Abstract
Fetal alcohol spectrum disorder (FASD) is a common neurodevelopmental problem, but neuropathologic descriptions are rare and focused on the extreme abnormalities. We conducted a retrospective survey (1980–2016) of autopsies on 174 individuals with prenatal alcohol exposure or an FASD diagnosis. Epidemiologic details and neuropathologic findings were categorized into 5 age groups. Alcohol exposure was difficult to quantify. When documented, almost all mothers smoked tobacco, many abused other substances, and prenatal care was poor or nonexistent. Placental abnormalities were common (68%) in fetal cases. We identified micrencephaly (brain weight <5th percentile) in 31, neural tube defects in 5, isolated hydrocephalus in 6, corpus callosum defects in 6 (including some with complex anomalies), probable prenatal ischemic lesions in 5 (excluding complications of prematurity), minor subarachnoid heterotopias in 4, holoprosencephaly in 1, lissencephaly in 1, and cardiac anomalies in 26 cases. The brain abnormalities associated with prenatal alcohol exposure are varied; cause–effect relationships cannot be determined. FASD is likely not a monotoxic disorder. The animal experimental literature, which emphasizes controlled exposure to ethanol alone, is therefore inadequate. Prevention must be the main societal goal, however, a clear understanding of the neuropathology is necessary for provision of care to individuals already affected.
Keywords: Autopsy, Epidemiology, Ethanol, Fetal alcohol, Human brain, Nicotine
INTRODUCTION
The potential for adverse effects of alcohol (ethanol) on the developing fetus has been recognized for centuries (1). The modern era of medical study began after the publications by Lemoine and coworkers in 1968 and by Jones and coworkers in 1973 (2, 3). They identified stillbirths, prematurity, growth retardation, cognitive delay, face and limb anomalies, microcephaly, and cardiac defects. The first autopsy description of presumed alcohol-related teratogenic effects documented microphthalmia, partially fused cerebral hemispheres with leptomeningeal hamartomas, simplified gyral pattern, large lateral ventricles, and agenesis of the corpus callosum (4). Images from this extreme case have been widely reproduced in publications and on the Internet. This was originally termed fetal alcohol syndrome (FAS). The more recent designation, fetal alcohol spectrum disorder (FASD), encompasses cases with functional abnormalities but not necessarily overt physical anomalies (5). However, the clinical diagnostic criteria (which include historical evidence of in utero alcohol exposure, facial anomalies, growth rate, head size, and neurodevelopmental features) are not unanimously agreed upon, with several different methods in use (6–9).
The prevalence of FASD has been difficult to determine, due to underreporting of maternal alcohol use and differences in diagnostic criteria. In the broad population up to 0.5% might be affected, while in certain geographic groups >20% might be affected (10). FASD is among the most common neurodevelopmental disorders (11, 12) and is associated with substantial costs to society because of lost productivity due to premature mortality and morbidity (13).
Despite the apparently high prevalence of FASD, neuropathological findings have been described in only 33 autopsy cases of suspected FASD (14–18). Reported abnormalities include micrencephaly, regional cortical dysgenesis, leptomeningeal heterotopias, hydrocephalus, holoprosencephaly, neural tube closure defects, partial or complete agenesis of the corpus callosum, cerebellum, and brainstem dysgenesis. In addition, ∼80 fetuses with a history of prenatal alcohol exposure (PNAE) have been described; the most convincing features are subtle abnormalities of the cortical vasculature (19–21) (see details of all known prior autopsy reports in Supplementary Data S1). Quantitative neuroimaging studies on people with FASD highlight the micrencephaly, abnormal gyral patterns, reductions in regional brain volume (primarily basal ganglia, diencephalon, and cerebellum), and abnormalities of the corpus callosum (22–25).
The reported autopsy material tends to document extreme abnormalities that are less frequently reported in the imaging literature. Therefore, our goals were (1) to assemble a community-based autopsy database of cases with reported PNAE and/or FASD diagnosis, (2) to document the epidemiological and neuropathological features of those cases, and (3) to assemble a collection of tissues to be used for future studies capable of validating pathogenetic hypotheses that have arisen from animal experimentation (26), including the potential association between PNAE and epigenetic modifications (27).
MATERIALS AND METHODS
Identification of Cases
Ethics approval was obtained from the Health Research Ethics Board of the University of Manitoba (approval #HS13161 – H2011:213). We conducted a retrospective survey of autopsies performed at the Health Sciences Centre (HSC) in Winnipeg, Canada from 1980 to 2016. HSC serves a population of about 1.3 million people. Manitoba has a relatively high prevalence of FASD (28) and has had a centralized diagnostic center for 2 decades (29). One of the coauthors (A.E.C.) has been a leader in developing diagnostic criteria for FASD (8, 30). Approximately 1100 autopsies are performed annually, among which ∼80% are medico-legal. The total includes 100–200 fetal/pediatric cases and ∼350 complete brain examinations per year. All pediatric autopsies since 1980 and all adult autopsies since 1996 (both medico-legal and family permission) are amenable to full-text search using DocFetcher software (SourceForge.net; open source software license from Eclipse Public License).
The senior author (M.R.D.) identified candidate cases by whole text search using a wide range of key words related to alcohol consumption during pregnancy or a clinical diagnosis of FASD; e.g. EtOH, alcohol, ethanol, booze, fetal alcohol, FASD, FAS, pFAS, FAE, ARND, ARBD, binge, drink, drank, drunk, liquor, beer, wine, vodka, whisky, home brew, substance abuse, etc. The clinical details of ∼500 reports were screened; ∼200 cases with probable PNAE or FASD were identified. Files predating 1994 had been scanned into searchable PDF format from microfilms, which at times did not allow for perfect text recognition. Candidate reports were thoroughly reviewed and summarized by the first author (J.S.J). Cases with a diagnosis or “suspected” FASD diagnosis were further investigated by review of records in the Program in Genetics and Metabolism database, the Manitoba FASD Centre (formerly the Clinic for Alcohol & Drug Exposed Children; established in 1999), or by hospital chart review. Cases that were not confirmed remained “suspected”, as a physician might have made the diagnosis without referral to the Manitoba FASD Centre, where a multidisciplinary team assessment is standard. The inclusion criteria were met by 174 cases.
Age Groupings and Descriptive Epidemiology
Cases were divided into 5 age groups: fetuses (stillborn/intrauterine death; n = 52); infants (1 day old to 12 months of age, including premature births; n = 65); children (13 months to 12 years; n = 32); teens (13–19 years; n = 14); and adults (20–65 years; n = 11). Details extracted from the reports included age, sex, birth characteristics (gestation, weight, method of birth, stillborn/liveborn), and maternal health and substance abuse during pregnancy (e.g. disease, infections, estimated alcohol use, tobacco use, other drug use, age at birth, prior abortions, parity and gravida, level of prenatal care, etc.). The maternal details were seldom available for children, teens, and adults. Additional information in these age groups included the history of FASD diagnosis, history of foster care or contact with Child & Family Services, history of epilepsy, and the individuals’ own substance abuse. Autopsy details included cause of death, brain weight, FASD facial anomalies (short palpebral fissures, smooth philtrum, and thin upper lip), other facial anomalies, abnormal palmar creases, and congenital abnormalities affecting other body regions (heart, kidneys, and skeletal). Brain weight percentiles were classified as <5th, >5th – ≤50th, >50th – ≤95th, and >95th percentile according to published autopsy databases (31–34). A brain weight <5th percentile was classified as micrencephalic. Microcephaly is usually defined as a head circumference of ≤3rd percentile, but it may not be a sensitive marker of PNAE (35). Because this is a retrospective analysis, almost all cases were missing some information. Percentage values only represent cases for which information was reported; they are not based on the totals.
Maternal alcohol ingestion was difficult to estimate because descriptors concerning amount, timing, and duration of alcohol exposure were varied. We broadly categorized alcohol ingestion as minimal/moderate use (“one episode of drinking”, “occasional alcohol”, “in the first trimester only”, etc.) or heavy/chronic use (“binge drinking”, “drinking heavily throughout pregnancy”, “arrived in hospital intoxicated and in labor”, etc.). Descriptors such as “drank during pregnancy”, “mother abuses alcohol”, or “mother has a history of alcohol abuse” were harder to classify. In rare cases, other children in the family had been diagnosed with FASD, but the mother denied current alcohol use.
Neuropathologic Examinations
Photographs of the brain were retrieved to verify gross abnormalities. Complete neuropathological assessment was conducted in 135 cases. Nine stillborn infants had autolytic liquefaction of the brain, which was not examined by microscopy. Among the teen and adult cases, the forensic pathologist cut 6 brains fresh with only 1 or 2 tissue samples saved for histology, and in 8 cases the brain was not examined. In general, brain samples had been fixed in 10% buffered formalin for 1–2 weeks, and then select regions were embedded in paraffin. Cases with a complete examination by a neuropathologist had, at a minimum, samples taken of parasagittal frontal lobe (level of caudate nucleus head), basal nuclei, hippocampus/medial temporal lobe, thalamus, midbrain, pons, and posterior cerebellum. In all cases, the glass slides (or recut slides) were reviewed by the senior author (M.R.D.), an experienced neuropathologist. The minimum histologic evaluation included hematoxylin and eosin staining of 5-µm-thick paraffin sections mounted on glass slides.
RESULTS
Complete epidemiological details of the 174 cases are presented in Supplementary Data S2. Details of the neuropathologic abnormalities are presented in Table 1.
TABLE 1.
