Abstract
We show that phytochromes modulate differentially various facets of light-induced ripening of tomato fruit (Solanum lycopersicum L.). Northern analysis demonstrated that phytochrome A mRNA in fruit accumulates 11.4-fold during ripening. Spectroradiometric measurement of pericarp tissues revealed that the red to far-red ratio increases 4-fold in pericarp tissues during ripening from the immature-green to the red-ripe stage. Brief red-light treatment of harvested mature-green fruit stimulated lycopene accumulation 2.3-fold during fruit development. This red-light-induced lycopene accumulation was reversed by subsequent treatment with far-red light, establishing that light-induced accumulation of lycopene in tomato is regulated by fruit-localized phytochromes. Red-light and red-light/far-red-light treatments during ripening did not influence ethylene production, indicating that the biosynthesis of this ripening hormone in these tissues is not regulated by fruit-localized phytochromes. Compression analysis of fruit treated with red light or red/far-red light indicated that phytochromes do not regulate the rate or extent of pericarp softening during ripening. Moreover, treatments with red or red/far-red light did not alter the concentrations of citrate, malate, fructose, glucose, or sucrose in fruit. These results are consistent with two conclusions: (a) fruit-localized phytochromes regulate light-induced lycopene accumulation independently of ethylene biosynthesis; and (b) fruit-localized phytochromes are not global regulators of ripening, but instead regulate one or more specific components of this developmental process.
Because photosynthetic organisms are dependent on photosynthetically active radiation as their source of energy, plant growth and development are intimately tied to changes in the light environment. Three classes of photoreceptors mediate light-induced development in response to light quantity, quality, directionality, and photoperiodicity. Phytochromes, the most thoroughly characterized class of plant photoreceptors, are chromoproteins that detect both red and far-red light. Individual plants contain several types of phytochromes, each of which is encoded by a distinct gene (Sharrock and Quail, 1989; Hauser et al., 1995). These red and far-red light receptors mediate numerous developmental processes throughout the plant's life cycle, including seed germination, de-etiolation, chloroplast development, stem elongation, anthocyanin biosynthesis, and photoperiodic flowering (Kendrick and Kronenberg, 1994).
Although attempts to define the role(s) of fruit-localized phytochrome in tomato (Solanum lycopersicum L.) ripening have been limited, existing evidence is consistent with the hypothesis that phytochromes play some regulatory role in this developmental process. An early study indicated that fruit-localized phytochromes regulate the accumulation of a “flavonoid-like” pigment in pericarp tissues (Piringer and Heinze, 1954). Subsequently it was reported that fruit-localized phytochromes mediate light-induced carotenoid biosynthesis in tomato (Khudairi and Arboleda, 1971; Thomas and Jen, 1975). A fourth investigation led to the conclusion that phytochrome-regulated carotenoid biosynthesis is correlated with red-light-induced ethylene production in tomato fruits, implying that fruit-localized phytochromes play a global regulatory role during the ripening of tomato fruit (Jen and Watada, 1977). Unfortunately, Jen and Watada (1977) used excessive irradiation treatments (14 h of high-fluence-rate red light) and neglected to address the far-red reversibility of red-light-induced ethylene production. Their conclusion that phytochromes regulate ethylene production in tomato fruits is thus only weakly supported at best.
These studies, as well as others that indicate lycopene consumption decreases the incidence of prostate cancer in men (Giovannucci et al., 1995), raise a number of important questions about the exact role(s) of fruit-localized phytochrome in tomato ripening. Does the expression of PHY loci in tomato fruit correlate with fruit ripening? Do fruit-localized phytochromes regulate lycopene accumulation during tomato ripening? Do fruit-localized phytochromes regulate ethylene biosynthesis during tomato ripening? Do fruit-localized phytochromes regulate other important components of fruit ripening, such as fruit softening or the accumulation of flavor components such as citrate, malate, Fru, Glc, and Suc? Results of experiments reported here help provide answers to these questions.
RESULTS
Differential Expression of PHYA in Tomato Fruit
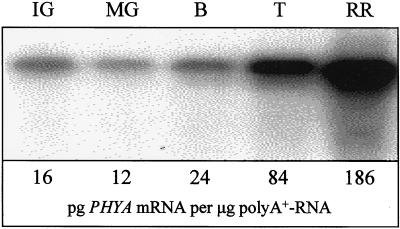
Although the expression of all five PHY loci (i.e. PHYA, PHYB1, PHYB2, PHYE, and PHYF) in tomato fruit was investigated, substantial differential expression during ripening was observed only for the PHYA locus (Fig. 1). An increase in the accumulation of PHYA mRNA was first detectable in breaker-stage fruit and continued throughout the ripening process (breaker, turning, and red-ripe stage fruit).
Figure 1.
