Abstract
Neurofibrillary tangles (NFTs) represent products of insoluble tau protein in the brains of patients with Alzheimer disease (AD). The cerebrospinal fluid (CSF) tau level is a biomarker in AD diagnosis. The soluble portion of tau protein in brain parenchyma is presumably the source for CSF tau but this has not previously been quantified. We measured CSF tau and soluble brain tau at autopsy in temporal and frontal brain tissue samples from 7 cognitive normal, 12 mild cognitively impaired, and 19 AD subjects. Based on the measured brain soluble tau, we calculated the whole brain tau load and estimated tau secretion factor. Our results suggest that the increase in NFT in AD is likely attributable to post-translational processes; the increase in CSF tau in AD patients is due to an accelerated carrier-based secretion. Moreover, cognitive dysfunction assessed by final Mini-Mental State Examination scores correlated with the secretion factor but not with the soluble tau.
Keywords: Alzheimer disease, Tau protein, Soluble tau
INTRODUCTION
Neurofibrillary tangles (NFTs) are one of the most important diagnostic criteria for the pathological diagnosis of Alzheimer disease (AD). Tangle formation progresses in a predictable spatial sequence, described by Braak Staging as transentorhinal stages (I–II), hippocampal/limbic stages (III–IV), and isocortical stages (V–VI), and is initiated by an abnormal tau protein conformation, which then sequesters additional normal soluble tau protein (1–3). Tangles have traditionally been identified at postmortem histological analysis, although emerging advancement in PET tracers that selectively bind to paired-helical filaments (PHFs) is a promising modality for longitudinal quantification and monitoring of NFT during AD progression (4, 5).
Tau levels in the cerebrospinal fluid (CSF) are significantly increased in AD patients and are the best single biomarker for AD diagnosis in terms of meta-analysis effect size and z-score (www.alzforum.org/alzbiomarker). CSF tau is a sensitive longitudinal marker of AD progression. CSF tau protein, and phosphorylated tau in particular, reflect axonal degeneration in AD patients (6). Previous immunohistochemical studies demonstrated that CSF tau most likely comes from brain soluble tau protein; PHF-tau is significantly increased but soluble tau is reduced in AD patients samples compared with cognitively normal (CN) control samples, particularly in frontal and temporal regions; this suggests the redistribution of tau from a soluble pool to a NFT pool (7). This process of redistribution seems to be unique to AD and is not part of normal aging processes (8). Because the conversion rate from soluble parenchymal tau into NFT-associated tau is presumably irreversible, this could be conceptualized as the precipitation factor. Meanwhile, the pool of soluble brain tau protein constitutes a source that can be released to the CSF (Fig. 1A). To supply the increases observed in NFT numbers and CSF tau concentrations, there must be continuous synthesis of brain parenchymal tau. The pool of soluble brain tau is not measurable in live patients and is not routinely measured at postmortem examination, but in tau transgenic mice the overall CSF tau levels mirror the expression and changes of brain tau (9). Reducing the amount of soluble brain tau effectively reversed the cognitive deficit in a preclinical study but pathological brain tau aggregates were not reduced (10), suggesting that soluble tau reduction may be a therapeutic target (11). Therefore, understanding soluble brain tau is crucial for using it as a biomarker and for testing therapeutic strategies for tau reduction. A previous human postmortem study reported that total brain parenchymal tau protein increased in both temporal and frontal lobe of AD brain tissues (12). However, it is necessary to measure brain tau and CSF tau in the same individuals to establish a quantitative model (Fig. 1A).
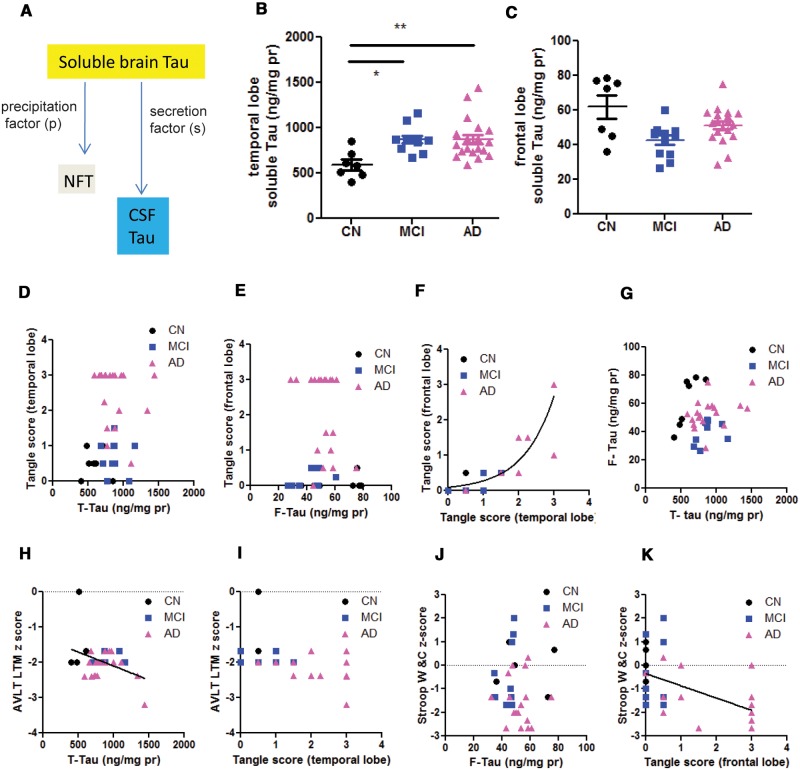
FIGURE 1.
