Abstract
Migration of dendritic cells (DC) to the central nervous system (CNS) is a critical event in the pathogenesis of multiple sclerosis (MS). While up until now, research has mainly focused on the transmigration of DC through the blood-brain barrier, experimental evidence points out that also the choroid plexus and meningeal vessels represent important gateways to the CNS, especially in early disease stages. On the other hand, DC can exit the CNS to maintain immunological tolerance to patterns expressed in the CNS, a process that is perturbed in MS. Targeting trafficking of immune cells, including DC, to the CNS has demonstrated to be a successful strategy to treat MS. However, this approach is known to compromise protective immune surveillance of the brain. Unravelling the migratory paths of regulatory and pathogenic DC within the CNS may ultimately lead to the design of new therapeutic strategies able to selectively interfere with the recruitment of pathogenic DC to the CNS, while leaving host protective mechanisms intact.
Keywords: Blood-brain barrier, Blood-CSF barriers, Central nervous system, Dendritic cell migration, Multiple sclerosis
INTRODUCTION
Although the central nervous system (CNS) is an immune-privileged site, its homeostasis critically depends on the presence of surveilling leukocytes and their migration into and out of the CNS. It is evident that, under physiological circumstances, recruitment of leukocytes to the CNS is restricted and tightly regulated at the physical barriers which form the interface between the CNS and peripheral immunity. Neuroinflammatory processes, on the other hand, are often associated with massive immune cell infiltration and CNS barrier breakdown, the one reinforcing the other. Of particular interest is the migration of dendritic cells (DC) into and out of the CNS. These antigen-presenting cells (APC) have the unique capacity to activate and polarize T cells, thereby determining the outcome of the adaptive immune response, that is, immunity or tolerance (1). Steady-state migration of DC out of the CNS to cervical lymph nodes has been reported to be essential in the maintenance of immune tolerance to brain-derived antigens (2). On the other hand, neuroinflammation associated with multiple sclerosis (MS) (3–7) or with experimental autoimmune encephalomyelitis (EAE) (8–10), the animal model for MS, is characterized by an enhanced recruitment of DC from the peripheral circulation to the CNS. This results in the accumulation of DC in the cerebrospinal fluid (CSF), meninges, perivascular lesions, and parenchyma, where they were shown to be critically involved in the inflammatory processes underlying autoimmune disease initiation and progression during MS (11–15).
In general, migration of DC through the body is coordinated by the specific set of chemokine receptors they express, which depends on the DC’s subtype and developmental stage. In their immature state, DC reside in the periphery where they scan the microenvironment for invading pathogens and other foreign as well as autologous cellular particles and proteins. Immature conventional DC (cDC) express a wide range of chemokine receptors, including C-C-chemokine receptor (CCR)1, CCR2, CCR3, CCR4, CCR5, CCR6, C-X-C-chemokine receptor (CXCR)2, and CXCR4 (16–22). This allows them to respond to constitutively expressed chemokines such as CXCL12, a CXCR4 ligand involved in lymphoid homing of DC (23). However, cDC are especially sensitive to so-called inducible chemokines, more specifically to CCL2, CCL3, CCL4, CCL5, CCL7, and CCL20 (16–22). Under homeostatic conditions, these chemokines are expressed at low levels in peripheral tissues, including the skin, lung, gut, and liver (24–28), and are involved in the basal recruitment of immature cDC into these organs for immune surveillance (29, 30). Upon an inflammatory insult, the expression of inducible chemokines is drastically increased (24–28, 31), facilitating the influx of additional immune cells, including immature DC. Once DC have taken up an antigen, they migrate to secondary lymphoid organs where the processed antigen is presented to T cells in an MHC-dependent manner (32). Depending on the context in which the antigen was captured, that is, in steady state or in the presence of molecular danger signals, DC induce tolerance or immunity, respectively (1). Upon encounter of a danger signal, cDC undergo a complex maturation process including the loss of CCR1-6 and CXCR2, while maintaining CXCR4 expression and strongly upregulating CCR7, concomitantly showing a strong chemotactic response toward CXCL12, and CCL19 and CCL21, respectively (19–22, 33). This will guide DC toward the draining lymph nodes. Although phenotypically, immature plasmacytoid DC (pDC) display a similar pattern of chemokine receptor expression as cDC, these receptors appear to be nonfunctional, because pDC lack migratory responsiveness to the respective inflammatory chemokine ligands in vitro and migrate toward CXCL12 only (22). This could explain the differential homeostatic distribution of pDC as compared to cDC. pDC mainly reside in the blood and lymphoid compartments and are only rarely found in healthy nonlymphoid tissues (34, 35). Interestingly, pDC also express chemokine-like receptor 1 (CMKLR1), the receptor for chemerin (36). Following proteolytic activation under inflammatory conditions, chemerin functions as a chemoattractant (37) allowing for specific recruitment of pDC to sites of inflammation. Upon maturation, pDC lose CXCL12 and chemerin responsiveness, upregulate CCR7, and strongly migrate in response to CCL19 and CCL21 (22, 38).
Recruitment of DC from the blood to peripheral tissues and their subsequent migration to draining lymph nodes has been studied extensively both in vitro (18–22, 38, 39) and in vivo in organs such as skin (40–45), lungs (29, 46, 47), and intestine (48–51) (reviewed by Alvarez et al [52]). However, given the long-standing previous paradigm that the CNS was an immune-privileged organ, completely devoid of peripheral immune cells, far less is known about DC trafficking to and from the CNS. Currently, it is recognized that the CNS is subject to continuous immune surveillance under homeostatic conditions, with peripherally derived DC located at strategic locations in the CNS. During neuroinflammation associated with MS, the number of DC in the CNS drastically increases and, in addition, DC invade the CNS parenchyma. Several studies have implicated DC as protagonists in the inflammatory processes underlying disease initiation and progression in MS (11–15). Indeed, it has been shown that the number of DC in the CNS correlates with disease severity. Pashenkov et al demonstrated that absolute numbers of both cDC and pDC in the CSF correlated with IgG indices in the CSF, a marker of neuroinflammation (3). Also in EAE, Sagar et al showed that accumulation of endogenous fluorescently labelled DC in the CNS correlated with EAE disease severity, using noninvasive near-infrared imaging (9). Furthermore, inducing increased numbers of DC in the CNS by systemic Flt3L treatment (14) or by intracerebral injection of immunostimulatory DC (15) significantly accelerated the onset and exacerbated the clinical course of EAE. Conversely, inhibition of Flt3 signaling induced DC apoptosis in vivo and was associated with a significant improvement of the clinical course of established EAE. Interestingly, Greter et al found CD11c+ cDC to be the only APC required to reactivate primed T cells in the CNS in situ and to consequently initiate EAE neuroinflammation (14). Additionally, epitope spreading, an essential feature of both disease exacerbations (53) and progression (54) in CNS autoimmunity, was shown to occur within the CNS and to be most likely mediated by peripherally derived cDC (55). Indeed, these cells were the only CNS-isolated APC capable of efficiently presenting endogenously acquired myelin antigens to activate naive myelin-specific T cells in vitro. In summary, DC are a rate-limiting factor for neuroinflammation in both early phases as well as during disease progression.
Here, we will summarize current understanding of DC trafficking to and from the CNS in health and during neuroinflammation in MS. Also, we will outline how current MS therapeutics affect these processes. Interfering with DC migration to and from the CNS in a specific and selective manner would represent an interesting future therapeutic strategy in combatting autoimmune neuroinflammation associated with MS.
OVERCOMING ENDOTHELIAL BARRIERS: GENERAL PRINCIPLES OF DC TRAFFICKING
In general, the classical multistep model of leukocyte transmigration (56, 57) also applies to circulating DC and DC precursors. Initial weak tethering and rolling interactions of DC with the endothelium are mainly mediated by P- and E-selectins. Circulating DC uniformly express P-selectin glycoprotein ligand-1 (PSGL-1) (40), the primary ligand for P-selectin, even though it is also able to bind E-selectin if correctly glycosylated (58). Additionally, monocyte-derived immature DC express sialyl Lewis x antigen, another P- and E-selectin ligand (59). Although PSGL-1 mediates tethering and rolling of DC over noninflamed endothelium (40), other selectin ligands appear to be involved in the recruitment of DC to sites of inflammation (60). For instance, the C-type lectin DC-SIGN (CD209) was shown to support tethering and rolling as well as subsequent adhesion of DC over both resting and activated endothelium in vitro by interacting with the adhesion molecule ICAM-2 on endothelial cells (61, 62). Although ICAM-2 is able to bind both DC-SIGN and the integrin LFA-1 expressed by DC, the DC-SIGN/ICAM-2 interaction is known to be of greater affinity (61, 63) and is able to resist shear stress (61). This suggests that the interaction of DC-SIGN with ICAM-2 may precede ICAM-2 binding to LFA-1 during adhesion. Indeed, while DC-SIGN/ICAM-2 interaction gradually slows down flowing cells, firm adhesion of DC to the endothelium is only possible after chemokine-mediated activation of integrins on the DC surface (64). Integrins expressed by circulating DC include the heterodimers α4β1 (VLA-4) (65), αLβ2 (LFA-1) (66), αMβ2 (Mac-1) (66), and α5β1 (VLA-5) (65). Their ligands are members of the immunoglobulin superfamily expressed by endothelial cells, namely VCAM-1 (for VLA-4), ICAM-1, -2 and -3 (for LFA-1), ICAM-1 (for Mac-1), or components of the extracellular matrix such as fibronectin (for VLA-5). Once firmly adhered, DC are guided through the endothelial barrier by gradients of chemokines and adhesion molecules. In steady state, this process appears to be mainly dependent on DC-SIGN and both β1- and β2-integrins, but it does not involve ICAM-1, VCAM-1 and PECAM-1 (38, 61), whereas under inflammatory circumstances, ICAM-1 and PECAM-1 do participate in DC transmigration through activated endothelium (38). Interestingly, different DC subpopulations have been shown to express a range of tight junction proteins, including JAM-A, occludin, and claudin-1 (67–70). These molecules may assist in the transmigration of DC through endothelial cell layers while maintaining barrier integrity by forming transient tight junction-like structures with surrounding endothelial cells during DC extravasation, as was shown for DC penetrating the gut epithelium to sample intraluminal antigens (67).
DC TRAFFICKING TO THE CNS
Origin of DC in the CNS
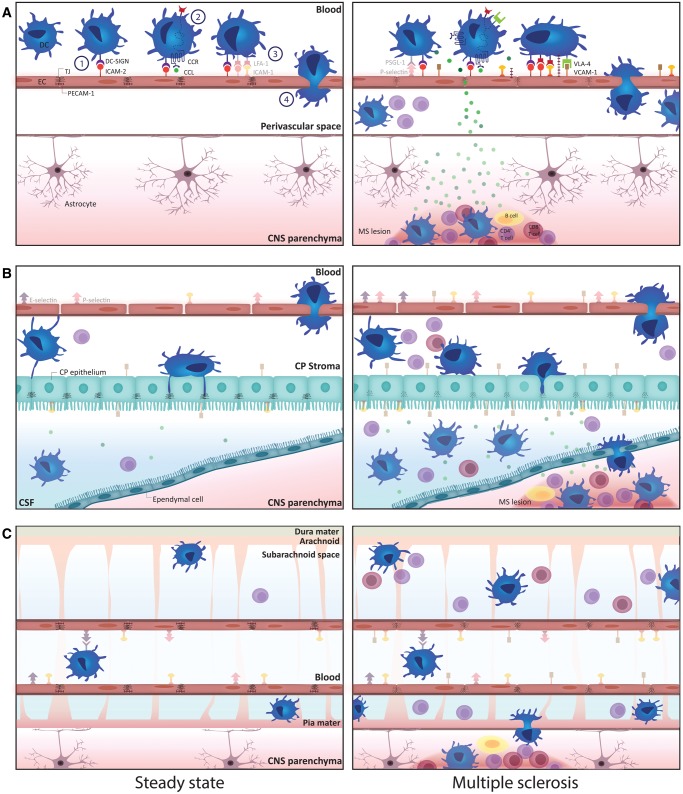
In the absence of inflammation, low numbers of DC are present in discrete areas of the CNS, more specifically in the meninges (71), in the choroid plexus (72, 73), in perivascular spaces (71), and in the CSF (3) (Fig. 1). The origin of these cells has been a topic of debate since their discovery. Although Fischer et al showed that DC-like cells can develop from microglia under the influence of GM-CSF in vitro (74), the majority of DC in the CNS are more likely to be derived from circulating bone marrow-derived precursor cells. While their preferential perivascular localization is indicative of a peripheral origin, this was further supported by a landmark study by Anandasabapathy et al (75). Using microarray analysis, they showed that the gene expression profile of murine DC isolated from the meninges and the choroid plexus closely resembles that of CD8+ splenic DC, but not that of microglia. Furthermore, in adoptive transfer experiments, the authors found that donor Flt3-positive bone marrow-derived DC precursors injected intravenously into nonirradiated Flt3-knock-out mice populated the meninges but not brain parenchyma of acceptor mice. The meningeal DC developed out of adoptively transferred monocyte and DC progenitors, common DC progenitors or pre-DC, but not from adoptively transferred monocytes (75). These findings support the notion that DC in the steady-state CNS derive from circulating precursors, which need to overcome at least one of the several barriers protecting the immune-privileged CNS in order to populate the brain at strategic positions for immune surveillance. Indeed, different routes for immune cell entry into the CNS have been described (76): Circulating immune cells can reach the CNS (1) by migrating through the choroid plexus, via which they gain access to the CSF; (2) by traversing the blood-brain barrier (BBB) into the parenchymal perivascular space; or (3) by transmigrating through postcapillary venules at the pial surface into subarachnoid and Virchow-Robin spaces (Fig. 1).
