied between the sociability and the SNP test (Silverman et al., 2010; Millan and Bales, 2013). To assess vulnerability to stress, on cocaine withdrawal day 40 within each group, approximately half of the mice were exposed to an elevated platform stressor (EPS) for 10 min and tested in the three‐chamber task 1 h later; the other half remained undisturbed in the home cage (no stress) prior to being tested in the three‐chamber task. Two days later (cocaine withdrawal day 42), the mice previously stressed were tested under no‐stress conditions and vice versa. The elevated platform was a square (10 × 10 cm) made of dark grey polypropylene, elevated 40 cm above the floor.
AVP and OT in situ hybridization histochemistry
To avoid the any effects the SNP testing may have on brain changes, the biochemistry studies were carried out using a fourth independent cohort of mice. In particular, wild‐type and CRF2−/− mice were treated with saline or cocaine, as described above. Then, 40 or 42 days after the last injection, they were exposed to the EPS for 10 min and killed by rapid cervical dislocation 1 h and 25 min later. Brains were rapidly removed, frozen in isopentane (−40°C) and stored at −80°C. The 1 h and 25 min interval was chosen to mimic the time elapsing between the EPS exposure and the end of the sociability phase during the three‐chamber tests. Brains were cut in coronal sections (12 μm) using a cryostat and thaw mounted onto gelatin‐coated slides to be processed for in situ hybridization. This was performed with oligonucleotide probes designed to recognize AVP (modified from Vacher et al., 2002) or OT (modified from Patisaul et al., 2003) mRNA, as previously described (Papaleo et al., 2007). Oligonucleotide probes were labelled by tailing with [35S]‐dATP (PerkinElmer SAS, Courtaboeuf, France) using terminal deoxynucleotide transferase (Promega, Madison, WI, USA). The specific activity of the oligonucleotide probes was 58 × 107 and 78 × 107 cpm·μg−1 for AVP or OT respectively. After being labelled, the probes were precipitated in absolute ethanol and 5 M sodium chloride, dried and resuspended at a concentration of 2 pg·μL−1 in the hybridization buffer (50% deionized formamide, 10% dextran sulphate, 20 mM Tris–HCl, 1 mM EDTA, 300 mM NaCl, 200 μg·mL−1 denatured salmon sperm DNA, 1% Denhardt, 0.1% SDS and 240 μg·mL−1 tRNA). The sections were fixed with 4% paraformaldehyde in phosphate buffer 0.1 M (pH 7.2) for 5 min at room temperature, rinsed twice for 30 min with 4× saline–sodium citrate (SSC; 1% Denhardt), acetylated into 4× SSC (0.25% acetic anhydride, 1.33% triethanolamine; pH 8) for 10 min at room temperature and then dehydrated in graded alcohol. The slides were then incubated horizontally overnight at 40°C with the hybridization solution containing the 35S‐labelled probe (45 μL per slide). At the end of the incubation, the slides were washed in decreasing concentrations of SSC (1× SSC at room temperature, 1× SSC at 40°C and 0.1× SSC at 40°C) and dehydrated in ethanol. The slides were then exposed at room temperature to Amersham Hyperfilms MP (Dominique Dutscher SAS, Brumath, France) over 2–4 days. Quantification of mRNA was performed by densitometry on X‐ray films. Optical density (OD) was measured within regions defining the PVN or the SON (bregma interval: −0.70/−0.94 mm), as identified using the Paxinos and Franklin mouse brain atlas (Paxinos and Franklin, 2001). For each mouse, two measurements were performed for each brain region, one per hemisphere. These measurements were then averaged, and the outcome was considered as a representative value. For statistical analysis, the data were expressed as OD × 103.
Statistical analysis
Each mouse was assigned a unique identification number that was used to conduct blind testing and data analysis. To prevent initial side preference from biasing the results, only animals exploring each side compartment of the three‐chamber apparatus for no more than 70% of the 10 min habituation phase were included in the study (inclusion criterion). To obtain sociability or SNP ratios, the percentage of time spent investigating the wire cage containing the unfamiliar or the novel mouse over the total time spent investigating the two wire cages was calculated respectively. Since under drug‐naïve conditions all of the animals met the inclusion criteria, a two‐way ANOVA with genotype (wild‐type, CRF2−/−) as a between‐subjects factor and repeated sociability tests or ITI as a within‐subject factor was used. However, after drug administration, repeated measure analyses could not be used because of missing values during one or more of the three‐chamber tests (i.e. animals not meeting the inclusion criterion). Therefore, within each test day, sociability, SNP ratios or entries were analysed using a two‐way ANOVA with genotype and treatment (saline and cocaine) as between‐subjects factors. Moreover, since three‐chamber performance is considered a yes‐or‐no result, within each experimental group, time spent by the subject mouse exploring the unfamiliar mouse or the object, the novel or the familiar mouse, during the sociability or the SNP tests, respectively, was compared using Student's paired t‐test, as previously suggested (Silverman et al., 2015; Kazdoba et al., 2016). Entries into the two side chambers of the apparatus were also measured to control for sedation or hyperactivity (Moy et al., 2013). A three‐way ANOVA was used to analyse body weight changes, with genotype and treatment as between‐subjects factors and days as a within‐subject factor. A two‐way ANOVA was used to analyse AVP and OT mRNA levels with genotype and treatment as between‐subjects factors. The accepted value for significance was P < 0.05. If main or interaction effects were significant, Fisher's least significant difference post hoc test was used for individual group comparisons. Statistical analyses were performed using the Statistica software (version 10, TIBCO Software Inc, Palo Alto, CA, USA). Data graphs were created using GraphPad Prism (GraphPad Software, La Jolla, CA, USA). The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017).
Results
Unaltered social behaviour in substance‐naïve CRF2 receptor‐deficient mice
The impact of CRF2 receptor deficiency upon social behaviour was first evaluated prior to cocaine administration. Although CRF2−/− mice showed a higher sociability ratio than wild‐type mice (P < 0.05; Table 1 and Figure 2A), either genotype spent more time exploring the unfamiliar mouse than the object (P < 0.05; Supporting Information Table S1 and Figure S1A). Repeated testing reduced entry into the two side chambers of the apparatus (P < 0.05; Table 1). Indeed, mice made more entries during the first than the second or the third three‐chamber test (P < 0.05; Figure 2B). During the SNP tests, social memory followed a genotype‐independent ITI‐linked decay (P < 0.05; Table 1). Indeed, when a 2 h ITI was applied between the sociability and the SNP test, mice showed lower or higher SNP ratios, as compared with no or a 4 h ITI respectively (P < 0.05; Figure 2C). Moreover, either genotype spent more time exploring the novel than the familiar mouse when no or a 2 h ITI was applied between the sociability and the SNP test (P < 0.05; Supporting Information Table S1 and Figure S1B). However, when a 4 h ITI was used, wild‐type and CRF2−/− mice spent similar time exploring the novel and the familiar mouse, indicating a genotype‐independent ITI‐induced SNP deficit (Supporting Information Table S1 and Figure S1B). During the SNP tests, neither genotype nor ITI influenced entry into the two side chambers of the apparatus (Table 1 and Figure 2D). Thus, under substance‐naïve conditions, CRF2 receptor deficiency does not alter either sociability or SNP.
Table 1.
Statistical analysis of sociability ratio, SNP ratio and entries into the two side compartments of the three‐chamber apparatus displayed by drug‐naïve wild‐type and CRF2−/− mice
| Sociability | SNP | |||
|---|---|---|---|---|
| Ratio | Entries | Ratio | Entries | |
| Geno | F 1, 64 = 4.33 | F 1, 64 = 3.51 | F 1, 64 = 0.89 | F 1, 64 = 3.43 |
| P < 0.05 | P = 0.07 | P = 0.34 | P = 0.07 | |
| RM | F 2, 128 = 0.07 | F 2, 128 = 6.50 | F 2, 128 = 79.19 | F 2, 128 = 1.27 |
| P = 0.93 | P < 0.005 | P < 0.05 | P = 0.28 | |
| Geno × RM | F 2, 128 = 1.24 | F 2, 128 = 1.41 | F 2, 128 = 1.42 | F 2, 128 = 0.45 |
| P = 0.29 | P = 0.25 | P = 0.25 | P = 0.64 | |
Geno, genotype; RM, repeated measure (sociability: tests 1, 2 and 3; SNP: 0, 2 and 4 h ITI between the sociability and the SNP test).