Analysis of soluble brain tau in temporal and frontal lobes. (A) Scheme of hypothesis: the increased synthesis of soluble brain tau provides the source for continuous NFT formation and CSF tau increases in AD. (B, C) A comparison of soluble tau in temporal lobes (t-tau) and frontal lobes (F-tau) in CN, MCI and AD patient samples. One-way ANOVA with post-hoc Tukey analysis: *p < 0.05; **p < 0.01 in Tukey group-pair test. Concentrations of soluble brain tau in the temporal lobe were significantly higher in AD and MCI subjects vs CN subjects. The concentrations of soluble brain tau in the frontal lobe were not significantly different among the 3 groups. (D, E) Correlation analysis of regional soluble tau and NFT in the same regions. There were no clear correlations between soluble tau and NFT tangle scores. (F) Cross-regional analysis of NFT. The frontal and the temporal tangle scores were fitted with equation Y = 0.089exp (1.135×), Goodness of Fit r2 = 0.8662. This relationship suggests that temporal NFT precede the growth of frontal NFT. (G) Cross-regional analysis of soluble tau. There is no clear correlation between frontal and temporal soluble tau. (H, I) AVLT LTM z scores inversely correlate with soluble tau in the temporal lobe (T-Tau) (H), but not with the NFT density (the temporal lobe tangle score) (I). The line in panel H indicates significant correlation in Spearman’s linear correlation model. (J, K) Stroop Word and Color test z score inversely correlated with the frontal lobe NFT density (tangle score, K), but not with the soluble Tau (F-tau, J). The line in panel K indicates significant correlation in Spearman’s linear correlation model.
In this study, we asked the following questions: 1) Are there increases in soluble tau in the brains of patients with AD or mild cognitive impairment (MCI) and do increases occur in all or only in specific regions? 2) Do levels of soluble tau correlate with the density of NFT, i.e. is a high concentration of soluble tau more likely to become incorporated into NFT? 3) Does CSF tau represent soluble brain tau globally or in a specific region? 4) How can a tau secretion factor be estimated? 5) Is the tau releasing process driven by an increased supply of brain tau protein? To address these questions, we measured CSF tau and soluble brain tau in temporal and frontal brain tissues from groups of CN, MCI, and AD subjects. Our initial hypothesis was that brain tau expression is enhanced in AD and leads to increased NFT formation and CSF tau levels (Fig. 1A).
MATERIALS AND METHODS
Subjects and Clinical Assessment
Approval for the study was provided by the Western Institutional Review Board. Written informed consent had been provided by the donors’ families. De-identified postmortem human cerebral cortex and CSF samples were obtained from the Arizona Alzheimer’s Disease Center and the Banner Sun Health Research Institute Brain and Body Donation Program, including 19 AD, 12 MCI, and 7 CN cases. All AD cases were selected as being intermediate or high probability for AD according to National Institute on Aging–Reagan criteria (National Institute on Aging–Alzheimer Association criteria) (13). The samples were free of other neurodegenerative disorders, including vascular dementia, Parkinson disease, dementia with Lewy bodies, frontotemporal dementia, hippocampal sclerosis, progressive supranuclear palsy, dementia lacking distinctive histologic features, multiple system atrophy, motor neuron disease with dementia, and corticobasal degeneration. The diagnosis of MCI was by consensus as a syndrome of cognitive impairment beyond age-adjusted norms that is not severe enough to impair daily function or fulfill clinical criteria for dementia (14–16). The Audiovisual Learning Test (AVLT) and the Stroop Word and Color test (Stroop W&C) were obtained within 1 year prior to death. The clinicopathologic data profile of the subjects is summarized in the Table and (17).
TABLE.
