Abstract
Perry syndrome is a rare atypical parkinsonism with depression, apathy, weight loss, and central hypoventilation caused by mutations in dynactin p150glued (DCTN1). A rare distal hereditary motor neuropathy, HMN7B, also has mutations in DCTN1. Perry syndrome has TAR DNA-binding protein of 43 kDa (TDP-43) inclusions as a defining feature. Other TDP-43 proteinopathies include amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD) with and without motor neuron disease (FTLD-MND). TDP-43 forms aggregates in neuronal cytoplasmic inclusions (NCIs), neuronal intranuclear inclusions, dystrophic neurites (DNs), as well as axonal spheroids, oligodendroglial cytoplasmic inclusions, and perivascular astrocytic inclusions (PVIs). We performed semiquantitative assessment of these lesions and presence of dynactin subunit p50 lesions in 3 cases of Perry syndrome and one of HMN7B. We compared them with 3 cases of FTLD-MND, 3 of ALS, and 3 of hippocampal sclerosis (HpScl). Perry syndrome had NCIs, DNs, and frequent PVIs and spheroids. Perry syndrome cases were similar, but different from ALS, FTLD-MND, and HpScl. TDP-43 pathology was not detected in HMN7B. Dynactin p50 inclusions were observed in both Perry syndrome and HMN7B, but not in the other conditions. These results suggest that Perry syndrome may be distinctive type of TDP-43 proteinopathy.
Keywords: Amyotrophic lateral sclerosis, DCTN1, Distal hereditary motor neuropathy 7B, Frontotemporal lobar degeneration with motor neuron disease, Perivascular astrocytic inclusions, Perry syndrome, TDP-43
INTRODUCTION
Perry syndrome is an autosomal dominant neurodegenerative disease characterized by parkinsonism, apathy, depression, weight loss, and central hypoventilation (1–8). At autopsy there is severe neuronal loss and gliosis in the substantia nigra without Lewy bodies or neurofibrillary tangles (1–6). In contrast, TAR DNA-binding protein of 43 kDa (TDP-43) pathology is found in the substantia nigra and other extrapyramidal nuclei in Perry syndrome (6). TDP-43 pathology can be classified by 6 distinct morphologic lesions: neuronal cytoplasmic inclusions (NCIs), neuronal intranuclear inclusions (NIIs), dystrophic neurites (DNs), axonal spheroids, oligodendroglial cytoplasmic inclusions (GCIs), and perivascular astrocytic inclusions (PVIs) (9). Given the fact that all published autopsied cases of Perry syndrome displayed TDP-43 pathology, Perry syndrome can be considered a TDP-43 proteinopathy (6).
TDP-43 was originally reported as a major pathognomonic protein in amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD) with TDP-43 inclusions (FTLD-TDP) (10, 11). Shortly after it was reported as specific to FTLD and ALS, Amador-Ortiz et al (12) reported TDP-43 in Alzheimer’s disease (AD) and hippocampal sclerosis (HpScl), widening the spectrum of TDP-43 proteinopathy. Subsequently, TDP-43 pathology has been also reported in other neurodegenerative diseases, including tauopathies and α-synucleinopathies (12–25). The morphology and distribution of TDP-43 pathology differs among each disease (26); however, there are no reports comparing the distribution of all 6 morphologic lesions of TDP-43 in various TDP-43 proteinopathies. The study by Josephs et al (27) evaluated the distinctive patterns of cortical and subcortical distribution of NCI, NII, and DNs in various subtypes of FTLD, but it did not include assessment of glial lesions or spheroids. It also did not include Perry syndrome. In this report we describe distribution of all major morphologic types of TDP-43 pathology in Perry syndrome, FTLD-TDP, ALS, and HpScl.
NCI composed of dynactin subunit p50 is another pathologic feature of Perry syndrome. DCTN1, which encodes the large subunit of the dynactin complex, p150glued, is essential for axoplasmic transport. Mutations in DCTN1 were etiologically linked to Perry syndrome (7). A different mutation in DCTN1 (G59S) causes distal spinal and bulbar muscular atrophy, known as distal hereditary motor neuropathy 7B (HMN7B) (28). Perry syndrome and HMN7B are genetically related disorders, but that have different neuropathologic features. Neuronal loss has been reported in the hypoglossal nucleus and anterior horn cells of the spinal cord in HMN7B (29). Although p50-positive NCIs have been reported, TDP-43 pathology has not been described in HMN7B. Furthermore, several variants of DCTN1 have been reported in frontotemporal dementia and ALS cases, although the pathogenicity of these variants has not been proved (30–36). To date, there are no studies on dynactin pathology in ALS or FTLD; therefore, it is necessary to clarify whether dynactin pathology, such as p50-positive NCIs, is specific to HMN7B or if it can also be found in in ALS and FTLD.
The aims of this study were to compare density and distribution of morphologic types of TDP-43 pathology in Perry syndrome and to determine whether dynactin pathology, namely p50-immunopositive NCI, is a specific finding to Perry syndrome and HMN7B. To accomplish our aims, we evaluated the distribution and morphology of TDP-43 and dynactin pathology in Perry syndrome, HMN7B, FTLD-motor neuron disease (MND), ALS, and HpScl.