Figure 2.
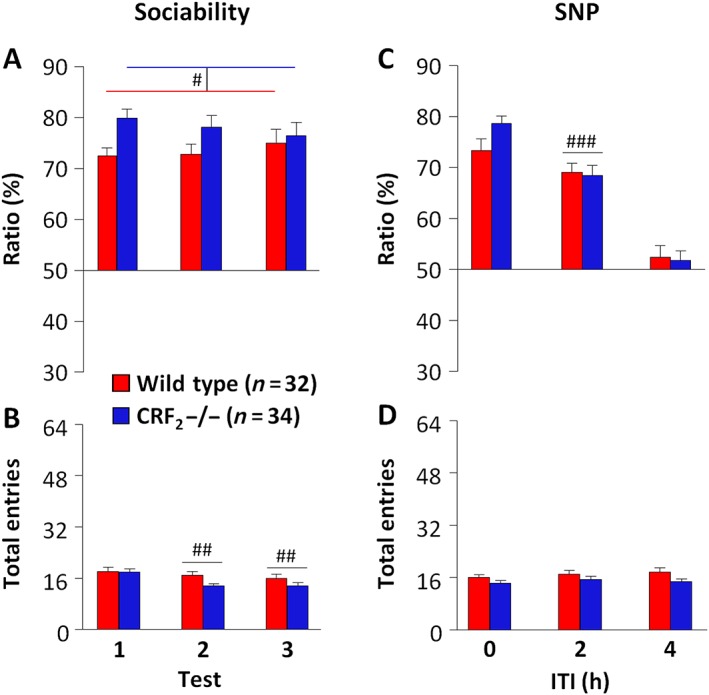
CRF2 receptor deficiency does not influence sociability or social recognition memory under basal drug‐naïve conditions. (A) Sociability and (C) SNP ratio (%) displayed by drug‐naïve wild‐type and CRF2−/− mice repeatedly tested (tests 1 to 3) once a week in the three‐chamber task. A 0, 2 or 4 h ITI was pseudorandomly applied between the sociability and the SNP test. The ratio was calculated as the percentage of time spent investigating the wire cage containing the unfamiliar or the novel mouse over the total time spent investigating the two wire cages during the sociability or the SNP test respectively. (B, D) Total entries made into the two side compartments of the three‐chamber apparatus during the sociability or the SNP test. Values represent mean ± SEM. # P < 0.05 versus wild‐type mice, genotype effect. ## P < 0.05 versus test 1, repeated measure effect. ### P < 0.05 versus 0 and 4 h ITI, repeated measure effect.
CRF2 receptor deficiency eliminates the sociability deficits associated with early cocaine withdrawal phases
The intermittent administration of escalating cocaine doses induced a genotype‐independent body weight loss (genotype effect: F 1, 60 = 1.79, P = 0.19; treatment effect: F 1, 60 = 24.77, P < 0.05; day effect: F 9, 540 = 25.91, P < 0.05; and treatment × day effect: F 9, 540 = 11.64, P < 0.05) that was evident starting 12 h after the 10th injection (P < 0.05, Supporting Information Figure S2). Analysis of sociability ratios immediately after the last cocaine injection revealed a genotype‐independent treatment effect (P < 0.05; Table 2). Indeed, cocaine‐treated mice showed a lower sociability ratio than saline‐treated mice (P < 0.05; Figure 3A). Notably, cocaine‐treated wild‐type and CRF2−/− mice spent less time investigating the unfamiliar mouse than the object, suggesting social avoidance (P < 0.05; Supporting Information Table S2 and Figure S3A). Cocaine‐treated mice also showed more entries than saline‐treated mice (P < 0.05; Table 2 and Figure 3B), suggesting an influence of hyperactivity on the lack of sociability. Seven days after the last administration, cocaine withdrawal affected sociability ratios in a genotype‐dependent manner (P < 0.05; Table 2). Notably, cocaine‐withdrawn wild‐type mice showed lower sociability ratios than cocaine‐withdrawn CRF2−/− and saline‐withdrawn mice (P < 0.05; Figure 3A). Indeed, unlike the sociability displayed by cocaine‐withdrawn CRF2−/− and saline‐withdrawn mice (P < 0.05), cocaine‐withdrawn wild‐type mice spent a similar time investigating the unfamiliar mouse and the object, further indicating sociability deficits (Supporting Information Table S2 and Figure S3A). The genotype‐dependent sociability observed on cocaine withdrawal day 7 was not due to locomotor activity since the experimental groups did not differ in the number of entries (Table 2 and Figure 3B). Finally, 14 days after the last injection, neither genotype nor treatment affected sociability ratios (Table 2 and Figure 3A). Indeed, similarly to cocaine‐withdrawn CRF2−/− and saline‐withdrawn mice, cocaine‐withdrawn wild‐type mice spent more time investigating the unfamiliar mouse than the object, indicating recovered sociability (P < 0.05; Supporting Information Table S2 and Figure S3A). Moreover, neither genotype nor treatment affected the number of entries (Table 2 and Figure 3B). These results indicate that CRF2 receptor deficiency eliminates the sociability deficits induced by cocaine withdrawal.
Table 2.
Statistical analysis of sociability ratio and entries into the two side compartments of the three‐chamber apparatus displayed by wild‐type and CRF2−/− mice immediately (0), 7 or 14 days after the last saline or cocaine administration
| Sociability | ||||||
|---|---|---|---|---|---|---|
| Days after the last administration | ||||||
| 0 | 7 | 14 | ||||
| Ratio | Entries | Ratio | Entries | Ratio | Entries | |
| Geno | F 1, 59 = 0.08 | F 1, 59 = 0.36 | F 1, 60 = 20.35 | F 1, 60 = 3.31 | F 1, 59 = 0.46 | F 1, 59 = 1.74 |
| P = 0.78 | P = 0.55 | P < 0.05 | P = 0.07 | P = 0.50 | P = 0.19 | |
| Treat | F 1, 59 = 69.46 | F 1, 59 = 34.91 | F 1, 60 = 7.99 | F 1, 60 = 1.83 | F 1, 59 = 0.21 | F 1, 59 = 0.45 |
| P < 0.05 | P < 0.05 | P < 0.05 | P = 0.18 | P = 0.65 | P = 0.50 | |
| Geno × Treat | F 1, 59 = 0.0003 | F 1, 59 = 0.007 | F 1, 60 = 4.19 | F 1, 60 = 0.03 | F 1, 59 = 1.53 | F 1, 59 = 0.03 |
| P = 0.98 | P = 0.93 | P < 0.05 | P = 0.87 | P = 0.22 | P = 0.87 | |
Geno, genotype; Treat, treatment (saline or cocaine).
Figure 3.
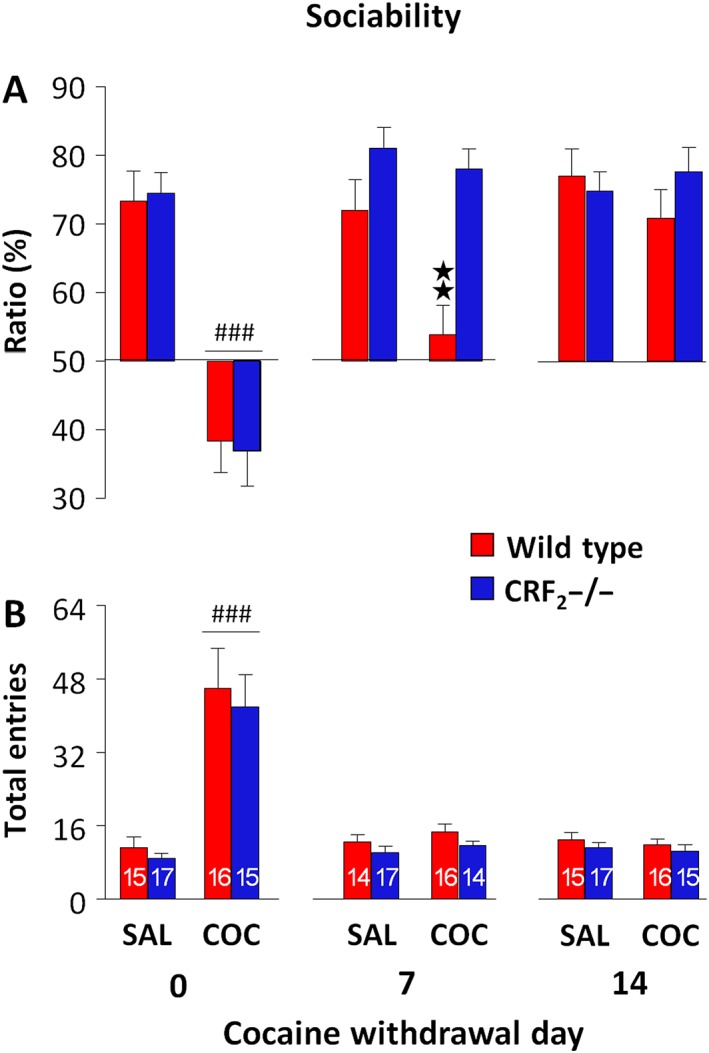
CRF2 receptor deficiency eliminates sociability deficits induced by cocaine withdrawal. (A) Sociability ratio (%) displayed by saline (SAL)‐ or cocaine (COC)‐treated wild‐type and CRF2−/− mice. The three‐chamber tests were carried out immediately (0), 7 and 14 days after the last saline or cocaine administration. (B) Total entries made into the two side compartments of the three‐chamber apparatus during the sociability test. The n for each experimental group is presented in the columns in panel B. Values represent mean ± SEM. **P < 0.05 versus all other groups on cocaine withdrawal day 7. ### P < 0.05 versus saline groups on day 0, genotype‐independent effect.