PHYA mRNA abundance increases in tomato fruit during ripening. Fruit (cv UC-82B) at five different developmental stages were harvested on the same day from plants in the greenhouse. mRNA was isolated from these fruit, separated electrophoretically, and blotted to nylon. RNA blots were subsequently hybridized with a 32P-labeled probe derived from a conserved region of PHYA, washed at high stringency, and exposed to a phosphor plate. Images were developed using a phosphor imager (Molecular Dynamics, Sunnyvale, CA). The lower panel shows the absolute abundance of PHYA mRNA. IG, Immature-green; MG, mature-green; B, breaker; T, turning/orange; RR, red-ripe.
The Red to Far-Red Light Ratio Increases in Pericarp Tissue during Ripening
Between 360 and 760 nm, as much as 1.4% of midday solar radiation passes through the epidermis and outer pericarp of ripening cv UC-82B fruit (data not shown). During the transition from the immature-green to the turning stage, the amount of red light that passed through these tissues increased 4-fold, while the amount of far-red light changed very little (Table I). During the transition from the turning stage to the red-ripe stage there was little if any change in the transmission of either red or far-red light. These changes in red and far-red light transmission thus lead to substantial changes in the red to far-red light ratio within the tissues of tomato fruits during ripening (Table I).
Table I.
The red- (R) to far-red-light (FR)a ratio inside tomato (cv UC-82B) pericarp increases during ripening
| Light Quality | Full Sunb | Stage of Tomato Fruit Development
|
||
|---|---|---|---|---|
| Immature-green | Turning | Red-ripe | ||
| Rc | 1.884 | 0.005 | 0.020 | 0.023 |
| FRd | 1.721 | 0.017 | 0.022 | 0.021 |
| R/FR ratio | 1.095 | 0.295 | 0.932 | 1.100 |
Values (μmol m−2 s−1 nm−1) for R and FR were obtained using a spectroradiometer under full sun. Nearly identical results were obtained in two independent experiments. Fruits were ripened on plants in the greenhouse and harvested on the same day at different stages of development.
As defined by Smith, 1982.
Athens, GA; August 1997, between noon and 1 pm.
Mean fluence rate (μmol m−2 s−1 nm−1; 665–675 nm).
Mean fluence rate (μmol m−2 s−1 nm−1; 725–735 nm).
Phytochromes Regulate Lycopene Accumulation in Tomato Pericarp during Ripening
Brief red-light treatments increased carotenoid accumulation in fruit from cv UC-82B, cv MoneyMaker, cv Mountain Pride, and cv Sweet 100 (Fig. 2, P < 0.001; data not shown). Red-light-induced carotenoid accumulation in all four of these cultivars was reversible by far-red light (Fig. 2, P < 0.001; data not shown), which is consistent with the suggestion that light-induced carotenoid accumulation in tomato fruits is mediated in part by phytochrome (Khudairi and Arboleda, 1971).
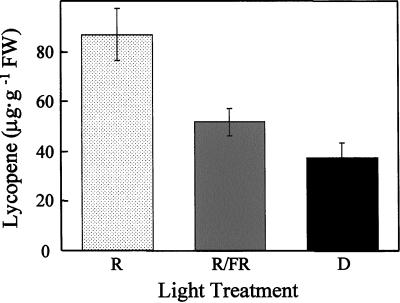
Figure 2.
Fruit-localized phytochromes mediate light-induced lycopene biosynthesis in fruit pericarp. Mature-green fruit (cv UC-82B) were harvested 45 DPA and, except for the indicated treatments, fully ripened in the dark (23°C, 80% relative humidity). After harvest, fruit received one of three light treatments daily: R, 5 min of red light followed immediately by a mock treatment of 15 min of far-red light (fruits were placed under the far-red-light source for 15 min without irradiation); R/FR, 5 min of red light followed immediately by 15 min of far-red light; D, mock treatment of 5 min of red light (fruits were placed under the red-light source for 5 min without irradiation) followed immediately by a mock treatment of 15 min of far-red light. Carotenoids were extracted from red-ripe fruit and quantified via RP-HPLC. se bars are shown (n = 10).
Consistent with the results of Fraser et al. (1994), RP-HPLC analysis of carotenoid extracts from red-ripe tomatoes indicated that greater than 90% of the pigment in these tissues is the red carotenoid lycopene (data not shown). Furthermore, compared with the dark control treatment (37.2 μg lycopene g−1 fresh weight; n = 10), lycopene accumulation in red-ripe fruit was induced 2.3-fold by red-light (86.6 μg lycopene g−1 fresh weight; P < 0.001, n = 10) and was reversible by far-red light (51.5 μg lycopene g−1 fresh weight; P < 0.01, n = 10). These data demonstrate that the accumulation of lycopene in these tissues is under the control of fruit-localized phytochrome (Fig. 2).