Patient Profiles
| CN | MCI | AD | p Valuea | |
|---|---|---|---|---|
| Number | 7 | 12 | 20 | |
| Gender (M/F) | 6/1 | 7/5 | 8/12 | 0.105b |
| Mean age (years, SD) | 87.0 (7.3) | 88.8 (5.3) | 81.5 (9.2) | 0.027 |
| Median PMI (25–75% percentile) | 2.75 (2.08, 3) | 2.76 (2.31, 3.42) | 3 (2.56, 4.12) | 0.514 |
| Frontal lobe plaque density—mean CERAD, (SD) | 0.8 (1.1) | 1.3 (1.2) | 2.6 (0.9) | <0.001 |
| Temporal lobe plaque density—Mean CERAD, (SD) | 1.1 (1.4) | 1.1 (1.3) | 2.7 (0.6) | <0.001 |
| Frontal lobe tangle score—Mean, (SD) | 0.1 (0.2) | 0.2 (0.2) | 2.0 (1.2) | 0.008 |
| Temporal lobe tangle score—mean (SD) | 0.5 (0.4) | 0.7 (0.5) | 2.5 (0.8) | <0.001 |
| Braak Stage—Median (25–75% percentile) | 4 (3,4) | 4 (3.3, 4) | 5.5 (4,6) | <0.001 |
| Last MMSE—mean (SD) | 27.7 (1.7) | 27.2 (2.4) | 13 (9.1) | 0.034 |
| AVLT-LT-z mean (SD)c | −1.43 (0.86) | −1.93 (0.13) | −2.13 (0.37) | 0.004 |
| Stroop WC z mean (SD)c | −0.06 (0.95) | −0.33 (1.41) | −1.57 (0.99) | 0.006 |
aAll p Values are derived from 1-way ANOVA, except for gender.
bwhich was a result of Chi-square test.
cZ scores were calculated to adjust for aging and educational level.
CN, cognitive normal; MCI, mild cognitive impairment; AD, Alzheimer disease; M, male; F, female; PMI, postmortem interval; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; MMSE, Mini-Mental State Examination; AVLT, Audiovisual Learning Test; Stroop WC, Stroop Word and Color test.
Neuropathological Assessment
At the time of brain removal, the cerebra were cut in the coronal plane into 1-cm-thick slices and then divided into left and right halves. The brainstems were sliced axially and the cerebella were sliced in parasagittal planes. The slices from the right half were frozen between slabs of dry ice while the slices from the left half were fixed by immersion in 4% buffered formaldehyde for 48 hours at 4 °C. Following cryoprotection in ethylene glycol and glycerol, selected 3 × 4 cm cerebral, cerebellar and brainstem blocks were sectioned at 40-µm thickness on a sliding freezing microtome. Sections were stained with hematoxylin and eosin, thioflavin S and enhanced silver methods for amyloid plaques and NFT using the Campbell-Switzer and Gallyas methods (18). Thioflavin S is one of the methods recommended and validated for neuritic plaque density grading by the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) (19); Braak NFT staging was originally described using the Gallyas stain (20). The validity and accuracy of this combination of stains for estimating the density of Aβ deposits has also been established through strong correlations with autoradiographic binding of the amyloid imaging ligand Florbetapir (Spearman rho = 0.95) to postmortem human brain sections from AD subjects (21), with biochemical measures (ELISA) of Aβ in human cerebral cortex extracts (Spearman rho = 0.89) and with quantitative immunohistochemical stains for Aβ (Spearman rho = 0.86) (22).
Histopathological scoring was performed blinded to clinical and neuropathological diagnosis. Amyloid plaque and NFT density were graded and staged at standard sites in frontal, temporal, parietal and occipital cortex, hippocampus and entorhinal cortex based on the aggregate impression from the 40-μm-thick sections stained with thioflavin S, Campbell-Switzer and Gallyas methods. Neuritic plaque density scores were obtained by assigning values of none, sparse, moderate and frequent, according to the CERAD templates (19). NFT abundance and distribution were also graded in the thick sections, using the CERAD templates for estimating tangle density, and the original Braak protocol for estimating topographical distribution (20).
Tissue Processing and Biochemical Assay
Brain and ventricular CSF samples were obtained through rapid autopsy, as previously described (23). Before tau protein quantification, the CSF was centrifuged in 10 000 rpm at 4 °C and an aliquot of supernatant was diluted as 1:50 to measure tau protein. The superior frontal gyrus and the middle temporal gyrus tissue was lysed in pre-cooled RIPA buffer 150 mM NaCl, 1.0% IGEPAL® CA-630, 0.5% sodium deoxycholate, 0.1% SDS, and 50 mM Tris, pH 8.0) supplemented with 1% mixed proteinase inhibitors (Sigma-Aldrich Inc. St Louis, MO, cat. number P8340), and 1% phosphatase inhibitor cocktail (Sigma-Aldrich Inc. cat. number P5726), and sonicated in 10 5-second strikes at 4 °C. Then the brain tissue lysates were centrifuged in 16000 x g; the supernatant was diluted to 1:50 to measure the total protein contents using Pierce bicinchoninic acid assay kit (ThermoFisher Scientific Inc. Waltham, MA, cat. number 23225). This approach only extracts detergent soluble tau protein but not tau protein aggregated in NFT (24). The brain supernatant (1:10 000 dilution) and CSF (1:50 dilution) were used to measure tau protein using a human total tau Elisa kit according to the manufacturer’s instruction (ThermoFisher Scientific Inc., Cat. number KHB0041).