Cocaine administration and withdrawal do not impair SNP
Immediately after the last injection, cocaine decreased SNP ratios, independently of genotype (P < 0.05; Table 3 and Figure 4A). However, all of the experimental groups investigated the novel more than the familiar mouse, indicating SNP (P < 0.05; Supporting Information Table S2 and Figure S3B). Cocaine treatment also genotype‐independently increased the number of entries (P < 0.05; Table 3 and Figure 4B). Consistently, on cocaine withdrawal day 7 or 14, neither genotype nor treatment affected SNP ratios (Table 3 and Figure 4A). Indeed, saline‐ or cocaine‐withdrawn mice of either genotype spent more time investigating the novel than the familiar mouse (P < 0.05; Supporting Information Table S2 and Figure S3B). Moreover, 7 and 14 days after the last administration, neither genotype nor treatment affected the number of entries (Table 3 and Figure 4B). These results indicate that cocaine administration and withdrawal do not affect the ability to detect social novel stimuli.
Table 3.
Statistical analysis of SNP ratio and entries into the two side compartments of the three‐chamber apparatus displayed by wild‐type and CRF2−/− mice immediately (0), 7 or 14 days after the last saline or cocaine administration
| SNP | ||||||
|---|---|---|---|---|---|---|
| Days after the last administration | ||||||
| 0 | 7 | 14 | ||||
| Ratio | Entries | Ratio | Entries | Ratio | Entries | |
| Geno | F 1, 59 = 0.60 | F 1, 59 = 0.84 | F 1, 60 = 0.75 | F 1, 60 = 1.15 | F 1, 59 = 0.57 | F 1, 59 = 0.27 |
| P = 0.44 | P = 0.36 | P = 0.39 | P = 0.29 | P = 0.45 | P = 0.60 | |
| Treat | F 1, 59 = 13.87 | F 1, 59 = 36.91 | F 1, 60 = 0.48 | F 1, 60 = 1.95 | F 1, 59 = 0.05 | F 1, 59 = 0.47 |
| P < 0.05 | P < 0.05 | P = 0.49 | P = 0.17 | P = 0.82 | P = 0.49 | |
| Geno × Treat | F 1, 59 = 1.23 | F 1, 59 = 0.00 | F 1, 60 = 1.98 | F 1, 60 = 0.00 | F 1, 59 = 0.10 | F 1, 59 = 2.88 |
| P = 0.27 | P = 1.00 | P = 0.16 | P = 1.00 | P = 0.75 | P = 0.09 | |
Geno, genotype; Treat, treatment (saline or cocaine).
Figure 4.
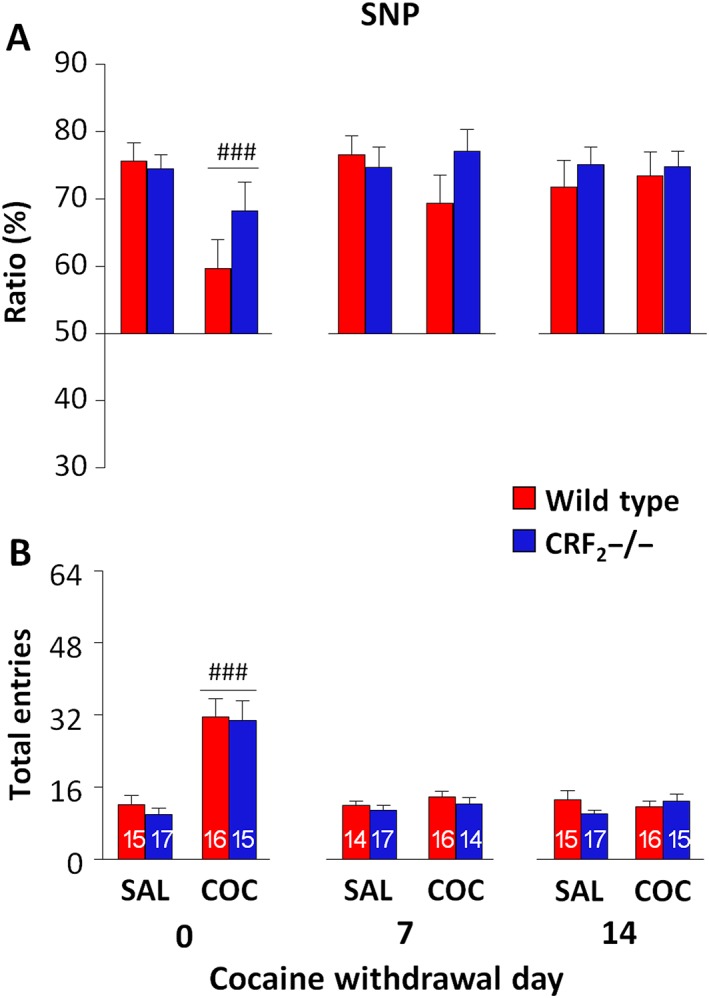
CRF2 receptor deficiency does not affect SNP. (A) SNP ratio (%) displayed by saline (SAL)‐ or cocaine (COC)‐treated wild‐type and CRF2−/− mice. The three‐chamber tests were carried out immediately (0), 7 and 14 days after the last saline or cocaine administration. (B) Total entries made into the two side compartments of the three‐chamber apparatus during the SNP test. The n for each experimental group is presented in panel B. Values represent mean ± SEM. ### P < 0.05 versus saline groups on day 0, genotype‐independent effect.
CRF2 receptor deficiency eliminates the long‐lasting vulnerability of social behaviour induced by cocaine withdrawal
Forty or 42 days after the last administration, under no‐stress conditions, neither genotype nor treatment affected sociability ratios (Table 4 and Figure 5A). Indeed, all of the experimental groups spent more time investigating the unfamiliar mouse than the object, indicating sociability (P < 0.05; Supporting Information Table S3 and Figure S4A). However, following exposure to the EPS, cocaine withdrawal affected sociability ratios in a genotype‐dependent manner (P < 0.05; Table 4). Indeed, cocaine‐withdrawn wild‐type mice showed lower sociability ratios than cocaine‐withdrawn CRF2−/− and saline‐withdrawn mice (P < 0.05; Figure 5A). Accordingly, cocaine‐withdrawn wild‐type mice spent similar time exploring the unfamiliar mouse and the object (Supporting Information Table S3 and Figure S4A), indicating a stress‐induced re‐emergence of sociability deficits. In contrast, cocaine‐withdrawn CRF2−/− mice exposed to the EPS spent more time investigating the unfamiliar mouse than the object (P < 0.05; Supporting Information Table S3 and Figure S4A), indicating resilience to stress. The EPS did not impair sociability in saline‐withdrawn wild‐type and CRF2−/− mice (P < 0.05; Supporting Information Table S3 and Figure S4A), suggesting a relatively mild nature of this stressor in substance‐naïve mice. Finally, both under no‐stress and stress conditions, neither genotype nor treatment affected the number of entries (Table 4 and Figure 5B), ruling out a role for locomotor activity in the stress‐induced sociability deficit displayed by cocaine‐withdrawn wild‐type mice. Thus, CRF2 receptor deficiency abolishes the long‐lasting social behaviour vulnerability to stress induced by cocaine withdrawal.