Ethylene Biosynthesis, Pericarp Softening, and the Accumulation of Citrate, Malate, Fru, Glc, and Suc during Ripening Are Not Regulated by Fruit-Localized Phytochromes
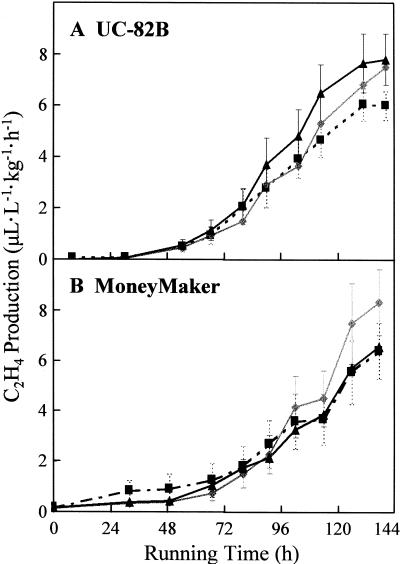
Compared with the dark control treatment (8.1 μL L−1 kg−1 h−1; n = 16), maximal rates of ethylene biosynthesis by ripening fruit (cv UC-82B) were not altered by treatment with red light (6.1 μL L−1 kg−1 h−1; P > 0.148, n = 16) or red/far-red light (7.5 μL L−1 kg−1 h−1; P > 0.563, n = 16; Fig. 3A). Furthermore, Figure 3A also indicates that red and red/far-red-light treatments did not influence the time of initiation of ethylene production or the rate at which ethylene production changes over time. Nearly identical results were observed with fruit from cv Money- Maker (Fig. 3B).
Figure 3.
Fruit-localized phytochromes do not regulate ethylene biosynthesis in cv UC-82B or cv MoneyMaker fruit. Mature-green fruit were harvested 45 (cv UC-82B) or 39 (cv MoneyMaker) DPA and, except for the indicated treatments, fully ripened in the absence of light (23°C, 80% relative humidity). During ripening, fruit received one of three light treatments daily: R (black-dashed line, ▪), 5 min of red light followed immediately by a mock treatment of 15 min of far-red light (fruits were placed under the far-red-light source for 15 min without irradiation); R/FR (gray line, ♦), 5 min of red light followed immediately by 15 min of far-red light; D (black line, ▴), mock treatment of 5 min of red light (fruits were placed under the red-light source for 5 min without irradiation) followed immediately by a mock treatment of 15 min of far-red light. Ethylene production was assayed approximately every 12 h and quantified by GC. se bars are shown (n = 16). A, cv UC-82B. B, cv MoneyMaker.
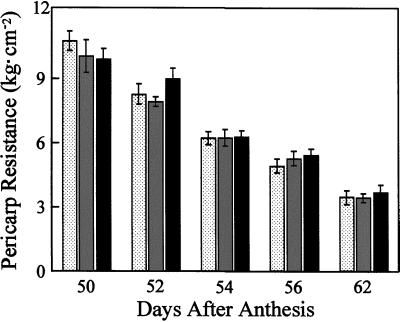
To determine if fruit-localized phytochrome regulate fruit softening, the effect of red or red/far-red-light treatments on pericarp firmness was investigated. No statistically significant differences between the red and the red/far-red-light treatments were observed at any point during the transition from the mature-green stage (Fig. 4, 50 DPA) to the red-ripe stage (Fig. 4, 62 DPA). These data imply that pericarp softening, and by extrapolation the extent of fruit softening, is not regulated by fruit-localized phytochromes.
Figure 4.
Fruit-localized phytochromes do not regulate fruit softening. Mature-green fruit (cv UC-82B) were harvested 45 DPA and, except for the indicated treatments, ripened in the absence of light (23°C, 80% relative humidity). During ripening, fruit received one of three light treatments daily: R (stippled bars), 5 min of red light followed immediately by a mock treatment of 15 min of far-red light (fruits were placed under the far-red-light source for 15 min without irradiation); R/FR (gray bars), 5 min of red light followed immediately by 15 min of far-red light; D (black bars), mock treatment of 5 min of red light (fruits were placed under the red-light source for 5 min without irradiation) followed immediately by a mock treatment of 15 min of far-red light. Fruit were analyzed for pericarp resistance at various stages during ripening. After removal of the epidermal tissue, pericarp resistance was measured using a penetrometer attached to a motorized drill press. Mature-green fruit have an average pericarp resistance of 11.9 ± 0.82 kg cm−2 (data not shown). se bars are shown (n = 8–16).
To determine if fruit-localized phytochromes regulate other biochemical components of tomato development, the effect of red and red/far-red-light treatments on the accumulation of citrate, malate, Fru, Glc, and Suc was investigated. Table II indicates that these treatments had no detectable effect on the concentrations of soluble citrate, malate, Fru, Glc, or Suc extracted from red-ripe fruit tissues.
Table II.