Data Analysis and Statistics
For each tissue sample from frontal and temporal lobe, the brain soluble tau protein level measured by Elisa approach was normalized to the total protein content in each sample, and reported as sample tau (ng/mg total protein). Then the whole brain tau is calculated as:
where y is the protein yield during brain tissue processing. The whole brain soluble tau estimated from frontal (f-tau) and temporal lobe (t-tau) were slightly different and are presented separately in the Results.
The whole CSF tau protein was calculated as:
where V is the total volume of CSF. Although the estimated volume is between 90 and 150 ml (25), we used the mean value of 120 ml.
Then, the tau secretion factor (s) was calculated as:
The average values from CN, MCI and AD groups were reported as mean ± SE and analyzed with 1-way ANOVA with post-hoc Tukey analysis. Pairs of parametric variables (soluble tau, CSF tau, Mini-Mental State Examination [MMSE]) were tested with Pearson linear correlation model; nonparametric variables (tangle score, Braak stage, z score) were tested with Spearman linear correlation model. The statistics were conducted using GraphPad Prism 5 (GraphPad Software, Inc. La Jolla, CA) or IBM SSPS (Chicago, IL). A p value < 0.05 was accepted as significant.
RESULTS
Regional Soluble Tau Does Not Predict NFT Density or Inter-Regional Progression
The soluble tau level in temporal parenchyma (t-tau) was found to be higher in AD and MCI compared with CN subject samples. The temporal tau level was 592.6 ± 57.3 ng/mg total protein in CN (n = 7), 871.0 ± 42.8 ng/mg in MCI (n = 12) and 874.5 ± 51.34 ng/mg in AD (n = 20, p = 0.0053, Fig. 1B). In contrast, the tau quantity in frontal parenchyma (f-tau) in AD did not significantly differ from that of CN subjects; f-tau in MCI was slightly reduced compared with CN (Fig. 1C). The frontal tau levels were 61.96 ± 6.78 ng/mg total protein in CN (n = 7), 42.67 ± 3.28 ng/mg in MCI (n = 12) and 51.31 ± 2.37 ng/ml in AD (n = 19, Fig. 1C). The temporal tau quantity did not correlate with the tangle density in the same region, which involves the aggregated tau protein (Spearman r = 0.1132, p = 0.5726, Fig. 1D) nor did the frontal tau quantity correlate with the tangle density in the frontal lobe (Spearman r0.1416, p = 0.3964, Fig. 1E). Thus, the numbers and densities of NFT are not driven by the quantity of soluble tau in those regions. Although we assume that NFT-tau is derived from soluble brain tau, the conversion rate (precipitation factor [p]) is independent of the available tau (Fig. 1A). Across the temporal and frontal regions, the frontal NFT density correlates with the temporal NFT density (Spearman r = 0.8963, p = 1.2 × 10−12, Fig. 1F). However, their relationship could be better fitted into an exponential growth curve (Goodness of Fit r2 = 0.8562, Fig. 1F). This exponential relation is consistent with Braak stage in that temporal tangles precede frontal tangles. In CN subjects only, the temporal soluble tau correlates with the frontal soluble tau (r = 0.8562, p = 0.0003, Fig. 1G, CN data point only). However, this correlation was lost in MCI and AD (p = 0.769, Fig. 1G), suggesting that the increased expression of tau protein in individuals may not be uniform in all brain regions. Therefore, soluble tau level is not predictive of the NFT formation. As such, the soluble tau level in regions inflicted in early Braak stage (e.g. temporal lobe) did not predict the tau level in regions of late Braak stage (e.g. frontal lobe). Furthermore, while Braak stage was defined based on the prevalence of NFT, temporal or frontal soluble total tau level did not correlates with Braak stage (p = 0.606 and 0.901, respectively), again suggesting that regional soluble tau level did not predict the progression of NFT. Therefore, we could not determine the precipitation factor based on a regression model.
Regional Soluble Tau and Regional Tangle Predict Regional Cognitive Performance in Different Ways
AVLT LT z score was inversely correlated with temporal lobe soluble tau concentration (Spearman r = −0.3754, p = 0.031, Fig. 1H). However, AVLT LT z score was not correlated with NFT numbers in the temporal lobe (Spearman r = −0.277, p = 0.1176, Fig. 1I). Stroop Word and Color Test z-score was not correlated with the soluble tau concentration in the frontal lobe (Spearman r = −0.1439, p = 0.4399, Fig. 1J), but strongly and inversely correlated with NFT in the frontal lobe (Spearman r = −0.5576, p = 0.0011, Fig. 1K). Thus, soluble tau predicted the temporal lobe regional cognitive function (AVLT) while NFT predicted the frontal lobe regional cognitive function (Stroop W&C).