MATERIALS AND METHODS
Case Material
We examined brain samples from 3 Perry syndrome cases (Case 1: West Virginia family, Case 2: Canada family, and Case 3: Columbia family) and 1 HMN7B case with DCTN1 mutations, compared with 3 FTLD-MND, 3 ALS, and 3 HpScl (Table 1). All cases, except the HMN7B case, had been processed in the Mayo Clinic brain bank for neurodegenerative disorders. The clinical history of patients with Perry syndrome have been previously reported (6, 8, 37), and the distribution of TDP-43 pathology in 2 of the Perry syndrome cases have also been reported (Cases 1 and 2) (6). Clinical and pathologic features of HMN7B, including description of dynactin p50-positive NCIs have been reported (29). HpScl was defined as neuronal loss in hippocampal CA1 and subiculum in the absence of other pathologic features of neurodegenerative disorders other than mild than age-associated changes (12). All autopsies were performed after consent of legal next-of-kin or an individual with power-of-attorney. Studies of autopsy samples are considered exempt from human subject research by the Mayo Clinic Institutional Review Board.
TABLE 1.
Demographic and Clinical Data in Perry Syndrome, FTLD-MND, ALS, HpScl, and HMN7B
| Case | Age | Sex | Brain Weight (g) | Clinical Diagnosis | Pathologic Diagnosis | DCTN1 Mutations |
|---|---|---|---|---|---|---|
| 1 | 44 | M | 1480 | Perry syndrome | Perry syndrome | T72P |
| 2 | 54 | M | 1420 | Perry syndrome | Perry syndrome | G71R |
| 3 | 60 | F | 1200 | Perry syndrome | Perry syndrome | G71R |
| 4 | 49 | M | 1420 | FTD | FTLD-MND | NA |
| 5 | 67 | F | 940 | ALS | FTLD-MND | NA |
| 6 | 61 | M | 1220 | FTD-MND | FTLD-MND | NA |
| 7 | 66 | M | 1260 | ALS | ALS | NA |
| 8 | 58 | F | 1220 | ALS | ALS | NA |
| 9 | 72 | F | 1060 | ALS | ALS | NA |
| 10 | 94 | F | 1160 | AD | HpScl | NA |
| 11 | 77 | F | 1020 | AD | HpScl | NA |
| 12 | 95 | F | 1000 | Normal | HpScl | NA |
| 13 | 76 | M | NA | HMN7B | HMN7B | G59S |
AD, Alzheimer disease; ALS, amyotrophic lateral sclerosis; F, female; FTD, frontotemporal dementia; FTLD-MND, frontotemporal lobar degeneration with motor neuron disease; HMN7B, distal hereditary motor neuropathy 7B; HpScl, Hippocampal sclerosis; M, male; NA, not available.
Immunohistochemistry
Paraffin-embedded 5-μm-thick sections from the frontal and temporal cortices, hippocampus, basal forebrain, brainstem, and spinal cord were processed for immunohistochemistry. Following deparaffinization in xylene and reagent alcohol, antigen retrieval was conducted by steaming slides in distilled water for 30 min. All immunohistochemistry was performed using DAKO EnVision reagents (DAKO, Carpinteria, CA) and a DAKO Autostainer as previously reported (38).
To assess TDP-43 and dynactin pathology, 17 regions of the central nervous system (motor cortex, hippocampal dentate fascia, CA1, entorhinal cortex, amygdala, putamen, globus pallidus, hypothalamus, nucleus basalis of Meynert, substantia nigra, midbrain tegmentum, locus coeruleus, pontine tegmentum, inferior olivary nucleus, hypoglossal nucleus, medullary tegmentum, and spinal cord anterior horn) were examined following TDP-43 immunohistochemistry. In the single case of HMN7B, only 5 regions were available for study (substantia nigra, midbrain tegmentum, locus coeruleus, pontine tegmentum, and spinal cord anterior horn). We performed immunohistochemistry with antibodies against TDP-43 (MC2085, Dr L. Petrucelli, Mayo Clinic, Jacksonville, FL, 1:3000) and p50 (BD Biosciences, San Jose, CA, 1:2500). All slides were reviewed simultaneously by 2 observers (D.W.D., T.M.), who agreed on the presence of TDP-43 immunoreactivity, defined as NCIs, DNs, PVIs, GCIs, NIIs, and spheroids in each region. The severity of TDP-43 lesions was graded semiquantitatively on a 5-point scale as 0 = absent; 0.5 = rarely observed; 1 = sparse; 2 = moderate; and 3 = frequent, as shown in Table 3 and Supplementary Data Table S1. p50 immunoreactivity was detected in only sparse NCIs and scored as present or absent in Tables 2 and 3.
TABLE 3.