Accumulation of citrate, malate, Fru, Glc, and Suc in tomato fruit is not regulated by fruit-localized phy
| Flavor Component | Light Treatment
|
||
|---|---|---|---|
| Ra | R/FRb | Darkc | |
| Citrate | 0.79 ± 0.05 | 0.85 ± 0.10 | 0.73 ± 0.07 |
| Malate | 0.16 ± 0.01 | 0.20 ± 0.02 | 0.17 ± 0.01 |
| Fru | 4.35 ± 0.20 | 4.27 ± 0.23 | 4.42 ± 0.13 |
| Glc | 3.61 ± 0.20 | 3.72 ± 0.31 | 3.61 ± 0.14 |
| Suc | 0.06 ± 0.00 | 0.07 ± 0.01 | 0.05 ± 0.02 |
Entries (μg g−1 fresh wt) are means ± se of four replicates.
Fruits were treated daily with 5 min of red light (R) followed immediately by a mock treatment with 15 min of far-red light (FR) (see “Materials and Methods”).
Fruits were treated daily with 5-min R followed immediately by 15-min FR.
Fruits were treated daily with a mock treatment of 5-min R followed immediately by a mock treatment with 15-min FR (see “Materials and Methods”).
DISCUSSION
Fruit-Localized Phytochromes Regulate Lycopene Accumulation in Tomato Fruit
Brief red-light treatments of harvested mature-green fruits stimulated carotenogenesis in cv UC-82B, cv MoneyMaker, cv Mountain Pride, and cv Sweet 100 fruit (Fig. 2; data not shown). The fact that red-light-induced pigmentation was reversible by far-red light in all four of these cultivars (Fig. 2; data not shown) confirms the hypothesis of Khudairi (1972) that phytochromes mediate light-induced pigmentation of tomato fruit, and is consistent with the recent discovery that the hp-2 mutant of tomato encodes a tomato homolog of the Det1 protein (Mustilli et al., 1999), which is an important component of phytochrome transduction chains. Moreover, because these experiments were conducted with detached fruit, it can be concluded that these phytochromes must exist within the fruits.
Red and far-red light penetrate the epidermis and pericarp of immature-green, turning, and red-ripe stage cv UC-82B fruit (Table I). Furthermore, during the transition from the immature-green stage to the red-ripe stage, the red to far-red light ratio (as defined by Smith, 1982) increased 3.7-fold inside these tissues (Table I). This increase inside tomato pericarp is due primarily to the degradation of chlorophyll, which preferentially absorbs red light. It is logical to assume that the increase in the red to far-red light ratio within these tissues leads to a concomitant shift in the photoequilibrium of fruit-localized phytochrome from Pr toward Pfr. Thus, it is not surprising that phytochrome-regulated components of this developmental process have arisen during the course of tomato evolution. Considering the fact that phytochromes regulate the accumulation of a variety of plant pigments (including carotenoids) in a variety of plant tissues (Kendrick and Kronenberg, 1994), it is also not surprising that fruit-localized phytochromes regulate the light-induced accumulation of lycopene in tomato fruit during ripening. While the lycopene measured here was extracted from epidermal and pericarp tissues, nothing is known about the tissue-specific distribution of phytochrome within tomato fruit. Consequently, it is impossible to determine whether the fruit-localized phytochrome that influences lycopene accumulation does so intracellularly or intercellularly.
PHYA mRNA accumulates 11.4-fold in cv UC-82B fruit during ripening (Fig. 1). Interestingly, this accumulation of PHYA mRNA is first detectable in fruit initiating carotenogenesis (breaker-stage fruit) and continues throughout the process of lycopene accumulation (breaker, turning, and red-ripe fruit). Thus, not only do these results demonstrate that the accumulation of PHYA mRNA increases significantly during ripening, but Figure 1 also indicates that the abundance of PHYA mRNA is correlated with the later stages of fruit development, perhaps with the initiation of light-induced carotenogenesis, and with the accumulation of lycopene. Despite these apparent correlations, it is not known if this increase in PHYA mRNA during ripening leads to an equivalent increase in functional phyA photoreceptors. Nor does convincing evidence exist to indicate that phyA regulates light-induced lycopene accumulation in these fruit. Preliminary results obtained in our laboratory (data not shown) indicate, however, that neither red nor red/far-red-light treatments influence the pigmentation of fruit obtained from a phyA− mutant (cv MoneyMaker; Lazarova et al., 1998a). These initial data are consistent with the hypothesis that fruit-localized phyA participates in light-induced lycopene accumulation in tomato fruit. Also consistent with this hypothesis is the recent report that phyA regulates far-red-light-dependent carotenoid accumulation in Arabidopsis, and that this regulation occurs at the level of phytoene synthase expression (Von Lintig et al., 1997).