Total Tau Protein in CSF Was Not Driven by Soluble Brain Tau
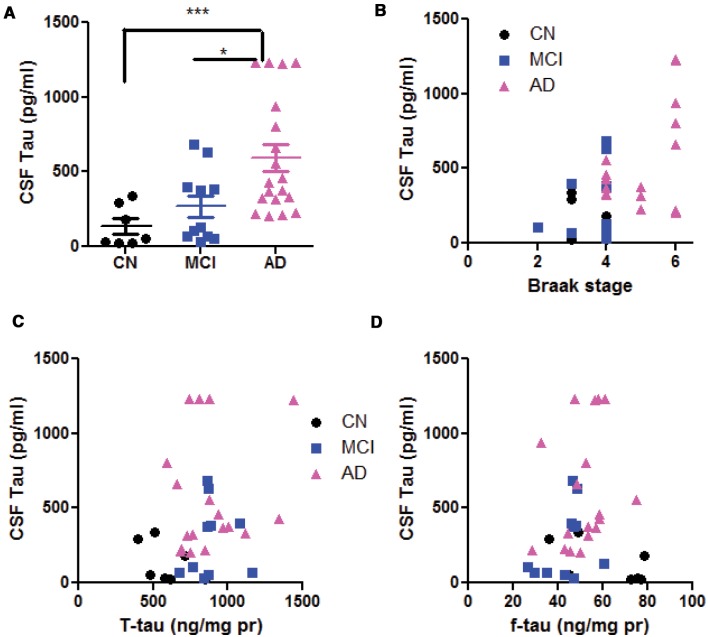
The increase in CSF total tau is a sensitive biomarker for human AD and other tauopathy. In our samples, CSF tau was 134.5 ± 51.2 pg/ml in CN, 267.5 ± 72.2 pg/ml in MCI and 596.4 ± 89.0 pg/ml in AD, showing a progressive increase from CN to AD (1-way ANOVA, p = 0.002, Fig. 2A). This average number range was slightly lower than CSF tau measured from live human subjects (26). CSF tau linearly correlates with Braak stage (Spearman r = 0.4153, p = 0.03, Fig. 2B). However, CSF tau did not correlate with soluble tau in temporal lobe (r = 0.2716, p = 0.094, Fig. 2C) or that in frontal lobe (r = 0.039, p = 0.82, Fig. 2D), suggesting that CSF tau was not determined by a specific regional brain parenchymal tau.
FIGURE 2.
CSF tau and its dependency on brain soluble tau. (A) Comparison of CSF tau in CN, MCI and AD subjects. One-way ANOVA with post-hoc Tukey analysis. *p < 0.05; ***p < 0.001 in Tukey group-pair test. Note that the average CSF tau level in AD is significantly higher than that in CN or MCI subjects; there is no significant difference between MCI and CN CSF tau. (B) Correlation analysis of CSF tau with Braak stage are significant (see text for detail). Correlation analysis of CSF tau with T-tau (C) and F-tau (D). There are no correlations between soluble brain tau and CSF tau.
Enhanced Tau Secretion from Brain Parenchyma to CSF in AD
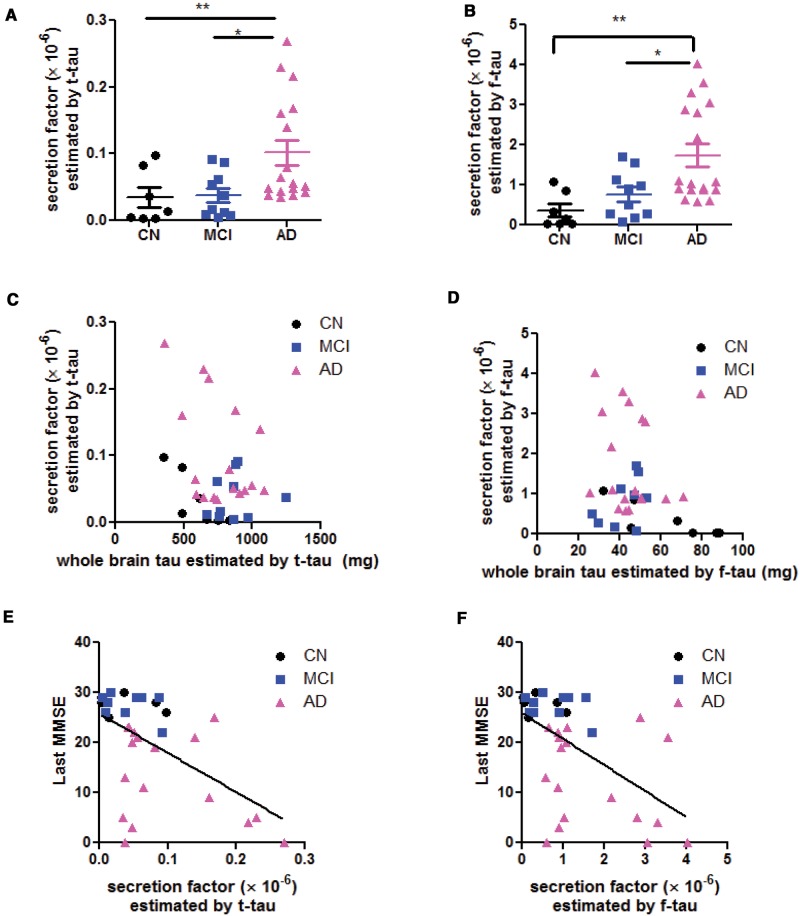
Based on the measured brain soluble tau, we calculated the whole brain tau load and estimate the secretion factor (see Materials and Methods section). Using temporal tau, the estimated secretion factors were (0.345 ± 0.151) × 10−5 in CN, (0.383 ± 0. 105) × 10−5 in MCI, and they were increased to (1.017 ± 0.19) × 10−5 in AD (1-way ANOVA, p = 0.016, with post-hoc Tukey analysis, Fig. 3A). Using the frontal tau, the estimated secretion factor was (0.361 ± 0.164) × 10−6 in CN, (0.758 ± 0. 184) × 10−6 in MCI, and increased to (1.740 ± 0.280) × 10−6 in AD (1-way ANOVA, p = 0.003, with post-hoc Tukey analysis, Fig. 3B). Thus, the absolute value of the calculated results was different depending on the estimation using temporal or frontal lobe data. However, the estimated secretion factor was higher in AD compared with CN or MCI when estimated either way. We hypothesized that the tau secretory process from brain parenchyma to CSF is a passive drainage (Fig. 1A). This indicates that the secretion factor is most likely proportional to the whole brain parenchymal tau and fits in a typical metabolic first-order kinetic model. Contrary to this hypothesis, however, the secretion factor estimated by temporal tau was not significantly correlated with the whole brain tau (r = −0.3565, p = 0.075, Fig. 3C); the secretion factor estimated by frontal tau was not significantly correlated with the whole brain tau either (r = −0.3132, p = 0.067, Fig. 3D). Therefore, instead of a passive first-order elimination process driven by the quantity of whole brain tau, the tau secretion process is likely an active process that is upregulated in AD.
FIGURE 3.