TDP-43 and p50 Staining in HMN7B
| Region | p50 | TDP-43 Pathology |
|||||
|---|---|---|---|---|---|---|---|
| NCIs | DNs | PVIs | GCIs | Spheroids | NIIs | ||
| Substantia nigra | + | − | − | − | − | − | − |
| Midbrain tegmentum | + | − | − | − | − | − | − |
| Locus coeruleus | + | − | − | − | − | − | − |
| Pontine tegmentum | + | − | − | − | − | − | − |
| Spinal cord anterior horn | + | − | − | − | − | − | − |
p50-positive NCIs were presence (+) or absence (−). TDP-43 pathology was scored on 5-point scale as—(0), absent; + (0.5), rarely observed; ++ (1), sparse; +++ (2), moderate and ++ ++ (3), frequent.
DNs, dystrophic neurites; GCIs, oligodendroglial cytoplasmic inclusions; HMN7B, distal hereditary motor neuropathy 7B; NCIs, neuronal cytoplasmic inclusions; NIIs, neuronal intranuclear inclusions; PVIs, perivascular astrocytic inclusions; TDP-43, TAR DNA-binding protein of 43 kDa.
TABLE 2.
Distribution of p50-Positive NCIs in Perry Syndrome, FTLD-MND, ALS and HpScl
| Region | Perry syndrome |
FTLD-MND |
ALS |
HpScl |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Motor cortex | − | − | − | − | − | − | − | − | − | − | − | − |
| Hippocampal dentate fascia | − | − | − | − | − | − | − | − | − | − | − | − |
| CA1 | − | − | − | − | − | − | − | − | − | − | − | − |
| Entorhinal cortex | − | − | − | − | − | − | − | − | − | − | − | − |
| Amygdala | − | − | − | − | − | − | − | − | − | − | − | − |
| Putamen | − | − | − | − | − | − | − | − | − | − | − | − |
| Globus pallidus | − | − | − | − | − | − | − | − | − | − | − | − |
| Hypothalamus | − | − | − | − | − | − | − | − | − | − | − | − |
| Nucleus basalis of Meynert | − | − | − | − | − | − | − | − | − | − | − | − |
| Substantia nigra | − | − | NE | − | − | − | − | − | − | − | − | − |
| Midbrain tegmentum | − | + | + | − | − | − | − | − | − | − | − | − |
| Locus coeruleus | + | − | − | − | − | − | − | − | − | − | − | − |
| Pontine tegmentum | − | + | − | − | − | − | − | − | − | − | − | − |
| Inferior olive nucleus | − | − | + | − | − | − | − | − | − | − | − | − |
| Hypoglossal nucleus | − | − | + | − | − | − | − | − | − | − | − | − |
| Medullary tegmentum | + | + | + | − | − | − | − | − | − | − | − | − |
| Spinal cord anterior horn | − | − | − | − | − | − | − | − | − | − | − | − |
p50-positive NCIs were presence (+) or absence (−).
ALS, amyotrophic lateral sclerosis; FTLD-MND, frontotemporal lobar degeneration with motor neuron disease; HpScl, Hippocampal sclerosis; NCIs, neuronal cytoplasmic inclusions; NE, not evaluated.
To characterize spheroids in Perry syndrome, we also performed immunohistochemistry with antibodies to phosphorylated neurofilament protein (SMI-31, BioLegend, San Diego, CA, 1:40 000) and amyloid precursor protein ([APP], EMD Millipore, Billerica, MA, 1:2000), which are frequently used as markers of a range of axonal lesions (39, 40).
Immunoelectron Microscopy
We used the subthalamic nucleus to perform immunoelectron microscopic evaluation of the fine structure of the inclusions in Perry syndrome. One millimetre cubes were dissected from the subthalamic nucleus of formalin-fixed autopsy brain (Case 3), and processed according to protocols published previously (41). Immunogold labeling was performed using a rabbit polyclonal antibody to TDP-43 (ProteinTech Group, Rosemont, IL), and affinity-purified rabbit polyclonal antibody to phospho-TDP-43 (pS409/410, Dr L. Petrucelli, Mayo Clinic, Jacksonville, FL). Goat-anti rabbit IgG conjugated with 18 nm colloidal gold particles was purchased from Jackson ImmunoResearch Laboratories, West Grove, PA. Thin sections were stained with uranyl acetate and lead citrate and examined with a Philips 208S electron microscope fitted with a Gatan 831 Orius digital camera (Gatan, Pleasanton, CA).