Table 4.
Statistical analysis of sociability ratio, SNP ratio and entries into the two side compartments of the three‐chamber apparatus displayed by saline‐ or cocaine‐treated wild‐type and CRF2−/− mice 40 or 42 days after the last administration
| Sociability | SNP | |||||||
|---|---|---|---|---|---|---|---|---|
| No stress | Stress | No stress | Stress | |||||
| Ratio | Entries | Ratio | Entries | Ratio | Entries | Ratio | Entries | |
| Geno | F 1, 57 = 1.22 | F 1, 57 = 0.42 | F 1, 58 = 5.64 | F 1, 58 = 1.20 | F 1, 57 = 0.03 | F 1, 57 = 0.10 | F 1, 58 = 0.78 | F 1, 58 = 0.54 |
| P = 0.27 | P = 0.52 | P < 0.05 | P = 0.28 | P = 0.84 | P = 0.75 | P = 0.38 | P = 0.47 | |
| Treat | F 1, 57 = 1.25 | F 1, 57 = 0.00 | F 1, 58 = 2.02 | F 1, 58 = 0.32 | F 1, 57 = 0.55 | F 1, 57 = 0.23 | F 1, 58 = 4.48 | F 1, 58 = 0.71 |
| P = 0.27 | P = 0.98 | P = 0.16 | P = 0.57 | P = 0.46 | P = 0.63 | P < 0.05 | P = 0.40 | |
| Geno × Treat | F 1, 57 = 0.01 | F 1, 57 = 0.41 | F 1, 58 = 4.37 | F 1, 58 = 2.10 | F 1, 57 = 0.86 | F 1, 57 = 0.48 | F 1, 58 = 0.31 | F 1, 58 = 1.75 |
| P = 0.92 | P = 0.53 | P < 0.05 | P = 0.15 | P = 0.36 | P = 0.49 | P = 0.58 | P = 0.19 | |
On cocaine withdrawal day 40 or 42, mice were exposed (stress) or not (no stress) to the EPS, using a within‐subject experimental design. Geno, genotype; Treat, treatment (saline or cocaine).
Figure 5.
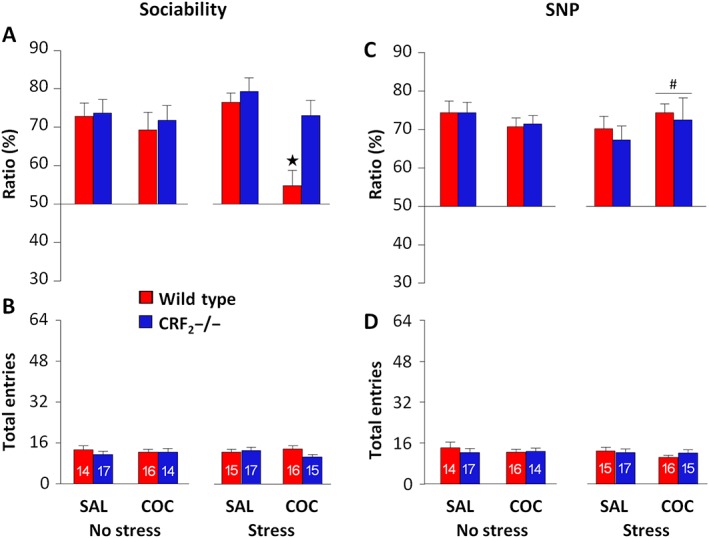
CRF2 receptor deficiency abolishes the long‐lasting social behaviour vulnerability to stress induced by cocaine (COC) withdrawal. (A) Sociability and (C) SNP ratio (%) displayed by saline (SAL)‐ or cocaine‐treated wild‐type and CRF2−/− mice exposed (stress) or not (no stress) to the EPS. The three‐chamber tests were carried out 40 or 42 days after the last administration using a within‐subject (stress vs. no stress) experimental design. (B, D) Total entries made into the two side compartments of the three‐chamber apparatus during the sociability or the SNP test. The n for each experimental group is presented in panels B and D. Values represent mean ± SEM. *P < 0.05 versus all other stressed groups. # P < 0.05 versus stressed saline groups, genotype‐independent effect.
Stress does not impair SNP in long‐term cocaine‐withdrawn mice
During the SNP tests carried out 40 or 42 days after the last administration, under no‐stress conditions, neither genotype nor treatment affected SNP ratios (Table 4 and Figure 5C). However, following exposure to the EPS, cocaine‐withdrawn mice showed higher SNP ratios than saline‐withdrawn mice, independently of genotype (P < 0.05; Table 4 and Figure 5C). Within‐group comparisons revealed that saline‐ and cocaine‐withdrawn wild‐type and CRF2−/− mice exposed or not to the EPS spent more time investigating the novel than the familiar mouse (P < 0.05; Supporting Information Table S3 and Figure S4B). Finally, neither genotype nor treatment affected the number of entries, both under no‐stress or stress conditions (Table 4, Figure 5D). These results indicate that stress does not alter the ability to detect social novel stimuli, either in substance‐naïve or in long‐term cocaine‐withdrawn mice.
CRF2 receptor deficiency abolishes the increased AVP and OT expression induced by cocaine withdrawal
Stressors increase AVP and OT activity in the PVN and the SON (Wotjak et al., 1998; Yang et al., 2012; Yan et al., 2014). Interestingly, the CRF2 receptor is expressed both in the PVN and the SON (Van Pett et al., 2000). However, to our knowledge, the role for the CRF system in brain AVP and OT activity induced by cocaine withdrawal remains unexplored. To address the latter issue, 40 or 42 days after the last saline or cocaine administration, wild‐type and CRF2−/− mice were exposed to the EPS, and brains were examined for AVP and OT mRNA expression. Cocaine withdrawal affected PVN‐AVP expression in a genotype‐dependent manner (genotype effect: F 1, 26 = 5.13, P < 0.05; treatment effect: F 1, 26 = 0.01, P = 0.91; and genotype × treatment interaction effect: F 1, 26 = 5.54, P < 0.05). Notably, cocaine‐withdrawn wild‐type mice had higher PVN‐AVP expression than cocaine‐withdrawn CRF2−/− mice (P < 0.05; Figure 6A, B). In contrast, cocaine‐withdrawn CRF2−/− mice did not differ from saline‐withdrawn mice (Figure 6A, B). However, neither genotype (F 1, 26 = 0.03, P = 0.85) nor treatment (F 1, 26 = 0.09, P = 0.76) affected SON‐AVP expression (genotype × treatment interaction effect: F 1, 26 = 1.11, P = 0.74; Figure 6A, B). Similarly, neither genotype (F 1, 26 = 0.85, P = 0.36) nor treatment (F 1, 26 = 2.84, P = 0.10) affected PVN‐OT expression (genotype × treatment interaction effect: F 1, 26 = 1.35, P = 0.25; Figure 6C, D). However, cocaine withdrawal affected SON‐OT expression in a genotype‐dependent manner (genotype effect: F 1, 26 = 0.88, P = 0.36; treatment effect: F 1, 26 = 0.39, P = 0.54; and genotype × treatment interaction effect: F 1, 26 = 6.54, P < 0.05). Indeed, cocaine‐withdrawn wild‐type mice showed higher SON‐OT expression than cocaine‐withdrawn CRF2−/− or saline‐withdrawn wild‐type mice (P < 0.05; Figure 6C, D). In contrast, cocaine‐withdrawn CRF2−/− mice did not differ from saline‐withdrawn mice (Figure 6C, D). These results indicate a brain region‐specific AVP or OT gene expression after a relatively long period of cocaine withdrawal. Moreover, they show that the cocaine withdrawal‐induced expression of AVP and OT is dependent on the CRF2 receptor.
Figure 6.