Figure 1.
Schematic overview of the paths for transmigration of DC to the CNS via the blood-brain barrier (A), the choroid plexus (B), and meningeal vessels (C) in the healthy (left panels) and the inflamed CNS (right panels). Transmigration of DC exiting the bloodstream occurs according to a well-defined multistep process (A, left panel). First, circulating DC gradually slow down through engaging in DC-SIGN-mediated tethering and rolling interactions with ICAM-2 [1]. After integrin activation through chemokine signaling [2], DC firmly adhere to the endothelium [3]. Finally, DC transmigrate through the endothelial layer [4]. Under steady-state conditions, DC reside in the perivascular space and do not infiltrate into the CNS parenchyma. Under inflammatory conditions, adhesion molecule expression by BBB endothelial cells and DC is increased, facilitating DC entry into the inflamed CNS (A, right panel). In the healthy CNS, DC can be identified in the choroid plexus stroma and in the CSF-filled ventricular spaces and subarachnoid space (B and C, left panels). Their numbers drastically increase during neuroinflammation (B and C, right panels). Moreover, DC in the CSF gain access to the CNS parenchyma during MS pathogenesis (B and C, right panels). Although several ligands of DC migratory molecules are expressed by the choroid plexus endothelium and epithelium (B) as well as by the meningeal vessel endothelium (C) and their expression is further increased under inflammatory conditions, it remains to be determined which of these are critical in guiding DC through these barriers during immune surveillance of the CNS as well as during neuroinflammation. (For opaque molecules, involvement in the process of DC migration to the CNS has been documented in scientific literature. Transparent molecules are described to be expressed by DC and at the CNS barriers and therefore could also be involved in DC migration to the CNS, but these interactions between the specific molecule pairs have not [yet] been proven to occur during DC migration to the CNS). BBB, blood-brain barrier; CCL, chemokine ligand; CCR, chemokine receptor; CNS, central nervous system; CP, choroid plexus; CSF, cerebrospinal fluid; DC, dendritic cell; DC-SIGN, dendritic cell-specific ICAM-grabbing nonintegrin; ICAM-1, intercellular adhesion molecule-1; ICAM-2, intercellular adhesion molecule-2; LFA-1, lymphocyte function-associated antigen-1; MS, multiple sclerosis; PECAM-1, platelet and endothelial cell adhesion molecule-1; PSGL-1, P-selectin glycoprotein ligand-1; VCAM-1, vascular cell adhesion molecule-1; VLA-4, very late antigen-4.
ROUTES OF ENTRY INTO THE CNS
Blood-Brain Barrier
Although the multistep paradigm of leukocyte trafficking also applies to immune cell migration to the CNS, the specialized structure of the BBB critically modulates this process (77–79). Its distinguished architecture comprising tight junctions, and its low basal expression of adhesion molecules ensure a limited but steady entry of immune cells, including DC, in normal physiological conditions, allowing for CNS immune surveillance (Fig. 1A). Indeed, under noninflammatory conditions, expression of P- and E-selectin could not be detected in the human cerebral vasculature postmortem (80), nor in Lewis rats (81), or in murine brain capillary endothelial cells cultured in vitro (82). In primary cultures of unstimulated human brain microvascular endothelial cells, on average only 6% of the cells expressed P-selectin (83). ICAM-1 is constitutively expressed at very low levels in the human cerebral microvasculature. In contrast, ICAM-2 and PECAM-1 are constitutively expressed at high levels in cerebral microvessels (80). However, it was shown that PECAM-1 concentrates at interendothelial junctions and is involved in maintaining BBB integrity rather than leukocyte recruitment. VCAM-1 and ICAM-3 are not expressed at the human BBB in steady-state conditions (80).
The healthy CNS parenchyma is devoid of DC and only rare perivascularly localized DC can be observed (64). An in vitro study focusing on the steady-state interaction between DC and the BBB confirmed that both immature and mature monocyte-derived DC minimally adhere to resting human brain microvascular endothelial cells. Adherence of DC to human microvascular endothelial cells was not affected by blocking ICAM-1, ICAM-2, PECAM-1, VCAM-1, or E-selectin on the endothelial cells or their respective ligands on DC (84), albeit that expression of these molecules by the resting endothelium was not investigated. In contrast, Wethmar et al demonstrated involvement of ICAM-2 in the transmigration process of immature murine bone marrow-derived DC through cerebromicrovascular endothelial monolayers (85). However, as ICAM-2-mediated transmigration was shown to occur independent of β2-integrins, it was suggested that LFA-1 expressed by DC is not involved or at least redundant for steady-state transmigration. Hence, other ligands for ICAM-2, such as DC-SIGN, are likely to be involved in this process.
BBB activation and breakdown during neuroinflammation are associated with massive infiltration of peripheral leukocytes into the CNS and several molecular changes also facilitate trafficking of DC from the peripheral circulation to the CNS in MS (Fig. 1A). These changes include (1) downregulated expression of tight junction proteins and strongly upregulated expression of cell adhesion molecules at the blood-CNS barriers; (2) enhanced secretion of chemokines by endothelial cells, perivascular immune cells, astrocytes, and other CNS-resident cells; and (3) enhanced trafficking molecule expression by circulating DC, as discussed hereafter.
Only a limited number of studies have focused on the interaction of DC with the BBB during autoimmune-mediated neuroinflammation in the context of MS. Using cytokine-activated human brain microvascular endothelial cells, Arjmandi et al studied adherence of DC to the inflamed BBB (84). Both mature and immature monocyte-derived DC adhered to the activated endothelial cells, but immature DC were significantly more efficient in doing so. Adherence of immature DC was mediated by ICAM-1, ICAM-2, VCAM-1, and PECAM-1 on the endothelial cells, whereas adherence of mature monocyte-derived DC was only affected by ICAM-1, but not ICAM-2, PECAM-1, VCAM-1, or E-selectin. Also, CD18 and DC-SIGN on both immature and mature DC were involved in this process. Interestingly, blocking E-selectin, its ligand sialyl Lewis x antigen, and VLA-4 did not affect the adherence of either immature or mature DC to activated endothelial cells in vitro. In agreement with this, other studies have shown that E- and P-selectins are not required for leukocyte recruitment across the BBB and development of EAE, despite elevated expression at the BBB during neuroinflammation (86, 87). However, this is contradicted by others (88), necessitating further investigation of the role of selectins in leukocyte and, more specifically, DC recruitment to the inflamed CNS.
Using intravital fluorescence video microscopy to visualize the initial interactions of infused, fluorescently labeled DC with the inflamed parenchymal microvessels in EAE, Jain et al confirmed that immature DC were far more efficient to firmly adhere to the endothelial cells as compared to mature DC (89). Immunofluorescence analysis of spinal cord sections revealed immature DC extravasating from the circulation into the perivascular space and in the spinal cord white matter parenchyma. In contrast, mature DC were unable to cross the BBB. They could, however, be detected in the meninges, similarly to immature DC, suggesting the meningeal blood-CSF barrier is more permissive for DC transmigration than the BBB under neuroinflammatory conditions. In contrast to the findings from Arjmandi et al (84), Jain et al demonstrated that the α4β1 integrin VLA-4, but not α4β7, is essential for recruitment of DC to the CNS in EAE by mediating firm adhesion to the inflamed vessel wall (89).
Several chemokines have been implicated in the recruitment of DC to the CNS in MS. Indeed, in brain lesions and CSF of MS patients, elevated levels of the DC-attracting chemokines CCL2 (90), CCL3 (91–93), CCL4 (92), CCL5 (92, 94, 95), CCL19 (96), and chemerin (6) are found, creating chemokine gradients that guide DC toward the site of inflammation. Interestingly, circulating DC of MS patients express increased levels of chemokine receptors as compared to DC of healthy controls. For instance, circulating cDC in patients with MS and optic neuritis demonstrate elevated expression of CCR5 as compared to patients with noninflammatory neurological diseases (NIND) and healthy controls (97). In addition, pDC were shown to display an increased capacity to upregulate CCR7 expression upon TLR9 stimulation in MS patients as compared to healthy individuals (98). Also in our hands, higher numbers of CCR5-expressing cDC and pDC in relapsing-remitting (RR)MS and chronic progressive MS, respectively, were found as compared to healthy controls, providing further evidence for aberrant expression of migration markers in MS. In addition, we found upregulated CCR7 expression on pDC of RRMS patients (99). Increased chemokine receptor expression was paralleled by an increased in vitro migratory capacity of DC from MS patients as compared to DC from healthy controls. Differences in migration-associated molecules between MS patients and healthy controls are not only observed in circulating peripheral blood DC but also at the level of in vitro generated monocyte-derived DC. Indeed, monocyte-derived DC of MS patients express higher levels of CCR7 (100) and DC-SIGN (101) as well as greater activity of the matrix metalloproteinases MMP-2 and MMP-9 as measured by zymography (102) as compared to monocyte-derived DC of healthy individuals.
The CCL2-CCR2 chemokine-receptor pair is hypothesized to be crucially involved in the development of autoimmune-mediated neuroinflammation, as CCR2-deficient mice are completely resistant to develop EAE (103) and CCL2-deficient mice show profoundly reduced disease severity (104). Interestingly, adoptive transfer of Cr2−/− T cells could induce EAE when transferred into wild-type mice, whereas wild-type T cells transferred into Cr2−/− mice were unable to do so (105). This suggests that CCR2 expression by another, most likely antigen-presenting, immune cell population is required for EAE susceptibility. Dogan et al confirmed that glial-derived CCL2 specifically recruits macrophages and cDC to the inflamed CNS, as chimeric mice lacking CCL2 expression in the CNS showed reduced accumulation of both CD45hiCD11b+CD11c- macrophages and CD11b+CD11c+ DC, but not of lymphocytes, as compared to wild-type mice or chimeric mice in which leukocytes lack CCL2 expression (106). In agreement with these findings, Clarkson et al recently reported an increased frequency of infiltrating CD45hiCD11b+ myeloid cells as well as CD45hiCD11b+CD11c+ DC in the CNS of mice receiving intracerebral injections of CCL2 (107). Furthermore, they showed that adoptive transfer of Cr2−/− bone marrow-derived DC failed to significantly accumulate in the CNS of mice with ongoing EAE, in contrast to transferred Cr2+/+ DC. Others confirmed that DC specifically accumulated at perivascular sites near CCL2-expressing lesions (9). In vitro, CCL2 was shown to stimulate transmigration of human LPS-matured DC, in a dose-dependent manner, by a p38-MAPK and ERK1/2-dependent process. Chemotaxis of immature DC was also driven by CCL2, and only dependent on ERK1/2 (22). However, opposed to the finding that DC downregulate inflammatory chemokine receptors upon maturation, mature DC demonstrated stronger chemotactic capacity compared to immature DC.