Major Malformative Neuropathologic Features in Cases With Documented Prenatal Alcohol Exposure (PNAE) or Clinical Diagnosis of Fetal Alcohol Spectrum Disorder (FASD)
| Age Group | Case | Age at Death | Clinical History | PNAE estimated amount | PNAE estimated duration | Tobacco | Other Drugs | Neuropathologic Findings | Facial anomalies | Cardiac anomalies | Organ/ Skeletal anomalies |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Case 4 | 20 weeks gestation male | Mother 17 years, hypothyroid (treated with levothyroxine), drank monthly, used cannabis daily, and smoked ∼10 cigarettes per day during pregnancy. Pregnancy terminated. | +++ | Entire | + | marijuana | Exencephaly with focal defect in calvarium, destruction of cerebrum, and secondary destructive changes in diencephalon and hindbrain; asymmetry of eyes and cleft palate are suggestive of amniotic band adhesion. | + | − | − |
| 1 | Case 13 | 24 weeks gestation female | Mother 30 years, occasional alcohol and cigarettes during pregnancy. Stillborn. | ++ | Entire | + | − | Anencephaly, vertebral and rib anomalies, absent kidneys, absent left lung, ambiguous female genitalia. | − | − | + |
| 1 | Case 14 | 23 weeks gestation male | Mother 37 years, drank during pregnancy. Pregnancy terminated. | Uncertain | Entire | ? | − | Brain 122 g (≥50th percentile). Lumbosacral myelomeningocele and Chiari type 2 malformation with distorted cerebellum. | − | − | − |
| 1 | Case 21 | 29 weeks gestation male | Mother 19 years, history of physical abuse during pregnancy. Liveborn by emergency caesarean section—died immediately | ++ | Entire | + | − | Brain 710 g (≥95th %ile). Moderately severe hydrocephalus due to aqueduct stenosis, partial agenesis of the posterior corpus callosum, misshapen midbrain with rudimentary cerebral peduncles, microscopic subventricular zone heterotopia on surface of lateral ventricle temporal horn, microscopic glial heterotopia in meninges ventral to thalamus and posterior to midbrain, asymmetric cerebellum, microphthalmia. | − | − | − |
| 1 | Case 27 | 33 weeks gestation male | Mother 30 years, type 2 diabetes, drank during pregnancy and smoked ∼4 cigarettes per day. Stillborn. | Uncertain | Entire | + | − | Brain 133 g (<5th percentile). Fused eyes, superior proboscis, alobar holoprosencephaly, horseshoe kidney with two ureters, arachnodactyly. Genetic analysis was not done. | + | − | + |
| 1 | Case 45 | 38 weeks gestation male | Mother (age not documented), drank and abused other drugs during pregnancy. Stillborn. | Uncertain | Entire | − | + (not specified) | Brain 233 g (<5th percentile). Sacral spina bifida with dermal sinus and filum terminale lipoma. | + | − | + |
| 2 | Case 60 | 3 days (32 weeks gestation) male | Mother 34 years, chronic alcohol abuser and smoked tobacco (1 package cigarettes per day). | +++ | ? | + | − | Brain 247 g (>50th %ile). Lumbosacral myelomeningocele with Chiari type 2 malformation, bilateral cystic kidneys. | − | − | + |
| 2 | Case 64 | 17 days (33 weeks gestation) male | Mother 21 years, drank heavily during first trimester (binged 3 times) and smoked tobacco (5–10 cigarettes per day). Died from complications of prematurity. | +++ | First trimester | + | − | Chromosomes normal. Abnormal face with micrognathia, small palpebral fissures, flat philtrum, tethered tongue. Brain 267 g (10th %ile). Absent posterior corpus callosum and slightly enlarged lateral ventricles, stenotic cerebral aqueduct and distorted pons, small cerebellum with deficiency of the inferior vermis, periventricular heterotopia on lateral ventricle walls (associated with no hemosiderin, no astroglial scar, and no buried ependymal cells), extensive leptomeningeal heterotopia erupting from posterior surface of midbrain | + | − | − |
| 2 | Case 71 | 17 days female | Mother 18 years, drank alcohol and sniffed solvents during pregnancy. Born at term, nondysmorphic, died from congenital Langerhans histiocytosis. | Uncertain | Entire | ? | solvent (toluene) inhalation | Brain 436 g (20th %ile), grossly normal; multiple small subarachnoid heterotopia on temporal lobe. | − | − | − |
| 2 | Case 72 | 13 days female | Born at 41 weeks. Hydrocephalus demonstrated by MR imaging at 4 days. Sudden death in bed. | Uncertain | Entire | + | − | Low set ears, small muscular ventricle septal defect in heart and polydactyly on one hand. Brain 540 g (>50th %ile). Moderately severe hydrocephalus, agenesis of posterior corpus callosum (likely not attributable to ventriculomegaly), and aqueduct stenosis | + | + | − |
| 2 | Case 76 | 22 days male | Mother 28 years, born at term, seizures. Died from complications due to congenital anomalies. | ++ | First trimester | − | − | Multiple anomalies: Bilateral cleft lip and palate, tetralogy of Fallot, single cystic kidney, bifid scrotum/hypospadias/cryptorchidism, upslanted eyes. Brain 387 g (<5th %ile). Small cysts with hemosiderin near interventricular foramen (probable in utero hemorrhage), small neuroglial heterotopia on ventricle wall near hippocampus, multiple small glial heterotopia in leptomeninges around midbrain | + | + | + |
| 2 | Case 79 | 28 days female | Mother (age not documented) drank heavily during pregnancy, possible viral illness ∼16th week. Baby born at term. Died from congenital cardiac malformation. | +++ | Entire | ? | − | Multiple anomalies; growth retarded, microcephaly, micrencephaly, complex cardiac defect (aortic isthmus hypoplasia, atrial and ventricular septal defects, large foramen ovale), thin lips, abnormal T7 vertebra, abnormal ovaries. Brain 276 g (<5th %ile), asymmetric temporal lobe gyri, agenesis posterior corpus callosum. | − | + | + |
| 2 | Case 83 | 1 month male | Mother 28 years, drank and smoked during pregnancy. Mother was drinking before going to bed with her infant (born at term). Died in bed with parent. | Uncertain | Entire | + | − | Nondysmorphic. The left hand showed a single transverse palmar crease. Brain 600 g (>50th %ile). Diplomyelia with splitting of central canal at mid lumbar level and duplication of sacral spinal cord (no external signs). | − | − | − |
| 2 | Case 85 | 5 weeks male | Born at term. Failure to thrive and dehydration. Multiple congenital anomalies. Died in bed. | Clinical diagnosis FAS | (denies use) | ? | − | Multiple anomalies: typical facial dysmorphism (low set ears, short palpebral fissures, poorly formed philtrum, thin upper lip, anteriorly tethered tongue), complex cardiac anomalies (subaortic VSD, partially closed by tricuspid valve tissue, ostium secundum ASD), dysplastic right kidney. Brain 421 g (10th %ile), grossly normal, rare heterotopia in frontal leptomeninges. | + | + | + |
| 2 | Case 91 | 7 weeks male | Mother had Grave’s disease (on propylthiouracil), Group B Streptococcus positive, poor prenatal care. Smoked during pregnancy and had alcohol early on. Born at term; died in bed with parent. | Uncertain | Early | + | − | Nondysmorphic. Brain 558 g (>50th %ile), grossly normal. Small glial heterotopia in leptomeninges around midbrain. | − | + | − |
| 2 | Case 109 | 4.5 months female | Born at term, infantile epilepsy treated with phenobarbital. MR imaging showed mild ventriculomegaly. Sudden unexplained death in bed. | Uncertain | Early | ? | marijuana | Brain 888 g (95th %ile). Mild hydrocephalus with no obvious histologic abnormalities. Site of CSF obstruction not identified. | − | − | − |
| 2 | Case 114 | 7 month male | Born at term. Mother smoked and consumed large amounts of alcohol during pregnancy. Sudden unexplained death in bed. | +++ | Early | + | − | Dolichocephalic skull (long head), wide spaced eyes, low set posteriorly rotated ears, high arched palate, abnormal right kidney, and arachnodactyly. MR of brain showed mild ventriculomegaly. Brain 1221 g (95th %ile), slightly abnormal gyri, and mild hydrocephalus (photographs were not available for review), no microscopic abnormalities. Site of CSF obstruction not identified. | + | − | + |
| 3 | Case 118 | 12.5 month male | Mother has history of drug and alcohol abuse. Born at term, nondysmorphic, Sudden unexplained death in bed. | Uncertain | Unknown | − | + (not specified) | Brain 930 g (<50th %ile), mid corpus callosum narrow (2 mm vs 4 mm on microscopy; no photographs available for review), old occipital lobe infarct (1 cm maximum dimension cavity with hemosiderin in wall), mild enlargement of lumbar central canal (hydromyelia). | − | − | + |
| 3 | Case 127 | 2 years female | No maternal details available. Severe neurological handicap, suspected fetal alcohol syndrome. Died from adenovirus pneumonia. | Clinical diagnosis FAS | Unknown | ? | ? | Dysmorphic face (microcephaly, smooth upper lip, epicanthic folds). Heart—ventricular septal defect, posterior overriding aorta. Brain weight not recorded, lissencephaly with abnormally thick neocortex, calretinin immunostain does not define lamination, poor distinction with underlying white matter. Miller–Dieker phenotype but no chromosomal or genetic analysis. | + | + | + |
| 3 | Case 132 | 3.5 years female | Mother sniffed gasoline regularly during pregnancy; full birth details not available (child in foster care). Severe cognitive delay. Died from pneumonia. | Clinical diagnosis FAS | Unknown | ? | gasoline inhalation | Scoliosis, congenital dysplasia of the left hip. Brain 420 g (<5th %ile), frontal ulegyria, occipital microgyria with laminar necrosis, cavitated infarct in thalamus, unilateral ventricular enlargement, hippocampal atrophy, severe Purkinje neuron loss. The pattern of destruction is suggestive of a prenatal insult. | − | − | + |
| 3 | Case 139 | 5.5 years male | Mother abused a variety of substances including alcohol. Born at term. Hydrocephalus. Unexplained episodic bradycardia. Died of sudden respiratory arrest. | Uncertain | Entire | ? | solvent (toluene) inhalation | Nondysmorphic. Brain weight not recorded. Moderately severe chronic hydrocephalus caused by midbrain venous malformation that compressed cerebral aqueduct. Secondary axon damage in periventricular white matter indicates acute exacerbation. No other malformations. | − | − | − |
| 3 | Case 141 | 5.5 years female | Mother 21 years, smoked and drank during pregnancy; birth at 29 weeks gestation. Multiple congenital anomalies and multiple corrective surgeries. Died from complications of severe hydrocephalus. | Uncertain | Entire | + | − | Multiple congenital anomalies including: hydrocephalus, vertebral defects, partial sacral agenesis, imperforate anus, cloacal outlet obstruction (VATER-H complex). Brain 1450 g (>95th %ile); very severe hydrocephalus due to aqueduct stenosis with secondary destructive changes in periventricular structures. | + | + | + |
| 3 | Case 143 | 7 years female | Maternal & birth details not available. Cerebral palsy, seizures, mild scoliosis. Died from pneumonia. | Clinical diagnosis FAS | ? | ? | ? | Microcephaly and hypertelorism. Brain 750 g (<5th %ile), atrophy and discoloration bilateral cerebral white matter with cystic destruction in left frontal lobe, hydrocephalus (predominantly ex vacuo), and severe neuron loss from hippocampi (mesial temporal sclerosis). The pattern of damage is suggestive of premature birth complications. | − | − | + |
| 3 | Case 146 | 9.5 years female | Mother abused various substances during pregnancy. Born at term, severe cognitive delay and epilepsy, severe hydrocephalus, died from pneumonia. | Clinical diagnosis FAS | ? | ? | + (not specified) | Abnormal head shape and scoliosis. Brain 1380 g (>95th %ile), moderately severe ventriculomegaly due to aqueduct stenosis and destructive changes in white matter secondary to the ventricular enlargement, and mesial temporal sclerosis. | − | − | + |
| 3 | Case 147 | 11 years female | Birth age not available. Female with severe cerebral palsy, spastic quadriparesis, severe developmental delay, and epilepsy. Died from pneumonia. | Uncertain | First trimester | + | − | Brain 385 g (<5th %ile). Bilateral symmetric destruction (likely ischemic) of cerebral hemispheres in distribution of middle cerebral arteries. Hippocampi were normal. The pattern of damage is suggestive of a midgestation in utero insult. | − | − | − |
| 4 | Case 157 | 15.5 years female | Female born at 29 weeks gestation. Mother smoked and drank alcohol during the pregnancy. In foster care. Small ventricular septal defect, atrial septal defect, and patent ductus arteriosus surgically repaired. Spastic quadriparesis (right > left), hearing and vision impaired, seizures treated with carbamazepine, and autistic-like features. Died from acute gastric volvulus. | Uncertain; clinical diagnosis FAE | Entire | + | – | Brain 1180 g (<50th %ile). The main neuropathological abnormality was an old infarct in the middle cerebral artery distribution involving the left temporal and parietal lobes. The lesion is more typical of an arterial ischemic lesion related to the cardiac anomaly, rather than a direct complication of premature birth. | – | + | – |
| 5 | Case 174 | 60 years male | Cognitive delay, epilepsy. Cause of death was atherosclerotic coronary artery disease | Clinical diagnosis FAS | ? | ? | Dysmorphic face (flat philtrum, wide spaced eyes), brain 1270 g (<5th percentile), mild ventriculomegaly, aqueduct stenosis at level of lower midbrain | + | − | − |
Tabular summary in each case shows age, clinical history and nonneurological anomalies (absent (–) or present (+)) possibly attributable to PNAE, estimated magnitude and duration of alcohol exposure in utero (+ = rare/minimal; ++ = occasional; +++ = regular/heavy, and uncertain), and other in utero substance exposures (admitted use (+), denied use (–), unknown (?). PNAE exposure amount is estimated from available history, which is imprecise and possibly an underestimate.