RESULTS
Morphology of Lesions With TDP-43 Immunohistochemistry
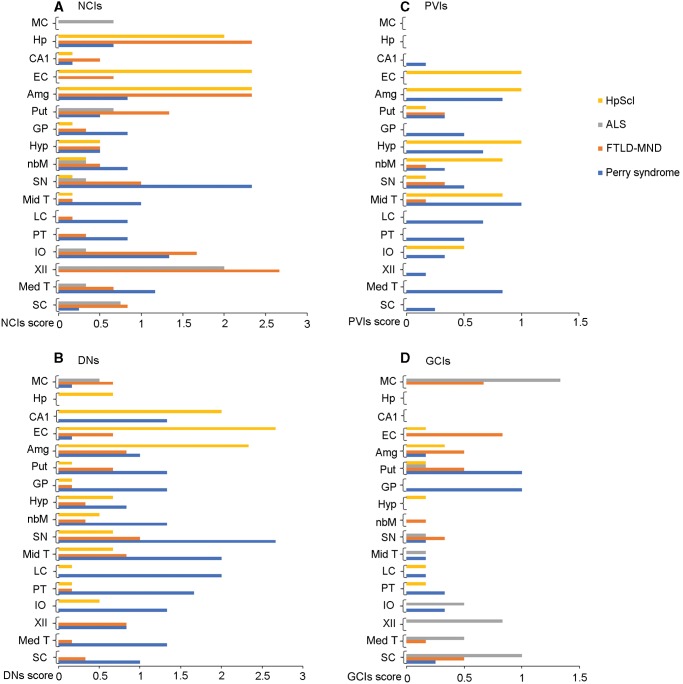
Graphical display of semiquantitative scores for NCIs, DNs, PVIs, and GCIs in Perry syndrome, FTLD-MND, ALS, and HpScl is found in Figure 1. Six types of TDP-43-immunopositive morphologic lesions (NCIs, DNs, PVIs, GCIs, spheroids, and NIIs) were observed in Perry syndrome (Fig. 2). The distribution and density of each morphologic lesion in Perry syndrome, FTLD-MND, ALS, and HpScl are summarized in Supplementary Data Table S1. Five morphologic lesions, except NIIs, were seen in all Perry syndrome cases. One case (Case 1) had NIIs in the nucleus basalis of Meynert and pontine tegmentum. The majority of TDP-43 lesions were NCIs and DNs, but PVIs were also relatively frequent. All Perry syndrome cases had TDP-43-positive spheroids that were most frequently detected in the substantia nigra, and less often in the globus pallidus. The spheroids were positive for SMI-31, APP, and p50 (Supplementary Data Fig. S1). Five types of lesions, except spheroids, were observed in FTLD-MND, with NCIs being the most frequent lesion type. In ALS, NCIs, and GCIs were frequent, but DNs were only seen in the motor cortex in ALS. PVIs, spheroids, and NIIs were not seen in ALS. All 6 morphologic lesions were seen in HpScl. The majority of TDP-43 lesions in HpScl were NCIs and DNs. In addition, PVIs were frequent in HpScl. One HpScl case had TDP-43-positive spheroids in the globus pallidus (Supplementary Data Table S1).
FIGURE 1.
Distribution and density of TDP-43 morphological lesions; neuronal cytoplasmic inclusions (NCIs) (A), dystrophic neurites (DNs) (B), perivascular astrocytic inclusions (PVIs) (C), and oligodendroglial cytoplasmic inclusions (GCIs) (D). MC, motor cortex; Hp, hippocampal dentate fascia; EC, entorhinal cortex; Amg, amygdala; Put, putamen; GP, globus pallidus; Hyp, hypothalamus; nbM, nucleus basalis of Meynert; SN, substantia nigra; Mid T, midbrain tegmentum; LC, locus coeruleus; PT, pontine tegmentum; IO, Inferior olive nucleus; XII, hypoglossal nucleus; Med T, medullary tegmentum; SC, spinal cord anterior horn; TDP-43, TAR DNA-binding protein of 43 kDa.
FIGURE 2.
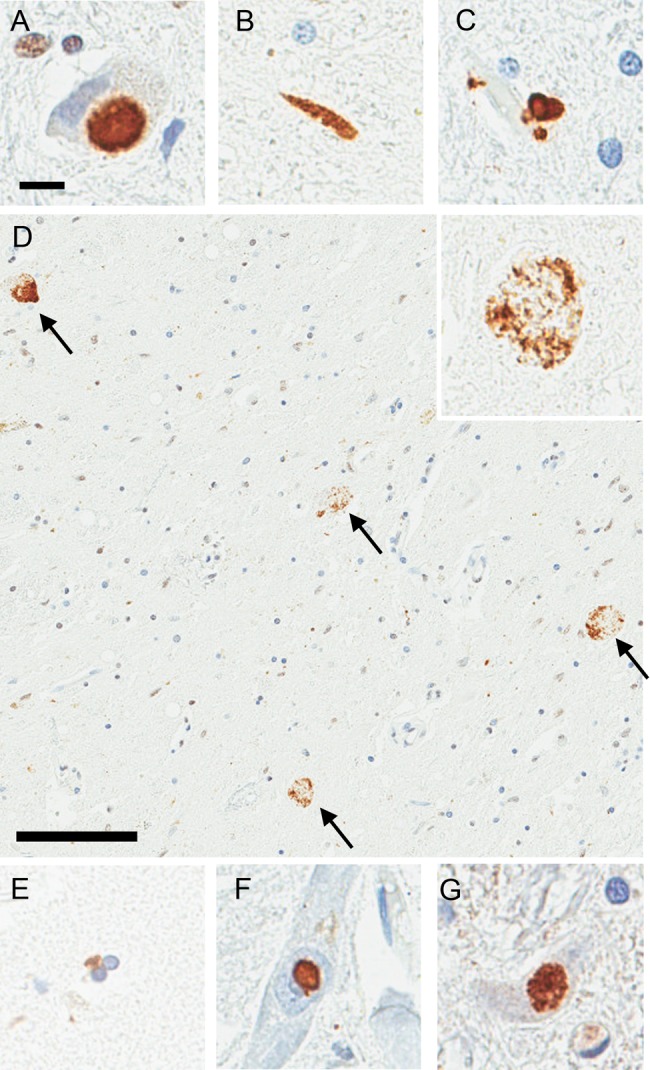
Immunohistochemistry for TDP-43 and p50 on tissue from 3 cases with Perry syndrome; neuronal cytoplasmic inclusions (NCIs) in the medullary tegmentum (A), dystrophic neurites in the substantia nigra (B), perivascular astrocytic inclusions in the locus coeruleus (C), spheroids in the substantia nigra (D), oligodendroglial cytoplasmic inclusions in the globus pallidus (E), neuronal intranuclear inclusions in the nucleus basalis of Meynert (F), and p50-positive NCIs in the medullary tegmentum (G). (D) TDP-43-positive spheroids are most frequently seen in the substantia nigra (arrows). High-magnification image is shown in the inset. Scale bars = 10 μm in (A); 100 μm in (D).