Also, CCL3 was found to attract DC to the inflamed CNS. In an in vitro model consisting of murine cerebral microvascular endothelial cell monolayers, CCL3 was shown to stimulate transmigration of mature bone marrow-derived DC in an MMP-dependent manner (108). After transmigration, these DC displayed an activated phenotype, evidenced by the upregulated expression of costimulatory molecules CD80, CD86, and CD40. Accordingly, migrated DC were superior to nonmigrated DC in stimulating antigen-specific T-cell activation. This underscores the importance of recruited DC in restimulation of local T cells residing in the perivascular spaces and their role in perpetuating autoimmune inflammation.
Choroid plexus
Because of the preferential perivascular localization of parenchymal lesions and the concomitant breach in BBB integrity, as visualized on contrast-enhanced MRI, the BBB was considered to be the major site of immune cell entry in the pathogenesis of CNS autoimmunity. However, more recent insights point toward an equally important role of leukocyte trafficking through the choroid plexus, at least in the initial phases of EAE.
In general, immune cells entering the CNS via the choroid plexus first extravasate from the capillary stromal vessels (Fig. 1B), which are fenestrated and express relatively high levels of P-selectin, E-selectin, and ICAM-1 (78, 109) and only low amounts of tight junction proteins (110), in contrast to microvessels of the BBB. Hence, these vessels exhibit a substantially higher degree of permeability. After entering the choroid plexus stroma, cells migrate toward the choroid plexus epithelium, which constitutes the actual blood-CSF barrier. In both rodents and humans, DC have been found in the choroid plexus stroma (73, 111–113) and between the epithelial cells at the ventricular surface of the choroid plexus (72, 114–116) under noninflammatory conditions. These DC display an immature phenotype (8, 116, 117). They extend their processes toward the stromal capillaries and between the epithelial cells of the choroid plexus (73, 116), indicative for their sentinel function sampling the microenvironment and the CSF for antigens. Furthermore, as most of the intraepithelial DC produce the immunosuppressive cytokine IL-10 (116), it is likely that these cells also actively contribute to the immunoquiescence in the CNS.
In order to reach the CSF, leukocytes have to cross the choroid plexus epithelial layer expressing several tight junction proteins, including occludin, zonula occludens-1 (ZO-1), and the claudins-1, -2, -3 and -11 (118–20). Interestingly, studies of the murine choroid plexus have shown that adhesion molecule expression at the blood-CSF barrier is highly polarized, with substantially higher constitutive expression of VCAM-1 and ICAM-1 on the apical aspect as compared to the basolateral side of the choroid plexus epithelium (78, 121). Noninflamed CSF is able to induce chemotaxis of immature monocyte-derived DC in vitro (122). Indeed, noninflamed CSF contains several chemokines including CCL2, CCL3, CCL4, CCL5, CCL20, CXCL10, CXCL11, and CXCL12 (95, 123, 124) that are known to attract immature DC. Also in vivo, low numbers of DC can be found in the human CSF (3, 97), where they constitute 1%–2% of the mononuclear cell fraction (3, 125). Despite the presence of DC in the choroid plexus and CSF under steady-state conditions, the precise mechanisms underlying homeostatic trafficking of DC to the CSF via the choroid plexus have not yet been studied extensively.
Although also in MS the pattern for DC recruitment through the choroid plexus remains elusive, several findings suggest that the choroid plexus is an important route of entry for DC, especially during early phases of the disease: (i) Murine CD11c+ DC accumulate rapidly in the choroid plexus following immunization with myelin oligodendrocyte glycoprotein, even prior to onset of EAE clinical signs (8, 126); (ii) there is a negative correlation between the number of DC in the CSF and MS duration, that is, patients with early MS show significantly higher numbers of DC in the CSF as compared to patients with longer disease duration (3); (iii) in MS and optic neuritis patients, CCR5 expression by cDC in the blood is significantly correlated with their numbers in the CSF (97) and CCR5 ligands are known to be upregulated in the CSF of MS patients (95); and (iv) increased numbers of pDC are found in the CSF of MS patients as compared to patients with NIND (3).
Moreover, cDC found in the CSF of MS patients display a more mature phenotype than their counterparts in blood, as demonstrated by increased expression of HLA-DR, CD86, CD80, and CD40 (3). Both cDC and pDC residing in the CSF express CCR5 (3) and about one-third of the MHC II+ DC population in the CSF expresses CCR7 (127). Interestingly, Although both CCR5 and CCR7 ligands are present in high levels in the CSF of MS patients (95, 128), their expression is also strongly upregulated or induced in the inflamed CNS parenchyma, in particular in MS lesions (91, 92, 94, 96), potentially providing traffic cues for DC to exit the CSF compartment and infiltrate the parenchyma. Indeed, intra-CSF injected DC are able to specifically infiltrate periventricular demyelinating lesions in EAE, and to reach parenchymal sites distant from the site of injection as well as perivascular inflammatory cuffs (129).
Meninges
DC are also present at meningeal sites in close contact with the CSF in the subarachnoid space, more specifically at the inner surface of the arachnoid layer and on the external surface of the pia mater, making them ideally positioned to sample antigens from the CSF (115) (Fig. 1C). Vass et al were the first to identify MHC class I and II-expressing dendriform cells, capable of taking up antigens from the CSF, in the leptomeninges of the healthy rat CNS, located predominantly around blood vessels (130). Later, the identity of these cells was confirmed by McMenamin et al, who described a discrete network of MHC class II-/OX62-expressing DC in the leptomeninges, dura mater and choroid plexus of healthy rats (114). Also in the healthy human CNS, DC-SIGN-positive DC were found in the meninges (71). Although meningeal microvessels exhibit a functional BBB which is characterized by high tight junction expression and consequently low paracellular permeability (131), subtle differences with the parenchymal BBB exist. Meningeal blood vessels lack direct ensheathment by astrocytic endfeet (131) and show a higher constitutive expression of cell adhesion molecules. Indeed, in contrast to the parenchymal BBB, meningeal endothelial cells constitutively express P- and E-selectin on their surface (86, 109). Additionally, ICAM-1 is abundantly expressed on endothelial cells of postcapillary venules in the noninflamed meninges (109). Altogether, this renders meningeal vessels more permissive for recruitment of immune cells, including DC. To date, however, studies focusing on homeostatic DC recruitment to the meninges are lacking.
During EAE induction, accumulation of DC in the meninges precedes their accumulation in the CNS parenchyma (132). It has been suggested that subsequently these DC migrate out of the meningeal compartment into the spinal cord parenchyma of EAE-affected mice (8). However, also in the meningeal compartment itself, accumulating DC were shown to interact with and activate CD4+ T cells, before the onset of clinical signs of EAE (133). Hence, the meninges not only represent an important gateway for DC, among other immune cells, to enter the CNS but also set the stage for primary interaction of infiltrated DC with myelin-specific T cells, before similar T-cell activation takes place in the brain parenchyma (133). It remains to be determined whether preferential accumulation of DC in the meninges prior to invasion of the CNS parenchyma is solely due to the higher constitutive adhesion molecule expression levels in meningeal vessels than in parenchymal vessels, or whether other mechanisms contribute to this phenomenon as well.
DC TRAFFICKING FROM THE CNS TO DRAINING LYMPH NODES
Immune surveillance of the CNS not only depends on migration of immune-competent cells into the CNS, it also requires drainage or trafficking of CNS-derived antigens to secondary lymphoid organs, more specifically to the cervical lymph nodes. In mice, exogenous antigens injected intracerebrally (134) or intracerebroventricularly (135) are capable of eliciting humoral and cellular immune responses in the cervical lymph nodes, providing evidence for an intact afferent immunity in the immune-privileged CNS. Nevertheless, Hatterer et al found that DC injected into the brain parenchyma of healthy rats demonstrate limited migration capacity and could not be detected in the cervical lymph nodes, whereas DC injected into the CSF did migrate to the cervical lymph nodes (136). However, others demonstrated that antigen-loaded bone marrow-derived DC injected intracerebrally into the CNS of naïve mice are capable of reaching the cervical lymph nodes, where they subsequently induce antigen-specific T-cell responses (137). Treatment of DC with pertussis toxin prior to intracerebral injection prevented their migration to the cervical lymph nodes, suggesting that an active, most likely chemokine receptor-driven pathway is responsible for this process, rather than passive drainage (137).
Although still rather controversial, 3 different routes for DC migration out of the CNS have been reported to date. Hochmeister et al demonstrated that bone marrow-derived DC injected into the striatum of rats migrate to the perivascular space, where they interact with the BBB endothelium and subsequently enter the blood vessel lumen (138). A second route was identified by Mohammad et al (2), as they described a CXCL12-CXCR4-dependent pathway for DC migration associated with the rostral migratory stream, which connects the olfactory bulb to the periventricular regions. Interestingly, interfering with DC migration along this rostral migratory stream pathway by targeted fingolimod treatment during EAE was shown to break immune tolerance and to increase EAE severity (2). A third exit route from the CNS comprises drainage via the CSF and the recently identified dural lymphatic vessels (139, 140). Indeed, CD11c+ cells with a DC-like morphology have been identified in the lumen of these meningeal lymphatic vessels (139). Migration along this route is probably coordinated in large part by the chemokines CCL19 (96, 128) and CCL21 (139) and their receptor CCR7 (127). In support of this hypothesis, Clarkson et al showed that Ccr7+/+ but not Ccr7−/− myelin-loaded DC reached the cervical lymph nodes after intracerebral injection into the CNS of EAE mice (141), or after recruitment to the inflamed CNS of mice inflicted with EAE (142).
EFFECTS OF MS THERAPEUTICS ON THE MIGRATORY CAPACITY OF DC
Current treatment for MS is based on the use of disease-modifying therapeutics. Most of these therapeutics have an anti-inflammatory mode of action and exert a wide spectrum of immunomodulatory effects affecting a broad range of immune cell types (recently reviewed by Torkildsen et al [143]). Also, the migration of immune cells, among other immune effector cell functions, is an important therapeutic target of several of the approved drugs for MS. Indeed, interferon-β (IFN-β), glatiramer acetate, fingolimod, natalizumab, and dimethyl fumarate are known to affect the migratory capacity of DC by modulating the expression of adhesion molecules, MMPs, and/or chemokine receptors, which will be discussed in more detail below and is summarized in the Table. In addition, MS therapeutics can also indirectly interfere with DC recruitment to the CNS by stabilizing the BBB and by decreasing the expression of adhesion molecules and chemokines by cells of the BBB and surrounding cells (extensively reported elsewhere [144–152]).
Table.