Stillbirth and Intrapartum Deaths
In the stillborn/intrapartum death cohort, we had 52 fetuses (20–41 weeks gestation, median 32 weeks gestation), with an almost equal ratio of male to females (25:27). Three pregnancies were electively terminated for malformation (neural tube defects for 2, kidney and bladder defects for 1), 5 cases were live born premature infants who died <1 day, and the remaining 44 were stillborn fetuses. Most (38/52) were premature (<37 weeks). Birth weight was below the 10th percentile (definition of small for gestational age) in 22 cases (11 of which were below the 5th percentile). The maternal age ranged from 15 to 39 years (median 25 years). Nine mothers had a vaginal or sexually transmitted infection at the time of birth and 5 had diabetes (Type I or II, 4 cases; gestational diabetes, 1 case). When indicated, >75% reported poor or no prenatal care during their pregnancy. Twelve cases had exclusively early/first trimester alcohol exposure (3 “minimal” and 4 “heavy”), 21 cases had the vague description of minimal use during pregnancy or history of alcohol use, and 19 had descriptors suggesting more substantial use into the second trimester or throughout pregnancy. In addition to alcohol ingestion, 86% of mothers smoked tobacco during the pregnancy and 77% abused other illicit drugs (when reported).
Anomalies were identified in the heart (n = 5), kidneys (n = 3), and skeletal system (n = 10). Diagnostic FASD facial anomalies were identified in 3 cases and other facial anomalies were identified in 10 cases. Placentas were examined in 50/52 cases; 16 were normal, 16 had chorioamnionitis / villis inflammation, 10 had hemorrhage or focal infarction, 4 had features or placental abruption and the remaining 4 cases has other findings.
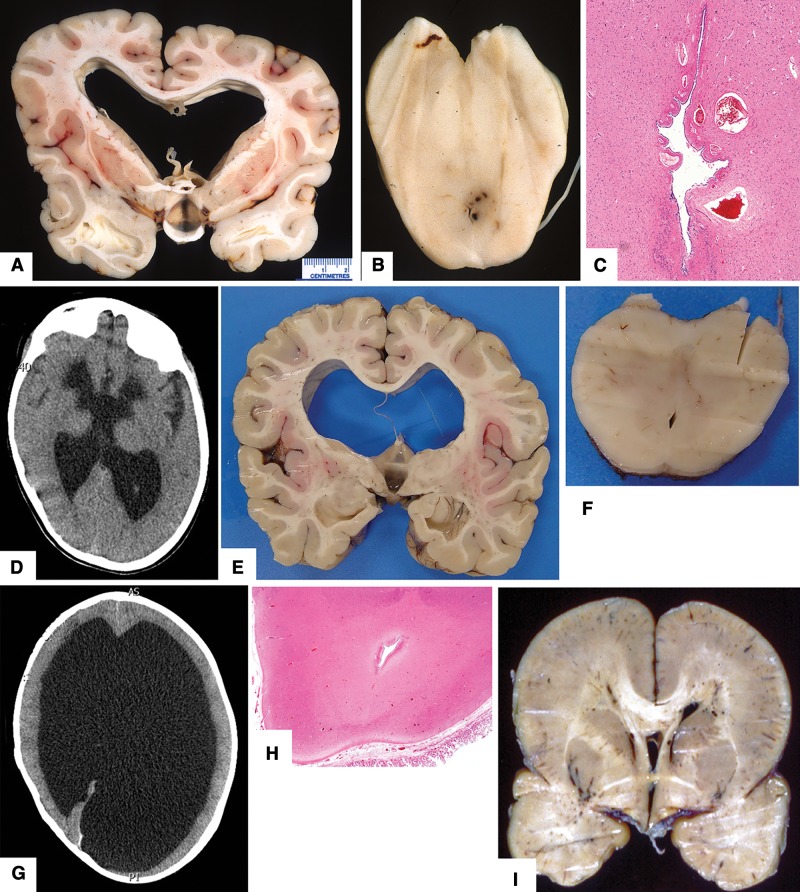
Brain weight was <5th percentile in 10 cases. Congenital neuropathological findings included 4 cases with neural tube defects (2 lumbosacral, 1 exencephaly (Fig. 1A, B), 1 anencephaly), 1 case with a complex brain malformation (moderately severe hydrocephalus due to aqueduct stenosis, partial agenesis of the posterior corpus callosum) (Fig. 1C-H), and 1 case with alobar holoprosencephaly. Multiple congenital anomalies were found in 2 cases. Acquired neuropathological findings included 12 cases with widespread hypoxic neuronal damage (typically apoptotic neurons in the hippocampus and pons), 2 cases with small periventricular hemorrhages, and 1 case with small foci of white matter necrosis (periventricular leukomalacia). Among the cases with placental anomalies, 12 brains had hypoxic neuron damage, 5 brains were not examined due to severe autolysis, 2 brains were not examined (for unknown reasons), 1 case had a neural tube defect, and 1 case had periventricular leukomalacia.
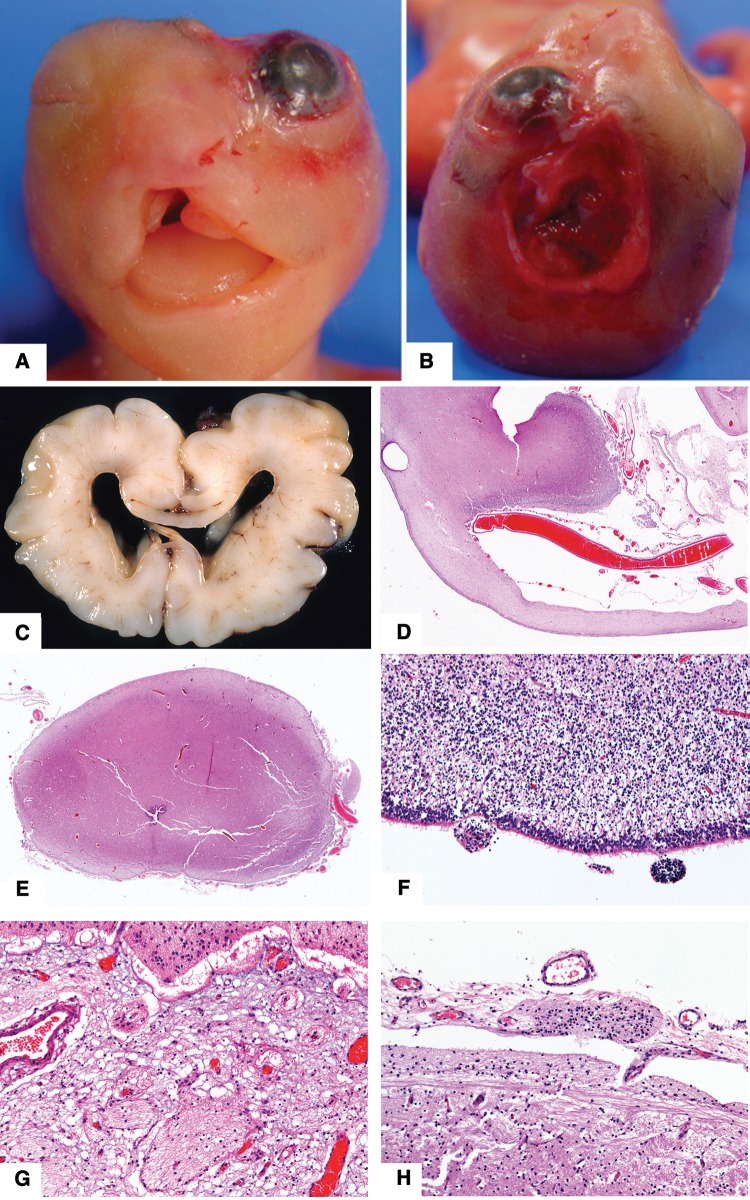
FIGURE 1.