Distribution of TDP-43 Pathology
The substantia nigra was the region most affected with respect to TDP-43 pathology in Perry syndrome. The motor cortex and entorhinal cortex were not affected in 2 cases of Perry syndrome (Cases 1 and 2), but 1 case (Case 3) had DNs in the motor cortex and entorhinal cortex. All 17 regions had TDP-43 pathology in FTLD-MND. The hippocampus, amygdala, and hypoglossal nucleus were the most severely affected. In ALS, the most affected regions were motor cortex, hypoglossal nucleus, and spinal cord anterior horn. ALS also had TDP-43 pathology of the medullary tegmentum, inferior olive nucleus, midbrain tegmentum, substantia nigra, nucleus basalis of Meynert, and putamen. The hippocampus, entorhinal cortex, and amygdala were severely affected in HpScl. In contrast, the motor cortex, hypoglossal nucleus, medullary tegmentum, and spinal cord anterior horn did not have TDP-43 lesions in HpScl.
TDP-43 Immunoelectron Microscopy
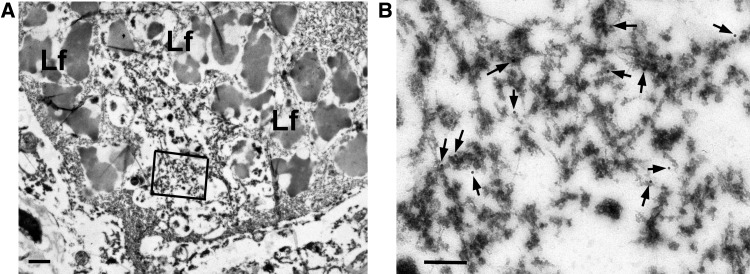
Using 2 different antibodies to TDP-43, we showed NCIs contained granule-associated filaments in the subthalamic nucleus (Fig. 3), similar to what we have previously reported in AD (41). The estimated diameter of the filaments was 10-nm and they were straight filaments.
FIGURE 3.
Immunoelectron microscopy with Perry syndrome. (A) A neuron from the subthalamic nucleus has a cytoplasmic inclusion (arrow) surrounded by lipofuscin (Lf). Boxed area is enlarged in (B). (B) The inclusion contains granule-associated filaments immunopositive for phospho-TDP-43 antibody (arrows point to gold particles). Scale bars = 1 μm in (A); 200 nm in (B).
Dynactin Immunohistochemistry
p50-positive NCIs were observed in the medullary tegmentum in all cases of Perry syndrome (Fig. 2; Table 2). The midbrain tegmentum, locus coeruleus, pontine tegmentum, inferior olive nucleus, and hypoglossal nucleus had p50-positive NCIs in Perry syndrome (Table 2). HMN7B had p50-positive NCIs in all regions evaluated (i.e. the substantia nigra, midbrain tegmentum, locus coeruleus, pontine tegmentum, and spinal cord anterior horn) (Table 3). In contrast, p50-positive NCIs were not detected in ALS, FTLD-MND, and HpScl (Table 2). These results suggest that of the cases included in this study, p50-positive NCIs are specific to those caused by mutations in DCTN1 (i.e. Perry syndrome and HMN7B).
DISCUSSION
In the present study, we describe distinctive distribution patterns of TDP-43 lesions among TDP-43 proteinopathies. PVIs and spheroids were more frequent in Perry syndrome than in other TDP-43 proteinopathies, and immunoelectron microscopy indicated that the ultrastructural characteristics of the inclusions in Perry syndrome were different from ALS or FTLD. Taken together, TDP-43 pathology in Perry syndrome represents a distinctive subtype of TDP-43 proteinopathy not captured in the current scheme (Types A–D) for FTLD (42) or the recently proposed Type E in a rare form of rapidly progressive FTLD (43).