DC Migration-Associated Molecules and Processes as Therapeutic Targets
| Target | Therapeutic Agent | Treatment | Type of DC | Species | Effect | Ref. |
|---|---|---|---|---|---|---|
| Chemokine receptors | ||||||
| CCR7 | Interferon-β | In vivo | pDC | Human | Normalized upregulation upon TLR9 stimulation | (98) |
| In vitro | BMDC | Mouse | Reduced upregulation upon maturation with pro-inflammatory cytokines, mediated through STAT-1 | (156) | ||
| In vivo | cDC, pDC | Human | No effect | (99) | ||
| CCR5 | In vivo | cDC, pDC | Human | No effect | (99) | |
| CCR7 | Natalizumab | In vivo | pDC | Human | Increased proportion of positive cells in treated vs untreated MS patients | (160) |
| CCR7 | Fingolimod | In vivo | CD11c+ DC | Mouse | Reduced expression as compared to DC of nontreated mice | (166) |
| In vitro | BMDC | Mouse | Reduced expression as compared to untreated BMDC | (166) | ||
| In vitro | moDC | Human | No effect | (163, 165) | ||
| CCR1, CCR3, CCR5, CXCR4 | In vitro | moDC | Human | No effect | (163, 165) | |
| CCR6 | Glatiramer acetate | In vivo | moDC | Human | Increased expression as compared to pretreatment levels | (157) |
| Matrix metalloproteinases | ||||||
| MMP-9 | Interferon-β | In vitro | moDC | Human | Decreased production and activity | (153) |
| In vitro | BMDC | Mouse | Abolished induction upon PGE2 stimulation, mediated through STAT-1 | (156) | ||
| Adhesion molecules | ||||||
| DC-SIGN | Interferon-β | In vitro | moDC | Human | Abolished induction during DC differentiation in vitro | (154) |
| CD62L | In vivo | cDC, pDC | Human | Reduced proportion of positive cells in treated vs nontreated MS patients | (99) | |
| VLA-4 | Natalizumab | In vivo | cDC, pDC | Human | Reduced proportion of positive cells as compared to pretreatment levels | (159) |
| In vivo | pDC | Human | Increased proportion but reduced staining intensity of positive cells in treated vs untreated MS patients | (160) | ||
| LFA-1 | In vivo | cDC | Human | Increased proportion of positive cells as compared to pretreatment levels | (159) | |
| β2-Integrin | Fingolimod | In vitro | moDC | Human | Reduced expression as compared to untreated moDC | (163) |
| αM-Integrin, PECAM-1, ICAM-1 | In vivo | CD11c+ DC | Mouse | Reduced expression as compared to untreated mice | (166) | |
| Actin polymerization | ||||||
| Actin | Fingolimod | In vitro | moDC | Human | Reduced actin polymerization | (163) |
| Signaling pathways | ||||||
| ERK1/2 | Dimethylfumarate | In vitro | BMDC | Mouse | Inhibited phosphorylation upon LPS stimulation | (170) |
| NF-κB | In vitro | BMDC | Mouse | Reduced p65 phosphorylation, resulting in reduced nuclear localization and transcriptional activity of p65 | (170) | |
BMDC, bone marrow-derived DC; CCR, C-C-chemokine receptor; CD62L, CD62 ligand; cDC, conventional DC; CXCR, C-X-C-chemokine receptor; DC-SIGN, dendritic cell-specific ICAM-grabbing nonintegrin; ERK1/2, extracellular signal-regulated kinases 1 and 2; ICAM-1, intercellular adhesion molecule-1; LFA-1, lymphocyte function-associated antigen-1; MMP-9, matrix metalloproteinase 9; moDC, monocyte-derived DC; NF-κB, nuclear factor kappa-B; pDC, plasmacytoid DC; PECAM-1, platelet and endothelial cell adhesion molecule-1; PGE2, prostaglandin E2; STAT-1, signal transducer and activator of transcription 1; TLR9, Toll-like receptor-9; VLA-4, very late antigen-4.
Interferon-β
IFN-β has a broad spectrum of biological activities which have been shown to be beneficial in the treatment of MS, including inhibitory effects on leukocyte proliferation, inducing a shift from a typical proinflammatory cytokine profile toward an anti-inflammatory one, and interfering with trafficking of inflammatory cells across the BBB. More specifically, IFN-β has been shown to modulate the expression of migration-associated molecules by DC, which is generally increased in MS patients. Indeed, upon IFN-β treatment, isolated pDC from MS patients normalized their capacity to upregulate CCR7 expression upon TLR9 stimulation to a similar extent as circulating pDC of healthy controls, that is, to lower levels than pDC from untreated MS patients (98). In addition, in vitro treatment of human monocyte-derived DC with IFN-β diminished MMP-9 production by DC (153) and inhibited the expression of DC-SIGN (154), but not that of the integrin ICAM-1 (155). Accordingly, Yen et al showed that IFN-β treatment inhibited CCR7 expression and MMP-9 production by murine myeloid DC in vitro and in vivo through STAT-1 signaling, leading to a reduced migratory capacity of these cells (156). In our hands, however, no effect of IFN-β or any other treatment could be demonstrated on CCR5 or CCR7 expression by circulating DC (99), albeit that a significantly lower number of circulating CD62L-expressing cDC and pDC was found in MS patients receiving IFN-β treatment as compared to untreated MS patients.
Glatiramer Acetate
Glatiramer acetate is a mixture of synthetic polypeptides composed of 4 amino acids designed to mimic myelin basic protein. The main immunomodulatory action of glatiramer acetate is skewing of the T-cell response toward a Th2-dominated one. Although glatiramer acetate has also been described to modulate the function of DC and directs these cells toward an anti-inflammatory state (148, 155), only few studies have investigated the effect of glatiramer acetate treatment on the migratory capacity of DC. In a 1-year follow-up study investigating the characteristics of DC in MS patients receiving glatiramer acetate treatment, Høglund et al found a consistent upregulation of CCR6 expression after 32 and 48 weeks of treatment as compared to pretreatment levels (157). The underlying mechanisms, however, remain to be unraveled.
Natalizumab
Natalizumab is a monoclonal antibody targeting the α4-subunit of the α4β1-integrin VLA-4, which is expressed by a broad range of leukocytes, including human DC (65). Reduced numbers of DC are found in the perivascular space of RRMS patients following treatment with natalizumab (158). In addition, a significant decrease in the proportion of α4β1-expressing cells within the circulating pDC and cDC population was demonstrated in RRMS patients receiving natalizumab, as soon as 48 hours after initiating therapy and lasting up to 12 months following the first administration of natalizumab, as compared to pretreatment levels (159). Kivisäkk et al, on the other hand, described an increased proportion but reduced staining intensity of α4-expressing pDC as compared to untreated MS patients (160). The reduced α4-integrin surface expression at the cellular level is probably due to internalization and/or shedding of α4-molecules, and not to decreased synthesis, similar to what was shown for T cells of natalizumab-treated MS patients (161). Interestingly, a significant upregulation of the integrin LFA-1 (αLβ2) on cDC (159) and of CCR7 on pDC (160) was reported following treatment with natalizumab.
Fingolimod
Fingolimod is a sphingosine-1-phosphate receptor (S1PR) modulator that causes the receptor to internalize upon binding. This reduces the cell’s responsiveness to S1P, a key regulator of immune cell trafficking. As S1P normally favors egress of CCR7-expressing lymphocytes from secondary lymphoid organs by overruling the CCR7-mediated retention signals, lymphocytes in fingolimod-treated individuals become trapped in the lymph nodes, preventing them to circulate to the inflamed CNS. As DC express S1PR isoforms 1-4 (162, 163), migration of DC is also affected by fingolimod. For instance, a dose-dependent reduction in chemotaxis by both immature and mature monocyte-derived DC was demonstrated following in vitro treatment with therapeutic doses of fingolimod (164). This effect was likely mediated by reduced actin polymerization, a prerequisite for cell migration, and not by reduced chemokine receptor expression by monocyte-derived DC, as the expression levels of CCR1, CCR3, CCR5, CCR7, and CXCR4 were not altered following treatment with fingolimod (164, 165). In contrast, Lan et al did report a significant reduction in CCR7 protein expression, and consequently a reduced in vitro migratory capacity toward the lymphoid chemokine CCL19, both in circulating DC of fingolimod-treated mice as well as in bone marrow-derived in vitro generated DC (166). In addition, a consistent downregulation of CD18 (integrin β2) expression in fingolimod-treated monocyte-derived DC (163) was demonstrated, whereas expression of the adhesion molecules CD11b (integrin αM), PECAM-1 and ICAM-1 was significantly downregulated on circulating DC in fingolimod-treated C57BL/10 mice (166). Overall, fingolimod interferes with inflammatory DC trafficking, indirectly by interfering with the process of actin polymerization, and directly by altering the expression of migration-associated molecules by DC. However, the effects of fingolimod on DC migratory capacity might also have adverse effects. In EAE, it was shown that fingolimod treatment interferes with DC migration out of the CNS, causing these cells to accumulate in the distal part of the rostral migratory stream (2). Accordingly, targeted delivery of this therapeutic to the rostral migratory stream resulted in a dose-dependent aggravation of EAE, associated with accumulation of DC in the CNS. Hence, although the overall effect of systemic fingolimod treatment is amelioration of neuroinflammation, this observation raises the question whether overall treatment efficacy of fingolimod would improve in case it would be BBB impermeable. Nevertheless, as fingolimod also exerts beneficial actions within the CNS (167), it remains to be determined which of these mechanisms—beneficial or detrimental—takes the overhand in the treatment of MS.
Dimethyl Fumarate
Dimethyl fumarate and its active metabolite monomethyl fumarate exert both neuroprotective effects through nuclear factor erythroid 2-related factor 2 (168) as well as immunomodulatory actions (169). Many of the agents’ immunomodulatory effects are mediated through perturbation of the nuclear factor kappa B (NF-κB) pathway (170). The NF-κB family of transcription factors regulates a broad range of both innate and adaptive immune cell functions, including cellular maturation, proliferation, and differentiation as well as production of cytokines and other immune effector molecules (171). Dimethyl fumarate impairs DC differentiation (172) and maturation (170) and alters their cytokine profile (170, 173, 174) in an NF-κB-dependent manner (170), thereby affecting their ability to stimulate and polarize T cells. Moreover, dimethyl fumarate was also shown to suppress ERK1/2 signaling in DC (170). Interestingly, ERK1/2 activation is involved in chemokine-driven migration of both mature and immature DC (9), suggesting that dimethyl fumarate-mediated inhibition of ERK1/2 function could result in impaired DC recruitment to the CNS.
Conclusion
Migration of DC to the CNS is a critical event in the pathogenesis of MS. Several studies reported a correlation between the number of DC in the CNS and the degree of neuroinflammation (3, 9, 14, 15). To date, studies investigating DC recruitment to the CNS in MS have focused on the transmigration of these cells through the BBB. However, experimental evidence points toward an equally important role of the choroid plexus (3, 8, 126) and meningeal vessels (132, 133) as a gateway for DC to the CNS, especially during early stages of disease. For this, future research efforts should focus not only on further elucidating the mechanisms underlying DC transmigration through the BBB but also on identifying the forces driving DC accumulation in the meninges, the choroid plexus, and subsequently in the CSF. Dynamic models of the blood-CSF and BBB in vitro combined with high magnification live cell imaging might be useful tools to perform initial screening for molecular determinants involved in immune cell transmigration. Subsequently, in vivo live cell imaging techniques can be used to verify the role of interesting target molecules in DC migration during EAE. Over the past few years, intravital multiphoton imaging techniques have become more user-friendly and increasingly popular for studying immune cell migration in experimental models, and the in vivo imaging field keeps rapidly evolving (175). For studying DC migration in particular, a photoconvertible reporter mouse strain was developed by Kitano et al, allowing them to distinctively visualize the migration of skin-resident XCR1-expressing DC to skin-draining lymph nodes (176). Although technically more challenging, a similar approach could be explored to elucidate the migratory behavior and role of DC exiting the CNS during immune surveillance as well as during neuroinflammation. Indeed, recent studies underscore the importance of DC migration out of the CNS in maintaining immune tolerance (2, 141), and several routes for DC migration from the CNS to the cervical lymph nodes have been identified. Although DC migrate out of the CNS, both in steady state conditions and during EAE in a CCR7-dependent manner (142), CCR7+ DC also accumulate in perivascular MS lesions (127), in which the CCR7 ligands CCL19 and CCL21 are highly expressed (96, 177). This led Kivisäkk et al to hypothesize that the afferent pathway of CNS immunity might be compromised (127), resulting in CCL19/CCL21-mediated retention of mature, immunostimulatory DC in the CNS during MS and EAE. Overall, autoimmune neuroinflammation associated with MS and EAE is characterized by an imbalance between efferent and afferent immunity, with an increased number of DC migrating to and accumulating in the CNS, whereas their migration out of the CNS is reduced. Restoring the balance might offer a valid therapeutic strategy for MS.
The therapeutic success of natalizumab constitutes the best proof-of-principle for leukocyte trafficking blockade as a valid approach for the treatment of neuroinflammatory disease. At the same time, the occurrence of progressive multifocal leukoencephalopathy in recipients provides the most salient caution about unexpected complications of this treatment strategy. More research is warranted to design therapeutic strategies able to selectively interfere with the recruitment of pathogenic leukocytes, including DC, to the CNS, while leaving host protective mechanisms intact. In this context, targeting migration-associated molecules such as chemokines and their receptors represents a valuable approach. In MS, neutralizing antibodies or small molecule inhibitors for CCR1 and CCR2 have been tested in several clinical trials (178–80), but the results are rather disappointing as these agents showed no or only modest efficacy (178, 180, 181). Also in other inflammatory diseases, chemokine receptor antagonism failed to meet the therapeutic expectations (178, 179). A potential explanation could be the high level of redundancy inherent to the chemokine-chemokine receptor system. Indeed, some chemokines are promiscuous binders, able to signal through different chemokine receptors. Chemokine receptors, on the other hand, can often bind more than one chemokine ligand (182). Moreover, inflammatory chemokines have overlapping actions, in that they all recruit leukocytes to sites of inflammation. Hence, interfering with signaling through one single chemokine receptor might be insufficient, as its ligand(s) possibly also target(s) other chemokine receptors. The use of broad-spectrum chemokine inhibitors (183) might overcome this problem, but it remains to be determined how efficacious these drugs are in vivo, and to which extent they still allow for homeostatic immune cell migration. Other possible targets for therapeutic intervention are adhesion molecules and proteinases. Recently, a new class of MMP inhibitors has become available, displaying both higher specificity and efficacy as compared to earlier MMP inhibiting compounds (184). Although still in an early phase of development, it might be interesting to test these next-generation MMP inhibitors as therapeutic compounds in inflammatory diseases such as MS.