Determination of tau secretion factor from brain to CSF. Secretion factor estimated based on temporal lobe soluble tau (t-tau) (A) and estimated based on frontal lobe soluble tau (f-tau) (B). One-way ANOVA with post-hoc Tukey analysis: *p < 0.05; **p < 0.01; ***p < 0.001 in Tukey group-pair test. (C, D) Linear regression test on the hypothesis that secretion factor is driven by brain soluble tau. Note that secretion factor (dependent factor) does not fit in linear model with whole brain tau (independent factor), suggesting that the secretion factor does not observe the first-order chemico-kinetic model. Last antemortem MMSE scores inversely correlate with the secretion factor, whether estimated by temporal tau (E) or frontal tau (F).
It is noteworthy, however, that the last antemortem MMSE (which were assessed within 1-year prior to death), inversely correlated with the secretion factor, whether it was estimated by the temporal tau (r = −0.5511, p = 0.0007, Fig. 3E) or by the frontal tau (r = −0.5575, p = 0.0005, Fig. 3F). Therefore, it is not the brain soluble tau level but the secretion factor that is associated with the cognitive outcome.
DISCUSSION
In response to the questions proposed in the Introduction, our data suggest the following: 1) Temporal lobe tau protein expression in AD and MCI is increased compared with CN patients, but there is no change in frontal lobe. 2) The level of soluble tau does not predict NFT formation; therefore, the conversion rate (precipitation factor) is not a function of total available tau. However, we were not able to quantitate the precipitation factor by regression. Soluble tau showed a correlation with regional cognitive performance in the temporal lobe. 3) We did not find a quantitative model to fit the relation between brain tau and CSF tau and could not use CSF tau to calculate brain tau retrospectively. 4) The tau secretion factor is estimated to be in the range of 10−5 to 10−6, depending on whether the estimation is based on frontal or temporal tau. In either case, the secretion factor in AD is approximately twice that in CN or MCI subjects. 5) Tau secretion into the CSF is likely an active and not passive process that is independent of brain tau level. 6) MMSE inversely correlates with the secretion factor but not the soluble tau in brain parenchyma.
The increase in temporal tau agrees with a previous report (12), but the lack of change in frontal tau differs from that study. This discrepancy in frontal tau measurements may result from heterogeneity in different patient populations. Brain tau protein is the likely source of both NFT and CSF tau and overexpression of tau protein enhances the formation of tau aggregation in vitro and promotes tangle formation in vivo (27–29). However, our data refute the hypothesis that precipitation factor is a function of the soluble tau, which is in continuous supply for NFT sequestration. In other words, the increased synthesis of soluble tau does not necessarily result in conversion into NFT in a predicable way. Alternatively, the conversion process must depend more on post-translational modification of tau (i.e. phosphorylation, the reduction of acetylation and glycosylation) than the amount of soluble tau; it is the quality but not the quantity of tau that determine the NFT formation.