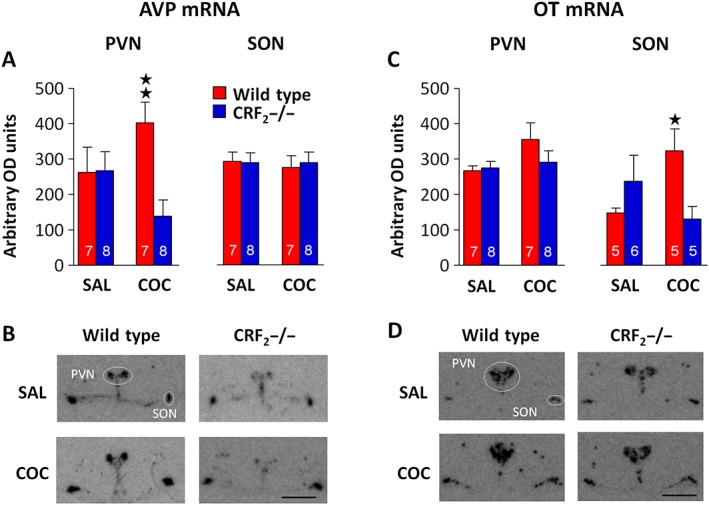
CRF2 receptor‐dependent expression of brain AVP and OT after long‐term cocaine (COC) withdrawal. (A, C) Optical density (OD) and (B, D) representative images of brain sections illustrating (A, B) AVP or (C, D) OT mRNA levels in the paraventricular (PVN) and the supraoptic (SON) nuclei of the hypothalamus in saline (SAL)‐ or cocaine‐withdrawn wild‐type and CRF2−/− mice following exposure to the EPS. Mice received chronic saline or cocaine treatment, as illustrated in Figure 1. On cocaine withdrawal day 40 or 42, the mice were exposed to the EPS for 10 min, and brains were rapidly collected 1 h and 25 min later. Bregma intervals are −0.70/−0.94 mm. Scale bar = 1 mm. The n for each experimental group is presented in panels A and C. Values represent mean ± SEM. Panel A: **P < 0.05 versus CRF2−/− cocaine‐withdrawn mice. Panel C: *P < 0.05 versus CRF2−/− cocaine‐withdrawn and wild‐type saline‐treated mice.
Discussion
The present study shows that cessation of intermittent administration of escalating doses of cocaine disrupts social behaviour in wild‐type, but not in CRF2−/− mice, revealing an essential role for the CRF2 receptor in sociability deficits induced by substance use and withdrawal. Furthermore, following a rather long 6 week period of cocaine withdrawal, exposure to a relatively mild stressor triggers the re‐emergence of sociability deficits in wild‐type, but not in CRF2−/− mice, emphasizing a critical role for the CRF2 receptor also in the long‐lasting vulnerability following substance withdrawal. Accordingly, in wild‐type, but not in CRF2−/− mice, long‐term cocaine withdrawal and stress exposure increase the hypothalamic expression of AVP and OT, signalling a vulnerable state. Interestingly, neither cocaine withdrawal nor stress susceptibility is associated with deficits in SNP, indicating preserved ability to detect and discern social stimuli.
To assess the impact of CRF2 receptor deficiency upon sociability and social memory span, prior to cocaine administration, wild‐type and CRF2−/− mice were repeatedly tested using a variable time interval between the sociability and the SNP tests. Wild‐type and CRF2−/− mice displayed similar sociability and social memory span. The latter results are in apparent contrast with a study showing that CRF2−/− mice exhibit a preference for familiar (nest mate) mice and avoid novel unknown mice, as compared with wild‐type mice (Shemesh et al., 2016). Differences in experimental conditions may underlie the discrepancies between the latter and the present study. Notably, in the latter study, a male ‘subject’ mouse could remain in a remote (non‐social) chamber or interact with a nest‐mate familiar sibling or a novel mouse. CRF2 receptor deficiency is known to increase anxiety‐like behaviour (Bale et al., 2000; Kishimoto et al., 2000). Thus, it is possible that in the latter study, the anxiogenic‐like effect of the CRF2 receptor null mutation contributed to the avoidance of the novel unknown, ethologically more threatening mouse. In contrast, the three‐chamber task used herein is thought to reliably measure social approach behaviour in mice, independently of emotional‐like or cognitive states (Silverman et al., 2010). Indeed, ethanol withdrawal decreased exploration of a three‐chamber apparatus, indicating increased anxiety‐like behaviour, without impairing sociability (Moy et al., 2013). Conversely, herein, cocaine withdrawal impaired sociability without affecting exploration, as assessed by entries into the two side compartments of the three‐chamber apparatus. Moreover, on cocaine withdrawal day 14, both genotypes displayed sociability and SNP. However, we previously reported that on cocaine withdrawal day 14, both genotypes display recognition memory deficits (Morisot et al., 2014), indicating that substance withdrawal‐induced cognitive dysfunction does not affect social behaviour, as assessed by the three‐chamber task. Accordingly, cocaine‐withdrawn mice did not show social behaviour deficits but displayed impaired novel object recognition memory, as determined, respectively, 12 and 13 days after the last drug injection (Georgiou et al., 2016b).
In wild‐type mice, the sociability deficits produced by the intermittent administration of escalating doses of cocaine lasted at least up to 7 days after substance discontinuation. In contrast, cocaine‐withdrawn CRF2−/− mice showed a social behaviour profile similar to substance‐naïve mice, indicating an essential role for the CRF2 receptor in cocaine‐induced sociability deficits. Noteworthy, despite disrupted sociability, wild‐type mice displayed SNP, indicating that the substance withdrawal‐induced sociability deficit was not due to alterations in the detection or processing of social stimuli. To our knowledge, no study has yet reported impaired sociability without a deficit in SNP. Indeed, prior studies reported SNP impairment alone or together with sociability deficits in mouse models of autism (Sala et al., 2011; Moy et al., 2013). This suggests that withdrawal from stimulant substances might produce a unique pattern of social dysfunction, namely, impaired sociability in spite of preserved social novelty recognition.
Herein, exposure to a mild environmental stressor that did not affect social behaviour of substance‐naïve mice induced the re‐emergence of sociability deficits in cocaine‐withdrawn wild‐type mice, even relatively long time (6 weeks) after the last substance administration. This observation parallels human patients where withdrawal from substances of abuse is followed by a strikingly long‐lasting period of vulnerability to stressful life events. Indeed, stress exposure is considered a major risk factor for relapse to substance intake in former substance users (Sinha, 2001). Accordingly, laboratory animal studies showed that physical, psychological or pharmacological stressors reinstate substance‐seeking behaviour and induce negative affective‐like states, even after relatively long periods of substance withdrawal (Shaham et al., 2003; Blatchford et al., 2005; Breese et al., 2005; Morisot et al., 2015). The present results extend to social behaviour the deleterious consequences of substance withdrawal and the associated long‐lasting vulnerability to stress. Most importantly, unlike wild‐type mice, following stress exposure, cocaine‐withdrawn CRF2−/− mice did not show sociability deficits, indicating an essential role for the CRF2 receptor in the long‐lasting vulnerability following discontinuation of stimulant substances. Together with the prior results showing a key role for the CRF2 receptor in the stress‐induced re‐emergence of cognitive dysfunction and up‐shifted motivational states in opiate‐withdrawn or cocaine‐withdrawn mice (Morisot et al., 2014; 2015; Morisot and Contarino, 2016), the present findings further point to the CRF2 receptor as a key neural substrate of the long‐lasting vulnerability induced by substances of abuse.
Investigations into the molecular mechanisms underlying the CRF2 receptor‐dependent sociability deficits in long‐term cocaine‐withdrawn mice revealed altered brain AVP and OT gene expression. Specifically, following stress exposure, cocaine‐withdrawn wild‐type mice displayed elevated expression of AVP in the PVN and OT in the SON, as compared with substance‐naïve mice. Repeated cocaine or nicotine administration has been shown to decrease OT and vasopressin peptide levels in the hypothalamus and hippocampus, to diminish vasopressin peptide levels in the amygdala and to increase http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=369 binding in the lateral septum or the amygdala (Sarnyai et al., 1992; Zanos et al., 2015; Georgiou et al., 2016b). Decreased OT peptide levels in the hypothalamus, increased OT receptor binding in the lateral septum and amygdala and decreased social behaviour have also been shown in morphine‐withdrawn mice (Zanos et al., 2014). Moreover, cocaine or methamphetamine withdrawal increased the expression of PVN‐AVP, OT receptor binding or immunoreactivity in the septum or the subthalamic nucleus, decreased novel object recognition memory and sucrose preference and increased anxiety‐like behaviour in mice or rats (Zhou et al., 2011; Baracz et al., 2016; Georgiou et al., 2016a,b). In addition, extensive research supports a major involvement of OT and AVP in social memory and social interaction (Neumann and Landgraf, 2012). However, the functional significance of the increased brain AVP and OT gene expression induced by substances of abuse remains largely unknown. One possibility is that withdrawal from substances of abuse induces a compensatory activation of AVP and OT systems in order to cope with this stressful and vulnerable condition. This hypothesis is supported by studies suggesting that AVP and OT are involved in coping with stress. Indeed, substance‐naïve rats exposed to the forced swim or the tail suspension stressors showed increased levels of AVP and OT in several hypothalamic nuclei, including the PVN and the SON, the frontal cortex and the amygdala (Yang et al., 2012; Yan et al., 2014). Moreover, rats repeatedly exposed to a forced swim stressor displayed increased AVP or OT release within the PVN or the SON respectively (Wotjak et al., 1998). Notably, functional antagonism of brain AVP and OT signalling by i.c.v. injection of AVP or OT receptor antagonists increased despair behaviour (immobility time) induced by the forced swim or the tail suspension stressors, suggesting that the increased AVP and OT activity served to attenuate the deleterious effects of stressful experiences (Yang et al., 2012; Yan et al., 2014). Within this framework, the elevated hypothalamic AVP and OT gene expressions observed herein in cocaine‐withdrawn mice might serve as valuable biomarkers of the long‐lasting vulnerability following substance withdrawal. However, since hypothalamic AVP and OT expressions were not investigated in non‐stressed animals, the present findings might conceivably have been a consequence of long‐term cocaine withdrawal, independently of EPS exposure.