This hypothesis differs from that of Hauser et al. (1997), who reported that PHYB2 and PHYF were preferentially expressed in tomato fruit compared with a variety of other organs, and hypothesized that one or both might therefore play a role in light-induced ripening events. Despite the difference in these two hypotheses, it is important to note that the data presented here do not contradict those of Hauser et al. (1997). The latter clearly document that although PHYA is not preferentially expressed in fruits, PHYA transcripts are in greater abundance in fruits than are transcripts from all other PHY, including PHYB2 and PHYF. Taken together, the data of Hauser et al. (1997) and those presented here raise the possibility that pigmentation of these fruit (and possibly other processes of fruit ripening) might be influenced by multiple phytochromes, perhaps in response to different illumination conditions. Future investigations with the available phyA (Lazarova et al., 1998a), phyB1 (Lazarova et al., 1998b), and phyB2 (Kerckhoffs et al., 1999) mutants should provide insights into the specific roles of these three phytochromes in tomato ripening.
The results presented here also provide new insights into the nature of phytochrome-regulated carotenogenesis in tomato fruit. To characterize the biochemical basis for red-light-induced carotenogenesis in these tissues, HPLC analysis was conducted on carotenoid extracts from red-light-, red/far-red-light-, and dark-treated fruit. Consistent with Fraser et al. (1994), our data demonstrate that greater than 90% of the carotenoids extracted from red-ripe fruit tissues are the red pigment lycopene (data not shown). In addition, brief red-light treatments of harvested mature-green fruit resulted in a 2.3-fold increase in the accumulation of lycopene in pericarp tissues (Fig. 2). Furthermore, red-light-induced lycopene accumulation was reversible by far-red light (Fig. 2). Collectively, these results indicate that the fruit color differences observed after white-light, red-light, red/far-red-light, and dark treatments (Boe and Salunkhe, 1967; Shewfelt and Halpin, 1967; Khudairi, 1972) are due primarily to phytochrome-regulated lycopene accumulation. Currently, it is not known whether light (via phytochrome photoreceptors) stimulates lycopene biosynthesis, inhibits lycopene degradation, or both.
Assuming that fruit-localized phytochromes do not regulate lycopene accumulation prior to the mature-green stage, our results also demonstrate that lycopene accumulation in tomato pericarp consists of light-independent and -dependent components. In other words, Figure 2 implies that light is not essential for the induction or accumulation of lycopene in tomato pericarp, at least after the immature-green stage. If light were necessary for the initiation and accumulation of this carotenoid in these tissues, lycopene would not accumulate in fruits receiving the dark treatment (see Fig. 2, D). Thus, it appears that fruit-localized phytochromes regulate the extent of lycopene accumulation and therefore the extent of fruit color development in tomato. Considering new evidence for a correlation between lycopene consumption and reduced rates of prostate cancer in adult males (Giovannucci et al., 1995), this observation is particularly relevant to the U.S. tomato industry, which typically conducts post-harvest ripening in the dark.
Fruit-Localized Phytochromes Are Not Global Regulators of Tomato Ripening
The work presented here demonstrates that the initiation, rate, or duration of ethylene biosynthesis during ripening of cv UC-82B and cv MoneyMaker fruit is influenced by red- or far-red-light treatments (Fig. 3). Contrary to the conclusions of Jen and Watada (1977), our results indicate that fruit-localized phytochromes do not regulate ethylene biosynthesis in ripening tomatoes, and therefore must regulate lycopene accumulation independently of ethylene biosynthesis, probably at a site downstream of the latter. One possible explanation for the discrepancy between the results presented here and those of Jen and Watada (1977) stems from differences in experimental irradiation conditions (see “Materials and Methods”). Jen and Watada irradiated fruit with red light for 14 h d−1, whereas the experiments presented here utilize red light for 5 min d−1. Perhaps red-light-induced ethylene biosynthesis is a high-irradiance response, and therefore does not occur after the low-irradiance red-light treatment used in these experiments. Alternatively, with 14 h d−1 of irradiation, contaminating light from other regions of the spectrum might have been responsible for their observations.
Even though fruit-localized phytochromes do not modify ethylene biosynthesis (Fig. 3), and thus do not mediate their effect via enhanced ethylene levels, the latter has been shown to play an important role in ripening-related accumulation of carotenoids. Not only does ethylene regulate phytoene synthase in tomato fruit (Bird et al., 1991), but fruit harboring mutations at a locus (Nr) postulated to encode an ethylene receptor (Yen et al., 1995) exhibit dramatically reduced carotenoid accumulation (Rick and Butler, 1956). It appears, therefore, that both ethylene and fruit-localized phytochromes modulate carotenogenesis in these fruit.
In addition to ethylene biosynthesis, red and red/far-red-light treatments influence neither pericarp softening (Fig. 4) nor the accumulation of citrate, malate, Fru, Glc, and Suc (Table II) in cv UC-82B fruit. These observations indicate that red and red/far-red-light treatments only regulate one or more specific components of tomato fruit development, including lycopene accumulation. Thus, fruit-localized phytochromes are not global regulators of tomato fruit development.