Although CSF total tau results from drainage of secreted tau from brain, we could not use CSF tau to estimate the tau load in the brain of live patients. Tau secretion is not likely to be a passive diffusion process. Tau release could be simply due to cell death when there is in brain trauma, AD or other neurodegenerative disease. However, CSF tau also increased along with aging in healthy humans with no evidence of neurodegeneration (30, 31). Alternatively, tau secretion from neurons is a carrier-facilitated release and the carrier could be exosomes (32). Enhanced tau secretion in AD could be an adaptive process for excreting hyperphosphorylated, misfolded or malfunctioning tau. A particularly interesting observation was that the MMSE scores inversely correlated with the calculated secretion factor but not with the soluble brain tau. The soluble tau may represent healthy tau protein while the secretion factor could be a parameter for excreting abnormal tau. Therefore, the secretion factor may be a better predictor of cognitive performance. Whereas p-tau is also a sensitive CSF marker, testing for it is unreliable in postmortem CSF and brain tissue because the ATP consuming phosphorylation process could be quickly reversed after death.
Although our approach of estimating CSF tau and parenchymal tau in the same subject has a unique advantage, it also has some intrinsic limitations. First, the ventricular CSF tau in this study was lower than spinal CSF tau measured from live patients (33–35). This difference might be attributable to different ELISA kits, heterogeneous patient populations or anatomic sources of CSF. Second, in the event of extensive cell death in AD, NFT could be fragmented and spill over into the pool of CSF tau resulting in an overestimation of the calculated secretion factor for AD. Despite these limitations, quantitative measurement of both CSF and parenchymal tau allowed us to construct and test a numerical model. Our quantitative analysis refuted the initial hypothesis that NFT and CSF tau depends on increased brain tau synthesis (Fig. 1A). The increase in NFT in AD is more likely attributable to post-translational processes. The increase in CSF tau is due to an accelerated carrier-based secretion. Furthermore, cognitive functions are correlated with this secretion factor but not with soluble tau.
ACKNOWLEDGEMENT
We thank Melissa Song (student volunteer) and Megan Nielson (student volunteer) for helping process the tissues.
Funding: Funding was provided by the Alzheimer Association (NIRG 14-322078 to P.H.), the Arizona Alzheimer’s Consortium (National Institutes of Health/National Institute on Aging grant P30 AG19610 and the state of Arizona), the Barrow Neurological Foundation, and the joint translational neuroscience grant from the Barrow Neurological Institute and University of Arizona, Phoenix. The Brain and Body Donation Program is supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research.
Disclosure/conflict of interest: The authors have no duality or conflicts of interest to declare.
REFERENCES
- 1. Iqbal K, Wang X, Blanchard J, et al. Alzheimer’s disease neurofibrillary degeneration: pivotal and multifactorial. Biochemical Soc Trans 2010;38:962–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iqbal K, Liu F, Gong CX, et al. Mechanisms of tau-induced neurodegeneration. Acta Neuropathol 2009;118:53–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Noble W, Hanger DP, Miller CC, et al. The importance of tau phosphorylation for neurodegenerative diseases. Front Neurol 2013;4:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. James OG, Doraiswamy PM, Borges-Neto S. PET Imaging of tau pathology in Alzheimer’s disease and tauopathies. Front Neurol 2015;6:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ishiki A, Okamura N, Furukawa K, et al. Longitudinal assessment of tau pathology in patients with Alzheimer’s Disease using [18F]THK-5117 positron emission tomography. PloS One 2015;10:e0140311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blennow K, Wallin A, Agren H, et al. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease?. Mol Chem Neuropathol 1995;26:231–45 [DOI] [PubMed] [Google Scholar]
- 7. Mukaetova-Ladinska EB, Harrington CR, Roth M, et al. Biochemical and anatomical redistribution of tau protein in Alzheimer's disease. Am J Pathol 1993;143:565–78 [PMC free article] [PubMed] [Google Scholar]
- 8. Mukaetova-Ladinska EB, Harrington CR, Roth M, et al. Alterations in tau protein metabolism during normal aging. Dementia 1996;7:95–103 [DOI] [PubMed] [Google Scholar]
- 9. Barten DM, Cadelina GW, Hoque N, et al. Tau transgenic mice as models for cerebrospinal fluid tau biomarkers. J Alzheimers Dis 2011;24(Suppl 2):127–41 [DOI] [PubMed] [Google Scholar]
- 10. Trojanowski JQ, Lee VM. Pathological tau: a loss of normal function or a gain in toxicity?. Nat Neurosci 2005;8:1136–7 [DOI] [PubMed] [Google Scholar]