Contrary to cocaine‐withdrawn wild‐type mice, following stress exposure, cocaine‐withdrawn CRF2−/− mice did not show any increase in the expression of AVP or OT in the PVN or the SON. As mentioned above, studies have suggested that the CRF system is involved in social interaction and social memory (reviewed in Hostetler and Ryabinin, 2013). The CRF2 receptor is expressed both in the PVN and the SON, major sources of brain AVP and OT (Van Pett et al., 2000). Moreover, double‐labelling in situ hybridization studies have shown co‐localization of CRF2 receptor mRNA with OT and AVP mRNA in the SON (Arima and Aguilera, 2000). However, to the best of our knowledge, no studies have yet demonstrated a functional link between the CRF system and the deregulated activity of AVP and OT systems induced by substances of abuse. In the present study, we report that CRF2 receptor deficiency eliminates both the stress‐induced re‐emergence of sociability deficits and the elevated hypothalamic expression of AVP and OT in long‐term cocaine‐withdrawn animals. This suggests that a relatively mild environmental stressor might activate brain CRF pathways which, via CRF2 receptors, might mediate vulnerability to stress and the hypothalamic expression of AVP and OT in order to counteract the long‐lasting cocaine withdrawal‐induced deleterious impact of stressors. Conversely, the lack of functional CRF2 receptors may confer stress resilience, which per se does not require AVP or OT elevations to cope with stressful events after substance withdrawal. These findings indicate a central role for the CRF2 receptor in stress vulnerability and the closely related induction of AVP and OT gene expression following cocaine discontinuation. Nevertheless, more studies are warranted to further explore the functional interplay between the stress‐responsive CRF, AVP and OT systems and their relative contribution to the long‐lasting vulnerable state of substance‐withdrawn individuals.
In conclusion, the present results demonstrate impaired social behaviour in mice withdrawn from chronic cocaine administration. Notably, CRF2 receptor deficiency eliminated the sociability deficits and the long‐lasting vulnerability to stress induced by cocaine discontinuation. Accordingly, CRF2 receptor deficiency also abolished the increased expression of the stress‐responsive AVP and OT systems that was provoked by cocaine withdrawal, further demonstrating a major role for the CRF2 receptor in the deleterious consequences of cocaine administration and withdrawal. Finally, the present findings suggest that pharmacological blockade of the CRF2 receptor might attenuate the social breakdown commonly observed in stimulant use disorders and help abstinent patients to refrain from reinitiating substance‐seeking and substance‐taking behaviour.
Author contributions
N.M. and A.C. designed the study; N.M. and R.M. conducted the behavioural and the in situ hybridization experiments; N.M., R.M., C.L.M. and A.C. analysed the data; and N.M., M.J.M. and A.C. wrote the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This http://onlinelibrary.wiley.com/doi/10.1111/bph.13405/abstract acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Table S1 Statistical analysis of sociability and social novelty preference (SNP) displayed by drug‐naïve wild‐type and CRF2−/− mice. Significant Ps mean higher exploration of the unfamiliar mouse vs. the object or the novel vs. the familiar mouse during the sociability or the SNP test, respectively. ITI: inter‐trial time interval between the sociability and the SNP test. N = 32–34/genotype.
Table S2 Statistical analysis of sociability and social novelty preference (SNP) displayed by wild‐type and CRF2−/− mice immediately (0), 7 or 14 days after the last saline or cocaine administration. Significant Ps mean higher or lower (*) exploration of the unfamiliar mouse vs. the object or the novel vs. the familiar mouse during the sociability or the SNP test, respectively. N = 15–17/group (see Figure 3 and Figure 4 for detailed group size). SAL: saline. COC: cocaine.
Table S3 Statistical analysis of sociability and social novelty preference (SNP) displayed by saline‐ or cocaine‐treated wild‐type and CRF2−/− mice 40 or 42 days after the last administration. On cocaine withdrawal day 40 or 42, mice were exposed (Stress) or not (No stress) to the elevated platform stressor (EPS), using a within‐subject experimental design. Significant Ps mean higher exploration of the unfamiliar mouse vs. the object or the novel vs. the familiar mouse during the sociability or the SNP test, respectively. N = 14–17/group (see Figure 5 for detailed group size). SAL: saline. COC: cocaine.
Figure S1 CRF2 receptor‐deficiency does not alter sociability or social memory span. Time (s) spent exploring the wire cages containing an object (O) or an unfamiliar mouse (U) during the (A) sociability test or a familiar (F) or a novel (N) mouse during the (B) social novelty preference (SNP) test by wild‐type and CRF2−/− mice repeatedly tested (tests 1 to 3) once a week in the 3‐chamber task. A 0, 2 or 4 h inter‐trial time interval (ITI) was applied between the sociability and the SNP test. Value represent mean ± S.E.M. *** P < 0.0005 vs. O or F, Student paired t‐test.
Figure S2 CRF2 receptor‐deficiency does not affect cocaine‐induced body weight loss. Body weight change, calculated as percentage of body weight recorded just prior (P) to the first injection, displayed by saline (SAL)‐ or cocaine (COC)‐ treated wild‐type and CRF2−/− mice. Represented is the body weight change as measured 12 h after the second injection of the cocaine dose indicated. Values represent mean ± S.E.M ### P < 0.0005 vs. SAL mice, genotype‐independent effect.
Figure S3 CRF2 receptor‐deficiency eliminates sociability deficits induced by cocaine withdrawal. Time (s) spent exploring the wire cages containing an object (O) or un unfamiliar mouse (U) during the (A) sociability test or a familiar (F) or a novel (N) mouse during the (B) social novelty preference (SNP) test by saline (SAL)‐ or cocaine (COC)‐treated wild‐type and CRF2−/− mice. The 3‐chamber tests were carried out immediately (0), 7 and 14 days after the last saline or cocaine administration. N of each experimental group is reported under the X axis. Values represent mean ± S.E.M. *P < 0.05 vs. O or F, Student paired t‐test.
Figure S4 CRF2 receptor‐deficiency abolishes the long‐lasting social behavior vulnerability to stress induced by cocaine withdrawal. Time (s) spent exploring the wire cages containing an object (O) or un unfamiliar mouse (U) during the (A) sociability test or a familiar (F) or a novel (N) mouse during the (B) social novelty preference (SNP) test by wild‐type or CRF2−/− mice exposed (Stress) or not (No Stress) to the elevated platform stressor (EPS). The 3‐chamber tests were carried out 40 or 42 days after the last administration of saline (SAL) or cocaine (COC). Moreover, the EPS was applied on cocaine withdrawal day 40 or 42, using a within‐subject experimental design. N of each experimental group is reported under the X axis. Values represent mean ± S.E.M. *P < 0.05 vs. O or F, Student paired t‐test.
Acknowledgements
The authors wish to thank Anne Fayoux and Stéphane http://www.inb.u-bordeaux2.fr/dev/FR2/ita.php?ita=St%C3%A9phane%20Lelgouach&id=145 (University of Bordeaux) for their valuable assistance with animal care. This study was supported by the Institut de Recherche Servier (IDRS), the Fondation pour la Recherche Médicale (FRM grant no. DPA20140629794 to A.C.), the University of Bordeaux and the Centre National de la Recherche Scientifique (CNRS), France. Funding sources had no involvement in the conduct of the research and/or the preparation of the manuscript.