Although NCIs were the most frequent type of TDP-43 pathology in all diseases studied, abundant PVIs and spheroids were characteristic features of Perry syndrome. TDP-43-positive PVIs have been described in a range of disorders, including Perry syndrome (6), FTLD-TDP, familial diffuse Lewy body disease (9), multiple system atrophy (44), progressive supranuclear palsy (45), and a patient with schizophrenia and superimposed dementia (46); however, they were not detected in our small series of ALS. This needs to be confirmed in a larger series of ALS cases. Although the pathologic role of PVIs remains unknown, the presence of TDP-43 pathology in astrocytic end-feet, demonstrated unequivocally by Lin et al (9) with double labeling immunoelectron microscopy for TDP-43 and glial fibrillary acidic protein, raises the possibility that neurodegenerative diseases with TDP-43 pathology may be associated with loss of blood–brain barrier integrity.
The different frequency of TDP-43-positive spheroids is a new finding of the present study. Neuroaxonal spheroids were frequent in Perry syndrome, but rare in HpScl and not found in this small series of ALS and FTLD-MND. Interestingly, spheroids in Perry syndrome (Supplementary Data Fig. S1) and HpScl (data not shown) were immunostained by the p50 antibody. The dynactin complex is essential for many axonal transport functions (47); therefore, p50 might be useful in demonstrating degenerating axons in certain disorders. While APP is the standard marker for demonstrating axonal transport interruption in a wide range of disorders (39), p50 may have more specificity. In addition to spheroids, Perry syndrome was characterized by many TDP-43-positive DNs; however, the morphology and distribution of the DNs were distinct from FTLD-TDP Type C, where abundant DNs are detected in temporal cortices, but minimal elsewhere (27). These findings suggest that axonal pathology may be a central component of the Perry syndrome pathogenesis.
Lin and Dickson (41) previously reported the ultrastructure of TDP-43-positive NCIs in various neurodegenerative diseases. In the present study, immunoelectron microscopic study revealed that the TDP-43-positive NCIs in Perry syndrome had similar ultrastructural features to those found in AD, but different from those found in FTLD-MND or ALS (41). In some AD cases, granule-coated filaments are present in neurons that also have neurofibrillary tangles with double immunogold labeling for tau (41). We speculate that the granule-associated filaments in Perry syndrome may contain dynactin, although we could not confirm this hypothesis. The ultrastructure of TDP-43-positive NCIs and unique pattern of TDP-43-positive PVIs and spheroids indicate that TDP-43 pathology in Perry syndrome is distinctive form of TDP-43 proteinopathy.
We found extensive TDP-43 inclusions in motor and entorhinal cortex of 1 Perry syndrome patient (Case 3) despite the lack of motor neuron symptoms, which differs from the minimal cortical pathology reported in Cases 1 and 2 by Wider et al (6). We hypothesize that longer disease duration in Case 3 may account for this difference in TDP-43 pathology distribution. The 6 years symptomatic duration of Case 3 was longer than that of both Cases 1 and 2 (3 years) (6). Indeed the average disease duration of Perry syndrome is only 5 years (8). To confirm this hypothesis, additional autopsy cases of Perry syndrome, especially those with long disease duration are needed.
We confirmed that all Perry syndrome cases have p50-positive NCIs, although we did not detect p50-positive NCIs in the substantia nigra, the most affected region in Perry syndrome. We speculate a factor that may contribute to the paucity of p50-positive NCIs is the severe neuronal loss in the substantia nigra in Perry syndrome. Not surprisingly, p50-positive NCIs were not observed in ALS and FTLD. This finding may suggest that DCTN1 does not contribute significantly to pathogenesis of ALS and FTLD. Despite the low frequency of p50-positive NCIs, they were detected in all Perry syndrome cases and thus they represent a pathologic hallmark of Perry syndrome. We previously reported colocalization of TDP-43 and p50 within inclusions was rare in Perry syndrome (7). It remains to be determined whether there is a direct link between TDP-43 and dynactin pathologies in Perry syndrome.
TDP-43 immunohistochemistry in a case of HMN7B was first demonstrated in this study and TDP-43 inclusions were not detected. We confirmed that Perry syndrome is pathologically different from HMN7B in distribution of neuronal loss and presence of TDP-43 pathology, although these are genetically related disorders.
There are some limitations of our study. First, the number of autopsy cases we examined was small. Since Perry syndrome is a rare neurodegenerative disease, only a few cases are available for neuropathologic research. This small sample size may decrease the power in determining pathologic differences between the disorders. Second, given the retrospective nature of this study, clinical information was limited. Third, ALS and FTLD-MND cases included in this study did not have screening for DCTN1 mutations. To clarify the possible relation between a variant of DCTN1 and TDP-43 pathology or clinical phenotype, ALS or FTLD-MND cases with the variant of DCTN1 should be analyzed. Additional studies with more cases harboring DCTN1 mutations will be required to elucidate the clinical consequences of TDP-43 pathology.