Neuropathologic features in fetal cases (see Table 1 for details). (A, B) Case 4. Photographs show face and vertex view of craniofacial defects associated with amniotic band. (C–H) Case 21. (C) Coronal slice showing moderately severe hydrocephalus. (D) Coronal section through the mid cingulate gyrus shows a thin corpus callosum, which was absent more posteriorly (original magnification: 12.5×). (E) Misshapen midbrain with rudimentary cerebral peduncles and a narrow cerebral aqueduct (original magnification: 12.5×). (F) Microscopic subventricular zone heterotopia are present on surface of lateral ventricle temporal horn (original magnification: 400×). (G) Microscopic glial heterotopia are present in meninges ventral to thalamus and (H) posterior to midbrain (original magnifications: both 400×). All hematoxylin and eosin stain.
Infants
In the infant cohort, 65 individuals died between the ages of ≥1 day and 12 months (median 5 weeks). Male sex predominated (65%), 24 (39%) were premature, and 4 had a birth weight <5th percentile. Mother’s age at birth ranged from 15 to 40 years of age (median 24 years). Eighteen reported poor or no prenatal care (no information was available for the remainder). At the time of birth, 5 mothers had a vaginal/sexually transmitted infection (or had been treated for one during the pregnancy). Two had diabetes (1 type II and 1 gestational), 1 had tuberculosis, 1 had hypertension, and 1 had Grave’s disease. In terms of PNAE, 17 (26%) cases had a maternal history of alcohol use/abuse, 19 (29%) reportedly drank alcohol during/throughout the pregnancy, 9 had minimal or occasional alcohol during pregnancy, 11 had documented drinking during a specific trimester (first: 6 cases, second: 4 cases, and third: 1 case) with the amount unspecified, and 7 had heavy/chronic use of alcohol during pregnancy (2 during the first trimester exclusively). Seven infants had a history of seizures/epilepsy; among these 3/7 had been born prematurely, and 2/7 had multiple congenital anomalies. The top 4 causes of death were sudden unexplained death in an infant (SUDI) (28%), complications due to malformations (23%), infection/sepsis (bacterial or viral) (19%), and unsafe sleeping environment (17%).
Fourteen cases had heart defects, 6 had kidney defects, and 4 had skeletal system defects. Twelve cases had typical FASD facial anomalies and 12 had other facial anomalies. Only 6 cases had placental examination; 3 had chorioamnionitis (no brain abnormalities), 1 had abruption (rare heterotopia in frontal leptomeninges), and 2 were normal.
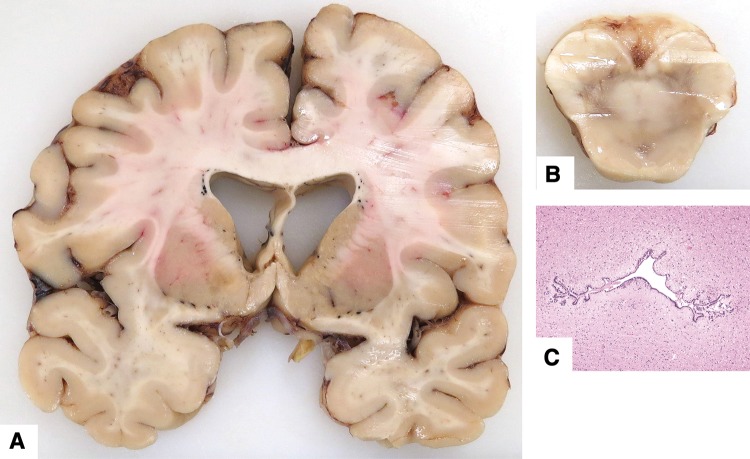
Eight cases (13%) had a brain weight below the 5th percentile (i.e. micrencephaly). One case had lumbosacral myelomeningocele with Chiari Type 2 malformation (NTD), 1 case had lumbar diplomyelia (NTD) (Fig. 3A, B), 4 cases had hydrocephalus (2 mild; 2 with agenesis of posterior corpus callosum (Fig. 2A-F and G-J)) and 1 case had agenesis of posterior corpus callosum and microcephaly (Fig. 2K). Four cases had only minor leptomeningeal heterotopias (Fig. 3C-G). Nonmalformative neuropathologic findings included 10 cases with hypoxic-ischemic neuronal changes or resolving brain hemorrhage (complications of premature birth), 1 case with periventricular leukomalacia (infant had been born at term), and 5 cases with bacterial meningitis. Among the 7 infants with a history of seizures or epilepsy, the hippocampi were normal.
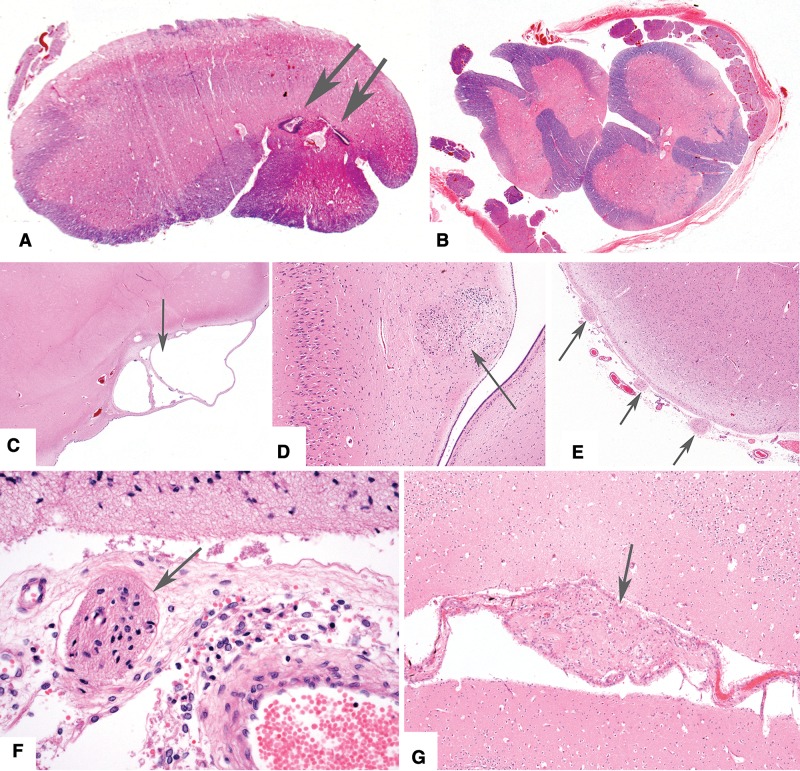
FIGURE 3.
Neuropathologic features in infant cases with other less severe anomalies (see Table 1 for details). (A, B) Case 83. Photomicrographs (solochrome cyanin stain; original magnification: 12.5×) showing diplomyelia with splitting of central canal at mid lumbar level (arrows) and duplication of sacral spinal cord. There were no external abnormalities. (C–E) Case 76. (C, Small cysts (arrow) with hemosiderin, likely caused by in utero hemorrhage, near the interventricular foramen (12.5×, hematoxylin and eosin stain). (D) Small neuroglial heterotopia on ventricle wall near hippocampus (100×). (E) Multiple small glial heterotopia in leptomeninges around midbrain (40×). (F) Case 71. Multiple tiny subarachnoid heterotopias (arrow) in leptomeninges over lateral temporal lobe. Some of these surround small blood vessels (400×). (G) Case 85. Rare small heterotopias (arrow) in frontal lobe sulcus (100×).
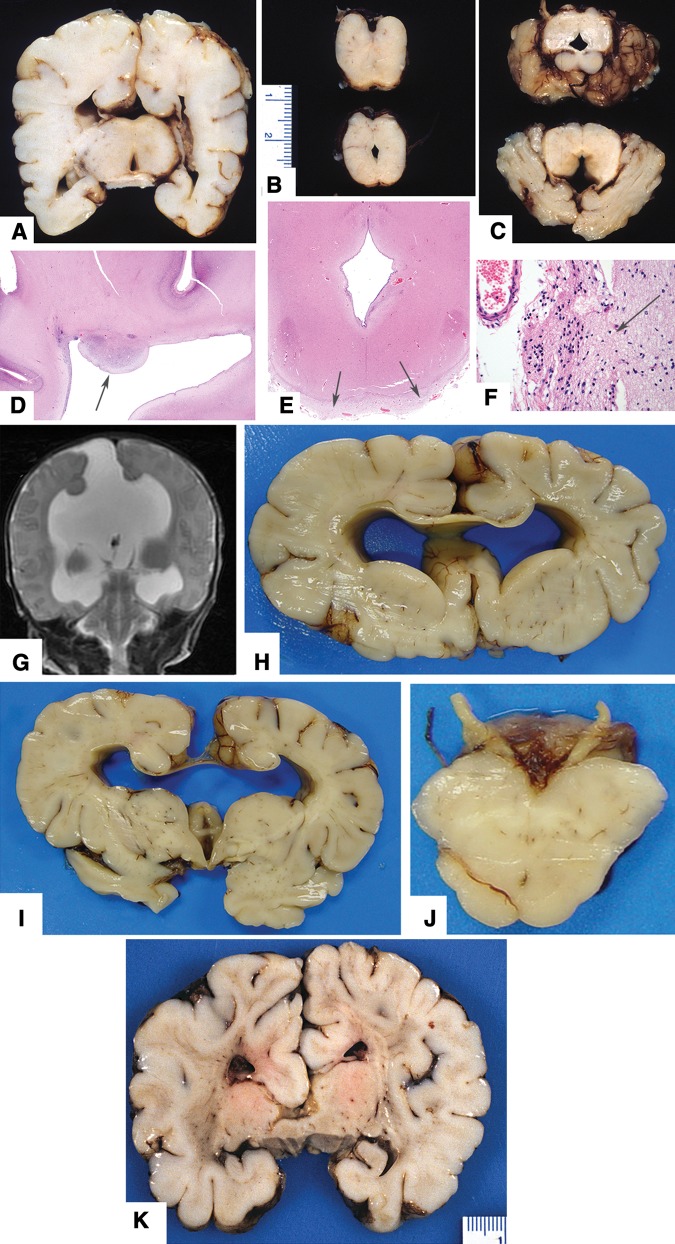
FIGURE 2.