Morisot, N. , Monier, R. , Le Moine, C. , Millan, M. J. , and Contarino, A. (2018) Corticotropin‐releasing factor receptor 2‐deficiency eliminates social behaviour deficits and vulnerability induced by cocaine. British Journal of Pharmacology, 175: 1504–1518. doi: 10.1111/bph.14159.
References
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM‐V) edn. American Psychiatric Association: Washington, DC. [Google Scholar]
- Arima H, Aguilera G (2000). Vasopressin and oxytocin neurones of hypothalamic supraoptic and paraventricular nuclei co‐express mRNA for type‐1 and type‐2 corticotropin‐releasing hormone receptors. J Neuroendocrinol 12: 833–842. [DOI] [PubMed] [Google Scholar]
- Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE et al (2000). Mice deficient for corticotropin‐releasing hormone receptor‐2 display anxiety‐like behaviour and are hypersensitive to stress. Nat Genet 24: 410–414. [DOI] [PubMed] [Google Scholar]
- Baracz SJ, Parker LM, Suraev AS, Everett NA, Goodchild AK, McGregor IS et al (2016). Chronic methamphetamine self‐administration dysregulates oxytocin plasma levels and oxytocin receptor fibre density in the Nucleus accumbens core and subthalamic nucleus of the rat. J Neuroendocrinol 28 https://doi.org/10.1111/jne.12337. [DOI] [PubMed] [Google Scholar]
- Basso AM, Spina M, Rivier J, Vale W, Koob GF (1999). Corticotropin‐releasing factor antagonist attenuates the “anxiogenic‐like” effect in the defensive burying paradigm but not in the elevated plus‐maze following chronic cocaine in rats. Psychopharmacology (Berl) 145: 21–30. [DOI] [PubMed] [Google Scholar]
- Blatchford KE, Diamond K, Westbrook RF, McNally GP (2005). Increased vulnerability to stress following opiate exposures: behavioral and autonomic correlates. Behav Neurosci 119: 1034–1041. [DOI] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ, Navarro M (2005). Prior multiple ethanol withdrawals enhance stress‐induced anxiety‐like behavior: inhibition by CRF1‐ and benzodiazepine‐receptor antagonists and a 5‐HT1a‐receptor agonist. Neuropsychopharmacology 30: 1662–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulbul M, Babygirija R, Cerjak D, Yoshimoto S, Ludwig K, Takahashi T (2011). Hypothalamic oxytocin attenuates CRF expression via GABAA receptors in rats. Brain Res 1387: 39–45. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deussing JM, Breu J, Kuhne C, Kallnik M, Bunck M, Glasl L et al (2010). Urocortin 3 modulates social discrimination abilities via corticotropin‐releasing hormone receptor type 2. J Neurosci 30: 9103–9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, Guptaa T, Cardillo S, Cho M, Carter CS (2002). Corticotropin‐releasing factor induces social preferences in male prairie voles. Psychoneuroendocrinology 27: 705–714. [DOI] [PubMed] [Google Scholar]
- Georgiou P, Zanos P, Garcia‐Carmona JA, Hourani S, Kitchen I, Laorden ML et al (2016a). Methamphetamine abstinence induces changes in μ‐opioid receptor, oxytocin and CRF systems: association with an anxiogenic phenotype. Neuropharmacology 105: 520–532. [DOI] [PubMed] [Google Scholar]
- Georgiou P, Zanos P, Hourani S, Kitchen I, Bailey A (2016b). Cocaine abstinence induces emotional impairment and brain region‐specific upregulation of the oxytocin receptor binding. Eur J Neurosci 44: 2446–2454. [DOI] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM (2003). International Union of Pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotropin‐releasing factor and their ligands. Pharmacol Rev 55: 21–26. [DOI] [PubMed] [Google Scholar]
- Herman JP, Tasker JG (2016). Paraventricular hypothalamic mechanisms of chronic stress adaptation. Front Endocrinol (Lausanne) 7: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler CM, Ryabinin AE (2013). The CRF system and social behavior: a review. Front Neurosci 7: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingallinesi M, Rouibi K, Le Moine C, Papaleo F, Contarino A (2012). CRF2 receptor‐deficiency eliminates opiate withdrawal distress without impairing stress coping. Mol Psychiatry 17: 1283–1294. [DOI] [PubMed] [Google Scholar]
- Jurek B, Slattery DA, Hiraoka Y, Liu Y, Nishimori K, Aguilera G et al (2015). Oxytocin regulates stress‐induced Crf gene transcription through CREB‐regulated transcription coactivator 3. J Neurosci 35: 12248–12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara M, Groenink L, Kas MJ, Bijlsma EY, Olivier B, Sarnyai Z (2011). Influence of transgenic corticotropin‐releasing factor (CRF) over‐expression on social recognition memory in mice. Behav Brain Res 218: 357–362. [DOI] [PubMed] [Google Scholar]
- Kazdoba TM, Hagerman RJ, Zolkowska D, Rogawski MA, Crawley JN (2016). Evaluation of the neuroactive steroid ganaxolone on social and repetitive behaviors in the BTBR mouse model of autism. Psychopharmacology (Berl) 233: 309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T, Radulovic J, Radulovic M, Lin CR, Schrick C, Hooshmand F et al (2000). Deletion of crhr2 reveals an anxiolytic role for corticotropin‐releasing hormone receptor‐2. Nat Genet 24: 415–419. [DOI] [PubMed] [Google Scholar]
- Koob GF (2008). A role for brain stress systems in addiction. Neuron 59: 11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong KC, Freeman LR, Berini CR, Ghee SM, See RE, Reichel CM (2017). Oxytocin reduces cocaine cued Fos activation in a regionally specific manner. Int J Neuropsychopharmacol 20: 844–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall C, Singer T (2012). The animal and human neuroendocrinology of social cognition, motivation and behavior. Nat Neurosci 15: 681–688. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Bales KL (2013). Towards improved animal models for evaluating social cognition and its disruption in schizophrenia: the CNTRICS initiative. Neurosci Biobehav Rev 37: 2166–2180. [DOI] [PubMed] [Google Scholar]
- Morisot N, Contarino A (2016). The CRF1 and the CRF2 receptor mediate recognition memory deficits and vulnerability induced by opiate withdrawal. Neuropharmacology 105: 500–507. [DOI] [PubMed] [Google Scholar]
- Morisot N, Le Moine C, Millan MJ, Contarino A (2014). CRF2 receptor‐deficiency reduces recognition memory deficits and vulnerability to stress induced by cocaine withdrawal. Int J Neuropsychopharmacol 17: 1969–1979. [DOI] [PubMed] [Google Scholar]
- Morisot N, Rouibi K, Contarino A (2015). CRF2 receptor deficiency eliminates the long‐lasting vulnerability of motivational states induced by opiate withdrawal. Neuropsychopharmacology 40: 1990–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nonneman RJ, Shafer GO, Nikolova VD, Riddick NV, Agster KL et al (2013). Disruption of social approach by MK‐801, amphetamine, and fluoxetine in adolescent C57BL/6J mice. Neurotoxicol Teratol 36: 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R (2012). Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci 35: 649–659. [DOI] [PubMed] [Google Scholar]
- Noel X, Brevers D, Bechara A (2013). A neurocognitive approach to understanding the neurobiology of addiction. Curr Opin Neurobiol 23: 632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, Ghozland S, Ingallinesi M, Roberts AJ, Koob GF, Contarino A (2008). Disruption of the CRF2 receptor pathway decreases the somatic expression of opiate withdrawal. Neuropsychopharmacology 33: 2878–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, Kitchener P, Contarino A (2007). Disruption of the CRF/CRF1 receptor stress system exacerbates the somatic signs of opiate withdrawal. Neuron 53: 577–589. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Scordalakes EM, Young LJ, Rissman EF (2003). Oxytocin, but not oxytocin receptor, is regulated by oestrogen receptor beta in the female mouse hypothalamus. J Neuroendocrinol 15: 787–793. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ (2001). The Mouse Brain in Stereotaxic Coordinates. Academic Press: San Diego. [Google Scholar]
- Preller KH, Herdener M, Schilbach L, Stampfli P, Hulka LM, Vonmoos M et al (2014). Functional changes of the reward system underlie blunted response to social gaze in cocaine users. Proc Natl Acad Sci U S A 111: 2842–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Epstein DH (2011). Stress in the daily lives of cocaine and heroin users: relationship to mood, craving, relapse triggers, and cocaine use. Psychopharmacology (Berl) 218: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter RM, Weiss F (1999). In vivo CRF release in rat amygdala is increased during cocaine withdrawal in self‐administering rats. Synapse 32: 254–261. [DOI] [PubMed] [Google Scholar]