In conclusion, we demonstrate that Perry syndrome has TDP-43 pathology with a predilection for the extrapyramidal motor system and characterized by abundant NCIs, DNs, PVIs, and spheroids. This pattern is clearly different from that in ALS, FTLD-MND, and HpScl. TDP-43 immunoelectron microscopic findings of Perry syndrome were also different from those of ALS or FTLD-TDP. Taken together, the results of our study indicate that Perry syndrome is a distinctive type of TDP-43 proteinopathy.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank the patients and their families who donated brains to help the understanding of neurodegeneration. The authors would also like to acknowledge Linda Rousseau and Virginia Phillips for histologic support. The authors wish to express their gratitude to Donald B. Calne, Susan Calne, Ludwig Gutmann, Mercedes Olaya C, and late Felipe Pretelt for recruiting patients.
REFERENCES
- 1. Perry TL, Bratty PJ, Hansen S, et al. Hereditary mental depression and Parkinsonism with taurine deficiency. Arch Neurol 1975;32:108–13 [DOI] [PubMed] [Google Scholar]
- 2. Roy EP III, Riggs JE, Martin JD, et al. Familial parkinsonism, apathy, weight loss, and central hypoventilation: successful long-term management. Neurology 1988;38: 637–9 [DOI] [PubMed] [Google Scholar]
- 3. Perry TL, Wright JM, Berry K, et al. Dominantly inherited apathy, central hypoventilation, and Parkinson’s syndrome: clinical, biochemical, and neuropathologic studies of 2 new cases. Neurology 1990;40:1882–7 [DOI] [PubMed] [Google Scholar]
- 4. Bhatia KP, Daniel SE, Marsden CD.. Familial parkinsonism with depression: a clinicopathological study. Ann Neurol 1993;34:842–7 [DOI] [PubMed] [Google Scholar]
- 5. Tsuboi Y, Wszolek ZK, Kusuhara T, et al. Japanese family with parkinsonism, depression, weight loss, and central hypoventilation. Neurology 2002;58:1025–30 [DOI] [PubMed] [Google Scholar]
- 6. Wider C, Dickson DW, Stoessl AJ, et al. Pallidonigral TDP-43 pathology in Perry syndrome. Parkinsonism Relat Disord 2009;15:281–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Farrer MJ, Hulihan MM, Kachergus JM, et al. DCTN1 mutations in Perry syndrome. Nat Genet 2009;41:163–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tacik P, Fiesel FC, Fujioka S, et al. Three families with Perry syndrome from distinct parts of the world. Parkinsonism Relat Disord 2014;20:884–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin WL, Castanedes-Casey M, Dickson DW.. Transactivation response DNA-binding protein 43 microvasculopathy in frontotemporal degeneration and familial Lewy body disease. J Neuropathol Exp Neurol 2009;68:1167–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arai T, Hasegawa M, Akiyama H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 2006;351:602–11 [DOI] [PubMed] [Google Scholar]
- 11. Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006;314:130–3 [DOI] [PubMed] [Google Scholar]
- 12. Amador-Ortiz C, Lin WL, Ahmed Z, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol 2007;61:435–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Josephs KA, Murray ME, Whitwell JL, et al. Staging TDP-43 pathology in Alzheimer’s disease. Acta Neuropathol 2014;127:441–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arai T, Mackenzie IR, Hasegawa M, et al. Phosphorylated TDP-43 in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol 2009;117:125–36 [DOI] [PubMed] [Google Scholar]
- 15. Bigio EH, Mishra M, Hatanpaa KJ, et al. TDP-43 pathology in primary progressive aphasia and frontotemporal dementia with pathologic Alzheimer disease. Acta Neuropathol 2010;120:43–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davidson YS, Raby S, Foulds PG, et al. TDP-43 pathological changes in early onset familial and sporadic Alzheimer’s disease, late onset Alzheimer’s disease and Down’s syndrome: association with age, hippocampal sclerosis and clinical phenotype. Acta Neuropathol 2011;122:703–13 [DOI] [PubMed] [Google Scholar]
- 17. Uryu K, Nakashima-Yasuda H, Forman MS, et al. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol 2008;67:555–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nelson PT, Schmitt FA, Lin Y, et al. Hippocampal sclerosis in advanced age: clinical and pathological features. Brain 2011;134:1506–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nag S, Yu L, Capuano AW, et al. Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Ann Neurol 2015;77:942–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakashima-Yasuda H, Uryu K, Robinson J, et al. Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol 2007;114:221–9 [DOI] [PubMed] [Google Scholar]
- 21. Yokota O, Davidson Y, Bigio EH, et al. Phosphorylated TDP-43 pathology and hippocampal sclerosis in progressive supranuclear palsy. Acta Neuropathol 2010;120:55–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aoki N, Murray ME, Ogaki K, et al. Hippocampal sclerosis in Lewy body disease is a TDP-43 proteinopathy similar to FTLD-TDP type A. Acta Neuropathol 2015;129:53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fujishiro H, Uchikado H, Arai T, et al. Accumulation of phosphorylated TDP-43 in brains of patients with argyrophilic grain disease. Acta Neuropathol 2009;117:151–8 [DOI] [PubMed] [Google Scholar]