Neuropathologic features in infant cases with complex anomalies including corpus callosum defects (see Table 1 for details). (A–F) Case 64. (A) Coronal slice through posterior frontal level shows absent posterior corpus callosum and slightly enlarged lateral ventricles. (B) Horizontal slices through upper brainstem showing stenotic cerebral aqueduct and distorted pons. (C) Horizontal slices showing small cerebellum with deficient inferior vermis. (D) Periventricular heterotopia on lateral ventricle walls (original magnification: 12.5×). Heterotopias were not associated with hemosiderin, astroglial scar, or buried ependymal cells. (E) Extensive leptomeningeal heterotopias cover the posterior surface of midbrain (12.5×) through interruptions in the pial surface (F, magnification 400×; arrows). (G–J) Case 72. (G) Magnetic resonance image (T2 weighted) at 4 days age shows hydrocephalus and absence of posterior corpus callosum. (H) Coronal slice through frontal lobes shows moderately severe hydrocephalus and thin corpus callosum. (I) Coronal slice of brain showing agenesis of posterior corpus callosum. (J) Horizontal slice through midbrain showing aqueduct stenosis. (K) Case 79. Coronal slice through posterior frontal level of a 28-day-old infant with no history of seizures shows asymmetric temporal lobes with incomplete rotation of the hippocampi (normal microscopic features) and agenesis of posterior corpus callosum.
Children
In the child cohort, 32 individuals died between 13 months and 12 years of age (median 3.5 years). Most were female (59%). A minority of autopsy reports documented maternal factors (18%) or details of the child’s birth (28%); this is likely because the details were less relevant to the child’s cause of death. Four cases had a confirmed FASD diagnosis, while 12 had a suspected FASD diagnosis. Seven cases had a seizure disorder or epilepsy. A bacterial or viral infection was the cause of death in 50%, followed by drowning or other environmental cause in 19%.
Six cases had a heart defect, 3 had kidney defects and 10 had skeletal anomalies. Seven cases had FASD facial anomalies, and 4 cases had other facial anomalies.
Six cases (21%) had a brain weight <5th percentile; 3 of these had ischemic brain lesions acquired in the perinatal period (Fig. 5A-G). One case, with a brain weight <10th percentile, had mild delay in cerebral myelination and mildly enlarged cerebral ventricles. Three cases had moderate to severe hydrocephalus (Fig. 4A-H), and 1 had Miller-Dieker type lissencephaly (Fig. 4I). Nonmalformative neuropathologic findings include 4 cases with residual damage due to hypoxic and hemorrhagic complications of premature birth, 1 with an old occipital lobe hemorrhagic infarct (Fig. 5H), 1 with severe acute brain trauma, 1 with acute meningitis, and 1 with chronic damage related to perinatal meningitis. Among the 7 cases with seizure disorder/epilepsy, 3 had hippocampi with neuron loss and reactive glial changes (“mesial temporal sclerosis”).
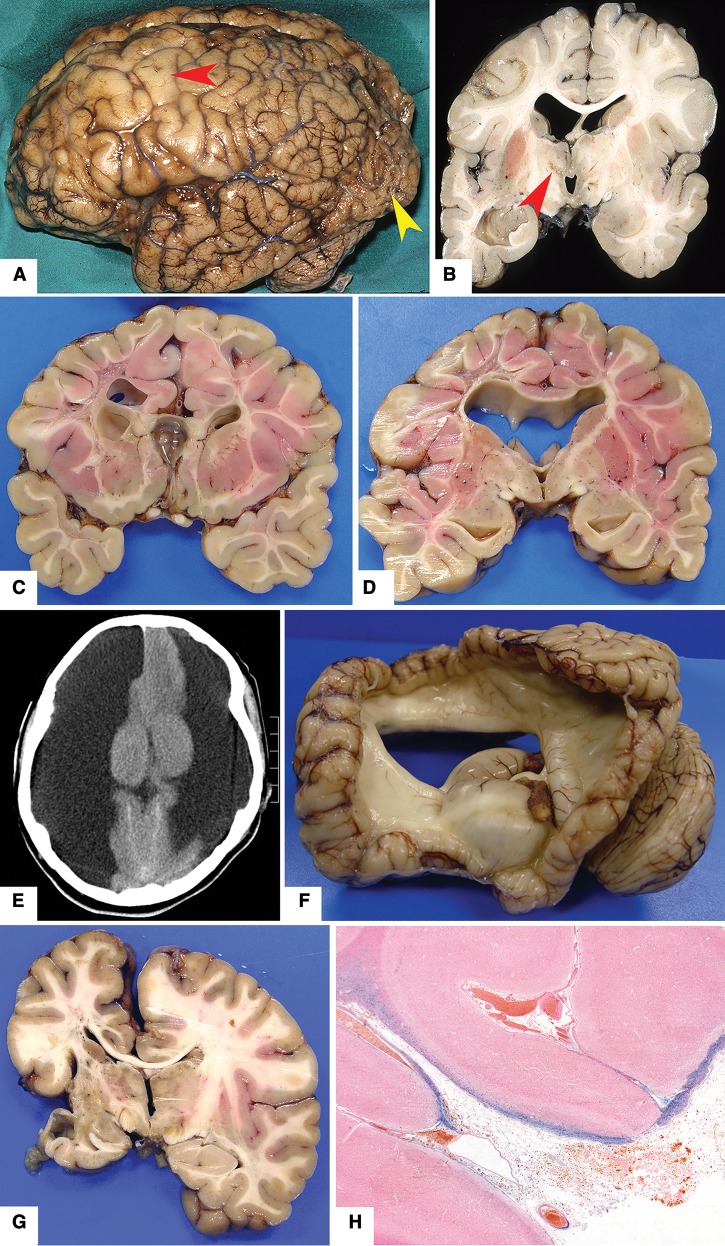
FIGURE 5.
Neuropathologic features in childhood cases with anomalies likely due to prenatal or perinatal ischemic brain insults (see Table 1 for details). (A, B) Case 132. Lateral view of the brain shows frontal ulegyria (red arrowhead) and occipital microgyria with laminar necrosis (yellow arrowhead). Coronal slice of the brain shows a cavitated infarct in the thalamus (red arrowhead) and unilateral ventricular enlargement. In addition, there was hippocampal atrophy and severe Purkinje neuron loss (not shown). The pattern of destruction is suggestive of a prenatal insult. (C, D) Case 143. Coronal slices through the brain show atrophy and discoloration bilateral cerebral white matter with cystic degeneration in left frontal lobe, and compensatory ventricular enlargement. In addition, there was >90% neuron loss from the hippocampi, especially the CA1 sectors (mesial temporal sclerosis). The pattern of damage is suggestive of premature birth complications. (E, F) Case 147. CT scan at 8 years age showed bilateral cerebral destruction. At autopsy, the brain exhibited bilateral symmetric loss of tissue in the distribution of the middle cerebral arteries. Hippocampi were preserved. The pattern of damage is suggestive of a midgestation in utero ischemic damage. (G) Case 157. Photograph showing an old ischemic infarct in the middle cerebral artery distribution involving the left temporal and parietal lobes; this is possibly related to the cardiac anomaly. (H) Case 118. Photomicrograph showing hemosiderin (blue) along the wall of a cavity extending from the surface of the occipital lobe to the ventricle wall (Perls’ Prussian blue stain; original magnification: 12.5×). This appears to be an old hemorrhagic infarct near the middle-posterior cerebral artery interface.
FIGURE 4.
Neuropathologic features in childhood cases with hydrocephalus and other anomalies due to malformations (see Table 1 for details). (A–C) Case 139. (A) Coronal slice showing moderately severe chronic hydrocephalus with disruption of the septum pellucidum. (B) The midbrain had a narrow cerebral aqueduct that was compressed by a venous malformation (C; original magnification: 40×; hematoxylin and eosin). Immunostain for amyloid precursor protein demonstrated damaged axons in the periventricular white matter (not shown), which is indicative of acute exacerbation of the ventriculomegaly. (D–F) Case 146. (D) CT scan of brain at 6 years age showed severe hydrocephalus. (E) Coronal slice showing moderately severe chronic hydrocephalus with disruption of the septum pellucidum. (F) Midbrain slice shows aqueduct stenosis. (G, H) Case 141. (G) CT scan at 5 years age showing extreme hydrocephalus in a child with vertebral defects, partial sacral agenesis, imperforate anus, and cloacal outlet obstruction (VATER-H complex). (H) Midbrain had aqueduct stenosis (12.5×). (I) Case 127. Photograph of the brain of a 2-year-old with severe lissencephaly; on morphologic grounds this was Miller–Dieker phenotype (poorly defined cortical lamination, no heterotopic overgrowth). No chromosomal or genetic analysis had been performed.
Teens
In the teen cohort 14 individuals died between 13 and 19 years (median 15.5 years). Ten were male and 4 were female. Eight had a confirmed FASD diagnosis, while 4 had a suspected FASD diagnosis. Four cases had history of personal substance abuse problems, and 3 cases had a seizure disorder/epilepsy. The causes/manners of death were suicide by hanging (6), accident (3), homicide (3), and complications of a chronic neurological disorder (2).
FASD facial anomalies were generally not described in these autopsy reports. No major somatic malformations were identified; 1 case had a minor cardiac defect.
No major brain malformations were identified. One case had a brain weight <5th percentile. Two cases had acute brain trauma, and 1 case had old damage related to hypoxic and hemorrhagic complications of premature birth at 29 weeks gestation. Among the 3 cases with a seizure disorder, the hippocampi were normal in all.
Adults
Eleven individuals died between 20 and 65 years (median 25 years); 10 were male. Two had a confirmed FASD diagnosis, while 9 had a suspected FASD diagnosis. Five cases had personal substance abuse problems. Five had psychiatric problems: 1 with multiple threats to commit suicide, 1 with depression, 1 with paranoid schizophrenia, 1 “heard voices”, and 1 had a criminal history of repeated car theft. Two cases had a seizure disorder/epilepsy. The causes/manners of death were no anatomical cause (n = 3), trauma due to homicide (n = 3), cardiac (n = 2), multidrug toxicity (n = 2), and suicide (n = 1).
As in the teenage group, facial features were not well described. No major somatic malformations were identified; 1 had mild thoracic scoliosis. Six cases (55%) were micrencephalic, and 1 had mild hydrocephalus due to aqueduct stenosis (Fig. 6). Other neuropathologic findings included acute brain trauma and old brain trauma (1 each).
FIGURE 6.
Case 174. 60-year-old man with cognitive delay and clinical diagnosis of fetal alcohol syndrome spectrum disorder (see Table 1 for details). (A) Coronal slice of brain shows mild ventriculomegaly. (B) Lower midbrain had a very narrow cerebral aqueduct. (C) Microscopic examination of the aqueduct showed a narrow, irregular channel lined by ependymal cells (40×; hematoxylin and eosin).