MATERIALS AND METHODS
Plant Materials
Tomato (redesignated Solanum lycopersicum by Spooner et al. [1993]) seeds were purchased from Sunseeds Genetics (Hollister, CA) (cv UC-82B) or were kindly provided by Richard Kendrick (cv MoneyMaker). Plants were cultivated in a greenhouse under supplemental lighting (400 W, sodium halide; 14-h light/10-h dark) using standard horticultural practices.
Tomato flowers were tagged at anthesis, and resultant fruits were allowed to develop synchronously for 39 d (cv MoneyMaker) or 45 d (cv UC-82B). Fruits were then harvested, sorted for uniform color and size, weighed, and treated with far-red light for 30 min. Far-red-light-treated fruits (MG3; Su et al., 1984) were ripened in the absence of light and exogenous ethylene (23°C, 100% relative humidity) until there were no further changes in pigmentation (typically 16 d). External ethylene was eliminated by constant passage of ethylene-free air over fruit during ripening. Ripening fruit were treated daily with one of three light treatments: (a) red-light treatment = 5 min of red light followed immediately by a mock treatment with 15 min of far-red light (fruits were placed under the far-red light source for 15 min without irradiation); (b) red/far-red light treatment = 5 min of red light followed immediately by 15 min of far-red light; or (c) dark treatment = a mock treatment with 5 min of red light (fruits were placed under the red light source for 5 min without irradiation) followed immediately by a mock treatment with 15 min of far-red light. The red and far-red light sources used here were described previously (Boeshore and Pratt, 1980, 1981).
RNA Blots
Poly(A+) RNA was purified from intact tomato fruits, separated electrophoretically, and blotted as described previously (Hauser et al., 1997). The PHY probe templates, methods for transcribing 32P-labeled complementary riboprobes, hybridization conditions, and the procedure for washing radiolabeled nylon membranes are also detailed in Hauser et al. (1997). All five PHY riboprobes (PHYA, PHYB1, PHYB2, PHYE, and PHYF) were hybridized with individual northern blots in independent experiments. Images of washed membranes were obtained with a phosphor imager (model 425F, Molecular Dynamics) and analyzed with ImageQuant software (version 4.2a, Molecular Dynamics). Phosphor screens were exposed for approximately 96 h. Absolute quantification of PHYA transcripts was conducted as described previously (Hauser et al., 1997).
Spectroradiometry
Tomato fruits (cv UC-82B) were ripened on plants in the greenhouse. Immature-green, turning, and red-ripe stage fruits were harvested for analysis on the same day. Equivalent samples of outer pericarp (approximately 2 cm in diameter) were carefully excised from the equatorial region of each fruit with a razor blade, and placed directly above a spectroradiometric detector (Li-Cor, Lincoln, NE) such that all solar radiation reaching the detector passed through the excised pericarp tissue. The quantity and quality of solar radiation that penetrated these pericarp samples was determined under a clear blue sky (in the absence of shadow), between noon and 1 pm in August of 1997. Measurements were conducted on the campus of The University of Georgia (Athens, GA).
Fruit Color
Carotenoids were extracted from pericarp tissue using a modification of a procedure described previously (Bushway, 1986). One gram of tissue was diced with a razor blade and pulverized in a Douce homogenizer with 10 mL of tetrahydrofuran (THF) containing 0.026% (w/w) butylated hydroxy toluene as an anti-oxidant. After carotenoid pigments were extracted completely from the tissue, the extract was passed through a 0.22-μm filter and diluted to 25 mL with THF. A 2.5-mL aliquot of this 25-mL extract was evaporated to dryness (40°C) under N2 and re-suspended in 0.5 mL of THF. To avoid carotenoid oxidation, the entire extraction procedure (except the evaporation step) was conducted on ice and under reduced illumination. If necessary, samples were stored at −20°C under N2 until reverse-phase high-performance liquid chromatography (RP-HPLC) analysis was conducted. Prior to RP-HPLC, samples were again passed through a 0.22-μm filter. Carotenoids were separated on a RP-C18 column (250 × 4.6 mm; Lichrosphere 5, Phenomenex, Torrance, CA) using a 30-min isocratic gradient of acetonitrile:MeOH:THF (58:35:7, v/v) and an HPLC solvent delivery system (model 2800, Bio-Rad Laboratories, Hercules, CA). After RP-HPLC separation, carotenoids were detected at 470 nm with a UV/VIS monitor (model 1706, Bio-Rad Laboratories). Lycopene was identified via co-migration with a tomato lycopene standard (model L-9879, Sigma-Aldrich, St. Louis), which was dissolved in THF prior to injection. Lycopene concentrations were determined by peak integration with the HPLC software (version 2.3, Bio-Rad Laboratories) and comparison with a standard curve of known lycopene concentrations.