In conclusion, DC can enter the CNS and position themselves at strategic locations for antigen sampling as part of CNS immune surveillance. On their turn, antigen-loaded DC can exit the CNS toward the draining cervical lymph nodes, a process which has been shown to be critical in maintaining CNS immune homeostasis. In MS, DC trafficking to, accumulation in, and migration from the CNS are perturbed. Correcting the imbalance might help to tackle MS-associated neuroinflammation.
REFERENCES
- 1. Cools N, Ponsaerts P, Van Tendeloo VFI, et al. Balancing between immunity and tolerance: An interplay between dendritic cells, regulatory T cells, and effector T cells. J Leukoc Biol 2007;82:1365–74. [DOI] [PubMed] [Google Scholar]
- 2. Mohammad MG, Tsai VWW, Ruitenberg MJ, et al. Immune cell trafficking from the brain maintains CNS immune tolerance. J Clin Invest 2014;124:1228–41http://dx.doi.org/10.1172/JCI71544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pashenkov M, Huang YM, Kostulas V, et al. Two subsets of dendritic cells are present in human cerebrospinal fluid. Brain 2001;124:480–92http://dx.doi.org/10.1093/brain/124.3.480 [DOI] [PubMed] [Google Scholar]
- 4. Plumb J, Armstrong MA, Duddy M, et al. CD83-positive dendritic cells are present in occasional perivascular cuffs in multiple sclerosis lesions. Mult Scler 2003;9:142–7http://dx.doi.org/10.1191/1352458503ms890oa [DOI] [PubMed] [Google Scholar]
- 5. Serafini B, Rosicarelli B, Magliozzi R, et al. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol 2004;14:164–74http://dx.doi.org/10.1111/j.1750-3639.2004.tb00049.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lande R, Gafa V, Serafini B, et al. Plasmacytoid dendritic cells in multiple sclerosis: Intracerebral recruitment and impaired maturation in response to interferon-[beta]. J Neuropathol Exp Neurol 2008;67:388–401http://dx.doi.org/10.1097/NEN.0b013e31816fc975 [DOI] [PubMed] [Google Scholar]
- 7. Longhini ALF, von Glehn F, Brandão CO, et al. Plasmacytoid dendritic cells are increased in cerebrospinal fluid of untreated patients during multiple sclerosis relapse. J Neuroinflammation 2011;8:2–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Serafini B, Columba-Cabezas S, Di Rosa F, Aloisi F.. Intracerebral recruitment and maturation of dendritic cells in the onset and progression of experimental autoimmune encephalomyelitis. Am J Pathol 2000;157:1991–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sagar D, Lamontagne A, Foss CA, et al. Dendritic cell CNS recruitment correlates with disease severity in EAE via CCL2 chemotaxis at the blood-brain barrier through paracellular transmigration and ERK activation. J Neuroinflammation 2012;9:245–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clarkson BD, Walker A, Harris M, et al. Mapping the accumulation of co-infiltrating CNS dendritic cells and encephalitogenic T cells during EAE. J Neuroimmunol 2014;277:39–49http://dx.doi.org/10.1016/j.jneuroim.2014.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bailey SL, Schreiner B, McMahon EJ, Miller SD.. CNS myeloid DCs presenting endogenous myelin peptides “preferentially” polarize CD4+ T(H)-17 cells in relapsing EAE. Nat Immunol 2007;8:172–80 [DOI] [PubMed] [Google Scholar]
- 12. Miller SD, McMahon EJ, Schreiner B, Bailey SL.. Antigen presentation in the CNS by myeloid dendritic cells drives progression of relapsing experimental autoimmune encephalomyelitis. Ann N Y Acad Sci 2007;1103:179–91http://dx.doi.org/10.1196/annals.1394.023 [DOI] [PubMed] [Google Scholar]
- 13. Dittel BN, Visintin I, Merchant RM, Janeway CA.. Presentation of the self antigen myelin basic protein by dendritic cells leads to experimental autoimmune encephalomyelitis. J Immunol 1999;163:32–9 [PubMed] [Google Scholar]
- 14. Greter M, Heppner FL, Lemos MP, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med 2005;11:328–34http://dx.doi.org/10.1038/nm1197 [DOI] [PubMed] [Google Scholar]
- 15. Zozulya AL, Ortler S, Lee J, et al. Intracerebral dendritic cells critically modulate encephalitogenic versus regulatory immune responses in the CNS. J Neurosci 2009;29:140–52http://dx.doi.org/10.1523/JNEUROSCI.2199-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Greaves DR, Wang W, Dairaghi DJ, et al. CCR6, a CC chemokine receptor that interacts with macrophage inflammatory protein 3 and is highly expressed in human dendritic cells. J Exp Med 1997;186:837–44http://dx.doi.org/10.1084/jem.186.6.837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Power CA, Church DJ, Meyer A, et al. Cloning and characterization of a specific receptor for the novel CC chemokine MIP-3 from lung dendritic cells. J Exp Med 1997;186:825–35http://dx.doi.org/10.1084/jem.186.6.825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin CL, Suri RM, Rahdon RA, et al. Dendritic cell chemotaxis and transendothelial migration are induced by distinct chemokines and are regulated on maturation. Eur J Immunol 1998;28:4114–22http://dx.doi.org/10.1002/(SICI)1521-4141(199812)28:12<4114::AID-IMMU4114>3.0.CO;2-C [DOI] [PubMed] [Google Scholar]
- 19. Sallusto F, Schaerli P, Loetscher P, et al. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol 1998;28:2760–9http://dx.doi.org/10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N [DOI] [PubMed] [Google Scholar]
- 20. Sozzani S, Allavena P, D’Amico G, et al. Differential regulation of chemokine receptors during dendritic cell maturation: A model for their trafficking properties. J Immunol 1998;161:1083–6 [PubMed] [Google Scholar]
- 21. Vecchi A, Massimiliano L, Ramponi S, et al. Differential responsiveness to constitutive vs. inducible chemokines of immature and mature mouse dendritic cells. J Leukoc Biol 1999;66:489–94 [DOI] [PubMed] [Google Scholar]
- 22. Penna G, Sozzani S, Adorini L.. Cutting edge: Selective usage of chemokine receptors by plasmacytoid dendritic cells. J Immunol 2001;167:1862–6http://dx.doi.org/10.4049/jimmunol.167.4.1862 [DOI] [PubMed] [Google Scholar]
- 23. Kabashima K, Shiraishi N, Sugita K, et al. CXCL12-CXCR4 engagement is required for migration of cutaneous dendritic cells. Am J Pathol 2007;171:1249–57http://dx.doi.org/10.2353/ajpath.2007.070225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schmuth M, Neyer S, Rainer C, et al. Expression of the C-C chemokine MIP-3 alpha/CCL20 in human epidermis with impaired permeability barrier function. Exp Dermatol 2002;11:135–42http://dx.doi.org/10.1034/j.1600-0625.2002.110205.x [DOI] [PubMed] [Google Scholar]
- 25. Reibman J, Hsu Y, Chen LC, et al. Airway epithelial cells release MIP-3alpha/CCL20 in response to cytokines and ambient particulate matter. Am J Respir Cell Mol Biol 2003;28:648–54http://dx.doi.org/10.1165/rcmb.2002-0095OC [DOI] [PubMed] [Google Scholar]
- 26. Banks C, Bateman A, Payne R, et al. Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn’s disease. J Pathol 2003;199:28–35 [DOI] [PubMed] [Google Scholar]
- 27. Reinecker H-C, Loh EY, Ringler DJ, et al. Monocyte-chemoattractant protein 1 gene expression in intestinal epithelial cells and inflammatory bowel disease mucosa. Gastroenterology 1995;108:40–50http://dx.doi.org/10.1016/0016-5085(95)90006-3 [DOI] [PubMed] [Google Scholar]
- 28. Maltby J, Wright S, Bird G, Sheron N.. Chemokine levels in human liver homogenates: Associations between GRO alpha and histopathological evidence of alcoholic hepatitis. Hepatology 1996;24:1156–60 [DOI] [PubMed] [Google Scholar]
- 29. Stumbles PA, Strickland DH, Pimm CL, et al. Regulation of dendritic cell recruitment into resting and inflamed airway epithelium: Use of alternative chemokine receptors as a function of inducing stimulus. J Immunol 2001;167:228–34http://dx.doi.org/10.4049/jimmunol.167.1.228 [DOI] [PubMed] [Google Scholar]
- 30. Bernardo D, Durant L, Mann ER, et al. Chemokine (C-C motif) receptor 2 mediates dendritic cell recruitment to the human colon but is not responsible for differences observed in dendritic cell subsets, phenotype, and function between the proximal and distal colon. Cell Mol Gastroenterol Hepatol 2016;2:22–39.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Proost P, Wuyts A, van Damme J.. The role of chemokines in inflammation. Int J Clin Lab Res 1996;26:211–23http://dx.doi.org/10.1007/BF02602952 [DOI] [PubMed] [Google Scholar]
- 32. Trombetta ES, Mellman I.. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol 2005;23:975–1028http://dx.doi.org/10.1146/annurev.immunol.22.012703.104538 [DOI] [PubMed] [Google Scholar]
- 33. Yanagihara S, Komura E, Nagafune J, et al. EBI1/CCR7 is a new member of dendritic cell chemokine receptor that is up-regulated upon maturation. J Immunol 1998;161:3096–102 [PubMed] [Google Scholar]
- 34. Collin M, McGovern N, Haniffa M.. Human dendritic cell subsets. Immunology 2013;140:22–30http://dx.doi.org/10.1111/imm.12117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chistiakov DA, Orekhov AN, Sobenin IA, Bobryshev YV.. Plasmacytoid dendritic cells: Development, functions, and role in atherosclerotic inflammation. Front Physiol 2014;5:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zabel BA, Silverio AM, Butcher EC.. Human blood plasmacytoid from myeloid dendritic cells in chemerin-directed chemotaxis distinguish chemokine-like receptor 1 expression and chemokine-like receptor 1 expression and chemerin-directed chemotaxis distinguish plasmacytoid from myeloid dendritic cells in human blood. J Immunol Ref 2005;174:244–51http://dx.doi.org/10.4049/jimmunol.174.1.244 [DOI] [PubMed] [Google Scholar]
- 37. Zabel BA, Allen SJ, Kulig P, et al. Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J Biol Chem 2005;280:34661–6http://dx.doi.org/10.1074/jbc.M504868200 [DOI] [PubMed] [Google Scholar]
- 38. de la Rosa G, Longo N, Rodríguez-Fernández JL, et al. Migration of human blood dendritic cells across endothelial cell monolayers : Adhesion molecules and chemokines involved in subset-specific transmigration. J Leukoc Biol 2003;73:639–49 [DOI] [PubMed] [Google Scholar]
- 39. Bianchi G, D’Amico G, Varone L, et al. In vitro studies on the trafficking of dendritic cells through endothelial cells and extra-cellular matrix. Dev Immunol 2000;7:143–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Robert C, Fuhlbrigge RC, Kieffer JD, et al. Interaction of dendritic cells with skin endothelium: A new perspective on immunosurveillance. J Exp Med 1999;189:627–36http://dx.doi.org/10.1084/jem.189.4.627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Villablanca EJ, Mora JR.. A two-step model for Langerhans cell migration to skin-draining LN. Eur J Immunol 2008;38:2975–80http://dx.doi.org/10.1002/eji.200838919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Martin-Fontecha A, Martin-Fontecha A, Lanzavecchia A, et al. Dendritic cell migration to peripheral lymph nodes. Handb Exp Pharmacol 2009;31–49 [DOI] [PubMed] [Google Scholar]