DISCUSSION
Mortality and PNAE/FASD
The risk of spontaneous abortion and stillbirth increases with the quantity of alcohol consumed during pregnancy (36–38). In utero PNAE deaths have been linked to placental insufficiency, which can result in hypoxia, and intrauterine growth restriction (39). In humans, alcohol consumption was positively correlated with placental hemorrhage and accelerated villous maturation (40). Administration of ethanol to Rhesus macaque monkeys daily for the first 60 days of gestation is associated with decreased placental blood flow and reduced fetal brain size at 110 days gestation (41). In the present study, placental abnormalities (including abruption) were documented in at least 68% of the fetal (and some infant) cases. Half of those cases were born prematurely and had a birth weight below the 50th percentile. Cigarette smoking, which is common among women who drink during pregnancy, is also a contributing factor to fetal hypoxia due to vasoconstrictor effects on the placental and umbilical cord vessels (42).
PNAE is also associated with increased risk of infant and child mortality (43). Among our infant and child cohorts, 32% had a documented history of premature birth, which increases the risk for adverse neurological outcome or early death (44). A search of the medical literature for information concerning mortality and FASD identified only 57 individuals, majority of whom died within the first year of life (54%) (45). Contributors to death were heart malformations (76%), brain malformations (51%), sepsis (14%), kidney malformations (14%), and cancer (8%). In our infant and child cohorts, 19% had infections and 23% had severe malformations that contributed to death. In the remainder, the cause of death was not obvious. Sudden unexplained death in infancy (SUDI) is defined as the death between the ages 1 day and 12 months that remains unexplained by autopsy (46). Risk factors for SUDI include premature birth, household tobacco smoke exposure, and unsafe sleep conditions (e.g. bed sharing with an adult; 17% in our infant cohort). Maternal alcohol use during pregnancy may be associated with continued use during the postnatal period and a risk for child neglect (47). In our PNAE/FASD cohorts, 19% of infants and 44% of children were either in foster care at the time of death or involved with child and family services (48).
Teens and young adults with FASD have disabilities such as mental illness, reckless and criminal behavior, and substance abuse problems (5), which put them at risk for suicide (7/25 in our combined teen and adult cohorts), homicide (6/25), and accidental traumatic or drug-related deaths (5/25).
Brain Abnormalities in PNAE and FASD
PNAE is postulated to interfere with normal neurological development through a range of mechanisms, including alteration of gene expression through epigenetic modifications (49), interference with neural crest cell migration (50), cell toxicity through reactive oxygen species (51), apoptosis of neurons (52), damage to radial glia and astrocytes (53), and inappropriate activation of microglia (54). In our cohorts collectively, the observed brain malformations were micrencephaly (31 cases), neural tube defects (6 cases), dysgenesis of the posterior corpus callosum combined with hydrocephalus (3 cases), isolated hydrocephalus of varied etiology and severity (5 cases), dysgenesis of the posterior corpus callosum combined with other minor anomalies (2 cases), isolated minor leptomeningeal heterotopias (4 cases), and ischemic/vascular brain lesions not attributable to a complication of birth or prematurity (6 cases) (see Table 2 for summary). The 3 youngest age groups had the most severe brain abnormalities. At least 2 factors might explain this: (1) the most severe malformations often involve multiple organ systems and are therefore likely to cause early death; or (2) among people who survived to adulthood, the ascertainment of FASD in the death investigation might be less reliable. In a meta-analysis of 127 clinical studies in which imaging was used to identify brain anomalies, 428 comorbid conditions were identified in individuals with FASD. These included microcephaly (62%), congenital hydrocephalus (58%), malformations of the corpus callosum (31%), epilepsy (21%), and spina bifida (<3%) (55). In a recent magnetic resonance (MR) imaging study of 62 children and teens with FASD, thin (hypoplastic) corpus callosum was found in 24, partial agenesis was found in 2, and cerebellar vermis hypoplasia in 11 (56). Also using MR imaging, small corpus callosum was identified in newborns with PNAE even after adjusting for brain volume (57, 58). In the absence of reliable normative data and photographs of mid-sagittal brain slices, subtle hypoplasia of the corpus callosum might be underreported in autopsy material. We identified a history of epilepsy or seizures in 19/122 (16%) cases (excluding stillbirths); among them 5 had micrencephaly, 4 had hydrocephalus, 3 had mesial temporal sclerosis, 3 had multiple congenital anomalies, and 2 had a history of meningitis. In the absence of an overt brain abnormality, epilepsy suggests that there may be subtle neuronal abnormalities in PNAE brains. Three-dimensional analysis of brain surface contours on MR images indicates that children and adolescents with FASD have significantly smoother cortices than controls (25). One MR imaging study showed reduced thickness of neocortex (males only), reduced regional brain volumes, and altered fractional anisotropy (“microstructure”) in white matter tracts of FASD children (59). However, another study showed increased cortical thickness in FASD subjects (60). Unfortunately, in the absence of postmortem imaging, it is difficult to mirror these quantitative parameters using conventional histopathological methods and, further, the structural correlate of fractional anisotropy changes is unclear.
TABLE 2.
Summary of Brain Abnormalities in Autopsy Cases With Prenatal Alcohol Exposure or Clinical Diagnosis of Fetal Alcohol Spectrum Disorder
| Stillbirths | Infants | Children | Teenage | Adults | |
|---|---|---|---|---|---|
| Total # of cases | 52 | 65 | 32 | 14 | 11 |
| Total # of brains examined | 34 | 65 | 31 | 12 | 7 |
| Micrencephaly | 10 | 8 | 6 | 1 | 6 |
| Neural tube defects | 4 | 2 | – | – | – |
| Corpus callosum dysgenesis + hydrocephalus | 1 | 2 | – | – | – |
| Corpus callosum dysgenesis + other minor anomaly | – | 1 | 1 | – | – |
| Hydrocephalus | – | 2 | 3 | – | 1 |
| Minor leptomeningeal heterotopia | – | 6 | 1 | – | – |
| Regional hypoxic– ischemic or hemorrhagic lesions unrelated to prematurity | 1 | 1 | 4 | – | – |
| Brain lesions related to prematurity | – | 2 | 4 | 1 | – |
| Probable genetic brain malformation | 1 | – | 1 | – | – |
| Mesial temporal sclerosis | – | – | 3 | – | – |
The possible contributions of PNAE to nervous system malformations must be considered in the context of developmental timing. Neural tube defects typically occur during weeks 3–4 of human gestation, although cranial defects due to amniotic band adhesions could develop later. Among our 6 cases with neural tube defects, drinking exclusively in the first trimester was reported in 1 case and drinking throughout pregnancy was reported in the others. Ethanol administered to pregnant rats on gestational days 6–12 is associated with delayed closure of the caudal neural tube (61), although the adverse effect of alcohol on neural tube closure in mice seems to be strain specific (62). In humans, pioneer axons of the corpus callosum genu appear during gestational weeks 11–12, with the posterior aspect (splenium) developing during weeks 16–18 (63). We identified 5 cases with corpus callosum dysgenesis (3 combined with hydrocephalus). The historical information was suggestive of high levels of PNAE (1 heavy drinking, 3 drinking throughout the pregnancy, and 1 binges in the first trimester). Corpus callosum anomalies in animal PNAE experiments are generally subtle. Sheep fetuses exposed to alcohol from gestational day 30–60 had activated microglia in the corpus callosum (64). Three-day-old male, but not female, rats exposed to alcohol from gestational day 6 to birth had a ∼20% reduction in corpus callosum area, however this was associated with overall reduction in brain volume (65). In contrast to humans, 3- to 5-year-old macaque monkeys with PNAE had enlarged corpus callosa (66). Neocortical leptomeningeal heterotopias, which reflect migration through the damaged glia limitans/pia mater, are postulated to occur in midgestation (67). Periventricular heterotopias reflect failed migration of germinal cells away from the ventricle wall. Although this is location-dependent, heterotopia along the roof of the ventricle likely indicate an insult before 20 weeks gestation (68). The timing of an insult that causes isolated hydrocephalus is difficult to determine; the cerebral aqueduct can be secondarily obstructed at any time during development (69). Among our 9 cases with hydrocephalus, 6 had aqueduct stenosis with some disorganization and chronic reactive astroglial changes, 1 had a venous anomaly compressing the aqueduct, and 2 had no obvious site of cerebrospinal fluid obstruction. Historical information did not identify a particular period of alcohol exposure. In a rat model of PNAE, a small proportion of offspring developed severe hydrocephalus with periventricular heterotopia; the site of cerebrospinal fluid obstruction was not identified (70).
The most common brain abnormality, micrencephaly (31 cases), cannot be attributed to a specifically timed insult. Hypotrophy of the brain could be caused by interference with progenitor cell proliferation, or through toxin-induced death of cells. Long projection neurons are generated in the first half of gestation, but interneuron and glial progenitors are generated well into the third trimester (71). Alternately, neurons of normal quantity could be small because they fail to mature properly (72). We found no evidence for an obvious defect in myelination.
Hypoxic–ischemic lesions were very common. In the stillborn population, scattered dead neurons are likely due to hypoxia immediately preceding death and 7 cases had lesions that could be attributed to complications of premature birth. Six cases had discrete regions of infarction that appeared to represent pre or perinatal insults. Three were arterial zone (one with cardiac anomaly) and one was deep periventricular. Compared to controls, alcohol-exposed human fetuses from 31 to 38 weeks gestation showed disorganized radial organization and angular branching of the cortical microvessels, which were immunolabeled with antibody to GluN1 (21). However, the pathogenesis of these large lesions, which correspond to the clinical entity known as perinatal arterial ischemic stroke, is difficult to explain on the basis of alcohol exposure alone. Other prenatal risk factors include prolonged rupture of amniotic membranes, preeclampsia, maternal smoking, infection, thrombophilia, and congenital heart disease (73).