Ethylene Production
Ethylene production was assayed during the experimental ripening period by placing individual fruits into sealed mason jars (0.946 L) for 1 h and then withdrawing 1-mL gas samples. Gas samples were collected approximately every 12 h and analyzed via gas chromatography (GC) (model 29 chromatograph, Fisher-Hamilton, Pittsburgh) using an activated alumina column (1.8 m × 12.7 mm) and a flame-ionization detection system. Ethylene was identified via co-migration with an ethylene standard and quantified with reference to a standard curve for ethylene concentration.
Fruit Softening
Fruit softening was assayed by measuring the pericarp resistance of whole tomato fruit after a small region (approximately 10 mm in diameter) of equatorial epidermal tissue was removed with a razor blade. Resistance was determined using a fruit penetrometer (McCormick Fruit Tech, Yakima, WA) fixed to a mechanical press. The plunger head on the penetrometer had a diameter of 3 mm, and the mechanical press was set to travel at 1.5 mm s−1.
Citrate, Malate, Fru, Glc, and Suc Content
Fruits were lyophilized immediately after the treatment period and powdered in a tissue grinder. Citrate, malate, Fru, Glc, and Suc were extracted from 100 mg of powdered tissue with 10 mL of 80% (v/v) MeOH and a mechanical tissue homogenizer (Tekmar-Dohrmann, Cincinnati). Phenyl-β-d-Glc was added to the resultant slurry as an internal standard. The slurry was then clarified by centrifugation, and 20 μL of each extract was subjected to the oxime-trimethyl silyl derivatization procedure described previously (Chapman and Horvat, 1989). Oxime-trimethyl silyl-derivatized citrate, malate, Fru, Glc, and Suc were separated by GC (HP 5890 series II, Hewlett-Packard, Palo Alto, CA) and detected with a flame-ionization detector. The GC column used was a 30-m × 0.32-mm (i.d.) fused silica, DB-5, 0.25-μm film, capillary column (J&W Scientific, Folsom, CA). Injector and detector temperatures were held at 250°C and 300°C, respectively. Helium was used as a carrier gas at a flow rate of 53.1 mL min−1. Air and hydrogen flow rates to the detector were held at 375 mL min−1 and 28 mL min−1, respectively. Nitrogen was used as a makeup gas at a flow rate of 30 mL min−1. The splitless mode was used throughout the analysis. Citrate, malate, Fru, Glc, and Suc were identified via co-migration with pure standards, and their concentrations were determined by peak integration with an integrator (HP 3396 series II, Hewlett-Packard) and comparison with standard curves for each of the pure standards.
ACKNOWLEDGMENTS
We gratefully acknowledge the technical assistance of Betty Schroeder, Carl Valenzano, Mark Zimmerman, and Andy Tull. Constructive discussions with Dr. Stanley J. Kays, Dr. James Giovannoni, and Dr. Hans van Doorn aided in the progress of this research. We also thank Dr. Stanley J. Kays for providing some of the research facilities for this work.
Footnotes
This work was supported by the National Science Foundation (grant no. IBN–9630195) and by a gift from Novartis Seeds (Enkhuizen, The Netherlands).
LITERATURE CITED
- Bird CR, Ray JA, Fletcher JD, Boniwell JM, Bird AS, Teulieres C, Blain I, Bramley PM, Schuch W. Using antisense RNA to study gene function: inhibition of carotenoid biosynthesis in transgenic tomatoes. Biotechnology. 1991;9:635–639. [Google Scholar]
- Boe AA, Salunkhe DK. Ripening tomatoes: ethylene, oxygen, and light treatments. Econ Bot. 1967;21:321–319. [Google Scholar]
- Boeshore ML, Pratt LH. Phytochrome modification and light-enhanced, in vivo-induced phytochrome pelletability. Plant Physiol. 1980;66:500–504. doi: 10.1104/pp.66.3.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeshore ML, Pratt LH. Characterization of a molecular modification of phytochrome that is associated with its conversion to the far-red-absorbing form. Plant Physiol. 1981;68:789–797. doi: 10.1104/pp.68.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushway RJ. Determination of α- and β-carotene in some raw fruits and vegetables by high-performance liquid chromatography. J Agric Food Chem. 1986;34:409–412. [Google Scholar]
- Chapman GW, Horvat RJ. The determination of nonvolatile acids and sugars by capillary GLC and GLC/MS. J Agric Food Chem. 1989;37:947–950. [Google Scholar]
- Fraser PD, Truesdale MR, Bird CR, Schuch W, Bramley PM. Carotenoid biosynthesis during tomato fruit development: evidence for tissue-specific gene expression. Plant Physiol. 1994;105:405–413. doi: 10.1104/pp.105.1.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst. 1995;87:1767–1776. doi: 10.1093/jnci/87.23.1767. [DOI] [PubMed] [Google Scholar]