- Rouibi K, Contarino A (2013). The corticotropin‐releasing factor receptor‐2 mediates the motivational effect of opiate withdrawal. Neuropharmacology 73: 41–47. [DOI] [PubMed] [Google Scholar]
- Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V et al (2011). Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol Psychiatry 69: 875–882. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G (1995). Brain corticotropin‐releasing factor mediates ‘anxiety‐like’ behavior induced by cocaine withdrawal in rats. Brain Res 675: 89–97. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Vecsernyes M, Laczi F, Biro E, Szabo G, Kovacs GL (1992). Effects of cocaine on the contents of neurohypophyseal hormones in the plasma and in different brain structures in rats. Neuropeptides 23: 27–31. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J (2003). The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 168: 3–20. [DOI] [PubMed] [Google Scholar]
- Shemesh Y, Forkosh O, Mahn M, Anpilov S, Sztainberg Y, Manashirov S et al (2016). Ucn3 and CRF‐R2 in the medial amygdala regulate complex social dynamics. Nat Neurosci 19: 1489–1496. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Pride MC, Hayes JE, Puhger KR, Butler‐Struben HM, Baker S et al (2015). GABAB receptor agonist R‐baclofen reverses social deficits and reduces repetitive behavior in two mouse models of autism. Neuropsychopharmacology 40: 2228–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN (2010). Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci 11: 490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R (2001). How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 158: 343–359. [DOI] [PubMed] [Google Scholar]
- Stoop R (2012). Neuromodulation by oxytocin and vasopressin. Neuron 76: 142–159. [DOI] [PubMed] [Google Scholar]
- Vacher CM, Fretier P, Creminon C, Calas A, Hardin‐Pouzet H (2002). Activation by serotonin and noradrenaline of vasopressin and oxytocin expression in the mouse paraventricular and supraoptic nuclei. J Neurosci 22: 1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C et al (2000). Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428: 191–212. [DOI] [PubMed] [Google Scholar]
- van Ree JM, Burbach‐Bloemarts EM, Wallace M (1988). Vasopressin neuropeptides and acquisition of heroin and cocaine self‐administration in rats. Life Sci 42: 1091–1099. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, Goldstein RZ (2011). Addiction: pulling at the neural threads of social behaviors. Neuron 69: 599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M (1998). Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: new insights into the secretory capacities of peptidergic neurons. Neuroscience 85: 1209–1222. [DOI] [PubMed] [Google Scholar]
- Yan Y, Wang Y‐L, Su Z, Zhang Y, Guo S‐X, Liu A‐J et al (2014). Effect of oxytocin on the behavioral activity in the behavioral despair depression rat model. Neuropeptides 48: 83–89. [DOI] [PubMed] [Google Scholar]
- Yang J, Pan Y‐J, Yin Z‐K, Hai G‐F, Lu L, Zhao Y et al (2012). Effect of arginine vasopressin on the behavioral activity in the behavior despair depression rat model. Neuropeptides 46: 141–149. [DOI] [PubMed] [Google Scholar]
- Zanos P, Georgiou P, Metaxas A, Kitchen I, Winsky‐Sommerer R, Bailey A (2015). Region‐specific up‐regulation of oxytocin receptor binding in the brain of mice following chronic nicotine administration. Neurosci Lett 600: 33–37. [DOI] [PubMed] [Google Scholar]
- Zanos P, Georgiou P, Wright SR, Hourani SM, Kitchen I, Winsky‐Sommerer R et al (2014). The oxytocin analogue carbetocin prevents emotional impairment and stress‐induced reinstatement of opioid‐seeking in morphine‐abstinent mice. Neuropsychopharmacology 39: 855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Litvin Y, Piras AP, Pfaff DW, Kreek MJ (2011). Persistent increase in hypothalamic arginine vasopressin gene expression during protracted withdrawal from chronic escalating‐dose cocaine in rodents. Neuropsychopharmacology 36: 2062–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F (2001). Changes in levels of regional CRF‐like‐immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 158: 374–381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Statistical analysis of sociability and social novelty preference (SNP) displayed by drug‐naïve wild‐type and CRF2−/− mice. Significant Ps mean higher exploration of the unfamiliar mouse vs. the object or the novel vs. the familiar mouse during the sociability or the SNP test, respectively. ITI: inter‐trial time interval between the sociability and the SNP test. N = 32–34/genotype.
Table S2 Statistical analysis of sociability and social novelty preference (SNP) displayed by wild‐type and CRF2−/− mice immediately (0), 7 or 14 days after the last saline or cocaine administration. Significant Ps mean higher or lower (*) exploration of the unfamiliar mouse vs. the object or the novel vs. the familiar mouse during the sociability or the SNP test, respectively. N = 15–17/group (see Figure 3 and Figure 4 for detailed group size). SAL: saline. COC: cocaine.
Table S3 Statistical analysis of sociability and social novelty preference (SNP) displayed by saline‐ or cocaine‐treated wild‐type and CRF2−/− mice 40 or 42 days after the last administration. On cocaine withdrawal day 40 or 42, mice were exposed (Stress) or not (No stress) to the elevated platform stressor (EPS), using a within‐subject experimental design. Significant Ps mean higher exploration of the unfamiliar mouse vs. the object or the novel vs. the familiar mouse during the sociability or the SNP test, respectively. N = 14–17/group (see Figure 5 for detailed group size). SAL: saline. COC: cocaine.
Figure S1 CRF2 receptor‐deficiency does not alter sociability or social memory span. Time (s) spent exploring the wire cages containing an object (O) or an unfamiliar mouse (U) during the (A) sociability test or a familiar (F) or a novel (N) mouse during the (B) social novelty preference (SNP) test by wild‐type and CRF2−/− mice repeatedly tested (tests 1 to 3) once a week in the 3‐chamber task. A 0, 2 or 4 h inter‐trial time interval (ITI) was applied between the sociability and the SNP test. Value represent mean ± S.E.M. *** P < 0.0005 vs. O or F, Student paired t‐test.
Figure S2 CRF2 receptor‐deficiency does not affect cocaine‐induced body weight loss. Body weight change, calculated as percentage of body weight recorded just prior (P) to the first injection, displayed by saline (SAL)‐ or cocaine (COC)‐ treated wild‐type and CRF2−/− mice. Represented is the body weight change as measured 12 h after the second injection of the cocaine dose indicated. Values represent mean ± S.E.M ### P < 0.0005 vs. SAL mice, genotype‐independent effect.
Figure S3 CRF2 receptor‐deficiency eliminates sociability deficits induced by cocaine withdrawal. Time (s) spent exploring the wire cages containing an object (O) or un unfamiliar mouse (U) during the (A) sociability test or a familiar (F) or a novel (N) mouse during the (B) social novelty preference (SNP) test by saline (SAL)‐ or cocaine (COC)‐treated wild‐type and CRF2−/− mice. The 3‐chamber tests were carried out immediately (0), 7 and 14 days after the last saline or cocaine administration. N of each experimental group is reported under the X axis. Values represent mean ± S.E.M. *P < 0.05 vs. O or F, Student paired t‐test.
Figure S4 CRF2 receptor‐deficiency abolishes the long‐lasting social behavior vulnerability to stress induced by cocaine withdrawal. Time (s) spent exploring the wire cages containing an object (O) or un unfamiliar mouse (U) during the (A) sociability test or a familiar (F) or a novel (N) mouse during the (B) social novelty preference (SNP) test by wild‐type or CRF2−/− mice exposed (Stress) or not (No Stress) to the elevated platform stressor (EPS). The 3‐chamber tests were carried out 40 or 42 days after the last administration of saline (SAL) or cocaine (COC). Moreover, the EPS was applied on cocaine withdrawal day 40 or 42, using a within‐subject experimental design. N of each experimental group is reported under the X axis. Values represent mean ± S.E.M. *P < 0.05 vs. O or F, Student paired t‐test.