PNAE and FASD Are Not Monofactorial Exposure Problems
In our PNAE cases with maternal information available, a large majority of mothers smoked tobacco (89%) or abused other drugs (83%) during their pregnancies. Furthermore, many had other diseases that can be associated with adverse pregnancy outcomes, including diabetes (8 cases), hypertension (1 case), obesity (1 case), and sexually transmitted infections (14 cases) (74). When reported, 88% of mothers had poor or no prenatal care. Poor nutrition is known to increase the risk of FASD (75). There is good evidence that harmful effects of toxins on the fetus may be either additive or synergistic, which confounds any attempt to attribute a causal effect to PNAE alone (76, 77). For example, administration of ethanol alone to pregnant rats on gestational day 9–12 had no adverse effects on the embryos, while the combination of ethanol and nicotine significantly retarded embryonic development (78). Nicotine has a constricting effect on the uterine arteries, which in turn reduces the amount of oxygenated blood the fetus receives (79). Maternal cigarette smoking causes episodic carbon monoxide and cyanide elevations in blood, which is associated with reduced head growth (80). MR imaging of female (but not male) adolescents showed that maternal cigarette smoking was associated with reduced size of posterior corpus callosum (81).
Limitations of the Study
As with any retrospective human autopsy study, this report has limitations. Maternal histories and details of PNAE were inconsistently reported and self-reported levels of consumption are frequently lower than reality (82). Therefore, our estimate of alcohol consumption is crude. There remains uncertainty whether low doses of alcohol have any adverse effect in pregnant humans (83). Because these autopsy cases were acquired over several decades, the FASD diagnostic criteria were not applied uniformly and the information collected at autopsy differed. Brain sampling also varied because several different neuropathologists did the examinations. Hopefully the prospective study being conducted by the Prenatal Alcohol in SIDS and Stillbirth Network will overcome some of these problems (84). There was likely a referral and ascertainment bias, particularly among the teenage and adult death groups. Only one of the teenage FASD brains available for study had micrencephaly, which is reportedly the most common abnormality. However, the circumstances of death were often associated with brain swelling; therefore, reliance on the brain weight could mask the presence of preexisting micrencephaly. The 2 cases with anomalies typically ascribed to genetic mutations (holoprosencephaly and Miller–Dieker lissencephaly) did not undergo genetic testing. Overall, we can only report on the associations between PNAE/FASD and human brain abnormalities; we cannot ascribe a cause–effect relationship.
Summary and Conclusions
This is the largest study of human PNAE and FASD autopsy brains reported. The information elicited is important, despite the deficiencies. There is no characteristic neuropathologic pattern associated with PNAE or FASD. Malformations must be distinguished from in utero vascular insults and complications of prematurity, which can contribute to the neurological phenotype but might be only indirectly related to PNAE. The almost ubiquitous concurrent use of tobacco is a critical point because of its direct association with placental dysfunction. Some of the brain features of PNAE/FASD might be teratogenic in the sense of a direct adverse effect of ethanol on brain development. However, we also provide indirect evidence that some of the brain damage might be caused by hypoxic/ischemic events. Although animal studies are necessary to understand the human abnormalities related to PNAE, the animal research community might be doing a disservice by focusing too much on fine details of ethanol-alone teratogenicity. Increased attention should be paid to combined toxicities, in particular ethanol plus nicotine or other vasoactive agents in tobacco. With respect to the clinical diagnosis of FASD, information more specific than “structural brain anomalies” should be provided, considering the diverse pathogenesis of these anomalies. Prevention through avoidance of alcohol, tobacco, and other substance abuse during pregnancy must be the main societal goal (85). Nevertheless, tissues accrued in the current study will be used to validate hypotheses about the pathogenesis of FASD that have been developed in animal experimentation.
Supplementary Material
ACKNOWLEGEMENT
We thank Susan Janeczko and Diane Legarta for technical assistance.
REFERENCES
- 1. Sanders JL. Were our forebears aware of prenatal alcohol exposure and its effects? A review of the history of fetal alcohol spectrum disorder. Can J Clin Pharmacol 2009;16:e288–e95 [PubMed] [Google Scholar]
- 2. Lemoine P, Harousseau H, Borteyru JP, et al. Les enfants de parents alcooliques. Anomalies observées. A propos de 127 cas. Ouest Med 1968;25:476–82 [Google Scholar]
- 3. Jones KL, Smith DW, Ulleland CN, et al. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet 1973;1:1267–71 [DOI] [PubMed] [Google Scholar]
- 4. Jones KL, Smith DW.. Recognition of the fetal alcohol syndrome in early infancy. Lancet 1973;302:999–1001 [DOI] [PubMed] [Google Scholar]
- 5. Senturias YS. Fetal alcohol spectrum disorders: an overview for pediatric and adolescent care providers. Curr Probl Pediatr Adolesc Health Care 2014;44:74–81 [DOI] [PubMed] [Google Scholar]
- 6. Coles CD, Gailey AR, Mulle JG, et al. A comparison among 5 methods for the clinical diagnosis of fetal alcohol spectrum disorders. Alcohol Clin Exp Res 2016;40:1000–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoyme HE, Kalberg WO, Elliott AJ, et al. Updated clinical guidelines for diagnosing fetal alcohol spectrum disorders. Pediatrics 2016;138:e20154256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cook JL, Green CR, Lilley CM, et al. Fetal alcohol spectrum disorder: a guideline for diagnosis across the lifespan. CMAJ 2016;188:191–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kable JA, Mukherjee RA.. Neurodevelopmental disorder associated with prenatal exposure to alcohol (ND-PAE): a proposed diagnostic method of capturing the neurocognitive phenotype of FASD. Eur J Med Genet 2017;60:49–54 [DOI] [PubMed] [Google Scholar]
- 10. Ospina M, Dennett L, Systematic Review On The Prevalence Of Fetal Alcohol Spectrum Disorders. Edmonton Canada: Institute of Health Economics; 2013 [Google Scholar]
- 11. Roozen S, Peters GJ, Kok G, et al. Worldwide prevalence of fetal alcohol spectrum disorders: a systematic literature review including meta-analysis. Alcohol Clin Exp Res 2016;40:18–32 [DOI] [PubMed] [Google Scholar]
- 12. Bishop DV. Which neurodevelopmental disorders get researched and why? PLoS One 2010;5:e15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Popova S, Lange S, Burd L, et al. The economic burden of fetal alcohol spectrum disorder in Canada in 2013. Alcohol Alcohol 2016;51:367–75 [DOI] [PubMed] [Google Scholar]
- 14. Clarren SK. Central nervous system malformations in two offspring of alcoholic women. Birth Defects Orig Artic Ser 1977;13:151–3 [PubMed] [Google Scholar]
- 15. Clarren SK, Alvord EC, Sumi SM, et al. Brain malformations related to prenatal exposure to ethanol. J Pediatr 1978;92:64–7 [DOI] [PubMed] [Google Scholar]
- 16. Peiffer J, Majewski F, Fischbach H, et al. Alcohol embryo- and fetopathy. Neuropathology of 3 children and 3 fetuses. J Neurol Sci 1979;41:125–37 [DOI] [PubMed] [Google Scholar]
- 17. Wisniewski K, Dambska M, Sher JH, et al. A clinical neuropathological study of the fetal alcohol syndrome. Neuropediatrics 1983;14:197–201 [DOI] [PubMed] [Google Scholar]
- 18. Roebuck TM, Mattson SN, Riley EP.. A review of the neuroanatomical findings in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res 1998;22:339–44 [DOI] [PubMed] [Google Scholar]
- 19. Konovalov HV, Kovetsky NS, Bobryshev YV, et al. Disorders of brain development in the progeny of mothers who used alcohol during pregnancy. Early Hum Dev 1997;48:153–66 [DOI] [PubMed] [Google Scholar]
- 20. Solonskii AV, Logvinov SV, Kutepova NA.. Development of brain vessels in human embryos and fetuses in conditions of prenatal exposure to alcohol. Neurosci Behav Physiol 2008;38:373–6 [DOI] [PubMed] [Google Scholar]
- 21. Jégou S, El Ghazi F, de Lendeu PK, et al. Prenatal alcohol exposure affects vasculature development in the neonatal brain. Ann Neurol 2012;72:952–60 [DOI] [PubMed] [Google Scholar]
- 22. Moore EM, Migliorini R, Infante MA, et al. Fetal alcohol spectrum disorders: recent neuroimaging findings. Curr Dev Disord Rep 2014;1:161–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nardelli A, Lebel C, Rasmussen C, et al. Extensive deep gray matter volume reductions in children and adolescents with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 2011;35:1404–17 [DOI] [PubMed] [Google Scholar]
- 24. Norman AL, Crocker N, Mattson SN, et al. Neuroimaging and fetal alcohol spectrum disorders. Dev Disabil Res Rev 2009;15:209–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hendrickson TJ, Mueller BA, Sowell ER, et al. Cortical gyrification is abnormal in children with prenatal alcohol exposure. Neuroimage Clin 2017;15:391–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Muralidharan P, Sarmah S, Zhou FC, et al. Fetal alcohol spectrum disorder (FASD) associated neural defects: complex mechanisms and potential therapeutic targets. Brain Sci 2013;3:964–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lussier AA, Weinberg J, Kobor MS.. Epigenetics studies of fetal alcohol spectrum disorder: where are we now? Epigenomics 2017;9:291–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Popova S, Lange S, Probst C, et al. Prevalence of alcohol consumption during pregnancy and Fetal Alcohol Spectrum Disorders among the general and Aboriginal populations in Canada and the United States. Eur J Med Genet 2017;60:32–48 [DOI] [PubMed] [Google Scholar]
- 29. Singal D, Brownell M, Hanlon-Dearman A, et al. Manitoba mothers and fetal alcohol spectrum disorders study (MBMomsFASD): protocol for a population-based cohort study using linked administrative data. BMJ Open 2016;6:e013330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chudley AE, Conry J, Cook JL, et al. Fetal alcohol spectrum disorder: Canadian guidelines for diagnosis. CMAJ 2005;172:S1–S21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maroun LL, Graem N.. Autopsy standards of body parameters and fresh organ weights in nonmacerated and macerated human fetuses. Pediatr Dev Pathol 2005;8:204–17 [DOI] [PubMed] [Google Scholar]