- 24. Arnold SJ, Dugger BN, Beach TG.. TDP-43 deposition in prospectively followed, cognitively normal elderly individuals: correlation with argyrophilic grains but not other concomitant pathologies. Acta Neuropathol 2013;126:51–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kouri N, Oshima K, Takahashi M, et al. Corticobasal degeneration with olivopontocerebellar atrophy and TDP-43 pathology: an unusual clinicopathologic variant of CBD. Acta Neuropathol 2013;125:741–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tan RH, Kril JJ, Fatima M, et al. TDP-43 proteinopathies: pathological identification of brain regions differentiating clinical phenotypes. Brain 2015;138:3110–22 [DOI] [PubMed] [Google Scholar]
- 27. Josephs KA, Stroh A, Dugger B, et al. Evaluation of subcortical pathology and clinical correlations in FTLD-U subtypes. Acta Neuropathol 2009;118:349–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Puls I, Jonnakuty C, LaMonte BH, et al. Mutant dynactin in motor neuron disease. Nat Genet 2003;33:455–6 [DOI] [PubMed] [Google Scholar]
- 29. Puls I, Oh SJ, Sumner CJ, et al. Distal spinal and bulbar muscular atrophy caused by dynactin mutation. Ann Neurol 2005;57:687–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Munch C, Sedlmeier R, Meyer T, et al. Point mutations of the p150 subunit of dynactin (DCTN1) gene in ALS. Neurology 2004;63:724–6 [DOI] [PubMed] [Google Scholar]
- 31. Munch C, Rosenbohm A, Sperfeld AD, et al. Heterozygous R1101K mutation of the DCTN1 gene in a family with ALS and FTD. Ann Neurol 2005;58:777–80 [DOI] [PubMed] [Google Scholar]
- 32. Takahashi Y, Seki N, Ishiura H, et al. Development of a high-throughput microarray-based resequencing system for neurological disorders and its application to molecular genetics of amyotrophic lateral sclerosis. Arch Neurol 2008;65:1326–32 [DOI] [PubMed] [Google Scholar]
- 33. Liu ZJ, Li HF, Tan GH, et al. Identify mutation in amyotrophic lateral sclerosis cases using HaloPlex target enrichment system. Neurobiol Aging 2014;35:2881. [DOI] [PubMed] [Google Scholar]
- 34. Steele JC, Guella I, Szu-Tu C, et al. Defining neurodegeneration on Guam by targeted genomic sequencing. Ann Neurol 2015;77:458–68 [DOI] [PubMed] [Google Scholar]
- 35. Cady J, Allred P, Bali T, et al. Amyotrophic lateral sclerosis onset is influenced by the burden of rare variants in known amyotrophic lateral sclerosis genes. Ann Neurol 2015;77:100–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vilarino-Guell C, Wider C, Soto-Ortolaza AI, et al. Characterization of DCTN1 genetic variability in neurodegeneration. Neurology 2009;72:2024–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pretelt F, Castaneda Cardona C, et al. Latin America’s first case of Perry syndrome and a new treatment option for respiratory insufficiency. J Neurol 2014;261:620–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koga S, Dickson DW, Bieniek KF.. Chronic traumatic encephalopathy pathology in multiple system atrophy. J Neuropathol Exp Neurol 2016;75:963–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Johnson VE, Stewart W, Weber MT, et al. SNTF immunostaining reveals previously undetected axonal pathology in traumatic brain injury. Acta Neuropathol 2016;131:115–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sundal C, Lash J, Aasly J, et al. Hereditary diffuse leukoencephalopathy with axonal spheroids (HDLS): a misdiagnosed disease entity. J Neurol Sci 2012;314:130–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lin WL, Dickson DW.. Ultrastructural localization of TDP-43 in filamentous neuronal inclusions in various neurodegenerative diseases. Acta Neuropathol 2008;116:205–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mackenzie IR, Neumann M, Baborie A, et al. harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 2011;122:111–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee EB, Porta S, Michael Baer G, et al. Expansion of the classification of FTLD-TDP: distinct pathology associated with rapidly progressive frontotemporal degeneration. Acta Neuropathol 2017. [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Geser F, Malunda JA, Hurtig HI, et al. TDP-43 pathology occurs infrequently in multiple system atrophy. Neuropathol Appl Neurobiol 2011;37:358–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Koga S, Sanchez-Contreras M, Josephs KA, et al. Distribution and characteristics of transactive response DNA binding protein 43 kDa pathology in progressive supranuclear palsy. Mov Disord 2017;32:246–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Geser F, Robinson JL, Malunda JA, et al. Pathological 43-kDa transactivation response DNA-binding protein in older adults with and without severe mental illness. Arch Neurol 2010;67:1238–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cianfrocco MA, DeSantis ME, Leschziner AE, et al. Mechanism and regulation of cytoplasmic dynein. Annu Rev Cell Dev Biol 2015;31:83–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.