- Hauser BA, Cordonnier-Pratt M-M, Daniel-Vedele F, Pratt LH. The phytochrome gene family in tomato includes a novel subfamily. Plant Mol Biol. 1995;29:1143–1155. doi: 10.1007/BF00020458. [DOI] [PubMed] [Google Scholar]
- Hauser BA, Pratt LH, Cordonnier-Pratt M-M. Absolute quantification of five phytochrome transcripts in seedlings and mature plants of tomato (Solanum lycopersicum L.) Planta. 1997;201:379–387. doi: 10.1007/s004250050080. [DOI] [PubMed] [Google Scholar]
- Jen JJ, Watada AE. Red light advances respiration and ethylene evolution in ripening tomatoes. Hortic Sci. 1977;12:459–460. [Google Scholar]
- Kendrick RE, Kronenberg GHM. Photomorphogenesis in Plants. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. [Google Scholar]
- Kerckhoffs LHJ, Kelmenson PM, Schreuder MEL, Kendrick CI, Kendrick RE, Hanhart CJ, Koornneef M, Pratt LH, Cordonnier-Pratt M-M. Characterization of the gene encoding the apoprotein of phytochrome B2 in tomato and identification of molecular lesions in two mutant alleles. Mol Gen Genet. 1999;261:901–907. doi: 10.1007/s004380051037. [DOI] [PubMed] [Google Scholar]
- Khudairi AK. The ripening of tomatoes. Am Sci. 1972;60:696–707. [Google Scholar]
- Khudairi AK, Arboleda OP. Phytochrome-mediated carotenoid biosynthesis and its influence by plant hormones. Physiol Plant. 1971;24:18–22. [Google Scholar]
- Lazarova GI, Kerckhoffs LHJ, Brandstädter J, Matsui M, Kendrick RE, Cordonnier-Pratt M-M, Pratt LH. Molecular analysis of PHYA in wild-type and phytochrome A-deficient mutants in tomato. Plant J. 1998a;14:653–662. doi: 10.1046/j.1365-313x.1998.00164.x. [DOI] [PubMed] [Google Scholar]
- Lazarova GI, Kubota T, Frances S, Peters JL, Hughes MJG, Brandstädter J, Széll M, Matsui M, Kendrick RE, Cordonnier-Pratt M-M, Pratt LH. Characterization of tomato PHYB1 and identification of molecular defects in four mutant alleles. Plant Mol Biol. 1998b;38:1137–1146. doi: 10.1023/a:1006068305454. [DOI] [PubMed] [Google Scholar]
- Mustilli AC, Fenzi F, Ciliento R, Alfano F, Bowler C. Phenotype of the tomato high pigment-2 mutant is caused by a mutation in the tomato homolog of DEETIOLATED1. Plant Cell. 1999;11:145–158. doi: 10.1105/tpc.11.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piringer AA, Heinze PH. Effect of light on the formation of a pigment in the tomato fruit cuticle. Plant Physiol. 1954;29:467–472. doi: 10.1104/pp.29.5.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick CM, Butler L. Phytogenetics of the tomato. Adv Genet. 1956;8:267–382. [Google Scholar]
- Sharrock RA, Quail PH. Novel phytochrome sequences in Arabidopsis thaliana: structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev. 1989;3:1745–1757. doi: 10.1101/gad.3.11.1745. [DOI] [PubMed] [Google Scholar]
- Shewfelt AL, Halpin JE. The effect of light quality on the rate of tomato color development. Proc Am Soc Hortic Sci. 1967;91:561–565. [Google Scholar]
- Smith H. Light quality photoperception and plant strategy. Annu Rev Plant Physiol. 1982;33:481–518. [Google Scholar]
- Spooner DM, Anderson GJ, Jansen RK. Chloroplast DNA evidence for the interrelationships of tomatoes, potatoes, and pepinos (Solanaceae) Am J Bot. 1993;80:676–688. [Google Scholar]
- Su L-Y, McKeon T, Grierson D, Cantwell M, Yang SF. Development of 1-aminocyclopropane-1-carboxylic acid synthase and polygalacturonase activities during the maturation and ripening of tomato fruit. Hortic Sci. 1984;19:576–578. [Google Scholar]
- Thomas RL, Jen JJ. Phytochrome-mediated carotenoid biosynthesis in ripening tomatoes. Plant Physiol. 1975;56:452–453. doi: 10.1104/pp.56.3.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Lintig J, Welsch R, Bonk M, Giuliano G, Batschauer A, Kleinig H. Light-dependent regulation of carotenoid biosynthesis occurs at the level of phytoene synthase expression and is mediated by phytochromes in Sinapis alba and Arabidopsis thaliana seedlings. Plant J. 1997;12:625–634. doi: 10.1046/j.1365-313x.1997.00625.x. [DOI] [PubMed] [Google Scholar]
- Yen H-C, Lee S, Tanksley SD, Lanahan MB, Klee HJ, Giovannoni JJ. The tomato Never-ripe locus regulates ethylene-inducible gene expression and is linked to a homolog of the Arabidopsis ETR1 gene. Plant Physiol. 1995;107:1343–1353. doi: 10.1104/pp.107.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]