- 43. Bajaña S, Roach K, Turner S, et al. IRF4 promotes cutaneous dendritic cell migration to lymph nodes during homeostasis and inflammation. J Immunol 2012;189:3368–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Idoyaga J, Fiorese C, Zbytnuik L, et al. Specialized role of migratory dendritic cells in peripheral tolerance induction. J Clin Invest 2013;123:844–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tomura M, Hata A, Matsuoka S, et al. Tracking and quantification of dendritic cell migration and antigen trafficking between the skin and lymph nodes. Sci Rep 2014;4:6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cook DN, Bottomly K.. Innate immune control of pulmonary dendritic cell trafficking. Proc Am Thorac Soc 2007;4:234–9http://dx.doi.org/10.1513/pats.200701-026AW [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hammad H, Lambrecht BN.. Lung dendritic cell migration. Adv Immunol 2007;93:265–78 [DOI] [PubMed] [Google Scholar]
- 48. Kobayashi H, Miura S, Nagata H, et al. In situ demonstration of dendritic cell migration from rat intestine to mesenteric lymph nodes: Relationships to maturation and role of chemokines. J Leukoc Biol 2004;75:434–42 [DOI] [PubMed] [Google Scholar]
- 49. Jang MH, Sougawa N, Tanaka T, et al. CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes. J Immunol 2006;176:803–10http://dx.doi.org/10.4049/jimmunol.176.2.803 [DOI] [PubMed] [Google Scholar]
- 50. Milling S, Yrlid U, Cerovic V, MacPherson G.. Subsets of migrating intestinal dendritic cells. Immunol Rev 2010;234:259–67http://dx.doi.org/10.1111/j.0105-2896.2009.00866.x [DOI] [PubMed] [Google Scholar]
- 51. Houston SA. The anatomical origins of migratory dendritic cells in the intestine. PhD Thesis, University of Glasgow, 2013
- 52. Alvarez D, Vollmann EH, von Andrian UH.. Mechanisms and consequences of dendritic cell migration. Immunity 2008;29:325–42http://dx.doi.org/10.1016/j.immuni.2008.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McRae BL. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J Exp Med 1995;182:75–85http://dx.doi.org/10.1084/jem.182.1.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tuohy VK, Yu M, Yin L, et al. The epitope spreading cascade during progression of experimental autoimmune encephalomyelitis and multiple sclerosis. Immunol Rev 1998;164:93–100http://dx.doi.org/10.1111/j.1600-065X.1998.tb01211.x [DOI] [PubMed] [Google Scholar]
- 55. McMahon EJ, Bailey SL, Castenada CV, et al. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med 2005;11:335–9http://dx.doi.org/10.1038/nm1202 [DOI] [PubMed] [Google Scholar]
- 56. Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell 1994;76:301–14http://dx.doi.org/10.1016/0092-8674(94)90337-9 [DOI] [PubMed] [Google Scholar]
- 57. Ley K, Laudanna C, Cybulsky MI, Nourshargh S.. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat Rev Immunol 2007;7:678–89http://dx.doi.org/10.1038/nri2156 [DOI] [PubMed] [Google Scholar]
- 58. Moore KL. Structure and function of P-selectin glycoprotein ligand-1. Leuk Lymphoma 1998;29:1–15http://dx.doi.org/10.3109/10428199809058377 [DOI] [PubMed] [Google Scholar]
- 59. Ebner S, Lenz A, Reider D, et al. Expression of maturation-/migration-related molecules on human dendritic cells from blood and skin. Immunobiology 1998;198:568–87http://dx.doi.org/10.1016/S0171-2985(98)80079-X [DOI] [PubMed] [Google Scholar]
- 60. Pendl GG, Robert C, Steinert M, et al. Immature mouse dendritic cells enter inflamed tissue, a process that requires E- and P-selectin, but not P-selectin glycoprotein ligand 1. Blood 2002;99:946–56http://dx.doi.org/10.1182/blood.V99.3.946 [DOI] [PubMed] [Google Scholar]
- 61. Geijtenbeek TB, Krooshoop DJ, Bleijs DA, et al. DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nat Immunol 2000;1:353–7http://dx.doi.org/10.1038/79815 [DOI] [PubMed] [Google Scholar]
- 62. García-Vallejo JJ, van Liempt E, da Costa Martins P, et al. DC-SIGN mediates adhesion and rolling of dendritic cells on primary human umbilical vein endothelial cells through LewisY antigen expressed on ICAM-2. Mol Immunol 2008;45:2359–69 [DOI] [PubMed] [Google Scholar]
- 63. Bleijs DA, Geijtenbeek TBH, Figdor CG, van Kooyk Y.. DC-SIGN and LFA-1: A battle for ligand. Trends Immunol 2001;22:457–63http://dx.doi.org/10.1016/S1471-4906(01)01974-3 [DOI] [PubMed] [Google Scholar]
- 64. Laudanna C, Kim JY, Constantin G, Butcher EC.. Rapid leukocyte integrin activation by chemokines. Immunol Rev 2002;186:37–46http://dx.doi.org/10.1034/j.1600-065X.2002.18604.x [DOI] [PubMed] [Google Scholar]
- 65. Brown KA, Bedford P, Leroy F, et al. Human blood dendritic cells: Binding to vascular endothelium and expression of adhesion molecules. Clin Exp Immunol 1997;54:601–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Freudenthal PS, Steinman RM.. The distinct surface of human blood dendritic cells, as observed after an improved isolation method. Proc Natl Acad Sci U S A 1990;87:7698–702http://dx.doi.org/10.1073/pnas.87.19.7698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rescigno M, Urbano M, Valzasina B, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2001;2:361–7http://dx.doi.org/10.1038/86373 [DOI] [PubMed] [Google Scholar]
- 68. Sung S-SJ, Fu SM, Rose CE, et al. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol 2006;176:2161–72http://dx.doi.org/10.4049/jimmunol.176.4.2161 [DOI] [PubMed] [Google Scholar]
- 69. Zimmerli SC, Hauser C.. Langerhans cells and lymph node dendritic cells express the tight junction component claudin-1. J Invest Dermatol 2007;127:2381–90http://dx.doi.org/10.1038/sj.jid.5700882 [DOI] [PubMed] [Google Scholar]
- 70. Ogasawara N, Kojima T, Go M, et al. Induction of JAM-A during differentiation of human THP-1 dendritic cells. Biochem Biophys Res Commun 2009;389:543–9http://dx.doi.org/10.1016/j.bbrc.2009.09.024 [DOI] [PubMed] [Google Scholar]
- 71. Serafini B, Rosicarelli B, Magliozzi R, et al. Dendritic cells in multiple sclerosis lesions: Maturation stage, myelin uptake, and interaction with proliferating T cells. J Neuropathol Exp Neurol 2006;65:124–41http://dx.doi.org/10.1093/jnen/65.2.124 [DOI] [PubMed] [Google Scholar]
- 72. Serot JM, Foliguet B, Béné MC, Faure GC.. Ultrastructural and immunohistological evidence for dendritic-like cells within human choroid plexus epithelium. Neuroreport 1997;8:1995–8 [DOI] [PubMed] [Google Scholar]
- 73. Hanly A, Petito CK.. HLA-DR-positive dendritic cells of the normal human choroid plexus: A potential reservoir of HIV in the central nervous system. Hum Pathol 1998;29:88–93http://dx.doi.org/10.1016/S0046-8177(98)90395-1 [DOI] [PubMed] [Google Scholar]
- 74. Fischer HG, Reichmann G.. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J Immunol 2001;166:2717–26http://dx.doi.org/10.4049/jimmunol.166.4.2717 [DOI] [PubMed] [Google Scholar]
- 75. Anandasabapathy N, Victora GD, Meredith M, et al. Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J Exp Med 2011;208:1695–705http://dx.doi.org/10.1084/jem.20102657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ransohoff RM, Kivisäkk P, Kidd G.. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol 2003;3:569–81 [DOI] [PubMed] [Google Scholar]
- 77. Hickey WF. Leukocyte traffic in the central nervous system: The participants and their roles. Semin Immunol 1999;11:125–37http://dx.doi.org/10.1006/smim.1999.0168 [DOI] [PubMed] [Google Scholar]
- 78. Kleine TO, Benes L.. Immune surveillance of the human central nervous system (CNS): Different migration pathways of immune cells through the blood-brain barrier and blood-cerebrospinal fluid barrier in healthy persons. Cytometry A 2006;69:147–51 [DOI] [PubMed] [Google Scholar]
- 79. Muldoon LL, Alvarez JI, Begley DJ, et al. Immunologic privilege in the central nervous system and the blood-brain barrier. J Cereb Blood Flow Metab 2013;33:13–21http://dx.doi.org/10.1038/jcbfm.2012.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Navratil E, Couvelard A, Rey A, et al. Expression of cell adhesion molecules by microvascular endothelial cells in the cortical and subcortical regions of the normal human brain: An immunohistochemical analysis. Neuropathol Appl Neurobiol 1997;23:68–80http://dx.doi.org/10.1111/j.1365-2990.1997.tb01187.x [PubMed] [Google Scholar]
- 81. Bernardes-Silva M, Anthony DC, Issekutz AC, Perry VH.. Recruitment of neutrophils across the blood-brain barrier: The role of E- and P-selectins. J Cereb Blood Flow Metab 2001;21:1115–24 [DOI] [PubMed] [Google Scholar]
- 82. Coisne C, Faveeuw C, Delplace Y, et al. Differential expression of selectins by mouse brain capillary endothelial cells in vitro in response to distinct inflammatory stimuli. Neurosci Lett 2005;392:216–20 [DOI] [PubMed] [Google Scholar]
- 83. Easton AS, Dorovini-Zis K.. The kinetics, function, and regulation of P-selectin expressed by human brain microvessel endothelial cells in primary culture. Microvasc Res 2001;62:335–45http://dx.doi.org/10.1006/mvre.2001.2350 [DOI] [PubMed] [Google Scholar]
- 84. Arjmandi A, Liu K, Dorovini-Zis K.. Dendritic cell adhesion to cerebral endothelium: Role of endothelial cell adhesion molecules and their ligands. J Neuropathol Exp Neurol 2009;68:300–13http://dx.doi.org/10.1097/NEN.0b013e31819a8dd1 [DOI] [PubMed] [Google Scholar]
- 85. Wethmar K, Helmus Y, Lühn K, et al. Migration of immature mouse DC across resting endothelium is mediated by ICAM-2 but independent of beta2-integrins and murine DC-SIGN homologues. Eur J Immunol 2006;36:2781–94 [DOI] [PubMed] [Google Scholar]
- 86. Döring A, Wild M, Vestweber D, et al. E- and P-selectin are not required for the development of experimental autoimmune encephalomyelitis in C57BL/6 and SJL mice. J Immunol 2007;179:8470–9 [DOI] [PubMed] [Google Scholar]
- 87. Sathiyanadan K, Coisne C, Enzmann G, et al. PSGL-1 and E/P-selectins are essential for T-cell rolling in inflamed CNS microvessels but dispensable for initiation of EAE. Eur J Immunol 2014;1–8 [DOI] [PubMed] [Google Scholar]
- 88. Kerfoot SM, Kubes P.. Overlapping roles of P-selectin and alpha 4 integrin to recruit leukocytes to the central nervous system in experimental autoimmune encephalomyelitis. J Immunol 2002;169:1000–6http://dx.doi.org/10.4049/jimmunol.169.2.1000 [DOI] [PubMed] [Google Scholar]
- 89. Jain P, Coisne C, Enzmann G, et al. Alpha4beta1 integrin mediates the recruitment of immature dendritic cells across the blood-brain barrier during experimental autoimmune encephalomyelitis. J Immunol 2010;184:7196–206http://dx.doi.org/10.4049/jimmunol.0901404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. McManus C, Berman JW, Brett FM, et al. MCP-1, MCP-2 and MCP-3 expression in multiple sclerosis lesions: An immunohistochemical and in situ hybridization study. J Neuroimmunol 1998;86:20–9http://dx.doi.org/10.1016/S0165-5728(98)00002-2 [DOI] [PubMed] [Google Scholar]
- 91. Balashov KE, Rottman JB, Weiner HL, Hancock WW.. CCR5+ and CXCR3+ T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci U S A 1999;96:6873–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Boven LA, Montagne L, Nottet HSLM, De Groot CJA.. Macrophage inflammatory protein-1alpha (MIP-1alpha), MIP-1beta, and RANTES mRNA semiquantification and protein expression in active demyelinating multiple sclerosis (MS) lesions. Clin Exp Immunol 2000;122:257–63http://dx.doi.org/10.1046/j.1365-2249.2000.01334.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Simpson J, Newcombe J, Cuzner M, Woodroofe M.. Expression of monocyte chemoattractant protein-1 and other β-chemokines by resident glia and inflammatory cells in multiple sclerosis lesions. J Neuroimmunol 1998;84:238–49 [DOI] [PubMed] [Google Scholar]