- 32. Dekaban AS, Sadowsky D.. Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights. Ann Neurol 1978;4:345–56 [DOI] [PubMed] [Google Scholar]
- 33. Phillips JB, Billson VR, Forbes AB.. Autopsy standards for fetal lengths and organ weights of an Australian perinatal population. Pathology 2009;41:515–26 [DOI] [PubMed] [Google Scholar]
- 34. Fracasso T, Vennemann M, Pfeiffer H, et al. Organ weights in cases of sudden infant death syndrome: a German study. Am J Forensic Med Pathol 2009;30:231–4 [DOI] [PubMed] [Google Scholar]
- 35. Treit S, Zhou D, Chudley AE, et al. Relationships between head circumference, brain volume and cognition in children with prenatal alcohol exposure. PLoS One 2016;11:e0150370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bailey BA, Sokol RJ.. Prenatal alcohol exposure and miscarriage, stillbirth, preterm delivery, and sudden infant death syndrome. Alcohol Res Health 2011;34:86–91 [PMC free article] [PubMed] [Google Scholar]
- 37. O'Leary C, Jacoby P, D'Antoine H, et al. Heavy prenatal alcohol exposure and increased risk of stillbirth. BJOG 2012;119:945–52 [DOI] [PubMed] [Google Scholar]
- 38. Salihu HM, Kornosky JL, Lynch O, et al. Impact of prenatal alcohol consumption on placenta-associated syndromes. Alcohol 2011;45:73–9 [DOI] [PubMed] [Google Scholar]
- 39. Tai M, Piskorski A, Kao JC, et al. Placental morphology in fetal alcohol spectrum disorders. Alcohol Alcohol 2017;52:138–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carter RC, Wainwright H, Molteno CD, et al. Alcohol, methamphetamine, and marijuana exposure have distinct effects on the human placenta. Alcohol Clin Exp Res 2016;40:753–64 [DOI] [PubMed] [Google Scholar]
- 41. Lo JO, Schabel MC, Roberts VH, et al. First trimester alcohol exposure alters placental perfusion and fetal oxygen availability affecting fetal growth and development in a non-human primate model. Am J Obstet Gynecol 2017;216:302.e1–e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Behnke M, Smith VC.. Prenatal substance abuse: short- and long-term effects on the exposed fetus. Pediatrics 2013;131:e1009–e24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Burd L, Klug MG, Bueling R, et al. Mortality rates in subjects with fetal alcohol spectrum disorders and their siblings. Birth Defects Res A Clin Mol Teratol 2008;82:217–23 [DOI] [PubMed] [Google Scholar]
- 44. Behrman RE, Stith Butler A, Committee on Understanding Premature Birth and Assuring Healthy Outcomes Preterm Birth: Causes, Consequences, and Prevention. Washington DC: National Academies Press; 2006 [PubMed] [Google Scholar]
- 45. Thompson A, Hackman D, Burd L.. Mortality in fetal alcohol spectrum disorders. Open J Pediatr 2014;4:21 [Google Scholar]
- 46. Byard RW. SUDI or “undetermined”: does it matter? Forensic Sci Med Pathol 2009;5:252–3 [DOI] [PubMed] [Google Scholar]
- 47. Burd L, Wilson H.. Fetal, infant, and child mortality in a context of alcohol use. Am J Med Genet C Semin Med Genet 2004;127C:51–8 [DOI] [PubMed] [Google Scholar]
- 48. Brownell M, Chartier M, Au W, et al. The Educational Outcomes of Children in Care in Manitoba. Winnipeg, MB: Manitoba Centre for Health Policy; 2015 [Google Scholar]
- 49. Liyanage VR, Curtis K, Zachariah RM, et al. Overview of the genetic basis and epigenetic mechanisms that contribute to FASD pathobiology. Curr Top Med Chem 2017;17:808–28 [DOI] [PubMed] [Google Scholar]
- 50. Smith SM, Garic A, Flentke GR, et al. Neural crest development in fetal alcohol syndrome. Birth Defects Res C Embryo Today 2014;102:210–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brocardo PS, Gil-Mohapel J, Christie BR.. The role of oxidative stress in fetal alcohol spectrum disorders. Brain Res Rev 2011;67:209–25 [DOI] [PubMed] [Google Scholar]
- 52. Creeley CE, Olney JW.. Drug-induced apoptosis: mechanism by which alcohol and many other drugs can disrupt brain development. Brain Sci 2013;3:1153–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wilhelm CJ, Guizzetti M.. Fetal alcohol spectrum disorders: an overview from the glia perspective. Front Integr Neurosci 2015;9:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Drew PD, Kane CJ.. Fetal alcohol spectrum disorders and neuroimmune changes. Int Rev Neurobiol 2014;118:41–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Popova S, Lange S, Shield K, et al. Comorbidity of fetal alcohol spectrum disorder: a systematic review and meta-analysis. Lancet 2016;387:978–87 [DOI] [PubMed] [Google Scholar]
- 56. Boronat S, Sanchez-Montanez A, Gomez-Barros N, et al. Correlation between morphological MRI findings and specific diagnostic categories in fetal alcohol spectrum disorders. Eur J Med Genet 2017;60:65–71 [DOI] [PubMed] [Google Scholar]
- 57. Jacobson SW, Jacobson JL, Molteno CD, et al. Heavy prenatal alcohol exposure is related to smaller corpus callosum in newborn MRI scans. Alcohol Clin Exp Res 2017;41:965–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yang Y, Phillips OR, Kan E, et al. Callosal thickness reductions relate to facial dysmorphology in fetal alcohol spectrum disorders. Alcohol Clin Exp Res 2012;36:798–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Treit S, Chen Z, Zhou D, et al. Sexual dimorphism of volume reduction but not cognitive deficit in fetal alcohol spectrum disorders: a combined diffusion tensor imaging, cortical thickness and brain volume study. Neuroimage Clin 2017;15:284–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang Y, Roussotte F, Kan E, et al. Abnormal cortical thickness alterations in fetal alcohol spectrum disorders and their relationships with facial dysmorphology. Cereb Cortex 2012;22:1170–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ross CP, Persaud TV.. Neural tube defects in early rat embryos following maternal treatment with ethanol and caffeine. Anat Anz 1989;169:247–52 [PubMed] [Google Scholar]
- 62. Becker HC, Diaz-Granados JL, Randall CL.. Teratogenic actions of ethanol in the mouse: a minireview. Pharmacol Biochem Behav 1996;55:501–13 [DOI] [PubMed] [Google Scholar]
- 63. Rakic P, Yakovlev PI.. Development of the corpus callosum and cavum septi in man. J Comp Neurol 1968;132:45–72 [DOI] [PubMed] [Google Scholar]
- 64. Watari H, Born DE, Gleason CA.. Effects of first trimester binge alcohol exposure on developing white matter in fetal sheep. Pediatr Res 2006;59:560–4 [DOI] [PubMed] [Google Scholar]
- 65. Zimmerberg B, Scalzi LV.. Commissural size in neonatal rats: effects of sex and prenatal alcohol exposure. Int J Dev Neurosci 1989;7:81–6 [DOI] [PubMed] [Google Scholar]
- 66. Miller MW, Astley SJ, Clarren SK.. Number of axons in the corpus callosum of the mature Macaca nemestrina: increases caused by prenatal exposure to ethanol. J Comp Neurol 1999;412:123–31 [PubMed] [Google Scholar]
- 67. Iida K, Hirano S, Takashima S, et al. Developmental study of leptomeningeal glioneuronal heterotopia. Pediatr Neurol 1994;10:295–8 [DOI] [PubMed] [Google Scholar]
- 68. Del Bigio MR. Cell proliferation in human ganglionic eminence and suppression after prematurity-associated haemorrhage. Brain 2011;134:1344–61 [DOI] [PubMed] [Google Scholar]
- 69. Del Bigio MR. Ependymal cells: biology and pathology. Acta Neuropathol 2010;119:55–73 [DOI] [PubMed] [Google Scholar]
- 70. Sakata-Haga H, Sawada K, Ohnishi T, et al. Hydrocephalus following prenatal exposure to ethanol. Acta Neuropathol 2004;108:393–8 [DOI] [PubMed] [Google Scholar]
- 71. Arshad A, Vose LR, Vinukonda G, et al. Extended production of cortical interneurons into the third trimester of human gestation. Cereb Cortex 2016;26:2242–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ernst C. Proliferation and differentiation deficits are a major convergence point for neurodevelopmental disorders. Trends Neurosci 2016;39:290–9 [DOI] [PubMed] [Google Scholar]
- 73. Lehman LL, Rivkin MJ.. Perinatal arterial ischemic stroke: presentation, risk factors, evaluation, and outcome. Pediatr Neurol 2014;51:760–8 [DOI] [PubMed] [Google Scholar]
- 74. Ornoy A, Reece EA, Pavlinkova G, et al. Effect of maternal diabetes on the embryo, fetus, and children: congenital anomalies, genetic and epigenetic changes and developmental outcomes. Birth Defects Res C Embryo Today 2015;105:53–72 [DOI] [PubMed] [Google Scholar]
- 75. Young JK, Giesbrecht HE, Eskin MN, et al. Nutrition implications for fetal alcohol spectrum disorder. Adv Nutr 2014;5:675–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chen WJA, Maier SE.. Combination drug use and risk for fetal harm. Alcohol Res Health 2011;34:27–8 [PMC free article] [PubMed] [Google Scholar]
- 77. Viteri OA, Soto EE, Bahado-Singh RO, et al. Fetal anomalies and long-term effects associated with substance abuse in pregnancy: a literature review. Am J Perinatol 2015;32:405–16 [DOI] [PubMed] [Google Scholar]
- 78. Woo ND, Persaud TV.. Rat embryogenesis following exposure to alcohol and nicotine. Acta Anat 1988;131:122–6 [DOI] [PubMed] [Google Scholar]
- 79. Scott-Goodwin AC, Puerto M, Moreno I.. Toxic effects of prenatal exposure to alcohol, tobacco and other drugs. Reprod Toxicol 2016;61:120–30 [DOI] [PubMed] [Google Scholar]
- 80. Phelan S. Smoking cessation in pregnancy. Obstet Gynecol Clin North Am 2014;41:255–66 [DOI] [PubMed] [Google Scholar]
- 81. Paus T, Nawazkhan I, Leonard G, et al. Corpus callosum in adolescent offspring exposed prenatally to maternal cigarette smoking. Neuroimage 2008;40:435–41 [DOI] [PubMed] [Google Scholar]
- 82. Bager H, Christensen LP, Husby S, et al. Biomarkers for the detection of prenatal alcohol exposure: a review. Alcohol Clin Exp Res 2017;41:251–61 [DOI] [PubMed] [Google Scholar]
- 83. Charness ME, Riley EP, Sowell ER.. Drinking during pregnancy and the developing brain: is any amount safe? Trends Cogn Sci 2016;20:80–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dukes KA, Burd L, Elliott AJ, et al. The Safe Passage Study: design, methods, recruitment, and follow-up approach. Paediatr Perinat Epidemiol 2014;28:455–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jonsson E, Salmon A, Warren KR.. The international charter on prevention of fetal alcohol spectrum disorder. Lancet Glob Health 2014;2:e135–e7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.