- 11. Dickey CA, Ash P, Klosak N, et al. Pharmacologic reductions of total tau levels; implications for the role of microtubule dynamics in regulating tau expression. Mol Neurodegener 2006;1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mukaetova-Ladinska EB, Abdel-All Z, Mugica ES, et al. Tau proteins in the temporal and frontal cortices in patients with vascular dementia. J Neuropathol Exp Neurol 2015;74:148–57 [DOI] [PubMed] [Google Scholar]
- 13. Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol 1997;56:1095–7 [DOI] [PubMed] [Google Scholar]
- 14. Petersen RC. Early diagnosis of Alzheimer's disease: is MCI too late?. Curr Alzheimer Res 2009;6:324–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vemuri P, Wiste HJ, Weigand SD, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: predicting future clinical change. Neurology 2009;73:294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vemuri P, Wiste HJ, Weigand SD, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: diagnostic discrimination and cognitive correlations. Neurology 2009;73:287–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Han P, Caselli RJ, Baxter L, et al. Association of pituitary adenylate cyclase-activating polypeptide with cognitive decline in mild cognitive impairment due to Alzheimer disease. JAMA Neurol 2015;72:333–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Braak H, Braak E. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol 1991;1:213–6 [DOI] [PubMed] [Google Scholar]
- 19. Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991;41:479–86 [DOI] [PubMed] [Google Scholar]
- 20. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–59 [DOI] [PubMed] [Google Scholar]
- 21. Choi SR, Schneider JA, Bennett DA, et al. Correlation of amyloid PET ligand florbetapir F 18 binding with Aβ aggregation and neuritic plaque deposition in postmortem brain tissue. Alzheimer Dis Assoc Dis 2012;26:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clark CM, Pontecorvo MJ, Beach TG, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol 2012;11:669–78 [DOI] [PubMed] [Google Scholar]
- 23. Beach TG, Adler CH, Sue LI, et al. Arizona study of aging and neurodegenerative disorders and brain and body donation program. Neuropathology 2015;35:354–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shiryaev N, Jouroukhin Y, Giladi E, et al. NAP protects memory, increases soluble tau and reduces tau hyperphosphorylation in a tauopathy model. Neurobiol Dis 2009;34:381–8 [DOI] [PubMed] [Google Scholar]
- 25. Karcher DS, McPherson RA, Cerebral, synovial, serous body fluids, and alternative specimens In: MacPherson RA, eds. Henry’s Clinical Diagnosis and Management by Laboratory Methods, 23rd ed.St.Louis, MO: Elsevier, Inc; 2017:481–508 [Google Scholar]
- 26. Tarawneh R, Head D, Allison S, et al. Cerebrospinal fluid markers of neurodegeneration and rates of brain atrophy in early Alzheimer disease. JAMA Neurol 2015;72:656–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu XL, Hu JY, Hu MY, et al. Sequence-dependent abnormal aggregation of human Tau fragment in an inducible cell model. Biochim Biophys Acta 2015;1852:1561–73. [DOI] [PubMed] [Google Scholar]
- 28. Harrington CR, Storey JM, Clunas S, et al. Cellular models of aggregation-dependent template-directed proteolysis to characterize tau aggregation inhibitors for treatment of Alzheimer disease. J Biol Chem 2015;290:10862–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Andorfer C, Kress Y, Espinoza M, et al. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem 2003;86:582–90. [DOI] [PubMed] [Google Scholar]
- 30. Sutphen CL, Jasielec MS, Shah AR, et al. Longitudinal cerebrospinal fluid biomarker changes in preclinical Alzheimer disease during middle age. JAMA Neurol 2015;72:1029–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Toledo JB, Zetterberg H, van Harten AC, et al. Alzheimer’s disease cerebrospinal fluid biomarker in cognitively normal subjects. Brain 2015;138:2701–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kalani A, Tyagi A, Tyagi N. Exosomes: mediators of neurodegeneration, neuroprotection and therapeutics. Molec Neurobiol 2014;49:590–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mielke MM, Haughey NJ, Bandaru VV, et al. Cerebrospinal fluid sphingolipids, β-amyloid, and tau in adults at risk for Alzheimer’s disease. Neurobiol Aging 2014;35:2486–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Samgard K, Zetterberg H, Blennow K, et al. Cerebrospinal fluid total tau as a marker of Alzheimer’s disease intensity. Int J Geriatr Psychiatry 2010;25:403–10 [DOI] [PubMed] [Google Scholar]
- 35. Yamamori H, Khatoon S, Grundke-Iqbal I, et al. Tau in cerebrospinal fluid: a sensitive sandwich enzyme-linked immunosorbent assay using tyramide signal amplification. Neurosci Lett 2007;418:186–9 [DOI] [PMC free article] [PubMed] [Google Scholar]