- 94. Hvas J, Mclean C, Justesen J, et al. Perivascular T cells express the pro-inflammatory chemokine RANTES mRNA in multiple sclerosis lesions. Scand J Immunol 1997;46:195–203http://dx.doi.org/10.1046/j.1365-3083.1997.d01-100.x [DOI] [PubMed] [Google Scholar]
- 95. Sørensen TL, Tani M, Jensen J, et al. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J Clin Invest 1999;103:807–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Krumbholz M, Theil D, Steinmeyer F, et al. CCL19 is constitutively expressed in the CNS, up-regulated in neuroinflammation, active and also inactive multiple sclerosis lesions. J Neuroimmunol 2007;190:72–9http://dx.doi.org/10.1016/j.jneuroim.2007.07.024 [DOI] [PubMed] [Google Scholar]
- 97. Pashenkov M, Teleshova N, Kouwenhoven M, et al. Elevated expression of CCR5 by myeloid (CD11c+) blood dendritic cells in multiple sclerosis and acute optic neuritis. Clin Exp Immunol 2002;127:519–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Aung LL, Fitzgerald-Bocarsly P, Dhib-Jalbut S, Balashov K.. Plasmacytoid dendritic cells in multiple sclerosis: Chemokine and chemokine receptor modulation by interferon-beta. J Neuroimmunol 2010;226:158–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Thewissen K, Nuyts AH, Deckx N, et al. Circulating dendritic cells of multiple sclerosis patients are proinflammatory and their frequency is correlated with MS-associated genetic risk factors. Mult Scler 2014;20:548–57http://dx.doi.org/10.1177/1352458513505352 [DOI] [PubMed] [Google Scholar]
- 100. Nuyts AH, Ponsaerts P, Van Tendeloo VFI, et al. Except for CCR7 expression, monocyte-derived dendritic cells from patients with multiple sclerosis are functionally comparable to those of healthy controls. Cytotherapy 2014;16:1024–30http://dx.doi.org/10.1016/j.jcyt.2014.02.016 [DOI] [PubMed] [Google Scholar]
- 101. Bartosik-Psujek H, Tabarkiewicz J, Pocinska K, et al. Immunomodulatory effects of vitamin D on monocyte-derived dendritic cells in multiple sclerosis. Mult. Scler 2010;16:1513–6 [DOI] [PubMed] [Google Scholar]
- 102. Kouwenhoven M, Ozenci V, Tjernlund A, et al. Monocyte-derived dendritic cells express and secrete matrix-degrading metalloproteinases and their inhibitors and are imbalanced in multiple sclerosis. J Neuroimmunol 2002;126:161–71http://dx.doi.org/10.1016/S0165-5728(02)00054-1 [DOI] [PubMed] [Google Scholar]
- 103. Izikson L, Klein RS, Charo IF, et al. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J Exp Med 2000;192:1075–80http://dx.doi.org/10.1084/jem.192.7.1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Huang DR, Wang J, Kivisakk P, et al. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med 2001;193:713–26http://dx.doi.org/10.1084/jem.193.6.713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Fife BT, Huffnagle GB, Kuziel WA, Karpus WJ.. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J Exp Med 2000;192:899–905http://dx.doi.org/10.1084/jem.192.6.899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Dogan R-NE, Elhofy A, Karpus WJ.. Production of CCL2 by central nervous system cells regulates development of murine experimental autoimmune encephalomyelitis through the recruitment of TNF- and iNOS-expressing macrophages and myeloid dendritic cells. J Immunol 2008;180:7376–84http://dx.doi.org/10.4049/jimmunol.180.11.7376 [DOI] [PubMed] [Google Scholar]
- 107. Clarkson B, Rayasam A, Walker A, et al. CCR2-dependent dendritic cell recruitment to CNS tissues during EAE promotes disease progression and inflammatory cytokine production by CNS T cells (CCR1P.243). J Immunol 2014;192(1 Suppl): 48 [Google Scholar]
- 108. Zozulya AL, Reinke E, Baiu DC, et al. Dendritic cell transmigration through brain microvessel endothelium is regulated by MIP-1 α chemokine and matrix metalloproteinases. J Immunol 2007;178:520–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kivisäkk P, Mahad DJ, Callahan MK, et al. Human cerebrospinal fluid central memory CD4+ T cells: Evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci U S A 2003;100:8389–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Strazielle N, Ghersi-Egea J-F.. Choroid plexus in the central nervous system: Biology and physiopathology. J Neuropathol Exp Neurol 2000;59:561–74http://dx.doi.org/10.1093/jnen/59.7.561 [DOI] [PubMed] [Google Scholar]
- 111. Matyszak MK, Lawson LJ, Perry VH, Gordon S.. Stromal macrophages of the choroid plexus situated at an interface between the brain and peripheral immune system constitutively express major histocompatibility class II antigens. J Neuroimmunol 1992;40:173–81http://dx.doi.org/10.1016/0165-5728(92)90131-4 [DOI] [PubMed] [Google Scholar]
- 112. Matyszak MK, Perry VH.. The potential role of dendritic cells in immune-mediated inflammatory diseases in the central nervous system. Neuroscience 1996;74:599–608http://dx.doi.org/10.1016/0306-4522(96)00160-1 [DOI] [PubMed] [Google Scholar]
- 113. Laperchia C, Allegra Mascaro AL, Sacconi L, et al. Two-photon microscopy imaging of thy1GFP-M transgenic mice: A novel animal model to investigate brain dendritic cell subsets in vivo. PLoS One 2013;8:e56144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. McMenamin PG. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J Comp Neurol 1999;405:553–62http://dx.doi.org/10.1002/(SICI)1096-9861(19990322)405:4<553::AID-CNE8>3.0.CO;2-6 [PubMed] [Google Scholar]
- 115. McMenamin PG, Wealthall RJ, Deverall M, et al. Macrophages and dendritic cells in the rat meninges and choroid plexus: Three-dimensional localisation by environmental scanning electron microscopy and confocal microscopy. Cell Tissue Res 2003;313:259–69http://dx.doi.org/10.1007/s00441-003-0779-0 [DOI] [PubMed] [Google Scholar]
- 116. Serot J-M, Béné M-C, Foliguet B, Faure GC.. Monocyte-derived IL-10-secreting dendritic cells in choroid plexus epithelium. J Neuroimmunol 2000;105:115–9 [DOI] [PubMed] [Google Scholar]
- 117. Quintana E, Fernández A, Velasco P, et al. DNGR-1+ dendritic cells are located in meningeal membrane and choroid plexus of the noninjured brain. Glia 2015;63:2231–48 [DOI] [PubMed] [Google Scholar]
- 118. Wolburg H, Wolburg-Buchholz K, Liebner S, Engelhardt B.. Claudin-1, claudin-2 and claudin-11 are present in tight junctions of choroid plexus epithelium of the mouse. Neurosci Lett 2001;307:77–80 [DOI] [PubMed] [Google Scholar]
- 119. Lippoldt A, Liebner S, Andbjer B, et al. Organization of choroid plexus epithelial and endothelial cell tight junctions and regulation of claudin-1, -2 and -5 expression by protein kinase C. Neuroreport 2000;11:1427–31http://dx.doi.org/10.1097/00001756-200005150-00015 [DOI] [PubMed] [Google Scholar]
- 120. Kooij G, Kopplin K, Blasig R, et al. Disturbed function of the blood–cerebrospinal fluid barrier aggravates neuro-inflammation. Acta Neuropathol 2013;128:267–77 [DOI] [PubMed] [Google Scholar]
- 121. Steffen BJ, Breier G, Butcher EC, et al. ICAM-1, VCAM-1, and MAdCAM-1 are expressed on choroid plexus epithelium but not endothelium and mediate binding of lymphocytes in vitro. Am J Pathol 1996;148:1819–38 [PMC free article] [PubMed] [Google Scholar]
- 122. Pashenkov M, Teleshova N, Kouwenhoven M, et al. Recruitment of dendritic cells to the cerebrospinal fluid in bacterial neuroinfections. J Neuroimmunol 2002;122:106–16http://dx.doi.org/10.1016/S0165-5728(01)00451-9 [DOI] [PubMed] [Google Scholar]
- 123. Semple BD, Bye N, Rancan M, et al. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): Evidence from severe TBI patients and CCL2–/– mice. J Cereb Blood Flow Metab 2010;30:769–82http://dx.doi.org/10.1038/jcbfm.2009.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Meeker RB, Poulton W, Markovic-Plese S, et al. Protein changes in CSF of HIV-infected patients: Evidence for loss of neuroprotection. J Neurovirol 2011;17:258–73http://dx.doi.org/10.1007/s13365-011-0034-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. De Graaf MT, Smitt PA, Luitwieler RL, et al. Central memory CD4+ T cells dominate the normal cerebrospinal fluid. Cytometry B Clin Cytom 2011;80B:43–50 [DOI] [PubMed] [Google Scholar]
- 126. Clarkson BD, Héninger E, Harris MG, et al. Innate-adaptive crosstalk: How dendritic cells shape immune responses in the CNS. Adv Exp Med Biol 2012;946:309–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kivisäkk P, Mahad DJ, Callahan MK, et al. Expression of CCR7 in multiple sclerosis: Implications for CNS immunity. Ann Neurol 2004;55:627–38 [DOI] [PubMed] [Google Scholar]
- 128. Pashenkov M, Söderström M, Link H.. Secondary lymphoid organ chemokines are elevated in the cerebrospinal fluid during central nervous system inflammation. J Neuroimmunol 2003;135:154–60 [DOI] [PubMed] [Google Scholar]
- 129. Hatterer E, Touret M, Belin MF, et al. Cerebrospinal fluid dendritic cells infiltrate the brain parenchyma and target the cervical lymph nodes under neuroinflammatory conditions. PLoS One 2008;3:e3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Vass K, Lassmann H, Wekerle H, Wisniewski HM.. The distribution of Ia antigen in the lesions of rat acute experimental allergic encephalomyelitis. Acta Neuropathol 1986;70:149–60http://dx.doi.org/10.1007/BF00691433 [DOI] [PubMed] [Google Scholar]
- 131. Allt G, Lawrenson JG.. Is the pial microvessel a good model for blood-brain barrier studies? Brain Res Brain Res Rev 1997;24:67–76 [DOI] [PubMed] [Google Scholar]
- 132. Barkauskas DS, Dixon Dorand R, Myers JT, et al. Focal transient CNS vessel leak provides a tissue niche for sequential immune cell accumulation during the asymptomatic phase of EAE induction. Exp Neurol 2015;266:74–85http://dx.doi.org/10.1016/j.expneurol.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kivisäkk P, Imitola J, Rasmussen S, et al. Localizing central nervous system immune surveillance: Meningeal antigen-presenting cells activate T cells during experimental autoimmune encephalomyelitis. Ann Neurol 2009;65:457–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ling C, Sandor M, Fabry Z.. In situ processing and distribution of intracerebrally injected OVA in the CNS. J Neuroimmunol 2003;141:90–8http://dx.doi.org/10.1016/S0165-5728(03)00249-2 [DOI] [PubMed] [Google Scholar]
- 135. Cserr HF, Harling-Berg CJ, Knopf PM.. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol 1992;2:269–76http://dx.doi.org/10.1111/j.1750-3639.1992.tb00703.x [DOI] [PubMed] [Google Scholar]
- 136. Hatterer E, Davoust N, Didier-Bazes M, et al. How to drain without lymphatics? Dendritic cells migrate from the cerebrospinal fluid to the B-cell follicles of cervical lymph nodes. Blood 2006;107:806–12http://dx.doi.org/10.1182/blood-2005-01-0154 [DOI] [PubMed] [Google Scholar]
- 137. Karman J, Ling C, Sandor M, Fabry Z.. Initiation of immune responses in brain is promoted by local dendritic cells. J Immunol 2004;173:2353–61http://dx.doi.org/10.4049/jimmunol.173.4.2353 [DOI] [PubMed] [Google Scholar]
- 138. Hochmeister S, Zeitelhofer M, Bauer J, et al. After injection into the striatum, in vitro-differentiated microglia- and bone marrow-derived dendritic cells can leave the central nervous system via the blood stream. Am J Pathol 2008;173:1669–81http://dx.doi.org/10.2353/ajpath.2008.080234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015;523:337–41http://dx.doi.org/10.1038/nature14432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Aspelund A, Antila S, Proulx ST, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 2015;212:991–9http://dx.doi.org/10.1084/jem.20142290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Clarkson B, Rayasam A, Walker A, et al. Migration of i.c. injected BMDCs from brain to deep cervical lymph nodes is CCR7 dependent but does not promote EAE disease progression. J. Immunol 2014;192(1 Suppl): 48: [Google Scholar]
- 142. Clarkson BD, Walker A, Harris MG, et al. CCR7 deficient inflammatory dendritic cells are retained in the central nervous system. Sci Rep 2017;7:42856.http://dx.doi.org/10.1038/srep42856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Torkildsen Ø, Myhr K-M, Bø L.. Disease-modifying treatments for multiple sclerosis—a review of approved medications. Eur J Neurol 2016;23:18–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Yong VW. Differential mechanisms of action of interferon- and glatiramer acetate in MS. Neurology 2002;59:802–8http://dx.doi.org/10.1212/WNL.59.6.802 [DOI] [PubMed] [Google Scholar]
- 145. Dhib-Jalbut S, Marks S.. Interferon-beta mechanisms of action in multiple sclerosis. Neurology 2010;74(Suppl 1):S17–24 [DOI] [PubMed] [Google Scholar]
- 146. Kraus J, Oschmann P.. The impact of interferon-β treatment on the blood-brain barrier. Drug Discov Today 2006;11:755–62 [DOI] [PubMed] [Google Scholar]
- 147. Cheng W, Chen G.. Chemokines and chemokine receptors in multiple sclerosis. Mediators Inflamm 2014;2014:659206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Begum-Haque S, Christy M, Wang Y, et al. Glatiramer acetate biases dendritic cells towards an anti-inflammatory phenotype by modulating OPN, IL-17, and RORγt responses and by increasing IL-10 production in experimental allergic encephalomyelitis. J Neuroimmunol 2013;254:117–24 [DOI] [PubMed] [Google Scholar]
- 149. Mellergård J, Edström M, Vrethem M, et al. Natalizumab treatment in multiple sclerosis: Marked decline of chemokines and cytokines in cerebrospinal fluid. Mult Scler 2010;16:208–17 [DOI] [PubMed] [Google Scholar]
- 150. Groves A, Kihara Y, Chun J.. Fingolimod: Direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy. J Neurol Sci 2013;328:9–18http://dx.doi.org/10.1016/j.jns.2013.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Spampinato SF, Obermeier B, Cotleur A, et al. Sphingosine 1 phosphate at the blood brain barrier: Can the modulation of S1P receptor 1 influence the response of endothelial cells and astrocytes to inflammatory stimuli? PLoS One 2015;10:e0133392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Kunze R, Urrutia A, Hoffmann A, et al. Dimethyl fumarate attenuates cerebral edema formation by protecting the blood-brain barrier integrity. Exp Neurol 2015;266:99–111http://dx.doi.org/10.1016/j.expneurol.2015.02.022 [DOI] [PubMed] [Google Scholar]
- 153. Bartholomé EJ, Van Aelst I, Koyen E, et al. Human monocyte-derived dendritic cells produce bioactive gelatinase B: Inhibition by IFN-beta. J. Interferon Cytokine Res 2001;21:495–501 [DOI] [PubMed] [Google Scholar]
- 154. Zang YCQ, Skinner SM, Robinson RR, et al. Regulation of differentiation and functional properties of monocytes and monocyte-derived dendritic cells by interferon beta in multiple sclerosis. Mult Scler 2004;10:499–506http://dx.doi.org/10.1191/1352458504ms1081oa [DOI] [PubMed] [Google Scholar]
- 155. Hussien Y, Sanna a, Söderström M, et al. Glatiramer acetate and IFN-beta act on dendritic cells in multiple sclerosis. J Neuroimmunol 2001;121:102–10 [DOI] [PubMed] [Google Scholar]
- 156. Yen J-H, Kong W, Ganea D.. Interferon beta inhibits dendritic cell migration through STAT-1 mediated transcriptional suppression of CCR7 and metalloproteinase 9. J Immunol 2010;184:3478–86http://dx.doi.org/10.4049/jimmunol.0902542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Høglund RA, Holmøy T, Harbo HF, et al. A one year follow-up study of natural killer and dendritic cells activities in multiple sclerosis patients receiving glatiramer acetate (GA). PLoS One 2013;8:e62237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. del Pilar Martin M, Cravens PD, Winger R, et al. Decrease in the numbers of dendritic cells and CD4+ T cells in cerebral perivascular spaces due to natalizumab. Arch Neurol 2008;65:1596–603 [DOI] [PubMed] [Google Scholar]
- 159. de Andrés C, Teijeiro R, Alonso B, et al. Long-term decrease in VLA-4 expression and functional impairment of dendritic cells during natalizumab therapy in patients with multiple sclerosis. PLoS One 2012;7:e34103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Kivisäkk P, Francois K, Mbianda J, et al. Effect of natalizumab treatment on circulating plasmacytoid dendritic cells: A cross-sectional observational study in patients with multiple sclerosis. PLoS One 2014;9:e103716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Börnsen L, Christensen JR, Ratzer R, et al. Effect of natalizumab on circulating CD4+ T-cells in multiple sclerosis. PLoS One 2012;7:e47578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Idzko M, Panther E, Corinti S, et al. Sphingosine 1-phosphate induces chemotaxis of immature and modulates cytokine-release in mature human dendritic cells for emergence of Th2 immune responses. FASEB J 2002;16:625–7 [DOI] [PubMed] [Google Scholar]
- 163. Müller H, Hofer S, Kaneider N, et al. The immunomodulator FTY720 interferes with effector functions of human monocyte-derived dendritic cells. Eur J Immunol 2005;35:533–45 [DOI] [PubMed] [Google Scholar]
- 164. Muller SL, Portwich M, Schmidt A, et al. The Tight junction protein occludin and the adherens junction protein α-catenin share a common interaction mechanism with ZO-1. J Biol Chem 2004;280:3747–56 [DOI] [PubMed] [Google Scholar]
- 165. Al-Jaderi Z, Maghazachi AA.. Effects of vitamin D3, calcipotriol and FTY720 on the expression of surface molecules and cytolytic activities of human natural killer cells and dendritic cells. Toxins (Basel) 2013;5:1932–47http://dx.doi.org/10.3390/toxins5111932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Lan YY, De Creus A, Colvin BL, et al. The sphingosine-1-phosphate receptor agonist FTY720 modulates dendritic cell trafficking in vivo. Am J Transplant 2005;5:2649–59http://dx.doi.org/10.1111/j.1600-6143.2005.01085.x [DOI] [PubMed] [Google Scholar]
- 167. Hunter SF, Bowen JD, Reder AT.. The direct effects of fingolimod in the central nervous system: Implications for relapsing multiple sclerosis. CNS Drugs 2016;30:135–47http://dx.doi.org/10.1007/s40263-015-0297-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Linker RA, Lee D-H, Ryan S, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011;134:678–92http://dx.doi.org/10.1093/brain/awq386 [DOI] [PubMed] [Google Scholar]
- 169. Linker RA, Gold R.. Dimethyl fumarate for treatment of multiple sclerosis: Mechanism of action, effectiveness, and side effects. Curr Neurol Neurosci Rep 2013;13:394.http://dx.doi.org/10.1007/s11910-013-0394-8 [DOI] [PubMed] [Google Scholar]
- 170. Peng H, Guerau-de-Arellano M, Mehta VB, et al. Dimethyl fumarate inhibits dendritic cell maturation via nuclear factor κB (NF-κB) and extracellular signal-regulated kinase 1 and 2 (ERK1/2) and mitogen stress-activated kinase 1 (MSK1) signaling. J Biol Chem 2012;287:28017–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Hayden MS, West AP, Ghosh S.. NF-κB and the immune response. Oncogene 2006;25:6758–80 [DOI] [PubMed] [Google Scholar]
- 172. Zhu K, Mrowietz U.. Inhibition of dendritic cell differentiation by fumaric acid esters. J Invest Dermatol 2001;116:203–8http://dx.doi.org/10.1046/j.1523-1747.2001.01159.x [DOI] [PubMed] [Google Scholar]
- 173. Ghoreschi K, Brück J, Kellerer C, et al. Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J Exp Med 2011;208:2291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Litjens NHR, Rademaker M, Ravensbergen B, et al. Monomethylfumarate affects polarization of monocyte-derived dendritic cells resulting in down-regulated Th1 lymphocyte responses. Eur J Immunol 2004;34:565–75http://dx.doi.org/10.1002/eji.200324174 [DOI] [PubMed] [Google Scholar]
- 175. Kawakami N. In vivo imaging in autoimmune diseases in the central nervous system. Allergol Int 2016;65:235–42http://dx.doi.org/10.1016/j.alit.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 176. Kitano M, Yamazaki C, Takumi A, et al. Imaging of the cross-presenting dendritic cell subsets in the skin-draining lymph node. Proc Natl Acad Sci U S A 2016;113:1044–9http://dx.doi.org/10.1073/pnas.1513607113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Alt C, Laschinger M, Engelhardt B.. Functional expression of the lymphoid chemokines CCL19 (ECL) and CCL 21 (SLC) at the blood-brain barrier suggests their involvement in G-protein-dependent lymphocyte recruitment into the central nervous system during experimental autoimmune encephalomyeli. Eur J Immunol 2002;32:2133–44http://dx.doi.org/10.1002/1521-4141(200208)32:8<2133::AID-IMMU2133>3.0.CO;2-W [DOI] [PubMed] [Google Scholar]
- 178. Pease J, Horuk R.. Chemokine receptor antagonists. J Med Chem 2012;55:9363–92http://dx.doi.org/10.1021/jm300682j [DOI] [PubMed] [Google Scholar]
- 179. Horuk R. Chemokine receptor antagonists: Overcoming developmental hurdles. Nat Rev Drug Discov 2009;8:23–33http://dx.doi.org/10.1038/nrd2734 [DOI] [PubMed] [Google Scholar]
- 180. Gladue RP, Brown MF, Zwillich SH.. CCR1 antagonists: What have we learned from clinical trials. Curr Top Med Chem 2010;10:1268–77http://dx.doi.org/10.2174/156802610791561237 [DOI] [PubMed] [Google Scholar]
- 181.EudraCT Number 2004-002567-24—Clinical trial results—EU Clinical Trials Register. Available at: https://www.clinicaltrialsregister.eu/ctr-search/trial/2004-002567-24/results. Accessed September 20, 2017
- 182. Mantovani A. The chemokine system: Redundancy for robust outputs. Immunol Today 1999;20:254–7http://dx.doi.org/10.1016/S0167-5699(99)01469-3 [DOI] [PubMed] [Google Scholar]
- 183. Fox DJ, Reckless J, Lingard H, et al. Highly potent, orally available anti-inflammatory broad-spectrum chemokine inhibitors. J Med Chem 2009;52:3591–5http://dx.doi.org/10.1021/jm900133w [DOI] [PubMed] [Google Scholar]
- 184. Levin M, Udi Y, Solomonov I, et al. Next generation matrix metalloproteinase inhibitors—Novel strategies bring new prospects. Biochim Biophys Acta 2017;1864:1927–39 [DOI] [PubMed] [Google Scholar]